Abstract
Current cancer diagnostic methods are challenged by low sensitivity, high false positive rate, limited tumor information, uncomfortable or invasive procedures, and high cost. Liquid biopsy that analyzes circulating biomarkers in body fluids represents a promising solution to these challenges. Exosomes are one of the promising cancer biomarkers for liquid biopsy because they are cell-secreted, nano-sized, extracellular vesicles that stably exist in all types of body fluids. Exosomes transfer DNAs, RNAs, proteins, and lipids from parent cells to recipient cells for intercellular communication and play important roles in cancer initiation, progression, and metastasis. Many liquid biopsy biosensors have been developed to offer non- or minimally-invasive, highly sensitive, simple, rapid, and cost-effective cancer diagnostics. This review summarized recent advances of liquid biopsy biosensors with a focus on the detection of exosomal proteins as biomarkers for cancer screening, diagnosis, and prognosis. We reviewed six major types of liquid biopsy biosensors including immunofluorescence biosensor, colorimetric biosensor, surface plasmon resonance (SPR) biosensor, surface-enhanced Raman scattering (SERS) biosensor, electrochemical biosensor, and nuclear magnetic resonance (NMR) biosensor. We shared our perspectives on future improvement of exosome-based liquid biopsy biosensors to accelerate their clinical translation.
Keywords: biosensor, cancer diagnosis, exosomes, liquid biopsy
INTRODUCTION
Cancer is a heavy socioeconomic burden. Worldwide cancer cases will increase by 50% from 14 million new cases in 2012 to 22 million new cases in 2030, and worldwide cancer deaths will increase by 60% from 8 million cancer deaths in 2012 to 13 million cancer deaths in 2030 (https://www.cancer.gov/about-cancer/understanding/statistics). Therefore, early detection of cancer is of particular importance in increasing the chance of successful treatment and reducing the mortality. For example, the 5-year survival rate for lung cancer plummets from 55% for early stage patients to 4% for late stage patients (1). However, current cancer early detection and screening procedures such as low-dose computed tomography (CT) in lung cancer, mammography and MRI in breast cancer, and colonoscopy in colorectal cancer are compromised by high false positive rate, low sensitivity, uncomfortable procedure, and high cost (2–8). Liquid biopsy that detects circulating biomarkers (such as circulating tumor cells, exosomes, circulating tumor DNA) in body fluids represents a promising strategy to address these challenges (9–13). Liquid biopsy is a simple, patient-friendly, non- or minimally invasive test with high detection sensitivity and specificity. In contrast to tissue biopsy that only provides a snapshot of tumor, liquid biopsy affords more comprehensive information of tumor at various time points, and therefore it is particularly useful in cancer screening, diagnosis, precision medicine, treatment response evaluation, and prognosis.
Exosomes are small (30–150 nm) extracellular vesicles secreted by cells. The generation of exosomes start with the inward budding of cell membrane to form intraluminal vesicles (ILVs) in early endosomes, which then evolve into multivesicular bodies (MVBs). MVBs fuse with cell membrane and release the ILVs as exosomes. Exosomes are present in many types of body fluids, such as blood, urine, breast milk, and saliva. Exosomes carry DNAs, RNAs, proteins, and lipids as cargos and they transfer these biomolecules from parent cells to recipient cells for cell-cell communication. Exosomes are actively involved in cancer development, metastasis, and the regulation of immune responses. More information about exosome biology and functions can be found in recent review articles (14–20).
Exosomal proteins have emerged as promising biomarkers for cancer diagnosis (21). For example, glypican-1 enriched in cancer cell-derived exosomes detects early stages of pancreatic cancer with 100% sensitivity and specificity (22). In lung cancer, exosomal proteins, such as EGFR, LRP1, and LG3BP, in patient sera and urine have been correlated with lung cancer stage and metastasis (23–27). The expression of exosomal proteins is typically measured by established techniques including enzyme-linked immunosorbent assay (ELISA), western blotting, immunobeads-based flow cytometry, and mass spectrometry (SELDI-MS or MALDI-MS). However, these methods are tedious, expensive, and time-consuming and thus are not readily adapted to clinical uses, especially for “point-of-care” tests. Liquid biopsy biosensors have been introduced as alternative technologies to overcome these disadvantages. These biosensors are miniaturized and portable sensing platforms. They consume small volume of samples and provide rapid, sensitive, high throughput, and cost-effective analysis of exosomal proteins. Many biosensors have demonstrated their great promise as cancer diagnostic tests. This review summarized recent advances of liquid biopsy biosensors with a special focus on the detection and characterization of exosomal proteins for cancer screening, diagnosis, and prognosis. Based on the detection methods, we divided the biosensors into six categories: immunofluorescence-based biosensors, colorimetric-based biosensors, surface plasmon resonance (SPR)-based biosensors, surface-enhanced Raman scattering (SERS)-based biosensors, electrochemical-based biosensors, and nuclear magnetic resonance (NMR)-based biosensors. Different from current reviews on similar topics (12,13), we focused more on the pre-clinical evaluation of the diagnostic performance of each biosensor, especially the sensitivity and specificity of exosomal protein biomarkers used by these biosensors in cancer detection. Table I summarized the sensing performance of these biosensors. Other emerging biosensors developed for exosome isolation and purification are not within the scope of this review.
Table I.
Summary of Biosensors and Their Sensing Performance
| Detection method | Biosensors | LOD | Sample (volume) | Cancer type | Biomarkers | Time | Reference |
|---|---|---|---|---|---|---|---|
| Fluorescence | Microfluidic biochip | 0.28–0.38 pg/mL | Plasma (30 μL) | Ovarian, lung | EpCAM, α-IGF-1R, CA125, CD9, CD81, and CD63 | ~ 90 min | He et al. Ref. (28) |
| ExoSearch | 750 exosomes/μL | Plasma (10 μL–10 mL) | Ovarian | CA-125, EpCAM, and CD24 | ~ 40 min | Zhao et al. Ref. (29) | |
| ExoScreen | NA | Serum (5 μL) | Colorectal | CD9, CD63, CD147, CEA, CA19–9 | ~120 min | Yoshioka et al. Ref. (30) | |
