Abstract
The micronemal protein 2 (MIC2) of Toxoplasma gondii shares sequence and structural similarities with a series of adhesive molecules of different apicomplexan parasites. These molecules accumulate, through a yet unknown mechanism, in secretory vesicles (micronemes), which together with tubular and membrane structures form the locomotion and invasion machinery of apicomplexan parasites. Our findings indicated that two conserved motifs placed within the cytoplasmic domain of MIC2 are both necessary and sufficient for targeting proteins to T. gondii micronemes. The first motif is based around the amino acid sequence SYHYY. Database analysis revealed that a similar sequence is present in the cytoplasmic tail of all transmembrane micronemal proteins identified so far in different apicomplexan species. The second signal consists of a stretch of acidic residues, EIEYE. The creation of an artificial tail containing only the two motifs SYHYY and EIEYE in a preserved spacing configuration is sufficient to target the surface protein SAG1 to the micronemes of T. gondii. These findings shed new light on the molecular mechanisms that control the formation of the microneme content and the functional relationship that links these organelles with the endoplasmic reticulum of the parasite.
Apicomplexan parasites cause a number of severe diseases of medical and veterinary importance, including malaria, toxoplasmosis, coccidiosis, and cryptosporidiosis. In these parasites, the invasion of host cells represents a crucial and obligatory step of the life cycle. This process involves a unique specialized subcellular structure, the apical complex that consists of a polar ring, subpellicular tubules, and regulated secretory vesicles known as micronemes and rhoptries. Morphological, biochemical, and functional evidence indicates that the micronemes are implicated in the initial process of parasite attachment to host cells and substrate-dependent motility (2, 9, 11, 13, 34, 36, 38, 43, 44, 46). Attachment to host cells has been shown to induce ultrastructural changes in a substantial fraction of micronemes at the apical complex, indicating that these secretory vesicles discharge their content in the early phase of the invasion process (9, 12). Such regulated exocytosis of micronemes could allow apicomplexan parasites to control the secretion of molecules involved in the invasion of host cells in a time-specific manner in response to signaling events (12). This notion has been supported by the observation that in Toxoplasma gondii, micronemes discharge their content through the extreme apical tip of the parasite in response to elevated intracellular Ca2+ (7, 8).
The micronemes of T. gondii, Eimeria tenella, Cryptosporidium parvum, and Plasmodium species have been shown to store a number of parasite-encoded molecules characterized by the presence of different combinations of adhesive domains. These structures include the thrombospondin (TSP) type I repeat, the Apple motif, the epidermal growth factor domain (D. Soldati and F. M. Tomley, submitted for publication; 6), and the integrin A domain. Proteins encompassing the TSP type I repeat and the A domain of integrins have been found in the micronemes of all apicomplexan parasites analyzed so far. Members of this protein family include Et100 (E. tenella) (39), TSP-related adhesive protein C1 (TRAP-C1; C. parvum) (37), micronemal protein 2 (MIC2; T. gondii) (42), NcMIC2 (Neospora caninum) (22), PfTRAP (Plasmodium falciparum) (27, 29), PySSP2 (P. yoelii) (30), and PbTRAP (P. berghei) (28). Experimental evidence, mainly derived from Plasmodium species, has revealed that the TSP-related molecules play a crucial role in two key processes during host cell invasion by parasites: specific recognition of host cell receptors and gliding motility. P. berghei sporozoites were shown to shed a trail of TRAP during gliding, and antibodies against this micronemal protein blocked parasite locomotion (36). Moreover, TRAP knockout sporozoites were not motile, failed to infect susceptible animals, and did not invade mosquito salivary glands (38). Recently, in vivo mutational analysis revealed that TRAP is implicated in the recognition and invasion of mosquito salivary glands by Plasmodium sporozoites and that this process is functionally distinct from its involvement in gliding motility and invasion of host hepatocytes (43).
All micronemal proteins identified so far in apicomplexan parasites have in common an amino-terminal hydrophobic sequence functioning as a signal peptide. A number of micronemal proteins including members of the TSP family have a highly conserved hydrophobic stretch of amino acids at the carboxyl-terminal end, displaying the features of a transmembrane (TM) domain. This region is followed by a putative acidic cytoplasmic tail of ∼43 to 45 amino acids. Identification of the microneme targeting signals will shed new light on the functional and structural relationship that links these parasite organelles with regulated secretory vesicles of higher eukaryotic cells. Combining molecular genetic identification of targeting signals with whole-genome analysis of the apicomplexan parasites T. gondii and P. falciparum is anticipated to provide a comprehensive view of the micronemal protein repertoire and the rationale for new vaccine design.
To identify the amino acid motifs regulating protein targeting to the micronemes, we have analyzed the subcellular localization in T. gondii tachyzoites of epitope-tagged constructs carrying amino acid substitutions or deletions at conserved residues of MIC2. The MIC2 targeting motifs were used to direct both heterologous and T. gondii surface proteins to the parasite micronemes.
MATERIALS AND METHODS
Host cells and parasite cultures.
Human foreskin fibroblast (HFF) and Vero cells were grown in Dulbecco's modified Eagle medium (Gibco) containing 10% NuSerum (Collaborative Biomedical Products). A single T. gondii line, the clonal isolate EP of the RH strain (31), was used in all manipulations described here. The parasites were propagated in vitro by serial passage on monolayers of HFF or Vero cells (31).
Expression of MIC2 and PbTRAP tagged constructs in T. gondii.
T. gondii tachyzoites were transfected using expression vectors generated from the basic plasmid pBluescript II SK+ (Stratagene) and containing a putative promoter sequence of the MIC2 gene spanning 1,480 nucleotides upstream of its starting codon and a 3′ untranslated region (UTR) of 1,200 nucleotides downstream of the MIC2 stop codon flanking the 5′ and 3′ of the recombinant coding sequences, respectively. Insertion of the epitope tag, introduction of the nucleotide substitutions in the MIC2 and PbTRAP sequences, and construction of the SAG1 chimeric variants were achieved by overlap PCR as described by Horton et al. (17). The following series of transfection vectors containing epitope insertions and amino acid substitutions were generated to investigate protein targeting.
pMIC2/Tag constructs.
pMIC2/Tag1 and pMIC2/Tag2 were designed to express a c-Myc-tagged MIC2 protein. The c-Myc epitope replaced nucleotides 2041 to 2070 (amino acids 680 to 690) and 2233 to 2262 (amino acids 745 to 754) of the MIC2 coding sequence in pMIC2/Tag1 and pMIC2/Tag2, respectively. The construct pMIC2/Tag3 contained a c-Myc/MIC2 version in which the c-Myc epitope replaced the last 13 C-terminal residues (amino acids 757 to 769).
pSAG1/MIC2 constructs.
