Abstract
COVID-19 (Coronavirus Disease 2019) pandemic highlighted the importance of air biosecurity because SARS-CoV-2 is mainly transmitted from person to person via airborne droplets. Preventing infectious droplets from entering the body is one of the best ways to protect against infection. This paper reviews the transmission patterns of airborne pathogens and air disinfection methods. A particular emphasis is put on studies devoted to the thermal inactivation of viruses. These reviews reveal that air heat treatment has not been seriously considered as a possible air disinfection approach. Simple calculations show that the energy input required for thermal disinfection of human’s air daily consumption is almost the same as for daily water consumption (by heat treatment from room temperature to 100 °C). Moreover, it is possible to organize a continuous heat recovery from the air already heated during disinfection to the inlet air, thus significantly increasing the energy efficiency. Therefore, I propose a solution for the thermal inactivation of airborne pathogens based on air heating and its subsequent cooling in a heat exchanger with heat recovery. Such a solution could be used to create mobile personal and stationary indoor air disinfectors, as well as heating, ventilation, and air conditioning systems. Thermal disinfection of air to breathe might one day be part of people’s daily life like thermal disinfection of drinking water. Aside from limiting infectious disease transmission, thermal inactivation might be the basis for developing inhaled vaccines using thermally inactivated whole pathogens.
1. Introduction
The COVID-19 (Coronavirus Disease 2019) pandemic has become one of humanity’s most significant challenges in recent decades. According to the World Health Organization (WHO), over 146 million cases of COVID-19 had been confirmed in the world in April 2021, the diseases claiming more than 3.1 million lives [1]. Coronavirus infection, which causes severe acute respiratory syndrome (SARS), often damages the respiratory system. However, the SARS-CoV-2 can also affect almost any organ in the body [2]. To my great sorrow, the coronavirus infection caused the death of our institute’s scientific director, Vladimir Fortov [3].
SARS-CoV-2 made a significant impact on the economy, forcing countries to maintain a balance between restrictive measures to contain the spread of the COVID-19 and efforts to preserve jobs [4]. Despite all the financial and monetary methods of economic regulation, world gross domestic product (GDP) should fall by 5.2 % in 2020 [5]. The pandemic has increased unemployment and decreased working hours for those who retained their jobs [6].
The COVID-19 pandemic has exacerbated social and psychological problems among the population [7]. Large numbers of people are afraid to stay in closed spaces, and people’s mobility was significantly limited. Restrictive measures have changed people’s everyday life.
The current COVID-19 pandemic bears some similarities to the 1918 Spanish Flu pandemic in terms of high infection rates, global spread, and death rates [8]. Outbreaks of Severe Acute Respiratory Syndrome (SARS) caused by the SARS-CoV in 2002–2003 and Middle East Respiratory Syndrome (MERS) caused by the MERS-CoV in 2012 showed higher lethalities than COVID-19. However, these outbreaks only spread locally, resulting in limited overall mortality [9].
The history of pandemics says that such disease outbreaks occur almost constantly [10]. COVID-19 is unlikely to be the last pandemic; an increase in the density of immunodeficient people and populations weakened by various pathologies (e.g., chronic diseases, accumulation of genetic defects, aging) can lead to cyclical pandemics caused by highly contagious pathogens (such as SARS-CoV-2) [11]. World population growth, as well as urbanization [12] and more mobility [13], increase the risks of new pandemics. New dangerous infectious disease outbreaks are fed by constant mutations of microorganisms in the natural environment [14].
The likelihood of a person becoming severely ill from an infectious disease is largely determined by two factors: the state of their immune system and the amount of pathogens received from the environment. One of the main methods to mitigate the risk is to use vaccines. Vaccines help develop immunity towards a particular pathogen, resulting in protection against infection and decreased subsequent pathogen load, which lowers disease severity. The use of vaccines against viruses, including coronaviruses, is associated, among other issues, with the risk of antibody-dependent enhancement of infection [15]. This effect may appear after some time when the concentration of antibodies drops to sub-neutralizing values. Those residual amounts of antibodies can trigger a severe infectious process when the human immune system re-meets the virus [16]. Another risk associated with vaccines is vaccine hesitancy [17]. To beat a virus, one needs herd immunity, which is very hard to reach. Vaccine hesitancy fails to achieve or sustain herd immunity. Therefore, along with vaccine development, it seems necessary to search for other solutions that can be effective against the spread of infectious diseases.
Another way to protect against infection is to prevent the pathogen from entering the body. The most appropriate protection mechanism depends on the pathogen’s predominant mode of penetration into the human body. According to WHO reports [18], SARS-CoV-2 is mainly transmitted from person to person by airborne droplets. Like other respiratory pathogens, including influenza and rhinovirus, coronavirus transmission usually occurs through sputum droplets produced by sneezing, coughing, and breathing [16]. Closed rooms and close contact between people facilitate the transmission. The period from infection to COVID-19 symptom onset can last up to 12.5 days [19]. Thus, the virus from an infected person can spread easily among families, colleagues, and medical staff. Measures ensuring the removal (decrease in concentration) of infectious particles in the air breathed by a healthy person will prevent the spread of airborne pathogen.
COVID-19 highlighted the importance of air biosecurity. Studying the mechanisms of the airborne pathogen spread and developing methods for air disinfection are becoming critical tasks.
In this paper, I propose a method based on the thermal inactivation of airborne pathogens. The idea arose from an analytical review of the literature devoted to transmission mechanisms of airborne pathogens and air disinfection methods, which I will summarize before describing the proposed method. I will also provide a review of the literature devoted to thermal pathogen inactivation. Particular attention will be given to studies devoted to viruses.
2. The transmission of viruses through the air
2.1. Sources of airborne viruses
Airborne contamination is one of the common transmission channels for infectious diseases, whether caused by viruses or bacteria. The main carriers of infections are infected animals [20]. The inflammation of the infected carrier’s respiratory tract facilitates the spread of pathogens. Infectious microorganisms are excreted with mucus droplets when coughing, sneezing, talking, crying, and screaming. One cough emits about 3 000 droplets while a sneeze produces about 40 000 droplets [21].
Virus-containing aerosols can also be generated from tissues of plants and animals killed by the virus. Hammond et al. even hypothesized that aerosols’ atmospheric transport could lead to intercontinental virus spread (for example, of influenza) [22]. Viruses can be transported in aerosols both by droplets of liquid and solid dust particles [23].
Coughing, sneezing, talking, and breathing releases a cloud of aerosol particles with a diameter from several millimeters to less than 1 µm [24]. Large droplets (over 50 μm in diameter) settle on the ground almost immediately, medium-sized droplets (from 10 to 50 μm in diameter) settle within a few minutes, and small droplets (about 10 μm in diameter), including the nuclei of evaporated larger droplets, may remain in the air for several hours and easily penetrate the respiratory tract. Airborne droplets may contain active viral particles, the size of which is usually from 20 to 400 nm [25]. Electron microscope images of SARS-CoV showed that the virion size ranges from 150 to 200 nm, with trimeric surface spikes measuring 10–20 nm [26]. Yao et al. proposed a molecular architecture of the SARS-CoV-2 with average diameters for the short, middle, and the long axis of the virus envelope measuring 64.8, 85.9, and 96.6 nm, respectively [27].
The composition and size of droplets emitted by an infected person depend on where they form. Larger droplets (about 100 μm) are formed in the oral cavity during speech and coughing, while smaller droplets (about 1 μm) are formed in the bronchioles during normal breathing and in the larynx during talking and coughing [28], [29], [30]. Yang et al. report that the size of droplets formed during coughing ranges from 0.62 to 15.9 µm (average size 8.35 µm) [31], and the size distribution depends on the presence of virions [32]. Xie et al. evaluated that in a stationary ambient air, droplets formed during sneezing (with an initial speed of 50 m/s) can travel more than 6 m, droplets formed during coughing (with an initial speed of 10 m/s), can travel more than 2 m, whereas droplets formed while breathing (with an initial speed of 1 m/s) travel no more than 1 m [33].
Feces, urine, and vomit can be another source of infectious bioaerosols, and Jones et al. showed that the SARS-CoV-2 virus could be detected both in stool samples and in urine samples [34]. However, compared to enteric viruses transmitted by the fecal-oral route (e.g., norovirus, adenovirus), the likelihood of SARS-CoV-2 transmission through feces or urine is much lower due to the relatively low viral load. A case of SARS-CoV-2 infection in a hotel corridor due to vomiting from an infected person has been described [35].
As a rule, people pay insufficient attention to airborne infection threats, probably because the threat is invisible due to the small size of pathogens and aerosol particles. However, as the literature analysis shows, the air can contain a fairly large number of potentially dangerous but invisible microorganisms, which can linger for a long time and move over long distances.
2.2. Infective dose of the virus
An important parameter is the minimum amount of pathogen required to infect the host, called the infective dose. The infective dose varies between pathogens and for different hosts. Different microorganisms will have different ways of overcoming the host’s defense mechanisms. Moreover, the infective dose depends on the state of the host's immune system and antibodies’ presence.
In literature, the virus’ infective dose is often expressed in TCID50 units (50 % Tissue Culture Infectious Dose). TCID50 is the amount of a pathogen that produces a cytopathic effect in 50 % of infected cells. Plaques being a typical cytopathic effect resulting from the virus’ action, plaque-forming units (PFU) are also often used to express the infective dose. The route of administration of the virus is very important in determining the infective dose. Table 1 shows the infective dose of some viruses.
Table 1.
Infective dose of some viruses.
| Virus | Host | Administration method | Dose | Effect | Source |
|---|---|---|---|---|---|
| Avian influenza virus A/Chicken/Shandong/01/2008 (H9N2) | Chicken | Aerosol | 42 151.59, 3 902.93, 390.29, 43.71 and 4.22 TCID50 |
Number infected: 10 of 10, 9 of 10, 4 of 10, 1 of 10, and 0 of 10, respectively (median dose 491 TCID50) | [39] |
| Influenza virus strain A2/Bethesda/10/63 | Men aged from 21 to 41 | Aerosol | For volunteers without neutralizing antibodies – 1 to 5 TCID50 | 3 illness among 9 volunteers | [40] |
| Influenza virus strain A/Kawasaki/9/86 (H1N1) | Volunteers aged from 18 to 50 | Intranasal | 107 TCID50 | 26 of 54 people became ill | [38] |
| Rhinovirus NIH 1734 | Men | Aerosol | 16, 20 and 66 TCID50 | all 8 volunteers became ill | [42] |
| Adenovirus type 4 | Men | Aerosol | 171, 5–11, 1–2 and 0.1 TCID50 | Number ill: 4 out of 4, 8 out of 9, 3 out of 5, 0 out of 3 volunteers, respectively | [41] |
| SARS-CoV-1 | Model (for apartment residents) | Aerosol | from 16 to 160 PFU | The ratio of the number of cases to the total number of residents ranged from 0.038 to 0.325, respectively | [47] |
| MERS-CoV mouse-adapted strain (icMERSma1) | Mouse | Intranasal | from 103 to 105 PFU | Such doses may cause severe respiratory disease | [45] |
| MERS-CoV strain EMC/2012 | hCD26/DPP4 transgenic mice | Intranasal | from 1.25 to 10 TCID50 and from 102 to 106 | doses of 1.25 to 10 TCID50 caused infection in 75–100 % of challenged mice, doses of 102 to 106 caused death in 100 % of challenged mice | [46] |
| SARS-CoV-2 | hACE2 transgenic mice | Close contact, via respiratory droplets and aerosol | 36 TCID50/min for aerosol method | Close contact infected 7 out of 13 animals, 3 out of 10 animals were infected through respiratory droplets, aerosol contamination needs time greater than 25 min | [44] |
| SARS-CoV-2 | Ferrets | Intranasal | 5·106, 5·104 and 5·102 PFU | viral RNA isolation in the upper respiratory tract in 6 out of 6 animals for high and medium doses, and 1 out of 6 for low dose | [43] |
For various influenza virus strains, the infective dose in TCID50 units varies from a few units to 1010 [36], [37]. Table 1 shows the infective doses of Influenza A/Kawasaki/9/86 (H1N1) administered by intranasal method [38], avian influenza virus A/Chicken/Shandong/01/2008 (H9N2), administered by aerosol [39], and influenza A2 virus administered to volunteers in the form of an aerosol [40].
For some influenza, rhinovirus, and adenovirus strains, infection of 50 % of the population occurred at doses inferior to 1 TCID50 [36]. Low infective doses have also been found for enteric viruses, norovirus, rotavirus, echovirus, poliovirus, and hepatitis A virus [36]. Teunis et al. showed that the influenza virus’ nasal inoculation is 20 times less infectious than when delivered in the form of an inhaled aerosol [37]. Yezli et al. also confirmed higher infective doses for nasal inoculation than for aerosol (up to several orders of magnitude) [36]. Infection dose for adenovirus type 4 was studied in [41]. Doses of rhinovirus NIH 1734 resulted in 100 % infection of men (8 volunteers) were defined [42].
