Abstract
The level of protection achieved by the standard two doses of COVID-19 mRNA vaccines in patients receiving maintenance hemodialysis (MHD) remains unclear. To study this we used the French Renal Epidemiology and Information Network (REIN) Registry to compare the incidence and severity of 1474 cases of COVID-19 diagnosed in patients receiving MHD after none, one or two doses of vaccine. Vaccination significantly reduce COVID-19 incidence and severity, but 11% of patients infected after two doses still died. Lack of vaccinal protection in patients naïve for SARS-CoV-2 could be due to defective Tfh response [38% of patients with negative spike-specific CD4+ T-cell interferon gamma release assay] and failure to generate viral neutralizing titers of anti-spike receptor binding domain (RBD) IgGs (63% of patients with titer at or under 997 BAU/ml, defining low/no responders) after two doses of vaccine. To improve protection, a third dose of vaccine was administered to 75 patients [57 low/no responders, 18 high responders after two doses] from the ROMANOV cohort that prospectively enrolled patients receiving MHD vaccinated with BNT162b2 (Pfizer). Tolerance to the third dose was excellent. High responders to two doses did not generate more anti-RBD IgGs after three doses but had more side effects. Importantly, 31 (54%) of low/no responders to two doses reached neutralizing titers of anti-RBD IgGs after three doses. A positive interferon gamma release assay and/or suboptimal titer of anti-RBD IgGs after two doses were the only predictive variables for response to three doses in multivariate analysis. Thus, the standard scheme of vaccination insufficiently protects patients receiving MHD. Anti-RBD IgG and specific CD4+ T-cell response after two doses can guide personalized administration of the third dose, which improves the humoral response of SARS-CoV-2–naïve patients receiving MHD.
Keywords: BNT162b2, COVID-19, hemodialysis, mRNA vaccine, SARS-CoV-2
Graphical abstract
Among the various alarms raised by the coronavirus disease 2019 (COVID-19) pandemic was its impact on the population of patients with end-stage kidney disease,1 , 2 particularly those requiring in-center hemodialysis. The logistical aspects of the technique indeed increase the risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections,3 which on the highly comorbid profile of patients on maintenance hemodialysis (MHD) then translates into a high rate of COVID-19–related death.1 , 4, 5, 6
Aiming at protecting this vulnerable population, French health authorities prioritized patients on MHD for vaccination.7 However, while 2 doses (2Ds) administered i.m. 3 weeks apart of BNT162b2, a lipid nanoparticle-encapsulated mRNA-based vaccine, induced both strong humoral and cellular immune responses against the spike protein of SARS-CoV-2 in the general population,8 our group9 and others10, 11, 12, 13, 14 have recently reported that patients on MHD, particularly those that were naïve for SARS-CoV-2, generated weaker responses than did healthy volunteers after this “standard” scheme of vaccination, raising questions about the actual level of protection provided by the vaccine.
The prospective observational Response of Hemodialyzed Patients to COVID-19 Vaccination (ROMANOV-II) study compared the severity of COVID-19 disease in patients on MHD according to their vaccination status and evaluated whether a third dose (3D) of BNT162b2 vaccine was safe and efficient to increase the generation of immune effectors.
Methods
Epidemiologic study
The Renal Epidemiology and Information Network (REIN) is the French national registry of all patients being treated by renal replacement therapy.15 Clinical, demographic, and laboratory data are collected at the start of renal replacement therapy along with dialysis modalities and are updated annually. Events such as death, transfer, withdrawal from dialysis, placement on a transplant waiting list, and kidney transplantation (from living or deceased donors), as well as COVID-19 diagnosis and severity are systematically reported in real time. Interrogation of the REIN registry was made on June 18, 2021, on the period from the February 1 to May 18, 2021.
To estimate the cumulative incidence of COVID-19 in patients on MHD, data from the REIN registry were cross-referenced with those of the Caisse Nationale d’Assurance Maladie (CNAM),16 which collects each week the cumulative number of patients on MHD that had received their first and second doses of mRNA vaccine.
Because protection of a vaccine dose was previously reported to be efficient from the 10th day following injection onward,17 patients were considered as “not vaccinated” until the 10th day after the first dose and remained in the group “1 dose of vaccine” until the 10th day after the second dose.
Severity of COVID-19 was graded as asymptomatic, mild, moderate, severe, critical, or death following the World Health Organization’s recommendations.18
The ROMANOV-II prospective observational study
In line with the French health authority’s recommendations,19 a third vaccine injection of mRNA BNT162b2 COVID-19 vaccine was proposed to all patients on MHD in the 2 centers of Lyon University Hospital who already received 2Ds of mRNA BNT162b2 and did not have any of the following contraindications: diagnosis of COVID-19 within the last 3 months, organ transplantation within the last 3 months, rituximab injection within the last 3 months, ongoing flare of vasculitis, acute sepsis, or major surgery within the last 2 weeks. Before the third injection, patients were informed of their serological status after 2Ds.
History of COVID-19 was defined as a positive polymerase chain reaction test in nasopharyngeal swab. The screening for infection was performed in patients in the presence of symptoms or because the patient had contact with a positive case. The same detection strategy was applied to patients on MHD and healthy volunteers (HVs).
All adult patients who received a third vaccine injection (within 3 months after the second vaccine injection) with BNT162b2 vaccine and who gave consent for the use of their blood were enrolled in this study. The samples were collected 10 to 14 days after the second and after the third vaccine injection for analysis of the postvaccinal immune response. This timing was selected based on previous reports demonstrating that both cellular and antibody responses are at their peak at this time point.8
Postvaccinal immune responses of patients on MHD were compared after the 2D and the 3D to those of a cohort of HVs, with blood sample collected at the same time point after the 2D of BNT162b2 for patients on MHD.
The ROMANOV-II study was conducted in accordance with the French legislation on biomedical research and the Declaration of Helsinki, and the protocol was evaluated by a national ethical research committee (ID-RCB 2021-A00325-36) and registered on clinicaltrial.gov as NCT04881396. The French national commission for the protection of digital information (Commission National de l’Informatique et des Libertés) authorized the conduction of the study.
Assessment of the tolerability and safety of vaccine injections
Local and systemic adverse events and use of anti-pyretic medications were collected retrospectively, based on a self-assessment questionnaire. Data collected correspond to adverse events within 7 days after the 2D and 3D, respectively.
