SUMMARY:
Associative learning allows animals to adapt their behavior in response to environmental cues. For example, sensory cues associated with food availability can trigger overconsumption even in sated animals. However, the neural mechanisms mediating cue driven non-homeostatic feeding are poorly understood. To study this, we recently developed a behavioral task in which contextual cues increase feeding even in sated mice (Stern et al., 2020). Here we show that an insular cortex to central amygdala circuit is necessary for conditioned overconsumption, but not for homeostatic feeding. This projection is marked by a population of glutamatergic nitric oxide synthase-1 (Nos1) expressing neurons, which are specifically active during feeding bouts. Finally, we show that activation of insular cortex Nos1 neurons suppresses satiety signals in the central amygdala. The data thus indicate that the insular cortex provides top-down control of homeostatic circuits to promote overconsumption in response to learned cues.
Keywords: Insular cortex, amygdala, feeding, Nitric Oxide Synthase-1
Graphical Abstract
eTOC Blurb
Feeding is a complex motivated behavior and environmental cues can potentiate Pavlovian feeding responses. Here, Stern et al. explore the neural mechanisms underlying this effect and find a population of nitric-oxide synthase-1 neurons in the insular cortex that project to the central amygdala and promote overconsumption.
INTRODUCTION
Obesity, which results from increased food intake and a state of positive energy balance, is a major public health problem in developed countries and a growing problem in the developing world (Hammond and Levine, 2010; Hurt et al., 2010). Feeding is a complex motivated behavior that is coordinated by homeostatic (e.g. alteration of metabolic and hormonal signals) and non-homeostatic environmental (e.g. hedonic, emotional and cognitive cues) factors (Berthoud, 2007; Berthoud et al., 2017; Lutter and Nestler, 2009; Rossi and Stuber, 2018; Saper et al., 2002). Environmental cues can be robust potentiators of feeding in both rodents and humans (Birch et al., 1989; Cornell et al., 1989; Petrovich, 2011; Weingarten, 1983), and learned associations between specific cues and food intake have even been invoked to explain the disparity in weight gain between different countries, cultures and socioeconomic classes (Belfort-DeAguiar and Seo, 2018). Thus, in patients, “context dependence,” defined as the over-reliance on environmental cues for decision-making, can contribute to increased consumption (Reynolds and Payne, 2017). Accordingly, increased television viewing is consistently associated with increased obesity in both children and adults, and obese individuals have altered responses to food-associated cues such as television advertising (Martin-Soelch et al., 2007). Despite this, little is known about the neurobiological mechanisms underlying non-homeostatic feeding, and in particular, how learned environmental cues can positively regulate food consumption.
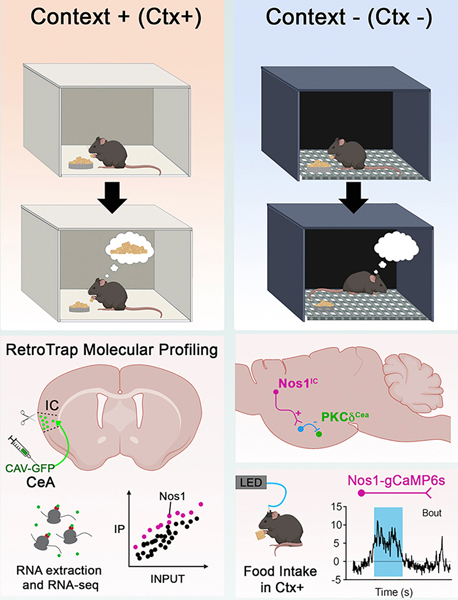
We recently began studies of the neural substrate for conditioned overconsumption using a behavioral paradigm in mice that employs appetitive Pavlovian conditioning to increase food intake specifically in response to contextual cues (Stern et al., 2020) (Figure 1A). In this paradigm, fasted mice are placed in a novel context (Ctx+) where food is available and allowed to eat for 30 minutes. We found that returning these same mice to this same environment at a later time led to increased food consumption even when they were sated. The trained mice did not show increased food intake when they were placed into a different environment (Ctx-) that had not been associated with food delivery. These experiments showed that the mice had been conditioned to associate sensory cues with a prior experience of hunger and food availability, leading them to later consume significant amounts of food when exposed to those cues, despite being satiated. This task is similar to that which was originally proposed by Weingarten in 1983 (Weingarten, 1983) and subsequently modified by Petrovich and colleagues (Holland and Petrovich, 2005), in which discrete cues (e.g. tone, light) were used to elicit conditioned overconsumption. In our related studies, we used contextual cues to shorten the total time required for animals to learn the task, enabling mechanistic studies of the underlying neural circuit that would be onerous using the original task. Consistent with previous reports implicating the insular cortex in encoding food-cue associations (Kusumoto-yoshida et al., 2014; Livneh et al., 2017; Pérez et al., 2011), pan-inhibition of the entire insular cortex blunted this conditioned overconsumption (Stern et al. 2020). However, the specific neurons in the insular cortex mediating this learned overconsumption had not been elucidated and the downstream circuit elements were not identified. We thus set out to map the expanded neural circuit responsible for this behavior.
Figure 1. The insular cortex is required for conditioned overconsumption, but not homeostatic feeding.

(A) Behavioral schematic of the conditioned overconsumption task, chemogenetic inhibition strategy and representative injection area.
(B-C) Food intake, measured in milligrams (mg) and plotted as cumulative intake over 30 minutes during training (B) or 20 minutes during testing in the conditioned overconsumption task (C). Mice were injected with either CNO (mCherry: dark blue bars, n=4; hM4Di: purple bars, n=10) or saline (hM4Di: light blue bars, n=5) before the testing session (two-way RM ANOVA, F(1,16)=14.04, **p=0.0018 followed by Holm’s-Sidak multiple comparisons).
(D) Food intake, measured in grams (g) and plotted as cumulative intake over a 24 hour period (Overnight feeding, n=4–8).
(E) Food intake, measured in g, and plotted as the cumulative intake in 1 hour following an overnight fast (Post-fast feeding, n=12).
(F) Time, measured in seconds (s) spent in the open arms of an elevated plus maze (EPM) (n=10–11).
(G) Velocity, measured in centimeters transversed per s (cm/s) (n=7).
See also Figure S1.
RESULTS
Insular cortex projection neurons are required for conditioned overconsumption, but not homeostatic feeding
We began by testing whether insular cortex projection neurons (rather than interneurons) mediate the effects on learned overconsumption (Figure 1A), as a prelude to establishing the identity of the key neural population and mapping the relevant target region. Projection neurons in the cortex are primarily glutamatergic and express CAMKIIa (Liu and Murray, 2012) and we therefore injected a CAMKIIa-hM4Di inhibitory DREADD (Designer Receptors Exclusively Activated by Designer Drugs) AAV into the insular cortex (Figure 1A). We specifically targeted the posterior part of the anterior insular cortex on a rostral-caudal axis (Figure S1) based on previous studies examining the role of the IC in food-cue associations (Kusumoto-yoshida et al., 2014; Livneh et al., 2017). In order to assess the effects of insular cortex inhibition on conditioned overconsumption, we trained mice in the task and administered saline or CNO 1 hour before the overconsumption test (Figure 1A) (Stern et al, 2020). During the training sessions, there was no difference in consumption among any of the groups immediately after a fast (Figure 1B). Consistent with our prior results, control mice (which received an AAV expressing mCherry but were given CNO) consumed significantly greater amounts of food when they were returned to the cage they previously associated with food delivery (Ctx+) compared to their intake in a cage which was not previously associated with food (Ctx-). In contrast, we found that inhibition of the insular cortex projection neurons prior to the overconsumption test blocked the increase in food intake seen in the controls (Figure 1C, right panel, dark blue bars, light blue bars *p<0.05; purple bars, n.s..). This reduction in food intake was limited to the overconsumption task, as inhibition of insular cortex projection neurons had no effect on the 24 hour food intake of mice kept in their home cage (Figure 1D; Overnight feeding, n.s.), nor did it affect food intake after refeeding (Figure 1E; Post-fast Feeding, n.s.), showing that these neurons do not regulate homeostatic feeding. In addition, separate controls expressing the AAV with hM4Di and given saline also ate more in Ctx+ during the test for overconsumption. Thus, there was no difference in feeding between the two control groups (hM4Di plus saline, or mCherry plus CNO), indicating that neither viral expression nor CNO alone had a behavioral effect. Inhibition of the projection neurons in the insular cortex also had no effect on anxiety behavior in an elevated plus maze task or general locomotor activity (Figure 1F–G, n.s.). These results indicate that glutamatergic projection neurons in the insular cortex mediate conditioned overconsumption, but do not regulate homeostatic feeding.
Insular Cortex ➜ CeA projection neurons are required for conditioned overconsumption
To identify the projection sites responsible for the effects of insular cortex on overconsumption, we injected an AAV expressing CaMKIIa-mCherry into the insular cortex and visualized the sites of mCherry-labeled nerve terminals. Several projection sites from the insular cortex were identified (Figure S2A–B), including a dense projection to the central amygdala (CeA) (Figure 2A, Figure S2A–B) which has been shown previously (Cai et al., 2014; Douglass et al., 2017; Schiff et al., 2018; Venniro et al., 2017). These results confirmed findings from a recent study showing that the insular cortex projects to all subdivisions of the CeA (Gehrlach et al., 2020), including CeL and CeM, and for this reason we did not differentiate between subregions in subsequent studies. We next confirmed this projection by showing a high level of GFP expression in the insula after injection of a retrograde-AAV expressing GFP into the CeA (Figure 2B). We tested whether this projection could activate neurons in the CeA by injecting a CAMKIIa-hM3Dq (an activating DREADD) into the insular cortex. We found increased Fos expression in the CeA after activating insular cortex projection neurons with CNO injections relative to saline (Figure 2C, ***p<0.001). While we also found that the insular cortex sends weak projections to the BLA (Gehrlach et al, 2020, see Figure S2A–B, Figure 2A), particularly to the ventrolateral portion, BLA Fos expression was not increased above the baseline level of Fos expression in the BLA following activation of the insular cortex projection neurons (Figure S2C).
Figure. 2: Insular Cortex ➜ CeA projection neurons are required for conditioned overconsumption.

(A) Representative images of terminal projection mapping (left) from CaMKII-mCherry expression in the insular cortex injection site (middle) and in axon terminals in the central amygdala (right).
(B) Representative image of retrograde tracing (left) from AAVretro-GFP expression in the central amygdala injection site (right) and labelled cell bodies in the insular cortex (middle).
