Abstract
Parathyroid hormone (PTH) is necessary for the regulation of calcium homeostasis and PTH (1–34) was the first approved osteoanabolic therapy for osteoporosis. It is well established that intermittent PTH increases bone formation and that bone remodeling and several cytokines and chemokines play an essential role in this process. Earlier, we had established that the chemokine, monocyte chemoattractant protein-1 (MCP-1/CCL2), was the most highly stimulated gene in rat bone after intermittent PTH injections. Nevertheless, MCP-1 function in bone appears to be complicated. To identify the primary cells expressing MCP-1 in response to PTH, we performed in situ hybridization of rat bone sections after hPTH (1–34) injections and showed that bone-lining osteoblasts are the primary cells that express MCP-1 after PTH treatment. We previously demonstrated MCP-1’s importance by showing that PTH’s anabolic effects are abolished in MCP-1 null mice, further implicating a role for the chemokine in this process. To establish whether rhMCP-1 peptide treatment could rescue the anabolic effect of PTH in MCP-1 null mice, we treated 4-month-old wild-type (WT) mice with hPTH (1–34) and MCP-1−/− mice with rhMCP-1 and/or hPTH (1–34) for 6 weeks. Micro-computed tomography (μCT) analysis of trabecular and cortical bone showed that MCP-1 injections for 6 weeks rescued the PTH anabolic effect in MCP-1−/− mice. In fact, the combination of rhMCP-1 and hPTH (1–34) has a synergistic anabolic effect compared with monotherapies. Mechanistically, PTH-enhanced transforming growth factor-β (TGF-β) signaling is abolished in the absence of MCP-1, while MCP-1 peptide treatment restores TGF-β signaling in the bone marrow. Here, we have shown that PTH regulates the transcription of the chemokine MCP-1 in osteoblasts and determined how MCP-1 affects bone cell function in PTH’s anabolic actions. Taken together, our current work indicates that intermittent PTH stimulates osteoblastic secretion of MCP-1, which leads to increased TGF-β signaling, implicating it in PTH’s anabolic actions.
Keywords: Osteoporosis, PTH, Bone, Chemokines, Monocyte chemoattractant protein-1
1. Introduction:
Osteoporosis is more common in the elderly population and causes morbidity and mortality. Parathyroid hormone is requisite for calcium homeostasis [1]. Although endogenous hyperparathyroidism is related to bone catabolism [2], PTH(1–34, teriparatide) was the first available prototypic osteoanabolic hormone for treating osteoporosis, when administered intermittently [3]. Despite its beneficial therapeutic potential in improving bone health and fracture healing, the exact mechanism of hPTH (1–34) action remains elusive. Elucidation of the mechanisms by which PTH can result in either osteoanabolic or catabolic effects has important implications for understanding and treating a broad range of metabolic bone disorders [4]. PTH has multiple actions, including indirect activation of the osteoclast through osteoblastic RANKL production resulting in increased bone resorption, as well as many direct changes in the functions of the osteoblast [5, 6]. It is well recognized that intermittent PTH increases bone formation and promotes bone remodeling [7]. Previously we have reported that intermittent treatment of rats with PTH (1–34) for 14 days highly induced several cytokines and chemokines, in particular, RANKL, IL-6, CXCL1, and CCL2 (MCP-1) [8]. Among all genes, the chemokine, MCP-1 (CCL2), was found to be the most highly stimulated, which suggested a potentially important role for MCP-1 in PTH’s anabolic effects [8]. Monocyte chemoattractant protein-1 (MCP-1 or CCL2), is a potent pro-inflammatory member of the CC motif chemokine family [9]. High expression of MCP-1 was reported in several bone pathophysiological conditions, such as prostate cancer bone metastasis and sites of osteoporotic bone [10–12]. MCP-1 is also expressed by osteoclasts and has a role in osteoclast formation [13–15]. CC chemokine receptor 2 (CCR2) is the one of the main receptors for MCP-1 and absence of CCR2 results in higher bone mass, further signifying an essential role for MCP-1 in bone metabolism [16].
We showed that PTH’s bone anabolic effects are abolished in MCP-1 null mice, further implicating an essential role for the chemokine [17]. We also found a significant increase in MCP-1 expression in osteoblasts. In addition, MCP-1 null mice did not show an increase in macrophage numbers, osteoclast surface, and osteoclast number, which were all observed in wild-type mice after daily PTH injections. We concluded that the abolition of PTH-mediated bone formation is due to lack of osteoclast and macrophage activity and that osteoblast MCP-1 expression is a key mediator of the anabolic effects of PTH on bone [17]. In addition, increased MCP-1 expression caused by PTH enhanced the osteoclast recruitment, differentiation, and fusion of pre-osteoclasts, suggesting that the PTH anabolic effect requires MCP-1-induced osteoclast recruitment [18].
Transforming growth factor-beta (TGF-β) is an essential growth regulator in bone and is produced by bone cells. TGF-β is abundantly present in bone matrix and regulates many biochemical processes within cells. TGF-β binds to the TGF-β type-2 receptor, which subsequently phosphorylates the type I receptor. The heterotetrameric TGF-β receptor complex, in turn, phosphorylates the Smads, especially Smad2. PTH can increase TGF-β activity via osteoclast activation, suggesting local systemic hormonal regulation of TGF-β [19, 20].
Herein, we have dissected the mechanism whereby MCP-1 mediates PTH’s anabolic effects on bone. We found that MCP-1, synthesized by osteoblasts and osteoblast-like cells in response to intermittent PTH stimulation, is essential for the recruitment of monocytes and pre-osteoclastic cells and administration could restore increased marrow macrophages. We found that MCP-1−/− mice did not demonstrate PTH-induced TGF-β signaling in bone marrow. This, as well as PTH’s anabolic effects on bone, could be restored by injections of rhMCP-1. Thus, TGF-β appears to serve as a coupling mediator for MCP-1’s role in PTH’s anabolic effects on bone.
2. Materials and Methods
2.1. Animal studies
All experimental animals were maintained under standard conditions with a 12-hour light/12-hour dark cycle with water ad libitum and standard rodent chow. All animal-related experiments using mice and rats were performed following the approved protocols of the Institutional Animal Care and Use Committee (IACUC) of New York University.
2.1.1. Rat model for in situ hybridization
Four-month-old male rats (4 in each group) were injected with hPTH (1–34) (80 μg/kg), or saline and bones were collected 1 h after injections. Femurs were processed by fixation in 4% paraformaldehyde, then demineralized in 10% EDTA for 3 weeks for in situ hybridization analysis (as detailed below).
2.1.2. Mouse model of 10 days MCP-1 treatment
Breeding pairs of wild-type (C57BL/6J) and MCP-1−/− mice on the same background were purchased from the Jackson Laboratories and bred for experimental animals. For pilot experiments, 4-month-old male MCP-1−/− mice (4 mice in each group) were injected with rhMCP-1 (0.25, 0.5 or 1 μg/mouse) daily for 10 days. Blood was collected daily 2 and 4 h after rhMCP-1 injections to measure the circulating concentrations of MCP-1 by ELISA. To achieve the desired increase in MCP-1 serum levels (the levels after PTH injections), wild-type 4-month-old male mice (4 mice in each group) were injected with hPTH (1–34) at 80 μg/kg/day or saline. After 10 days of daily injections to all described groups, mice were killed (2 h after the last injection) and tibiae, femora, and blood collected. One femur was fixed in 70% ethanol and a tibia in 4% paraformaldehyde. μCT was performed on the femur while the tibia was processed for immunohistochemistry for p-Smad2.
2.1.3. Long-term (6 weeks) MCP-1 treatment in mice
Male wild-type and MCP-1−/− mice at 4 months of age were randomly distributed to saline or treatment groups (n=8 per group). Wild-type mice were injected with hPTH (1–34) at 80 μg/kg/day or saline daily, while MCP-1−/− mice were injected with saline, hPTH, rhMCP-1 (0.25μg) or rhMCP-1 plus hPTH daily for 6 weeks. Two doses of calcein (10 mg/kg) were given to all groups by intraperitoneal injection, at days 7 and 2 before euthanasia. After 6 weeks of daily injections to all described groups, mice were killed and tibiae, femora and blood collected. One femur was fixed in 70% ethanol for at least 2 days. One tibia was fixed in 4% paraformaldehyde. μCT and histomorphometry were performed on the femur while the tibia was processed for immunohistochemistry for p-Smad2 and Mac-3.
2.2. In situ hybridization
In situ hybridization detection of MCP-1-mRNA was performed on rat bone sections after PTH injection. Briefly, 5 μm paraffin sections were washed in buffer, dehydrated in alcohol, and hybridized with digoxigenin-labeled sense and antisense MCP-1 riboprobes (plasmid kindly provided by Dr. Richard Miller at Northwestern University School of Medicine) at a concentration of 2 ng/μl in hybridization solution (DIG Northern starter kit, Roche, Basel, Switzerland) at 37°C for 12 h. Samples were incubated with anti-digoxigenin-antibodies coupled to alkaline phosphatase. After rigorous washes (4 times with wash buffer, provided with Roche kit), the sections were incubated with the NBT/BCIP substrate complex for 1 h at room temperature. The sense MCP-1 probe was used as a negative control.
2.3. RNA Isolation and Quantitative Real-time PCR Analysis
Total RNA was prepared from the metaphyseal region of the femurs or cultured cells using a TRIzol kit (Invitrogen) according to the manufacturer’s protocol. Of the total RNA, 1 μg was used to synthesize cDNA by using reverse transcriptase TaqMan® (Life Technologies, Inc.). We used SYBR® Green Master Mix for quantitative real-time RT-PCR detection [21]. Relative mRNA or fold changes in expression were calculated using a formula reported previously [22]. All mRNA expression levels were normalized to β-actin and expressed as fold values compared with the WT saline-injected mice. The details of primers used for mRNA expression are given in supplementary table 1.