As previously described,17 pain at the injection site was assessed according to the following scale: mild, does not interfere with activity; moderate, interferes with activity; severe, prevents daily activity; and critical, emergency department visit or hospitalization. Redness and swelling were measured according to the following scale: mild, 2.0 to 5.0 cm in diameter; moderate, >5.0 to 10.0 cm in diameter; severe, >10.0 cm in diameter; and critical, necrosis or exfoliative dermatitis (for redness) and necrosis (for swelling). Fever categories were mild, 38.0 °C to 38.4 °C; moderate >38.4 °C to 38.9 °C; severe, >38.9 °C to 40 °C; and critical, >40 °C. Medication use was not graded. Additional scales were as follows: fatigue, headache, chills, new or worsened muscle pain, new or worsened joint pain (mild: does not interfere with activity; moderate: some interference with activity; or severe: prevents daily activity), vomiting (mild: 1 to 2 times in 24 hours; moderate: >2 times in 24 hours; or severe: requires intravenous hydration), and diarrhea (mild: 2 to 3 loose stools in 24 hours; moderate: 4 to 5 loose stools in 24 hours; or severe: 6 or more loose stools in 24 hours); critical for all events indicated an emergency department visit or hospitalization.
Assessment of anti-SARS-CoV-2 humoral response
In vitro neutralization assay
SARS-CoV-2 (BetaCoV/France/IDF0571/2020 virus [Global Initiative on Sharing Avian Influenza Data Accession ID = EPI_ISL_411218]) was isolated in Vero E6 from a nasal swab of one of the first patients who was found to be COVID-19-positive in France and was kindly provided by Dr. Olivier Terrier and the Virpath lab (Centre International de Recherche en Infectiologie–Lyon). To generate virus stocks, Vero E6 cells were inoculated with virus at a multiplicity of infection of 0.01. Supernatant fluid was harvested at 72 hours postinfection, clarified by low-speed centrifugation, aliquoted, and stored at −80 °C. Virus stock was quantified by classic limiting dilution plaque assay on Vero E6 cells (kindly provided by Dr. F-L. Cosset, Centre International de Recherche en Infectiologie–Lyon).
Two-fold dilutions of serum in 50 μl of Dulbecco’s modified Eagle’s medium, containing 2X penicillin/streptomycin, were incubated with 200 plaque-forming units of SARS-CoV-2 in 50 μl of Dulbecco’s modified Eagle’s medium for 15 minutes at room temperature. Aliquots of 100 μl of Dulbecco’s modified Eagle’s medium + 4% fetal bovine serum containing 2.5 × 104 Vero E6 cells were added to achieve a final dilution of sera from 1:100 to 1:12,800 (4 wells per dilution). Cells were incubated for 5 days at 37 °C, 5% CO2. After 15 minutes of fixation in paraformaldehyde 4% in phosphate buffered saline 1X, cytopathic effect was revealed by crystal violet staining and scored by a researcher (CM) blinded to the study design and sample identity. Neutralization endpoint titers were expressed as the value of the last serum dilution that completely inhibited a virus-induced cytopathic effect.
Anti-RBD IgG response
The IgG antibodies directed against the receptor binding domain (RBD) of the spike glycoprotein of the SARS-CoV-2 were detected by a chemiluminescence technique, using the Maglumi SARS-CoV-2 S-RBD IgG test (Snibe Diagnostic) on a Maglumi 2000 analyzer (Snibe Diagnostic), according to the manufacturer’s instructions. Briefly, 10 μl of serum were incubated in the appropriate buffer with magnetic microbeads covered with spike RBD recombinant antigen to form immune complexes. After precipitation in a magnetic field and washing, N-(4-aminobutyl)-N-ethylisoluminol–stained anti-human IgG antibodies were added to the samples. After a second magnetic separation and washing, the appropriate reagents were added to initiate a chemiluminescence reaction. When necessary, sera were diluted sequentially up to 1:1000.
As recommended by the World Health Organization,20 the titers are expressed as binding arbitrary units/ml (BAU/ml).
Assessment of the anti-SARS-CoV-2 spike cellular immune responses
Enumeration of SARS-CoV-2 spike-specific T CD4+, Tfh, and CD8+ cytotoxic cells
Peripheral blood mononuclear cells were collected and isolated by centrifugation on a Ficoll density gradient. The cells were then frozen in fetal calf serum supplemented with 10% dimethylsulfoxide (Sigma).
CD8+ and CD4+ T cells specific for SARS-CoV-2 spike protein were enumerated using the technique reported by Grifoni et al.21 SARS-CoV-2 spike-specific T follicular helper (Tfh) cells were enumerated according to a technique developed by our team and previously published.22 Briefly, after thawing, cells were concentrated at 107 cells/ml in Roswell Park Memorial Institute complete medium and left to rest overnight at 37 °C and 5% CO2 in a 96-well round-bottom plate, 106 cells/well. The next day, the Roswell Park Memorial Institute medium was changed, and the cells were cultured for 24 hours in the presence of a pool of overlapping peptides covering the entire sequence of the spike protein of SARS-CoV-2 (PepMixTM, JPT Peptides Technologies GmbH). The final concentration of the peptides was 1 μg/ml. Cells cultured with dimethylsulfoxide (Sigma) alone (1/250) were used as negative controls. Cells were then rinsed and incubated at room temperature with a Fixable Viability Dye (eBiosciences) and 1 of the 2 following fluorescent antibodies panels for 30 minutes. Panel 1: CD3 (UHCT1), CD8 (SK1), from BD Biosciences; CD4 (SK3), CD69 (FN50), CD137 (4B4-1), CD134 (OX-86) from BioLegend. Panel 2: CD4 (SK3) from BioLegend, CD3 (UHCT1) CXCR5 (RF8B2), CD25 (2A3), from BD Biosciences. Cells were fixed with 2% methanol-free formaldehyde. Sample acquisitions were made on a BD LSR Fortessa 4L flow cytometer (BD Biosciences). The gating strategies used for these analyses are shown in Supplementary Figure S1A to C.
Interferon-γ release assay
Spike-specific CD4+ T-cell responses were quantified in the circulation of the HVs and patients on MHD using the QuantiFERON SARS-CoV-2 test (Qiagen), a commercially available interferon-γ release assay (IGRA), according to the manufacturer’s instructions.
Briefly, 1 ml blood was distributed in each tube of the assay: (i) uncoated tube: negative control/background noise, (ii) tube coated with mitogen: positive control, (iii) tube coated with human leukocyte antigen II–restricted 13-mers peptides derived from the entire SARS-CoV-2 spike glycoprotein used to stimulate CD4+ T cells. After 20 hours of culture at 37 °C, tubes were centrifugated 15 minutes at 2500g and stored at 4 °C before interferon-γ quantification in the supernatant by enzyme-linked immunosorbent assay.
The CD4+ T-cell assay value was the difference between tube (iii) and the negative control (i).
Statistical analysis
All the analyses were carried out using R software version 4.0.4 (R Foundation for Statistical Computing) and or GraphPad Prism version 8.0 (GraphPad Software). Categorical variables were expressed as percentages and compared with the chi-squared test. Continuous variables were expressed as mean ± SD and compared using one-way analysis of variance and multiple t-tests post hoc analyses or as median and interquartile range (IQR) and compared using Mann-Whitney U test for variables with nonnormal distribution.