(C) DREADD-based activity mapping strategy (left). Quantification (middle) of cfos+ cells in the central amygdala in mice injected with saline compared to CNO and representative images (right). (Nested t-test: t=6.98, df =11, ****P<0.0001). Cfos within the CeA is denoted by white arrows.
(D) Food intake, measured in mg, of mice expressing hM4Di in the insular cortex and injected with CNO or saline into the CeA prior to the overconsumption test (n=4–5, two-way ANOVA followed by Holm-Sidak’s multiple comparison test, CNO: F(1,14)=5.2, *P=0.04, CS: F(1,14)=11.0, **P=0.005, Interaction: F(1,14)=4.9, *P=0.04,.)
(E) Food intake, measured in g, and plotted as cumulative intake of mice expressing hM4Di in the insular cortex and injected with CNO or saline into the CeA prior to refeeding for 1 hour (n=11–12).
(F) The percentage of time spent (%) in the stimulated side of an RTPP chamber in mice expressing Arch3.0 in insular cortex ➜ CeA projection neurons (n=6, unpaired t-test, t=2.3, df=10, *P=0.04).
(A-F) Scale bars, 400µm. IC: Insular Cortex; BLA: Basolateral Amygdala; CeA: Central Amygdala. Anatomical borders are denoted by dashed white lines.
See also Figure S2.
We next tested the functional role of inhibiting this insular cortex ➜.CeA projection on conditioned overconsumption by injecting the CAMKIIa-hM4Di AAV into the insular cortex and injecting CNO locally at the nerve terminals in the CeA. As expected, we found that control mice ate significantly more in the Ctx+ than the Ctx- during the conditioned overconsumption task (Figure 2D, blue bars, *p<0.05). In contrast, inhibition of the insular cortex ➜ CeA projection significantly reduced food intake when the trained mice were placed in Ctx+, and the mice now ate a comparable amount to what was consumed in Ctx- (Figure 2D, purple bars, n.s.). Inhibition of the insular cortex ➜ CeA projection had no effect on refeeding after an overnight fast (Figure 2E), anxiety or locomotion (Figure S2D–E). We also performed a real-time place preference (RTPP) test after inhibiting the insular cortex ➜ CeA projection by injecting Cre-mediated retrograde expression of the inhibitory optogenetic opsin, Arch3.0 into the CeA. We found that inhibition of the insular cortex ➜ CeA projection by placing an optical fiber in the insular cortex led mice to avoid the side of the RTPP chamber paired with light (Figure 2F). This suggests that inhibition of the insular cortex ➜ CeA projection may prevent overconsumption by decreasing the rewarding aspects of the cue-food association.
Molecular identification of Insular Cortex ➜ CeA projection neurons
Having established that the insular cortex projection to the CeA is necessary for conditioned overconsumption, we next set out to identify the IC neuronal cell type(s) responsible for this effect using retro-TRAP, a method that enables molecular profiling of neurons based on their sites of projection (Ekstrand et al., 2014). As previously reported, we injected the retrograde viral tracer, CAV-GFP, into the CeA of SYN-NBL10 mice, which express a camelid nanobody to GFP fused to the L10 subunit of the ribosome in neurons. We then dissected the insular cortex and performed an immunoprecipitation (IP) to pull down mRNAs associated with the GFP-bound fraction, which corresponds to markers expressed in insular cortex CeA projection neurons (Figure 3A). The enrichment of each gene was calculated as the number of reads in the precipitated RNA relative to the total (IP/input [INP]) and we confirmed these findings using qPCR and later, in situ hybridization. Gfp, a positive control, was highly enriched in the IP fraction, whereas glial markers Gfap and Mal were depleted (Figure 3A, bottom left). We then generated a dataset of significantly enriched and depleted genes (q-value <0.05) and identified molecular markers that were significantly enriched in the insular cortex ➜ CeA projection relative to the entire population of insular cortex neurons (Figure 3B). We found ~2,500 significantly enriched genes out of ~18,000 that were detectable, and ~2,000 of those had an enrichment of 2-fold or greater (Table S1).
Figure 3: Molecular identification of Insular Cortex ➜ CeA projection neurons.

(A) Retro-TRAP experimental strategy. Syn-NBL10 mice were injected with CAV-GFP into the central amygdala and polysomes bound by GFP in the insular cortex were precipitated out, representing the insular cortex CeA projection neurons. Lower left, the fold change of positive (Gfp, green) and negative (Gfap and Mal, black) controls run by qPCR.
(B) Plot depicting the average IP value and average Input values (log2) of all genes detected during sequencing. Genes significantly enriched with a q-value <0.05 are represented in green. Genes significantly depleted with a q-value <0.05 are represented in yellow. Selected genes of interest are highlighted in magenta.
(C) Representative in situ hybridization images and accompanying average values in FPKM of the Input and IP fractions of genes enriched in the Insular Cortex Layer II/III region (courtesy of Allen Brain Atlas). Individual data points represent individual FPKM values. Significance is noted as differential gene expression q-values (n=3, pooled groups of 4–5 mice each).
(D) Enrichment of additional Nitric Oxide – cGMP pathway genes in the IP fraction compared to the Input. Individual data points represent individual FPKM values. Significance is noted as differential gene expression q-values (n=3, pooled groups of 4–5 mice each).
Many of the genes that were enriched in the immunoprecipitated fraction are expressed predominantly in the layer II/III area of the insular cortex. including neuronal nitric oxide synthase-1 (nNos1, or Nos1), Matn2, Slc16a2, Wfs1, Ptpro and Cdh13 (Lein et al., 2007) (Figure 3C). We also found enrichment of other genes in the Nitric Oxide gCMP pathway (Paul et al., 2017), including Slc7a3, Pde1a, Gucy1a3 and Gucy1b3 (Paul et al., 2017) (Figure 3D, *P<0.05, **P<0.01). Together, these data suggested that Nos1 might be a molecular marker for the insular cortex ➜ CeA projection.
In the cortex, Nos1 has previously been shown to be expressed in GABAergic interneuron populations (Paul et al., 2017). However, other data have shown that there is also a separate population of long-range Nos1 projection neurons (Tricoire and Vitalis, 2012), including excitatory pyramidal cells (Gerashchenko et al., 2008). We found a depletion of markers for inhibitory interneurons including Sst, Pvalb, Vip, Npy, Cort, Calb2, and Cck in the retro-TRAP dataset (Figure S3A), suggesting that the profiling data was unlikely to be contaminated by interneurons. We also found enrichment of additional markers which have also been previously reported to be expressed in Nos1 long-range projection neurons (Paul et al., 2017), such as Rgsp, Syt4, Hhip, Trpc5 and Tacr1 (Figure S3B). Overall, the data thus suggested that Nos1 neurons are expressed in insular cortex projection neurons and represent a potential novel marker for insular cortex ➜ CeA projection neurons. We next confirmed this using a separate set of neural tracing and functional studies.
Glutamatergic Insular Cortex Nos1 neurons project to the CeA and are necessary for conditioned overconsumption
To confirm that a subset of insular cortex Nos1 neurons project to the CeA, we used several neuronal tracers including an AAV-FLEX-GFP tracing virus (Figure 4A) and a transsynaptic anterograde tracing herpes simplex (H129ΔTK-TT) virus (Lo and Anderson, 2011) (Figure 4B). Both were injected into the insular cortex of Nos1-Cre mice and showed marker expression in the CeA, confirming that insular cortex Nos1 neurons project to CeA. To quantitate these data, we injected a color flipping retrograde AAV into the CeA of Nos1-Cre mice (Figure 4C). This AAV expresses GFP in the presence of Cre, and tdTomato in its absence. We then analyzed expression of GFP vs. tdTomato in the insular cortex and found that ~71.6% of the total number of insular cortex neurons that project to the CeA express Nos1 (Figure 4C). Nearly all Nos1 ➜ CeA projection neurons (~90%) mapped using this virus were located in more superficial layers 2–3 of the insular cortex, with sparse expression in deeper layers (Figure 4C).
Figure 4. Glutamatergic Insular Cortex Nos1 neurons project to the CeA and are necessary for conditioned overconsumption.
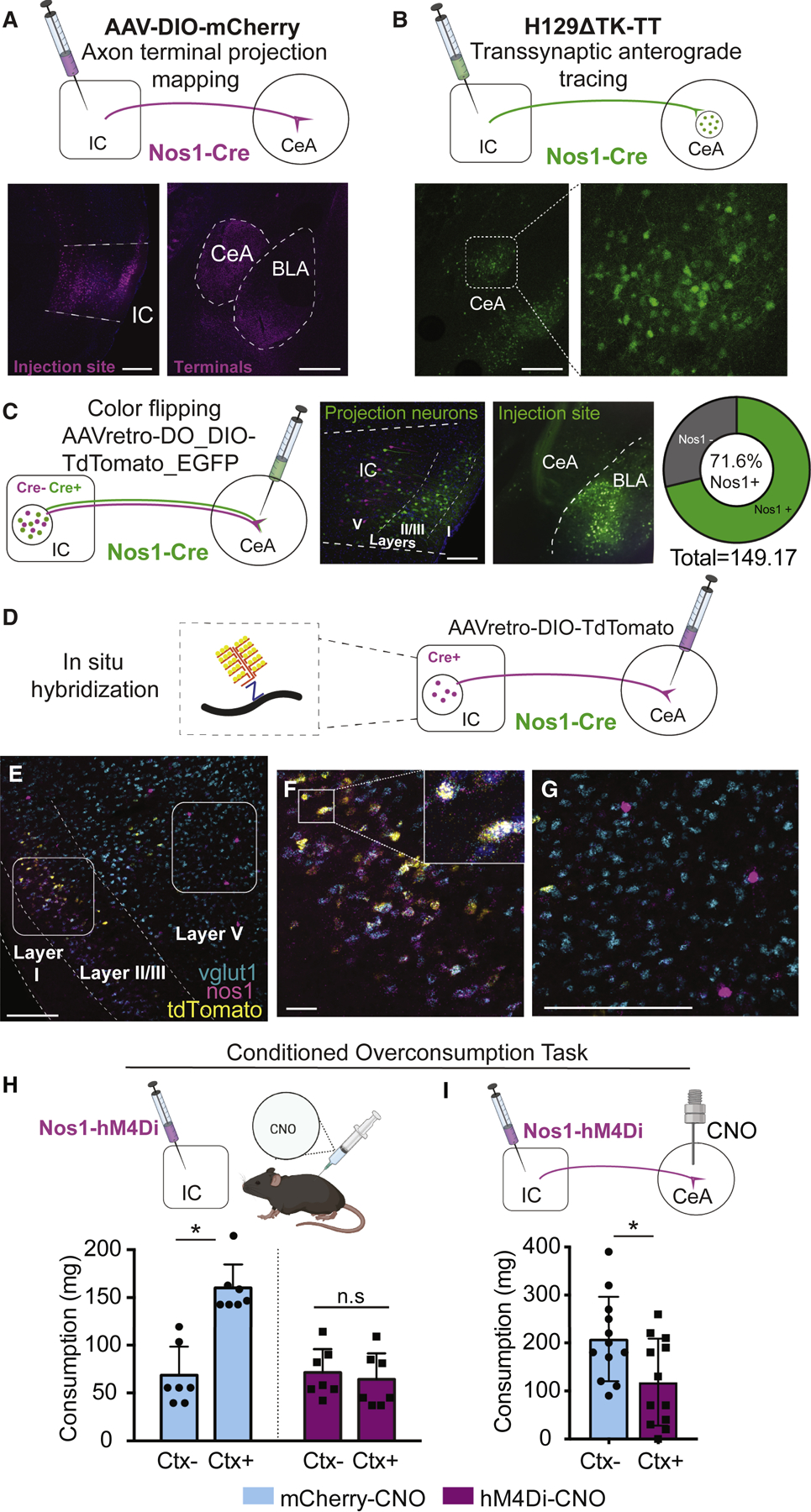
(A) Representative images of terminal projection mapping (top) from DIO-mCherry expression in the insular cortex injection site of Nos1-Cre mice (left) and axon terminals in the CeA (right). Scale bar, 400µm.