2.4. Micro-Computed Tomography (μCT)
The animals were euthanized and femurs were dissected, cleaned of soft tissue, and fixed and stored in 70% ethanol prior to high-resolution μCT analysis. The Bruker SkyScan 1172 μCT scanner (SkyScan, Ltd., Kartuizersweg, Kontich, Belgium) was used to perform the μCT analyses. All samples were scanned in batches of six at a nominal resolution (pixels) of 9.7 μm. Images were obtained using the following parameters: 60 kV, 167 uA, and pixel size of 9.7 μm, matrix size of 2000 × 1000, 0.3 degrees’ rotation, 6 averages, a movement correction factor of 10, and aluminum filter. NRECON (SkyScan) was used to reconstruct all images using thresholding of 0–0.065, ring artifact correction of 7, beam-hardening correction of 40, and Gaussian smoothing (factor 1). The reconstructed data were binarised using thresholding of 79–255. CTAn software (SkyScan) was used for 3-D and 2-D volumetric analyses of trabecular and cortical bone, respectively. All parameters of μCT measurements follow the guidelines reported by Bouxsein et al.[23]. The trabecular analysis was performed in the region starting 25 slices from the end of the growth plate and extending 258 slices towards the distal femur [17]. To maintain the consistency of cortical parameters, 100 slices were chosen in the cortical region, omitting 250 slices as offset from the start of the growth plate (to exclude the trabecular region) as a reference point [2].
2.5. Histomorphometric analyses
For histomorphometric analyses, femurs were fixed in 70% ethanol, dehydrated, and embedded in methyl methacrylate (Polysciences, Warrington, PA). Longitudinal tissue sections (5 and 10 μm thick) were cut on a Leica RM2265 microtome. Histomorphometry of femurs was performed following an established protocol [17, 24, 25]. Five μm thick sections were stained with toluidine blue to detect osteoclasts. Unstained sections (10 μm thick) were used to assess dynamic parameters using the fluorescent calcein labels (mineral apposition rate (MAR), mineralized surface and bone formation rate (BFR)). BioQuant image analysis system (Nashville, TN) was used for quantitative analyses. All histomorphometry measurements were calculated and performed following the standard nomenclature approved by the American Society for Bone and Mineral Research [24, 25]. All analyses were done blinded.
2.6. Immunohistochemistry
Paraformaldehyde-fixed tibiae were demineralized in 10% EDTA, paraffin-embedded and cut as 5 μm sections. For p-Smad2 immunohistochemistry, paraffin sections were deparaffinized, rehydrated and immunostained with a 1:75 dilution of rabbit anti-phospho-Smad2 antibody (Cell Signaling) using the ABC staining system (Santa Cruz Biotechnology) as described previously [26]. Briefly, anti-phospho-Smad2 primary antibody was incubated overnight at 4°C followed by 30 min incubation with peroxidase conjugated secondary antibody at room temperature or using the HistoMouse Max AEC detection kit (Invitrogen). For Mac3 immunohistochemistry, sections were incubated with a 1:100 dilution of Mac-3 antibody or isotype control (Serotec, Oxford, UK) for 60 min at room temperature followed by a 10-min incubation with secondary antibody and using the HistoMouse Max AEC detection kit. P-Smad2 and Mac-3 positive cells were counted in the secondary spongiosa region of the tibia. Twenty fields were captured in a raster pattern and Image-J software was used for quantification [2].
2.7. Serum biomarkers and cytokine measurements
At the end of 10 days or 6 weeks of hPTH or rhMCP-1 injections, blood samples were collected by cardiac puncture. Sera were obtained after centrifugation at 5000 rpm for 10 min. Serum MCP-1 concentrations were measured by using the Quantikine ELISA (R&D Systems). Serum C-terminal telopeptide of collagen (CTX, Immunodiagnostic Systems Inc.) and P1NP (MyBiosource.com), were measured by rodent-specific ELISAs as described previously [2, 27].
2.8. Mineralization of bone marrow stromal cells (BMSCs)
Bone marrow cells from WT and MCP-1−/− mice after 6 weeks of PTH or saline injections were isolated and cultured according to a previous publication [28]. Briefly, the femora were excised, cleaned of soft tissues, epiphyses removed and the marrow flushed out with 20 ml of culture medium consisting of α-MEM, supplemented with 10% fetal bovine serum, 10−7 M dexamethasone, 50 μg/ml ascorbic acid, and 10 mM β-glycerophosphate. Released BMSCs were collected and plated (2 × 106 cells/well) in 12-well plates in the same culture medium. They were cultured for 21 days at 37 °C in a humidified atmosphere of 5% CO2. The culture medium was changed every second day. After 21 days of culture, cells were fixed in 4% paraformaldehyde, rinsed with PBS and stained with 40 mM Alizarin Red S. Stained cells were first photographed, and alizarin stain was then extracted in cetylpyridinium chloride (CPC) at pH 7.0 followed by colorimetric detection at 570 nm.
2.9. Statistical Analyses
Statistical analyses were performed by one-way or two-way ANOVA followed by post hoc Newman–Keuls multiple comparison tests of significance using GraphPad Prism 3.02 software. Data are expressed as the mean ± standard error of the mean with p < 0.05 considered statistically significant.
3. Results
3.1. PTH-enhanced TGF-β signaling is abolished in the marrow in the absence of MCP-1
Previously we have shown that PTH profoundly increased MCP-1 mRNA and protein expression in bone and serum in rats [8]. To further confirm the cell types that express MCP-1, we performed in situ hybridization using bones of 4-month-old male rats. Rats were used for this analysis because the stimulation by PTH is so profound in this species, even 50-fold with 1 injection of PTH [18]. Rats were administered 1 injection of hPTH (1–34) or saline and tibiae collected 1 h after injection followed by in situ hybridization with digoxigenin-labeled sense and antisense MCP-1 riboprobes. PTH stimulates bone-lining and bone marrow stromal cells to express MCP-1 (Figure 1A). As seen in Figure 1B, PTH injection increased the number of MCP-1 positive cells almost 3 fold compared with saline-injected rat bones within 1 h of PTH injection. These observations confirmed that the rapid and substantial increase in MCP-1 mRNA in bones [8] is from osteoblast-lineage cells and not myeloid lineage cells, although these express MCP-1 at later times [17]. The changes in RNA expression precede the immunohistochemical staining for MCP-1 seen in the same bone-lining cells after 2 PTH injections [17]. These data suggest that PTH regulates MCP-1 transcription in stromal and bone lining cells in vivo.
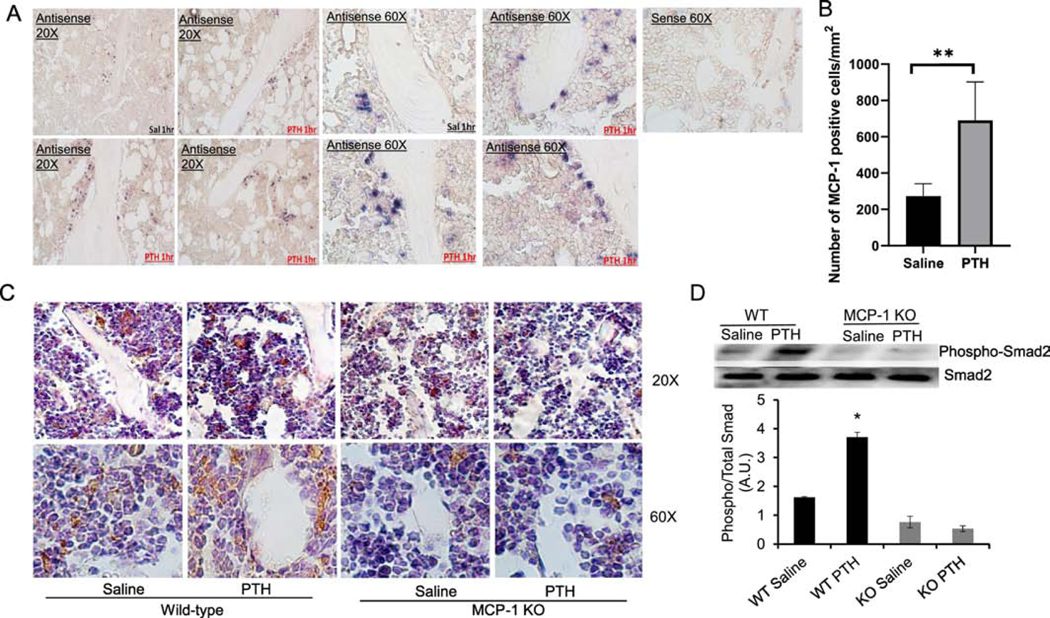
Figure 1: MCP-1 null mice have an attenuated PTH-mediated increase in Smad2 phosphorylation:
In situ hybridization shows PTH stimulates MCP-1 mRNA in bone-lining cells. Male 4-month-old rats were injected with 80 μg/kg hPTH (1–34) or saline and the bones collected 1 h after the injections. (A) In situ hybridization was accomplished with digoxigenin-labeled sense and antisense MCP-1 riboprobes. (B) Quantification of in situ staining in cells lining bone and in marrow showed increased expression 1 h after the injection (n=4 rats per group, 9 fields per section).C) Four-month-old male WT and MCP-1−/− mice were injected daily with 80 μg/kg hPTH (1–34) or saline vehicle for 6 weeks and bones were collected 2 h after the last injection. Tibial sections were immunostained for pSmad2. All magnifications are 60X. (D) Representative Western blot for phospho-Smad2 and total Smad2 in bone marrow after 6 weeks of hPTH (1–34) injections in wild-type and MCP-1−/− mice and quantitative analysis of Western blot for phospho-Smad2/total Smad2. * p<0.05 vs. saline-injected wild-type mice.
We previously demonstrated MCP-1’s importance by showing that the anabolic effects of daily injections of PTH are abolished in MCP-1 null mice [17]. Nevertheless, MCP-1 function appears to be complex. We also showed that the MCP-1−/− mice are unable to recruit osteoclasts or macrophages in response to daily injected PTH (1–34) [17]. Yet, the mediator between these two events was unknown. A body of literature has shown that TGF-β is abundantly present in bone matrix in a latent form [29]; is released and activated by osteoclastic action [30]; and is able to recruit BMSCs to osteoclastic resorption sites through Smad 2/3 signaling [31]. Thus, TGF-β couples bone resorption to formation. It has been shown that PTH increases TGF-β concentrations and mRNA in human osteoblasts [32, 33]. To examine whether the requirement of MCP-1 in the anabolic effect of PTH involves TGF-β signaling in vivo, we stained for TGF-β action in sections of bone from wild-type or MCP-1−/− mice that had received PTH or saline. We used the phosphorylation of Smad2 as a marker of TGF-β action. As is evident in Fig. 1C, there is minimal staining for phosphorylated Smad2 in marrows of the MCP-1 null mice, especially after PTH treatment, whereas this staining is enhanced in marrows of wild-type mice. It is notable in the wild-type mice that there is minimal staining for phospho-Smad2 associated with cells on bone surfaces, suggesting that the majority of TGF-β signaling occurs in BMSCs in the marrow. Our results suggest that MCP-1 null mice show no PTH-stimulated increase in Smad2 phosphorylation. Further, we have isolated bone marrow from the same mice and performed Western blots to examine the expression of phospho-Smad2. The results show that PTH significantly increased Smad-2 phosphorylation in the bone marrow of wild-type mice. Deletion of MCP-1 prevented the PTH-mediated increase in Smad-2 phosphorylation, suggesting that MCP-1 is required for PTH-mediated TGF-β activation and signaling (Fig 1D).