Logistic regression models were used in both univariate and multivariate analyses. All the explanatory variables significantly associated with outcomes in univariate analyses (P < 0.10) were included in multivariate models. Stepwise regression analyses with bidirectional elimination were then performed, using Akaike information criterion to select the most fitting final multivariate models.
Results
Patients on MHD are insufficiently protected against COVID-19 after 2 doses of mRNA vaccine
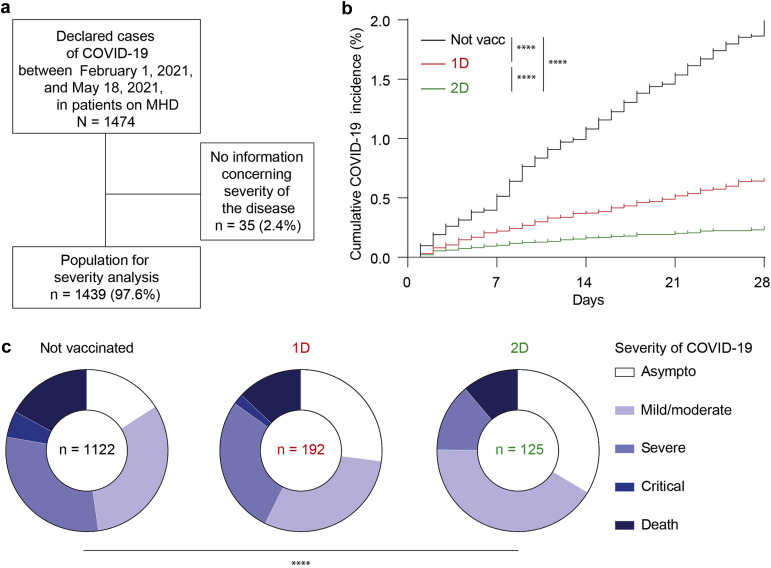
To evaluate the level of protection conferred by COVID-19 mRNA vaccination to patients on MHD who are naïve for the virus the French national registry Renal Epidemiology and Information Network (REIN)23 was interrogated to identify all the cases of COVID-19 diagnosed in patients on MHD from February 1 to May 18, 2021, the period during which MHD population was prioritized for vaccination in France (Figure 1 a). During this period, the virus circulation rate in France was moderate (estimated ∼200 cases/week per 100,000 people) and the large majority of COVID-19 cases were related to either to the original coronavirus strain detected in Wuhan, or the alpha variant (Supplementary Figure S2).24
Figure 1.
Severity of coronavirus disease 2019 (COVID-19) in patients on maintenance hemodialysis (MHD) according to their vaccination status. (a) Flowchart of the epidemiologic study conducted through the Renal Epidemiology and Information Network (REIN) network. (b) Cumulative incidence of the cases of COVID-19 that occurred over the study period in patients on MHD before vaccination or up to 10 days after the first dose (Not vacc, black curve), from 10 days after the first dose to 10 days after the second dose (1D, red curve), or more than 10 days after the second dose of vaccine (2D, green curve). Log-rank test; ∗∗∗∗P < 0.0001. (c) Severity of COVID-19 was color coded and the distribution was compared between the groups of patients on MHD defined according to their vaccination status. Chi-square test; ∗∗∗∗P < 0.0001. Asympto, asymptomatic; Not vacc, not vaccinated.
The cumulative incidence of COVID-19 at 28 days was 1.98% in patients on MHD who are virus-naïve and nonvaccinated. Although vaccination reduced this number to, respectively, 0.65% after the first dose (1D) and to 0.25% after the 2D (log-rank P < 0.0001) (Figure 1b), this level of protection remains largely inferior to what was reported in the general population.17 , 25
Over the study period, a total of 1474 cases of COVID-19 were reported. For the 1439 patients on MHD who were infected (97.6%), for whom the information was available, the severity of disease was analyzed according to whether the diagnosis of COVID-19 was made before vaccination (not vaccinated, n = 1122; Figure 1b, black), 10 days after the 1D (n = 192; Figure 1b, red), or 10 days after the 2D (n = 125; Figure 1b, green) of vaccine. The characteristics of the population are presented in Table 1 . Patients’ characteristics were similar in the 3 groups with exception of age, cardiopathy, and time in MHD, which were all higher in patients who developed COVID-19 after the 2D of vaccine, probably because the patients with the more comorbid profile were vaccinated with the highest priority (Table 1).
Table 1.
Characteristics of COVID-19–infected patients on MHD according to their vaccination status
| Variables | Whole cohort (N = 1439) | Not vaccinated (n = 1122) | 1D (n = 192) | 2D (n = 125) | P |
|---|---|---|---|---|---|
| Sex ratio, M/F | 1.37 (833/606) | 1.32 (639/483) | 1.49 (115/77) | 1.72 (79/46) | 0.338 |
| Age, yr | 69.6 ± 15.0 | 68.5 ± 15.2 | 71.7 ± 14.3 | 74.0 ± 13.5 | <0.0001 |
| BMI, kg/m2 | 27.4 ± 6.30 | 27.5 ± 6.24 | 27.0 ± 6.48 | 27.3 ± 6.52 | 0.676 |
| Comorbidities | |||||
| Diabetes | 732 (51) | 581 (52) | 89 (46) | 62 (50) | 0.364 |
| Cardiopathy | 572 (40) | 420 (37) | 84 (44) | 68 (54) | 0.0006 |
| Vascular disease | 380 (26) | 280 (25) | 58 (30) | 42 (34) | 0.051 |
| Respiratory disease | 269 (19) | 204 (18) | 42 (22) | 23 (18) | 0.477 |
| Malignancy | 148 (10) | 108 (10) | 25 (13) | 15 (12) | 0.289 |
| HD parameters | |||||
| Time in HD, mo | 5.5 ± 6.6 | 5.2 ± 6.2 | 6.4 ± 7.7 | 6.0 ± 7.6 | 0.046 |
| Time HD/wk, h | 11.6 ± 1.63 | 11.6 ± 1.62 | 11.8 ± 1.70 | 11.7 ± 1.60 | 0.196 |
1D, first dose; 2D, second dose; BMI, body mass index; COVID-19, coronavirus disease 2019; HD, hemodialysis; MHD, maintenance hemodialysis.
Values are n (%) or mean ± SD.