The study of the infective dose of SARS-CoV-2 for ferrets showed that high (5 × 106 PFU) and medium (5 × 104 PFU) doses administered intranasally released viral RNA in the upper respiratory tract of 6 out of 6 animals. However, only 1 out of 6 ferrets showed symptoms after infection with a low dose (5 × 102 PFU) [43]. Seven out of 13 transgenic hACE2 mice were contaminated after close contact with 3 infected animals [44]. Three of 10 animals became infected through respiratory drops (in neighboring cages), and aerosol infection required at least 25 min at a dose rate of 36 TCID50/min.
Douglas et al. created a MERS-CoV strain adapted to mice (maM35c4) which caused severe respiratory diseases at doses ranging from 103 to 105 PFU [45]. MERS-CoV caused illness and death in 50 % of challenged mice at doses of <1 and 10 TCID50, respectively [46].
Watanabe et al. created a dose–response model for SARS-CoV-1 and showed that doses of 43 and 280 PFU caused disease in 10 % and 50 % of cases, respectively [47]. Van Damme et al. made the hypothesis that COVID-19 severity depended on the viral dose in inoculums and was related to transmission potential [48]: a person with asymptomatic infection or mild disease will, on average, spread a lower dose of virus and is less likely to contaminate another. When they do, the newly infected person is more likely to have a mild disease than a person infected by a severely ill individual who spreads, on average, higher doses of the virus.
The concentration of viral genome copies in the oral cavity of a patient infected with COVID-19 can be up to 1011 per ml of sputum and varies throughout the disease [49], [50], [51], [52]. Viral loads in respiratory samples (nasal, throat swabs, and sputum) taken from 80 patients at different stages of COVID-19 ranged from 641 to 1.34 × 1011 copies/ml [49]; the mean values for throat and sputum samples were 7.99 × 104 and 7.52 × 105 copies/ml. A patient who exhibited a sputum viral load of 1.34 × 1011 copies/ml 8 days after the onset of COVID-19 symptoms subsequently died. Asymptomatic SARS-CoV-2 cases also exhibited relatively high viral loads of 108 copies/ml. During the first days of the disease onset, the viral load can be 108-109 copies/ml. Buonanno et al. determined that a person infected with SARS-CoV-2 can release more than 100 infective doses per hour in the form of sputum droplets simply by speaking [53].
Viral loads have also been studied for other coronavirus infections. The maximum viral load in the nasopharynx of patients infected with SARS-CoV-1 ranged from 105 to 108 copies/ml [54]. For patients with severe MERS, the peak viral load in sputum or tracheal aspirate ranged from 106 to 109 RNA copies/ml, while in mild cases, it was at least 100 times lower [55]. The viral load for common respiratory coronaviruses of such strains as NL63 and OC43 is typically 1 000–10 000 times lower than the peak values for SARS-CoV-1, SARS-CoV-2, and MERS [56].
2.3. The content of viruses in the air
Després et al. estimated the mass of viruses in 1 m3 of the atmosphere [57]. The concentration of viral particles in the air was taken as 3·104 m−3, which corresponds to the maximum concentration of influenza virus particles in outdoor pet markets in Taiwan (an endemic area for this virus) [58]. Assuming that the mass of one viral particle is 2·10-8 μg, which corresponds to one of the heaviest known viruses (vaccinia virus), the estimated mass of viruses in 1 m3 of the atmosphere was 6·10-4 μg. This value is approximately four orders of magnitude smaller than the atmosphere’s total biogenic substance content [59].
The concentration of viral particles in the air increases when approaching the sources of their formation. As such, viral aerosols near infected animals and humans have often become the subject of research. Table 2 shows the values of the concentration of viruses in the air near infected animals. For pigs infected with the classical swine fever virus, the value was about 104 m−3 [60]. A viral aerosol released at a concentration of 103 –105 is typical for other animals’ infections such as foot-and-mouth disease, Newcastle disease, and Aujeszky's disease virus [61], [62], [63].
Table 2.
The concentration of viruses in the air near infected animals.
Table 3 presents the concentration of human viruses in the air under various conditions. Studies [64], [65] show that the concentration of SARS-CoV-2 viral particles is less than 1 per 1 m3 of outdoor air. Studies [66], [67] show that the concentration of viral genome copy in the air of a room under negative pressure hosting patients with confirmed COVID-19 is 1000–10,000 times higher. The same level of concentrations of RNA copies was observed in [68] in the air in a health center, a day-care facility, and onboard airplanes during an influenza epidemic. Using these data, one can estimate the infectious concentrations of airborne viruses.
Table 3.
The concentration of human viruses in the air under various conditions.
| Virus | Conditions | Content in 1 m3 of air, viral genome copies | Source |
|---|---|---|---|
| Influenza | in a health center at the Virginia Institute of Technology (USA) during the 2009–2010 flu season | 5.8·103-1.6·104 | [68] |
| in a day-care facility in Blacksburg, Virginia (USA), during the 2009–2010 flu season | 1.6·104-3.7·104 | ||
| on board at Roanoke Airport, Virginia (USA) during the 2009–2010 flu season | 1.1–1.4·104 | ||
| SARS-CoV-2 | outdoors in northern and southern Italy during the 2020 pandemic | <0.8 | [64] |
| SARS-CoV-2 | outdoors in northern Italy during the 2020 pandemic | <1 | [65] |
| SARS-CoV-2 | in hospital rooms with infected patients | 1-2·103 | [66] |
| SARS-CoV-2 | in the rooms and corridor of the hospital | 2·103 | [67] |
| near an infected patient | 4·103 |
2.4. The viability of airborne viruses
The presence of viral particles in the air does not mean they pose an infectious hazard. Over time, viruses lose their activity.
According to [69], the inactivation rate of viruses in an aerosol follows the equation:
where C is the concentration of active viruses at time t, C0 is the initial concentration of active viruses (at time t = 0), k is an empirically determined inactivation rate factor, and n is the viability decay rate (recommended value n = 0.5 [69]). For the most stable viruses, the coefficient k is about 0.01 min−1 [58], which corresponds to a half-life of about 1 h [70], [71]. Such viruses are almost completely inactivated in aerosols in 1 day. The inactivation rate of some viruses in an aerosol may be higher, but it strongly depends on environmental conditions [72], [73]. Table 4 shows the inactivation rate of some viruses in aerosols.
Table 4.
The inactivation rate of viruses in aerosols. T - temperature, RH - relative humidity.
| Virus | Conditions | Half-life | Source |
|---|---|---|---|
| Swine fever virus | T = 21–22 °C, RH = 60–74 % | from 4.5 to 15 min | [60] |
| Vaccinia virus | T = 10.5–11.5 °C, RH = 20 % | more than 23 h | [80] |
| T = 21–23 °C, RH = 48–51 % | about 6 h | ||
| T = 31.5–33.5 °C, RH = 50 % | about 1 h | ||
| T = 31.5–33.5 °C, RH = 80–83 % | about 0.5 h | ||
| Influenza virus type A, strain PR 8 | T = 7–8 °C, RH = 23–25 % | more than 23 h | |
| T = 20.5–24 °C, RH = 50–51 % | about 0.5 h | ||
| T = 20.5–24 °C, RH = 81 % | about 5 min | ||
| T = 32 °C, RH = 20 % | from 0.5 to 1 h | ||
| T = 32 °C, RH = 81 % | about 5 min | ||
| Poliovirus type I | T = 20 ± 1 °C, RH = 80 ± 5 % | 9.07 ± 1.82 h | [78] |
| Human coronavirus 229E | T = 6 ± 1 °C, RH = 30 ± 5 % | 34.46 ± 3.21 h | |
| T = 6 ± 1 °C, RH = 50 ± 5 % | 102.53 ± 9.38 h | ||
| T = 6 ± 1 °C, RH = 80 ± 5 % | 86.01 ± 5.28 h | ||
| T = 20 ± 1 °C, RH = 30 ± 5 % | 26.76 ± 6.21 h | ||
| T = 20 ± 1 °C, RH = 50 ± 5 % | 67.33 ± 8.24 h | ||
| T = 20 ± 1 °C, RH = 80 ± 5 % | 3.34 ± 0.16 h | ||
| SARS-CoV-1 | T = 21–23 °C, RH = 65 % | about 1.2 h | [79] |
| SARS-CoV-2 | T = 21–23 °C, RH = 65 % | about 1.1 h |
The inactivation rate increases with the temperature [74]. The number of active viruses in aerosols also decreases when exposed to increasing ultraviolet radiation doses [71], [75], [76]. The impact of air’s relative humidity on the inactivation rate depends on the virus. For example, the survival of influenza virus in aerosol is highest at high relative humidity, while survival of foot and mouth disease virus is highest at medium relative humidity [72], [74], [77].
Ijaz et al. studied human coronavirus 229E inactivation rate in aerosols at different temperatures and different relative humidity [78]. They found that at 20 °C, the highest half-life, about 27 h, is achieved at 50 % relative humidity, while at 80 % relative humidity, the half-life is reduced to 3 h. At 6 °C the maximum half-life, 102 h, is also achieved at 50 % relative humidity, while the minimum half-life, 34 h, is achieved at 30 % relative humidity.
According to [79], SARS-CoV-1 and SARS-CoV-2 average half-lives are approximately the same, 1.1 and 1.2 h, respectively.
3. Air disinfection methods
Various methods are used today for reducing the concentration of active viral particles in the air and, consequently, the risk of infection.
3.1. Ultraviolet germicidal irradiation
One of the widespread methods for inactivating pathogens in the air is ultraviolet germicidal irradiation (UVGI). UVGI damages pathogens’ DNA/RNA, decreasing their reproduction rate and leading to their extinction.
The germicidal effect of ultraviolet radiation primarily depends on irradiation intensity and duration of exposure [81]. An increase of both irradiation intensity and duration of exposure leads to higher values of viral inactivation.
An important parameter influencing the effectiveness of UVGI is air’s relative humidity. An increase in the air’s relative humidity decreases the effectiveness of UVGI for both bacteria [82] and viruses [83].
The germicidal effect of ultraviolet irradiation also depends on the nature of the virus. According to [83], the doses for 90% inactivation of airborne single-stranded RNA, single-stranded DNA, double-stranded RNA, and double-stranded DNA viruses were 339–423, 444–494, 662–863, and 910–1196 μW s/cm2, respectively. Almost twice these doses were required to reach 99% inactivation.
Studies of the effectiveness of inactivation of SARS-CoV-2 virions in the air are still ongoing [84], [85], [86], [87], [88]. So far, they recommend using UVGI in crowded places, especially in enclosed spaces.
However, the application of UVGI devices has raised concerns of potential injury to people, specifically to eyes and skin [89]. To protect themselves, special personal protective equipment might be needed (glasses with light filters, face masks, gloves, overalls). Indeed, exposing eyes and skin to ultraviolet radiation can cause burns of different degrees. In addition, ultraviolet radiation sources are relatively complex and bulky, and their operation at certain wavelengths is accompanied by the release of ozone which can exceed authorized threshold concentrations [90]. To eliminate ozone generation, a soft glass coating is used to filter out the ozone-forming irradiance and avoid potential ozone hazards in the air [91].
3.1.1. Mercury-based ultraviolet lamps
Mercury-based ultraviolet lamps, which are filled with mercury and a starting gas (typically argon), are the most common UVGI devices [91]. The most common low-pressure lamps use a gas discharge in mercury vapor, with an emission spectrum near the wavelength of 253.7 nm [92].
Despite the extensive use of conventional mercury-type devices, they have the following disadvantages: the short lifespan and frequent replacement (4000–10000 h), large size light fixture, uncertain lamp surface temperature, a requirement of warm-up time (about 5 min), and mercury as a toxic environmental contaminant [93], [94]. Mechanical damage to the lamp, which uses a gas discharge in mercury vapor, can release hazardous mercury or mercury-containing compounds into the environment.
3.1.2. LED-based ultraviolet lamps
Light-emitting diodes (LEDs) are alternative materials to replace conventional mercury-containing ultraviolet lamps. LED-based ultraviolet disinfection solves some of the limitations of mercury lamps: a reduced size allows for a greater field of application and the absence of a warm-up time, as they are minimally affected by operating temperatures, allows for faster times of use, and avoids the heating of irradiated materials [95]. The effectiveness of LED-based ultraviolet technology against the SARS-CoV-2 was shown in [96]. However, at present, LED-based ultraviolet in-duct air disinfection systems exist in the prototype stage only, mainly due to limited LED output power [91].
3.1.3. Other ultraviolet-based solutions
Alternative ultraviolet-based solution can be represented by xenon pulsed light devices, which generate, in comparison with other devices, a wider ultraviolet spectrum (200–280 nm), which some authors consider to be more efficient [97]. However, they require more energy, which can lead to reduced lamp life.
Ultraviolet chip technology is another solution that aims to overcome the limitations of older lamps and has been proposed as a viable alternative to LEDs [98]. This technology provides
high-power and improves the light energy density [99]. Ultraviolet chip has low current, and is less influenced by temperature compared to an LED. However, the heat dissipation problem is also one of the key issues for this technology [100]. At present, this technology is at the prototype stage only.
3.2. Filtration
Filtration is to separate aerosolized infectious particles from contaminated air. This method can be used to create personal protective measures, indoor air cleaners as well as heating, ventilation, and air conditioning (HVAC) systems.