3.2. MCP-1 is required for PTH-mediated osteogenic gene expression in bone.
PTH’s anabolic effects are attributed to the stimulation of osteogenic genes, which favor the differentiation of precursor cells into mature osteoblasts [34]. In fact, we and others have shown that daily injections of PTH (1–34) regulate expression of many osteoblastic genes [8]. To determine the requirement of MCP-1 in PTH-mediated osteogenic gene expression, 4-month-old male wild-type and MCP-1−/− mice were injected daily with 80 μg/ kg hPTH (1–34) or saline for 6 weeks and bones were collected 2 h after the last injection. qPCR analysis was performed to assess the expression of osteogenic genes such as alkaline phosphatase, Runx2, Osterix, Fos, Col1a1, osteocalcin (Ocn), Ccr2 and Tgfb1 (Figure 2A–H). Intermittent PTH failed to induce the expression of osteogenic genes in bones of MCP-1−/− mice while there was an increase in the expression of these genes in wild-type mice (Fig 2A–H).
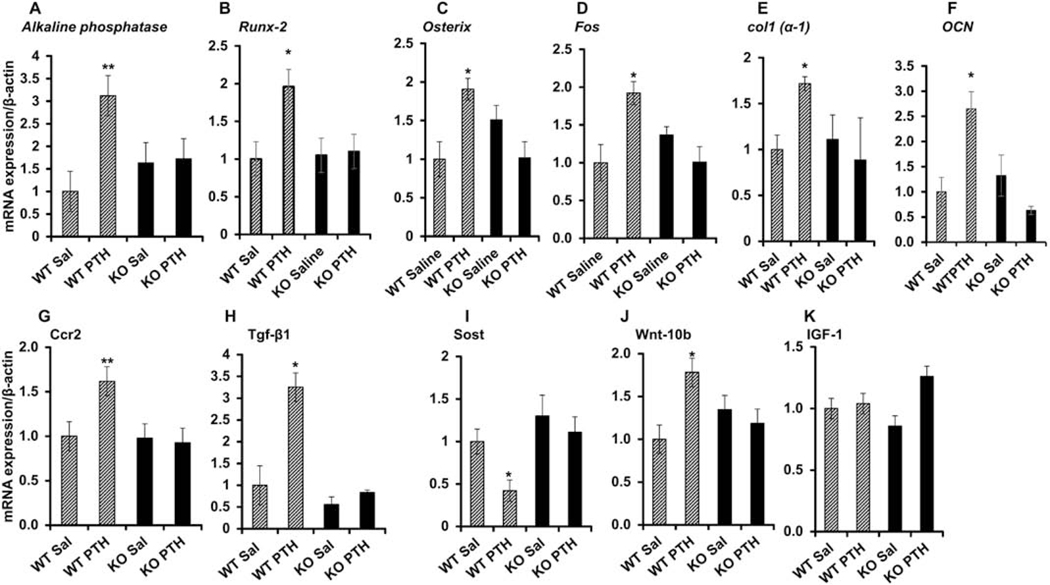
Figure 2: MCP-1 is required for PTH-mediated osteogenic gene expression in bone.
(A-H) Four-month-old male WT and MCP-1−/− mice were injected daily with 80 μg/kg hPTH (1–34) or saline for 6 weeks and bones were collected 2 h after the last injection. qPCR analysis was performed to determine the expression of genes such as alkaline phosphatase, Runx2, Osterix, Fos, Col1a1, osteocalcin (Ocn), Ccr2 and Tgfb1. Intermittent PTH failed to induce the expression of these genes in bones of MCP-1−/− mice. (I-J) MCP-1 null mice show no PTH regulation of the Wnt pathway, Sost and Wnt10-b. (K) Igf-1 mRNA was unchanged after PTH injection in both genotypes. All bones were collected 2 h after the last injection and RNA isolated from the distal femurs for qRT-PCR, (n=3 in each group). *p < 0.05 and **p < 0.01 compared to saline-injected WT mice.
In addition to modulating TGF-β expression and action, PTH also regulates bone remodeling by coordinating signaling of other local factors, such as Wnts, bone morphogenetic proteins, and IGF-1. In fact, we found that PTH stimulation of Wnt10b and inhibition of Sost was ablated in the absence of MCP-1, while regulation of both genes was seen in wild-type mice (Fig. 2I and J). We have not observed any changes in IGF-1 mRNA after PTH injection in both genotypes (Fig. 2K). To investigate the role of MCP-1 on osteoblastogenesis and mineralization, bone marrow cells from WT and MCP-1−/− mice after 6 weeks of daily PTH or saline injections were cultured in bone marrow mineralization medium for 21 days as shown in Supplementary Fig 1A. hPTH injections enhanced mineralization of cells from WT bone marrows compared with the saline group (Supplementary Fig 1B and C). The deletion of MCP-1 abolished the hPTH-mediated bone marrow mineralization, indicating that MCP-1 is required for bone marrow osteoblastogenesis.
3.3. MCP-1 treatment of MCP-1 deficient mice mimics PTH’s anabolic effects on trabecular bone
To determine whether MCP-1 replacement would rescue the PTH-mediated bone anabolic effect in MCP-1−/− mice, we injected rhMCP-1 peptides to 4-month-old MCP-1−/− male mice daily. Preliminary experiments were done with 3 different doses of MCP-1 (0.25, 0.5 and 1 μg/mouse) for 6 days and blood collected daily after 2 h to measure the circulating concentrations of MCP-1 by ELISA. We found that all doses significantly elevated the serum MCP-1 levels in a dose-dependent fashion (Fig 3A and B). Higher doses of rhMCP-1 (0.5 and 1 μg/mouse) injections significantly increased most of the bone anabolic parameters even after 10 days of injections compared with the saline-injected wild-type mice (Supplementary Fig 2A). These higher doses also increased the trabecular bone structure and TGF-β signaling in the bone marrow to those comparable to PTH treatment of wild-type mice (Supplementary Fig 2A, B and C). The 0.25 μg/mouse dose of rhMCP-1 treatment mimics the serum MCP-1 levels seen in wild-type animals after PTH treatment with the transient doubling of basal levels (Fig 3A and Supplementary Fig 3A and B). Thus, we used this dose for extended studies.
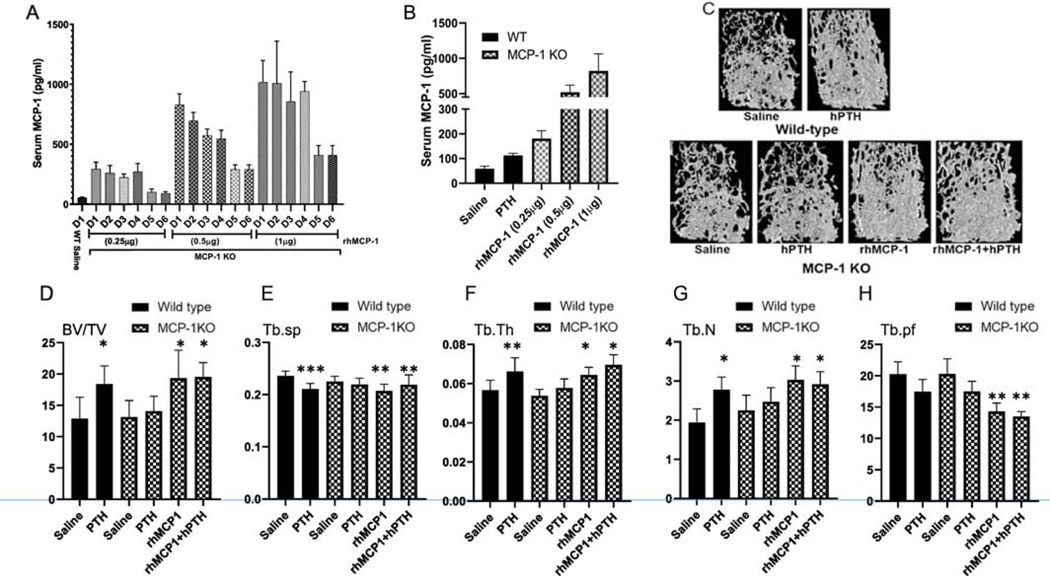
Figure 3: MCP-1 treatment mimics the PTH anabolic effect in trabecular bone of MCP-1 deficient mice.
All mice were 4-month old males at the start of the injections. (A) Serum MCP-1 levels after different doses of rhMCP-1 injections (0.25, 0.5 and 1 μg/mice) given daily for six days and blood collected 2 h after each of the injections to measure the circulating concentrations of MCP-1 by ELISA. (B) Serum MCP-1 levels after 10 days of daily injections for the conditions shown in the figure (hPTH; hPTH (1–34) at 80 ug/kg to WT mice) and blood collected 2 h after the last injection. (C) Representative μCT images of the trabecular bone of femurs after 6 weeks of the different daily treatment regimes as shown. hPTH (1–34) was given at 80 μg/kg; rhMCP-1 was given at 0.25 μg /mouse. (D–H) μCT analyses of the distal femoral trabecular bone of the treatments in (C) showing BV/TV (%), Tb.Sp (mm), Tb.Th (mm), Tb.N (1/mm) and Tb.Pf (1/mm). Values are expressed as mean ± SEM (n = 8 mice/group). *p < 0.05 and **p < 0.01 compared to saline-injected WT mice.