The distribution of patients across the 5 stages of severity (asymptomatic, mild, severe, critical, or death26) of COVID-19 as defined by the World Health Organization was statistically different among the 3 groups (Figure 1c). However, despite an increased proportion of less severe forms (asymptomatic or mild or moderate) of COVID-19 in patients who were vaccinated, 11% of patients on MHD that had received 2Ds of mRNA vaccine still died from COVID-19 (Figure 1c). The latter result is drastically different from that reported in the pivotal studies conducted in the general population17 and demonstrates that vaccination with the 2D “standard” scheme is insufficient to protect all patients on MHD.
Standard 2D scheme of vaccination induces flawed humoral immune responses in patients on MHD who are virus-naïve
Among the 150 patients on MHD who were dialyzing at Lyon University Hospital, 38 (25.3%) refused the vaccine or had contraindications. Of the 112 who received 2Ds of BNT162b2 mRNA vaccine, 106 (14 of whom had a previous history COVID-19, black circle) gave consent for analysis of the postvaccinal immune response and were enrolled in ROMANOV study (Figure 2). To understand why patients on MHD who were virus-naïve and were insufficiently protected by COVID-19 mRNA vaccination, the humoral and cellular immune responses of the latter were compared to that of 30 HVs (4 of whom had a previous history COVID-19, black triangle).
Figure 2.
Flowchart of the Response of Hemodialyzed Patients to COVID-19 Vaccination (ROMANOV) prospective study. 2Ds, 2 doses; 3Ds, 3 doses; COVID-19, coronavirus disease 2019; MHD, maintenance hemodialysis.
Enumeration of SARS-CoV-2 spike-specific CD8+ T cells was made after 2Ds by flow cytometry using the activation induced marker technique21 (Figure 3 a). Although the percentage of circulating spike-specific CD8+ T cells was more heterogeneous and slightly reduced in patients on MHD as compared with in HVs (median: 0.15 [IQR: 0.05–0.57] vs. 0.31 [IQR: 0.21–0.45]; P = 0.042; Figure 3b), this mild difference was unlikely to be the sole explanation to the major difference in protection against COVID-19 observed in the 2 vaccinated populations.
Figure 3.
Comparison of the immune responses of patients on maintenance hemodialysis (MHD) and healthy volunteers (HVs) after 2 doses (2Ds) of BNT162b2. Spike-specific cellular and humoral immune responses were evaluated 10 to 14 days after the 2D of vaccine in the circulation of 77 patients on MHD (circles; among which 14 had a previous history of coronavirus disease 2019, black circles) and 30 HVs (triangles; among which 4 had a previous history of coronavirus disease 2019, black triangles). (a,b) Enumeration of spike-specific CD8+ T cells by the activation-induced markers technique. (a) Gating strategy is shown on representative flow cytometry profiles. (b) Histogram showing individual values for HVs and patients on MHD. (c,d) Evaluation of viral neutralization capacity of the serum by in vitro functional assay. (c) Schematic representation of the methodology. (d) Histogram showing individual values for HVs and patients on MHD. (e,f) Enumeration of spike-specific CD4+ T follicular helper (Tfh) cells. (e) Gating strategy is shown on representative flow cytometry profiles. (f) Histogram showing individual values for HVs and patients on MHD, the latter being distributed in 2 groups (Neutral[+] or Neutral[−]) according to the viral neutralization capacity of their serum. Mann-Whitney U test; not significant (NS), P > 0.05; ∗P ≤ 0.05; ∗∗P < 0.01; ∗∗∗∗P < 0.0001. (g) The relation between the titers of anti–receptor binding domain (RBD) IgG measured in antigen-binding assay and the viral neutralization capacities evaluated in the in vitro functional assay shown in c was analyzed by linear regression. The threshold of anti-RBD IgG titer (997 binding arbitrary units [BAU]/ml) above which all sera had viral neutralization capacity is indicated by a vertical dashed line and was used to defined high responders (High-R) versus low or no responders (Low- or no-R) to 2Ds of vaccine. (h) The relation between the result of spike-specific CD4+ T-cell interferon-γ release assay (IGRA) and the percentage of spike-specific CD4+ Tfh cells enumerated as shown in e was analyzed by linear regression. FSC-A, forward scatter area; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
We next went on analyzing the viral-neutralizing capacity of patients’ sera after 2Ds using an in vitro functional assay (Figure 3c). While the serum of patients on MHD with previous history of COVID-19 (Figure 3d, black circles) had similar viral neutralizing capacity as the sera of HVs, this serum characteristic was profoundly depressed in patients on MHD who were vaccinated and naïve for the virus (Figure 3d, open circles).
Viral neutralization capacity of serum depends on the presence of high titers of IgGs directed against the spike protein. The generation of IgGs against a protein antigen requires a particular subset of CD4+ T cells, the Tfh cells, which are specialized in providing the help to B cells and are necessary to B cells’ differentiation into antibody-producing plasma cells.27 , 28 Enumeration of SARS-CoV-2 spike-specific CD4+ T and Tfh cells was performed in the circulation after the 2D using 2 distinct techniques.21 , 22 In contrast to patients on MHD who developed neutralizing IgG titers (neutral+), patients on MHD whose serum lacks viral neutralizing capacity (neutral−) had reduced levels of both spike-specific CD4+ T cells (Supplementary Figure S1D and E) and spike-specific Tfh cells in their circulation (Figure 3f).
Surrogate assays to monitor neutralizing antibodies and spike-specific Tfh cells in routine clinical practice
Viral-neutralizing antibodies, the generation of which depends on spike-specific Tfh cells, seem important to provide protection against COVID-19 after vaccination. Monitoring of these immune effectors after vaccination could therefore be interesting to identify patients on MHD who are insufficiently protected. Unfortunately, neither in vitro neutralizing assay, nor the enumeration of spike-specific Tfh cells in the circulation can be performed in routine clinical practice.
Antigen-binding assays are convenient and widely used in routine clinical care for monitoring of antibody response. Comparing the titer of IgG directed against the RBD of the spike glycoprotein of the SARS-CoV-2 (anti-RBD IgG) measured with chemoluminescence assay and the neutralizing capacity of the serum, we observed a highly significant (P < 0.0001) and strong (r 2 = 0.67) positive correlation (Figure 3g). Hence, we could establish that a titer of anti-RBD IgGs ≥997 BAU/ml (Figure 3g, vertical dashed line) was systematically associated with viral neutralizing capacity of the serum. This threshold was therefore used in the rest of the study to define “high” (anti-RBD IgG ≥ 997 BAU/ml; n = 39 of 106, 36.8%) vs. “low or no” (anti-RBD IgG < 997 BAU/ml; n = 67 of 106, 63.2%) response to vaccine (Figure 2). An indirect validation of this functional threshold is provided by the fact that almost all HVs, who are efficiently protected against COVID-19 by the vaccination, had anti-RBD IgG titers ≥997 BAU/ml after 2Ds of vaccine (Figure 3d and g).
