Abstract
OBJECTIVE
Diabetes-related end-stage kidney disease (ESKD-D) disproportionately affects U.S. racial/ethnic minority populations compared with whites. However, from 1996 to 2013, ESKD-D incidence among American Indians and Alaska Natives (AIANs) and blacks declined. We assessed recent ESKD-D incidence data to determine whether trends by race/ethnicity have changed since 2013.
RESEARCH DESIGN AND METHODS
U.S. Renal Data System data from 2000 to 2016 were used to determine the number of whites, blacks, AIANs, Asians, and Hispanics aged ≥18 years with newly treated ESKD-D (with diabetes listed as primary cause). Using census population estimates as denominators, annual ESKD-D incidence rates were calculated and age adjusted to the 2000 U.S. standard population. Joinpoint regression was used to analyze trends and estimate an average annual percent change (AAPC) in incidence rates.
RESULTS
For adults overall, from 2000 to 2016, age-adjusted ESKD-D incidence rates decreased by 53% for AIANs (66.7–31.2 per 100,000, AAPC −4.5%, P < 0.001), by 33% for Hispanics (50.0–33.3, −2.1%, P < 0.001), and by 20% for blacks (56.2–44.7, −1.6%, P < 0.001). However, during the study period, age-adjusted ESKD-D incidence rates did not change significantly for Asians and increased by 10% for whites (15.4–17.0, 0.6%, P = 0.01). In 2016, ESKD-D incidence rates in AIANs, Hispanics, and blacks were ~2.0–2.5 times higher than whites.
CONCLUSIONS
ESKD-D incidence declined for AIANs, Hispanics, and blacks and increased for whites. Continued efforts might be considered to reverse the trend in whites and sustain and lower ESKD-D incidence in the other populations.
In the U.S., diabetes is the leading cause of end-stage kidney disease (ESKD) (i.e., kidney failure requiring dialysis or transplantation), accounting for 47% of newly treated cases of ESKD in 2016 (1). This proportion varies among racial and ethnic groups, ranging from 43% among blacks to 74% among American Indians and Alaska Natives (AIANs) (1). Although diabetes burden remains highest among AIANs (2), their diabetes-related ESKD (ESKD-D) incidence declined by 54% between 1996 and 2013, more than other racial or ethnic groups (3). ESKD-D incidence during this period also declined significantly by 18% for blacks (3). Between 1996 and 2013, the ratio in age-adjusted ESKD-D incidence compared with whites was reduced from 4.7 times to 1.7 times higher in AIAN adults and from 4.3 times to 2.8 times higher in black adults.
In the U.S. population with diabetes overall, after declining for more than a decade, the incidence of ESKD-D has leveled off since 2010 (4,5). Furthermore, after increasing for many years, the prevalence of diagnosed diabetes has declined in AIANs and leveled off in other U.S. racial/ethnic groups (6,7). Thus, we wanted to explore whether recent trends in ESKD-D incidence by race or ethnicity have changed.
RESEARCH DESIGN AND METHODS
Data from the U.S. Renal Data System (USRDS) were analyzed to assess trends in the incidence of ESKD-D among U.S. adults from 2000 to 2016. The USRDS (funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health) is a surveillance system for ESKD that is based on clinical and claims data reports to the Centers for Medicare & Medicaid Services (CMS) (1). The CMS Medicare program covers ESKD treatment for beneficiaries regardless of age and pays >80% of the cost of ESKD treatment in the U.S. (1). Data from the CMS Medical Evidence Report form (CMS 2728) collected by USRDS include demographic (e.g., age, sex, race, ethnicity) and ESKD-related (e.g., first date of treatment, primary cause of ESKD) information. Renal care providers are required to complete the CMS Medical Evidence Report for each new patient with ESKD, regardless of Medicare eligibility status.
For each year studied, USRDS data were used to determine the number of adults aged ≥18 years in the U.S. who began treatment (dialysis or kidney transplantation) for ESKD-D (i.e., with diabetes listed as the primary cause of ESKD). Data were analyzed for adults overall and by age-group for whites, blacks, AIANs, Asians, and Hispanics. Racial groups included people of Hispanic and non-Hispanic origin; the Hispanic group included people of any race. For each year from 2000 to 2016, ESKD-D incidence was calculated as the number of newly treated adults divided by the respective age- and race- or ethnicity-specific resident population estimates from the U.S. census. ESKD-D incidence for adults overall was age adjusted by the direct method to the 2000 U.S. population using age-groups 18–44, 45–64, 65–74, and ≥75 years (8). Trends were examined using Joinpoint Regression Program version 4.3.1.0 software (Statistical Methodology and Applications Branch and Data Modeling Branch, Surveillance Research Program, National Cancer Institute) (9). Joinpoint regression uses permutation tests to identify points where linear trends change significantly in either direction or magnitude. Each trend segment is described by an annual percent change (APC) with a 95% CI; the overall trend is described by an average APC (AAPC) with its corresponding 95% CI. The rate of change for linear trends was tested to determine whether it was significantly different from 0, and results were considered statistically significant with a two-sided P < 0.05.
RESULTS
Selected characteristics of the study population are shown in Table 1. For AIANs, the absolute number of adults starting ESKD-D treatment declined by 6% from 878 in 2000 to 825 in 2016. In contrast, for the other racial/ethnic groups, the number starting ESKD-D treatment increased from 2000 to 2016 (27,306–38,794 [42%] for whites, 11,355–13,742[21%] for blacks, 6,897–10,187 [48%] for Hispanics, 1,141–2,618 [129%] for Asians) (Table 1).