The MCP-1 null mice were injected with this chosen dose (0.25 μg/mouse) daily or with the saline vehicle; half of the animals in each group also received daily PTH (80 μg/kg) injections, while the other half received the saline vehicle for six weeks. Wild type animals also received PTH or saline for comparison. As we and others have reported [17, 35], hPTH injections increased trabecular bone mass in WT mice (Fig. 3D and Supplementary Fig. 2A). However, PTH was unable to increase trabecular bone mass in MCP-1−/− mice compared with the vehicle-treated KO mice, as we have previously observed. In particular, hPTH increased trabecular bone volume (BV/TV) (Fig. 3D), trabecular thickness (Tb.Th) (Fig. 3F), trabecular number (Tb.N) (Fig 3G) and decreased trabecular separation (Tb.Sp) (Fig. 3E) and trabecular pattern formation (Tb.pf) in WT mice compared with the vehicle-treated WT mice. However, MCP-1−/− mice failed to show the hPTH-mediated bone anabolic effects. Daily exogenous MCP-1 injections to MCP-1 null mice rescued the trabecular bone anabolic parameters to those seen in WT mice treated with hPTH (Figure 3D–H). We found that rhMCP-1 injections alone increased trabecular bone parameters suggesting that this chemokine is sufficient for PTH’s anabolic effects.
3.4. MCP-1 injections restored hPTH anabolic effects of the bone microarchitecture in the cortical compartment
Similar to our previous observations, μCT analysis of cortical bone (midshaft femur) showed that 6 weeks of hPTH injections significantly increased the cortical bone volume (BV/TV) (Fig 4B), total area (T.Ar) (Fig 4C), bone perimeter (B.Pm) (Fig 4D), total cortical thickness (Cs.Th) (Fig 4E), bone area (B.Ar) (Fig 4F) and cortical area/ total area (Fig 4G) in WT mice. However, hPTH injections did not change any of these parameters in MCP-1−/− mice, and nor were MCP-1 injections alone able to have an effect on cortical bone. In contrast, daily rhMCP-1 (0.25 μg/mouse) injections, together with PTH injections, significantly increased the cortical bone microarchitectural parameters in MCP-1−/− mice (Fig 4B–G). The combination of rhMCP-1 along with hPTH showed a higher anabolic effect on BV/TV compared to hPTH injection of WT mice. These data suggest that MCP-1 replacement, along with PTH administration, is required and beneficial for the anabolic effect of hPTH in cortical bone. It also suggests that, in cortical bone, PTH must elicit other pathways, as well as require MCP-1, for its anabolic effects.
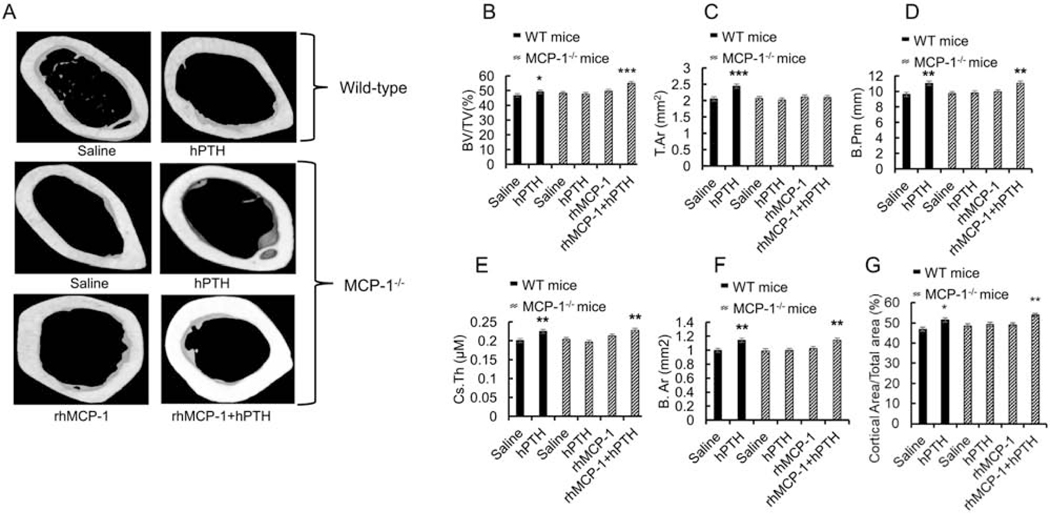
Figure 4: MCP-1 injections restored hPTH anabolic effects in the bone microarchitecture in the cortical compartment.
MicroCT analysis of the cortical bone of the femurs of wild-type and MCP-1−/− mice following PTH and/or MCP-1 daily injections for 6 weeks. A). Representative images of cortical bone in the femoral diaphysis from each treatment group of wild-type and MCP-1−/− mice are shown. (B-G) μCT analyses of cortical bone in the femurs of mice after 6 weeks of injections showing BV/TV (%), T.Ar (mm2), B.Pm. (mm), Cs.Th. (μm), B.Ar (mm2) and cortical area/total area (%). All values are expressed as mean ±SEM (n=8 mice/group). *p < 0.05, **p < 0.01 and ***p < 0.001 compared to saline-treated WT mice.
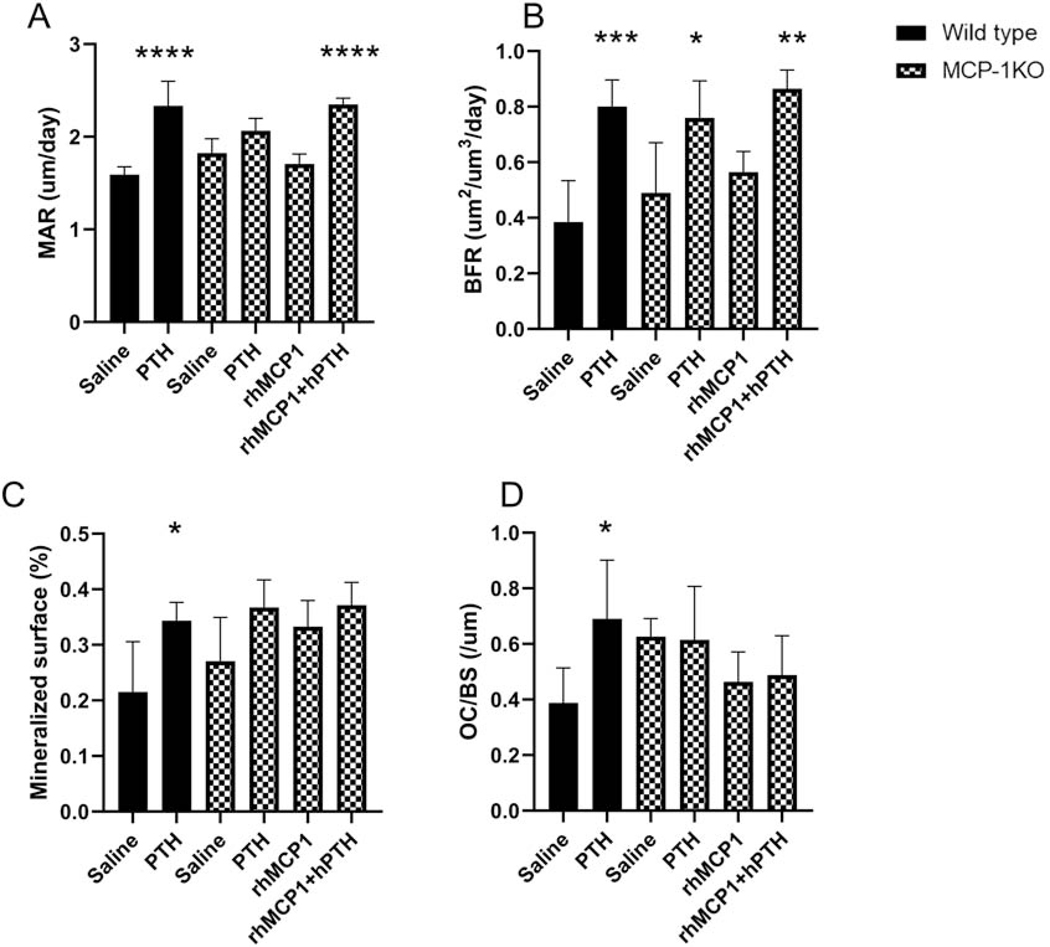
3.5. MCP-1 injections augment the PTH mediated dynamic histomorphometry parameters in MCP-1−/− mice
Dynamic histomorphometric analysis revealed that 6 weeks of hPTH injections significantly increased the mineral apposition rate (MAR) and mineralized surface in wild type mice compared with vehicle-treated mice but failed to show the effect in MCP-1 null mice (Fig 5A and C). However, the bone formation rate (BFR) increased in wild-type and MCP-1−/− mice after hPTH treatment, which we have previously observed [17]. In contrast, daily rhMCP-1 (0.25 μg/mouse) injections, together with PTH injections, significantly increased the MAR and BFR in MCP-1−/− mice while not changing the mineralized surface (Fig 5A, B and C). As we have previously observed, 6 weeks of daily hPTH injections significantly increased the osteoclast number to bone surface (Fig 5D) while no changes were observed in the PTH-treated MCP-1−/− mice. Surprisingly, no changes were observed in the mice that received rhMCP-1, either alone or with PTH. This is in distinct contrast to the changes observed in marrow monocyte and macrophage numbers (see Fig. 6B below).
Figure 5: MCP-1 injections augment the PTH mediated dynamic histomorphometry indices in MCP-1−/− mice.
Dynamic histomorphometric analysis of the femurs of wild-type and MCP-1−/− mice following PTH and/or MCP-1 daily injections for 6 weeks. (A) Mineral apposition rate (MAR), (B) Bone formation rate (BFR), (C) Mineralized surface of femurs of WT and MCP-1−/− mice after 6 weeks of the various treatments. (D) Osteoclast numbers/Bone surface of the same animals. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to saline-treated WT mice. All values are expressed as mean ±SEM (n=5 mice/group).