IGRAs are already used in clinical practice to monitor the T-cell response against Mycobacterium tuberculosis.29 The results obtained with a commercially available SARS-CoV-2 CD4+ T-cell IGRA were compared to the enumeration of antigen-specific Tfh cells by flow cytometry (Figure 3h). The highly significant (P < 0.0001) and strong (r 2 = 0.65) positive correlation observed suggests that CD4+ T-cell IGRA can be used as a surrogate assay to flow cytometry for the monitoring of spike-specific Tfh-cell response.
Prospective observational study on the third dose of mRNA vaccine in patients on MHD
In an attempt to improve vaccine protection against COVID-19 in patients on MHD, French health officials authorized the administration of a 3D of vaccine in this population from mid-April 2021 onward.19
A 3D of BNT162b2 mRNA vaccine was therefore offered to all 67 patients on MHD in the ROMANOV study with low or no anti-RBD IgG response and was effectively administered to 57 of them (85.1%) (Figure 2). In absence of clear consensus, the administration of the 3D of vaccine was not limited to patients on MHD with low or no anti-RBD IgG titers, and 18 of 39 patients on MHD with high IgG response (46.2%; P < 0.0001) also accepted a 3D of vaccine (Figure 2). The characteristics of the 75 patients on MHD that received 3Ds of vaccine are presented Table 2 .
Table 2.
Clinical and biological characteristic of patients on MHD who were injected with a 3D of BNT162b2
| Variables | Whole cohort (N = 75) |
|---|---|
| Male | 48 (64) |
| Age, yr | 65.8 ± 14.4 |
| BMI, kg/m2 | 26.8 ± 6.4 |
| Comorbidities | |
| Diabetes | 35 (47) |
| Cardiopathy | 36 (48) |
| Respiratory disease | 6 (8) |
| Hepatic disease | 4 (5) |
| Cause of renal failure | |
| Vascular | 17 (23) |
| Diabetes | 27 (36) |
| Glomerulonephritis | 7 (9) |
| Hereditary | 3 (4) |
| Uropathy | 0 (0) |
| Others | 21 (28) |
| Previous SOT | 16 (21) |
| IS therapy | 8 (11) |
| History of COVID-19 | 3 (4) |
| Time in HD, mo | 56 ± 69 |
| HD parameters | |
| Time HD/wk, h | 10.6± 2.77 |
| Kt/V | 1.56 ± 0.43 |
| Biological characteristics | |
| Hemoglobinemia, g/l | 106 ± 16 |
| C-reactive protein, mg/l | 13.4 ± 20.9 |
| Albuminemia, g/l | 36.4 ± 6.7 |
3D, third dose; BMI, body mass index; COVID-19, coronavirus disease 2019; HD, hemodialysis; IS, immunosuppressive; Kt/V, quantification of dialysis adequacy by the formula: dialysis clearance of urea (K) multiplied by t (dialysis time) divided by the volume of distribution of urea (V); MHD, maintenance hemodialysis; SOT, solid organ transplantation.
Values are n (%) or mean ± SD.
Reactogenicity to the 3D of mRNA vaccine in patients on MHD
Among included patients on MHD, tolerability data were available for 82 of 106 patients after the 2D and 63 of 75 after the 3D. Overall tolerance to the 3D of BNT162b2 mRNA vaccine was good in patients on MHD (Figure 4 a and b). No patients developed critical side effects requiring hospitalization. Forty percent of these patients with a 3D (25 of 63) developed local sides effects, the most frequently reported being pain at the injection site (40%). Forty-six percent of these patients with a 3D (29 of 63) reported systemic side effects, including fatigue (32%), chills (16%), and soreness (16%). In almost all cases (74 of 83, 89%), the intensity of the symptoms was mild or moderate (Figure 4a and b).
Figure 4.
Reactogenicity to the third dose (3D) of mRNA vaccine in patients on maintenance hemodialysis (MHD). (a) Proportion of patients on MHD who developed local and systemic adverse events after the second dose (2D) and the 3D of vaccine are represented. Severity of the adverse event is color-coded (0–4) according to the scale detailed in the Methods section. (b) The number and the severity of local and systemic adverse events that occurred after the 2D and 3D of vaccine are compared. Chi-square test. (c) Proportion of patients on MHD who developed local and systemic adverse events after the 3D of vaccine according to the viral neutralization capacity of their serum after the 2D (high: neutralization+ vs. low or no: neutralization−). (d) The number and the severity of local and systemic adverse events that occurred after 3D of vaccine were compared between patients who were high responders and those who were low or no responders. Chi-square test. ∗∗∗P < 0.0001. NS, not significant.
When local and systemic side effects of vaccine were compared between the 2D and the 3D, no significant difference was found, neither regarding the frequency nor severity of symptoms (Figure 4a and b). However, when the profile of tolerance was compared between patients on MHD according to the intensity of the humoral response after 2Ds of vaccine, a significant trend for more side effects was observed in patients with high response (Figure 4c and d).
Impact of the 3D of mRNA vaccine on humoral response of patients on MHD
When the whole cohort of patients on MHD (n = 75) was considered, a significant increase in the median titer of anti-RBD IgG was observed after the 3D of vaccine (309.8 [IQR: 36.5–996.3 vs. 2212 [IQR: 394.9–3247] BAU/ml after the 2D and 3D, respectively; P < 0.0001; Figure 5 a). However, this global positive result hides major interindividual heterogeneity.
Figure 5.
Evolution of anti–receptor binding domain (RBD) IgG titers and the results of spike-specific CD4+ T-cell interferon-γ release assay (IGRA) between the second dose (2D) and the third dose (3D) of vaccine in patients on maintenance hemodialysis (MHD). (a–c) Anti-RBD IgG titers expressed in binding arbitrary units (BAU/ml) were measured 10 to 14 days after the 2D and 3D of vaccine. Upper dashed line represents the threshold (997 BAU/ml) above which all sera have viral neutralization capacity. This limit was used to define high versus low or no responders to the 2D. Lower dotted line indicates the limit of detection of the assay. (a) Results of the whole cohort of patients on MHD are plotted. (b,c) Evolution of anti-RBD IgG titers between the 2D and 3D of vaccine were compared for high responders (n = 18; b) and low or no responders (n = 57; c) only. Wilcoxon test. (d–f) Result of spike-specific CD4+ T-cell IGRA were measured 10 to 14 days after the 2D and 3D of vaccine. Lower dashed line indicates the limit of positivity of the assay. (d) Results of the whole cohort of patients on MHD are plotted. (e,f) Evolution of the results of spike-specific CD4+ T-cell IGRA between the 2D and 3D of vaccine were compared for high responders (n = 18; e) and low or no responders (n = 57; f) only. Wilcoxon test. The proportion of positive IGRA is indicated in the pie chart. (g) Forest plot of the results of the multivariate analysis conducted to identify the variables independently associated with the generation of anti-RBD IgG titers ≥997 BAU/ml after the 3D. (h) The proportion of patients on MHD that generated anti-RBD IgG titers ≥997 BAU/ml after the 3D is shown according to the presence of anti-RBD IgG and the result of spike-specific CD4+ T-cell IGRA after the 2D. Chi-square test. NS, not significant: P > 0.05; ∗P ≤ 0.05; ∗∗∗∗P < 0.0001. CI, confidence interval.