Table 1.
Number of US adults aged ≥18 years beginning treatment for diabetes-related end-stage kidney disease, by race or ethnicity,* sex, age, and treatment modality, 2000 and 2016.
| AIANs | Asians | Blacks | Whites | Hispanics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2016 | 2000 | 2016 | 2000 | 2016 | 2000 | 2016 | 2000 | 2016 | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Total | 878 | 825 | 1,141 | 2,618 | 11,355 | 13,742 | 27,306 | 38,794 | 6,897 | 10,187 |
| Sex | ||||||||||
| Male | 397 | 440 | 602 | 1,546 | 4,800 | 7,054 | 14,282 | 22,755 | 3,451 | 5,890 |
| (45.2) | (53.3) | (52.8) | (59.1) | (42.3) | (51.3) | (52.3) | (58.7) | (50.0) | (57.8) | |
| Female | 481 | 385 | 539 | 1,072 | 6,555 | 6,688 | 13,024 | 16,039 | 3,446 | 4,297 |
| (54.8) | (46.7) | (47.2) | (40.9) | (57.7) | (48.7) | (47.7) | (41.3) | (50.0) | (42.2) | |
| Age (years) | ||||||||||
| 18–44 | 88 | 124 | 61 | 156 | 1,195 | 1,741 | 2,644 | 2,776 | 640 | 1,053 |
| (10.0) | (15.0) | (5.3) | (6.0) | (10.5) | (12.7) | (9.7) | (7.2) | (9.3) | (10.3) | |
| 45–64 | 514 | 422 | 438 | 944 | 5,450 | 6,294 | 10,848 | 15,665 | 3,372 | 4,927 |
| (58.5) | (51.2) | (38.4) | (36.1) | (48.0) | (45.8) | (39.7) | (40.4) | (48.9) | (48.4) | |
| 65–74 | 210 | 205 | 384 | 789 | 3,086 | 3,639 | 8,214 | 12,161 | 1,924 | 2,667 |
| (23.9) | (24.8) | (33.7) | (30.1) | (27.2) | (26.5) | (30.1) | (31.3) | (27.9) | (26.2) | |
| ≥75 | 66 | 74 | 258 | 729 | 1,624 | 2,068 | 5,600 | 8,192 | 961 | 1,540 |
| (7.5) | (9.0) | (22.6) | (27.8) | (14.3) | (15.0) | (20.5) | (21.1) | (13.9) | (15.1) | |
| Treatment modality | ||||||||||
| Hemodialysis | 802 | 754 | 1,030 | 2,241 | 10,698 | 12,689 | 24,361 | 34,800 | 6,413 | 9,290 |
| (91.3) | (91.4) | (90.3) | (85.6) | (94.2) | (92.3) | (89.2) | (89.7) | (93.0) | (91.2) | |
| Peritoneal dialysis | 70 | 68 | 97 | 356 | 586 | 1,000 | 2,457 | 3,636 | 433 | 845 |
| (8.0) | (8.2) | (8.5) | (13.6) | (5.2) | (7.3) | (9.0) | (9.4) | (6.3) | (8.3) | |
| Transplant | NA† | NA† | NA† | 20 | 22 | 48 | 299 | 318 | 17 | 46 |
| (0.8) | (0.2) | (0.3) | (1.1) | (0.8) | (0.2) | (0.5) | ||||
| Unknown | NA† | NA† | NA† | --† | 49 | --† | 189 | 40 | 34 | --† |
| (0.4) | (0.7) | (0.1) | (0.5) | |||||||
Racial groups include people of Hispanic and non-Hispanic origin; Hispanics may be of any race.
Cell size ≤10.
AIANs=American Indians and Alaska Natives; NA=not available.
ESKD-D Incidence Trends From 2000 to 2016
From 2000 to 2016, the age-adjusted ESKD-D incidence rates for adults overall decreased for AIANs by 53% from 66.7 to 31.2 per 100,000 (AAPC −4.5%, P < 0.001), decreased for Hispanics by 33% from 50.0 to 33.3 per 100,000 (−2.1%, P < 0.001), and decreased for blacks by 20% from 56.2 to 44.7 per 100,000 (−1.6%, P < 0.001) (Table 2 and Fig. 1). For Asians, the age-adjusted ESKD-D incidence rates did not change significantly and remained ~20.0 per 100,000 throughout the study period. On the other hand, for whites, the age-adjusted ESKD-D incidence rates increased by 10% from 15.4 per 100,000 in 2000 to 17.0 per 100,000 in 2016 (0.6%, P = 0.01).
Table 2.
Incidence rates of diabetes-related end-stage kidney disease and incidence trend analysis among adults aged ≥18 years, by race or ethnicity and age group — United States, 2000–2016.