(B) Representative images of central amygdala neurons (top) of mice injected with the transsynaptic anterograde tracer H129∆TK-TT in the insular cortex of Nos1-Cre mice (left, inset is magnified to the right). Scale bar, 400µm.
(C) Representative images of Nos1-Cre mice injected with a color-flipping Cre-dependent retrograde AAV in the CeA (left). Nos1+ cells (green) and Nos1- cells (magenta) are labeled in the insular cortex and the CeA injection side is shown with Nos1+ fibers (middle). Scale bar, 150µm. Quantification of the percentage of Nos1+ neurons in the CeA projecting population (right) averaged from n=4 mice.
(D) Viral tracing and in situ hybridization strategy.
(E) Representative in situ hybridization in the insular cortex of vGlut1 (cyan), Nos1 (magenta) and tdTomato (yellow) from Nos1-Cre mice injected with AAVretrograde-FLEX-tdTomato into the CeA. Scale bar, 200µm. Left inset of (E) is magnified in (F), depicting layer II/III neurons expressing all three markers. Scale bar, 40µm The right inset of (E) is magnified in (G) depicting putative Nos1 interneurons neurons that do not express vGlut1. Scale bars, 200µm.
(H) Nos1-Cre mice injected with AAVs encoding hM4Di or control vector (left) in the insular cortex were tested for their overconsumption response in either the Ctx+ or Ctx- (right). Food intake, measured in mg, of Nos1-Cre mice expressing hM4di (purple bars) or mCherry (blue bars) and injected with CNO prior to the overconsumption test. (Interaction: F(1,12)=4.34, P=0.059; CS: F (1, 12) = 3.173, P=0.1002; DREADD: F(1, 12) = 2.546, P=0.1366, with Sidak’s multiple comparisons post-hoc test, *P<0.05, n=7).
(I) Nos1-Cre mice injected with AAVs encoding hM4Di or control vector (left) in the insular cortex and implanted with cannula over the CeA for local CNO infusion. They were tested for their overconsumption response in either the Ctx+. Food intake, measured in mg, of Nos1-Cre mice expressing hM4di (purple bars) or mCherry (blue bars) and injected with CNO prior to the overconsumption test (Unpaired t-test: t=2.47, df =22, *P=0.022, n=11–12).
These findings suggest that Nos1 marks a specific population of neurons that project from the insular cortex ➜ CeA, but does not establish whether they are GABAergic or glutamatergic. To confirm their neurotransmitter identity, we performed in situ hybridization of brains from Nos1-Cre mice after injection with a retrograde Cre-dependent AAV expressing tdTomato into the CeA to label Nos1 insular cortex ➜ CeA projection neurons (Figure 4D–E). We found that the population of insular cortex Nos1 neurons in Layers II/III that project to the CeA, and express tdTomato, exclusively expressed vGlut1 (Figure 4F). In contrast, a separate population of neurons expressing high levels of Nos1, but not tdTomato (indicating that they did not project to amygdala), was observed in layers V/VI (and thus did not project to CeA). These neurons did not express vGlut1 and likely correspond to GABAergic interneurons (Figure 4G). Consistent with this, we found that a smaller population of Nos1 neurons expressing vGAT in deeper layers do not co-express tdTomato and therefore do not project to the CeA (Figure S4). These data therefore indicate that there are two distinct populations of Nos1 neurons in the insular cortex: a specific population of glutamatergic Nos1 neurons in Layers II/III of the insular cortex that project to the central nucleus of the amygdala and the canonical Nos1 GABAergic interneurons.
While Nos1 neurons in hypothalamus have been implicated in feeding behavior (Leshan et al., 2012; Sutton et al., 2014), less is known about the function of Nos1 neurons in cortex, particularly with respect to feeding. Since Nos1 marks this insular cortex ➜ CeA projection, we then tested whether the insular cortex Nos1 neurons are functionally required for the conditioned overconsumption response. We inhibited insular cortex Nos1 neurons by injecting a Cre-dependent AAV expressing hM4Di into the insular cortex of Nos1-Cre mice. In line with our previous results, inhibition of the insular cortex Nos1 neurons prior to assaying conditioned overconsumption completely blocked the overconsumption in fed mice in Ctx+ relative to their intake in Ctx- (Figure 4H). Inhibition of these neurons did not alter homeostatic feeding, locomotion or anxiety (Figure S5A–C). Furthermore, specific inhibition of the insular cortex Nos1 terminals in the CeA by injecting CNO there also prevented conditioned overconsumption (Figure 4I), without an effect on homeostatic feeding (Figure S5D–E). Together, these data show that conditioned overconsumption is mediated by a projection from insular cortex Nos1 neurons to the central amygdala, but that this projection is not necessary for homeostatic feeding.
Insular Cortex Nos1 neurons are activated during consumption bouts
We next set out to monitor the activity of insular cortex Nos1 neurons using fiber photometry (Gunaydin et al., 2014) to record intracellular Ca2+ levels during the overconsumption task. We injected an AAV expressing FLEX-GCaMP6s into the insular cortex of Nos1-Cre mice and recorded from Nos1 neurons during all phases of training and testing. As expected, consumption was significantly increased in the Ctx+ compared to the Ctx- during the overconsumption test (Figure S6D). During training sessions, we found that insular cortex Nos1 neurons are strongly activated during bouts of food consumption (Figure 5A–C) and that their activity was particularly high during the first bout (Figure S6C). We found a similar increase of activity during the overconsumption test in the Ctx+ when fully sated mice continued to overeat (Figure 5B–C, Figure S6D). In contrast, there was no increase in activity when mice investigated but did not consume food either during training or testing sessions (Figure S6A–B). There was also was little change in Nos1 neural activity during habituation or when mice were placed in Ctx- and did not consume food (Figure 5B–D).
Figure. 5: Insular Cortex Nos1 neurons are activated during consumption bouts.

(A) Sample trace of Nos1:GCaMP6s fluorescence of one mouse during the training session of the conditioned overconsumption task. Food consumption bouts are noted by blue bars above the trace. Selected bouts are magnified in the insets below.
(B) Heatmap of peri-event time plots (PETP) of Nos1:GCaMP6s fluorescence around investigation of the empty food cup (Habituation), consumption bouts (Training and Testing, Ctx+) and investigation of food Testing, Ctx-). Individual bouts are depicted in each row. Bouts from individual mice are denoted by lefthand brackets (n=4 mice). Blue arrows and white dashed lines represent the onset of consumption or investigation. Corresponding PETP of Nos1:GCaMP6s fluorescence depicted in Zscore is shown below each heatmap. Dark line and lighter shading indicates mean ± SEM of change in fluorescence for that group of mice. Blue arrows represent the onset of consumption or investigation.
Quantification of the area under the curve (AUC) before and after the commencement of the relevant behavior is inset into the Z-score graph. (n=4, *p<0.05).
See also Figure S6.
Because the aforementioned GABAergic insular cortex Nos1 neurons could have been contributing to the signals detected after GCaMP6s injections, we also monitored activity in the axons of glutamatergic Nos1 projection neurons. We injected an axon-targeted AAV (Broussard et al., 2018) expressing a cre-dependent GCaMP6s into the insular cortex of Nos1-cre mice and inserted the optical fiber into the CeA in order to record the activity of the glutamatergic Nos1 projection neurons that terminate in the CeA. Consistent with the recordings made in the insular cortex, there was significantly increased activity of the Nos1 projection neurons during bouts of consumption (Figure S6E), including during the training and testing phases of the overconsumption task as well as during home cage feeding of normal chow. We conclude that, consistent with their role in inducing overconsumption in sated animals, CeA-projecting, insular cortex Nos1 neurons are activated during bouts of food consumption.
Insular Cortex Nos1 neurons regulate CeA Pkcδ activity to control food intake
Finally, we evaluated the effect of Nos1 projection neurons on a key population of CeA neurons expressing Protein Kinase C, delta (Pkcδ) (Cai et al., 2014) The terminals of the insular cortex Nos1 projection neurons are localized in the lateral portion of the CeA, which contains a heterogeneous population of neurons each expressing one or more of several different markers (Kim et al., 2017). Examination of in situ hybridizations of these markers in the lateral CeA compared to Nos1 axons filled with an AAV expressing mCherry revealed that the Nos1 terminals overlapped with the soma of Pkcδ neurons more consistently than with other neural populations (Figure 6A). Consistent with this, injection of an AAV expressing a Flex-GFP-synaptophysin construct into the insular cortex of Nos1-Cre mice showed dense synaptophysin expression in nerve terminals in the same, lateral region of the CeA as Pkcδ neurons (Figure 6B). Furthermore, an injection of the transsynaptic anterograde tracer, H129ΔTK-TT into the insular cortex of Nos1-Cre mice labeled Pkcδ neurons in the CeA (Figure 6C, white arrows). This virus serially infects neurons across synapses and thus labels both direct and indirect targets of the insular cortex Nos-1 neurons. Pkcδ neurons are a key anorexigenic population in the CeA and are activated by homeostatic satiety signals, including refeeding, cholecystokinin and lithium chloride (Cai et al., 2014). We therefore hypothesized that insular cortex Nos1 projection neurons, which are glutamatergic, might promote conditioned overconsumption by indirectly inhibiting Pkcδ neurons in the CeA.