pSAG1/TM-CTMIC2 was engineered to encode a chimeric protein containing the following elements: (i) the first 289 amino acids of the major surface antigen SAG1; (ii) a region encompassing a short amino acid sequence (305 to 328) of the human membrane cofactor (CD46); and (iii) the TM and cytoplasmic domains of MIC2 containing the c-Myc epitope placed in the same position as in pMIC2/Tag2. The rationale of developing the pSAG1/TM-CTMIC2 construct was based on previous observations showing that SAG1 was correctly folded and targeted to the surface of T. gondii parasites after the natural glycosylphosphatidylinositol (GPI) anchor was replaced by the human CD46 TM region and cytoplasmic tail (33). The CD46 sequence also included 24 amino acids upstream of the TM region that were introduced in the SAG1/TM-CTMIC2 sequence at the junction between SAG1 and the MIC2 TM sequences. The SAG1 coding sequence was amplified from T. gondii genomic DNA. A synthetic DNA sequence coding the 23 amino acids of human CD46 was fused to a PCR fragment amplified from pMIC2/TAG2 encompassing nucleotides 2092 to 2310 of the MIC2 coding sequence by overlap PCR. The forward primer used in this amplification reaction was designed to contain the restriction enzyme PstI at the 5′ end. A second PstI restriction site was already present at the 3′ the end of the amplified SAG1 sequence and used to fuse the CD46/MIC2 fragment with the SAG1 sequence. Briefly, both fragments were digested with PstI, ligated together, and amplified by PCR using a forward primer (SAG1fusPrMIC2) and a reverse primer (cMIC2revPacI) overlapping the first 20 bp of SAG1 coding sequence and the last 20 bp of MIC2 coding sequence, respectively. The PCR chimeric product was linked to the 3′ end of the MIC2 promoter by overlap PCR and cloned in the PacI site upstream to the 3′ UTR of the MIC2 gene. A SalI restriction site was inserted in the sequence coding the 23 CD46 amino acids during construction of the chimeric sequence. pSAG1/TMCD46-CTMIC2 and all of the pSAG1/MIC2mut variants were generated by PCR overlap using the pSAG1/TM-CTMIC2 vector, digested by SalI and PacI restriction enzymes, as cassette.
pPbTRAP.
pPbTRAP contains (i) a 1,480-nucleotide sequence encompassing the promoter of the T. gondii MIC2 gene; (ii) the sequence coding the PbTRAP protein, and (iii) a 1,200-nucleotide UTR flanking the 3′ end of the MIC2 gene. The coding sequence (nucleotides 1 to 1818) of PbTRAP was amplified from P. berghei genomic DNA. The PCR fragment was inserted downstream of the MIC2 promoter by overlap PCR amplification.
pPbTRAP/MIC2.
After digestion of pPbTRAP with restriction enzymes NsiI and PacI, the nucleotide sequence encoding the cytoplasmic tail was replaced by the corresponding region of MIC2 obtained by PCR and in which the NsiI and PacI restriction sites had been introduced during amplification, creating pPbTRAP/MIC2. All PCR amplifications were carried out using Pfu DNA polymerase, which exhibits the lowest error rate of any thermostable DNA polymerase (10). The PCRs reactions were performed in 100 μl for 25 cycles of 96°C (1 min), 55 to 60°C (1 min), and 72°C (1.5 min) in a thermal cycler. All constructs were sequenced using a T7 sequencing Kit (Pharmacia Biotech).
T. gondii transfection experiments.
Freshly harvested tachyzoites (2 × 107) were centrifuged to remove the culture medium, and the resulting pellet was resuspended in cytomix (120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4-KH2PO4 [pH 7.6], 25 mM HEPES [pH 7.6], 2 mM EGTA, 5 mM MgCl2, 2 mM ATP, 5 mM glutathione). Parasites were resuspended in 700 μl of cytomix containing 50 μg of supercoiled plasmid DNA. The entire mixture was then transferred to an electroporation cuvette (4-mm gap) and exposed to an electric pulse with an electroporator (BTX Electro Cell Manipulator 600) in the high-voltage mode (charging voltage and resistance set at 2.0 kV and 48 W, respectively). Electroporated parasites were transferred to fresh HFF monolayers and incubated at 37°C for 24 h.
Antisera and MAbs used in immunofluorescence (IF) assays.
The mouse monoclonal antibody (MAb) 7E4 (S. Naitza et al., unpublished data) was raised against a recombinant polypeptide encompassing amino acids 263 to 429 of PbTRAP. MAb 9E10 directed against the c-Myc epitope (EQKLISEEDL) was purchased from Sigma and used diluted 1:500. MAb T10 1F7 directed against MIC1 was kindly provided by J. F. Dubremetz and used diluted 1:1,000.
Immunoblotting.
T. gondii parasites were lysed in 1× radioimmunoprecipitation assay buffer, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose filters by semidry electroblotting. Nonspecific adsorption of antibodies to the nitrocellulose was prevented by incubating the filters for 1 h at 37°C with 5% nonfat dry milk in 2× TNT (20 mM Tris-HCl [pH 8], 300 mM NaCl, 0.1% Tween 20). The filters were probed with specific antisera or MAbs, washed extensively with 2× TNT, and incubated with alkaline phosphatase (AP)-conjugated goat anti-mouse immunoglobulin G (1:5,000 dilution; Sigma). The filters were developed with nitroblue tetrazolium (0.3 mg/ml) and 5-bromo-4-chloro-3-indolylphosphate (0.15 mg/ml) in 100 mM Tris-HCl (pH 9.5)–100 mM NaCl–5 mM MgCl2 (AP buffer).
IF assay.
IF assays were performed on intracellular parasites, using monolayers of HFF cells grown in 60-mm-diameter petri dishes and infected with tachyzoites. At 24 h after infection with tachyzoites, the monolayers were fixed for 20 min in 3.7% formaldehyde–phosphate-buffered saline (PBS), air dried, and kept at −20°C until use. The cells were permeabilized with 0.5% Triton X-100 for 20 min, blocked for 1 h in 2% NuSerum–PBS, and then incubated at room temperature for 1 h with either MAb 9E10 or MAb 7E4. After three washings with PBS, the samples were incubated 30 min with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse immunoglobulin diluted 1:128 (Sigma). The stained monolayers were washed three times with PBS, mounted with Vectashield (Vector), and examined under a Bio-Rad 600 confocal fluorescence microscope.
Double IF was performed by adding, after the first incubation with MAb T10 1F7 and the secondary FITC-labeled antibody, biotin-conjugated MAb 9E10 and tetramethylrhodamine isothiocyanate-conjugated streptavidin (Jackson ImmunoResearch Laboratories). The samples were washed three times in PBS, air dried, mounted with Vectashield (Vector), and observed under a Bio-Rad 600 confocal fluorescence microscope. MAb 9E10 (Boehringer) was biotinylated using sulfo–N-hydroxysuccinimide–biotin according to the protocol provided by the manufacturer (Pierce).
RESULTS
Localization of tagged MIC2 variants in transiently transfected T. gondii tachyzoites.