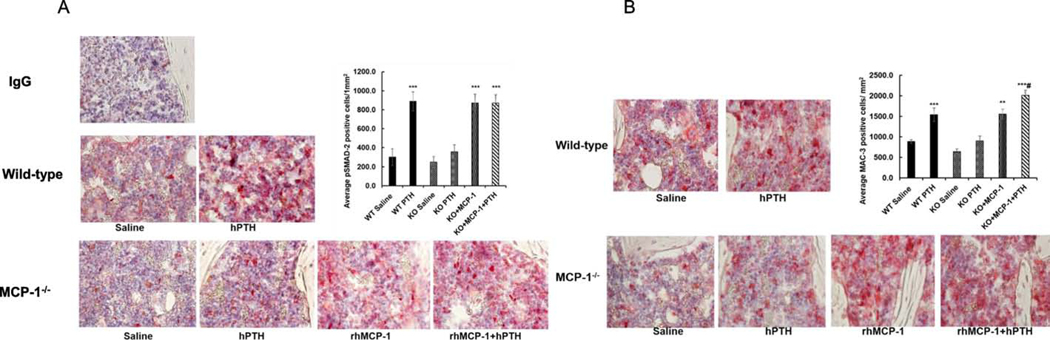
Figure 6: Restoration of increased macrophage numbers and enhanced TGF-β signaling with injected MCP-1 in MCP-1-deficient mice.
(A) Immunohistochemistry of phospho-Smad2 in the tibiae after 6 weeks of hPTH (1–34; 80 ug/kg) and/or rhMCP-1 (0.25μg/mouse) injections to wild-type and MCP-1−/− mice. rhMCP-1 injections rescued the p-Smad2 staining in MCP-1−/− mice to comparable to hPTH-injected wild-type mice by quantitation of stained cells. (B) Mac3 immunostaining of sections of tibiae of WT and MCP-1−/− mice after 6 weeks of the various treatments. There is a modest expression of Mac-3 in MCP-1 null mice, with and without hPTH injections and rhMCP-1 injections increased the Mac-3 positive cells in the tibiae of MCP-1−/− mice, as seen by the quantitation of stained cells. IgG served as a negative control. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to vehicle-injected WT mice and #p < 0.05 compared to PTH-injected WT mice by two-way ANOVA.
3.6. Restoration of increased macrophage numbers and enhanced TGF-β signaling with injected MCP-1 in MCP-1-deficient mice.
To investigate whether rhMCP-1 injection would restore PTH-mediated TGF-ꞵ signaling, we injected rhMCP-1 or hPTH for 6 weeks in MCP-1−/− mice and performed immunostaining for phospho-Smad-2 in tibial sections of MCP-1−/− and wild-type mice. rhMCP-1 injection restored Smad-2 phosphorylation in MCP-1−/− mice to that seen in wild-type mice after PTH injections (Fig. 6A). These data suggest that MCP-1 expression is vital for PTH-mediated TGF-β activation and signaling. In addition, we have found significant increases in numbers of cells stained for Mac-3 (mouse macrophage-specific marker) in the secondary spongiosae after rhMCP-1 injections in MCP-1−/− mice similar to that seen in wild-type PTH-injected mice, establishing that MCP-1 is required for monocyte and macrophage recruitment in bone marrow (Fig. 6B).
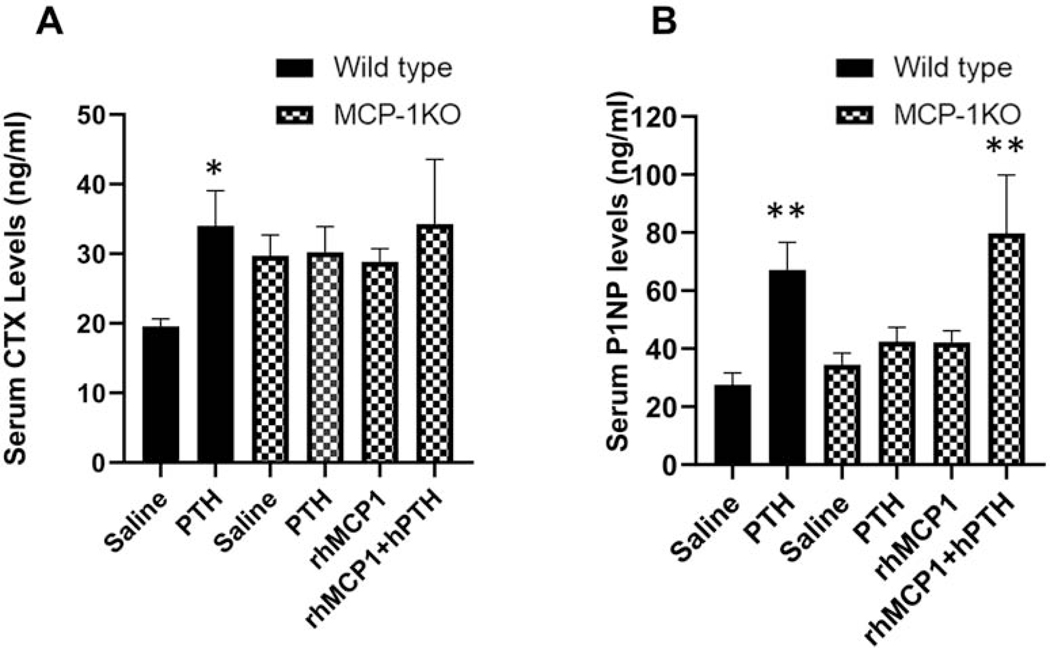
3.7. Effects of MCP-1 injections on biochemical markers of bone turnover
Based on the differential effects of PTH in wild-type and MCP-1−/− mice on many bone parameters as determined by micro-CT and immunohistochemistry, we next examined alterations in the serum levels of biochemical markers of bone turnover. Specifically, P1NP and CTX-1, markers of bone formation and bone resorption respectively were determined by ELISA. Wild-type mice showed an increase in CTX and P1NP levels after 10 days of PTH injections and rhMCP-1 (1μg) injections increased serum P1NP almost 2-fold in MCP-1−/− mice (Supplementary Fig 4A and B). As shown in Fig 7A, intermittent PTH injections for 6 weeks resulted in increased serum levels of CTX-1 in wild-type mice but no significant changes in MCP-1−/− mice, even with rhMCP-1 administration. Likewise, PTH injections increased P1NP in wild-type mice and failed to show an effect in MCP-1−/− mice while rhMCP-1 (0.25 ug) injections rescued the hPTH-mediated stimulation of serum P1NP in MCP-1−/− mice (Fig 7B). These data are similar to the μCT and histomorphometry observations.
Figure 7: Determination of biochemical markers of bone formation and bone resorption.
Sera were isolated from wild-type and MCP-1−/− mice after 6 weeks of saline, hPTH (1–34) and/or rhMCP-1 injections. The levels of carboxy-terminal cross-linking telopeptides of type I collagen (CTX, A), a marker of bone resorption and P1NP (B), a marker of bone formation, were determined by ELISAs. Data represent mean ± SEM of 8 mice per group. *p < 0.05 and **p<0.01 compared with vehicle-treated WT mice.
4. Discussion
Although intermittent PTH (1–34) treatment is a current anabolic therapy to treat osteoporosis, the exact mechanism of action remains elusive. MCP-1, also known as CCL2, is a member of the C-C chemokine family and has a potent chemotactic property for monocytes. Our previously published in vitro and in vivo data established a vital role for MCP-1 in PTH action on bone [36, 37]. Here we report that in vivo intermittent PTH treatment increases MCP-1 mRNA expression by bone-lining mononuclear cells. Furthermore, MCP-1 appears necessary for PTH-enhanced TGF-β action and this can be restored by MCP-1 replacement. We think the TGF-β signaling pathway facilitates the recruitment of mesenchymal cells towards the active bone surface. Daily injections of rhMCP-1 rescued the bone anabolic effect of hPTH and TGF-β signaling in MCP-1 null mice. These data suggest that MCP-1 is essential for the PTH-mediated bone anabolic effect through increased signaling of TGF-β.
It is well established that chemokines have a diverse effect on bone and its cells. CXC and CC subtypes of chemokines promote the migration of osteoclast precursor cells and assist osteoclast formation and bone resorption [38]. MCP-1, a potent chemokine for monocytes and macrophages, plays a dynamic role in PTH-induced bone resorption [2]. The source of MCP-1 can be diverse with many cell types, such as osteoblasts, endothelial cells, fibroblasts, epithelial cells, smooth muscle cells and monocytes, producing it. In vitro and in vivo studies demonstrated increased expression of MCP-1 by rat osteoblasts following exposure to PTH, leading to bone resorption via chemoattraction of pre-osteoclasts and finally, an increase in osteoclastogenesis [18].
In our previous studies, we have reported that MCP-1 is the most highly up-regulated gene after intermittent hPTH (1–34) injections in rats [8, 18]. In addition to increased mRNA expression in bone, serum MCP-1 levels almost double after 14 days of hPTH injections [17] and we have shown the same in mice (Supplementary Fig 3). Furthermore, MCP-1 is necessary for hPTH’s bone anabolic effects [17]. In another study from our group, we have found that MCP-1 is also necessary for PTH’s catabolic effects [2]. Continuous infusion of PTH fails to induce monocyte and macrophage recruitment and bone loss in MCP-1 null mice [2]. Together, these show that MCP-1 is required for both anabolic and catabolic responses to PTH in bone and MCP-1 may provide a pharmacological target for PTH-induced bone disease [2, 17].
Human PTH (1–34) injections increased total bone mRNA for MCP-1. The RNA was obtained from the trabecular-rich metaphyseal region of the distal femur, which is an osteoblast-rich site. It was important to identify the cells in the bone which express MCP-1 mRNA after PTH treatment. However, MCP-1 is a secreted protein and not clearly detected by immunohistochemistry in the early time period after the first PTH injection. To address this issue, we performed in situ hybridization for MCP-1 to provide unambiguous information of the primary cell of its origin in response to PTH. Here in this present study, the in situ hybridization of MCP-1 clearly showed that PTH stimulates initial expression of MCP-1 in bone lining mononuclear cells.
Although it was established that MCP-1 is necessary for PTH’s anabolic effects on bone and that the recruitment of macrophages and osteoclasts is abolished in MCP-1 null mice [17], we did not know the mediators that couple these two events. We have found that TGF-β signaling increased after hPTH injections in wild-type mice but not in MCP-1−/− mice, indicating that TGF-β is one of the critical mediators. A body of literature has shown that TGF-β is especially enriched in bone matrix [39], is released and activated by osteoclastic action [30], and is able to recruit bone marrow stromal cells (BMSCs) to osteoclastic resorption sites through Smad 2/3 signaling [31]. Thus, an MCP-1-mediated increase in TGF-β may act as the coupling factor for bone resorption to formation. However, we do not know the cell types that express increased pSmad 2 after PTH treatment. This finding is similar to the data from previous studies that suggest that inhibition of bone resorption, followed by decreased bone turnover, decreases PTH-induced bone formation, implying a requirement for the osteoclast in PTH’s anabolic effects. Bisphosphonates, known inhibitors of bone resorption, can blunt the PTH-mediated anabolic effect, suggesting that active bone resorption is prerequisite for the anabolic actions of PTH [40, 41].