Figure 6: Insular Cortex Nos1 neurons regulate CeA Pkcδ activity to control food intake.
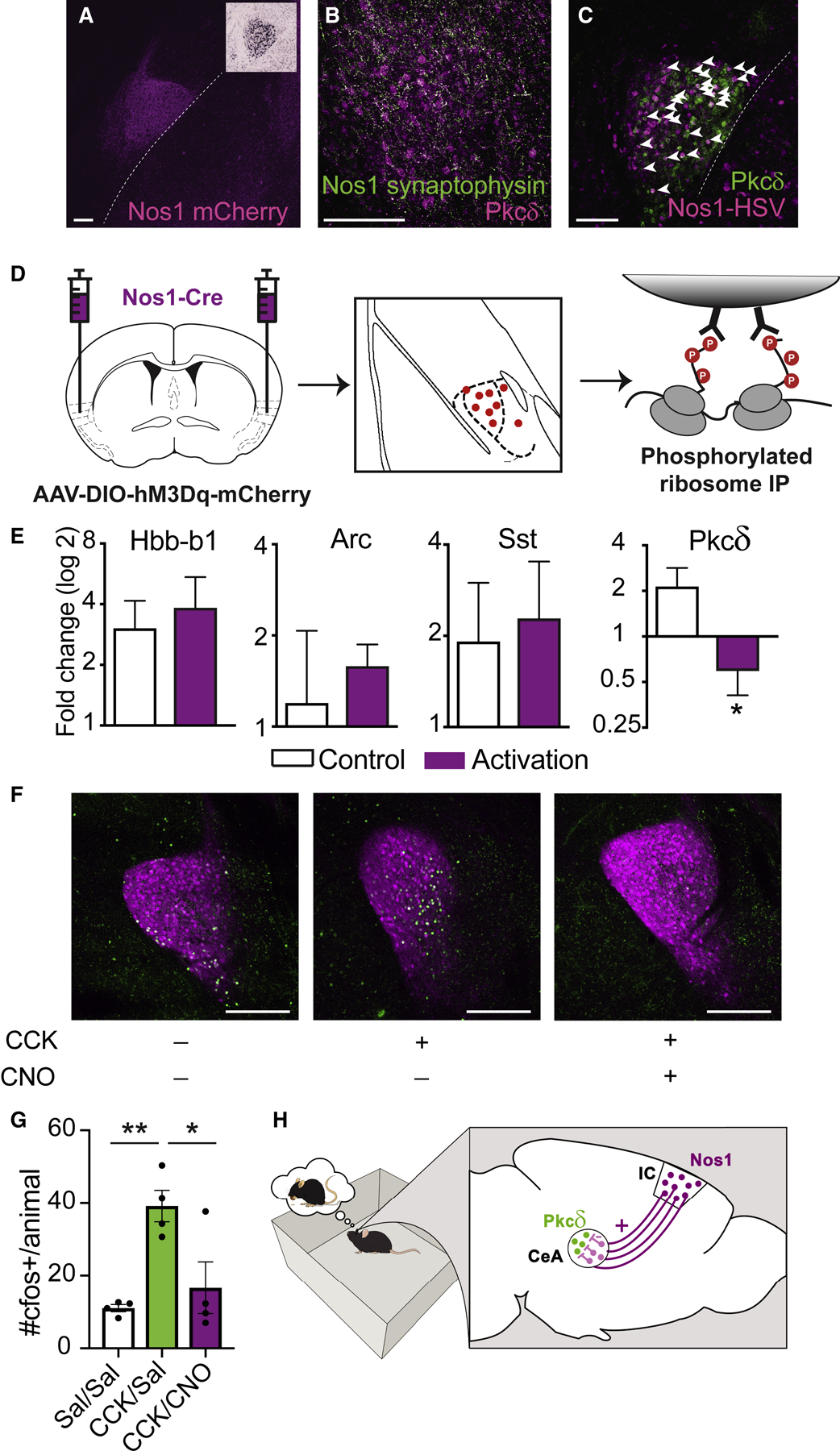
(A) Representative image of Nos1 terminals in the CeA (magenta). Inset depicts Pkcδ in the CeA (Allen Brain Atlas).
(B) Representative image of synaptophysin-labeled terminals (green) from the insular cortex of Nos1-Cre mice, localized in the same location as Pkcδ neurons (magenta) in the CeA.
(C) Representative image of anterograde tracing (magenta) revealing insular cortex Nos1 neurons that are synaptically connected to Pkcδ neurons (green) in the CeA (white arrows). (A-C) Scale bars, 150µm.
(D) Phospho-TRAP experimental scheme. Insular cortex Nos1 neurons were activated with hM3Dq DREADDs and pS6+ neurons in the CeA were immunoprecipitated out one hour later.
(E) Fold-change determined by qPCR of quality control (Arc, Hbb-b1) and CeA, (Sst, Pkcδ) markers. (Paired t-test: t=4.822, df=2, *P=0.04)
(F) Representative images depicting co-expression of Pkcδ (magenta) and cfos (green) in Nos1-Cre mice injected with AAV-DIO-hM3Dq-mCherry into the insular cortex and injected with saline+saline (left), Cck+Saline (middle) or Cck+CNO (right). Scale bars, 250µm.
(G) Quantification of (F) (nested One-Way ANOVA, **p=0.0039 followed by Tukey’s Multiple Comparison Test, **p<0.01, *p,0.05).
(H) Model of learned overconsumption in which mice are placed in the food-associated context, leading to Nos1 inhibition of satiety signals in Pkcδ neurons in the CeA.
See also Figure S7.
To test whether insular cortex Nos1 neurons can modulate the activity of CeA Pkcδ neuron function, we first used phospho-TRAP (phospho-translating ribosome affinity purification) (Knight et al., 2012) to show depletion of Pkcδ in the CeA after activation of the insular cortex Nos1 neurons. While there are no markers for neural inhibition (analogous to cFos for activation), depletion of RNAs after phospho-TRAP is an indicator of neural inhibition (Knight et al., 2014). To that end, we activated insular cortex Nos1 neurons by treating mice infected with an AAV expressing Cre-dependent hM3Dq with CNO, after which we immunoprecipitated polysomes from CeA using a phospho-S6 (pS6) antibody (Figure 6D). Consistent with inhibition of Pkcδ neurons, the gene for Pkcδ, Prkcd, was significantly depleted after Nos1 neural activation (~4-fold compared to saline-injected controls, *p<0.05) (Figure 6E). We did not find depletion of RNAs for a set of other lateral CeA markers including Pnoc, Sst and Nts (Cai et al., 2014; Hardaway et al., 2019; Kim et al., 2017) suggesting that the Prkcd population is the only known population in this region that is inhibited following activation of insular cortex Nos1 neurons (Figure 6E, Fig S7A).
To confirm that Pkcδ neurons are inhibited following the activation of insular cortex Nos1 projection neurons, we injected mice intraperitoneally with the satiety-inducing peptide cholecystokinin (CCK), which leads to cFos expression and activation of Pkcδ neurons (Cai et al., 2014, Kim et al., 2017). We found that activating Nos1 neurons using an hM3Dq activatory DREADD completely blocked the CCK-induced increase in cFos in Pkcδ neurons (Figure 6F–G).
To further address the connectivity between insular cortex Nos1 neurons and neurons in the CeA, we utilized a Cre-conditional GFP monosynaptic rabies tracer (Kohara et al., 2014; Lavin et al., 2020) to map the inputs from the insular cortex onto CeA neurons expressing Somatostatin (Sst) and Pkcδ neurons in Sst-Cre and Pkcd-Cre mice. Specifically, we evaluated whether retrograde tracing of inputs to these neurons would label Nos1 starter neurons in the insular cortex. In both cases we observed GFP labeling of insular cortex Nos1 neurons, showing that there are projections to both Sst and Pkcδ neurons in the CeA (Figure S7B). Because most CeA neurons are GABAergic (Duvarci and Pare, 2014), the aggregate data suggest that insular cortex Nos1 neurons project to a heterogeneous subset of CeA neurons that in turn inhibit the overall Pkcδ neuronal population to promote overconsumption (Figure 6H).
DISCUSSION
Here we show that glutamatergic Nos1 neurons in the insular cortex comprise a top-down neural pathway that mediates conditioned overconsumption via a projection to the central amygdala. In contrasts, this projection is not required for homeostatic feeding, which has been shown previously in other studies testing the effect of general insular cortex inhibition (Baldo et al., 2016; Livneh et al., 2017) as well as for the insular cortex ➜ CeA projection (Gehrlach et al., 2019). This identification of a molecularly defined neural circuit that specifically links environmental cues to increased food intake, even when animals are satiated, provides a basis for understanding how visual and other sensory inputs, potentially analogous to advertising in humans, can lead to increased body weight.
The contextual version of the conditioning task that we used to elicit overconsumption is similar to the cued task employed by Petrovich and others. Despite this, there are important differences between contextual and cued learning, which have been well-established in other behavioral domains. For example, contextual fear conditioning requires an intact hippocampus and amygdala, whereas auditory fear conditioning requires only an intact amygdala (Phillips and LeDoux, 1992). Petrovich and colleagues have established that the BLA is required for the cued version of the overconsumption task (Petrovich et al., 2005). While our data do not exclude a role for the BLA in mediating the contextual task we employed, the finding that specifically inhibiting the insular cortex ➜ CeA projection blocks overconsumption suggests that the BLA is not required. Future work will be necessary to establish the extent to which the mechanisms underlying contextual and cued overconsumption are distinct and whether our results regarding the role of the insular cortex and central amygdala can be extended more generally to other overconsumption tasks.
Our findings that the insular cortex is required for conditioned overconsumption are also consistent with prior reports showing that the insular cortex is required for learning Pavlovian cue-food associations during periods of hunger (Kusumoto-yoshida et al., 2014; Livneh et al., 2017). While these studies, which monitored neural activity, suggested a possible role for the insular cortex in guiding food seeking based on cued information, they did not establish a functional role for the insular cortex in consumption, nor was the relevant cell type identified. Here we show that learned, sensory inputs regulate food intake by activating a specific population of Nos1 neurons in the insular cortex which promote food intake in response to contextual cues, even in sated animals.