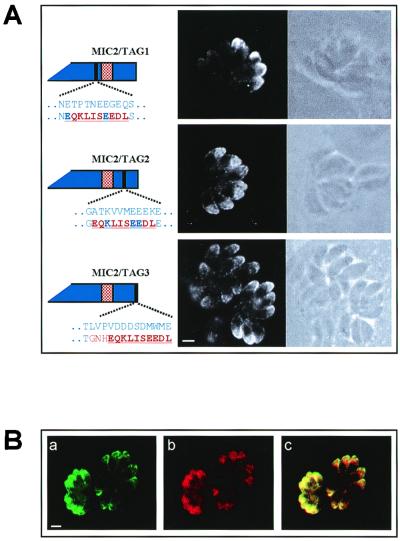
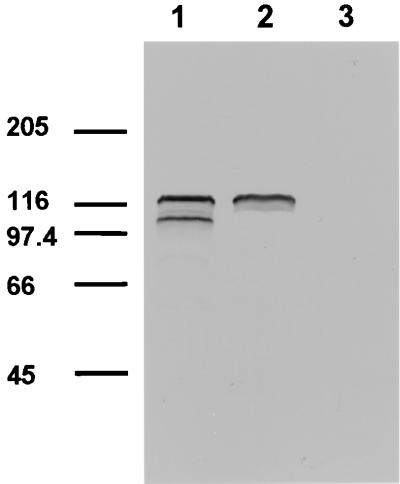
Gene knockout and structure-function analyses of TRAP in Plasmodium parasites have revealed that this micronemal protein plays a vital role in parasite motility and invasion of host cells (18, 38, 43). These findings suggest that replacement of endogenous MIC2 with mutants compromised in targeting may be lethal for the parasite. To circumvent this possible limitation, we investigated the targeting of MIC2 in T. gondii by transiently transfecting the parasites with constructs encoding tagged MIC2 variants. The use of transiently transfected tachyzoites was dictated by the difficulty encountered in establishing stable transfected T. gondii lines expressing some of the MIC2 variants. The expression of genes encoding c-Myc-tagged MIC2 protein was achieved by using as promoter a DNA sequence encompassing 1,480 nucleotides upstream of the ATG codon of MIC2. This strategy allowed the expression of a series of recombinant MIC2 mutated proteins that could be easily distinguished by IF and immunoblot analyses from the T. gondii endogenous MIC2 molecule. In MIC2/Tag1, the c-Myc epitope was placed seven amino acids upstream of the putative MIC2 TM domain (amino acids 680 to 690), causing the replacement of eight amino acids (Fig. 1A). The insertion of c-Myc in MIC2/Tag2 (amino acids 745 to 754) generated seven amino acid changes, while in MIC2/Tag3 the c-Myc sequence replaced the last 13 C-terminal amino acids of MIC2 (Fig. 1A). Protein lysates of infected HFF cells containing tachyzoites transfected with pMIC2/Tag1 were analyzed in immunoblot assay using MAb 9E10. Two bands migrating with apparent molecular masses of 120 and 100 kDa were found in the lysate of tachyzoites transfected with pMIC2/Tag1 (Fig. 2), whereas only the 120-kDa band was observed in tachyzoites transfected with pMIC2/Tag2 (Fig. 2). These findings are in agreement with the expected molecular weight of MIC2 and of its processed products (1, 42) and would suggest that the processing cleavage site is localized between the two c-Myc epitopes of MIC2/Tag1 and MIC2/Tag2. Alternatively the insertion of the c-Myc epitope in construct MIC2/Tag2 could have destroyed the cleavage site.
FIG. 1.
(A) Immunolocalization of c-Myc-tagged MIC2 proteins, shown in confocal fluorescence (dark-field) and transmission (bright-field) photomicrographs of T. gondii tachyzoites transfected with constructs pMIC2/Tag1, pMIC2/Tag2, and pMIC2/Tag3. Intracellular parasites were incubated with MAb 9E10 directed against the c-Myc epitope and FITC-conjugated secondary antibody. The chimeric proteins encoded by the constructs are schematically shown. The blue and the red stippled boxes indicate the MIC2 sequence and the position of its TM region, respectively. The c-Myc epitope is represented as a black bar. The amino acid substitutions introduced by the insertion of the c-Myc epitope are indicated in red below the wild-type amino acid residues shown as blue letters. The amino acid sequence of the c-Myc epitope is underlined. Magnification of ×630, plus zoom factor of 2 for image acquisition; scale bar = 2 μm. (B) Confocal fluorescence photomicrographs showing the colocalization of MIC1 and the MIC2/Tag2. Intracellular pMIC2/Tag2-transfected tachyzoites were sequentially incubated with MAb T10 1F7 directed against the micronemal protein MIC1 and FITC-labeled secondary antibody (a) and biotin-labeled MAb 9E10 and rhodamine-labeled streptavidin (b). To precisely visualize the localization of MIC1 and MIC2/Tag2 within the tachyzoites, the two fluorescence photomicrographs were merged (c) using the software CoMOS version 7.0a (confocal microscope operating software) from Bio-Rad. Magnification of ×630, plus zoom factor of 2 for image acquisition; scale bar = 2 μm.
FIG. 2.
Immunoblot analysis of T. gondii parasites expressing different c-Myc-tagged MIC2 proteins. Protein lysates corresponding to 107 parasites were separated by SDS-PAGE on a 10% gel under reducing conditions and immunoblotted with MAb 9E10 directed against the c-Myc epitope and AP-conjugated secondary antibody. Lanes 1 and lane 2 contain T. gondii lysates from pMIC2/TAG1- and pMIC2/TAG2-transfected parasites, respectively; lane 3 contains nontransfected tachyzoites as a control. Migration positions of the high-molecular-weight standards (Sigma) are indicated in kilodaltons.
To investigate the intracellular localization of the tagged MIC2 proteins, we analyzed transiently transfected tachyzoites by IF using MAb 9E10 directed against the c-Myc epitope. This analysis revealed that the recombinant proteins MIC2/Tag1 to -3 were efficiently expressed by a high proportion of parasites and were mainly localized at the apical tip of the tachyzoites, facing the parasitophorous vacuole membrane (Fig. 1A). This localization pattern is identical to that reported for endogenous MIC2 as well as for different T. gondii micronemal proteins (1, 26), including MIC1, thus suggesting that the recombinant MIC2/Tag1, -2, and -3 proteins were correctly targeted to the parasite micronemes. To confirm the micronemal localization of these proteins, we carried out colocalization experiments using MAb T10 1F7 directed against MIC1 (1). This analysis was performed by IF confocal microscopy to maximize the resolution of morphological details. MAbs 9E10 and T10 1F7 showed the same staining pattern in transfected parasites (Fig. 1B). In particular, we observed a complete overlap in the distribution of the fluorescent signals emitted by the immunological probes used to localize the tagged recombinant MIC2 proteins and endogenous MIC1. The colocalization analysis was extended to all constructs used in these studies.
The putative cytoplasmic tail of MIC2 contains a micronemal targeting sequence.
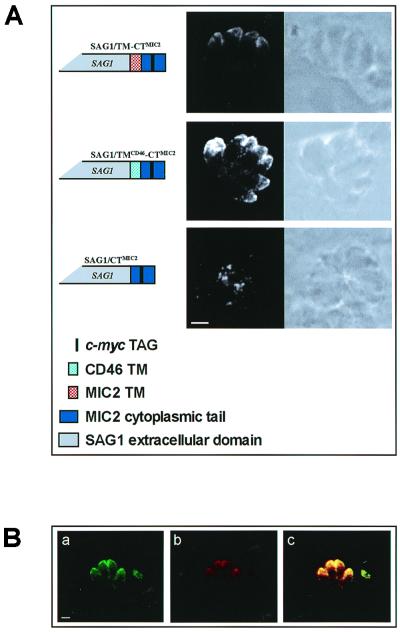
The cytoplasmic tails from different proteins of distantly related organisms have been shown to contain a variety of amino acid motifs responsible for targeting polypeptides to specific subcellular compartments (3, 4, 14, 40, 45). This notion together with the observation that all putative transmembrane micronemal proteins identified so far share a number of conserved residues in their cytoplasmic tails prompted us to investigate whether the corresponding region of MIC2 contained a microneme targeting sequence. We generated a construct, pSAG1/TM-CTMIC2, that expressed a polypeptide encompassing the coding sequence of the T. gondii surface protein SAG1 (amino acids 1 to 289), without the GPI anchor signal, linked to the TM region and cytoplasmic tail (amino acids 698 to 769) of MIC2 (Fig. 3A). The c-Myc epitope was inserted in the cytoplasmic tail of MIC2 at positions 745 to 754, where it was shown not to affect the localization of MIC2/Tag2 to the apical tip of the parasites. To analyze the expression and localization of the protein encoded by pSAG1/TM-CTMIC2, transiently transfected tachyzoites were processed in IF using MAb 9E10. This analysis revealed that chimeric SAG1 polypeptides showed the same subcellular distribution as endogenous MIC1 in IF confocal microscopy (Fig. 3), thus suggesting that the MIC2-derived sequences contained the parasite micronemal targeting determinants. To assess the contribution of the TM region of MIC2 to microneme targeting, we generated two additional constructs (Fig. 3A) in which the TM domain of SAG1/TM-CTMIC2 was either replaced with that from human CD46 (SAG1/TMCD46-CTMIC2) or deleted (SAG1/CTMIC2). Our results indicated that SAG1/TMCD46-CTMIC2 protein was efficiently delivered to the micronemes whereas the SAG1/CTMIC2 variant lacking a membrane-spanning region appeared to accumulate at the posterior end of the parasites in the parasitophorous vacuole, probably secreted through the dense granules (Fig. 3A). The micronemal localization of SAG1/TMCD46-CTMIC2 was confirmed by colocalization with the endogenous micronemal protein MIC1 (Fig. 3B). These findings indicated that the cytoplasmic tail of MIC2 is sufficient to target the chimeric protein to the microneme provided that is correctly oriented across the parasite intracellular membranes.