Since the TGF-β pathway is only one of several pathways involved in bone anabolism, we investigated whether MCP-1 may have effects on additional pathways. We found that deletion of MCP-1 prevented PTH effects on two parts of the Wnt pathway, which has a major role in bone acquisition and metabolism. MCP-1−/− mice injected with hPTH showed no decline in mRNA levels for the Wnt pathway inhibitor, Sost, and no stimulation of Wnt 10b, while both effects were seen in wild-type mice (Fig. 8). Lack of MCP-1 may prevent release of active TGF-β from the bone matrix in MCP-1 null mice and thus, fail to inhibit Sost and stimulate Wnt10b expression respectively in MCP-1−/− mice. It should be noted that Igf1 mRNA levels were unchanged in any treatment groups. Thus, concerning PTH’s anabolic effects, MCP-1 appears to be upstream of both the TGF-β and Wnt pathways, broadening its importance as a required mediator of PTH action.
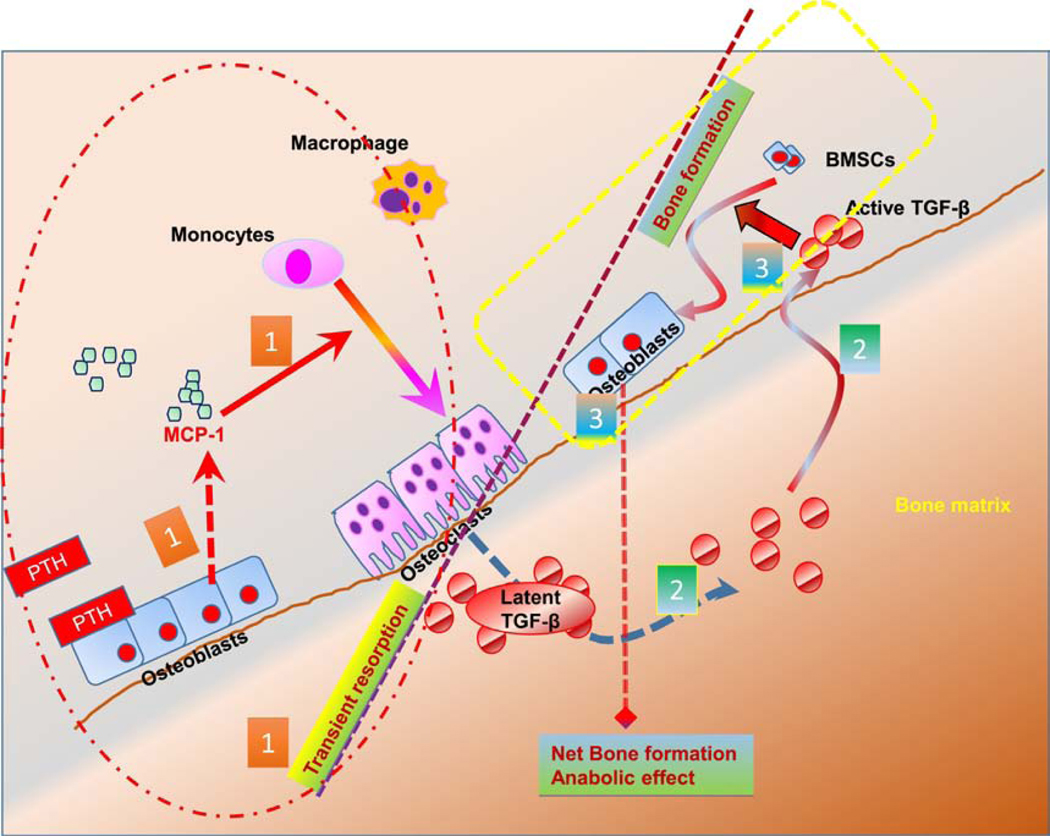
Figure 8: Schematic diagram of MCP-1 signaling and PTH mediated bone anabolic effects.
(1) Intermittent PTH rapidly increases MCP-1 expression in the osteoblast. Osteoblastic MCP-1 is necessary for recruitment of monocytes to macrophages and osteoclasts after PTH treatment and the latter’s transient enhanced breakdown of bone. (2) The bone matrix contains abundant latent TGF-β. Short-term osteoclastogenesis increases the release of active TGF-β from bone matrix. (3) Finally, the active TGF-β then acts on bone marrow stromal cells (BMSCs) and recruits them to osteoclastic resorption sites where they initiate new bone formation.
Quantitative RT-PCR data showed that the intermittent hPTH regime increased the expression of bone-forming markers such as alkaline phosphatase, collagen type A1 (Col-1A1), osteocalcin and osteoblast-specific transcription factors (Runx2 and Osterix) in the bones of the wild-type mice while failing to increase these in MCP-1 null mice, further suggesting the importance of MCP-1 for PTH-mediated increase in anabolic genes. In addition, we have also found that hPTH injections significantly increased Fos expression in bone and enhanced bone marrow mineralization compared with the saline group in wild-type but not in MCP-1 null mice suggesting that MCP-1 is required for bone marrow mesenchymal cell recruitment and their osteoblastogenesis.
Similar to an earlier study, PTH showed bone anabolic effects in wild-type mice but not in MCP-1−/−mice. As reported earlier, due to redundancy in the chemokine system and because CCR2 has other ligands than MCP-1 [42, 43], we have not seen any basal developmental bone phenotypes in MCP-1 null mice [2, 17] although this has been observed by Binder et al [16]. Since we have seen that hPTH injections failed to induce the bone anabolic effect in MCP-1−/− mice, we were interested in determining whether daily rhMCP-1 injection would rescue the PTH-mediated bone anabolic effect in these mice. Daily injections of the rhMCP-1 peptide (0.25, 0.5 and 1μg/mice) for 1 week significantly elevated serum MCP-1 levels dose-dependently. Interestingly, even the lower dose (0.25μg/mice) of rhMCP-1 restored MCP-1 serum levels in MCP-1−/− mice comparable to PTH-injected wild-type mice. Higher doses of rhMCP-1 (0.5 and 1 μg/mouse) injections significantly increased the bone architecture and most of the bone anabolic parameters even after only 10 days compared with the saline-injected wild-type mice (Supplementary Fig 2A). TGF-β signaling also increased after rhMCP-1 (0.5 and 1 μg/mouse) injections in MCP-1−/− mice and comparable to wild-type mice treated with PTH (Supplementary Fig 2B, C). The lower dose of exogenous MCP-1 (0.25 μg/mice) rescued an increase in trabecular bone anabolic parameters in MCP-1 null mice (Figure 3). Similarly, we have seen that rhMCP-1 injections for 6 weeks enhanced hPTH-mediated bone microarchitecture in the cortical compartment of MCP-1 null mice. Interestingly, we have observed a prominent effect of rhMCP-1 in trabecular bone, but it is less effective on cortical bone. These μCT data confirm that an increase in serum MCP-1 level is required for the functional anabolic effect of hPTH.
Increase in Mac-3 staining in the secondary spongiosae after rhMCP-1 injections in MCP-1−/− mice suggest that MCP-1 is a prerequisite for macrophage and monocyte recruitment in the bone marrow. Immunohistochemistry of tibial sections of rhMCP-1 injected mice showed an increase in Smad-2 phosphorylation in MCP-1−/− mice further indicating that a PTH-mediated increase in MCP-1 enhanced TGF-β signaling in the bone marrow, which may facilitate the recruitment of BMSCs (precursors of osteoblasts) towards the bone remodeling area, and couple bone resorption to bone formation. We have already reported that MCP-1 has direct effects on the pre/osteoclastic cell and on monocytes/macrophages, and MCP-1 enables the PTH-mediated bone loss independently of RANKL and OPG in conditions of continuous infusion, clearly demonstrating an important independent, entirely separate role from the RANKL/OPG axis [2].
Together, these findings confirm the additive osteoanabolic effect of MCP-1 and intermittent PTH on bone. It has been reported that both continuous and intermittent PTH treatment increased MCP-1 expression, probably via the PKA pathway, in the femur of rats and rat osteoblastic cells [8]. However, the amount and duration of MCP-1 expression determine the outcome of PTH actions (anabolic vs. catabolic) in bone. In the catabolic protocol, the upregulation of MCP-1 was moderate but sustained continuously leading to bone resorption overwhelming bone formation with a net result of bone loss. Similarly, patients with primary hyperparathyroidism demonstrated increased serum MCP-1 levels [44]. However, intermittent PTH administration leads to a transient increase, followed by a rapid decline in MCP-1 levels. We postulate that the initial spike in MCP-1 expression results in a short-term increase in bone resorption. This MCP-1-mediated transient bone resorption may be responsible for TGF-β activation from bone matrix and a subsequent increase in bone formation or PTH’s anabolic effect in the bone. Similarly, several clinical and preclinical studies reported the active involvement of initial osteoclastic resorption for the full anabolic effect of PTH on bone [41, 45]. For all these reasons, MCP-1 appears to be an important target for elucidating PTH’s actions on bone.
In conclusion, we demonstrated that MCP-1 is a highly and rapidly regulated chemokine in bone from an anabolic protocol of PTH injections. The MCP-1 null mice cannot display a PTH-stimulated increase in bone mass and osteoclasts and macrophages nor show PTH-enhanced TGF-β signaling, as seen in wild-type mice. We think that the latent TGF-β1 released from bone matrix augments the recruitment of BMSCs towards remodeling sites through TGF-β type-2 receptor present on bone marrow stromal cells and facilitates osteoblast differentiation and bone formation (Fig. 8). However, this can only be unequivocally proven by studies with TGF-β receptor knockout mice. Nevertheless, exogenous injections of the MCP-1 peptide in MCP-1 null mice rescued the anabolic effect and PTH’s stimulation of TGF-β signaling. These accumulated data define a novel mechanism of PTH’s anabolic actions on bone and the essential role of MCP-1 in this process. In patients with postmenopausal osteoporosis, serum MCP-1 serves as a potential biomarker reflecting disease severity and therapeutic interventions that target MCP-1 and its related signaling pathways in order to delay osteoporosis warrant additional study [46].