Our studies targeted a portion of the insular cortex aligned along the middle of the anterior-posterior axis, which has also been referred to as the posterior portion of the anterior insula or the mid-insula (see Figure S1). The gustatory portion of the insular cortex has a well-established role in taste-responsiveness (Kosar et al., 1986; Schier et al., 2016) and corresponds to the anterior to the ventral mid-insula. The visceral portion of the insular cortex has been less well-studied and extends from dorsal mid-insula to the posterior insula. Although it is well known that the insular cortex is required for conditioned taste aversion (CTA) (Yiannakas and Rosenblum, 2017) and taste neophobia (Roman and Reilly, 2007), Schier et al. (2016), showed that anterior gustatory cortex was not required for CTA in rats, and rather that only the portion that overlaps with visceral cortex is required (e.g. the posterior part of the anterior insula). These data together with ours provide further evidence that the mid-insular cortical region can regulate food intake in response to specific learned cues positively in the case of learned overconsumption and negatively in the case of CTA. It will thus be interesting to establish whether the effect of conditioned taste aversion is also mediated by Nos1 neurons or by a different neural population.
A recent study delineated a role for the posterior insular cortex in aversive behaviors (Gehrlach et al., 2019). Together with our data and others showing the role of the anterior to mid-insular cortex in mediating food-seeking, this implies that the function of the insular cortex differs along the anterior-posterior axis with regards to valence, similar to the transition between sweet and bitter along the same axis (Fletcher et al., 2017; Wang et al., 2018). Indeed, one recent study showed that the middle portion of the insular cortex did not respond in a characteristic way to either sweet or bitter tastants (Wang et al., 2018). Future studies will be required to define the functional role and possible gradients of function across the insular cortex.
We focused on the projection from insular cortex to the central amygdala, in part, because of the density of this projection and also because the central amygdala is a well established regulator of feeding (Cai et al., 2014; Douglass et al., 2017). Previous studies have shown differing roles for different CeA subpopulations, with Pkcδ neurons having an inhibitory effect on food intake (Cai et al., 2014, Kim et al., 2017), while Htr2a and Pnoc neurons have a stimulatory effect on food intake (Douglass et al., 2017, Hardaway et al., 2018). Inhibitory effects on food intake have also been shown via a projection from the parabrachial nucleus (PBN) to the CeA (Palmiter, 2018). The PBN also projects weakly to the insular cortex, but a possible effect of inputs from the PBN to the insular cortex on feeding has not been reported. Furthermore, all of these projections are bidirectional, indicating that there is ongoing feedback regulating feeding behaviors.
Overall, our data indicate that learned responses regulating feeding are controlled by top-down inputs from cortex to subcortical pathways that also play a more general role in the control of homeostatic feeding. These studies thus provide a potential framework for understanding how prior experience can regulate a basic behavior. Specifically, our data suggest that insular cortex Nos1 neurons may mediate overconsumption by suppressing homeostatic satiety signals in the central amygdala. This suggests that top-down signals respond to sensory cues which then modulate subcortical circuits that are largely controlled by interoceptive inputs. However, our finding that inhibition of the insular cortex to CeA projection prevents overconsumption further suggests that these top-down inputs may not be able to override subcortical orexigenic pathways. Top-down inhibition of satiety appears to involve an effect on Pkcδ neurons as directly shown by our finding that insular cortex Nos1 neurons can diminish the neural effects of CCK, a satiety signal, to activate Pkcδ neurons. The microcircuit within the central amygdala is complex but previous evidence has suggested that Pkcδ neurons are an important output mediating satiety (Cai et al., 2014; Kim et al., 2017). There are a number of possibilities for how suppression of Pkcδ neurons by insular cortex Nos1 neurons could occur. While insular cortex Nos1 projection neurons are glutamatergic, the vast majority of central amygdala neurons are GABAergic (Duvarci and Pare, 2014). This suggests that there is likely to be an intermediate neuron in the central amygdala that is activated by Nos1 neurons that in turn inhibits Pkcδ neurons. One possibility is that insular cortex Nos1 neurons project to another previously defined CeA population such as Sst, Htr2a, Nts or Pnoc, and that these in turn inhibit Pkcδ neurons. Our retrograde rabies tracing data showing inputs from insular cortex Nos1 neurons to CeA Sst neurons are consistent with this possibility, though further studies will be required to confirm this. However, we also saw inputs from insular cortex Nos1 neurons to CeA Pkcδ neurons. It is therefore also possible that a subset of Pkcδ neurons could themselves be direct targets, as, for example, one study showed that most CeA Pkcδ neurons are extensively interconnected (Li et al., 2013). Thus, insular cortex Nos1 neurons could project to a small subset of Pkcδ neurons which in turn inhibits the entire Pkcδ population. A third possibility is that multiple populations are targeted by insular cortex Nos1 neurons to directly and indirectly inhibit CeA Pkcδ neurons. Although our rabies tracing study suggests that the third possibility is likely, further studies to distinguish among these possibilities are needed.
Finally, we find that Nos1 neurons are active during consumption bouts both during training, when mice are fasted, during testing, when mice are sated and yet still overeat, and even during home cage feeding of chow. Because Nos1 neurons are not required for homeostatic feeding, these data suggest that Nos1 neurons encode information about the environment during bouts of food consumption and guide future consumption by a learned association linking environmental cues to internal state. However, a limitation of the fiber photometry experiments is that because there is an implanted fiber, the fact that the animals are in a room with different visual and other cues that differentiate it from the normal home cage feeding, additional studies will be needed to determine specifically which sensory inputs are being encoded by Nos1 neurons. Taken together, the functional data and the finding that Nos1 neurons are active during consumption bouts indicates that they are likely key cortical output neurons to the CeA promoting food intake. The data further suggest that the activity of the neurons is regulated by learned sensory cues, consistent with recent studies (Kusumoto-yoshida et al., 2014; Livneh et al., 2017). Further work will be necessary to elucidate the specific circuit changes in the conditioned state that promote overconsumption.
Overall, these studies provide new insight into how the brain links diverse environmental (sensory) stimuli to modulate innate behavioral responses under the aegis of specific environmental cues. Our studies invoke the insular cortex as playing an important functional role in these conditioned responses. Consistent with this, human imaging studies have suggested that the insular cortex activity is altered in patients with obesity (Bruce et al., 2012; Frank et al., 2013; Hogenkamp et al., 2016). These findings provide potential insights into how specific cues in human, such as advertising or eating in front of the television, can lead to increased food intake in specific circumstances. This work is thus highly relevant to understanding how environmental factors can regulate food intake potentially leading to obesity.
Limitations of the Study
In these experiments we employed a paradigm in which sated animals overconsume food in response to contextual sensory cues. The method requires minimal training, taking only 2 trials over the course of the entire 5 day task. The use of this approach was advantageous compared to traditional appetitive conditioning to a single sensory cue because it enabled us to complete mechanistic studies in a timeframe that would not otherwise have been possible. However, the fact that we used a specific novel context instead of discrete sensory cues prevented us from using in vivo imaging to determine whether or not Nos1 neurons encode a specific cue.
It is also important to note that our behavioral studies were conducted in male mice only, as was common practice when the studies began, as the use of female mice can be confounded by hormonal variation during the estrous cycle. We have since aligned laboratory practice with the latest guidelines indicating use of both males and females for behavioral studies. Future studies on this topic will test the results in female mice as well.
Lastly, because we found that Nos1 neurons make up ~70% of the central amygdala projecting population from the insular cortex, we cannot rule out that there is also a subpopulation of Nos-1 neurons that actually lead to overconsumption in response to the novel context or that there is another population of central amygdala-projecting insular cortex neurons involved in this process. Future studies utilizing single-cell sequencing techniques, rather than retro-TRAP, which provides relative abundances over the entire tissue sample, may help to address this question.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by either one of the corresponding authors; The Lead Contact is Jeffrey M. Friedman (friedj@mail.rockefeller.edu).
Materials Availability:
This study did not generate new unique reagents
Data and code availability:
RNA sequencing data has been submitted to GEO (GSE GSE163743). All other data that support the findings of this study are available from the corresponding author upon reasonable request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
C57BL/6J, Nos1-Cre and Sst-Cre mice were obtained from Jackson Laboratories. SYN-NBL10 mice were generated in our laboratory (Ekstrand et al. 2014). Pkcδ-Cre mice were generated at Rockefeller University through the GENSAT project and are available through MMRRC. Mice were housed according to the guidelines of the Rockefeller’s University Animal Facility. Males and females at age 8–20 weeks were used throughout this study and all animal experiments were approved by the Rockefeller University IACUC, according to NIH guidelines. Male mice were used for behavioral experiments, but both male and female mice were used for anatomical studies, including TRAP. Mice were kept on a 12-h/12-h light/dark cycle (lights on at 7:00 a.m.) and had access to food and water ad libitum, except when noted otherwise. Mice were socially housed in groups of 3–5 whenever possible, except in the cases of cannula and optogenetic implants, in which case mice were housed individually. For fiber photometry, mice were housed in groups of 2–3 mice.
METHOD DETAILS
Viral vectors
All AAVs used in this study were purchased from UNC Vector Core or Addgene, except where noted. AAV5-CaMKIIa-hM4Di-mCherry, AAV5-DIO-hM4Di-mCherry, AAV-DIO-Arch3.0-EGFP were used for inhibition studies. AAV5-CaMKIIa-hM3Dq-mCherry or AAV5-DIO-hM3Dq-mCherry were used for activation studies. Control AAV-CaMKIIa-mCherry, AAV-DIO-mCherry or AAV-DIO-EGFP viruses were used for comparison in Cre+ littermate control mice. pAAV-Ef1a-DO_DIO-TdTomato_EGFP-WPRE-pA (color-flipping virus), AAVretro-CAG-GFP and AAVretro-FLEX-tdTomato and AAV-FLEX-SynatophysinGFP were used for tracing studies. The retrograde tracer CAV-GFP was obtained from Montpelier and used for retro-TRAP experiments. H129ΔTK-TT was a gift from David Anderson. AAV-FLEXGCaMP6s and AAV-FLEX-axon-GCaMP6s were used for fiber photometry studies. For tracing with AAV, mice were euthanized 2–4 weeks after injections. For tracing with H129ΔTK-TT, mice were monitored and euthanized within 5 days of injection as previously described (Lo et al. 2011). Rabies tracing was conducted exactly as previously described (Lavin et al 2020). pAAV-syn-FLEX-splitTVA-EGFP-tTA and pAAV-TREtight-mTagBFP2-B19G were obtained from Addgene. G-deleted Rabies virus was obtained from the Salk Viral Vector Core. For behavior, we waited a minimum of 3 weeks following viral injections.