| EV array | 2.5 × 104 exosomes per sensing spot | Plasma (10 μL) | Healthy controls | Up to 60 biomarkers | ~ 3 days | Jorgensen et al. Ref. (31–33) | |
| Nano-IMEX | ~ 50 exosomes/μL | Plasma (2 μL) | Ovarian | CD9, CD63, CD81, EpCAM | NA | Zhang et al. Ref. (34) | |
| Colorimetric | Double-filtration microfluidic biochip | NA | Urine (8 mL) | Bladder | CD63 | NA | Liang et al. Ref. (35) |
| Microfluidic biochip with AC-EHD-induced nanoshearing | ~ 2760 exosomes/μL | Serum (500 μL) | Breast, prostate | HER2, CD9, PSA | NA | Vaidyanathan et al. Ref. (36) | |
| s-SWCNT aptasensor | 5.2 × 105 exosomes/μL | Cell-derived exosomes | Breast | CD63 | NA | Xia et al. Ref. (37) | |
| SPR | Microfluidic biochip | 2070 exosomes/μL | Serum | Breast | HER2 | NA | Sina et al. Ref. (38) |
| SPRi | ~ 4.87 × 107 exosomes/cm2 | Cell-derived exosomes | Liver, melanoma | CD9, CD41, CD63, CD82, EpCAM, and E-cadherin | NA | Zhu et al. Ref. (39) | |
| nPLEX | ~ 3000 exosomes | Ascites | Ovarian | Up to 12 biomarkers. CD24, CD41, CD45, CD63, CA125, CA19–9, D2–40, EpCAM, EGFR, HER2, CLDN3, and MUC18 | ~ 30 min | Im et al. Ref. (40) | |
| SERS | Magnetic nanobeads and SERS nanoprobe | ~ 1200 exosomes | Cell-derived exosomes | Breast | CD63 and HER2 | ~ 120 min | Zong et al. Ref. (41) |
| Electrochemical | EFIRM | NA | Serum saliva (10 μL) | Lung | CD63 | NA | Wei et al. Ref. (42) |
| iMEX | 3 × 104 exosomes | Plasma (10 μL/marker) | Ovarian | Up to 8 biomarkers. EpCAM, EGFR, CA-125, HER2, MUC18, CD24, CD63, CD9, and CD81 | ~ 60 min | Jeong et al. Ref. (43) | |
| Sandwich immunosensor | 200 exosomes/μL | Cell-derived exosomes (1.5 μL) | Breast | CD9 | NA | Doldan et al. Ref. (44) | |
| Aptasensor | 103 exosomes/μL | Cell-derived exosomes | Liver | CD63 | NA | Zhou et al. Ref. (45) | |
| μNMR | Microfluidic biochip combined microfiltration with NMR | ~ 104 exosomes | Plasma | Brain | CD63, EGFR, EGFRvIII, PDPN, IDH1, and R132H | NA | Shao et al. Ref. (46) |
IMMUNOFLUORESCENCE-BASED EXOSOMAL PROTEIN DETECTION
Immunofluorescence technique has been widely used for exosomal protein detection because of its capability of multiplexed sensing and simplicity to be integrated with microfluidic biochips. Generally, there are two strategies for immunofluorescence-based exosomal protein detection. One strategy is to use antibody-conjugated magnetic beads to capture exosomes; therefore, the exosomes can be easily retained and released in the microfluidic channels by applying and removing the magnet. Then, the expression of protein markers of captured exosomes is measured via immunofluorescence approaches. He et al. developed a microfluidic biochip that was able to directly isolate and enrich exosomes from plasma samples and complete the in situ measurement of both surface and intra-vesicular proteins of target exosomes (28). As shown in Fig. 1, a cascading microchannel circuit was designed to realize exosome immunomagnetic isolation (first stage capture), on-chip exosome lysis to release proteins, protein immunoprecipitation (second stage capture), and characterization via chemifluorescence-assisted immunoassays. This microfluidic biochip analyzed the levels of exosome surface proteins (EpCAM, α-IGF-1R, CA125, CD9, CD81, and CD63) in plasma samples from two lung cancer patients, two ovarian cancer patients, and three healthy controls. The levels of all exosome surface proteins except CD63 were significantly higher in plasma samples from cancer patients than healthy controls. The expression of exosomal CD63 was high in ovarian cancer but low in lung cancer, suggesting that exosomal CD63 might be a biomarker to discriminate between cancer types. The microfluidic biochip also measured the expression of surface protein IGF-1R and intra-vesicular protein phosphorylated IGF-1R (p-IGF-1R) in EpCAM+ exosomes of lung cancer patients and healthy controls. An evident overexpression of IGF-1R was observed in EpCAM+ exosomes from lung cancer patients when compared with healthy controls. However, the expression of EpCAM+ exosomal p-IGF-1R was not able to distinguish lung cancer patients from healthy controls, which was confirmed by ELISA analysis. Besides, the limit of detection (LOD) of this biochip were 0.281 and 0.383 pg/mL for IGF-1R and p-IGF-1R protein standards, respectively, which were about 100-fold lower than commercial ELISA kits. Overall, this microfluidic biochip was a highly sensitive and one-stop analytical platform that enabled selective isolation of a subpopulation of exosomes from as low as 30-μL plasma samples and quantitative analysis of exosome surface and intra-vesicular proteins in ~ 1.5 h.
Fig. 1.
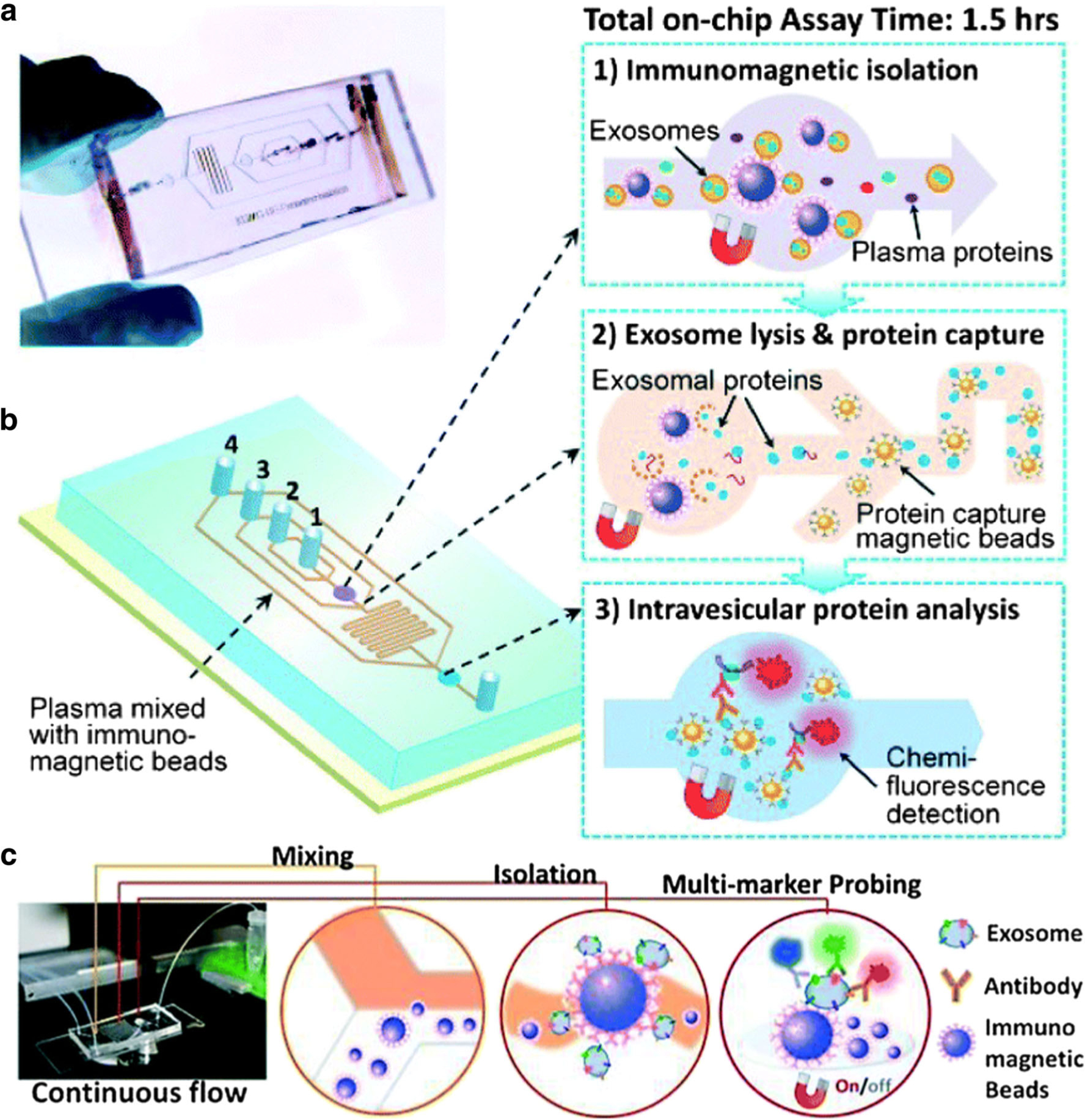
The photo (a) and the schematic diagram (b) of the microfluidic biochip for exosome immunomagnetic isolation (1), on-chip exosome lysis, and protein immunoprecipitation (2) and intra-vesicular protein detection via chemifluorescence-assisted immunoassays (3). c The setup and detection mechanism of ExoSearch biochip. Antibody-conjugated magnetic beads were used to capture exosomes from plasma samples. Multiplexed exosomal protein detection was realized by multi-color fluorescence imaging. Figures are adapted from Refs. (28) and (29) with permission from the Royal Society of Chemistry.
To increase the throughput and realize multiplexed biomarker detection, the He team improved the design of microfluidic circuits and developed the ExoSearch biochip (Fig. 1c) (29). Instead of off-chip premixing of plasma samples with antibody-conjugated magnetic beads to capture exosomes, the ExoSearch biochip realized on-chip continuous-flow mixing, which offered the capability to process up to 10-mL plasma samples. A cocktail of antibodies labeled with different fluorescent dyes were used to afford multiplexed exosome surface protein detection via multi-color fluorescence imaging without additional exosome lysis process. The expression levels of CA-125, EpCAM, and CD24 on exosomes in plasma samples from ovarian cancer patients (n = 15) were compared with healthy controls (n = 5). The distinguishing performances of these exosomal protein biomarkers were evaluated by receiver operating characteristic (ROC) analysis and the area under the curve (AUC) was around 1.0. When EpCAM was used as the biomarker, the ExoSearch biochip achieved LOD of 750 exosomes/μL, which was 1000-fold more sensitive than ELISA. The promising results from the preliminary study demonstrated that ExoSearch biochip may be a useful tool in analyzing exosomal proteins for cancer diagnosis.