Buising et al. suggested indoor air cleaners based on HEPA-filters to increase the clearance of aerosols from the air in COVID-19 clinical spaces [101]. They showed that with two domestic air cleaners in a single patient room of a hospital ward, 99% of aerosols could be cleared within 5.5 min.
The main measure to prevent the spread of infections via aerosols is to wear medical masks. Wearing conventional surgical masks reduces on average the outward particle emission rates while speaking and coughing by 90% and 74%, respectively [102]. However, the pore size of conventional medical masks’ filter material is usually too large to trap small viral particles effectively [103].
FFP2, FFP3, and N95 face masks provide better protection against airborne pathogens [103]. These masks are equipped with thick filters bearing tiny pores to trap the smallest viral particles. For example, N95 masks display 95% efficiency to filter 0.1 to 0.3 µm NaCl particles and reach 99.5% or higher for 0.75 µm ones [104]. However, small pores create resistance to airflow and significantly obstruct breathing [105]. Enhanced protection and comfort could be provided by ventilated face masks [106]. However, respirators are relatively expensive in limited supplies. Some populations might not have sufficient access during pandemics.
Applying special antiviral compounds to their surface can improve the antiviral properties of conventional filters. In particular, filters containing acids, such as citric acid or polyacrylic acid, might inactivate viruses [104]. Filters containing calcined and hydrated dolomites, hydroxyapatite crystals, tungsten oxide, iodide particles (palladium (II), silver (I), copper (I)), or activated carbon are also better at adsorbing and inactivating viruses from the air [104]. Borkow et al. proposed a respirator containing copper oxide to protect against the influenza virus [107]. Kawabata et al. proposed using a composite microporous membrane based on a pyridine-type polymer to remove viruses from the air [108], while in [109] the filters were chemically modified with polyethyleneimine.
However, the use of filters causes some worries. For example, a clinical study [110] showed decreased blood oxygen content and increased pulse rate in surgeons after wearing surgical masks during operations. Moreover, the increased use of personal protective equipment, including masks, cause a serious waste disposal problem [111].
3.3. Nanomaterials
Different nanomaterials can help with air disinfection. Nanoparticles, such as Ag, Cu, Au, Fe, present broad-spectrum antiviral properties [112]. The virus can be adsorbed on the metal surface – its glycoproteins interacting with the metal particles – and become inactive. Metal nanoparticles may also enter cells and interact with viral nucleic acids to exert their antiviral capability. Another type of nanomaterial with antiviral properties is nanocarbon. These carbon-containing nanomaterials include fullerenes, carbon nanotubes, graphene-based materials, graphitic carbon nitrides [112].
However, the use of nanomaterials for air disinfection has its limitations. Nanomaterials may break up and disperse into the air, reducing air quality. Like viruses, some nanoparticles can penetrate lung or skin epithelia and enter the circulatory and lymphatic systems, reaching tissues and organs, potentially disrupting cellular processes and causing disease [113].
3.4. Ventilation
Ventilation is the process of supplying outdoor air to a room or building naturally or using mechanical means. In mechanically ventilated buildings, ventilation air is usually provided by HVAC systems. By [114], the main types of ventilation systems are hybrid/mixing ventilation systems, displacement ventilation systems, wind-driven ventilation systems, mechanical ventilation with heat recovery systems.
Ventilation systems that are commonly used change the droplet concentration, temperature, and humidity of the indoor air environment [115]. Evaluation of displacement and mixed ventilation systems and their effects on indoor air is of interest to human health and comfort [116], [117]. Indoor airflow regimes, room pressurization, and filtration for hospitals and other infectious-bounded spaces were studied by Mirzaie et al., Barbosa et al., and Liu et al. [115], [118], [119].
Ventilation plays an essential role in removing exhaled air containing viruses, reducing their total concentration in the air and, therefore, the amount inhaled by humans [120]. The risk of increased infectious disease transmission is larger in spaces with low ventilation rates. In particular, public spaces such as shops, offices, schools, kindergartens, libraries, restaurants, cruise ships, elevators, conference rooms, or public transport may not have any specific ventilation systems and rely only on open doors and windows.
The deployment of industrial systems for indoor controlled ventilation, air conditioning, and equipment disinfection is mandatory in medical institutions and some industrial enterprises but is associated with significant investments. As a way to prevent the spread of viral diseases, ventilation presents several limitations associated, among other things, with the air distribution pathways and thermal comfort of people [92]. Decreasing the concentration of harmful substances in the air by one order of magnitude requires a similar increase in the air exchange rate.
3.5. Photocatalytic oxidation
Photocatalysis accelerates a chemical reaction with the combined action of a catalyst and irradiation with light. The photocatalytic activity forms oxygen-containing radicals with a high oxidizing capacity [121]. TiO2 is normally used as a catalyst for the oxidation of organic compounds in the air [122], [123], [124].
Oxygen-containing radicals generated by photocatalysis destroy the envelope or capsid of viruses, releasing viral genetic material, minerals, and proteins [121]. The organic compounds of the viruses can be completely degraded, leading to their inactivation. Zhang et al. suggested three mechanisms of virus inactivation during photocatalysis: physical damage to viruses, the toxicity of photocatalysts’ metal ions, and chemical oxidation by oxygen-containing radicals [125].
The efficiency of photocatalytic systems depends on the duration of contact with the catalyst and may decrease due to the accumulation of inactivated residues masking the catalyst. Moreover, active oxygen-containing radicals can trigger the formation of secondary chemical compounds (aldehydes, ketones, etc.) which above certain levels decrease air quality [126].
3.6. Plasma inactivation
This virus inactivation relies on the formation of active radicals in the plasma discharge. Filipić argued that virus inactivation is achieved through the reaction of viral nucleic acids or capsid proteins with reactive oxygen or nitrogen species [127].
Although low-temperature plasma is considered a promising tool for a wide range of biomedical applications, understanding the molecular mechanisms underlying biochemical reactions in plasma remains a serious scientific challenge [128]. Assessing the impact of this method, in particular of reactive oxygen or nitrogen species, on air quality and human health is required.
3.7. Essential oils
Essential oils, as well as individual compounds derived from them such as terpenes, terpenoids, and aromatic compounds, exhibit antimicrobial activity against a broad class of pathogens [129], [130], [131]. Essential oils have also proven themselves as antiviral agents [132], [133].
Essential oils’ antimicrobial effects rely on their cytotoxic effect, which damages the cell membrane. Essential oils’ compounds are lipophilic and therefore pass through the cell wall and cytoplasmic membrane [134]. They disrupt the structure of the polysaccharides, fatty acids, and phospholipids layers, making the membranes permeable [135].
However, essential oils are not selective for pathogens and can also negatively affect eukaryotic cells [136]. Essential oils may cause allergic contact dermatitis [137], [138] and may be associated with respiratory symptoms [139].
4. Which relatively simple method is not used today?
We have seen above that today, reducing the risk of transmission of infectious diseases by airborne droplets is achieved by UVGI, filtration, photocatalytic oxidation, plasma inactivation, different nanomaterials, essential oils, as well as ventilation. All these methods, to one degree or another, reduce the viral load of the air that a person breathes. However, all these methods present certain disadvantages and limitations (Table 5 ). None of the methods completely solve the problem of airborne virus transmission.
Table 5.
Methods used today to eliminate airborne pathogens: advantages and disadvantages
| Method | Advantages | Disadvantages and limitations | |
|---|---|---|---|
| Ultraviolet germicidal irradiation (UVGI) | Mercury-based lamps | - mature technology; - suitable for various applications. |
- the short lifespan and frequent replacement; - large size light fixture; - possible release of ozone above the permissible concentration; - the likelihood of burns to the skin and eyes; - possible release of hazardous mercury and mercury-containing compounds, particularly if the lamp is mechanically damaged. |
| LED-based lamps | - suitable for various applications; - rapid start. |
- limited LED output power; - heat dissipation problem; - prototype stage. |
|
| Alternative ultraviolet-based solutions (xenon pulsed light, chip technology) | - suitable for various applications; - rapid start; - high-power system. |
- uncertain lamp life; - heat dissipation problem; - prototype stage. |
|
| Filtration | - easily accessible (face masks); - improves air quality concerning dust particles. |
- low efficacy of conventional medical masks; - resistance to breathing; - the high cost of FFP2, FFP3, and KN95 respirators; - long-term use of masks and respirators can adversely affect human health (hypoxia, etc.). - waste disposal problem. |
|
| Ventilation | - high reliability; - provide high air quality; - environmentally friendly. |
- large investments; - difficulties in providing thermal comfort for people; - bulky structures. |
|
| Photocatalytic oxidation | - non-toxicity; - wide area of application; - simple, light apparatus. |
- decreasing efficiency due to deposits of inactivated residues on the catalyst; - decrease in air quality due to secondary reactions of the catalyst. |
|
| Plasma inactivation | - shortened size and mass of a device; - does not depend on the strain of the pathogen. |
- negative impact of reactive oxygen or nitrogen radicals on air quality and human health. | |
| Nanomaterials | - broad-spectrum antiviral properties - safe method. |
- decrease in air quality; - possible toxic effect. |
|
| Essential oils | - easily accessible; - simple usage. |
- negative impact of essential oil components on the human organism during prolonged use. | |
A question arises from the above analysis: what method is not yet used to inactivate airborne pathogens? I will propose my answer to this question later. Before that, I want to talk about another substance that, like air, is vital for humans – water. The most known, accessible, and widespread inactivation method of harmful pathogens in water is its thermal treatment. WHO recommends that water be heated to boiling temperature before drinking; after the water has reached a rolling boil, it should be removed from the heat, allowed to cool naturally, without the addition of ice, and protected from post-treatment re-contamination during storage [140].
A healthy adult consumes on average 2–3 L (2–3 kg) of water per day [141] and inhales on average 7,000–11,000 L (10–14 kg) of air [142]. Therefore, the mass of air consumed by a person significantly exceeds the mass of water consumed by a person. However, only for water do we normally use a multistage purification system that includes one or more of the methods described in the previous paragraph for air and ends, as a rule, with heat treatment. None of the methods of purification and protection from pathogens listed in the previous section is routinely used on air. Moreover, heat treatment has not been seriously considered as a possible way to neutralize harmful airborne microorganisms.
5. About the influence of high temperatures on viruses
Temperature-based inactivation of pathogens (viruses) is the subject of active scientific research. High temperatures can lead to thermal destruction of the constituent parts of the virions, for instance, thermal denaturation of the surface proteins. Jonges et al. showed that thermal inactivation of influenza viruses (human influenza H3N2 and avian influenza H7N3) is associated with the loss of functionality of the surface hemagglutinin and other glycoproteins [143]. Virus inactivation is considerably enhanced above 50 °C [144].
Zou et al. studied thermal inactivation of the avian influenza A (H7N9) virus [145]. The results showed that the virus in the solution can be effectively inactivated by treatment at 56 °C for 30 min, at 65 °C for 10 min, and at 70 °C and above for no more than 1 min. The virus remained active when treated for 1 min at 56 °C or 5 min at 65 °C. Shahid et al. obtained similar results, inactivating the H5N1 avian influenza virus with heat treatments at 56 °C for 30 min [146].
Tomasula et al. studied thermal inactivation of the foot and mouth disease virus in milk at temperatures ranging from 72 to 95 °C, for 18.6 or 36 s [147]. The virus concentration in the raw material was 104 PFU. Thermal inactivation reduced it by 4 logs to a value below the detection limit.
Chin et al. studied SARS-CoV-2 stability at various temperatures [148]. The initial value was 6–8 log TCID50/ml. After 14 days at 4 °C, the virus titer had decreased by only 0.7 logs. When the temperature was raised to 70 °C, the virus was inactivated after 5 min (the virus detection limit was 100 TCID50/ml). In addition, SARS-CoV-2 was also shown to be more resistant to heat on smooth surfaces, while being sensitive to standard disinfection methods (soap solutions).
Pastorino et al. studied SARS-CoV-2 thermal inactivation at different temperatures and treatment times (56 °C for 30 min, 60 °C for 60 min, 92 °C for 15 min) using infected cell culture, blood serum, and a saliva sample from the nasopharynx of an infected person [149]. In all cases, they observed a drop in TCID50 by four orders of magnitude. The 56 °C/30 min and 60 °C/60 min protocols had little impact on the RNA copies detection, whereas 92 °C/15 min drastically reduced the detection limit. Treatment of serum samples at 56 °C for 30 min had a negligible impact on IgG detection results using a commercial ELISA test, whereas a drastic decrease in neutralizing titers was observed.
Batéjat et al. heated a cell culture, samples from the nasopharynx, and serums, containing SARS-CoV-2 [150]. They showed that SARS-CoV-2 was inactivated in less than 30 min, 15 min and 3 min at 56 °C, 65 °C, and 95 °C, respectively. All samples initially contained 6 logs TCID50/ml of SARS-CoV-2. The virus detection limit was 0.67 log TCID50/ml.
Gamble et al. showed that heating conditions also have a significant effect on the rate and efficiency of virus inactivation [151]. In particular, they found that the inactivation time for the SARS-CoV-2 virus in cell culture can vary by almost two orders of magnitude at the same temperature (70 °C) – from 0.86 min when using closed tubes to 37 min in open cups in a dry oven. They concluded that placing samples in open containers reduces the efficiency of heat inactivation drastically.