Pharmacology
CNO (Sigma) was dissolved in saline with 0.1% DMSO and injected either intraperitoneally (i.p) at 3 mg/kg or through a cannula at 500uM. Behavioral tests were performed 30–40 minutes after drug injection. Cholecystokinin (CCK) (Tocris) was injected i.p. at 5 μg/kg. Mice were euthanized 1 hour following CCK or CNO injection for cfos analysis.
Stereotaxic Injections.
Mice were anesthetized with isoflurane, placed in a stereotaxic frame (Kopft Instruments) and bilaterally injected in either the insular cortex or the central amygdala using the following coordinates relative to bregma: Insular Cortex (AP: +0.26; ML: 3.85; DV: −3.9); Central Amygdala (AP: −1.4; M/L: 2.8; DV: −4.72) (Paxinos and Franklin, 2019). A total of 200–400 nL of virus at high titer concentrations (at least 1011) were injected per site at a rate of 100 nL/min in Hamilton Neurosyringes. The injection needle was then left in place for 7–10 minutes to account for virus diffusion. For pharmacological experiments, cannula were implanted in the central amygdala at the same coordinates as above (Plastics One). For fiber photometry experiments, optic fiber implants (Doric) were implanted in the insular cortex at the same coordinates as the viral injection. For optogenetics experiments, optic fiber implants (Thor Labs) were implanted over the CeA at the following coordinates relative to bregma: AP: −1.4; ML: 2.8; DV: −4.4 (Paxinos and Franklin, 2019). Implants were inserted slowly and secured to the mouse skull using two layers of Metabond (Parkell Inc) followed by a layer of dental cement. Mice were single housed and monitored in the first weeks following optic implantation. For all surgeries, mice were monitored for 72h to ensure full recovery; two weeks later mice were used in experiments and sacrificed following behavioral studies to confirm viral expression and fiber placement using immunohistochemistry.
Conditioned Overconsumption
Procedures were followed similar to that previously described in Stern et al., 2020. Prior to habituation, mice were given 5–10 chocolate flavored 20mg Precision Pellets during each day of handling to prevent neophobia. Mice were habituated to two different contexts. Contexts were easily distinguishable based on shape, size, floor texture and were in different rooms. Habituation consisted of 20 min exposure to a novel context. Mice were returned to their home cages after habituation, and one hour later, all cages were changed to prevent food dust on the floor from being consumed and food removed for the next 18–24 hours. The next day mice designated at Ctx+ were trained in the habituation context, and mice designated at Ctx- remained in their home cage. Training consisted of 30 min exposure to the context with 2g of Precision Pellets freely available in a food well. Pellets were counted after the training session to determine food consumption. Mice were refed ad lib for 24h and then fasted again for 18–24h before a second training session. One hour after the second training session, all mice were returned to ad libitum feeding for 48h, at which time testing was conducted. Testing consisted of both Ctx+ and Ctx- mice undergoing a 20 min exposure to the habituation context with 2g of Precision Pellets freely available in a food well. Pellets were again counted after testing to determine food consumption. In these experiments, mice were injected with CNO or saline 1 hour prior to the overconsumption test. Ordering during all behavioral procedures was counterbalanced to control for satiety effects.
Overnight Feeding
Mice were injected with CNO or saline 1 hour prior to dark phase onset with pre-measured food available in the food hopper of their home cage. Chow was measured at 1h, 2h, 4h, 12h and 24h post injection.
Post-Fast Feeding
Mice were fasted for 24h starting 3–4h after the light-phase onset in their home cage. Mice were subsequently injected with CNO or saline and then refed. Food was measured for 1h after refeeding.
Elevated Plus Maze
Mice were placed at the center of a cross-shaped, elevated maze in which two arms are closed with dark walls and two arms are open and allowed to explore for 10 min. Mice were injected with CNO (1mg/kg) 1h before testing. After, mice were returned to their home cage and the maze floor was cleaned in between subjects. All subjects were recorded using a camera and behavior (time spent in open and closed arms, distance and velocity) were analyzed using Ethovison 9.0 (Noldus).
Real Time Place Preference (RTPP)
Mice were habituated to optic patch cables for 3–5 days for ~3 minutes each. The amount of time spent in each compartment of the RTPP chamber was recorded using video tracking software (Ethovision 9, Noldus). On the testing day, one side was designated as light-paired in which active entry triggered photostimulation (inhibition: constant light, 5–10 mW), using lasers controlled by a Mini IO box from Noldus and a waveform generator (Keysight). Sessions lasted for 20 min and the amount of time spent in each compartment was recorded.
Retro-TRAP
The Retro-TRAP profiling experiments were performed according to Ekstrand et al., 2014. Mice were injected with CAV-GFP into the CeA and fourteen days later mice were sacrificed by cervical dislocation and the insular cortex was rapidly dissected in ice-cold Buffer B (1xHBSS, 4 mM NaHCO3, 2.5 mM HEPES [pH 7.4], 35 mM Glucose) with 100 mg/ml cycloheximide (Sigma). The dissected pieces were pooled in 3 groups of 5–6 brains each and transferred to a glass homogenizer (Kimble Kontes 20), and homogenized in 1.5 ml ice-cold Buffer C (10 mM HEPES [pH 7.4], 150 mM KCl, 5 mM MgCl2) with 0.5 mM DTT (Sigma), 80 U/ml RNasin Plus (Promega), 40U/ml Superase-In (Life Technologies), 100 mg/ml cycloheximide, protease inhibitor cocktail (Roche) and 100 ng/ml GFP-Trap Protein (ChromoTek). Tissue samples were homogenized three times at 250 rpm and ten times at 750 rpm on a variable-speed homogenizer (Glas-Col) at 4°C. Homogenates were transferred to microcentrifuge tubes and clarified at 2,000xg for 10 min at 4°C. 140 μl each of 10% IGEPAL CA-630 (NP-40; Sigma) and 1,2-diheptanoyl-sn-glycero-3-phospho-choline (DHPC at 100 mg/0.69 ml; Avanti Polar Lipids) was added to the supernatant. The solutions were mixed and centrifuged again at 17,000xg for 15 min at 4°C. The resulting supernatants were transferred to new tubes and 50 μl of each cleared lysate was mixed with 50 μl Lysis Buffer (0.7 μl β-mercaptoethanol/100 μl Lysis Buffer; Agilent Absolutely RNA Nanoprep Kit) and stored at −80° for later preparation as input RNA. The remaining lysates (approximately 1.5 ml) were used for immunoprecipitation. The beads incubating with GFP antibodies were washed twice in Buffer A with 0.5 mM DTT, 80 U/ml RNasin Plus and 100 mg/ml cycloheximide before the cleared brain lysates were added. The immunoprecipitation was allowed to run at 4°C for 40 min. Beads were washed four times with Buffer D (10 mM HEPES [pH 7.4], 350 mM KCl, 5 mM MgCl2, 1% NP40) with 0.5 mM DTT, 80 U/ml RNasin Plus and 100 mg/ml cycloheximide. Before removing the last wash solution the beads were moved to a new tube. After the final wash, RNA was eluted by adding 100 μl Lysis Buffer and purified using the Absolutely RNA Nanoprep Kit (Agilent). For qPCR analysis cDNA was prepared with the QuantiTect Reverse Transcription Kit (QIAGEN). RNA quality was checked using a RNA PicoChip on a bioanalyzer. RIN values > 8 were used.
RNA Sequencing
cDNA was amplified using SMARTer Ultralow Input RNA for Illumina Sequencing Kit and sequenced on an Illumina HiSeq2500 platform. RNA sequencing raw data was uploaded and analyzed using BaseSpace apps (TopHat and Cufflinks; Illumina) using an alignment to annotated mRNAs in the mouse genome (UCSC, Mus musculus assembly mm10). The average immunoprecipitated (IP) and Input value of each enriched and depleted genes with a q-value lower than 0.05 were plotted using GraphPad Prism (GraphPad).
Phospho-TRAP
The Phospho-TRAP profiling experiments were performed according to Knight et al., 2012. Briefly, mice injected with AAV5-DIO-hM3Dq in the insular cortex (coordinates above) were separated into groups of 4–6 mice per group (termed control or activation). Control mice were given a saline injection and Activation mice were given an injection of CNO (3mg/kg). Mice were euthanized 1 hour post-injection, brains were removed, and the central amygdala were dissected on ice and pooled into 3 replicates per group of 4–6 mice each. Tissue was homogenized and clarified by centrifugation. Ribosomes were immunoprecipitated by using 4 ug of polyclonal antibodies against pS6 (Invitrogen) previously conjugated to Protein A-coated magnetic beads (Thermofisher). A small amount of tissue RNA was saved before the immunoprecipitation (Input) and both input and immunoprecipitated RNA (IP) were then purified using RNAeasy Mini kit (QIAGEN). RNA quality was checked using a RNA PicoChip on a bioanalyzer. RIN values > 8 were used. For qPCR analysis cDNA was prepared with the QuantiTect Reverse Transcription Kit (QIAGEN).
qPCR analysis
qPCR using predesigned Taqman probes (idtDNA) were used. The abundance of these genes in IP and Input RNA was quantified using Taqman Gene Expression Master Mix (Applied Biosystems). Transcript abundance was normalized to beta-actin. Fold of Change were calculated using standards or with the ∆∆Ct method if there was not enough material to make standards.
Fiber Photometry
Mice were acclimated to patch cables for 5 min for 3 days before commencing conditioned overconsumption procedures. Mice underwent recording during habituation to the two contexts, training in Ctx+, testing in Ctx+ and testing in Ctx-. Consumption was measured during all of the behavioral phases. Behavioral video files and fiber photometry data were time-locked via a TTL pulse sent upon commencement of behavioral video recording (Noldus). Analysis of the signal was done using fiber photometry system and processor (RZ5P) from TDT (Tucker-Davis Technologies), which includes the Synapse software (Tucker-Davis Technologies). Postrecording analysis was performed using custom-written MATLAB codes. The bulk fluorescent signals from each channel were normalized to compare across animals and experimental sessions. The 405 nm LED emission was used as the isosbestic control. GCaMP6s signals that are recorded at this wavelength are not calcium-dependent; thus, changes in signal can be attributed to autofluorescence, bleaching, and fiber bending. Accordingly, any fluctuations that occurred in the 405 control channels were removed from the 473 channel before analysis. Change in fluorescence (ΔF) was calculated as (473 nm signal − fitted 405 nm signal), adjusted so that a ΔF/F was calculated by dividing each point in ΔF by the 405 nm curve at that time. Z-score and plot traces were calculated for all experiments using MATLAB. A blind experimenter scored videos for consumption bouts and for investigation of the food and/or food cup, which was defined as nose-pokes into the food cup. We then analyzed fluorescence for 10 seconds before and after consumption/investigation, which was used to construct peri-event time plots (PETP). We then calculated the area under the curve (AUC) in MATLAB both before and after consumption/investigation and then calculated the change in the AUC after consumption/investigation commenced. For statistical purposes, we then compared the change in AUC between groups.