In addition to micro-sized magnetic beads (2.8 μm) used by the He team (28,29), Fang et al. developed a microfluidic biochip that used nano-sized magnetic beads (400 nm) to accomplish the immunocapture of exosomes via anti-CD63 antibody and the detection of exosomal EpCAM and HER2 by immunofluorescence staining (47). Significantly higher levels of exosomal EpCAM was detected in plasma samples from breast cancer patents (n = 6) compared to normal controls (n = 3). Exosomal HER2 levels also agreed well with the tissue HER2 expression, i.e., exosomal HER2 levels increased accordingly from HER2- negative patients (n = 5) to HER2 moderate expression patients (n = 11) to HER2 strong expression patients (n = 3). These results suggested that the microfluidic biochip may be used for breast cancer diagnosis and molecular classification.
Besides magnetic beads, Yoshioka et al. developed an ExoScreen assay that used AlphaScreen™ photosensitizer beads to detect exosomal proteins for colorectal cancer detection (Fig. 2) (30). In ExoScreen assay, the biotinylated antibody and the acceptor beads conjugated with a second antibody were directly mixed with the serum samples without any purification step to capture exosomes that carry both proteins on the surface. Then, the streptavidin-coated donor beads were added to interact with biotin and establish the donor bead-exosome-acceptor bead complexes. The expression of exosomal proteins were measured by detecting the fluorescence signals from the acceptor beads. The ExoScreen detected significantly higher levels of CD147/CD9 double-positive exosomes in sera from colorectal cancer patients (n = 194) than healthy controls (n = 191). The levels of CD147/CD9 double-positive exosomes were reduced in patient sera after surgical removal of the tumor. The diagnostic performance of CD147/CD9 double-positive exosomes was much better than CEA and CA19–9, which are the most commonly used biomarkers for colorectal cancer diagnosis. The ExoScreen assay operates in 96- and 384-well plates, only requires 5 μL serum samples, can be completed in 2 h, and shows higher detection sensitivity than ELISA. This mix-and-read, high throughput assay is a simple and fast method to analyze exosomal proteins for cancer diagnosis and surveillance.
Fig. 2.
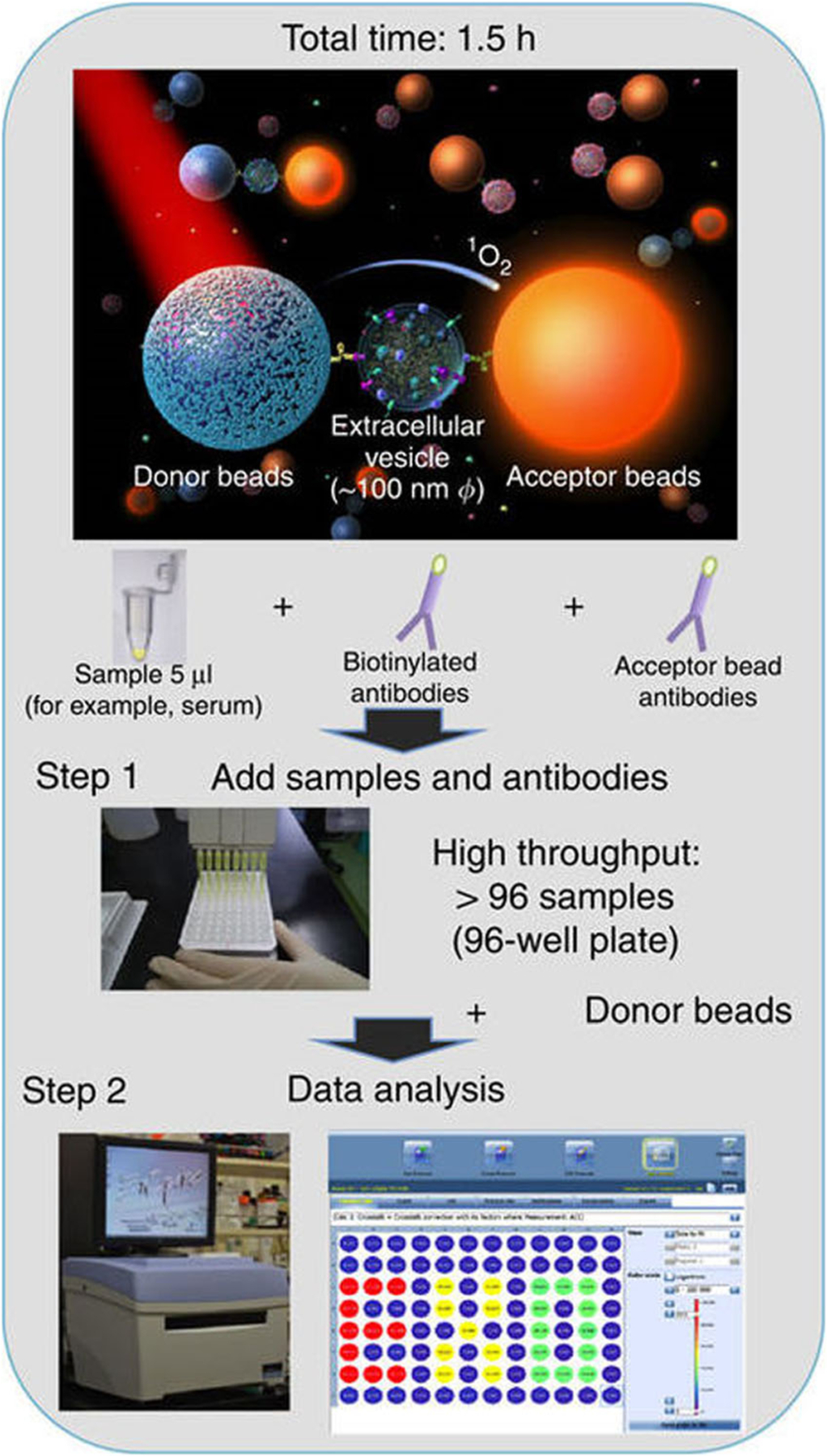
Sensing mechanism and workflow of ExoScreen assay that used AlphaScreen™ photosensitizer beads to detect exosomal proteins. Exosomal proteins connected donor beads and acceptor beads and thus induced fluorescent signals from acceptor beads. Figures are adapted from Ref. (30) with permission from the Nature Publishing Group.
The other strategy relies on the immobilization of antibodies on the surface of microfluidic biochips. After the immunoisolation of exosomes, immunofluorescence technique is used to characterize the expression of exosomal proteins. With this strategy, a panel of antibodies can be printed on the biochip surface to achieve multiplexed, automated, and rapid detection of exosomal proteins. Jorgensen et al. developed an extracellular vesicle (EV) microarray which was capable of detecting up to 60 exosomal proteins from 10 μL plasma (Fig. 3) (31–33). A panel of total 60 captured antibodies recognizing generic exosome markers (such as CD9, CD63) and tumor-associated markers (such as EpCAM, HER2, p53) were printed on epoxy-coated slides. Ten-microliter plasma samples were applied on the slides to capture exosomes based on their surface proteins. A mixture of biotinylated anti-CD9, CD63, and CD81 antibodies were then applied to exclude other extracellular vesicles and to ensure that only exosomes were detected on the EV array. Using exosomes derived from LS180 colon cancer cells, the LOD of EV array was 2.5 × 104 exosomes for each detection spot. The EV array detected similar levels of exosomal CD9 and CD81 in the plasma from healthy donors (n = 7); however, the expression of other exosomal proteins showed quite large donor-to-donor variations, suggesting that more investigations with healthy controls are required to identify the normal ranges of exosomal proteins before they are used as biomarkers for cancer diagnosis.
Fig. 3.