Supplementary Material
Supplementary Figure 1: MCP-1 is required for bone marrow osteoblastogenesis. (A) Four-month-old male WT and MCP-1−/− mice were injected daily with 80 μg/kg hPTH (1-34) or saline for 6 weeks and bones were collected 2 h after the last injection. Bone marrow cells from these bones were cultured in bone marrow mineralization medium for 21 days. hPTH (1-34) injections significantly enhanced the mineralization of the bone marrow cells compared with the saline group. The deletion of MCP-1 blunted the hPTH-mediated bone marrow cell mineralization. (B) Alizarin red-S staining. (C) Quantification of alizarin red-S staining with cetylpyridinium chloride (CPC) extraction at pH 7.0 and quantification. *p<0.05 compared to saline-injected WT mice.
Supplementary Figure 2:: Short-term high dose rhMCP-1 injections increased the bone parameters in MCP-1−/− mice. (A) μCT parameters of the trabecular region of femurs after the various treatment regimes for 10 days showing BV/TV (%), Tb.Th (mm), Tb.Sp (mm), and Tb.N (1/mm) and Tb.Pf (1/mm). (n = 4 mice/group). *p < 0.05 compared to saline-injected WT mice. Stippled bars are MCP-1−/− mice. (B) H&E staining of the sections of tibiae of WT and MCP-1−/− mice after 10 days of treatment with hPTH (1-34) and rhMCP-1 (0.5-1 μg/mouse) respectively. (C) Immunohistochemistry of pSmad-2 in the tibia after 10 days of daily hPTH (80μg/kg) and/or rhMCP-1 (0.5-1μg/mouse) injections to wild-type and MCP-1−/− mice, respectively. rhMCP-1 injections rescued the pSmad-2 staining in MCP-1−/− mice to be comparable to hPTH-injected wild-type mice.
Supplementary Figure 3: PTH increased MCP-1 serum levels in mice in vivo. (A) Four month old male mice were injected with vehicle (saline), or 80 μg/kg hPTH(1-34) and blood was collected as shown. A single injection of hPTH increased MCP-1 levels to double at 2 h. (B) MCP-1 levels 2 h after each injection. On day 2 and 3 of PTH injections, the serum MCP-1 levels were elevated to 4-fold but declined at later days, although still being double the baseline values.
Supplementary Figure 4:: Effect of short-term high dose rhMCP-1 injections on bone biochemical markers in MCP-1−/− mice. Sera were isolated 2 h after the last injection from wild-type and MCP-1−/− mice after 10 days of daily PTH or rhMCP-1 (0.5-1μg/mouse) injections. The levels of carboxy-terminal cross-linking telopeptides of type I collagen (CTX), a marker of bone resorption and P1NP, a marker of bone formation, were determined by ELISA. hPTH increases both serum CTX and P1NP in wild-type mice while rhMCP-1 (1μg/mouse) only increases P1NP to that comparable to the PTH-injected wild-type mice. (n = 4 mice/group). *p < 0.05, compared to vehicle-treated WT mice.
Table 1.
The mouse-specific primer sequences used for RT-qPCR analysis
| Gene name | Primer sequence | Accession number |
|---|---|---|
| β-actin | F 5′-TCC TCC TGA GCG CAA GTA CTC T-3′ R 5′-CGG ACT CAT CGT ACT CCT GCT T-3′ |
NM_007393.5 |
| ALP | F 5′-ATCTTTGGTCTGGCTCCCATG −3′ R 5′- TTTCCCGTTCACCGTCCAC −3′ | NM_007431.3 |
| Runx2 | F 5′- AGTCCCAACTTCCTGTGCTCC-3 R 5′- CGGTAACCACAGTCCCATCTG-3′ | NM_001146038.2 |
| Osx | F 5′- ACCTAACAGGAGGATTTTGGTTTG −3′ R 5′- GCCTTTGCCCACCTACTTTTT-3′ | XM_006520519.4 |
| Col 1a1 | F 5′- CCTTCATGTCCAAGCAGGA −3′ R 5′- GCGCCGGAGTCTGTTCACTA −3′ | NM_007742.4 |
| Osteocalcin | F 5′- GCAATAAGGTAGTGAACAGACTCC −3′ R 5′- GTTTGTAGGCGGTCTTCAAGC −3′ | NM_001032298.3 |
| Tgfb1 | F 5′- ACAAGAGCAGTGAGCGCTGAA-3′ R 5′- GTGTGGAGCAACATGTGGAAC-3′ | NM_011577.2 |
| SOST | F 5′- AGCCTTCAGGAATGATGCCAC-3′ R 5′- TTTGGCGTCATAGGGATGGT-3′ | NM_024449.6 |
| CCR2 | F 5′- TTTGTTTTTGCAGATGATTCAA-3′ R 5′- TGCCATCATAAAGGAGCCAT-3′ | NM_009915.2 |
| Wnt10b | F 5′- ACGACATGGACTTCGGAGAGAAGT −3′ R 5′- CATTCTCGCCTGGATGTCCC −3′ | XM_006520891.3 |
| c-fos | F 5′- AATGGTGAAGACCGTGTCAGGA −3′ R 5′- CCCTTCGGATTCTCCGTTTCT −3′ | NM_010234.3 |
| IGF-1 |
Highlights:
PTH-enhanced TGF-β signaling is abolished in the marrows of MCP-1 knockout mice.
MCP-1 expression is necessary for the PTH recruitment of monocytes/macrophages.
rhMCP-1 peptide treatment rescues PTH’s anabolic effects in MCP-1 deficient mice.
Acknowledgements
This work was supported in part by the National Institutes of Health CTSA grant UL1 TR001445 from the National Center for Advancing Translational Sciences and by S10 OD010751 (to N.C.P.).
Footnotes
Declarations of Interest: None
Present address: Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center, Omaha, NE, 68198–5870
Credit Author Statement
Authors’ roles: Conceptualization: NCP. Data curation: JAS, CLH, ZH and JJ. Formal analysis: JAS, CLH, ZH, JJ and NCP. Funding acquisition: NCP. Investigation: JAS and CLH. Methodology: JAS, CLH, ZH, JJ and DBR. Project administration: NCP. Roles/Writing – original draft: JAS, ZH, JJ, DBR and NCP. Writing – review and editing: JAS, CLH, ZH, JJ, DBR and NCP. JAS takes responsibility for the integrity of the data analyses.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Thompson DD, Seedor JG, Fisher JE, Rosenblatt M, Rodan GA, Direct action of the parathyroid hormone-like human hypercalcemic factor on bone, Proc Natl Acad Sci U S A 85(15) (1988) 5673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siddiqui JA, Johnson J, Le Henaff C, Bitel CL, Tamasi JA, Partridge NC, Catabolic Effects of Human PTH (1–34) on Bone: Requirement of Monocyte Chemoattractant Protein-1 in Murine Model of Hyperparathyroidism, Sci Rep 7(1) (2017) 15300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R, Anabolic actions of parathyroid hormone on bone, Endocr Rev 14(6) (1993) 690–709. [DOI] [PubMed] [Google Scholar]
- [4].Silva BC, Costa AG, Cusano NE, Kousteni S, Bilezikian JP, Catabolic and anabolic actions of parathyroid hormone on the skeleton, J Endocrinol Invest 34(10) (2011) 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ, Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation, Cell 93(2) (1998) 165–76. [DOI] [PubMed] [Google Scholar]
- [6].Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T, Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL, Proc Natl Acad Sci U S A 95(7) (1998) 3597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH, Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis, N Engl J Med 344(19) (2001) 1434–41. [DOI] [PubMed] [Google Scholar]
- [8].Li X, Liu H, Qin L, Tamasi J, Bergenstock M, Shapses S, Feyen JH, Notterman DA, Partridge NC, Determination of dual effects of parathyroid hormone on skeletal gene expression in vivo by microarray and network analysis, J Biol Chem 282(45) (2007) 33086–97. [DOI] [PubMed] [Google Scholar]
- [9].Rahimi P, Wang CY, Stashenko P, Lee SK, Lorenzo JA, Graves DT, Monocyte chemoattractant protein-1 expression and monocyte recruitment in osseous inflammation in the mouse, Endocrinology 136(6) (1995) 2752–9. [DOI] [PubMed] [Google Scholar]
- [10].Hopwood B, Tsykin A, Findlay DM, Fazzalari NL, Gene expression profile of the bone microenvironment in human fragility fracture bone, Bone 44(1) (2009) 87–101. [DOI] [PubMed] [Google Scholar]
- [11].Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, Roodman GD, Zhang J, Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption, Cancer Res 67(8) (2007) 3646–53. [DOI] [PubMed] [Google Scholar]
- [12].Lu Y, Cai Z, Xiao G, Liu Y, Keller ET, Yao Z, Zhang J, CCR2 expression correlates with prostate cancer progression, J Cell Biochem 101(3) (2007) 676–85. [DOI] [PubMed] [Google Scholar]
- [13].Kim MS, Day CJ, Morrison NA, MCP-1 is induced by receptor activator of nuclear factor-{kappa}B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation, J Biol Chem 280(16) (2005) 16163–9. [DOI] [PubMed] [Google Scholar]
- [14].Kim MS, Day CJ, Selinger CI, Magno CL, Stephens SR, Morrison NA, MCP-1-induced human osteoclast-like cells are tartrate-resistant acid phosphatase, NFATc1, and calcitonin receptor-positive but require receptor activator of NFkappaB ligand for bone resorption, J Biol Chem 281(2) (2006) 1274–85. [DOI] [PubMed] [Google Scholar]
- [15].Miyamoto K, Ninomiya K, Sonoda KH, Miyauchi Y, Hoshi H, Iwasaki R, Miyamoto H, Yoshida S, Sato Y, Morioka H, Chiba K, Egashira K, Suda T, Toyama Y, Miyamoto T, MCP-1 expressed by osteoclasts stimulates osteoclastogenesis in an autocrine/paracrine manner, Biochem Biophys Res Commun 383(3) (2009) 373–7. [DOI] [PubMed] [Google Scholar]
- [16].Binder NB, Niederreiter B, Hoffmann O, Stange R, Pap T, Stulnig TM, Mack M, Erben RG, Smolen JS, Redlich K, Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis, Nat Med 15(4) (2009) 417–24. [DOI] [PubMed] [Google Scholar]
- [17].Tamasi JA, Vasilov A, Shimizu E, Benton N, Johnson J, Bitel CL, Morrison N, Partridge NC, Monocyte chemoattractant protein-1 is a mediator of the anabolic action of parathyroid hormone on bone, J Bone Miner Res 28(9) (2013) 1975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li X, Qin L, Bergenstock M, Bevelock LM, Novack DV, Partridge NC, Parathyroid hormone stimulates osteoblastic expression of MCP-1 to recruit and increase the fusion of pre/osteoclasts, J Biol Chem 282(45) (2007) 33098–106. [DOI] [PubMed] [Google Scholar]