| Incidence Rate (per 100,000 adults) | Percent change | Overall trend | Initial trend | Subsequent trends | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 2000 | 2016 | AAPC (95% CI) | P | Years | APC (95% CI) | P | Years | APC (95% CI) | P | |
| AIANs* | |||||||||||
| ≥18† | 66.7 | 31.2 | −53 | −4.5 (−5.9, −3.0) | <0.001 | 2000–13 | −5.6 (−6.4, −4.8) | <0.001 | 2013–16 | 0.7 (−7.2, 9.3) | 0.85 |
| 18–44 | 7.7 | 7.6 | −1 | −0.8 (−7.2, 6.0) | 0.81 | 2000–09 | 1.1 (−2.3, 4.5) | 0.49 | 2009–12 | −10.8 (−38.1, 28.4) | 0.49 |
| 2012–16 | 2.9 (−8.3, 15.5) | 0.58 | |||||||||
| 45–64 | 108.3 | 46.6 | −57 | −4.5 (−6.2, −2.8) | 0.002 | 2000–13 | −5.8 (−6.7, −4.8) | <0.001 | 2013–16 | 1.2 (−8.0, 11.4) | 0.79 |
| 65–74 | 231.2 | 92.8 | −60 | −5.8 (−7.7, −3.8) | <0.001 | 2000–12 | −7.6 (−9.0, −6.2) | <0.001 | 2012–16 | −0.0 (−7.7, 8.3) | 0.99 |
| ≥75 | 119.3 | 61.9 | −48 | −4.2 (−5.7, −2.8) | <0.001 | 2000–16 | −4.2 (−5.7, −2.8)‡ | <0.001 | -- | -- | -- |
| Asians* | |||||||||||
| ≥18† | 19.8 | 19.7 | −1 | −0.0 (−2.1, 2.1) | 1.00 | 2000–09 | 0.0 (−1.0, 1.1) | 0.94 | 2009–12 | −2.4 (−12.9, 9.3) | 0.63 |
| 2012–16 | 1.7 (−1.9, 5.4) | 0.31 | |||||||||
| 18–44 | 1.2 | 2.0 | 64 | 4.3 (3.0, 5.5) | <0.001 | 2000–16 | 4.3 (3.0, 5.5)‡ | <0.001 | -- | -- | -- |
| 45–64 | 19.6 | 21.0 | 7 | −0.5 (−1.1, 0.2) | 0.17 | 2000–16 | −0.5 (−1.1, 0.2)‡ | 0.17 | -- | -- | -- |
| 65–74 | 75.6 | 61.1 | −19 | −1.3 (−2.0, −0.7) | 0.001 | 2000–16 | −1.3 (−2.0, −0.7)‡ | 0.001 | -- | -- | -- |
| ≥75 | 81.2 | 85.7 | 6 | 0.3 (−0.2, 0.8) | 0.24 | 2000–16 | 0.3 (−0.2, 0.8)‡ | 0.24 | -- | -- | -- |
| Blacks* | |||||||||||
| ≥18† | 56.2 | 44.7 | −20 | −1.6 (−2.4, −0.8) | <0.001 | 2000–08 | −0.7 (−1.3, −0.0) | 0.048 | 2008–12 | −4.3 (−7.1, −1.4) | 0.009 |
| 2012–16 | −0.6 (−2.5, 1.3) | 0.49 | |||||||||
| 18–44 | 8.0 | 10.3 | 30 | 1.8 (1.1, 2.6) | <0.001 | 2000–07 | 4.2 (2.7, 5.7) | <0.001 | 2007–16 | 0.1 (−0.9, 1.0) | 0.91 |
| 45–64 | 82.0 | 60.2 | −27 | −2.0 (−2.8, −1.1) | <0.001 | 2000–08 | −1.5 (−2.2, −0.8) | 0.001 | 2008–12 | −4.6 (−7.7, −1.4) | 0.01 |
| 2012–16 | −0.2 (−2.2, 1.9) | 0.82 | |||||||||
| 65–74 | 188.0 | 129.8 | −31 | −2.6 (−3.5, −1.6) | <0.001 | 2000–08 | −1.3 (−2.1, −0.5) | 0.005 | 2008–12 | −5.8 (−9.2, −2.3) | 0.005 |
| 2012–16 | −1.7 (−4.0, 0.6) | 0.12 | |||||||||
| ≥75 | 131.9 | 118.8 | −10 | −1.1 (−1.6, −0.7) | <0.001 | 2000–16 | −1.1 (−1.6, −0.7)‡ | <0.001 | -- | -- | -- |
| Hispanics* | |||||||||||
| ≥18† | 50.0 | 33.3 | −33 | −2.1 (−2.4, −1.8) | <0.001 | 2000–16 | −2.1 (−2.4, −1.8)‡ | <0.001 | -- | -- | -- |
| 18–44 | 3.9 | 4.4 | 12 | 1.1 (0.1, 2.0) | 0.03 | 2000–03 | −1.4 (−5.3, 2.7) | 0.46 | 2003–09 | 4.0 (2.2, 5.9) | 0.001 |
| 2009–16 | −0.4 (−1.5, 0.7) | 0.42 | |||||||||
| 45–64 | 68.6 | 44.1 | −36 | −2.2 (−2.4, −1.9) | <0.001 | 2000–16 | −2.2 (−2.4, −1.9)‡ | <0.001 | -- | -- | -- |
| 65–74 | 176.9 | 110.9 | −37 | −2.9 (−3.2, −2.6) | <0.001 | 2000–16 | −2.9 (−3.2, −2.6)‡ | <0.001 | -- | -- | -- |
| ≥75 | 143.4 | 97.9 | −32 | −1.7 (−2.3, −1.2) | <0.001 | 2000–16 | −1.7 (−2.3, −1.2)‡ | <0.001 | -- | -- | -- |
| Whites* | |||||||||||
| ≥18† | 15.4 | 17.0 | 10 | 0.6 (0.3, 1.0) | 0.01 | 2000–06 | 0.8 (0.2, 1.3) | 0.01 | 2006–12 | −0.5 (−1.2, 0.2) | 0.16 |
| 2012–16 | 2.1 (1.1, 3.2) | 0.001 | |||||||||
| 18–44 | 3.0 | 3.2 | 9 | 0.7 (−0.0, 1.4) | 0.06 | 2000–05 | −1.3 (−3.3, 0.7) | 0.19 | 2005–16 | 1.6 (1.0, 2.2) | <0.001 |
| 45–64 | 20.7 | 23.3 | 13 | 0.8 (0.6, 1.1) | <0.001 | 2000–11 | 0.3 (0.1, 0.6) | 0.009 | 2011–16 | 1.9 (1.1, 2.7) | <0.001 |
| 65–74 | 51.3 | 50.7 | −1 | −0.3 (−0.8, 0.3) | 0.34 | 2000–06 | 0.2 (−0.5, 1.0) | 0.54 | 2006–12 | −2.0 (−2.9, −1.0) | 0.002 |
| 2012–16 | 1.6 (0.2, 3.1) | 0.03 | |||||||||
| ≥75 | 37.3 | 46.2 | 24 | 1.4 (0.7, 2.0) | <0.001 | 2000–05 | 3.6 (2.3, 4.9) | <0.001 | 2005–12 | −0.7 (−1.6, 0.3) | 0.16 |
| 2012–16 | 2.2 (0.3, 4.1) | 0.03 | |||||||||
AIANs=American Indians and Alaska Natives; APC=annual percentage change; AAPC=average annual percentage change; CI=confidence interval.