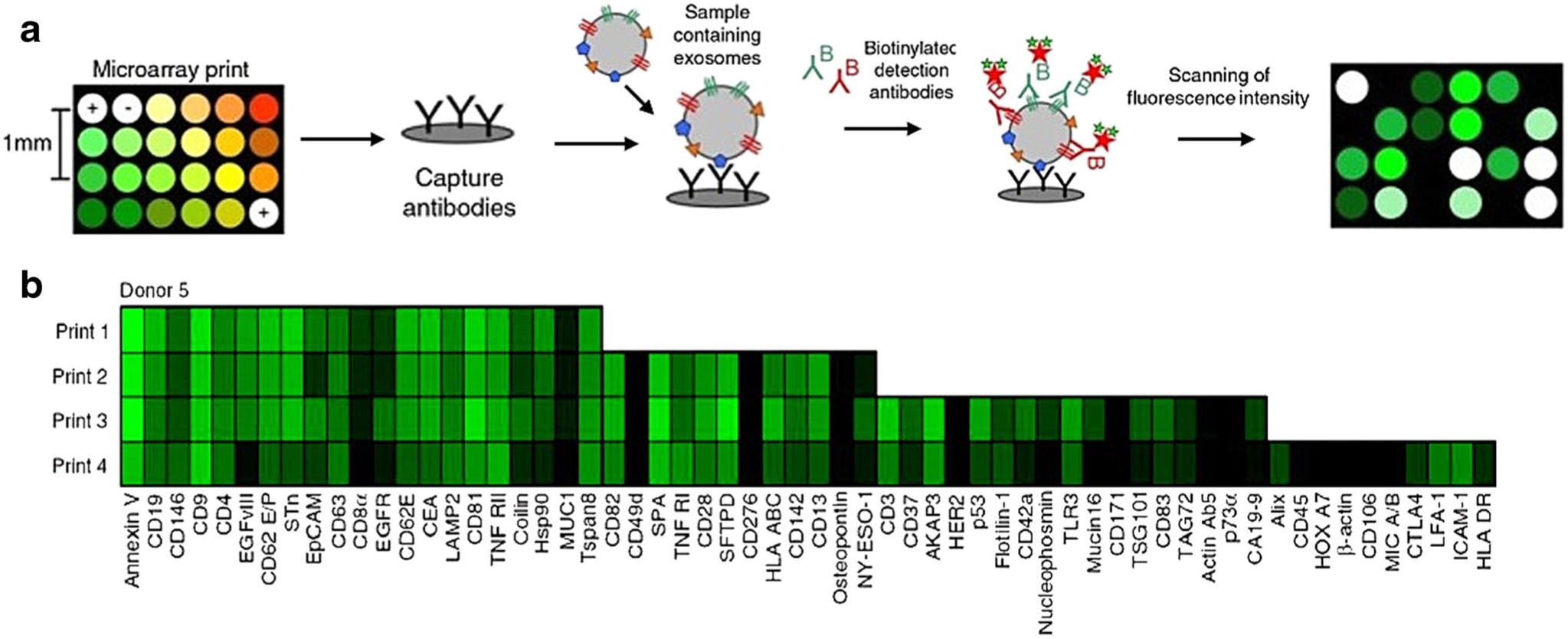
a Schematic overview of EV array detection of exosomal proteins. b Representative exosome phenotyping results using the EV array printed with 21 (print 1), 33 (print 2), 50 (print 3), and 60 (print 4) capturing antibodies. Figures are adapted from Refs. (31) and (32) with permission from Taylor and Francis.
Recently, micro/nanostructures have been introduced to the microfluidic design to further improve the antibody immobilization density and enhance the exosome capture efficiency. Zhang et al. reported a nano-interfaced microfluidic exosome (nano-IMEX) biosensor to detect exosomal proteins for ovarian cancer diagnosis (34). As shown in Fig. 4, the surface of the nano-IMEX biosensor was coated with graphene oxide (GO) and polydopamine (PDA) to generate nanostructures, which greatly increased the surface area and the antibody immobilization density. The nano-IMEX biosensor also contained Y-shaped microposts to facilitate the interaction between exosomes and antibodies and thus enhance the exosome capture efficiency. These micro/nanostructures significantly increased the detection sensitivity of the nano-IMEX biosensor. With exosomes from COLO-1 colon cancer cells, the LOD of nano-IMEX biosensor was ~ 50 exosomes/μL, which was 1000-fold higher than ELISA. The nano-IMEX biosensor was able to detect exosomes directly from 2 μL plasma samples with no need for sample processing. Using exosomal CD9, CD81, and EpCAM as the biomarkers, the nano-IMEX biosensor successfully distinguished ovarian cancer patients (n = 7) from healthy controls (n = 5).
Fig. 4.

a The structure of nano-IMEX biochip. b The mechanism of exosome capturing and analysis on nano-IMEX biochip. Graphene oxide and polydopamine coating-generated nanostructures on the surface and increased the antibody immobilization density. The Y-shaped microposts facilitated the interaction between exosomes and antibodies and thus enhanced the exosome capture efficiency. Exosomal protein levels were measured by immunofluorescence. Figures are adapted from Ref. (34) with permission from the Royal Society of Chemistry.
COLORIMETRIC-BASED EXOSOMAL PROTEIN DETECTION
Colorimetric approaches rely on the color change (from colorless to blue color) of 3,3′,5,5′-tetramethylbenzidine (TMB) oxidation reaction to detect exosomal proteins through visual inspection or UV-Vis spectrophotometry. Colorimetric assays are simple, fast, and cost-effective detection methods which can be easily operated and do not require sophisticated sensing equipment. Liang et al. developed a double-filtration microfluidic biochip that first isolated exosomes (30–200 nm) from urine using size exclusion method and then quantified the expression of exosomal CD63 by on-chip ELISA (Fig. 5) (35). Briefly, exosomes with size between 30 and 200 nm were enriched by flowing through two filter membranes with pore sizes of 200 and 30 nm. Horseradish peroxidase (HRP)-labeled antibodies were used to capture exosomes based on CD63 expression. Then, TMB was added, oxidized by HRP, and changed color from colorless to blue color. The results were captured by a cellphone and the images were analyzed to quantify the levels of exosomal CD63. This method detected an elevated expression of exosomal CD63 in urine samples from bladder cancer patients (n = 15) compared with healthy controls (n = 8). The ROC analysis showed that when the AUC was 0.96, the biochip had 81.3% sensitivity and 90% specificity in distinguishing bladder cancer patients from healthy controls.
Fig. 5.

Schematic (a) and image (b) of the double-filtration microfluidic device for exosome isolation and detection. Based on size exclusion, exosomes with sizes between 30 and 200 nm were collected. c Schematic of on-chip ELISA for exosomal CD63 detection. d The oxidation of TMB by HRP turned the solution from colorless to blue, which was imaged using a smart phone and analyzed by ImageJ. Figures are adapted from Ref. (35) with permission from Nature Publishing Group.
Vaidyanathan et al. developed a colorimetric biosensor that achieved LOD of ~ 2760 exosomes/μL (36). Such high detection sensitivity was realized by alternating current electrohydrodynamic (ac-EHD)-induced nanoshearing, which brought exosomes toward the surfaces, improved the interaction between exosomes and surface bound antibodies, and therefore enhanced exosome capture efficiency. Moreover, the ac-EHD-induced nanoshearing removed weakly bound molecules from the surfaces and reduced non-specific binding. After the capture of exosomes, HRP-labeled detection antibodies were used to recognize target proteins. TMB was added to react with HRP, and the color of final solution changed from colorless to blue color. The color change was proportional to the expression of exosomal proteins, which was either qualitatively detected by naked eyes or quantitatively measured by UV-Vis spectroscopy. The anti-HER2 functionalized biosensor detected higher levels of HER2 in exosomes derived from HER2(+) BT-474 breast cancer cells and HER2(+) breast cancer patient serum than those from HER2(−) MDA-MB-231 breast cancer cells and HER2(−) patient serum. However, with anti-CD9 functionalized biosensor, similar levels of exosomal CD9 were detected in exosomes derived from both HER2(+) BT-474 cells and HER2(−) MDA-MB-231 cells and from both HER2(+) patient serum and HER2(−) patient serum. These results demonstrated that the biosensor was able to selectively capture target exosomes and quantify exosomal proteins. The biosensor also demonstrated its capability of multiplexed sensing by simultaneous detection of exosomal HER2 from BT-474 cells and exosomal PSA from PC3 prostate cancer cells.