Immunohistochemistry, quantifications and imaging.
Mice were perfused and brains were postfixed for 24h in 10% formalin. Brain slices were taken using a vibratome (Leica), blocked for 1h with 0.3% Triton X-100, 3% bovine serum albumin (BSA), and 2% normal goat serum (NGS) and incubated in primary antibodies for 24h at 4ºC. Then, free-floating slices were washed three times for 10 min in 0.1% Triton X-100 in PBS (PBS-T), incubated for 1h at room temperature with secondary antibodies, washed in PBS-T and mounted in Vectamount with DAPI (Southern Biotech). Antibodies used here were: anti-cfos (1:500; Cell Signaling Cat#2250S, RRID:AB_2247211), anti-phospho-S6 (Invitrogen, Cat#44–923G, RRID:AB_2533798), anti-mCherry (1:1000; Abcam, Cat# ab205402), anti-GFP (1:1000, Abcam, Cat#ab13970, RRID:AB_300798), anti-Pkcδ (1:500, Abcam, Cat#ab182126) goat-anti-rabbit (Alexa 488 or Alexa 594, Alexa646 1:1000; Thermo Scientific), goat anti-chicken Alexa488, Alexa594, Alexa 647 (1:1000; Thermo Scientific). Images were taken using an LSM780 confocal (Zeiss) and images were processed using ImageJ software (NIH). Cfos counts were conducted for 2–3 sections / animal with a n=2–4 animals / group.
Fluorescent In Situ Hybridization
For examination of gene expression, tissue samples of mice injected with AAVretro-FLEX-tdTomato underwent single molecule fluorescent in situ hybridization (smFISH). Isoflurane anesthetized mice were decapitated, and brains were harvested and flash frozen on aluminum foil on dry ice. Brains were then immediately stored at −80°C. Prior to sectioning, brains were equilibrated to −16°C in a cryostat for 30 min. Brains were sectioned coronally at 15 μm and thaw-mounted onto Superfrost Plus slides (25×75 mm, Fisherbrand). Slides were air-dried for 60 to 90 min prior to storage at −80°C. smFISH for all genes examined - Nos1 (Cat# 437691), mCherry (Cat# 431202), Vglut1 ( Cat# 416631) and Vgat (Cat# 319191) - was performed using RNAscope Fluorescent Multipex Kit (Advanced Cell Diagnostics) according to the manufacturer’s guidelines. Slides were counterstained for the nuclear marker DAPI using Vectashield mounting medium with DAPI (ThermosFisher). Sections were imaged using an LSM780 confocal (Zeiss) and processed using ImageJ software (NIH).
QUANTIFICATION AND STATISTICAL ANALYSIS
All results are presented as mean ± s.e.m. and were analyzed with Prism software or in Matlab. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications. Normality tests and F tests for equality of variance were performed before choosing the statistical test. Unless otherwise indicated, statistics were based on unpaired-t tests or Two-Way ANOVAs with Sidak’s posthoc comparison. P < 0.05 was considered significant (*P < 0.05, **P < 0.01, ***P < 0.001). Nested t-tests were used when multiple measures were taken per animal, e.g. immunohistochemistry and fiber photometry. Animals in the same litter were randomly assigned to different treatment groups and blinded to experimenters in the various experiments. Injection sites and viral expression were confirmed for all animals. Mice showing incorrect injection sites or optic fiber placement were excluded from the data analysis.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-phospho ribosomal protein 6 pSer244/pSer247 | Invitrogen | Cat#44–923G, RRID:AB_2533798 |
| Chicken anti-GFP | Abcam | Cat#ab13970, RRID:AB_300798 |
| Chicken anti-mCherry | Abcam | Cat# ab205402 |
| Rabbit anti-cfos | Cell Signaling | Cat#2250S, RRID:AB_2247211 |
| Goat anti-rabbit IgG Alexa 488 | Invitrogen | Cat#A11008, RRID:AB_143165 |
| Goat anti-rabbit IgG Alexa 594 | Invitrogen | Cat#A11072, RRID:AB_142057 |
| Goat anti-chicken IgG Alexa 488 | Invitrogen | Cat#A11039, RRID:AB_142924 |
| Goat anti-chicken IgG Alexa 594 | Invitrogen | Cat#A11042, RRID:AB_142083 |
| Rabbit Anti-PKC delta | Abcam | Cat#ab182126 |
| Goat anti-rabbit IgG Alexa 647 | Invitrogen | Cat#A21245, RRID:AB_2535813 |
| Bacterial and Virus Strains | ||
| AAV-FLEX-GFP | Addgene | Cat#28304 |
| pAAV-hSyn-DIO-hM3D(Gq)-mCherry | Addgene | Cat#44361 |
| AAV-hSyn-DIO-mCherry | Addgene | Cat#50459 |
| pAAV-hSyn-DIO-hM4D(Gi)-mCherry | Addgene | Cat#44362 |
| AAV5-CaMKIIa-hM3D(Gq)-mCherry | UNC Vector Core | N/A |
| AAV5-CaMKIIa-hM4D(Gi)-mCherry | UNC Vector Core | N/A |
| AAV5-hSynapsin1-FLEX-axon-GCaMP6s | Addgene | Cat#112010 |
| H129ΔTK-TT | Lo et al., 2011 | N/A |
| retroAAV-CAG-GFP | Addgene | Cat#37825-AAVrg |
| AAV1-phSyn1(S)-FLEX-tdTomato-T2A-SypEGFP-WPRE (Synaptophysin) | Salk | N/A |
| CAV-GFP | Montpelier | N/A |
| pAAV-Ef1a-DO_DIO-TdTomato_EGFP-WPRE-pA (color flipping) | Addgene | Cat#37120-AAVrg |
| AAVrg-CAG-FLEX-tdTomato | Addgene | Cat#28306-AAVrg |
| AAV5-CAMKIIa-mCherry | UNC Vector Core | N/A |
| pAAV.Syn.Flex.GCaMP6s.WPRE.SV40 | Addgene | Cat#100845 |
| pAAV-FLEX-ArchT-tdTomato | Addgene | Cat#28305 |
| AAV-pgk-Cre | Addgene | Cat#24593 |
| pAAV-syn-FLEX-splitTVA-EGFP-tTA | Addgene | Cat#100798 |
| pAAV-TREtight-mTagBFP2-B19G | Addgene | Cat#100799 |
| G-deleted Rabies | Salk Viral Vector Core | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Clozapine-N-Oxide | Tocris Bioscience | Cat#4936; CAS:34233–69-7 |
| CCK | Tocris Bioscience | Cat#1166 |
| Critical Commercial Assays | ||
| Agilent Absolutely RNA Nanoprep Kit | Agilent | Cat#400753 |
| RNAeasy Mini kit | Qiagen | Cat#74104 |
| RNAscope Fluorescent Multiplex Kit | Advanced Cell Diagnostics (ACD) | Cat# 320850 |
| RNAscope® Probe-Nos1 | ACD | Cat#437691 |
| RNAscope® Probe-mCherry | ACD | Cat#431202 |
| RNAscope® Probe-Vglut1 | ACD | Cat#416631 |
| RNAscope® Probe-Vgat | ACD | Cat#319191 |
| QuantiTect Reverse Transcription Kit | Qiagen | Cat#205311 |
| SMARTer Ultralow Input RNA | Illumina | Cat#634936 |
| Taqman Gene Expression Master Mix | Applied Biosystems | Cat#4369016 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J | Jackson Laboratories | Cat#000664 |
| Mouse: B6.129-Nos1tm1(cre)Mgmj/J | Jackson Laboratories | Cat#017526 |
| Mouse: SYN-NBL10 | Ekstrand et al., 2014 | N/A |
| Mouse: B6J.Cg-Ssttm2.1(cre)Zjh/MwarJ | Jackson Laboratories | Cat#028864 |
| Mouse: Tg(Prkcd-glc-1/CFP,-cre)EH124Gsat/Mmucd | GENSAT; MMRRC | 011559-UCD |
| Oligonucleotides | ||
| Primetime Standard qPCR Assay for Egfp | Life Technologies | Mr04329676 |
| Primetime Standard qPCR Assay for Mal | idtDNA | Mm.PT.42.13360338 |
| Primetime Standard qPCR Assay for Gfap | idtDNA | Mm.PT.58.31297710 |
| Primetime Standard qPCR Assay for Hbb-b1 | idtDNA | N008220.1.pt.Hbb-b1 |
| Primetime Standard qPCR Assay for Pkcd | idtDNA | Mm.PT.58.10644503 |
| Primetime Standard qPCR Assay for Sst | idtDNA | N009215.1.pt.Sst |
| Primetime Standard qPCR Assay for Nts | idtDNA | Mm.Pt.42.10471851 |
| Primetime Standard qPCR Assay for Arc | idtDNA | Mm.PT.58.5865502.g |
| Primetime Standard qPCR Assay for Pnoc | idtDNA | Mn.PT.47.19064242 |
| Primetime Standard qPCR Assay for Actb | idtDNA | Mm.PT.39a.22214843.g |
| Software and Algorithms | ||
| Tophat | Basespace | https://www.illumina.com/products/by-type/informatics-products/basespace-sequence-hub/apps/tophat-alignment.html |
| Cufflinks | Basespace | https://www.illumina.com/products/by-type/informatics-products/basespace-sequence-hub/apps/cufflinks-assembly-de.html |
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| PRISM 8.0 | Graphpad | https://www.graphpad.com/scientific-software/prism/ |
| MATLAB 2019a | Mathworks | https://www.mathworks.com/products/matlab.html |
| Allen Brain Atlas | Allen Institute | https://portal.brain-map.org/ |
| Ethovision 9.0 | Noldus | https://www.noldus.com/ethovision-xt |
| Synapse | TDT | https://www.tdt.com/component/synapse-software/ |
| Deposited Data | ||
| Retro-TRAP (IC to CeA) | This paper | GEO: GSE163743 |
Highlights.