FIG. 3.
(A) Immunolocalization of a SAG1-MIC2 chimeric protein and variants in which the MIC2 TM region was either deleted or replaced with the TM region of human CD46. The structure of the chimeric proteins is schematically shown. Confocal fluorescence (dark-field) and transmission (bright-field) photomicrographs show intracellular tachyzoites transfected with the constructs pSAG1/TM-CTMIC2, pSAG1/TMCD46-CTMIC2, and pSAG1/CTMIC2. Intracellular parasites were incubated with MAb 9E10 and FITC-conjugated secondary antibody. Magnification of ×630, plus zoom factor of 2 for image acquisition; scale bar = 2 μm. (B) Confocal fluorescence photomicrographs showing the colocalization of MIC1 and the chimeric protein SAG1/TMCD46-CTMIC2. Magnification of ×630, plus zoom factor of 2 for image acquisition; scale bar = 2 μm.
A tyrosine-rich motif is implicated in microneme targeting.
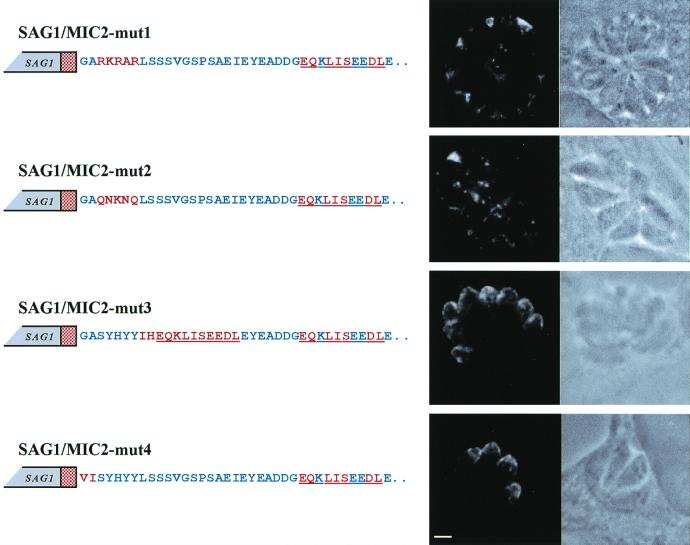
Previous reports have shown that membrane-spanning proteins utilize a variety of tyrosine- and leucine-based motifs, usually localized in their cytoplasmic tails, to be targeted to specific membrane-bounded compartments (4, 20, 24, 32). This notion prompted us to investigate the involvement in microneme targeting of the motif SYHYY based around tyrosine residues Y722, Y724, and Y725. This motif is placed in the cytoplasmic tail of MIC2 immediately downstream of the TM domain. We generated two constructs, SAG1/MIC2-mut1 and SAG1/MIC2-mut2, in which the SYHYY motif of MIC2 motif was replaced by either the TM stop-transfer signal from P-selectin (RKRAR) (25) or a random amino acid sequence (QNKNQ) that was predicted to maintain the hydrophilic pattern of the SYHYY sequence. IF analysis of intracellular tachyzoites expressing either SAG1/MIC2-mut1 or SAG1/MIC2-mut2 revealed that these two recombinant proteins were found outside the parasite body, suggesting that they were secreted into the parasitophorous vacuole (Fig. 4). As a control, we analyzed the localization of SAG1/MIC2 chimeric polypeptides containing amino acid substitutions in different regions of the cytoplasmic tail flanking the SYHYY sequence. In the construct SAG1/MIC2-mut3, a second c-Myc epitope was introduced immediately after Y725, causing a 12-amino-acid exchange. In the SAG1/MIC2-mut4 construct, the first two amino acids of the cytoplasmic tail, a glycine and an alanine, were replaced with a valine and an isoleucine, respectively (Fig. 4). IF analysis of transfected tachyzoites showed that both pSAG1/MIC2-mut3- and pSAG1/MIC2-mut4-encoded polypeptides were localized at the apical tip of intracellular tachyzoites (Fig. 4).
FIG. 4.
Confocal fluorescence and transmission photomicrographs showing the immunolocalization of SAG1/MIC2 chimeric proteins containing amino acid substitutions in the cytoplasmic tail of MIC2. Intracellular tachyzoites, transfected with the constructs pSAG1/MIC2-mut1, pSAG1/MIC2-mut2, pSAG1/MIC2-mut3, and pSAG1/MIC2-mut4, were incubated with MAb 9E10 and FITC-conjugated secondary antibody. The red stippled box represents the MIC2 TM domain. The wild-type MIC2 amino acid residues are indicated as blue letters in the schematic representation of the constructs. Amino acid substitutions are shown in red letters, and the c-Myc epitope is underlined. Magnification of ×630, plus zoom factor of 2 for image acquisition; scale bar = 2 μm.