Leclercq et al. studied MERS-CoV thermal inactivation in cell culture [152]. They showed that it takes 25 and 1 min to decrease TCID50 by four orders of magnitude at 56 and 65 °C, respectively. There was no significant titer decrease after 2 h at 25 °C.
Garrido et al. studied MS2 virus inactivation in a vessel with water and aqueous salt solutions, through which preheated (up to 150 °C) air bubbles were passed [153]. They showed that inactivation by almost 2 logs could be achieved at a solution temperature of about 47 °C.
Jiang et al. studied virus thermal inactivation on a laboratory flow-type setup [154]. The virus solution passed through a stainless-steel capillary, sequentially through a hot zone, where the capillary was immersed in heated oil, and a cold zone, where the capillary was immersed in a solution with ice. Using the mouse hepatitis virus (MHV), they found that a temperature of 85.2 °C for 0.48 sec reduced the number of plaques by 5 logs (initial titer of 5 × 107 PFU/ml) while a temperature of 83.4 °C for 0.95 sec completely inactivated the virus (decrease in titer by 6 logs).
Yap et al. presented the results of calculations predicting the time of coronavirus inactivation as a function of temperature [155]. The paper considered SARS-CoV-1, SARS-CoV-2, MERS-CoV, the transmissible gastroenteritis virus, the mouse hepatitis virus, and the swine epidemic diarrhea virus at temperatures up to 100 °C. The results showed that at 70 °C, concentration decreases by 3 log (99.9%) are reached in 3 min on average for all viruses. At temperatures above 80 °C, the estimated time for which SARS-CoV-1 and SARS-CoV-2 concentrations decrease by 3 orders of magnitude is less than 1 min. However, SARS-CoV-2 appears to be slightly more stable than SARS-CoV-1. The model’s limitations were that it did not consider the relative humidity and the environment (material) in which the virions reside. Guillier et al. presented another model for predicting the time of coronavirus inactivation on surfaces and in suspension as a function of temperature [156]. Morris et al. introduced a model for predicting the stability of SARS-CoV-2 on an inert surface at various temperatures and humidity [157]. In particular, while the average virus half-life was more than 24 h at 10 °C and 40 % relative humidity, it was only about 1.5 h at 27 °C and 65 % relative humidity.
Grinshpun et al. tested MS2 virus inactivation by passing a viral aerosol in a chamber where an axial heater warmed up the air flow [158]. The diameter and length of the cylindrical chamber were 50 and 320 mm, respectively, the diameter and length of the cylindrical axial heater were 10 and 230 mm, respectively. Two air flow rates were used, 18 and 36 l/min. They observed a decrease of the PFU by three orders of magnitude both with an average air temperature of 150 °C at a flow rate of 18 l/min and an average air temperature of 230 °C at a flow rate of 36 l/min. Inactivation levels of more than 99,996% were achieved at temperatures over 170 and 250 °C, at flow rates of 18 and 36 l/min, respectively.
Yu et al. developed filters based on foamed nickel for disinfection by heating air up to 200 °C [159]. The prototype device was tested with an aerosol containing SARS-CoV-2. Air passed through six curved strips of foamed nickel with the size of 24 cm × 4 cm and a depth (hot zone length) of 1.6 cm. The air flow rate was 10 l/min. They showed that one pass of aerosol containing SARS-CoV-2 was sufficient to inactivate 99.8 % of the viruses. The same method achieved an inactivation efficiency of 99.9 % with the airborne bacterium Bacillus anthracis.
Overall, the number of works devoted to virus thermal inactivation is quite limited. Table 6 recapitulates recent studies. I found only two exploratory experimental studies devoted to viral aerosol thermal inactivation [158], [159]. Hence, we can conclude that this area remains poorly studied and requires additional scientific research.
Table 6.
Inactivation of viruses at high temperatures.
| Virus | Temperature, time | Medium/conditions | Inactivation efficiency | Source |
|---|---|---|---|---|
| Human influenza virus A/Wisconsin/67/2005 (H3N2) | 35.0, 38.3, 43.7, 49.6, 55.6, 61.3, 66.7 and 70 °C during 30 min | Cell culture supernatants (concentration 104.8 TCID50/ml) | The virus is completely inactivated at temperatures ≥ 55.6 °C | [143] |
| Avian influenza virus A/Mallard/NL/12/00 (H7N3) | 35.0, 38.3, 43.7, 49.6, 55.6, 61.3, 66.7 and 70 °C during 30 min | Cell culture supernatants (concentration 105.3 TCID50/ ml) | The virus is completely inactivated at temperatures ≥ 55.6 °C | |
| Avian influenza viruses A/Anhui/1/2013 and A/Shanghai/1/2013 (H7N9) | 56, 65, 70, 75, 100 °C during 1–60 min | The solution containing 107.7 50% of the infectious dose of eggs per ml | Viruses are completely inactivated at 56 °C for 30 min, at 65 °C for 10 min, at 70 °C, 75 °C, and 100 °C for 1 min | [145] |
| Avian influenza virus H5N1 | 4, 28 and 56 °C | Suspension | The virus remained active at 4 °C after 100 days, inactivated at 28 °C after 24 h, and at 56 °C after 30 min | [146] |
| Foot and mouth disease virus | from 72 to 95 °C, treatment time 18.6 or 36 s | Milk of infected cow (virus concentration 104 PFU) | Reduction of titer by 4 logs (below the detection limit) | [147] |
| SARS-CoV-2 | 4, 22 and 70 °C | Culture with virus content 6–8 log TCID50/ ml (detection limit 100 TCID50/ml) | The virus titer decreased 0.7 logs at 4 °C after 14 days. At 70 °C, the virus was inactivated after 5 min. | [148] |
| SARS-CoV-2 | Protocols: 56 °C and 30 min, 60 °C and 60 min, 92 °C and 15 min | Cell culture supernatants, blood serum, and saliva samples from the nasopharynx of an infected person (initial concentration 105 to 106 TCID50/ml, detection limit 100.5 TCID50/ml) | In all cases, a 5 log drop in TCID50 was observed. The activity was retained only in cell culture supernatants at 56 °C/30 min and 60 °C/60 min. | [149] |
| SARS-CoV-2 | 56 °C, 65 °C, and 95 °C, time to 60 min | Cell culture supernatants, nasopharyngeal and serum samples (concentration 106 TCID50/ml, detection limit 100.67 TCID50/ml) | The virus is inactivated after 30 min, 15 min, and 3 min at 56 °C, 65 °C, and 95 °C, respectively. | [150] |
| SARS-CoV-2 | 70 °C, time to 90 min | Cell culture (concentration 105 TCID50/ml, detection limit 100.5 TCID50/ml) | Virus half-life 0.86 min when using closed tubes and 37 min in open dishes in a dry oven | [151] |
| MERS-CoV | 25, 56 and 65 °C, time to 120 min | Cell culture (concentration 105.59 TCID50/ml, detection limit 100.67 TCID50/ml) | Inactivation at 56 °C for 60 min, at 65 °C for 15 min. It takes 25 and 1 min to decrease TCID50 by 4 orders of magnitude at 56 and 65 °C, respectively. At 25 °C, no decrease in titer is observed after 2 h. | [152] |
| Virus MS2 ATCC 15597-B1 | Bubble temperature 150 °C, the temperature of the solution in the vessel 47 °C | A vessel with water and aqueous solutions of salts, through which preheated air bubbles were passed. The volume of the liquid (solution) is 250 ml. The total content of the MS2 virus 7.25·105 | Drop in virus concentration by 2 log | [153] |
| Mouse hepatitis virus MHV-A59 | from 55 ℃ to 170 ℃ | Cell culture (concentration 5·107 PFU/ml). The virus solution is passed through a stainless-steel capillary sequentially through the hot zone and the cold zone. Heating occurs in that part of the capillary that is immersed in heated oil, cooling occurs in that part of the capillary that is immersed in a solution with ice. | At a temperature of 85.2 °C for 0.48 s, the PFU decreases by 5 logs, at a temperature of 83.4 °C for 0.95 s, the virus is completely inactivated | [154] |
| SARS-CoV, SARS-CoV-2, MERS-CoV and others | Up to 100 °C | Modeling | At a temperature of 70 °C, a decrease in concentration by 3 logarithms averaged for all viruses is achieved in 3 min. At temperatures above 80 °C, the estimated time for which the concentration of SARS-CoV-1 and SARS-CoV-2 decreases by 3 orders of magnitude is less than 1 min. | [155] |
| Virus MS2 | Up to 250 °C, time 0.1–1 s | Flow chamber with an axial heater. Two air flows – 18 and 36 l/min. The diameter and length of the chamber are 50 mm and 320 mm, respectively, the diameter and length of the heater are 10 mm and 230 mm, respectively. | A drop in the concentration of the virus in the air by 1 log at 90 °C (18 l/min) and 140 °C (36 l/min). A drop in the concentration of the virus in the air by 3 logs at 150 °C (18 l/min) and 230 °C (36 l/min). | [158] |
| SARS-CoV-2 | Up to 200 °C | Air with viruses was passed through six curved strips of foamed nickel, the size of each strip is 24 cm × 4 cm, and the depth (length of the hot zone) is 1.6 cm. The air flow rate – 10 l/min. | The inactivation efficiency by one pass is 99.8 %. | [159] |
6. The proposed solution
It may seem that heating air to high temperatures to inactivate pathogens requires high energy inputs. To estimate the value of this energy input, let compare the energy inputs required for disinfecting the daily norms of human consumption of water and air by heating them to a temperature of 100 °C. In the calculations, we will use the daily norm of human consumption of water, 3 kg, and the daily norm of human consumption of air, 14 kg.
The amount of energy required to heat 3 kg of water from 20 °C to 100 °C is:
where – heat capacity of water (4.2 kJ/(kg·К) [160]), – mass of water (3 kg), – temperature difference.
The amount of energy required to heat 14 kg of air from 20 °C to 100 °C is:
where CAir – heat capacity of air (1.005 kJ/(kg·К) [161]), – mass of air (14 kg), – temperature difference.
Thus, the amount of heat required to disinfect the daily consumption of air is comparable to the amount of heat required to disinfect the daily consumption of water. The difference is only 10 % at maximum value for the daily norm of human consumption of air. Airborne viruses are deactivated at a wide range of temperatures. Therefore, heating the air to a higher temperature (above 100 °C) requires higher energy. However, air disinfection is needed only in situations associated with a high risk of infection (for example, in medical centers, crowded places, and inside poorly ventilated rooms). Therefore, round-the-clock air disinfection may rarely be required. Moreover, unlike water, a person consumes air constantly. Therefore, it is possible to set up a continuous heat recovery from the air already heated by disinfection to the air coming in for disinfection from the inlet. This heat recovery can save a significant part of the energy spent on heating.
Unlike the water that can be thermally disinfected and stored in a container, the flowing air is difficult to restrict in a confined space and wait for people to breathe. So, one needs to thermal disinfect all the surrounding air of a human being, which would make it much larger than 14 kg/day. This is why heat recovery is very important for improving the energy efficiency of thermal air disinfection.
The cost of thermal disinfection of air can be evaluated using the cost of electrical energy if the last is used to heat the air. If the cost of electrical energy is 10 ¢ per kWh [162], then the cost of disinfection for the daily norm of human consumption of air is as follows:
Here, 0.9 is the efficiency of conversion of electrical to thermal energy; 0.313 kWh is 1126 kJ. The obtained value of thermal disinfection of air should be decreased if heat recovery is applied. It could also be decreased if cheaper heat sources than electricity is used. At the same time, this cost could be increased if the consumption of disinfected air exceeds the daily consumption rate. The cost of thermal disinfection of air is increased if additional cooling is used to adjust the comfortable temperature to breathe. The exact cost depends on the design of the disinfection device and the operating conditions.
Fig. 1 presents a schematic description of the proposed method for air heat treatment. Air enters through an air supply device. The inlet air passes through a filter, either coarse or fine. Inlet air is then brought to the heat exchanger, after which it travels to the heater. The heater warms the air up to the specified maximum temperature. The heat exchanger aims to heat the inlet air from the air already heated. Thus, the heat exchanger provides cooling of the outlet air, as well as heat recovery.
Fig. 1.
Scheme of a proposed solution for thermal inactivation of airborne pathogens. 1 – filter, either coarse or fine; 2 - air supply device; 3 - heat exchanger for heat recovery (to warm the inlet air and cool the outlet air); 4 - heater to warm the air up to the specified maximum temperature; 5 - thermal insulation.
Pathogens should be inactivated while going through the heat exchanger and the heater. Based on the results of previous studies, the maximum temperature is probably in the range of 50–250 °C.
Grinshpun et al. and Yu et al. showed that thermal inactivation provided a 3 log reduction of active viral particles in aerosol [158], [159]. At the same time, we know that the concentration of viral genome copies in hospitals and near infected patients is three times higher than that in outdoor air (Table 3). The detected concentration of viral genome copies is higher than the concentration of active viral particles because active viral particles consist of the viral genome and not all viral particles are active. Therefore, inactivation with a 3-fold reduction in the concentration of active viral particles should make the infectious air in hospital rooms and around infected patients as safe as outdoor air. By [158], [159], thermal inactivation provides such improvement of air safety. So, thermal inactivation should effectively prevent the spread of diseases caused by airborne pathogens, including influenza viruses and coronaviruses.