An insula to central amygdala projection is required for conditioned overconsumption
The insula to central amygdala projection is marked by nitric oxide synthase-1 (Nos1)
Insular cortex Nos1 neurons are specifically active during food consumption bouts
Activation of insula Nos1 neurons suppresses activation of central amygdala Pkcδ
Acknowledgments:
We thank Ravi Tolwani and the staff of the Comparative Biosciences Center, Connie Zhao and the staff at the Genomics Resource Center and Alison North and the staff at the Bioimaging Resource Center at Rockefeller University for technical assistance. We thank David Anderson for providing the H129ΔTK-TT virus. We thank Christin Kosse for providing Matlab codes and for fiber photometry advice and discussions. We thank Inna Piscitello and Kristina Hedbacker for technical support. This work was funded by an F32DK107077, K99DA048749, a NARSAD Young Investigator Award, and a Kavli NSI Pilot Grant (S.A.S), the Klarman Family Foundation and the JPB Foundation (J.M.F).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: The authors declare no competing interests.
Inclusion and Diversity: One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community. One or more of the authors of this paper received support from a program designed to increase minority representation in science.
References
- Baldo BA, Spencer RC, Sadeghian K, and Mena JD (2016). GABA-Mediated Inactivation of Medial Prefrontal and Agranular Insular Cortex in the Rat: Contrasting Effects on Hunger- and Palatability-Driven Feeding. Neuropsychopharmacology 41, 960–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort-DeAguiar R, and Seo D (2018). Food Cues and Obesity: Overpowering Hormones and Energy Balance Regulation. Curr. Obes. Rep 7, 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H-R (2007). Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol. Behav 91, 486–498. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R, Münzberg H, and Morrison CD (2017). Blaming the Brain for Obesity: Integration of Hedonic and Homeostatic Mechanisms. Gastroenterology 152, 1728–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL, McPhee L, Sullivan S, and Johnson S (1989). Conditioned meal initiation in young children. Appetite 13, 105–113. [DOI] [PubMed] [Google Scholar]
- Broussard GJ, Liang Y, Fridman M, Unger EK, Meng G, Xiao X, Ji N, Petreanu L, and Tian L (2018). In vivo measurement of afferent activity with axon-specific calcium imaging. Nat. Neurosci 21, 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce JM, Hancock L, Bruce A, Lepping RJ, Martin L, Lundgren JD, Malley S, Holsen LM, and Savage CR (2012). Changes in brain activation to food pictures after adjustable gastric banding. Surg. Obes. Relat. Dis 8, 602–608. [DOI] [PubMed] [Google Scholar]
- Cai H, Haubensak W, Anthony TE, and Anderson DJ (2014). Central amygdala PKC-δ(+) neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci 17, 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell CE, Rodin J, and Weingarten H (1989). Stimulus-induced eating when satiated. Physiol. Behav 45, 695–704. [DOI] [PubMed] [Google Scholar]
- Douglass AM, Kucukdereli H, Ponserre M, Markovic M, Gründemann J, Strobel C, Alcala Morales PL, Conzelmann K-K, Lüthi A, and Klein R (2017). Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat. Neurosci 20, 1384–1394. [DOI] [PubMed] [Google Scholar]
- Duvarci S, and Pare D (2014). Amygdala microcircuits controlling learned fear. Neuron 82, 966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand MI, Nectow AR, Knight Z. a., Latcha KN, Pomeranz LE, and Friedman JM (2014). Molecular profiling of neurons based on connectivity. Cell 157, 1230–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Ogg MC, Lu L, Ogg RJ, and Boughter JD Jr (2017). Overlapping Representation of Primary Tastes in a Defined Region of the Gustatory Cortex. J. Neurosci 37, 7595–7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Kullmann S, and Veit R (2013). Food related processes in the insular cortex. Front. Hum. Neurosci 7, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrlach DA, Dolensek N, Klein AS, Chowdhury RR, Matthys A, Junghänel M, Gaitanos TN, Podgornik A, Black TD, Vaka NR, et al. (2019). Aversive state processing in the posterior insular cortex. Nat. Neurosci 22, 1424–1437. [DOI] [PubMed] [Google Scholar]
- Gehrlach DA, Weiand C, Gaitanos TN, Cho E, Klein AS, Hennrich AA, Conzelmann K-K, and Gogolla N (2020). A whole-brain connectivity map of mouse insular cortex. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Wisor JP, Burns D, Reh RK, Shiromani PJ, Sakurai T, de la Iglesia HO, and Kilduff TS (2008). Identification of a population of sleep-active cerebral cortex neurons. Proc. Natl. Acad. Sci. U. S. A 105, 10227–10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin L. a., Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky K. a., et al. (2014). Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RA, and Levine R (2010). The economic impact of obesity in the United States. Diabetes Metab. Syndr. Obes 3, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Andrew Hardaway J, Halladay LR, Mazzone CM, Pati D, Bloodgood DW, Kim M, Jensen J, DiBerto JF, Boyt KM, et al. (2019). Central Amygdala Prepronociceptin-Expressing Neurons Mediate Palatable Food Consumption and Reward. Neuron 102, 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenkamp PS, Zhou W, Dahlberg LS, Stark J, Larsen AL, Olivo G, Wiemerslage L, Larsson E-M, Sundbom M, Benedict C, et al. (2016). Higher resting-state activity in rewardrelated brain circuits in obese versus normal-weight females independent of food intake. Int. J. Obes 40, 1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, and Petrovich GD (2005). A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol. Behav 86, 747–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt RT, Kulisek C, Buchanan LA, and McClave SA (2010). The obesity epidemic: challenges, health initiatives, and implications for gastroenterologists. Gastroenterol. Hepatol 6, 780–792. [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, and Tonegawa S (2017). Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron 93, 1464–1479.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight Z. a., Tan K, Birsoy K, Schmidt S, Garrison JL, Wysocki RW, Emiliano A, Ekstrand MI, and Friedman JM (2012). Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell 151, 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight ZA, Schmidt SF, Birsoy K, Tan K, and Friedman JM (2014). A critical role for mTORC1 in erythropoiesis and anemia. Elife 3, e01913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K, Pignatelli M, Rivest AJ, Jung H-Y, Kitamura T, Suh J, Frank D, Kajikawa K, Mise N, Obata Y, et al. (2014). Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat. Neurosci 17, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, and Norgren R (1986). Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res 379, 329–341. [DOI] [PubMed] [Google Scholar]
- Kusumoto-yoshida I, Liu H, Chen BT, Fontanini A, and Bonci A (2014). Central role for the insular cortex in mediating conditioned responses to anticipatory cues [DOI] [PMC free article] [PubMed]
- Lavin TK, Jin L, Lea NE, and Wickersham IR (2020). Monosynaptic tracing success depends critically on helper virus concentrations. Front. Synaptic Neurosci 12, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, and Myers MG Jr (2012). Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat. Med 18, 820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, and Li B (2013). Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci 16, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-B, and Murray KD (2012). Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: location, location, location. Epilepsia 53 Suppl 1, 45–52. [DOI] [PubMed] [Google Scholar]
- Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, Goldey GJ, Diaz VE, Jikomes N, Resch JM, et al. (2017). Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 546, 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo L, and Anderson DJ (2011). A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron 72, 938–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, and Nestler EJ (2009). Homeostatic and hedonic signals interact in the regulation of food intake. J. Nutr 139, 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C, Linthicum J, and Ernst M (2007). Appetitive conditioning: neural bases and implications for psychopathology. Neurosci. Biobehav. Rev 31, 426–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD (2018). The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci 41, 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Crow M, Raudales R, He M, Gillis J, and Huang ZJ (2017). Transcriptional Architecture of Synaptic Communication Delineates GABAergic Neuron Identity. Cell 171, 522–539.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, and Franklin KBJ (2019). Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates (Academic Press; ). [Google Scholar]
- Pérez CA, Stanley SA, Wysocki RW, Havranova J, Ahrens-Nicklas R, Onyimba F, and Friedman JM (2011). Molecular annotation of integrative feeding neural circuits. Cell Metab 13, 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD (2011). Forebrain circuits and control of feeding by learned cues. Neurobiol. Learn. Mem 95, 152–158. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Holland PC, and Gallagher M (2005). Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J. Neurosci 25, 8295–8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, and LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci 106, 274–285. [DOI] [PubMed] [Google Scholar]
- Reynolds AJ, and Payne CR (2017). Understanding the Relationship Between Context Dependence and Susceptibility to Consumption Cues: A Structured Abstract. In Creating Marketing Magic and Innovative Future Marketing Trends, (Springer International Publishing; ), pp. 719–723. [Google Scholar]
- Roman C, and Reilly S (2007). Effects of insular cortex lesions on conditioned taste aversion and latent inhibition in the rat: Insular cortex and latent inhibition. Eur. J. Neurosci 26, 2627–2632. [DOI] [PubMed] [Google Scholar]
- Rossi MA, and Stuber GD (2018). Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab 27, 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, and Elmquist JK (2002). The need to feed: homeostatic and hedonic control of eating. Neuron 36, 199–211. [DOI] [PubMed] [Google Scholar]
- Schier LA, Blonde GD, and Spector AC (2016). Bilateral lesions in a specific subregion of posterior insular cortex impair conditioned taste aversion expression in rats. J. Comp. Neurol 524, 54–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff HC, Bouhuis AL, Yu K, Penzo MA, Li H, He M, and Li B (2018). An Insula-Central Amygdala Circuit for Guiding Tastant-Reinforced Choice Behavior. J. Neurosci 38, 1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern SA, Doerig KR, Azevedo EP, Stoffel E, and Friedman JM (2020). Control of non-homeostatic feeding in sated mice using associative learning of contextual food cues. Mol. Psychiatry 25, 666–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton AK, Pei H, Burnett KH, Myers MG Jr, Rhodes CJ, and Olson DP (2014). Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J. Neurosci 34, 15306–15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire L, and Vitalis T (2012). Neuronal nitric oxide synthase expressing neurons: a journey from birth to neuronal circuits. Front. Neural Circuits 6, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, Cifani C, Marchant NJ, Yizhar O, Bossert JM, et al. (2017). The Anterior Insular Cortex→Central Amygdala Glutamatergic Pathway Is Critical to Relapse after Contingency Management. Neuron 96, 414–427.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gillis-Smith S, Peng Y, Zhang J, Chen X, Salzman CD, Ryba NJP, and Zuker CS (2018). The coding of valence and identity in the mammalian taste system. Nature 558, 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten HP (1983). Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science 220, 431–433. [DOI] [PubMed] [Google Scholar]
- Yiannakas A, and Rosenblum K (2017). The Insula and Taste Learning. Front. Mol. Neurosci 10, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing data has been submitted to GEO (GSE GSE163743). All other data that support the findings of this study are available from the corresponding author upon reasonable request.