In addition to antibodies, aptamer was also used in the colorimetric detection of exosomal proteins. Xia et al. designed a single-walled carbon nanotube (s-SWCNT)-based colorimetric aptasensor (37). In the aptasensor, s-SWCNT was capped with aptamer DNA strands that recognized CD63. In the absence of exosomes, the aptamer DNA strands bound to s-SWCNT and enhanced the peroxidase activity of s-SWCNT, which catalyzed TMB-H2O2 and induced the color change of the solution (from colorless to blue color). In the presence of exosomes, the binding between aptamer DNA strands and exosomal CD63 released aptamers from s-SWCNT due to conformational changes. Without the aptamers, the catalytic ability of s-SWCNT was reduced significantly, leading to the color change of solution from deep blue to moderate blue. By targeting the CD63 on MCF-7 cell-derived exosomes, the LOD of this assay was 5.2×105 exosomes/μL. Compared with antibody-based colorimetric biosensors, this aptamer-based assay was simpler and cheaper with acceptable sensitivity.
SPR-BASED EXOSOMAL PROTEIN DETECTION
Surface plasmon resonance (SPR) is a real-time and label-free optical biosensing technology that has been widely used to detect the binding of biomolecules onto met al surfaces (48). In SPR, the receptor molecules are immobilized on met al surfaces, such as gold surfaces. The binding of target biomolecules with receptor molecules changes the local refractive index, affects the optical properties of the SP modes, and allows the optical detection of target biomolecules by measuring the SP signal changes. Depending on the antibodies immobilized on the surface, SPR platforms can selectively measure the subpopulation of exosomes. The high sensitivity, subpopulation selectivity, reduced sample volume, and short assay time have made SPR a new way to analyze exosomal proteins for cancer screening and diagnosis, especially with rare and dilute samples.
Rupert et al. used the Biacore 2000 SPR instrument (GE healthcare) to measure the CD63 expression of exosomes derived from the human mast cell line, HMC-1.2 (49). The concentration of CD63-positive exosomes quantified by SPR was 6.3 ± 0.3 μg/mL, which was higher than the BCA assay (4.1 ± 0.2 μg/mL) because SPR measured the total mass of exosomes, including proteins, lipids, and nucleotides. When the exosome concentrations were in the picomolar range, the SPR showed high signal-to-noise ratio of ~ 100, which was comparable with ELISA assay. Grasso et al. used the Biacore 3000 SPR instrument (GE healthcare) to detect the surface protein profile of exosomes derived from three human breast cancer cell lines, MCF-7, BT-474, and MDA-MB-231, representing the luminal A, luminal B, and claudin low breast cancer, respectively (50). The expression levels of six exosomal proteins (CD63, CD9, CD24, CD44, EpCAM, and HER2) were measured. The expression of exosomal EpCAM successfully distinguished MDA-MB-231 cells from MCF-7 and BT-474 cells. In addition, the authors successfully measured these six exosomal proteins using 0.5-mL plasma samples from healthy controls, demonstrating its potential as a bioanalytical procedure for clinical applications. Sina et al. utilized a custom-built SPR platform to quantify the proportion of tumor-derived exosome subpopulation from the total exosome population (38). The total exosome population was isolated using antibodies against exosomal CD9 and CD63. Then, anti-HER2 antibody was used to detect HER2+ exosome subpopulation. The LOD of this approach was 2070 exosomes/μL and the linear dynamic range of detection was 2070 to 3300 exosomes/μL. The amount of HER2+ exosomes was > 10-fold higher in serum samples from HER2+ patients (n = 6) than HER2− patients (n = 2) and healthy controls (n = 2). In HER2+ serum samples, HER2+ exosome subpopulation was about 14–35% of total exosome population.
In order to realize multiplexed sensing, Zhu et al. combined SPR imaging (SPRi) with antibody microarray to simultaneously quantify multiple exosome transmembrane proteins (39). As shown in Fig. 6, antibodies against exosomal proteins were printed on the gold surface by SmartArrayer 48 microarray printer (capitalBio, China). The capture of exosomes by antibody microarray affected the refractive index, changed the reflected laser intensities, and allowed the measurement of exosomal proteins by SPRi (PlexAray HT, Plexera Bioscience). The antibody microarray SPRi biosensor detected the expression of CD9, CD41b, CD63, CD82, EpCAM, and E-cadherin on exosomes derived from MHCC97H human hepatocellular carcinoma cells. To evaluate the detection sensitivity of the SPRi biosensor, MHCC97H cells were treated with Rab27a-siRNA to decrease the exosome secretion and monensin to increase the exosome secretion. The expression of exosomal CD9 and CD41b was significantly reduced after Rab27a-siRNA treatment and increased after monensin treatment. Based on these results, the LOD of SPRi biosensor was estimated to be ~ 4.87 × 107 exosomes/cm2 assuming the diameter of exosomes is ~ 70 nm. The antibody microarray SPRi biosensor detected much higher exosomal CD9 and CD41b levels in exosomes secreted from cells with high metastatic potential (i.e., MHCC97H and B16-F10 cells) than those secreted from cells with low metastatic potential (i.e., MHCC97L and B16-F1 cells), demonstrating the possibility of using antibody microarray SPRi biosensor in cancer metastasis detection.
Fig. 6.
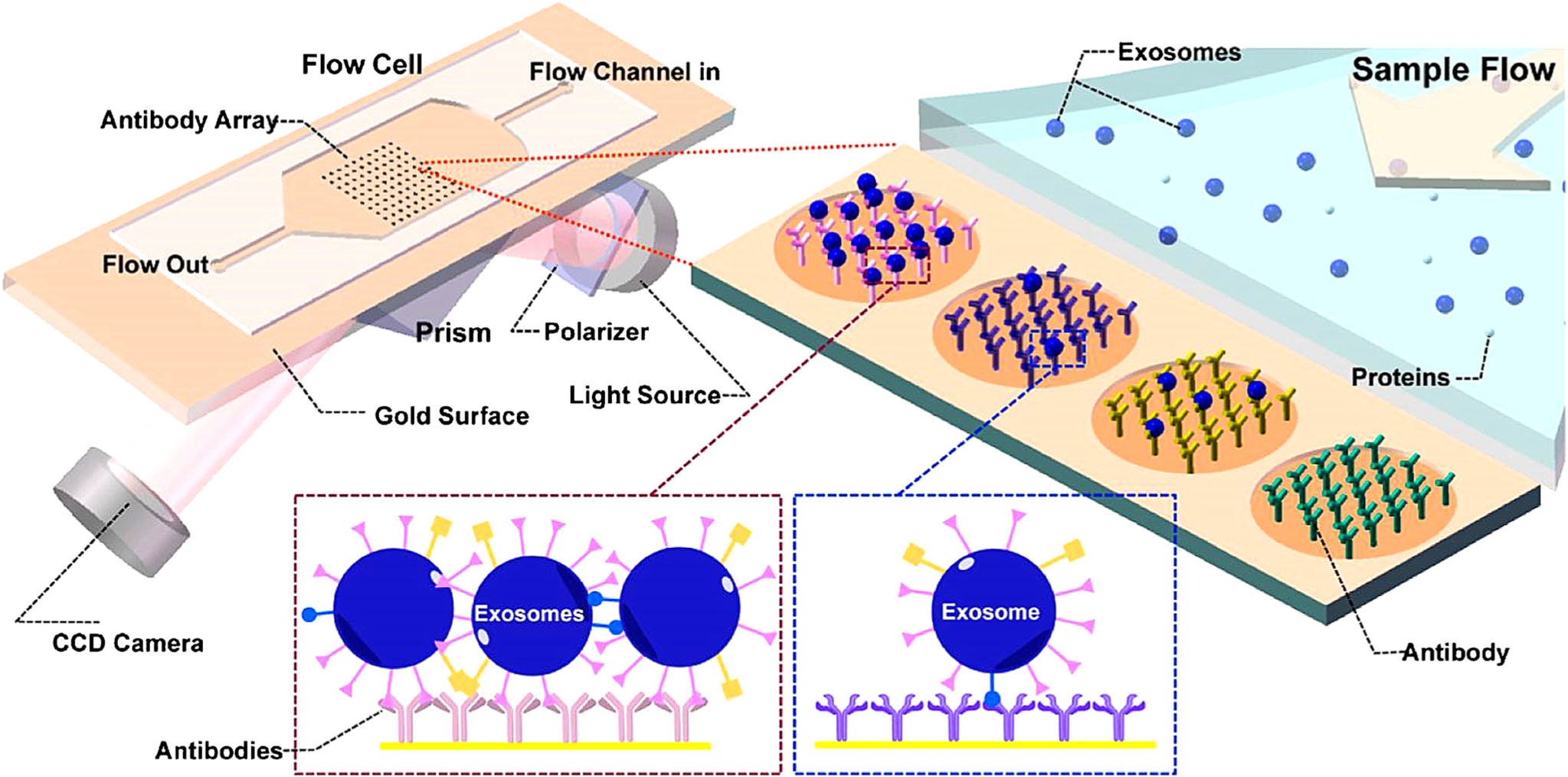
Schematic of SPR imaging in combination with antibody microarray to capture exosomes and characterize exosomal proteins. Antibodies specific to exosome membrane proteins were printed on gold surface. Purified exosomes were flowed through the device and captured by the antibodies. The changes of reflected laser light intensity after exosome binding were recorded to characterize the expression of exosome membrane proteins. Figures are adapted from Ref. (39) with permission from American Chemical Society.