The efficiency of applying the proposed thermal disinfection on bioaerosols depends mainly on two parameters: the maximum heating temperature and the exposure time. The recommended values for these parameters, as well as the inactivation effectiveness, should be determined empirically. More experimental work is required in this direction.
One of the key elements of the proposed solution is a heat exchanger. Its use significantly reduces the energy input required to heat the air to a specified temperature. The heat exchanger also lowers the temperature of outlet air close to the temperature of ambient air. Without a heat exchanger, the air coming out of the outlet would be hot, and all the heat generated for the disinfection would be warming the ambient air. With a heat exchanger, the warming of the ambient air will be minimal.
According to Fig. 1, the outlet air is cooled by the inlet air via passing through the heat exchanger. It is well known that air has poor thermal conductivity (unlike water), so it is really difficult to cool the outlet air to an ambient temperature in such an air-air heat exchanger. To cool the outlet air to a temperature close to the ambient temperature, a heat exchanger with a large heat exchange surface, and therefore dimensions may be required. The efficiency and size of the heat exchanger will depend on its design, material, purpose, and operating conditions. To cool the outlet air, additional coolers could be used. It could be a radiator that cools with an excess flow of ambient air. Additional air conditioning refrigeration systems can also be used to adjust the temperature of outlet air. The use of an additional cooling system increases the cost of the device and the disinfection process, but it provides a complete product for air disinfection and conditioning. To increase the efficiency of the proposed method, the compression of the air may be also applied. It would improve the thermal conductivity of air and lead to a decrease in the size of a device.
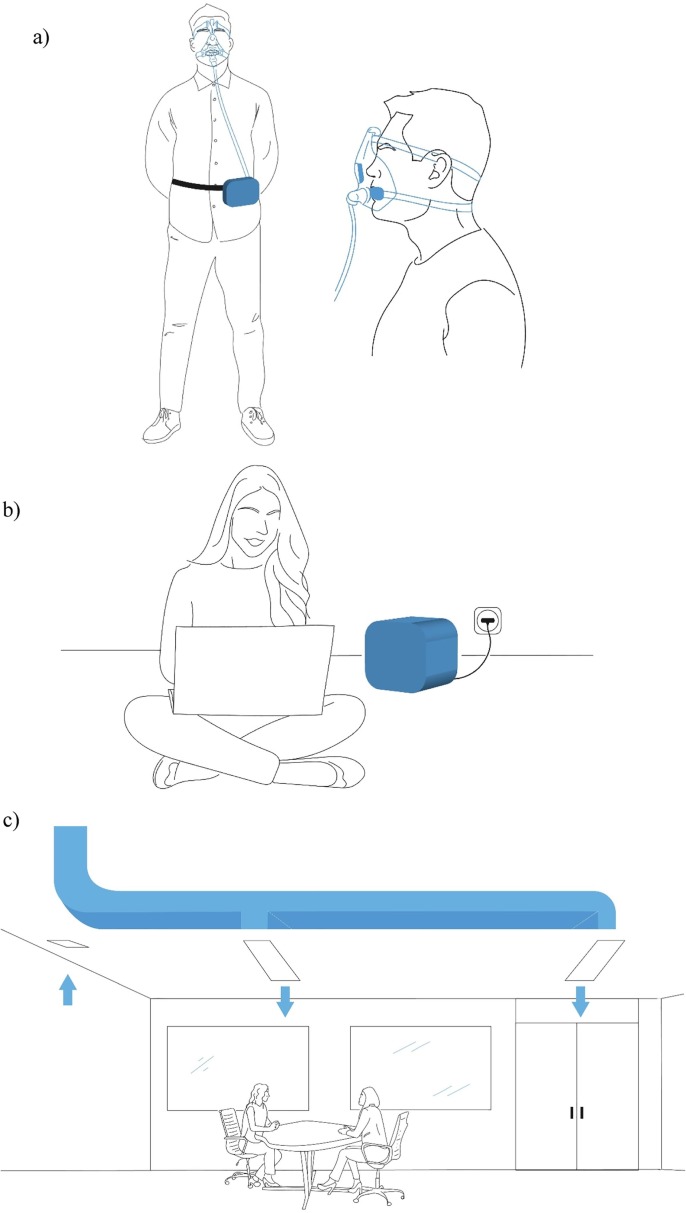
The proposed method of airborne pathogens thermal inactivation can be used to create mobile personal and stationary indoor air disinfectors, as well as HVAC systems. Fig. 2 shows conceptual sketches of three possible applications.
Fig. 2.
Conceptual sketches of three possible applications of the proposed solution for thermal inactivation of pathogens: a) mobile personal air disinfector, b) stationary indoor air disinfector, and c) within the heating, ventilation, and air conditioning (HVAC) system.
A personal air disinfector is a portable device that supplies disinfected air directly to the face area (e.g., into the face mask). It can be worn when visiting places presenting high infectious risks and at the workplace, if this entails close contact with many people (e.g., hospitals, schools, shops, transport). Personal air disinfectors can also help prevent the spread of the disease within a group of people who live in the same room or flat. They can be used to disinfect both the air exhaled by a sick person and the air inhaled by a healthy person. The discussion about personal ventilation as a control measure for airborne transmissible disease spread goes ahead in the literature [92], [163], [164]. Personal ventilation is proposed as a means to provide clean air to the breathing zone of an occupant, thus improving perceived air quality and safety. Various filters or UVGI devices have already been proposed to improve personal ventilation systems. However, to the best of my knowledge, thermal inactivation has not yet been proposed in that context.
A stationary indoor air disinfector is a device that processes the ambient air inside a room. They can be used in residential apartments, offices, or medical centers. Airborne infectious droplets are inactivated when the air circulates through the device. Stationary indoor air disinfectors based on thermal inactivation can be used instead of UVGI systems and filter-based air cleaners.
HVAC systems containing a high-temperature disinfection setup should improve the safety of both supply and exhaust air. Using such HVAC systems is particularly urgent in medical centers and other public places with a potentially high risk of spreading infection. Rezaei et al. suggested using the hot air of an HVAC condenser to eliminate SARS-CoV-2 from its exhaust air [165]. The proposed system produces exhaust air at 50–80 °C, which would facilitate virus inactivation.
The source of heat for thermal inactivation could be electrical energy. Heat for disinfection could be also obtained from the direct combustion of fuels. The heat from renewable energy sources and waste heat could be also utilized by thermal inactivation. Study [166] proposed an innovative concept for the combination of solar heating and thermal air disinfection using the Trombe wall for buildings.
One of the main issues in the thermal inactivation of airborne viruses is how to provide high energy efficiency and a comfortable temperature to breathe. These issues should be solved by thermal engineering. Effective use of thermal inactivation requires solving a number of thermal engineering issues (e.g. development of appropriate thermal equipment like heaters, thermal exchangers, HVAC systems etc.). It is also important to find materials for the devices, especially those materials that accelerate the inactivation process. Additional research and development should be done in this direction. Applied thermal engineers are expected to focus on thermal inactivation of airborne pathogens in the future.
The total energy efficiency of a proposed solution depends on the heat exchanger design. The heat exchanger design also impacts the weight and size of the air disinfector. To maintain a high and stable temperature in the heater, both the heater and the heat exchanger must be well insulated, and heat dissipation towards the external environment minimized. The heat exchange between incoming and outgoing air flows must be efficient in the exchanger. Air-to-air heat exchangers can adopt different designs: plate, shell, and tube, thin-walled tube in tube, etc. First of all, the heat exchanger design should provide high heat transfer efficiency and low heat dissipation. In particular, the heat exchanger design may ensure that the hottest zone is inside its 3D volume. Additive manufacturing [167], [168] can be used for that purpose.
The exposure time of air at high temperatures is an important parameter for thermal disinfection. The duration of air exposure to elevated temperatures depends on the internal volume of the heat exchanger and the heater. To heat the air for 5 s, a personal disinfector with an airflow rate of about 130 cm3/s (11500 l/day which corresponds to a person’s breathing rate) should have an internal volume of about 660 cm3. A stationary indoor air disinfector should have approximately the same flow rate as UVGI-based device (per unit volume of the room). With an air flow rate of 10 m3/h, a 28 L heat exchanger will provide an exposure time at elevated temperatures for about 10 s. Potential disadvantages of the proposed disinfectors include noise and vibration from the air supply device. These problems should be solved with the help of mechanical engineering.
The proposed solution could ensure people’s biological safety in emergencies and pandemics and serve as a tool against bioterrorism. It would provide a simple and scalable solution for air disinfection in public and industrial spaces featuring significant people and personnel densities. It could be deployed widely in medical centers, urban and industrial buildings. On a smaller scale, it could be used in homes when a family member is infected. Passengers, e.g. onboard airplanes or trains, could use thermal pathogen inactivation to protect themselves and others.
It is important to note that thermal inactivation may be applied against a variety of pathogens. Overall, essential advantages of the proposed method of thermal pathogen inactivation are as follows:
-
-
it does not hinder breathing;
-
-
it does not affect air quality;
-
-
it provides high inactivation efficiency;
-
-
it provides high energy efficiency (when heat recovery is applied);
-
-
it does not depend on the strain of the pathogen.
7. Discussion
The importance of air’ biological safety has significantly gained prominence due to the COVID-19 pandemic. One of the possible and promising ways to ensure air’s biological safety and prevent the spread of infectious diseases is the thermal inactivation of pathogens. Among the prospective advantages of thermal pathogen inactivation are the simplicity of its implementation and its high efficiency.
Regular and responsible use of thermal air disinfection could reduce the burden on intramuscular seasonal viral disease vaccine companies. Using thermal air disinfection near sources of pathogens decreases the body’s viral load. Mostly inactivated microorganisms enter the body. I hypothesize that this thermal pathogen inactivation method could become the basis for developing inhalation vaccines for various infectious diseases, using inactivated whole pathogens. Historical documents indicate that vaccination against smallpox by inhalation of powder made from dried pieces of damaged skin from recovering patients was practiced in ancient China (as early as the 11th century) [169]. Today, inhaled vaccines are considered promising against several infectious diseases, including influenza viruses [170], measles [171], [172], and others. Pulmonary delivery of the vaccine has also been suggested as a promising approach against COVID-19 [173]. Aside from pathogens, the thermal inactivation method may also be used to denature some allergens thus limiting the body’s allergic reactions. However, additional research is needed in this area.
Thermal inactivation might also be tested during the treatment of diseases of viral infections transmitted by airborne droplets. Breathing with a sterile pathogen-free air could slow the spread of the pathogen in the body. If the infectious droplets exhaled by a sick person are inactivated, then when inhaled, these viral particles don’t attack new areas of the surface of the respiratory organs.
Thermal disinfection of air might enter people’s daily life like thermal disinfection of drinking water today. Man learned to boil water a long time ago. Herodotus’ notes say that “the king (Cyrus) drinks only her (water from Choaspes) and nothing else. This water is boiled, and very many four wheeled waggons drawn by mules carry it in silver vessels, following the king whithersoever he goes at any time” [174]. However, this water disinfection method only became widespread at the beginning of the 20th century [175]. Today, boiling water is the most accessible known disinfection method for residents of both developed and developing countries, where it became a life-style [176].
Aside from water, heat treatment has progressively been used against pathogens in various situations and processes, such as thermal treatment of many drinks and foods (e.g., wine, milk, etc.). This process is called pasteurization in honor of Louis Pasteur, who proposed the technology.
Thermal inactivation of airborne microbes could gain a comparable spread in both research and commercial applications. In the context of the recent pandemic, this seems viable. The growing population of the planet and the degree of urbanization reinforce the need for such a technology. Figuratively speaking, thermal disinfection of air might become a life-style.
Funding
No specific funding was used for the presented work, which is a personal initiative.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The proposed method was patented (RU patent 2750412, Applicant and Inventor: M.S. Vlaskin).
Acknowledgments
The author thanks Dr. Nicolas Gambardella (aSciStance Ltd) for proofreading, and Maria Stepanova for the sketches.
References
- 1.World Health Organization WHO Coronavirus (COVID-19) Dashboard. 2020. https://covid19.who.int/ accessed April 12, 2021.
- 2.Jain U. Effect of COVID-19 on the Organs. Cureus. 2020;12(8):e9540. doi: 10.7759/cureus.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonitz M., Ebeling W., Filinov V.S. In memoriam Vladimir Evgenevich Fortov. CoPP. 2021;61(1) [Google Scholar]
- 4.Yoo S., Managi S. Global mortality benefits of COVID-19 action. Technol. Forecast Soc. Change. 2020;160 doi: 10.1016/j.techfore.2020.120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The World Bank, The Global Economic Outlook During the COVID-19 Pandemic: A Changed World (2020). https://www.worldbank.org/en/news/feature/2020/06/08/the-global-economic-outlook-during-the-covid-19-pandemic-a-changed-world.
- 6.International Labour Organization. (2021). COVID-19 Pandemic in the World of Work ILO. Monitor: COVID-19 and the world of work. 7th editionhttps://www.ilo.org/global/topics/coronavirus/impacts-and-responses/WCMS_767028/lang--en/index.htm.