Racial groups include people of Hispanic and non-Hispanic origin; Hispanics may be of any race.
Age-adjusted based on the 2000 US standard population.
APC=AAPC (i.e., 0 joinpoints).
Figure 1—
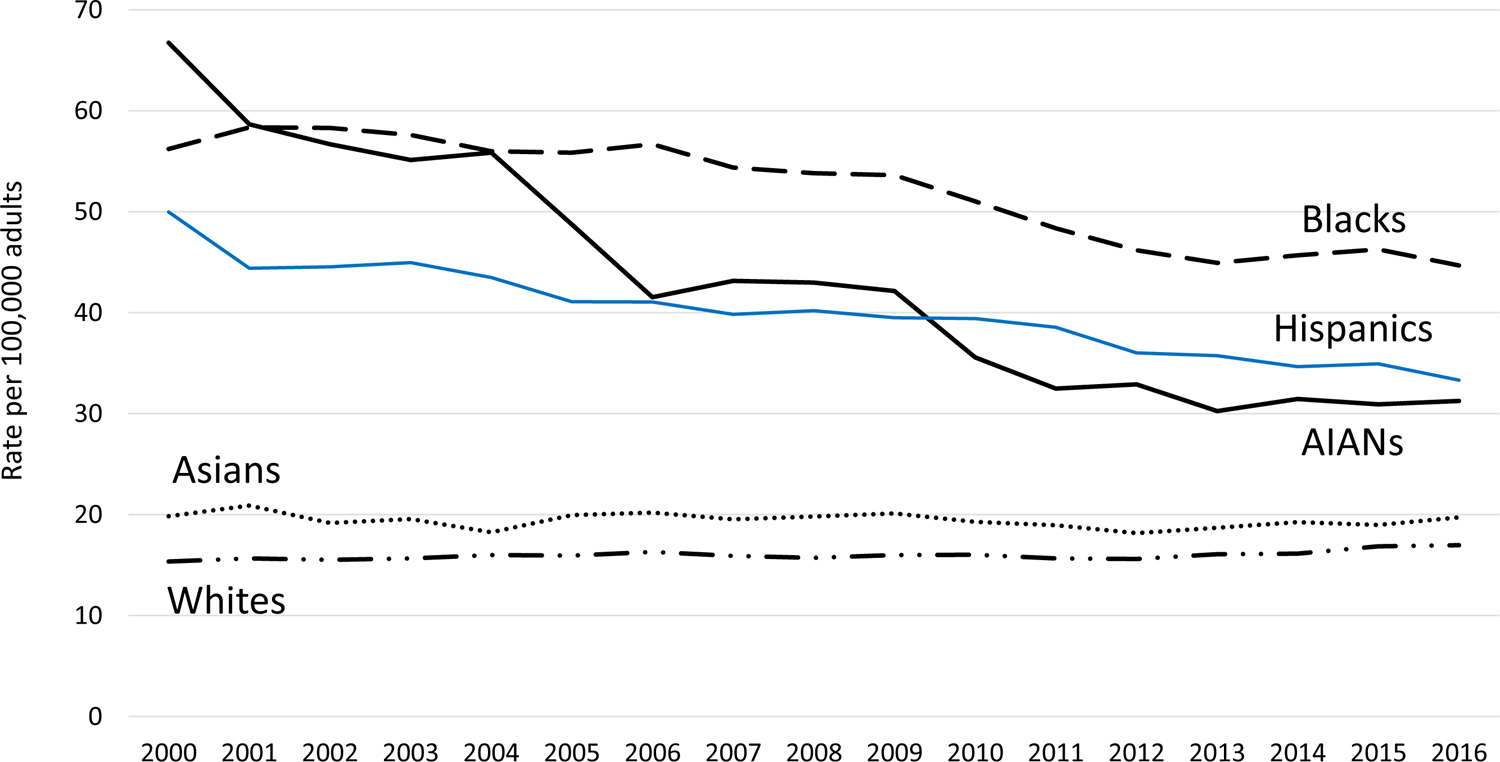
Incidence of diabetes-related ESKD among adults aged ≥18 years by race or ethnicity, 2000–2016. Age-adjusted based on the 2000 U.S. standard population. Racial groups include people of Hispanic and non-Hispanic origin; Hispanics may be of any race.