Patients on MHD with high humoral response after 2Ds of vaccine (n = 18), all maintained high levels of anti-RBD IgG after the 3D but without significant increase of their titer (median: 2757 [IQR: 1869–4365] vs. 3619 [IQR: 2127–11035] BAU/ml after the 2D and 3D, respectively; P = 0.154; Figure 5b). In contrast, patients on MHD with low or no humoral response after 2Ds experienced a significant increase of anti-RBD IgG after the 3D (median: 10.5 [IQR: 1.05–69.9] vs. 353.1 [IQR: 36.2–2592] BAU/ml; P < 0.0001; Figure 5c). However, there was again significant interindividual heterogeneity in the response and only 31 of 57 of low or no responders to 2Ds (54.4%) reached optimal titer (i.e., ≥ 997 BAU/ml; Figure 5c, dashed line) of anti-RBD IgG after a 3D.
Impact of the 3D of mRNA vaccine on cellular response of patients on MHD
The impact of a 3D on antigen-specific Tfh-cell response was indirectly monitored using spike-specific CD4+ T-cell IGRA. Globally, the 3D of vaccine did not result in a significant increase in spike-specific Tfh-cell response in the circulation of patients on MHD neither when the amount of interferon-γ (median: 0.127 [IQR: 0.014–1.040] vs. 0.261 [IQR: 0.025–0.820] IU/ml; P = 0.517) nor the proportion of patients on MHD with positive IGRA (57% vs. 64%; P = 0.50) were considered (Figure 5d). The result remained unchanged when the analysis was made within the subpopulations of patients on MHD with high versus low or no humoral response after 2Ds of vaccine (Figure 5e and f).
Unexpectedly, we noticed that some patients on MHD experienced a reduction in their CD4+ T-cell IGRA result between the 2D and 3D (Figure 5d–f). This proportion was not different between high and low or no responders to 2Ds (8 of 18, 44% vs. 24 of 57, 42%; P > 0.99). Although we do not have definitive explanation for this observation, it could be due to technical limitations of the assay and/or it could indicate interindividual heterogeneity in the durability of the CD4+ T-cell response.
Defining the subpopulation of patients on MHD that should receive a 3D of vaccine
Because it was less well tolerated in these patients (Figure 4c and d) and did not improve their immune response (Figure 5b and f), we concluded that the subpopulation of patients on MHD with already high humoral response after 2Ds should not receive a 3D of vaccine.
To identify among the patients on MHD with low or no response after the standard scheme of vaccination those who would benefit from a 3D of vaccine, the characteristics of the patients on MHD who reached high titer of anti-RBD IgG after the 3D (responders to the 3D: n = 31 of 57, 54.4%) were compared to that of the rest of the cohort (nonresponders to 3D: n = 26 of 57, 45.6%). The former group was less exposed to immunosuppressive drugs and had more often detectable anti-RBD IgG and positive spike-specific CD4+ T-cell IGRA after 2Ds (Table 3 ). In multivariate analysis, the only variables that predicted an immune response to the 3D was the presence of low titers of anti-RBD IgG (odds ratio: 10.1 [95% confidence interval: 1.3–216.5]; P = 0.054) and a positive spike-specific CD4+ T-cell IGRA (odds ratio: 9.25 [95% confidence interval: 2.44–40.7]; P = 0.002) after 2Ds (Figure 5g). Furthermore, combining this information, we observed that the probability to respond to the 3D in patients on MHD that were low or no responders to 2Ds was the highest in patients positive for both tests (82%) (Figure 5h). The response rate decreased to 41% in patients on MHD with only low anti-RBD IgG after the 2D and dropped to 0% in those in whom both tests were negative after the 2D (Figure 5h).
Table 3.
Univariate and multivariate analysis used to identify predictive factors of response to the 3D
| Variables | No response to 3D (n = 26) | Response to 3D (n = 31) | OR univariate (95% CI; P) | OR multivariate (95% CI; P) |
|---|---|---|---|---|
| Male | 21 (81) | 19 (61) | 0.38 (0.10–1.22; 0.115) | |
| Age, yr | 69.2 ± 13 | 66.6 ± 14 | 0.99 (0.95–1.02; 0.455) | |
| BMI, kg/m2 | 25.8 ± 4.7 | 27.7 ± 7.3 | 1.06 (0.97–1.18; 0.256) | |
| Comorbidities | ||||
| Diabetes | 13 (50) | 15 (48) | 0.94 (0.33–2.67; 0.903) | |
| Cardiopathy | 16 (62) | 13 (42) | 0.45 (0.15–1.29; 0.143) | |
| Respiratory disease | 2 (8) | 3 (10) | 1.29 (0.20–10.4; 0.792) | |
| Hepatic disease | 3 (12) | 1 (3) | 0.26 (0.01–2.14; 0.251) | |
| Previous SOT | 6 (23) | 3 (10) | 0.36 (0.07–1.52; 0.179) | |
| IS drug | 6 (23) | 2 (6) | 0.23 (0.03–1.11; 0.090) | 1.18 (0.10–13.2; 0.890) |
| History of COVID-19 | 1 (4) | 2 (6) | 1.72 (0.16–38.4; 0.664) | |
| Time in HD, d | 1135 ± 1022 | 1266 ± 1511 | 1.00 (1.00–1.00; 0.705) | |
| HD parameters | ||||
| Time HD/wk, h | 683 ± 94 | 685 ± 78 | 1.00 (0.99–1.01; 0.926) | |
| Kt/V | 1.5 ± 0.3 | 1.4 ± 0.5 | 0.74 (0.20–2.63; 0.644) | |
| Biological | ||||
| Hemoglobinemia, g/l | 108 ± 10 | 107 ± 16 | 1.00 (0.96–1.04; 0.940) | |
| C-reactive protein, mg/l | 19.0 ± 30.1 | 9.0 ± 8.4 | 0.97 (0.93–1.00; 0.124) | |
| Albuminemia, g/l | 34.6 ± 4.5 | 36.6 ± 5.7 | 1.08 (0.97–1.20; 0.167) | |
| Anti–SARS-CoV-2 response after 2Ds | ||||
| Detectable anti-RBD IgG | 15 (58) | 30 (97) | 22.0 (3.8–421.7; 0.005) | 10.1 (1.3–216.5; 0.054) |
| CD4+ T-cell IGRA (+) | 5 (19) | 24 (77) | 14.4 (4.26–57.6; <0.001) | 9.25 (2.44–40.7; 0.002) |
2Ds, 2 doses; 3D, third dose; BMI, body mass index; CI, confidence interval; COVID-19, coronavirus disease 2019; HD, hemodialysis; IGRA, interferon-γ release assay; IS, immunosuppressive; Kt/V, the quantification of dialysis adequacy by the formula: dialysis clearance of urea (K) multiplied by t (dialysis time) divided by the volume of distribution of urea (V); OR, odd ratio; RBD, receptor binding domain; SOT, solid organ transplantation.