Commercial SPR instrument can provide high detection sensitivity, for example, a minimum detectable surface concentration of ~ 0.1 pg/mm2 (51). However, their large detection spot and the need of bulky prisms limit their effectiveness for compact and miniaturized biosensing, especially for point-of-care testing. Nano-plasmonic biosensors employing nanoscale topographies are attractive miniaturized platforms for label-free, high throughput, and sensitive detection of biomolecules (52–54). Im et al. developed a nano-plasmonic exosome (nPLEX) biosensor, which consisted of arrays of periodic nanohole patterns in the met al film (Fig. 7) (40). The capture of exosomes by affinity binding with antibodies caused the spectral shifts or intensity changes of the laser light, which were proportional to the levels of exosomal proteins. The LOD of nPLEX biosensor was ~ 3000 exosomes, which was 10,000- and 100-fold higher than western blot and ELISA, respectively. Ascites samples from ovarian cancer patients (n = 20) and noncancerous cirrhosis patients (the controls, n = 10) were applied on the nPLEX biosensor with 12 × 3 nanohole arrays. The expression of exosomal EpCAM and CD24 was measured via a portable imager system. The levels of exosomal EpCAM and CD24 were significantly higher in ascites samples from ovarian cancer patients than the controls. The diagnosis accuracy was 93% for EpCAM, 87% for CD24, and further increased to 97% when EpCAM and CD24 were combined. In addition, the expression of exosomal EpCAM and CD24 or both was decreased in ascites samples from ovarian cancer patients who were responders to chemotherapy; however, for nonresponders, the levels of these biomarkers were increased. These results demonstrated that nPLEX biosensor may serve as a useful assay for cancer diagnosis and treatment response evaluation.
Fig. 7.

a A SEM image of periodic nanoholes in the nPLEX biochip. b A SEM image of exosomes captured by antibodies modified on nPLEX biochip surface. c Representative responses (transmission spectra shifts and intensity increases) to the binding of PEG, antibody and exosomes on the nPLEX biochip. d A picture of nPLEX biochip integrated with a multichannel microfluidic cell. Figures are adapted from Ref. (40) with permission from Nature Publishing Group.
SERS-BASED EXOSOMAL PROTEIN DETECTION
Zong et al. developed a SERS-based method for exosomal protein measurement (41). Magnetic nanobeads and SERS nanoprobes were conjugated with two different antibodies specific for two proteins on exosomes. When exosomes were added, the magnetic nanobead-exosome-SERS nanoprobe complexes were formed and then precipitated by a magnet. SERS was used to analyze the exosomal proteins. The LOD of the SERS assay was ~ 1200 exosomes, and the total assay time was ~ 2 h. With exosomal CD63 and HER2 as the biomarkers, the SERS signal was 3.8 times higher for exosomes derived from SKBR3 breast cancer cells than those from MRC5 normal lung fibroblasts, demonstrating its potential for breast cancer diagnosis.
ELECTROCHEMICAL-BASED EXOSOMAL PROTEIN DETECTION
Electrochemical biosensors represent a promising detection platform for exosome analysis because they provide high detection sensitivity, fast and multiplexed sensing, and compact and automated setup, which make them quickly adapt to clinical settings. Electrochemical biosensors generally use HRP to label target biomolecules and then rely on the reaction between HRP and TMB to generate redox signals, which can be detected by amperometric method and used to calculate the concentrations of target biomolecules. Wei et al. developed an electrical field-induced release and measurement (EFIRM) biosensor for exosomal protein detection (42). In EFIRM biosensor, exosomes were directly captured from serum or saliva by magnetic beads conjugated with anti-CD63 antibodies and enriched on electrodes by a magnet. The exosome surface proteins were detected by HRP-labeled detection antibodies. TMB was then added to react with HRP to generate electrical signals. The EFIRM biosensor was evaluated using 10 μL serum and saliva samples from xenograft mice bearing H460 human lung cancer cell line that stably expressed green fluorescence protein (GFP). The EFIRM biosensor detected CD63-GFP-positive exosomes in both the serum and the saliva from all tumor-bearing mice, but not in mice with no tumors. The detection sensitivity was comparable to western blot. This pioneer work demonstrated the feasibility of using electrochemical method to directly detect exosomal proteins in biological samples.
Later, Jeong et al. advanced the electrochemical detection method and developed a portable integrated magnetic-electrochemical exosome (iMEX) biosensor for multiplexed exosomal protein detection (Fig. 8) (43). The iMEX biosensor used multichannel design and offered simultaneous detection of eight markers within 1 h and each marker only required 10 μL plasma. The LOD of iMEX biosensor was 3×104 exosomes, which was ~ 100-fold more sensitive than ELISA. The iMEX biosensor detected much higher levels of exosomal EpCAM and CD24 in plasma samples from ovarian cancer patients (n = 11) than healthy controls (n = 5). The expression of exosomal EpCAM and CD24 was decreased in patients who were responders (n = 2) to drug treatment but remained unchanged (EpCAM) or increased (CD24) in the non-responders (n = 2).
Fig. 8.
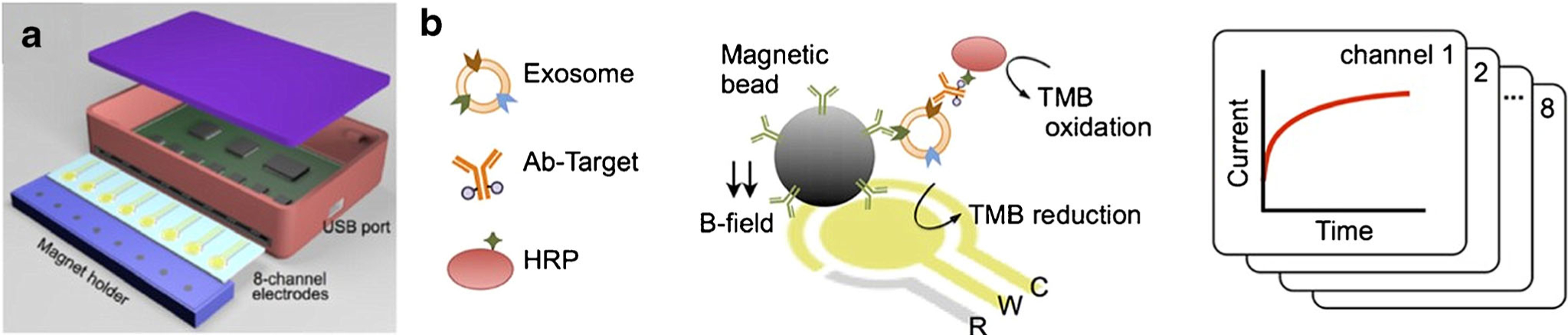
Schematic (a) and sensing mechanism (b) of iMEX biosensor. Exosomes were captured by antibody-conjugated magnetic beads and enriched on electrodes by a magnet. The target exosome surface proteins were detected by HRP-labeled detection antibodies. TMB was then added to react with HRP to generate electrical signals. Figures are adapted from Ref. (43) with permission from American Chemical Society.