- 7.Saladino V., Algeri D., Auriemma V. the psychological and social Impact of Covid-19: new perspectives of well-being. Front. Psychol. 2020;11:1–6. doi: 10.3389/fpsyg.2020.577684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein J.L. The Spanish 1918 Flu and the COVID-19 disease: the art of remembering and foreshadowing pandemics. Cell. 2020;183(2):285–289. doi: 10.1016/j.cell.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Z., Lian X., Su X., Wu W., Marraro G.A., Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respirat. Res. 2020;21(1):224. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anversa D. History of pandemics: the definitive guide to discover the worst and deadliest epidemics and pandemics that changed our world. from the Roman empire to the modern era. Independently Published. 2020 [Google Scholar]
- 11.Supotnitskiy M.V. COVID-19 pandemic as an indicator of «blank spots» in epidemiology and infectious pathology. J. NBC Protect. Corps. 2020;4(3):338–372. [Google Scholar]
- 12.Chaolin G.U. In: International Encyclopedia of Human Geography. second ed. Kobayashi A., editor. Elsevier; Oxford: 2020. Urbanization; pp. 141–153. [Google Scholar]
- 13.Schafer A., Victor D.G. The future mobility of the world population. Transport. Res. A: Pol. Pract. 2000;34(3):171–205. [Google Scholar]
- 14.Karlsson E.K., Kwiatkowski D.P., Sabeti P.C. Natural selection and infectious disease in human populations. Nat. Rev. Genet. 2014;15(6):379–393. doi: 10.1038/nrg3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee W.S., Wheatley A.K., Kent S.J., DeKosky B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020;5(10):1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Supotnitskiy M.V. Novel coronavirus SARS-CoV-2 in the context of global epidemiology of coronavirus infections. J. NBC Protect. Corps. 2020;4(1):32–64. [Google Scholar]
- 17.Jacobson R.M., St Sauver J.L., Finney Rutten L.J. Vaccine Hesitancy. Mayo Clin Proc. 2015;90(11):1562–1568. doi: 10.1016/j.mayocp.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions: scientific brief, 09 July 2020. World Health Organization (2020). https://apps.who.int/iris/handle/10665/333114. License: CC BY-NC-SA 3.0 IGO.
- 19.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones A.M., Harrison R.M. The effects of meteorological factors on atmospheric bioaerosol concentrations—a review. Sci. Total Environ. 2004;326(1):151–180. doi: 10.1016/j.scitotenv.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Dhand R., Li J. Coughs and sneezes: their role in transmission of respiratory viral infections, including SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020;202(5):651–659. doi: 10.1164/rccm.202004-1263PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammond G.W., Raddatz R.L., Gelskey D.E. Impact of atmospheric dispersion and transport of viral aerosols on the epidemiology of influenza. Rev. Infect Dis. 1989;11(3):494–497. doi: 10.1093/clinids/11.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen P.-S., Tsai F., Lin C., Yang C.-Y., Chan C.-C., Young C.-Y., Lee C.-H. Ambient influenza and avian influenza virus during dust storm days and background days. Environ. Health Perspect. 2010;118:1211–1216. doi: 10.1289/ehp.0901782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blachere F.M., Lindsley W.G., Pearce T.A., Anderson S.E., Fisher M., Khakoo R., Meade B.J., et al. Measurement of airborne influenza virus in a hospital emergency department. Clin. Infect. Dis. 2009;48(4):438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- 25.D. Knipe, P. Howley, D. Griffin, R. Lamb, M. Martin, B. Roizman, S. Straus et al., Fundamental Virology, 4th Edition; and Fields Virology, 4th Edition, Volumes I and II: Fundamental Virology, 4th Edition; Fields Virology, 4th Edition, Volumes I and II. Clinical Infectious Diseases - CLIN INFECT DIS. 2002. vol. 34, pp. 1029-1030.
- 26.Lin Y., Yan X., Cao W.-C., Wang C., Feng J., Duan J., Xie S. Probing the structure of the SARS coronavirus using scanning electron microscopy. Antiviral Therapy. 2004;9:287–289. [PubMed] [Google Scholar]
- 27.Yao H., Song Y., Chen Y., Wu N., Xu J., Sun C., Zhang J., et al. Molecular architecture of the SARS-CoV-2 virus. Cell. 2020;183(3):730–738.e713. doi: 10.1016/j.cell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmgren H., Ljungström E. Influence of film dimensions on film droplet formation. J. Aeros. Med. Pulmonary Drug Del. 2012;25(1):47–53. doi: 10.1089/jamp.2011.0892. [DOI] [PubMed] [Google Scholar]
- 29.Propagation and Breakup of Liquid Menisci and Aerosol Generation in Small Airways. J. Aerosol Med. Pulmonary Drug Delivery 22(4) 2009 341–353. [DOI] [PMC free article] [PubMed]
- 30.Fabian P., Brain J., Houseman E.A., Gern J., Milton D.K. Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. Drug Del. 2011;24(3):137–147. doi: 10.1089/jamp.2010.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Size and Concentration of Droplets Generated by Coughing in Human Subjects. J Aerosol Med. 20(4) (2007) 484-494. [DOI] [PubMed]
- 32.Hersen G., Moularat S., Robine E., Géhin E., Corbet S., Vabret A., Freymuth F. Impact of health on particle size of exhaled respiratory aerosols: case-control study. Clean: Soil Air Water. 2008;36(7):572–577. doi: 10.1002/clen.200700189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie X., Li Y., Chwang A.T.Y., Ho P.L., Seto W.H. How far droplets can move in indoor environments? Revisiting the Wells evaporation? Falling curve. Indoor Air. 2007;17(3):211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 34.Jones D.L., Baluja M.Q., Graham D.W., Corbishley A., McDonald J.E., Malham S.K., Hillary L.S., et al. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air 16(5) (2006) 335–347. [DOI] [PubMed]
- 36.Yezli S., Otter J. Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food Environ. Virol. 2011;3:1–30. doi: 10.1007/s12560-011-9056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teunis P.F.M., Brienen N., Kretzschmar M.E.E. High infectivity and pathogenicity of influenza A virus via aerosol and droplet transmission. Epidemics. 2010;2(4):215–222. doi: 10.1016/j.epidem.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Doyle W.J., Skoner D.P., Alper C.M., Allen G., Moody S.A., Seroky J.T., Hayden F.G. Effect of rimantadine treatment on clinical manifestations and otologic complications in adults experimentally infected with influenza A (H1N1) virus. J. Infect. Diseases. 1998;177(5):1260–1265. doi: 10.1086/515294. [DOI] [PubMed] [Google Scholar]
- 39.Yao M., Lv J., Huang R., Yang Y., Chai T. Determination of infective dose of H9N2 avian influenza virus in different routes: aerosol, intranasal, and gastrointestinal. Intervirology. 2014;57(6):369–374. doi: 10.1159/000365925. [DOI] [PubMed] [Google Scholar]
- 40.Alford R.H., Kasel J.A., Gerone P.J., Knight V. Human influenza resulting from aerosol Inhalation. Proc. Soc. Exp. Biol. Med. 1966;122(3):800–804. doi: 10.3181/00379727-122-31255. [DOI] [PubMed] [Google Scholar]
- 41.Couch R.B., Knight V., Douglas R.G., Jr., Black S.H., Hamory B.H. The minimal infectious dose of adenovirus type 4; the case for natural transmission by viral aerosol. Trans. Am. Clin. Climatol. Assoc. 1969;80:205–211. [PMC free article] [PubMed] [Google Scholar]
- 42.Cate T.R., Couch R.B., Fleet W.F., Griffith W.R., Gerone P.J., Knight V. Production of tracheobronchitis in volunteers with rhinovirus in a small-particle aerosol1. Am. J. Epidemiol. 1965;81(1):95–105. doi: 10.1093/oxfordjournals.aje.a120501. [DOI] [PubMed] [Google Scholar]
- 43.Ryan K.A., Bewley K.R., Fotheringham S.A., Slack G.S., Brown P., Hall Y., Wand N.I., et al. Dose-dependent response to infection with SARS-CoV-2 in the ferret model and evidence of protective immunity. Nat. Commun. 2021;12(1):81. doi: 10.1038/s41467-020-20439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bao L., Gao H., Deng W., Lv Q., Yu H., Liu M., Yu P., et al. Transmission of severe acute respiratory syndrome coronavirus 2 via close contact and respiratory droplets among human angiotensin-converting enzyme 2 mice. J. Infect. Diseases. 2020;222(4):551–555. doi: 10.1093/infdis/jiaa281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douglas M.G., Kocher J.F., Scobey T., Baric R.S., Cockrell A.S. Adaptive evolution influences the infectious dose of MERS-CoV necessary to achieve severe respiratory disease. Virology. 2018;517:98–107. doi: 10.1016/j.virol.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao X., Garron T., Agrawal A.S., Algaissi A., Peng B.-H., Wakamiya M., Chan T.-S., et al. Characterization and demonstration of the value of a lethal mouse model of middle east respiratory syndrome coronavirus infection and disease. J. Virol. 2016;90(1):57–67. doi: 10.1128/JVI.02009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe T., Bartrand T.A., Weir M.H., Omura T., Haas C.N. Development of a dose-response model for SARS coronavirus. Risk Anal. 2010;30(7):1129–1138. doi: 10.1111/j.1539-6924.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Damme W., Dahake R., van de Pas R., Vanham G., Assefa Y. COVID-19: Does the infectious inoculum dose-response relationship contribute to understanding heterogeneity in disease severity and transmission dynamics? Med. Hypotheses. 2021;146:110431. doi: 10.1016/j.mehy.2020.110431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. The Lancet Infect. Diseases. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., Zimmer T., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.To K.-K.-W., Tsang O.-T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C., Yip C.-C.-Y., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Diseases. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleiboeker S., Cowden S., Grantham J., Nutt J., Tyler A., Berg A., Altrich M. SARS-CoV-2 viral load assessment in respiratory samples. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buonanno G., Stabile L., Morawska L. Estimation of airborne viral emission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020;141 doi: 10.1016/j.envint.2020.105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L.M., Law K.I., et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. The Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh M.-D., Park W.B., Choe P.G., Choi S.-J., Kim J.-I., Chae J., Park S.S., et al. Viral load kinetics of MERS coronavirus infection. N Engl. J. Med. 2016;375(13):1303–1305. doi: 10.1056/NEJMc1511695. [DOI] [PubMed] [Google Scholar]
- 56.Lassaunière R., Kresfelder T., Venter M. A novel multiplex real-time RT-PCR assay with FRET hybridization probes for the detection and quantitation of 13 respiratory viruses. J. Virol. Methods. 2010;165(2):254–260. doi: 10.1016/j.jviromet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Després V., Huffman J.A., Burrows S.M., Hoose C., Safatov A., Buryak G., Fröhlich-Nowoisky J., et al. Primary biological aerosol particles in the atmosphere: a review. Tellus B: Chem. Phys. Meteorol. 2012;64(1):15598. [Google Scholar]
- 58.P.S. Chen, F.T. Tasi, W.C. Kuo, Airborne influenza and avian influenza viruses from long term transportation and its health effect. The 2008 European Aerosol Conference, Thessaloniki, Greece, August 24-292008.