During the study period, among people aged 18–44 years, ESKD-D incidence rates remained stable for AIANs (AAPC −0.8%, P = 0.81) and for whites (0.7%, P = 0.06) but increased for Asians from 1.2 to 2.0 per 100,000 (4.3%, P < 0.001), for blacks from 8.0 to 10.3 per 100,000 (1.8%, P < 0.001), and for Hispanics from 3.9 to 4.4 per 100,000 (1.1%, P = 0.03) (Table 2). In the age-groups >18–44 years, ESKD-D incidence rates from 2000 to 2016 declined for AIANs, blacks, and Hispanics (Table 2 and Fig. 2). For Asians, ESKD-D incidence rates throughout the study period remained stable for those aged 45–64 years and ≥75 years and decreased for those aged 65–74 years from 75.6 to 61.1 per 100,000 (−1.3%, P = 0.001). On the other hand, for whites, ESKD-D incidence rates increased for those aged 45–64 years from 20.7 to 23.3 per 100,000 (0.8%, P < 0.001) and ≥75 years from 37.3 to 46.2 per 100,000(1.4%, P < 0.001) and remained stable for those aged 65–74 years (Table 2).
Figure 2—
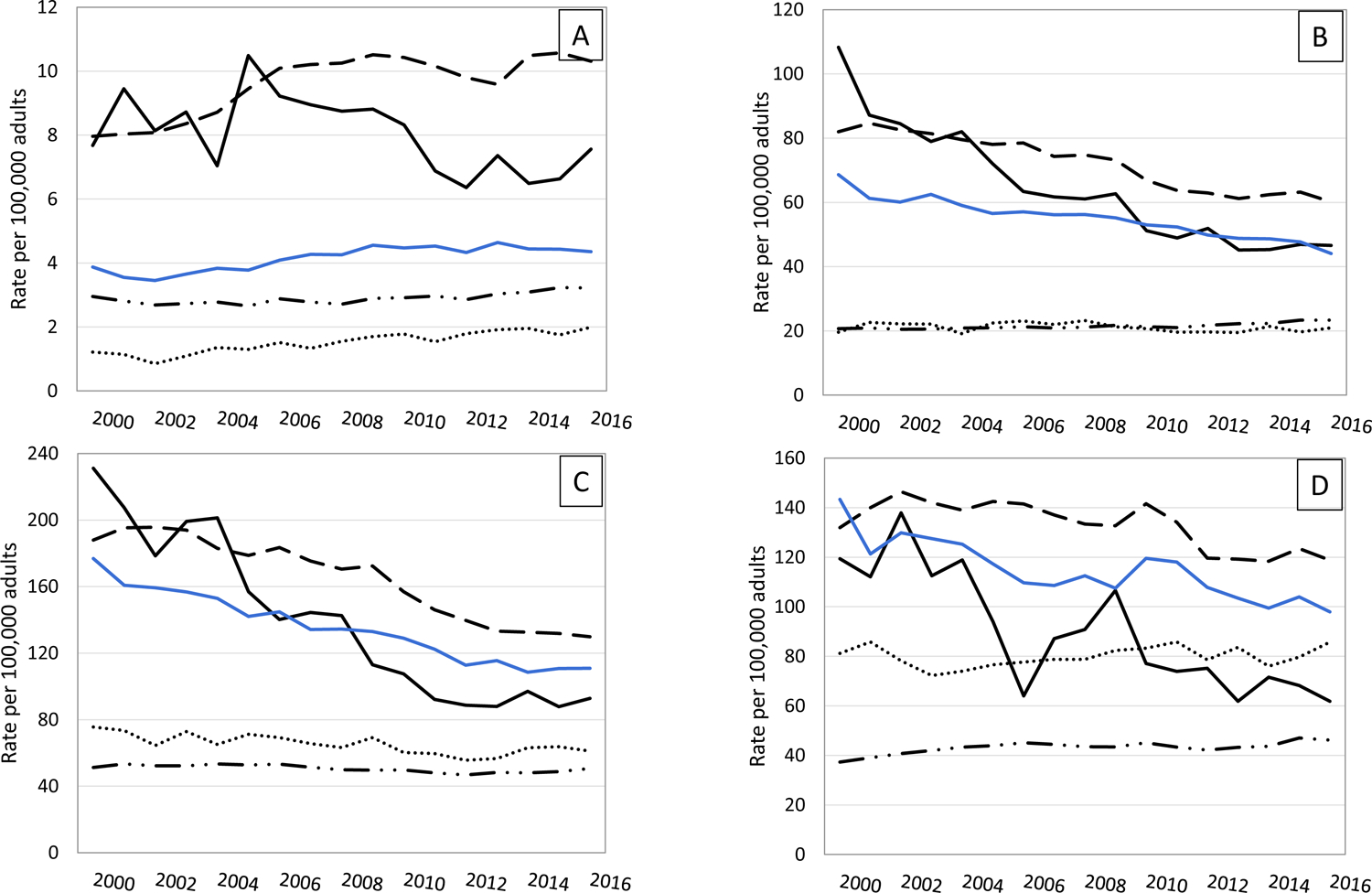
Incidence of diabetes-related ESKD among adults aged ≥18 years by age and race or ethnicity, 2000–2016. Racial groups include people of Hispanic and non-Hispanic origin; Hispanics may be of any race. A: Aged 18–44 years. B: Aged 45–64 years. C: Aged 65–74 years. D: Aged ≥75 years. Dashed line, blacks; solid black line, AIANs; solid blue line, Hispanics; dotted line, Asians; combination dashed/dotted line, whites.