The sensitivity of electrochemical detection method was further improved by adapting the sandwich-type ELISA format. Doldan et al. developed an electrochemical sandwich immunosensor in which capturing antibodies were immobilized on the biochip surface (44). The surface proteins of captured exosomes were detected by HRP-labeled detection antibodies and quantified by electrical signals generated after TMB treatment. The biosensor successfully detected CD9 on exosomes from MCF7 breast cancer cells. The sensing performance was not affected by the presence of diluted fet al bovine serum (0.1% or higher dilution), demonstrating the potential of the biosensor in analyzing exosomes from unprocessed clinical serum samples. The biosensor offered high detection sensitivity with LOD of 200 exosomes/μL and worked well with sample volume as low as 1.5 μL.
Besides antibodies, aptamers can also be applied on electrochemical biosensors. Zhou et al. developed an electrochemical-based aptasensor which coated CD63 aptamer DNA strand on the electrode surface (45). A methylene blue-labeled antisense nucleic acid strand paired with the aptamer strand before loading the exosomes on the biosensor. After applying the exosomes on the electrode, the aptamers bound with exosome surface CD63 proteins, which caused the release of methylene blue-labeled probes and changed the redox signals. Exosomes derived from HepG2 liver cancer cells were used to validate the aptasensor. The LOD of the aptasensor was 103 exosmes/μL, which was 100-fold lower than ELISA.
MICRONUCLEAR MAGNETIC RESONANCE-BASED EXOSOMAL PROTEIN DETECTION
Shao et al. developed a miniaturized, microfluidic micronuclear magnetic resonance (μNMR) biosensor that combined microfiltration with NMR, enabling the simultaneous quantification of exosomes number concentration and exosomal protein expression (46). As shown in Fig. 9, the exosomes were labeled with 7 nm target-specific magnetic nanoparticles (MNPs) in the mixer section of the microfluidic μNMR biosensor. The MNP-labeled exosomes were then washed and concentrated after they passed through a filter membrane. MNP labeling gave exosomes superparamagnetic property and resulted in the decay of 1H NMR signal. Because the signal decay rate correlates with the concentrations of MNPs, the expression of exosomal proteins was quantitatively measured. When the expression of exosomal CD63 was analyzed, the μNMR biosensor showed high reproducibility and superior detection sensitivity with LOD of ~104 exosomes, which was 100-, 1000-, and 10,000-fold more sensitive than Nanosight LM10 nanoparticle characterization system, ELISA, and western blotting, respectively. The μNMR biosensor distinguished glioblastoma multiforme (GBM) patients (n = 24) from normal controls (n = 8) with detection accuracy higher than 90% with four exosomal biomarkers (EGFR, EGFRvIII, PDPN, and IDH1 R132H). The μNMR biosensor successfully predicted the treatment outcome of two GBM drugs, temozolomide, and geldanamycin, in xenograft GBM mouse model, and the responses to the temozolomide and radiation combination therapy in GBM patients. With further optimization, the μNMR biosensor could realize a simple, fast, and portable point-of-care test for the diagnosis and prognosis of GBM and other diseases.
Fig. 9.

a Circulating microvesicles, mainly exosomes, were labeled with MNPs via a two-step labeling procedure. Exosomal proteins were first labeled with monoclonal antibodies conjugated with trans-cyclooctene (mAb-TCO), and then coupled with MNP modified with 1,2,4,5-tetrazine (TZ). b Schematic and a photo of the microfluidic μNMR biosensor. Exosomes were mixed with mAb-TCO and MNP-TZ in the chanotic mixer, and then were concentrated by passing through the filter membrane. The on-chip NMR detection of exosomal proteins was realized by the integrated microcoil. Figures are adapted from Ref. (46) with permission from Nature Publishing Group.
CONCLUSIONS AND FUTURE DIRECTIONS
Exosomes, used to be considered as cellular trash bags, have shown tremendous value in understanding the molecular mechanisms of cancer biology. Abundantly and stably present in body fluids, tumor-derived exosomes have become attractive liquid biopsy-based cancer biomarkers for diagnosis and precision medicine. Recently significant progress has been made in liquid biopsy biosensors to realize fast, highly sensitive, and high throughput analysis of exosomes. The successful detection of exosomal biomarkers in biospecimens (serum, plasma, ascites fluids) from cancer patients has demonstrated the feasibility of using biosensors in clinical settings. In the future, continuous efforts are still required to develop exosome-based biosensors into routine clinical diagnostic assays. First of all, the sensing performance of biosensors needs to be further improved to handle biological samples, such as blood and urine. The LODs of many biosensors mentioned above are generally determined using pre-enriched, cell-derived exosomes, which have not considered the complexity of biological samples. Biological samples contain thousands of components, such as cells, proteins, nucleic acids, lipids, and extracellular vesicles. The biosensors are required to identify exosomes from all other components and to achieve high detection sensitivity and specificity with the presence of these contaminant components. Besides, other extracellular vesicles, such as microvesicles and apoptotic bodies, interfere with the exosome detection due to overlapped sizes and shared surface markers; therefore, the biosensors should also achieve more effective purification of exosomes from body fluids. Secondly, the detection multiplexity of biosensors needs to be improved. Depending on detection methods, no or limited multiplexity has been seen with majority of the biosensors. The best multiplexity has been realized by the EV array, which can detect up to 60 biomarkers. Moreover, most biosensors only detect exosome surface proteins. Integration of exosome surface protein biomarkers with intra-vesicular protein biomarkers and other types of intra-vesicular biomarkers such as DNAs and RNAs, may provide a more comprehensive profile of tumor and further enhance the diagnostic value of exosomes. Thirdly, the biosensors need to be validated in large cohort of patients. Currently, the development of many biosensors is still in the feasibility test phase with biological samples from small cohorts of patients. The large-scale validation studies are required to justify the clinical benefits before the benchtop-to-bedside transition. Fourthly, to obtain FDA approval for clinical use, the biosensors need to meet regulatory requirements and address challenges such as repeatability and reliability. Repeatability is a key factor in technical measurements. The biosensors need to achieve target coefficient of variation (CV) and provide the most reproducible and least biased results. Reliability is another important factor we have to consider when handling biological samples. The use of external spike is an effective way to normalize the sample processing procedures. However, the biological fluctuations of the levels of exosomal biomarkers in the same individual or among a group of people who share similar characteristics (age, gender, race, smoking status, disease status, etc.) represent a major bottleneck in establishing reliable assays. To overcome this challenge, single or multiple internal normalization factors should be included in the assays. Fifthly, the adaptability of biosensors to clinical settings should be improved to address challenges faced in commercialization. The setups of many microfluidic biosensors are quite complicated and the operation of these biosensors require intensive training and expensive supporting equipment, which greatly limited their applications in clinical settings. The design of biosensors should incorporate more user-friendly and cost-effective concepts to speed up the commercialization and clinical translation. Last but not least, the establishment of technical standards to evaluate the detection sensitivity and specificity of biosensors is urgently needed. The sensing performances of biosensors developed by different groups have been evaluated using different model systems (exosomes from various sources, different sample processing procedures, and different biomarkers), which has made the comparison between different biosensors quite challenging. Unified guidelines for sample sources, sample handling protocols, and biomarkers are critically important for the community to compare and validate the exosome-based biosensors and promote their clinical applications.
In summary, exosome-based liquid biopsy biosensors are exciting technologies that may serve as companion and complementary tests in cancer screening, diagnosis, personalized therapy, treatment response monitoring, and prognosis. Although these biosensors are still in infancy and many challenges need to be overcome for wide clinical use, repaid advancement in this field makes us believe that they will eventually have a significant impact on cancer care.
ACKNOWLEDGMENTS
Authors acknowledge the funding support from National Cancer Institute of the National Institutes of Health under award number 5R33CA191245. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Cancer Treatment &Survivorship Facts & Figures 2016–2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2016-2017.pdf.