- 59.Jaenicke R. Abundance of cellular material and proteins in the atmosphere. Science. 2005;308(5718):73. doi: 10.1126/science.1106335. [DOI] [PubMed] [Google Scholar]
- 60.Weesendorp E., Landman W.J., Stegeman A., Loeffen W.L. Detection and quantification of classical swine fever virus in air samples originating from infected pigs and experimentally produced aerosols. Vet. Microbiol. 2008;127(1–2):50–62. doi: 10.1016/j.vetmic.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 61.Donaldson A.I., Wardley R.C., Martin S., Ferris N.P. Experimental Aujeszky's disease in pigs: excretion, survival and transmission of the virus. Vet. Rec. 1983;113(21):490–494. doi: 10.1136/vr.113.21.490. [DOI] [PubMed] [Google Scholar]
- 62.Donaldson A.I., Alexandersen S. Predicting the spread of foot and mouth disease by airborne virus. Rev. Sci. Tech. 2002;21(3):569–575. doi: 10.20506/rst.21.3.1362. [DOI] [PubMed] [Google Scholar]
- 63.Li X., Chai T., Wang Z., Song C., Cao H., Liu J., Zhang X., et al. Occurrence and transmission of Newcastle disease virus aerosol originating from infected chickens under experimental conditions. Vet. Microbiol. 2009;136(3):226–232. doi: 10.1016/j.vetmic.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Chirizzi D., Conte M., Feltracco M., Dinoi A., Gregoris E., Barbaro E., La Bella G., et al. SARS-CoV-2 concentrations and virus-laden aerosol size distributions in outdoor air in north and south of Italy. Environ. Int. 2021;146 doi: 10.1016/j.envint.2020.106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Belosi F., Conte M., Gianelle V., Santachiara G., Contini D. On the concentration of SARS-CoV-2 in outdoor air and the interaction with pre-existing atmospheric particles. Environ. Res. 2021;193 doi: 10.1016/j.envres.2020.110603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K., Lim X.F., et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11(1):2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santarpia J.L., Rivera D.N., Herrera V.L., Morwitzer M.J., Creager H.M., Santarpia G.W., Crown K.K., et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Scientific Reports. 2020;10(1):12732. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang W., Elankumaran S., Marr L.C. Concentrations and size distributions of airborne influenza A viruses measured indoors at a health centre, a day-care centre and on aeroplanes. J. Roy. Soc. Interf. 2011;8(61):1176–1184. doi: 10.1098/rsif.2010.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Posada J.A., Redrow J., Celik I. A mathematical model for predicting the viability of airborne viruses. J. Virol. Methods. 2010;164(1):88–95. doi: 10.1016/j.jviromet.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 70.Donaldson A.I. The influence of relative humidity on the stability of foot-and-mouth disease virus in aerosols from milk and faecal slurry. Res. Vet. Sci. 1973;15(1):96–101. [PubMed] [Google Scholar]
- 71.McDevitt J.J., Milton D.K., Rudnick S.N., First M.W. Inactivation of poxviruses by upper-room UVC light in a simulated hospital room environment. PLOS ONE. 2008;3(9) doi: 10.1371/journal.pone.0003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donaldson A.I. The influence of relative humidity on the aerosol stability of different strains of foot-and-mouth disease virus suspended in saliva. J. Gen. Virol. 1972;15(1):25–33. doi: 10.1099/0022-1317-15-1-25. [DOI] [PubMed] [Google Scholar]
- 73.Donaldson A.I., Ferris N.P. Airborne stability of swine vesicular disease virus. Vet. Rec. 1974;95(1):19–22. doi: 10.1136/vr.95.1.19. [DOI] [PubMed] [Google Scholar]
- 74.Weber T.P., Stilianakis N.I. A note on the inactivation of influenza A viruses by solar radiation, relative humidity and temperature. Photochem Photobiol. 2008;84(6):1601–1602. doi: 10.1111/j.1751-1097.2008.00416.x. author reply 1603–1604. [DOI] [PubMed] [Google Scholar]
- 75.Jensen M.M. Inactivation of airborne viruses by ultraviolet irradiation. Appl Microbiol. 1964;12(5):418–420. doi: 10.1128/am.12.5.418-420.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sagripanti J.L., Lytle C.D. Inactivation of influenza virus by solar radiation. Photochem Photobiol. 2007;83(5):1278–1282. doi: 10.1111/j.1751-1097.2007.00177.x. [DOI] [PubMed] [Google Scholar]
- 77.Schaffer F.L., Soergel M.E., Straube D.C. Survival of airborne influenza virus: effects of propagating host, relative humidity, and composition of spray fluids. Arch Virol. 1976;51(4):263–273. doi: 10.1007/BF01317930. [DOI] [PubMed] [Google Scholar]
- 78.Ijaz M.K., Brunner A.H., Sattar S.A., Nair R.C., Johnson-Lussenburg C.M. Survival characteristics of airborne human coronavirus 229E. J. Gen. Virol. 1985;66(12):2743–2748. doi: 10.1099/0022-1317-66-12-2743. [DOI] [PubMed] [Google Scholar]
- 79.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harper G.J. Airborne micro-organisms: survival tests with four viruses. J. Hygiene. 1961;59(4):479–486. doi: 10.1017/s0022172400039176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ko G., First M.W., Burge H.A. The characterization of upper-room ultraviolet germicidal irradiation in inactivating airborne microorganisms. Environ. Health Perspect. 2002;110(1):95–101. doi: 10.1289/ehp.0211095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peccia J., Werth H.M., Miller S., Hernandez M. Effects of relative humidity on the ultraviolet induced inactivation of airborne bacteria. Aerosol. Sci. Technol. 2001;35(3):728–740. [Google Scholar]
- 83.Tseng C.-C., Li C.-S. Inactivation of virus-containing aerosols by ultraviolet germicidal irradiation. Aerosol. Sci. Technol. 2005;39(12):1136–1142. [Google Scholar]
- 84.Nardell E.A., Nathavitharana R.R. Airborne spread of SARS-CoV-2 and a potential role for air disinfection. JAMA. 2020;324(2):141–142. doi: 10.1001/jama.2020.7603. [DOI] [PubMed] [Google Scholar]
- 85.García de Abajo F.J., Hernández R.J., Kaminer I., Meyerhans A., Rosell-Llompart J., Sanchez-Elsner T. Back to normal: an old physics route to reduce SARS-CoV-2 transmission in indoor spaces. ACS Nano. 2020;14(7):7704–7713. doi: 10.1021/acsnano.0c04596. [DOI] [PubMed] [Google Scholar]
- 86.Heßling M., Hönes K., Vatter P., Lingenfelder C. Ultraviolet irradiation doses for coronavirus inactivation - review and analysis of coronavirus photoinactivation studies. GMS Hyg. Infect. Control. 2020;15:Doc08. doi: 10.3205/dgkh000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beggs C.B., Avital E.J. Upper-room ultraviolet air disinfection might help to reduce COVID-19 transmission in buildings: a feasibility study. PeerJ. 2020;8 doi: 10.7717/peerj.10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schuit M., Gardner S., Wood S., Bower K., Williams G., Freeburger D., Dabisch P. The influence of simulated sunlight on the inactivation of influenza virus in aerosols. J. Infect. Diseases. 2020;221(3):372–378. doi: 10.1093/infdis/jiz582. [DOI] [PubMed] [Google Scholar]
- 89.E.A. Nardell, S.J. Bucher, P.W. Brickner, C. Wang, R.L. Vincent, K. Becan-McBride, M.A. James, et al., Safety of upper-room ultraviolet germicidal air disinfection for room occupants: results from the Tuberculosis Ultraviolet Shelter Study. Public health reports (Washington, DC: 1974). 2008. vol. 123, 1, pp. 52-60. [DOI] [PMC free article] [PubMed]
- 90.Szeto W., Yam W.C., Huang H., Leung D.Y.C. The efficacy of vacuum-ultraviolet light disinfection of some common environmental pathogens. BMC Infect Dis. 2020;20(1):127. doi: 10.1186/s12879-020-4847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo H., Zhong L. Ultraviolet germicidal irradiation (UVGI) for in-duct airborne bioaerosol disinfection: review and analysis of design factors. Build Environ. 2021;197 doi: 10.1016/j.buildenv.2021.107852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bolashikov Z.D., Melikov A.K. Methods for air cleaning and protection of building occupants from airborne pathogens. Build. Environ. 2009;44(7):1378–1385. doi: 10.1016/j.buildenv.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nunayon S.S., Zhang H., Lai A.C.K. Comparison of disinfection performance of UVC-LED and conventional upper-room UVGI systems. Indoor Air. 2020;30(1):180–191. doi: 10.1111/ina.12619. [DOI] [PubMed] [Google Scholar]
- 94.Shin J.-Y., Kim S.-J., Kim D.-K., Kang D.-H., Elkins C.A. Fundamental characteristics of deep-UV light-emitting diodes and their application to control foodborne pathogens. Appl. Environ. Microbiol. 2016;82(1):2–10. doi: 10.1128/AEM.01186-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim D.-K., Kang D.-H., Schottel J.L. UVC LED irradiation effectively inactivates aerosolized viruses, bacteria, and fungi in a chamber-type air disinfection system. Appl. Environ. Microbiol. 2018;84(17):e00944–00918. doi: 10.1128/AEM.00944-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shimoda H., Matsuda J., Iwasaki T., Hayasaka D. Efficacy of 265-nm ultraviolet light in inactivating infectious SARS-CoV-2. J. Photochem. Photobiol. 2021;7 doi: 10.1016/j.jpap.2021.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simmons S.E., Carrion R., Alfson K.J., Staples H.M., Jinadatha C., Jarvis W.R., Sampathkumar P., et al. Deactivation of SARS-CoV-2 with pulsed-xenon ultraviolet light: Implications for environmental COVID-19 control. Infect Control Hosp. Epidemiol. 2020;42(2):127–130. doi: 10.1017/ice.2020.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Messina G., Della C.A., Ferraro P., Amodeo D., Corazza A., Nante N., Cevenini G. An emerging innovative UV disinfection technology (Part II): virucide activity on SARS-CoV-2. Int. J. Env. Res. Public Health. 2021;18(8):3873. doi: 10.3390/ijerph18083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu H.-H., Lin K.-H., Lin S.-T. A study on the heat dissipation of high power multi-chip COB LEDs. Microelectron. J. 2012;43(4):280–287. [Google Scholar]
- 100.Liang S., Hao R., Fan D., Li B., Huang J., Zhang Y., Wu J., et al. Structural optimization and numerical thermal analysis of ultraviolet light-emitting diodes with high-power multi-chip arrays. Optik. 2020;222 [Google Scholar]
- 101.K.L. Buising, R. Schofield, L. Irving, M. Keywood, A. Stevens, N. Keogh, G. Skidmore, et al., Use of portable air cleaners to reduce aerosol transmission on a hospital COVID-19 ward. medRxiv : the preprint server for health sciences. 2021, pp. 2021.2003.2029.21254590.
- 102.Asadi S., Cappa C.D., Barreda S., Wexler A.S., Bouvier N.M., Ristenpart W.D. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Scientific Reports. 2020;10(1):15665. doi: 10.1038/s41598-020-72798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Derrick J.L., Li P.T.Y., Tang S.P.Y., Gomersall C.D. Protecting staff against airborne viral particles: in vivo efficiency of laser masks. J. Hosp. Infect. 2006;64(3):278–281. doi: 10.1016/j.jhin.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 104.Qian Y., Willeke K., Grinshpun S.A., Donnelly J., Coffey C.C. Performance of N95 respirators: filtration efficiency for airborne microbial and inert particles. Am. Ind. Hyg. Assoc. J. 1998;59(2):128–132. doi: 10.1080/15428119891010389. [DOI] [PubMed] [Google Scholar]
- 105.Li Y., Tokura H., Guo Y.P., Wong A.S.W., Wong T., Chung J., Newton E. Effects of wearing N95 and surgical facemasks on heart rate, thermal stress and subjective sensations. Int. Arch. Occup. Environ. Health. 2005;78(6):501–509. doi: 10.1007/s00420-004-0584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huo S., Zhang T. Ventilation of ordinary face masks. Build. Environ. 2021;205 doi: 10.1016/j.buildenv.2021.108261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Borkow G., Zhou S.S., Page T., Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PLOS ONE. 2010;5(6) doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kawabata N., Ujino I. Removal of virus from air by filtration using a composite microporous membrane made of crosslinked poly(N-benzyl-4-vinylpyridinium chloride) React. Funct. Polym. 1998;37(1):213–218. [Google Scholar]
- 109.Tiliket G., Sage D.L., Moules V., Rosa-Calatrava M., Lina B., Valleton J.M., Nguyen Q.T., et al. A new material for airborne virus filtration. Chem. Eng. J. 2011;173(2):341–351. [Google Scholar]
- 110.Beder A., Büyükkoçak Ü., Sabuncuoğlu H., Keskil Z.A., Keskil S. Preliminary report on surgical mask induced deoxygenation during major surgery. Neurocirugia. 2008;19(2):121–126. doi: 10.1016/s1130-1473(08)70235-5. [DOI] [PubMed] [Google Scholar]
- 111.Sangkham S. Face mask and medical waste disposal during the novel COVID-19 pandemic in Asia. Case Stud. Chem. Environ. Eng. 2020;2:100052. doi: 10.1016/j.cscee.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li R., Cui L., Chen M., Huang Y.u. Nanomaterials for airborne virus inactivation. A short review. Aeros. Sci. Eng. 2021;5(1):1–11. [Google Scholar]
- 113.Buzea C., Pacheco I.I., Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4) doi: 10.1116/1.2815690. pp. Mr17-71. [DOI] [PubMed] [Google Scholar]
- 114.Elsaid A.M., Mohamed H.A., Abdelaziz G.B., Ahmed M.S. A critical review of heating, ventilation, and air conditioning (HVAC) systems within the context of a global SARS-CoV-2 epidemic. Process Saf. Environ. Prot. 2021;155:230–261. doi: 10.1016/j.psep.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mirzaie M., Lakzian E., Khan A., Warkiani M.E., Mahian O., Ahmadi G. COVID-19 spread in a classroom equipped with partition – a CFD approach. J. Hazard. Mater. 2021;420 doi: 10.1016/j.jhazmat.2021.126587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van Hooff T., Blocken B., Aanen L., Bronsema B. A venturi-shaped roof for wind-induced natural ventilation of buildings: wind tunnel and CFD evaluation of different design configurations. Build. Environ. 2011;46(9):1797–1807. [Google Scholar]
- 117.Gilani S., Montazeri H., Blocken B. CFD simulation of stratified indoor environment in displacement ventilation: Validation and sensitivity analysis. Build. Environ. 2016;95:299–313. [Google Scholar]
- 118.Barbosa B.P.P., Brum N.d.C.L. Validation and assessment of the CFD-0 module of CONTAM software for airborne contaminant transport simulation in laboratory and hospital applications. Build Environ. 2018;142:139–152. [Google Scholar]
- 119.Liu W., Liu D., Gao N. CFD study on gaseous pollutant transmission characteristics under different ventilation strategies in a typical chemical laboratory. Build Environ. 2017;126:238–251. [Google Scholar]
- 120.Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., Cao J., et al. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142 doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Habibi-Yangjeh A., Asadzadeh-Khaneghah S., Feizpoor S., Rouhi A. Review on heterogeneous photocatalytic disinfection of waterborne, airborne, and foodborne viruses: can we win against pathogenic viruses? J. Colloid Interface Sci. 2020;580:503–514. doi: 10.1016/j.jcis.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hand D.W., Perram D.L., Crittenden J.C. Destruction of DBP precursors with catalytic oxidation. J. AWWA. 1995;87(6):84–96. [Google Scholar]
- 123.Matsunaga T., Tomoda R., Nakajima T., Nakamura N., Komine T. Continuous-sterilization system that uses photosemiconductor powders. Appl. Environ. Microbiol. 1988;54(6):1330–1333. doi: 10.1128/aem.54.6.1330-1333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cho M., Chung H., Choi W., Yoon J. Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl. Environ. Microbiol. 2005;71(1):270–275. doi: 10.1128/AEM.71.1.270-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang C., Li Y., Shuai D., Shen Y., Wang D. Progress and challenges in photocatalytic disinfection of waterborne viruses: a review to fill current knowledge gaps. Chem. Eng. J. 2019;355:399–415. [Google Scholar]
- 126.Sæbjörnsson K.O., Fang L. Laboratory study on incomplete oxidation of a photocatalytic oxidation air purifier. HB 2006 - Healthy Buildings: Creating a Healthy Indoor Environment for People. Proceedings. 2006;2:271–276. [Google Scholar]
- 127.Filipić A., Gutierrez-Aguirre I., Primc G., Mozetič M., Dobnik D. Cold plasma, a new hope in the field of virus inactivation. Trends Biotechnol. 2020;38(11):1278–1291. doi: 10.1016/j.tibtech.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lunov O., Zablotskii V., Churpita O., Chánová E., Syková E., Dejneka A., Kubinová Š. Cell death induced by ozone and various non-thermal plasmas: therapeutic perspectives and limitations. Scientific Reports. 2014;4(1):7129. doi: 10.1038/srep07129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bassolé I.H., Juliani H.R. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17(4):3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Friedman M., Henika P.R., Mandrell R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002;65(10):1545–1560. doi: 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- 131.I.b. Jantan, B.A. Karim Moharam, J. Santhanam, J.A. Jamal, Correlation between chemical composition and antifungal activity of the essential oils of eight cinnamomum. Species. Pharm Biol. 46(6) (2008) 406-412.