ESKD-D Incidence Trends Between 2000 and 2016
For AIANs overall, the decline in rates occurred between 2000 and 2013 (APC−5.6%, P < 0.001), and then rates leveled off through 2016 (Table 2 and Fig. 2). Between 2000 and 2016, ESKD-D incidence rates remained stable for AIANs aged 18–44 years. For AIANs aged 45–64 years, ESKD-D rates declined from 2000 to 2013 (−5.8%, P < 0.001) and then leveled off to 46.6 per 100,000 in 2016. For AIANs aged 65–74 years, rates declined from 2000 to 2012 (−7.6%, P < 0.001) and then leveled off to 92.8 per 100,000 in 2016. For AIANs aged ≥75 years, rates declined consistently throughout the study period from 119.3 per 100,000 in 2000 to 61.9 per 100,000 in 2016 (−4.2%, P < 0.001).
ESKD-D incidence among blacks declined from 2000 to 2008 (APC −0.7%, P = 0.048) and more steeply from 2008 to 2012 (−4.3%, P = 0.009) before leveling off from 2012 to 2016 (Table 2). This trend pattern—an initial period of decline followed by a stabilization in rates—was also seen among blacks aged 18–44, 45–64, and 65–74 years (Table 2 and Fig. 2). Among blacks aged ≥75 years, ESKD-D incidence declined steadily throughout the study period from 131.9 to 118.8 per 100,000 (−1.7%, P < 0.001). ESKD-D incidence also declined throughout the study period for Hispanics aged 45–64, 65–74, and ≥75 years (Table 2). However, among Hispanics aged 18–44 years, ESKD-D incidence remained unchanged between 2000 and 2003, increased between 2003 and 2009 (4.0%, P = 0.001), and then leveled off between 2009 and 2016.
Among Asians overall, ESKD-D incidence between 2000 and 2016 did not change significantly, and trends by age-group were similar to the overall trend (Table 2 and Fig. 2). For whites overall, ESKD-D incidence increased from 2000 to 2006 (APC 0.8%, P = 0.01), leveled off from 2006 to 2012, and increased again from 2012 to 2016 (2.1%, P = 0.001) (Table 2). However, somewhat different trend patterns were seen by age-group. ESKD-D incidence increased for whites aged 18–44 years from 2005 to 2016 (1.6%, P < 0.001), for those aged 45–64 years from 2000 to 2011 (0.3%, P = 0.009), and more steeply from 2011 to 2016 (1.9%, P < 0.001); for those aged 65–74 years from 2012 to 2016 (1.6%, P = 0.03); and for those aged ≥75 years from 2000 to 2005 (3.6%, P < 0.001) and then again from 2012 to 2016 (2.2%, P = 0.03) (Table 2).
CONCLUSIONS
The ESKD-D incidence rate among AIAN adults overall declined by 53% from 2000 to 2016, more than for other race and ethnicity groups and similar to a previous report for this population (3). During the study period, the ratio in incidence rates between AIANs and whites decreased by more than one-half, from 4.3 times in 2000 to 1.8 times in 2016. Although not as steep as the decline seen in AIANs, ESKD-D incidence rates declined by 20% for blacks and by 33% for Hispanics, also narrowing the gap with whites in these two population groups. Despite these declines, ESKD-D incidence rates in 2016 for AIANs, Hispanics, and blacks remained nearly twice as high or higher compared with whites. Furthermore, after an initial significant decline, ESKD-D incidence rates leveled off for AIANs from 2013 to 2016 and for blacks from 2009 to 2016, a finding that is consistent with the recent plateau in ESKD-D incidence in the overall U.S. diabetic population (4,5). This plateau in ESKD-D incidence after about a decade of continued decline may be due in part to the disproportionately higher prevalence of diagnosed diabetes in AIANs and blacks compared with whites (2) in combination with the reduction in diabetes-related mortality in AIANs (10,11). Because incidence of ESKD-D was defined by people beginning ESKD treatment in a given year, other factors may have affected incidence, including changes in treatment and care practices (e.g., when to begin dialysis) (1,12).
The significant reduction in ESKD-D incidence among AIANs parallels sustained improvements in glycemic, lipid, and blood pressure control for AIANs with diabetes (3,13–16). In 2014, among AIANs with diabetes, 76% were prescribed ACE inhibitors or angiotensin II receptor blockers (ARBs) compared with 56% of U.S. adults with diabetes during 2009–2014 (3,17). Among AIAN patients with diabetes and hypertension or chronic kidney disease (CKD), prescription of ACE inhibitors or ARBs between 2003 and 2015 was ~80% (3). Furthermore, in 2015, blood pressure levels among AIANs with diabetes and hypertension were well controlled (mean 133/76 mmHg) (3,13). These advances, in turn, likely resulted from improvements in patient care provided by the Indian Health Service (IHS), tribal, and urban Indian health facilities and services funded by the Special Diabetes Program for Indians (SDPI) (3,13,15,16). Starting in the mid-1980s, IHS, tribal, and urban Indian health facilities implemented population health and team-based approaches to diabetes care, including CKD testing and CKD case management as part of diabetes care, with the goal of reducing risk factors for diabetes complications (3,15). These efforts were complemented and supported, in part, by the SDPI grant programs that have successfully implemented evidence-based and community-driven strategies to prevent and treat diabetes since 1997 (13,15,16).