Values are n (%) or mean ± SD.
Discussion
This prospective observational study demonstrates that, in contrast with what reported in the general population,17 the “standard” 2D scheme with BNT162b2 vaccine provides insufficient protection against the severe forms of COVID-19 in patients on MHD who are naïve for the virus.
This problem could be due to the fact that patients on MHD develop a flawed humoral response after 2Ds of vaccine, as illustrated by the very limited viral neutralizing capacity of their serum as compared with that of HVs. This could be the consequence of the deleterious impact of uremic toxins30 , 31 on the generation of spike-specific Tfh cells, a crucial subset to generate high titer of IgGs27 that was detected in reduced number in patients on MHD who fail to respond to the vaccination. These conclusions are in line with recent studies that reported that patients on MHD who are naïve for SARS-CoV-2 develop impaired humoral and cellular immune responses after 2Ds of BNT162b2.9, 10, 11, 12, 13, 14
Based on the observations that (i) patients on MHD with a previous history of COVID-19 had a response to vaccine indistinguishable from that of HVs,9 and (ii) previous studies with protein-based vaccine (such as hepatitis B vaccine) reported acceptable response rates when dosing and/or number of administrations were increased,32 a 3D of BNT162b2 vaccine was offered to patients on MHD in France.19 Although, the safety of the 3D of BNT162b2 vaccine was excellent and comparable to that of the 2D with no critical local or systemic side-effect reported, the tolerance was worst in patients with already high humoral response, who did not improve significantly their immune response against the spike protein of SARS-CoV-2 after this additional injection. Furthermore, while not identified in our cohort, some cases of (re)activation of autoimmune disorders have been reported after mRNA vaccines in the literature.33, 34, 35 This threat further supports avoiding useless additional vaccine injection in patients already protected after 2Ds.
In contrast, after a 3D, 91% of patients on MHD who are virus-naïve with low or no response after 2Ds experienced an increase of anti-RBD-IgG titer, 54% of whom up to an optimal (≥997 BAU/ml) level that was associated with viral neutralization. This latter subgroup could be identified after 2Ds (preemptively) by the fact that they had a positive CD4+ T-cell IGRA (a surrogate for the presence of spike-specific Tfh cells) and/or suboptimal titers (detectable but <997 BAU/ml) of anti-RBD IgGs in antigen-binding assay. Importantly, both assays are commercially available and easy to implement in routine clinical practice, which could pave the way for the personalization of the vaccination strategy in patients on MHD.
Our study has some limitations. Even if the size of the ROMANOV cohort is at least comparable to what is currently reported in the literature,10 , 11 , 14 our observations were made on a limited number of patients from a single university hospital. Furthermore, all these patients were receiving in-center MHD and were therefore characterized by a highly comorbid profile (Table 2). This shall be kept in mind because this bias may limit the generalizability of our conclusions.
Based on the findings presented herein, we propose the following strategy to optimize the protection of patients on MHD who are naïve for SARS-CoV-2. All these vulnerable patients should be offered the standard scheme of vaccination in priority. Anti-RBD IgG and spike-specific CD4+ T cells should be monitored in their circulation 10 to 14 days after the 2D, resulting in the definition of 3 subgroups with distinct needs: (i) patients with high anti-RBD IgG titer (≥997 BAU/ml) do not require further intervention; (ii) patients with low or no anti-RBD IgG titer but positive CD4+ T-cell IGRA, whom are the most likely to respond, should be offered a 3D of vaccine; and (iii) patients with neither detectable anti-RBD IgG nor positive CD4+ T-cell IGRA after 2Ds, whom will not respond to a 3D, might rather receive infusion of monoclonal antibodies as means to induce passive immunization. Future prospective studies are urgently needed to confirm the validity of such personalized anti-COVID-19 vaccination strategy in patients on MHD who are naïve for SARS-CoV-2.
Appendix
List of collaborators from the REIN Registry: Chantrel François, Reydit Mathilde, Tiple Aurélien, Bechade Clémence, Bemrah Abdelkader, Vigneau Cécile, Sautenent Bénédicte, Kazes Isabelle, Courivaud Cécile, Gabriel Jean-Marc, Edet Stéphane, Mercadal Lucile, Moranne Olivier, Toure Fatouma, Laurain Emmanuelle, Ranlin Alex, Longlune Nathalie, Glowacki François, Tivollier Jean-Michel, Brunet Philippe, Lavainne Fréderic, Berard Etienne, Sarraj Ayman, Bauwens Marc, Testevuide Pascale, Vacher Coponat Henri, Galland Roula, Schauder Nicole, Salmi Louis -Rachid, Cerasuolo Damiano, Tendron-Franzin Anaïs, Bayat Sahar, Halimi Jean Michel, Wolak Aurore, Gentile Stéphanie, Devictor Bénédicte, Monnet Elisabeth, Boucaut Maitre Denis, Nacher Mathieu, Merle Véronique, Jais Jean-Philippe, Daures Jean-Pierre, Vergnenegre Alain, Loos-Ayav Carole, Merle Sylvie, Hazzan Marc, Gervolino Shirley, Nguyen Jean-Michel, and Iacobelli Silvia.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors are indebted to the members of the Groupe de Recherche Clinique (GREC: Céline Dagot, Farah Pauwels, Fatiha M’Raiagh, and Daniel Sperandio) and Lise Siard, Claudine Lecuelle, and Philippe Favre from Eurofins Biomnis for their precious help during the conduction of the study.
ME is supported by the Hospices Civils de Lyon (Année Médaille d’Or) and by Institut National de la Santé et de la Recherche Médicale (Poste Accueil). XC is supported by the Société Française de Transplantation. OT is supported by Fondation pour la Recherche Médicale (PME20180639518) and the Etablissement Français du Sang.
ME, XC, TB, EM, and OT are members of the Lyon Immunopathology Federation of the Hospices Civils de Lyon.
Footnotes
Figure S1. Flow cytometry analyses of anti-spike T-cell responses.
Figure S2. Epidemiology of coronavirus disease 2019 pandemic in France during the study period.