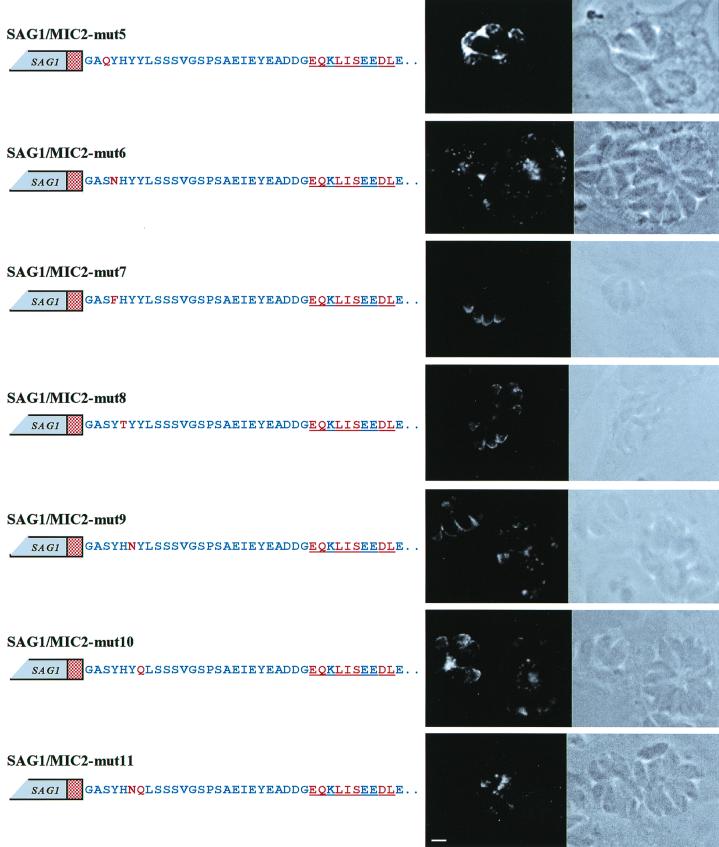
The contribution of each residue of the SYHYY motif in forming the targeting signal was investigated by introducing single and multiple amino acid substitutions. To minimize effects due to structural changes, the wild-type residues were replaced by amino acids showing similar hydrophilic properties. The replacement of Y722 with an asparagine abolished the targeting (construct SAG1/MIC2-mut6) to the apical tip of the parasites and redirected the chimeric protein to the parasitophorous vacuolar space, while replacement of this residue with a phenylalanine (construct SAG1/MIC2-mut7) had no effect (Fig. 5). This analysis revealed that residues Y724 and Y725 contributed to the formation of the targeting signal. Tachyzoites transfected with pSAG1/MIC2-mut9 and pSAG1/MIC2-mut10, in which one of the residues Y724 and Y725 was replaced by an asparagine or glutamine, showed only a partial localization at the apical tip of the tachyzoites together with a strong staining of the parasitophorous vacuole. The substitution of both tyrosine residues (construct SAG1/MIC2-mut11) resulted in a complete loss of apical targeting and in secretion of the chimeric polypeptide into the parasitophorous vacuole (Fig. 5). The substitution of S721 with a glutamine (construct SAG1/MIC2-mut5) drastically reduced the apical localization of the SAG1/MIC2 polypeptide in transfected parasites, suggesting the involvement of this residue in the formation of the targeting signal. Amino acid H723 appeared not be involved in targeting, as shown by the localization of the polypeptide encoded by the SAG1/MIC2-mut8 construct (Fig. 5).
FIG. 5.
Confocal fluorescence and transmission photomicrographs showing the immunolocalization of SAG1/MIC2 chimeric proteins containing amino acid substitutions in the tyrosine-based motif of the MIC2 cytoplasmic tail. Wild-type MIC2 amino acids and mutated residues are indicated in the schematic representation of the constructs as blue and red letters, respectively. The red stippled box represents the MIC2 TM domain. The sequence of the c-Myc epitope is underlined. Magnification of ×630, plus zoom factor of 2 for image acquisition; scale bar = 2 μm.
The SYHYY sequence is necessary but not sufficient for microneme targeting.
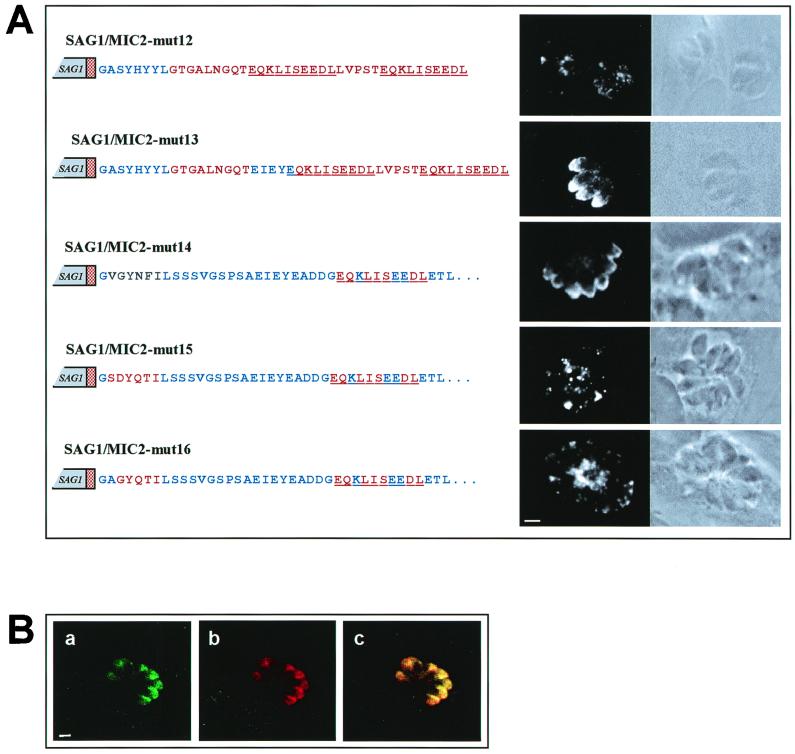
To assess whether the SYHYY motif alone was sufficient to target a heterologous polypeptide to the micronemes, we investigated the localization of the SAG1 chimeric protein encoded by construct pSAG1/MIC2-mut12. This protein contained in its cytoplasmic tail the SYHYY motif followed by an artificial sequence, carrying two c-Myc epitopes and mimicking the hydrophilic profile of the cytoplasmic tail of MIC2 (Fig. 6A). IF analysis clearly showed that this protein was not targeted to the apical tip of intracellular tachyzoites but secreted mainly into the parasitophorous vacuole (Fig. 6A). This finding indicated that other sequences in addition to the SYHYY motif were implicated in the process leading to protein accumulation in the micronemes. Analysis of the residues that were left unchanged by insertion of the c-Myc epitope at different positions in the cytoplasmic tail of MIC2 indicated that this additional motif ought to be placed within the sequence EIEYEADDGE. This notion was further supported by the observation that this sequence encompassed the conserved acidic motif EXEY/FE, found in the cytoplasmic tails of all micronemal membrane-spanning proteins from T. gondii and N. caninum. To investigate the function of this second motif, we developed a construct (pSAG1/MIC2-mut13) that encoded a SAG1 chimeric protein containing an artificial cytoplasmic tail that encompassed both the SYHYY and EIEYE motifs separated by a linker amino acid sequence. IF analysis showed that the protein encoded by this construct was localized at the apical tip of the tachyzoites as a typical micronemal protein (Fig. 6A). This conclusion was also supported by the observation that SAG1/MIC2mut13 showed the localization pattern of endogenous MIC1 in IF confocal microscopy (Fig. 6B). These results demonstrated that two different cytoplasmic determinants, a tyrosine-based and acidic motif, are required and sufficient to target proteins to the parasite micronemes.
FIG. 6.
(A) Confocal fluorescence and transmission photomicrographs showing the immunolocalization of chimeric proteins containing the MIC2 tyrosine-based motif alone (SAG1/MIC2-mut12) or in combination with a short glutamate-rich conserved sequence of the MIC2 cytoplasmic tail (SAG1/MIC2-mut13). The parasites were also transfected with constructs in which the MIC2 tyrosine motif had been replaced with the homologous region of PbTRAP (SAG1/MIC2-mut14) or with the endocytic targeting signals of rat TGN38 and human LAMP1 (SAG1/MIC2-mut15 and SAG1/MIC2-mut16). Wild-type MIC2 amino acids and mutated residues are indicated in the schematic representation of the constructs as blue and red letters, respectively. The red stippled box represents the MIC2 TM domain. The c-Myc epitope is underlined. PbTRAP-derived residues are indicated in green. Magnification of ×630, plus zoom factor of 2 for image acquisition; scale bar = 2 μm. (B) Confocal fluorescence photomicrographs showing the colocalization of MIC1 and the chimeric protein SAG1/MIC2-mut13. Magnification of ×630, plus zoom factor of 2 for image acquisition; scale bar, = 2 μm.