In addition to declines in ESKD-D incidence in AIAN people, diabetes prevalence and mortality, diabetic retinopathy, and hospitalizations for uncontrolled diabetes have decreased, and prevalence of childhood obesity has stabilized (6,10,11,18–20). Furthermore, ESKD prevalence in the AIAN population has declined by 36% since 2000 (1), the only instance of a decline in ESKD prevalence for a major racial group since the beginning of the Medicare ESKD program in 1973. Taken together, these trends are promising signs for the future of diabetes complications in AIAN people.
In 2016, use of ACE inhibitors or ARBs was >70% among AIANs, Asians, blacks, whites, and Hispanics aged ≥65 years with diabetes and CKD (21) and 76% among Hispanics aged 18–74 years with diabetes and hypertension between 2008 and 2011 (22). In people with diabetes and CKD, blood pressure control with ACE inhibitors or ARBs has been shown to slow CKD progression to ESKD (23,24). However, not all racial and ethnic groups experienced decreasing trends in ESKD-D incidence. During the study period, ESKD-D incidence rates did not change significantly for Asians and increased for whites. Although reasons for the increase in ESKD-D incidence in whites are unclear, this finding is consistent with documented and previously reported ESKD-D incidence trends in this population (3,5). Furthermore, the lack of decline in ESKD-D incidence in all racial and ethnic groups aged 18–44 years is of particular concern and may be due in part to poorer risk factor control in young adult diabetic populations compared with people with diabetes in older age-groups (25). In 2016, the Medicare expenditure per person per year for patients with ESKD-D on hemodialysis was $95,563, and the total Medicare spending for ESKD-D was $16.1 billion, about one-half (46%) of the $35.4 billion Medicare spending for ESKD overall (1). Barring countervailing forces, a decrease in ESKD-D incidence in the U.S. population would foreseeably reduce or lower total Medicare expenditures for ESKD and diabetes in general. An analysis of the impact of the reduced ESKD-D incidence in AIANs as a result of the SDPI and other IHS efforts estimated the potential Medicare cost savings between $174 and $520 million over a 10-year period from the reduction of ~2,256–2,602 new ESKD-D cases (26).
The major strength of this study is that the reported results are based on data from the USRDS, a national registry of virtually all patients being treated for ESKD. On the other hand, the findings in this report are subject to a few limitations. First, people who refused treatment, died before receiving treatment, or whose treatment was not reported to CMS were not included. Second, diabetes as the primary cause of ESKD could be over as certained because it is based on the physician’s assessment of the patient at the time of the indication for ESKD treatment (27). Third, differential classification of race in the USRDS and the U.S. census (physician reported vs. self-identified) could result in over- or underestimation of the actual incidence of ESKD-D in racial- or ethnic-specific groups. Finally, because of a lack of data on type or duration of diabetes, we could not examine the impact of these factors on the risk of developing ESKD-D.
Managing risk factors such as diabetes and high blood pressure has been shown to help to prevent or delay CKD, and CKD treatment can slow disease progression and reduce the risk of complications, including ESKD (28). In late 2017, the CDC announced a new 5-year cooperative agreement to scale up the National Diabetes Prevention Program (National DPP) in underserved areas and to reach populations currently underrepresented in the program relative to their risk. These populations include Medicare beneficiaries, men, African Americans, Asian Americans, Hispanics, AIANs, Pacific Islanders, and noninstitutionalized people with visual impairment or physical disabilities (29,30). The CDC’s National DPP is a partnership of public and private organizations working together to build a nationwide delivery system for a 12-month lifestyle change program proven to prevent or delay the onset of type 2 diabetes (29). The CDC also supports state health departments’ efforts to make the National DPP and evidence-based diabetes management interventions available to high-burden populations and communities and supports tribes and tribal organizations directly to expand access to and participation in the National DPP lifestyle change program.
In July 2019, the U.S. Department of Health and Human Services launched the Advancing American Kidney Health initiative outlining government strategies and activities to reduce the risk of kidney failure, among other goals (30). This initiative aims to increase efforts to prevent, detect, and slow the progression of kidney disease, including addressing risk factors like diabetes and high blood pressure. Integrating public health, clinical, and community-based approaches to deliver evidence-based interventions aimed at reducing ESKD-D risk factors, such as has been done by the IHS, may prevent ESKD-D and decrease incidence (3,13,15). Continued efforts to monitor trends and racial/ethnic disparity gaps in ESKD, CKD, and their risk factors, in addition to tracking other factors such as awareness, pre-ESKD care, and risk factor control, will be crucial to assessing the success of these interventions (1,31).
Acknowledgments.
The data reported here have been supplied by the USRDS. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the IHS.