- 132.Asif M., Saleem M., Saadullah M., Yaseen H.S., Al Zarzour R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology. 2020;28(5):1153–1161. doi: 10.1007/s10787-020-00744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.J.K.R.d. Silva, P.L.B. Figueiredo, K.G. Byler, W.N. Setzer, Essential oils as antiviral agents. potential of essential oils to treat SARS-CoV-2 Infection: an in-silico investigation, Int. J. Mol. Sci. 21(10) (2020) 3426. [DOI] [PMC free article] [PubMed]
- 134.Brochot A., Guilbot A., Haddioui L., Roques C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. MicrobiologyOpen. 2017;6(4) doi: 10.1002/mbo3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 136.Carson C.F., Hammer K.A., Riley T.V. Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006;19(1):50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schaller M., Korting H.C. Allergic airborne contact dermatitis from essential oils used in aromatherapy. Clin. Exp. Dermatol. 1995;20(2):143–145. doi: 10.1111/j.1365-2230.1995.tb02719.x. [DOI] [PubMed] [Google Scholar]
- 138.Sindle A., Martin K. Essential oils – Natural products not necessarily safe. Int J Women's Dermatol. 2020 doi: 10.1016/j.ijwd.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gibbs J.E. Essential oils, asthma, thunderstorms, and plant gases: a prospective study of respiratory response to ambient biogenic volatile organic compounds (BVOCs) J. Asthma All. 2019;12:169–182. doi: 10.2147/JAA.S193211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.World Health Organization Technical Brief. Boil. Water. 2015;WHO/FWC/WSH/15.02 https://www.who.int/water_sanitation_health/dwq/Boiling_water_01_15.pdf [Google Scholar]
- 141.Annobil E. In: A Prescription for Healthy Living. Short E., editor. Academic Press; 2021. Chapter 25 - Water: how much should be consumed and what are its health benefits? pp. 281–286. [Google Scholar]
- 142.Nazaroff W.W. The air around us. Indoor Air. 2018;28(1):3–5. doi: 10.1111/ina.12423. [DOI] [PubMed] [Google Scholar]
- 143.Jonges M., Liu W.M., van der Vries E., Jacobi R., Pronk I., Boog C., Koopmans M., et al. Influenza virus inactivation for studies of antigenicity and phenotypic neuraminidase inhibitor resistance profiling. J. Clin. Microbiol. 2010;48(3):928. doi: 10.1128/JCM.02045-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bertrand I., Schijven J.F., Sánchez G., Wyn-Jones P., Ottoson J., Morin T., Muscillo M., et al. The impact of temperature on the inactivation of enteric viruses in food and water: a review. J. Appl. Microbiol. 2012;112(6):1059–1074. doi: 10.1111/j.1365-2672.2012.05267.x. [DOI] [PubMed] [Google Scholar]
- 145.Zou S., Guo J., Gao R., Dong L., Zhou J., Zhang Y., Dong J., et al. Inactivation of the novel avian influenza A (H7N9) virus under physical conditions or chemical agents treatment. Virol. J. 2013;10:289. doi: 10.1186/1743-422X-10-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shahid M.A., Abubakar M., Hameed S., Hassan S. Avian influenza virus (H5N1); effects of physico-chemical factors on its survival. Virol. J. 2009;6:38. doi: 10.1186/1743-422X-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tomasula P.M., Kozempel M.F., Konstance R.P., Gregg D., Boettcher S., Baxt B., Rodriguez L.L. Thermal inactivation of foot-and-mouth disease virus in milk using high-temperature, short-time pasteurization. J. Dairy Sci. 2007;90(7):3202–3211. doi: 10.3168/jds.2006-525. [DOI] [PubMed] [Google Scholar]
- 148.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1) doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R.N. Heat inactivation of different types of SARS-CoV-2 samples: what protocols for biosafety, molecular detection and serological diagnostics? Viruses. 2020;12(7):735. doi: 10.3390/v12070735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.C. Batéjat, Q. Grassin, J.-C. Manuguerra, I. Leclercq, Heat inactivation of the Severe Acute Respiratory Syndrome Coronavirus 2. bioRxiv. 2020, pp. 2020.2005.2001.067769. [DOI] [PMC free article] [PubMed]
- 151.A. Gamble, R.J. Fischer, D.H. Morris, K.C. Yinda, V.J. Munster, J.O. Lloyd-Smith, Heat-treated virus inactivation rate depends strongly on treatment procedure. bioRxiv. 2020, pp. 2020.2008.2010.242206. [DOI] [PMC free article] [PubMed]
- 152.Leclercq I., Batéjat C., Burguière A.M., Manuguerra J.C. Heat inactivation of the Middle East respiratory syndrome coronavirus. Influenza Other Respirat. Viruses. 2014;8(5):585–586. doi: 10.1111/irv.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Garrido A., Pashley R.M., Ninham B.W. Low temperature MS2 (ATCC15597-B1) virus inactivation using a hot bubble column evaporator (HBCE) Colloids Surf B Biointerfaces. 2017;151:1–10. doi: 10.1016/j.colsurfb.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 154.Y. Jiang, H. Zhang, J.A. Wippold, J. Gupta, J. Dai, P. de Figueiredo, J.L. Leibowitz, et al., Sub-second heat inactivation of coronavirus. bioRxiv. 2020. vol, p. 2020.2010.2005.327528.
- 155.Yap T.F., Liu Z., Shveda R.A., Preston D.J. A predictive model of the temperature-dependent inactivation of coronaviruses. Appl. Phys. Lett. 2020;117(6) doi: 10.1063/5.0020782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guillier L., Martin-Latil S., Chaix E., Thébault A., Pavio N., Le Poder S., Batéjat C., et al. Modeling the inactivation of viruses from the coronaviridae family in response to temperature and relative humidity in suspensions or on surfaces. Appl. Environ. Microbiol. 2020;86(18):e01244–01220. doi: 10.1128/AEM.01244-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.D.H. Morris , K.C. Yinda, A. Gamble, F.W. Rossine, Q. Huang, T. Bushmaker, R.J. Fischer, et al. The effect of temperature and humidity on the stability of SARS-CoV-2 and other enveloped viruses. bioRxiv. 2020, pp. 2020.2010.2016.341883.
- 158.Grinshpun S.A., Adhikari A., Li C., Yermakov M., Reponen L., Johansson E., Trunov M. Inactivation of aerosolized viruses in continuous air flow with axial heating. Aerosol. Sci. Technol. 2010;44(11):1042–1048. [Google Scholar]
- 159.Yu L., Peel G.K., Cheema F.H., Lawrence W.S., Bukreyeva N., Jinks C.W., Peel J.E., et al. Catching and killing of airborne SARS-CoV-2 to control spread of COVID-19 by a heated air disinfection system. Mater. Today Phys. 2020;15 doi: 10.1016/j.mtphys.2020.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.IAPWS Industrial Formulation 1997 for the Thermodynamic properties of Water and Steam. International Association for the Properties of Water and Steam. Thermophysical Properties Division, National Institute of Standards and Technology, Colorado 80305, USA.
- 161.I. Dincer, M. Kanoǧlu, Refrigeration Systems and Applications, Second Edition. Refrigeration Systems and Applications, Second Edition. 2010.
- 162.U.S. Energy Information Administration, Electric Power Monthly, Table 5.3, February 2021, preliminary data. 2021.
- 163.Pantelic J., Sze-To G.N., Tham K.W., Chao C.Y.H., Khoo Y.C.M. Personalized ventilation as a control measure for airborne transmissible disease spread. J. Roy. Soc. Interface. 2009;6(suppl_6):S715–S726. doi: 10.1098/rsif.2009.0311.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu C., Wei X., Liu L., Su L., Liu W., Wang Y., Nielsen P.V. Effects of personalized ventilation interventions on airborne infection risk and transmission between occupants. Build. Environ. 2020;180 doi: 10.1016/j.buildenv.2020.107008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rezaei N., Jafari M., Nazari A., Salehi S., Talati F., Torab R., Nejad-Rahim R. A novel methodology and new concept of SARS-CoV-2 elimination in heating and ventilating air conditioning systems using waste heat recovery. AIP Adv. 2020;10(8) doi: 10.1063/5.0021575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xie H., Yu B., Wang J., Ji J. A novel disinfected Trombe wall for space heating and virus inactivation: concept and performance investigation. ApEn. 2021;291 doi: 10.1016/j.apenergy.2021.116789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Attaran Mohsen. The rise of 3-D printing: the advantages of additive manufacturing over traditional manufacturing. Bus Horiz. 2017;60(5):677–688. [Google Scholar]
- 168.Cooke S., Ahmadi K., Willerth S., Herring R. Metal additive manufacturing: technology, metallurgy and modelling. J. Manufact. Process. 2020;57:978–1003. [Google Scholar]
- 169.Cao X. Immunology in China: the past, present and future. Nat. Immunol. 2008;9(4):339–342. doi: 10.1038/ni0408-339. [DOI] [PubMed] [Google Scholar]
- 170.Tomar J., Biel C., de Haan C.A.M., Rottier P.J.M., Petrovsky N., Frijlink H.W., Huckriede A., et al. Passive inhalation of dry powder influenza vaccine formulations completely protects chickens against H5N1 lethal viral challenge. Eur. J. Pharm. Biopharm. 2018;133:85–95. doi: 10.1016/j.ejpb.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Higginson D., Theodoratou E., Nair H., Huda T., Zgaga L., Jadhav S.S., Omer S.B., et al. An evaluation of respiratory administration of measles vaccine for prevention of acute lower respiratory infections in children. BMC Public Health. 2011;11(3):S31. doi: 10.1186/1471-2458-11-S3-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.LiCalsi C., Christensen T., Bennett J.V., Phillips E., Witham C. Dry powder inhalation as a potential delivery method for vaccines. Vaccine. 1999;17(13):1796–1803. doi: 10.1016/s0264-410x(98)00438-1. [DOI] [PubMed] [Google Scholar]
- 173.Abdellatif Ahmed A.H., Tawfeek Hesham M., Abdelfattah Ahmed, El-Saber Batiha Gaber, Hetta Helal F. Recent updates in COVID-19 with emphasis on inhalation therapeutics: nanostructured and targeting systems. J. Drug. Deliv Sci. Technol. 2021;63:102435. doi: 10.1016/j.jddst.2021.102435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Herodotus (Translated by Godley A.D. The Persian Wars, Volume I. Loeb Classical Library, 1920.
- 175.A Brief History of Water and Health from Ancient Civilizations to Modern Times. IWA Publishing. https://www.iwapublishing.com/news/brief-history-water-and-health-ancient-civilizations-modern-times.
- 176.Rosa G., Clasen T. Estimating the scope of household water treatment in low- and medium-income countries. Am. Soc. Trop. Med. Hygiene. 2010;82(2):289–300. doi: 10.4269/ajtmh.2010.09-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]