- [19].Centrella M, Canalis E, McCarthy TL, Stewart AF, Orloff JJ, Insogna KL, Parathyroid hormone-related protein modulates the effect of transforming growth factor-beta on deoxyribonucleic acid and collagen synthesis in fetal rat bone cells, Endocrinology 125(1) (1989) 199–208. [DOI] [PubMed] [Google Scholar]
- [20].Centrella M, McCarthy TL, Canalis E, Parathyroid hormone modulates transforming growth factor beta activity and binding in osteoblast-enriched cell cultures from fetal rat parietal bone, Proc Natl Acad Sci U S A 85(16) (1988) 5889–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nakatani T, Chen T, Johnson J, Westendorf JJ, Partridge NC, The Deletion of Hdac4 in Mouse Osteoblasts Influences Both Catabolic and Anabolic Effects in Bone, J Bone Miner Res 33(7) (2018) 1362–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pfaffl MW, A new mathematical model for relative quantification in real-time RT-PCR, Nucleic Acids Res 29(9) (2001) e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R, Guidelines for assessment of bone microstructure in rodents using micro-computed tomography, J Bone Miner Res 25(7) (2010) 1468–86. [DOI] [PubMed] [Google Scholar]
- [24].Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM, Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee, J Bone Miner Res 28(1) (2013) 2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee, J Bone Miner Res 2(6) (1987) 595–610. [DOI] [PubMed] [Google Scholar]
- [26].Zheng L, Pi C, Zhang J, Fan Y, Cui C, Zhou Y, Sun J, Yuan Q, Xu X, Ye L, Cao X, Zhou X, Aberrant activation of latent transforming growth factor-beta initiates the onset of temporomandibular joint osteoarthritis, Bone Res 6 (2018) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Le Henaff C, Ricarte F, Finnie B, He Z, Johnson J, Warshaw J, Kolupaeva V, Partridge NC, Abaloparatide at the Same Dose Has the Same Effects on Bone as PTH (1–34) in Mice, J Bone Miner Res 35(4) (2020) 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Siddiqui JA, Swarnkar G, Sharan K, Chakravarti B, Sharma G, Rawat P, Kumar M, Khan FM, Pierroz D, Maurya R, Chattopadhyay N, 8,8”-Biapigeninyl stimulates osteoblast functions and inhibits osteoclast and adipocyte functions: Osteoprotective action of 8,8”-biapigeninyl in ovariectomized mice, Mol Cell Endocrinol 323(2) (2010) 256–67. [DOI] [PubMed] [Google Scholar]
- [29].Dallas SL, Rosser JL, Mundy GR, Bonewald LF, Proteolysis of latent transforming growth factor-beta (TGF-beta )-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix, J Biol Chem 277(24) (2002) 21352–60. [DOI] [PubMed] [Google Scholar]
- [30].Oreffo RO, Mundy GR, Seyedin SM, Bonewald LF, Activation of the bone-derived latent TGF beta complex by isolated osteoclasts, Biochem Biophys Res Commun 158(3) (1989) 817–23. [DOI] [PubMed] [Google Scholar]
- [31].Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X, TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation, Nat Med 15(7) (2009) 757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wu Y, Kumar R, Parathyroid hormone regulates transforming growth factor beta1 and beta2 synthesis in osteoblasts via divergent signaling pathways, J Bone Miner Res 15(5) (2000) 879–84. [DOI] [PubMed] [Google Scholar]
- [33].Wu X, Pang L, Lei W, Lu W, Li J, Li Z, Frassica FJ, Chen X, Wan M, Cao X, Inhibition of Sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling, Cell Stem Cell 7(5) (2010) 571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kuo SW, Rimando MG, Liu YS, Lee OK, Intermittent Administration of Parathyroid Hormone 1–34 Enhances Osteogenesis of Human Mesenchymal Stem Cells by Regulating Protein Kinase Cdelta, Int J Mol Sci 18(10) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Siddiqui JA, Partridge NC, Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement, Physiology (Bethesda) 31(3) (2016) 233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gilchrist A, Stern PH, Editorial: Chemokines and Bone, Front Endocrinol (Lausanne) 9 (2018) 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Siddiqui JA, Partridge NC, CCL2/Monocyte Chemoattractant Protein 1 and Parathyroid Hormone Action on Bone, Front Endocrinol (Lausanne) 8 (2017) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Khan UA, Hashimi SM, Bakr MM, Forwood MR, Morrison NA, CCL2 and CCR2 are Essential for the Formation of Osteoclasts and Foreign Body Giant Cells, J Cell Biochem 117(2) (2016) 382–9. [DOI] [PubMed] [Google Scholar]
- [39].Balooch G, Balooch M, Nalla RK, Schilling S, Filvaroff EH, Marshall GW, Marshall SJ, Ritchie RO, Derynck R, Alliston T, TGF-beta regulates the mechanical properties and composition of bone matrix, Proc Natl Acad Sci U S A 102(52) (2005) 18813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM, The effects of parathyroid hormone, alendronate, or both in men with osteoporosis, N Engl J Med 349(13) (2003) 1216–26. [DOI] [PubMed] [Google Scholar]
- [41].Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ, Pa THSI, The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis, N Engl J Med 349(13) (2003) 1207–15. [DOI] [PubMed] [Google Scholar]
- [42].Gong X, Gong W, Kuhns DB, Ben-Baruch A, Howard OM, Wang JM, Monocyte chemotactic protein-2 (MCP-2) uses CCR1 and CCR2B as its functional receptors, J Biol Chem 272(18) (1997) 11682–5. [DOI] [PubMed] [Google Scholar]
- [43].Wain JH, Kirby JA, Ali S, Leucocyte chemotaxis: Examination of mitogen-activated protein kinase and phosphoinositide 3-kinase activation by Monocyte Chemoattractant Proteins-1, −2, −3 and −4, Clin Exp Immunol 127(3) (2002) 436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Patel H, Trooskin S, Shapses S, Sun W, Wang X, Serum monocyte chemokine protein-1 levels before and after parathyroidectomy in patients with primary hyperparathyroidism, Endocr Pract 20(11) (2014) 1165–9. [DOI] [PubMed] [Google Scholar]
- [45].Koh AJ, Demiralp B, Neiva KG, Hooten J, Nohutcu RM, Shim H, Datta NS, Taichman RS, McCauley LK, Cells of the osteoclast lineage as mediators of the anabolic actions of parathyroid hormone in bone, Endocrinology 146(11) (2005) 4584–96. [DOI] [PubMed] [Google Scholar]
- [46].Yang XW, Wang XS, Cheng FB, Wang F, Wan L, Wang F, Huang HX, Elevated CCL2/MCP-1 Levels are Related to Disease Severity in Postmenopausal Osteoporotic Patients, Clin Lab 62(11) (2016) 2173–2181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: MCP-1 is required for bone marrow osteoblastogenesis. (A) Four-month-old male WT and MCP-1−/− mice were injected daily with 80 μg/kg hPTH (1-34) or saline for 6 weeks and bones were collected 2 h after the last injection. Bone marrow cells from these bones were cultured in bone marrow mineralization medium for 21 days. hPTH (1-34) injections significantly enhanced the mineralization of the bone marrow cells compared with the saline group. The deletion of MCP-1 blunted the hPTH-mediated bone marrow cell mineralization. (B) Alizarin red-S staining. (C) Quantification of alizarin red-S staining with cetylpyridinium chloride (CPC) extraction at pH 7.0 and quantification. *p<0.05 compared to saline-injected WT mice.
Supplementary Figure 2:: Short-term high dose rhMCP-1 injections increased the bone parameters in MCP-1−/− mice. (A) μCT parameters of the trabecular region of femurs after the various treatment regimes for 10 days showing BV/TV (%), Tb.Th (mm), Tb.Sp (mm), and Tb.N (1/mm) and Tb.Pf (1/mm). (n = 4 mice/group). *p < 0.05 compared to saline-injected WT mice. Stippled bars are MCP-1−/− mice. (B) H&E staining of the sections of tibiae of WT and MCP-1−/− mice after 10 days of treatment with hPTH (1-34) and rhMCP-1 (0.5-1 μg/mouse) respectively. (C) Immunohistochemistry of pSmad-2 in the tibia after 10 days of daily hPTH (80μg/kg) and/or rhMCP-1 (0.5-1μg/mouse) injections to wild-type and MCP-1−/− mice, respectively. rhMCP-1 injections rescued the pSmad-2 staining in MCP-1−/− mice to be comparable to hPTH-injected wild-type mice.
Supplementary Figure 3: PTH increased MCP-1 serum levels in mice in vivo. (A) Four month old male mice were injected with vehicle (saline), or 80 μg/kg hPTH(1-34) and blood was collected as shown. A single injection of hPTH increased MCP-1 levels to double at 2 h. (B) MCP-1 levels 2 h after each injection. On day 2 and 3 of PTH injections, the serum MCP-1 levels were elevated to 4-fold but declined at later days, although still being double the baseline values.
Supplementary Figure 4:: Effect of short-term high dose rhMCP-1 injections on bone biochemical markers in MCP-1−/− mice. Sera were isolated 2 h after the last injection from wild-type and MCP-1−/− mice after 10 days of daily PTH or rhMCP-1 (0.5-1μg/mouse) injections. The levels of carboxy-terminal cross-linking telopeptides of type I collagen (CTX), a marker of bone resorption and P1NP, a marker of bone formation, were determined by ELISA. hPTH increases both serum CTX and P1NP in wild-type mice while rhMCP-1 (1μg/mouse) only increases P1NP to that comparable to the PTH-injected wild-type mice. (n = 4 mice/group). *p < 0.05, compared to vehicle-treated WT mice.