Footnotes
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Prior Presentation. Parts of this study were presented at the 2019 Kidney Week Annual Meeting of the American Society of Nephrology, Washington, DC, 7–10 November 2019 and at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
References
- 1.United States Renal Data System. 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA, Centers for Disease Control and Prevention, US Department of Health and Human Services, 2020 [Google Scholar]
- 3.Bullock A, Burrows NR, Narva AS, et al. Vital Signs: decrease in incidence of diabetes-related end-stage renal disease among American Indians/Alaska Natives - United States, 1996–2013. MMWR Morb Mortal Wkly Rep 2017;66:26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregg EW, Hora I, Benoit SR. Resurgence in diabetes-related complications. JAMA 2019;321: 1867–1868 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. United States Diabetes Surveillance System [Internet]. Available from https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html. Accessed 21 January 2020
- 6.Bullock A, Sheff K, Hora I, et al. Prevalence of diagnosed diabetes in American Indian and Alaska Native adults, 2006–2017. BMJ Open Diabetes Res Care 2020;8:e001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benoit SR, Hora I, Albright AL, Gregg EW. New directions in incidence and prevalence of diagnosed diabetes in the USA. BMJ Open Diabetes Res Care 2019;7:e000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes 2001;(20):1–9 [PubMed] [Google Scholar]
- 9.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for Joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–351 [DOI] [PubMed] [Google Scholar]
- 10.Cho P, Geiss LS, Burrows NR, Roberts DL, Bullock AK, Toedt ME. Diabetes-related mortality among American Indians and Alaska Natives, 1990–2009. Am J Public Health 2014;104(Suppl. 3):S496–S503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics. Health, United States, 2017–data finder [Internet], 2018. Available from https://www.cdc.gov/nchs/hus/contents2017.htm. Accessed 21 January 2020
- 12.O’Hare AM, Choi AI, Boscardin WJ, et al. Trends in timing of initiation of chronic dialysis in the United States. Arch Intern Med 2011;171:1663–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Indian Health Service. Special Diabetes Program for Indians—2014 report to Congress. Changing the course of diabetes: turning hope into reality [Internet],2014. Available from https://www.ihs.gov/sites/newsroom/themes/responsive2017/display_objects/documents/RepCong_2016/SDPI_2014_Report_to_Congress.pdf. Accessed 21 January 2020 [PubMed]
- 14.Pratte KA, Johnson A, Beals J, Bullock A, Manson SM, Jiang L; Special Diabetes Program for Indians Diabetes Prevention Program. Regression to normal glucose regulation in American Indians and Alaska Natives of a diabetes prevention program. Diabetes Care 2019;42:1209–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narva A Population health for CKD and diabetes: lessons from the Indian Health Service. Am J Kidney Dis 2018;71:407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang L, Johnson A, Pratte K, Beals J, Bullock A, Manson SM; Special Diabetes Program for Indians Diabetes Prevention Program. Long-term outcomes of lifestyle intervention to prevent diabetes in American Indian and Alaska Native communities: the Special Diabetes Program for Indians Diabetes Prevention Program. Diabetes Care 2018;41:1462–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA 2016; 316:602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bursell SE, Fonda SJ, Lewis DG, Horton MB. Prevalence of diabetic retinopathy and diabetic macular edema in a primary care-based teleophthalmology program for American Indians and Alaskan Natives. PLoS One 2018;13: e0198551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agency for Healthcare Research and Quality. Hospital admissions for uncontrolled diabetes improving among American Indians and Alaska Natives [Internet], 2018. (AHRQ Publ. no. 18(19)-0033–7-EF). Available from https://www.ahrq.gov/data/infographics/aian-diabetes.html. Accessed 20 February 2020
- 20.Bullock A, Sheff K, Moore K, Manson S. Obesity and overweight in American Indian and Alaska Native children, 2006–2015. Am J Public Health 2017;107:1502–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Office of Disease Prevention and Health Promotion; Healthy People 2020. Chronic kidney disease [Internet]. Available from https://www.healthypeople.gov/2020/data-search/Search-the-Data#objid=4097. Accessed 28 April 2020
- 22.Casagrande SS, Avilés-Santa L, Corsino L, et al. Hemoglobin A1C, blood pressure, and LDL-cholesterol control among Hispanic/Latino adults with diabetes: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Endocr Pract 2017;23:1232–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; The Collaborative Study Group. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329: 1456–1462 [DOI] [PubMed] [Google Scholar]
- 24.Lewis EJ, Hunsicker LG, Clarke WR, et al. ; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 25.Ali MK, Bullard KM, Gregg EW, Del Rio C. A cascade of care for diabetes in the United States: visualizing the gaps. Ann Intern Med 2014;161: 681–689 [DOI] [PubMed] [Google Scholar]
- 26.Department of Health and Human Services; Office of the Assistant Secretary for Planning and Evaluation. Issue Brief Office of Health Policy. The Special Diabetes Program for Indians: estimates of Medicare Savings [Internet], 2019. Available from https://aspe.hhs.gov/system/files/pdf/261741/SDPI_Paper_Final.pdf. Accessed 21 January 2020
- 27.Yuan CM, Nee R, Ceckowski KA, Knight KR, Abbott KC. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clin Kidney J 2017;10:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2019. Atlanta, GA, US Department of Health and Human Services, Centers for Disease Control and Prevention, 2019 [Google Scholar]
- 29.Centers for Disease Control and Prevention. National Diabetes Prevention Program [Internet]. Available from https://www.cdc.gov/diabetes/prevention/index.html. Accessed 21 January 2020
- 30.Department of Health and Human Services; Office of the Assistant Secretary for Planning and Evaluation. Advancing American Kidney Health [Internet], 2019. Available from https://aspe.hhs.gov/system/files/pdf/262046/AdvancingAmericanKidneyHealth.pdf. Accessed 21 January 2020
- 31.Centers for Disease Control and Prevention. Chronic Kidney Disease (CKD) Surveillance System [Internet]. Available from https://nccd.cdc.gov/CKD/default.aspx. Accessed 21 January 2020