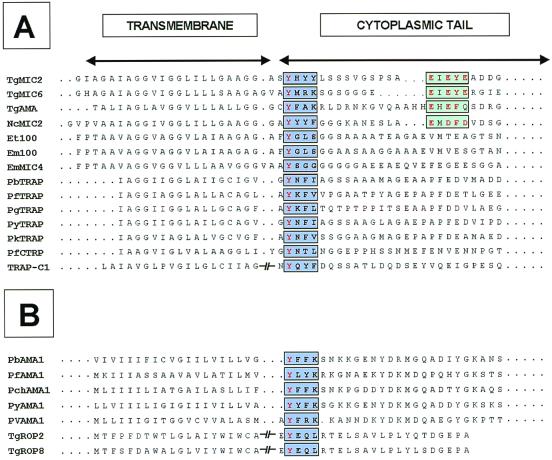
We have compared the cytoplasmic tails of micronemal proteins of different apicomplexan parasites to identify structural features common to the motifs of MIC2 involved in protein targeting to the micronemes (Fig. 7A). This analysis revealed that the first tyrosine residue of the SYHYY motif is present in all known micronemal proteins of Plasmodium, Eimeria, Toxoplasma, Neospora, and Cryptosporidium species as well as in the rhoptry proteins AMA1 of Plasmodium parasites and ROP2 and ROP8 of T. gondii (Fig. 7B). This tyrosine is usually separated by one amino acid from a second aromatic residue. The acidic motif EXEY/FE is found only in the micronemal proteins from T. gondii and in NcMIC2 from N. caninum (Fig. 7A).
FIG. 7.
Sequence alignment of the putative TM sequences and cytoplasmic domains of micronemal proteins of different apicomplexan parasites. (A) Micronemal proteins. The tyrosine and glutamate motifs are indicated by light blue and green boxes, respectively. Identical or conserved residues are indicated in red. (B) Rhoptry proteins. A tyrosine-based motif is also found in the cytoplasmic tail of membrane-spanning proteins localized in the rhoptries of different Plasmodium species and T. gondii. Tg, T. gondii; Nc, N. caninum; Et, E. tenella; Em, E. maxima; C, C. parvum; Pb, P. berghei; Pf, P. falciparum; Pg, P. gallinaceum; Py, P. yoelii; Pk, P. knowlesi; Pch, P. chabaudi; Pv, P. vivax. GenBank accession numbers: TgMIC2, U62660; TgMIC6, AF110270; TgAMA, AF010264; NcMIC2, AFO61273; Et100, AF032905; Em100, M99058; PbTRAP, U67763; PfTRAP, X13022; PgTRAP, U64899; PyTRAP, M84732; PkTRAP, U64900; PfCTRP, U34363; TRAP-C1, AF017267; PbAMA1, AAC47192; PfAMA1, AF061332; PchAMA1, M25248; PyAMA1, AAC47193; PvAMA1, AAC16731; TgROP2, Z36906; TgROP8, AF011377.
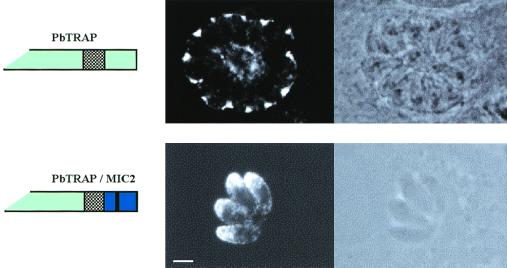
We investigated whether tyrosine-based sequences found in molecules from other apicomplexan parasites and distantly related organisms could function as micronemal targeting signals in T. gondii. Our data showed that the SAG1 chimeric protein SAG1/MIC2-mut14, in which the SYHYY motif was replaced by the VGYNFI sequence of PbTRAP, was localized at the apical tip of intracellular tachyzoites (Fig. 6A). In contrast, insertion of the internalization tyrosine-based signals SDYQRL and AGYQTI from rat TGN38 (5) and human LAMP1 (15) abolished the apical localization of the molecules encoded by the constructs pSAG1/MIC2-mut15 and pSAG1/MIC2-mut16 (Fig. 6A). Notably, when the entire PbTRAP was expressed in transiently transfected T. gondii, the Plasmodium protein was exclusively localized outside the tachyzoites, suggesting that PbTRAP was secreted in the parasitophorous vacuole rather than being targeted to the micronemes (Fig. 8). Replacement of the cytoplasmic tail of PbTRAP with the corresponding sequence of MIC2, containing a c-Myc epitope, was sufficient to target the PbTRAP/MIC2 chimeric protein to the micronemes (Fig. 8). These findings together indicate that the tyrosine-based motif VGYNFIL of PbTRAP functions as a microneme targeting sequence in T. gondii. However, sequence alignment suggests that PbTRAP lacks a typical EXEY/FE motif with crucial residue spacing, thus providing an explanation for the mistargeting of PbTRAP when expressed in T. gondii.
FIG. 8.
Immunolocalization of PbTRAP and PbTRAP/MIC2. Confocal fluorescence (dark-field) and transmission (bright-field) photomicrographs show T. gondii tachyzoites transfected with the constructs pPbTRAP and pPbTRAP/MIC2. Intracellular parasites were incubated with MAb 7E4 directed against PbTRAP and FITC-conjugated secondary antibody. The proteins encoded by the constructs are schematically shown. Magnification of ×630, plus zoom factor of 2 for image acquisition; scale bar = 2 μm.
DISCUSSION
To identify the amino acid sequences that function as targeting signals for micronemes, we have analyzed in T. gondii the subcellular localization of recombinant c-Myc-tagged MIC2 variants and SAG1/MIC2 chimeric proteins. We have demonstrated that a protein in which the GPI anchor of the surface molecule SAG1 was replaced by the TM sequence of human CD46 followed by the cytoplasmic domain of MIC2 (SAG1/TMCD46-CTMIC2) localized at the apical tip of intracellular tachyzoites. The ultrastructural localization of the tagged MIC2 constructs by immunoelectron microscopy was hampered by the use of transiently transfected parasites. Moreover, processing of the sample for immunoelectron microscopy dramatically reduced the ability of MAb 9E10 to recognize the c-Myc epitopes in the MIC2 constructs. However, a colocalization experiment by IF confocal microscopy showed that the subcellular distributions of SAG1/TMCD46-CTMIC2 and the endogenous micronemal protein MIC1 were identical, indicating that the SAG1 chimeric protein was delivered to the parasite micronemes. Notably, a previous report showed that replacement of the SAG1 GPI anchor with the TM and cytoplasmic domains of human CD46 did not affect the localization of SAG1 to the surface of T. gondii tachyzoites (33), whereas insertion of the cytoplasmic tail of MIC2 redirected the targeting of this membrane-spanning protein from the cell surface to the micronemes. On the basis of these findings, we concluded that the cytoplasmic tail of MIC2 contained all of the sequence information necessary and sufficient for targeting this parasite protein to the micronemes.
Mutation analysis revealed that the MIC2 targeting sequences consisted of two distinct amino acid motifs. The first motif spans the sequence SYHYY (amino acids 721 to 725), which is located close to the putative membrane-spanning domain. Tyrosine 722 was shown to be crucial for the targeting function of this motif. Substitution of either tyrosine 724 or tyrosine 725 had only a mild effect on the subcellular localization of a SAG1/MIC2 chimeric molecule. In contrast, the substitution of both tyrosine 724 and tyrosine 725 with asparagine and glutamine, respectively, resulted in the complete loss of micronemal targeting. The staining was mainly localized in the parasitophorous vacuole, indicating that these chimeric proteins were redirected into the parasite default secretory pathway, which has been reported to be mediated by dense granules (19).