Contributor Information
in collaboration with the REIN Registry:
Chantrel François, Reydit Mathilde, Tiple Aurélien, Bechade Clémence, Bemrah Abdelkader, Vigneau Cécile, Sautenent Bénédicte, Kazes Isabelle, Courivaud Cécile, Gabriel Jean-Marc, Edet Stéphane, Mercadal Lucile, Moranne Olivier, Toure Fatouma, Laurain Emmanuelle, Ranlin Alex, Longlune Nathalie, Glowacki François, Tivollier Jean-Michel, Brunet Philippe, Lavainne Fréderic, Berard Etienne, Sarraj Ayman, Bauwens Marc, Testevuide Pascale, Vacher Coponat Henri, Galland Roula, Schauder Nicole, Salmi Louis -Rachid, Cerasuolo Damiano, Tendron-Franzin Anaïs, Bayat Sahar, Halimi Jean Michel, Wolak Aurore, Gentile Stéphanie, Devictor Bénédicte, Monnet Elisabeth, Boucaut Maitre Denis, Nacher Mathieu, Merle Véronique, Jais Jean-Philippe, Daures Jean-Pierre, Vergnenegre Alain, Loos-Ayav Carole, Merle Sylvie, Hazzan Marc, Gervolino Shirley, Nguyen Jean-Michel, and Iacobelli Silvia
Supplementary Material
References
- 1.Thaunat O., Legeai C., Anglicheau D., et al. IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT) Kidney Int. 2020;98:1568–1577. doi: 10.1016/j.kint.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quintaliani G., Reboldi G., Di Napoli A., et al. Exposure to novel coronavirus in patients on renal replacement therapy during the exponential phase of COVID-19 pandemic: survey of the Italian Society of Nephrology. J Nephrol. 2020;33:725–736. doi: 10.1007/s40620-020-00794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng J.H., Hirsch J.S., Wanchoo R., et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98:1530–1539. doi: 10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jager K.J., Kramer A., Chesnaye N.C., et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilbrands L.B., Duivenvoorden R., Vart P., et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haute Autorité de Santé Vaccins Covid-19: quelle stratégie de priorisation à l’initiation de la campagne? https://www.has-sante.fr/jcms/p_3221237/fr/vaccins-covid-19-quelle-strategie-de-priorisation-a-l-initiation-de-la-campagne
- 8.Sahin U., Muik A., Derhovanessian E., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 9.Espi M., Charmetant X., Barba T., et al. The ROMANOV study found impaired humoral and cellular immune responses to SARS-Cov-2 mRNA vaccine in virus unexposed patients receiving maintenance hemodialysis. Kidney Int. 2021;100:928–936. doi: 10.1016/j.kint.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertrand D., Hamzaoui M., Lemée V., et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:2147–2152. doi: 10.1681/ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danthu C., Hantz S., Dahlem A., et al. Humoral response after SARS-Cov-2 mRNA vaccine in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32:2153–2158. doi: 10.1681/ASN.2021040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speer C., Göth D., Benning L., et al. Early humoral responses of hemodialysis patients after COVID-19 vaccination with BNT162b2. Clin J Am Soc Nephrol. 2021;16:1073–1082. doi: 10.2215/CJN.03700321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grupper A., Sharon N., Finn T., et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16:1037–1042. doi: 10.2215/CJN.03500321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rincon-Arevalo H., Choi M., Stefanski A.-L., et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 15.Couchoud C., Stengel B., Landais P., et al. The renal epidemiology and information network (REIN): a new registry for end-stage renal disease in France. Nephrol Dial Transplant. 2006;21:411–418. doi: 10.1093/ndt/gfi198. [DOI] [PubMed] [Google Scholar]
- 16.L’Assurance Maladie Données vaccination par pathologie et département/région. https://datavaccin-covid.ameli.fr/explore/dataset/donnees-vaccination-par-pathologie/explore/information
- 17.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;83:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization COVID-19 Clinical management: living guidance. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 [PubMed]
- 19.DGS-Urgent Precisions sur la vaccination COVID-19: modalities d’administration des rappels et vaccination des personnes immunodeprimes er de leurs proches. https://solidarites-sante.gouv.fr/IMG/pdf/dgs_urgent_52_precisions_sur_la_vaccination_imd.pdf
- 20.Mattiuzzo G., Bentley E.M., Hassall M., et al. Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody. https://www.who.int/publications/m/item/WHO-BS-2020.2403
- 21.Grifoni A., Weiskopf D., Ramirez S.I., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahdal S., Saison C., Valette M., et al. Residual activatability of circulating Tfh17 predicts humoral response to thymodependent antigens in patients on therapeutic immunosuppression. Front Immunol. 2018;9:3178. doi: 10.3389/fimmu.2018.03178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agence de la Biomédecine. R.E.I.N. (Réseau Epidémiologique et Information en Néphrologie) https://www.agence-biomedecine.fr/R-E-I-N-Reseau-Epidemiologique-et-Information-en-Nephrologie
- 24.Santé Publique France Coronavirus: circulation des variants du SARS-CoV-2. https://www.santepubliquefrance.fr/dossiers/coronavirus-covid-19/coronavirus-circulation-des-variants-du-sars-cov-2
- 25.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institutes of Health COVID-19 Treatment Guidelines: clinical spectrum of SARS-CoV-2 Infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 27.Chen C.-C., Koenig A., Saison C., et al. CD4+ T cell help is mandatory for naive and memory donor-specific antibody responses: impact of therapeutic immunosuppression. Front Immunol. 2018;9:275. doi: 10.3389/fimmu.2018.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50:1132–1148. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest. 2007;131:1898–1906. doi: 10.1378/chest.06-2471. [DOI] [PubMed] [Google Scholar]
- 30.Espi M., Koppe L., Fouque D., Thaunat O. Chronic kidney disease-associated immune dysfunctions: impact of protein-bound uremic retention solutes on immune cells. Toxins. 2020;12:300. doi: 10.3390/toxins12050300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betjes M.G.H. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 32.Mast E.E., Weinbaum C.M., Fiore A.E., et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55:1–33. quiz CE1–4. [PubMed] [Google Scholar]
- 33.Masset C., Kervella D., Kandel-Aznar C., et al. Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine. Kidney Int. 2021;100:465–466. doi: 10.1016/j.kint.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Agati V.D., Kudose S., Bomback A.S., et al. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. 2021;100:461–463. doi: 10.1016/j.kint.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanna C., Hernandez L.P.H., Bu L., et al. IgA nephropathy presenting as macroscopic hematuria in 2 pediatric patients after receiving the Pfizer COVID-19 vaccine. Kidney Int. 2021;100:705–706. doi: 10.1016/j.kint.2021.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.