- 2.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aberle DR, Abtin F, Brown K. Computed tomography screening for lung cancer: has it finally arrived? Implications of the national lung screening trial. J Clin Oncol. 2013;31(8):1002–8. 10.1200/JCO.2012.43.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patz EF, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174:269–74. 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chudgar NP, Bucciarelli PR, Jeffries EM, Rizk NP, Park BJ, Adusumilli PS, et al. Results of the national lung cancer screening trial: where are we now? Thorac Surg Clin. 2015;25:145–53. 10.1016/j.thorsurg.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan F, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–91. 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599–614. 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray C, Bell LN, Liang H, Collins D, Yale SH. Colorectal cancer screening. WMJ. 2017;116:27–33. [PubMed] [Google Scholar]
- 9.Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–48. 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 10.Gingras I, Salgado R, Ignatiadis M. Liquid biopsy: will it be the ‘magic tool’ for monitoring response of solid tumors to anticancer therapies. Curr Opin Oncol. 2015;27:560–7. 10.1097/CCO.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 11.Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–38. 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 12.Contreras-Naranjo JC, Wu HJ, Ugaz VM. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip. 2017;17:3558–77. 10.1039/c7lc00592j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He M, Zeng Y. Microfluidic exosome analysis toward liquid biopsy for cancer. J Lab Autom. 2016;21:599–608. 10.1177/2211068216651035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–25. 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–42. 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–32. 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 18.ELA S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–57. 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 19.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.HR M, Bayraktar E, KH G, Abd-Ellah MF, Amero P, Chavez-Reyes A, et al. Exosomes: from garbage bins to promising therapeutic targets. Int J Mol Sci. 2017;18 10.3390/ijms18030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Li C, Zhou T, Liu X, Liu X, Li X, et al. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16:145. 10.1186/s12943-017-0706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–82. 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakobsen KR, Paulsen BS, Baek R, Varming K, Sorensen BS, Jorgensen MM. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles. 2015;4:26659. 10.3402/jev.v4.26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita T, Kamada H, Kanasaki S, Maeda Y, Nagano K, Abe Y, et al. Epidermal growth factor receptor localized to exosome membranes as a possible biomarker for lung cancer diagnosis. Pharmazie. 2013;68:969–73. 10.1691/ph.2013.3599. [DOI] [PubMed] [Google Scholar]
- 25.Clark DJ, Fondrie WE, Yang A, Mao L. Triple SILAC quantitative proteomic analysis reveals differential abundance of cell signaling proteins between normal and lung cancer-derived exosomes. J Proteome. 2016;133:161–9. 10.1016/j.jprot.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Li XJ, Hayward C, Fong PY, Dominguez M, Hunsucker SW, Lee LW, et al. A blood-based proteomic classifier for the molecular characterization of pulmonary nodules. Sci Transl Med. 2013;5:207ra142. 10.1126/scitranslmed.3007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Zhang Y, Qiu F, Qiu Z. Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis. 2011;32:1976–83. 10.1002/elps.201000598. [DOI] [PubMed] [Google Scholar]
- 28.He M, Crow J, Roth M, Zeng Y, Godwin AK. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip. 2014;14:3773–80. 10.1039/c4lc00662c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Z, Yang Y, Zeng Y, He M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. 2016;16:489–96. 10.1039/c5lc01117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun. 2014;5:3591. 10.1038/ncomms4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorgensen M, Baek R, Pedersen S, Sondergaard EK, Kristensen SR, Varming K. Extracellular vesicle (EV) array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J Extracell Vesicles. 2013;2 10.3402/jev.v2i0.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jorgensen MM, Baek R, Varming K. Potentials and capabilities of the extracellular vesicle (EV) array. J Extracell Vesicles. 2015;4:26048. 10.3402/jev.v4.26048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baek R, Jorgensen MM. Multiplexed phenotyping of small extracellular vesicles using protein microarray (EV Array). Methods Mol Biol. 2017;1545:117–27. 10.1007/978-1-4939-6728-58. [DOI] [PubMed] [Google Scholar]
- 34.Zhang P, He M, Zeng Y. Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating. Lab Chip. 2016;16:3033–42. 10.1039/c6lc00279j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang LG, Kong MQ, Zhou S, Sheng YF, Wang P, Yu T, et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci Rep. 2017;7:46224. 10.1038/srep46224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaidyanathan R, Naghibosadat M, Rauf S, Korbie D, Carrascosa LG, Shiddiky MJ, et al. Detecting exosomes specifically: a multiplexed device based on alternating current electrohydrodynamic induced nanoshearing. Anal Chem. 2014;86:11125–32. 10.1021/ac502082b. [DOI] [PubMed] [Google Scholar]
- 37.Xia Y, Liu M, Wang L, Yan A, He W, Chen M, et al. A visible and colorimetric aptasensor based on DNA-capped single-walled carbon nanotubes for detection of exosomes. Biosens Bioelectron. 2017;92:8–15. 10.1016/j.bios.2017.01.063. [DOI] [PubMed] [Google Scholar]
- 38.Sina AA, Vaidyanathan R, Dey S, Carrascosa LG, Shiddiky MJ, Trau M. Real time and label free profiling of clinically relevant exosomes. Sci Rep. 2016;6:30460. 10.1038/srep30460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu L, Wang K, Cui J, Liu H, Bu X, Ma H, et al. Label-free quantitative detection of tumor-derived exosomes through surface plasmon resonance imaging. Anal Chem. 2014;86:8857–64. 10.1021/ac5023056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Im H, Shao H, Park YI, Peterson VM, Castro CM, Weissleder R, et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat Biotechnol. 2014;32(5):490–5. 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zong S, Wang L, Chen C, Lu J, Zhu D, Zhang Y, et al. Facile detection of tumor-derived exosomes using magnetic nanobeads and SERS nanoprobes. Anal Methods. 2016;8:5001–8. 10.1039/c6ay00406g. [DOI] [Google Scholar]
- 42.Wei F, Yang J, Wong DT. Detection of exosomal biomarker by electric field-induced release and measurement (EFIRM). Biosens Bioelectron. 2013;44:115–21. 10.1016/j.bios.2012.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeong S, Park J, Pathania D, Castro CM, Weissleder R, Lee H. Integrated magneto-electrochemical sensor for exosome analysis. ACS Nano. 2016;10:1802–9. 10.1021/acsnano.5b07584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doldan X, Fagundez P, Cayota A, Laiz J, Tosar JP. Electrochemical sandwich immunosensor for determination of exosomes based on surface marker-mediated signal amplification. Anal Chem. 2016;88:10466–73. 10.1021/acs.analchem.6b02421. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Q, Rahimian A, Son K, Shin DS, Patel T, Revzin A. Development of an aptasensor for electrochemical detection of exosomes. Methods. 2016;97:88–93. 10.1016/j.ymeth.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012;18:1835–40. 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang S, Tian H, Li X, Jin D, Li X, Kong J, et al. Clinical application of a microfluidic chip for immunocapture and quantification of circulating exosomes to assist breast cancer diagnosis and molecular classification. PLoS One. 2017;12:e0175050. 10.1371/journal.pone.0175050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh P SPR biosensors: historical perspectives and current challenges. Sensors Actuators B Chem. 2016;229:110–30. 10.1016/j.snb.2016.01.118. [DOI] [Google Scholar]
- 49.Rupert DL, Lasser C, Eldh M, Block S, Zhdanov VP, Lotvall JO, et al. Determination of exosome concentration in solution using surface plasmon resonance spectroscopy. Anal Chem. 2014;86:5929–36. 10.1021/ac500931f. [DOI] [PubMed] [Google Scholar]
- 50.Grasso L, Wyss R, Weidenauer L, Thampi A, Demurtas D, Prudent M, et al. Molecular screening of cancer-derived exosomes by surface plasmon resonance spectroscopy. Anal Bioanal Chem. 2015;407:5425–32. 10.1007/s00216-015-8711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Homola J Surface plasmon resonance sensors for detection of chemical and biological species. Chem Rev. 2008;108:462–93. 10.1021/cr068107d. [DOI] [PubMed] [Google Scholar]
- 52.Stewart ME, Anderton CR, Thompson LB, Maria J, Gray SK, Rogers JA, et al. Nanostructured plasmonic sensors. Chem Rev. 2008;108:494–521. 10.1021/cr068126n. [DOI] [PubMed] [Google Scholar]
- 53.Anker JN, Hall WP, Lyandres O, Shah NC, Zhao J, Van Duyne RP. Biosensing with plasmonic nanosensors. Nat Mater. 2008;7:442–53. 10.1038/nmat2162. [DOI] [PubMed] [Google Scholar]
- 54.Im H, Shao H, Weissleder R, Castro CM, Lee H. Nano-plasmonic exosome diagnostics. Expert Rev Mol Diagn. 2015;15:725–33. 10.1586/14737159.2015.1041378. [DOI] [PMC free article] [PubMed] [Google Scholar]