The SYHYY motif shares structural similarities and localization on the cytoplasmic tail with tyrosine-based sorting sequences previously described for higher eukaryotic organisms (20, 23–25, 32). Tyrosine-based signals are widely distributed and consist of a continuous sequence of four to six amino acids containing a critical aromatic residue that is usually a tyrosine placed in a degenerate context characterized by the presence of a single amino acid with large hydrophobic side chains (⊘) (40). Previous reports have shown that membrane-spanning proteins are sorted in the endocytic and exocytic pathways by tyrosine-based signals placed in their cytoplasmic tails (32, 40). Surprisingly, two typical tyrosine-based internalization signals SDYQRL and AGYQTI were not able to functionally complement the deletion of the SYHYY sequence, suggesting that the YXX⊘ consensus motif is not recognized by the targeting machinery of T. gondii. The observation that the targeting sequence in T. gondii requires at least the presence of two aromatic residues or nonhydrophobic amino acids in position 4 of the YXX⊘ motif would support this conclusion and highlight important differences with the targeting motifs of higher eukaryotic organisms. Sequence comparison of micronemal proteins revealed the presence of potential tyrosine-based motifs in their cytoplasmic tails, suggesting the presence of common targeting pathways in apicomplexan parasites. The ability of the putative tyrosine-based signal VGYNFI from PbTRAP to efficiently complement the SYHYY sequence in a SAG1/MIC2 chimeric protein supports this hypothesis.
Attempts to deliver SAG1 to the micronemes by using the SYHYY motif alone were not successful, thus revealing the involvement of additional MIC2 sequences in the targeting function. These additional sequences were identified in the motif EIEYE that spanned the conserved consensus sequence EXEY/FE found only in the cytoplasmic tails of micronemal proteins from T. gondii and N. caninum. In higher eukaryotic organisms, amino acid motifs based on acid residues have been reported to function in conjunction with tyrosine-based targeting signals to direct the low-density lipoprotein receptor to the basolateral membrane (23) and mammalian furin in the trans-Golgi network (41).
Very little is known on the molecular mechanisms regulating protein trafficking in T. gondii; only recently have clathrin-coated vesicles been observed in this parasite (16, 19, 21). The well-established relationship between tyrosine-based signals and the μ chains of the clathrin-associated adapter complexes AP-2, AP-1, and AP-3 suggest that similar adapter complexes and coated vesicles may interact with the tyrosine-based determinant found within the cytoplasmic tail of MIC2 and mediate intracellular trafficking from the trans-Golgi network to micronemes. Putative tyrosine-based motifs are also found in the cytoplasmic tails of other proteins from apicomplexan parasites, such as the rhoptry proteins AMA1 and ROP2 from Plasmodium and T. gondii, respectively. Accordingly, tyrosine motifs could have a more general role in directing proteins to parasite secretory organelles, whereas additional sequences such as the EXEY/FE motif could be implicated at branch points of the targeting pathway to direct parasite proteins to the micronemes.
Together, these findings provide a novel framework for understanding the molecular mechanisms involved in the targeting of membrane-spanning proteins into parasite micronemes. In addition, the identification of a microneme signature in combination with the genomic information originating from sequencing projects of several apicomplexan parasites including P. falciparum, T. gondii, and C. parvum will help in recognizing novel genes involved in crucial steps of the parasite life cycle.
ACKNOWLEDGMENTS
We thank Furio Spano, Federico Giannoni, Bruno Arca′, and David Roos for helpful discussions.
This work was supported by a grant from the Wellcome Trust to A.C. M.D. has been supported by a short-term EMBO fellowship and by the TMR program of the European Union.
REFERENCES
- 1.Achbarou A, Mercereau-Puijalon O, Autheman J M, Fortier B, Camus D, Dubremetz J F. Characterization of microneme proteins of Toxoplasma gondii. Mol Biochem Parasitol. 1991;47:223–233. doi: 10.1016/0166-6851(91)90182-6. [DOI] [PubMed] [Google Scholar]
- 2.Adams J H, Hudson D E, Torii M, Ward G E, Wellems T E, Aikawa M, Miller L H. The Duffy receptor family of Plasmodium knowlesiis located within the micronemes of invasive malaria merozoites. Cell. 1990;63:141–153. doi: 10.1016/0092-8674(90)90295-p. [DOI] [PubMed] [Google Scholar]
- 3.Andersson A M, Melin L, Bean A, Pettersson R F. A retention signal necessary and sufficient for Golgi localization maps to the cytoplasmic tail of a Bunyaviridae(Uukuniemi virus) membrane glycoprotein. J Virol. 1997;71:4717–4727. doi: 10.1128/jvi.71.6.4717-4727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aroeti B, Okhrimenko H, Reich V, Orzech E. Polarized trafficking of plasma membrane proteins: emerging roles for coats, SNAREs, GTPases and their link to the cytoskeleton. Biochim Biophys Acta. 1998;1376:57–90. doi: 10.1016/s0304-4157(98)00005-7. [DOI] [PubMed] [Google Scholar]
- 5.Bos K, Wraight C, Stanley K K. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 1993;12:2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown P J, Billington K J, Bumstead J M, Clark J D, Tomley F M. A microneme protein from Eimeria tenellawith homology to the Apple domains of coagulation factor XI and plasma pre-kallikrein. Mol Biochem Parasitol. 2000;107:91–102. doi: 10.1016/s0166-6851(00)00179-1. [DOI] [PubMed] [Google Scholar]
- 7.Carruthers V B, Moreno S N, Sibley L D. Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii. Biochem J. 1999;342:379–386. [PMC free article] [PubMed] [Google Scholar]
- 8.Carruthers V B, Sibley L D. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol Microbiol. 1999;31:421–428. doi: 10.1046/j.1365-2958.1999.01174.x. [DOI] [PubMed] [Google Scholar]
- 9.Carruthers V B, Sibley L D. Sequential protein secretion from three distinct organelles of Toxoplasma gondiiaccompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- 10.Cha R S, Tilly W G. PCR primer. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1995. [Google Scholar]
- 11.Dessens J T, Beetsma A L, Dimopoulos G, Wengelnik K, Crisanti A, Kafatos F C, Sinden R E. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 1999;18:6221–6227. doi: 10.1093/emboj/18.22.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Entzeroth R, Kerckhoff H, Konig A. Microneme secretion in Coccidia: confocal laser scanning and electron microscope study of Sarcocystis murisin cell culture. Eur J Cell Biol. 1992;59:405–413. [PubMed] [Google Scholar]
- 13.Fang X D, Kaslow D C, Adams J H, Miller L H. Cloning of the Plasmodium vivaxDuffy receptor. Mol Biochem Parasitol. 1991;44:125–132. doi: 10.1016/0166-6851(91)90228-x. [DOI] [PubMed] [Google Scholar]
- 14.Gough N R, Fambrough D M. Different steady state subcellular distributions of the three splice variants of lysosome-associated membrane protein LAMP-2 are determined largely by the COOH-terminal amino acid residue. J Cell Biol. 1997;137:1161–1169. doi: 10.1083/jcb.137.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarnieri F G, Arterburn L M, Penno M B, Cha Y, August J T. The motif Tyr-X-X-hydrophobic residue mediates lysosomal membrane targeting of lysosome-associated membrane protein 1. J Biol Chem. 1993;268:1941–1946. [PubMed] [Google Scholar]
- 16.Hager K M, Striepen B, Tilney L G, Roos D S. The nuclear envelope serves as an intermediary between the ER and Golgi complex in the intracellular parasite Toxoplasma gondii. J Cell Sci. 1999;112:2631–2638. doi: 10.1242/jcs.112.16.2631. [DOI] [PubMed] [Google Scholar]
- 17.Horton R M, Hunt H D, Ho S N, Pullen J K, Pease L R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 18.Kappe S, Bruderer T, Gantt S, Fujioka H, Nussenzweig V, Menard R. Conservation of a gliding motility and cell invasion machinery in Apicomplexan parasites. J Cell Biol. 1999;147:937–944. doi: 10.1083/jcb.147.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karsten V, Qi H, Beckers C J, Reddy A, Dubremetz J F, Webster P, Joiner K A. The protozoan parasite Toxoplasma gondiitargets proteins to dense granules and the vacuolar space using both conserved and unusual mechanisms. J Cell Biol. 1998;141:1323–1333. doi: 10.1083/jcb.141.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Borgne R, Hoflack B. Protein transport from the secretory to the endocytic pathway in mammalian cells. Biochim Biophys Acta. 1998;1404:195–209. doi: 10.1016/s0167-4889(98)00057-3. [DOI] [PubMed] [Google Scholar]
- 21.Lingelbach K, Joiner K A. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J Cell Sci. 1998;111:1467–1475. doi: 10.1242/jcs.111.11.1467. [DOI] [PubMed] [Google Scholar]
- 22.Lovett J L, Howe D K, Sibley L D. Molecular characterization of a thrombospondin-related anonymous protein homologue in Neospora caninum. Mol Biochem Parasitol. 2000;107:33–43. doi: 10.1016/s0166-6851(99)00228-5. [DOI] [PubMed] [Google Scholar]
- 23.Matter K, Yamamoto E M, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 25.Modderman P W, Beuling E A, Govers L A, Calafat J, Janssen H, von dem Borne A E, Sonnenberg A. Determinants in the cytoplasmic domain of P-selectin required for sorting to secretory granules. Biochem J. 1998;336:153–161. doi: 10.1042/bj3360153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrissette N S, Bedian V, Webster P, Roos D S. Characterization of extreme apical antigens from Toxoplasma gondii. Exp Parasitol. 1994;79:445–459. doi: 10.1006/expr.1994.1106. [DOI] [PubMed] [Google Scholar]
- 27.Robson K J, Hall J R, Jennings M W, Harris T J, Marsh K, Newbold C I, Tate V E, Weatherall D J. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988;335:79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- 28.Robson K J, Naitza S, Barker G, Sinden R E, Crisanti A. Cloning and expression of the thrombospondin related adhesive protein gene of Plasmodium berghei. Mol Biochem Parasitol. 1997;84:1–12. doi: 10.1016/s0166-6851(96)02774-0. [DOI] [PubMed] [Google Scholar]
- 29.Rogers W O, Malik A, Mellouk S, Nakamura K, Rogers M D, Szarfman A, Gordon D M, Nussler A K, Aikawa M, Hoffman S L. Characterization of Plasmodium falciparumsporozoite surface protein 2. Proc Natl Acad Sci USA. 1992;89:9176–9180. doi: 10.1073/pnas.89.19.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers W O, Rogers M D, Hedstrom R C, Hoffman S L. Characterization of the gene encoding sporozoite surface protein 2, a protective Plasmodium yoeliisporozoite antigen. Mol Biochem Parasitol. 1992;53:45–51. doi: 10.1016/0166-6851(92)90005-5. [DOI] [PubMed] [Google Scholar]
- 31.Roos D S, Donald R G, Morrissette N S, Moulton A L. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 32.Sandoval I V, Bakke O. Targeting of membrane proteins to endosomes and lysosomes. Trends Cell Biol. 1994;4:292–297. doi: 10.1016/0962-8924(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 33.Seeber F, Dubremetz J F, Boothroyd J C. Analysis of Toxoplasma gondiistably transfected with a transmembrane variant of its major surface protein, SAG1. J Cell Sci. 1998;111:23–29. doi: 10.1242/jcs.111.1.23. [DOI] [PubMed] [Google Scholar]
- 34.Sim B K, Toyoshima T, Haynes J D, Aikawa M. Localization of the 175-kilodalton erythrocyte binding antigen in micronemes of Plasmodium falciparummerozoites. Mol Biochem Parasitol. 1992;51:157–159. doi: 10.1016/0166-6851(92)90211-2. [DOI] [PubMed] [Google Scholar]
- 35.Soldati D, Boothroyd J C. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science. 1993;260:349–352. doi: 10.1126/science.8469986. [DOI] [PubMed] [Google Scholar]
- 36.Spaccapelo R, Naitza S, Robson K J, Crisanti A. Thrombospondin-related adhesive protein (TRAP) of Plasmodium bergheiand parasite motility. Lancet. 1997;350:335. doi: 10.1016/S0140-6736(97)24031-6. [DOI] [PubMed] [Google Scholar]
- 37.Spano F, Putignani L, Naitza S, Puri C, Wright S, Crisanti A. Molecular cloning and expression analysis of a Cryptosporidium parvumgene encoding a new member of the thrombospondin family. Mol Biochem Parasitol. 1998;92:147–162. doi: 10.1016/s0166-6851(97)00243-0. [DOI] [PubMed] [Google Scholar]
- 38.Sultan A A, Thathy V, Frevert U, Robson K J, Crisanti A, Nussenzweig V, Nussenzweig R S, Menard R. TRAP is necessary for gliding motility and infectivity of Plasmodiumsporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 39.Tomley F M, Clarke L E, Kawazoe U, Dijkema R, Kok J J. Sequence of the gene encoding an immunodominant microneme protein of Eimeria tenella. Mol Biochem Parasitol. 1991;49:277–288. doi: 10.1016/0166-6851(91)90071-d. [DOI] [PubMed] [Google Scholar]
- 40.Trowbridge I S, Collawn J F, Hopkins C R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 41.Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks M S, Peters P J, Bonifacino J S. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan K L, Carruthers V B, Sibley L D, Ajioka J W. Molecular characterisation of an expressed sequence tag locus of Toxoplasma gondiiencoding the micronemal protein MIC2. Mol Biochem Parasitol. 1997;84:203–214. doi: 10.1016/s0166-6851(96)02796-x. [DOI] [PubMed] [Google Scholar]
- 43.Wengelnik K, Spaccapelo R, Naitza S, Robson K J, Janse C J, Bistoni F, Waters A P, Crisanti A. The A-domain and the thrombospondin-related motif of Plasmodium falciparumTRAP are implicated in the invasion process of mosquito salivary glands. EMBO J. 1999;18:5195–5204. doi: 10.1093/emboj/18.19.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wertheimer S P, Barnwell J W. Plasmodium vivaxinteraction with the human Duffy blood group glycoprotein: identification of a parasite receptor-like protein. Exp Parasitol. 1989;69:340–350. doi: 10.1016/0014-4894(89)90083-0. [DOI] [PubMed] [Google Scholar]
- 45.West A E, Neve R L, Buckley K M. Identification of a somatodendritic targeting signal in the cytoplasmic domain of the transferrin receptor. J Neurosci. 1997;17:6038–6047. doi: 10.1523/JNEUROSCI.17-16-06038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuda M, Sawai T, Chinzei Y. Structure and expression of an adhesive protein-like molecule of mosquito invasive-stage malarial parasite. J Exp Med. 1999;189:1947–1952. doi: 10.1084/jem.189.12.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]