Abstract
There is lack of evidence on the role of blood eosinophil count (BEC) as a predictor of treatment response in patients with chronic cough. The study aimed to evaluate BEC as a predictor of treatment response in all non-smoking adults with chronic cough and normal chest radiograph referred to cough clinic and in a subgroup of patients with chronic cough due to asthma or non-asthmatic eosinophilic bronchitis (NAEB).
This prospective cohort study included 142 consecutive, non-smoking patients referred to our cough centre due to chronic cough. The management of chronic cough was performed according to the current recommendations. At least a 30-mm decrease of 100-mm visual analogue scale in cough severity and a 1.3 points improvement in Leicester Cough Questionnaire were classified as a good therapeutic response.
There was a predominance of females (72.5%), median age 57.5 years with long-lasting, severe cough (median cough duration 60 months, severity 55/100 mm). Asthma and NAEB were diagnosed in 47.2% and 4.9% of patients, respectively. After 12–16 weeks of therapy, a good response to chronic cough treatment was found in 31.0% of all patients. A weak positive correlation was demonstrated between reduction in cough severity and BEC (r=0.28, p<0.001). Area under the curve for all patients with chronic cough was 0.62 with the optimal BEC cut-off for prediction of treatment response set at 237 cells·µL−1 and for patients with chronic cough due to asthma/NAEB was 0.68 (95% CI 0.55–0.81) with the cut-off at 150 cells·µL−1.
BEC is a poor predictor of treatment response in adults with chronic cough treated in the cough centre.
Short abstract
Evaluation of blood eosinophil count has limited value in prediction of therapeutic response in patients with difficult-to-treat chronic cough https://bit.ly/3EjaYsZ
Introduction
Cough is not only a defensive physiological reflex of the airways but also a very common symptom of various pulmonary and extrapulmonary diseases. When acute, it usually points to a transient underlying disease, such as acute airway infection or asthma exacerbation and suggests a good prognosis in terms of its resolution. In contrast, chronic cough, defined in adults as the cough lasting >8 weeks, is considered to be much more cumbersome, at least in some patients. Chronic cough has been reported in as many as 5–10% of the adult population and is one of the most common reasons for seeking medical advice from a respiratory specialist [1–3]. The significance and the prognosis in chronic cough is largely related to the underlying disease. Chronic cough frequently results from smoking-related conditions, i.e. chronic bronchitis, COPD or lung cancer. Cause-oriented management, including smoking cessation and inhalation therapy with bronchodilators in combination with inhaled corticosteroids (ICS), is relatively effective in the alleviation of cough [4–6].
On the other hand, the most frequent causes (or rather triggers) of chronic cough in non-smoking patients with a normal chest radiograph are four common conditions: asthma, non-asthmatic eosinophilic bronchitis (NAEB), rhinitis and rhinosinusitis (which, along with chronic cough, constitute an entity described as upper airway cough syndrome (UACS)) and gastro-oesophageal reflux (GER) [1, 7]. Diagnosis and treatment of chronic cough may be challenging, in particular the latter has been shown to have limited efficacy. Despite different therapeutic attempts, the reduction in cough severity is incomplete and regarded unsatisfactory in even up to 46% of patients with chronic cough with persistent impairment of quality of life (QoL) [8, 9]. If causal treatment is ineffective, refractory chronic cough (RCC) should be diagnosed. While thorough diagnostics do not allow to identify any chronic cough reason, unexplained chronic cough (UCC) is diagnosed [8]. Both RCC and UCC are commonly associated with hypersensitivity of cough reflex, which is a key component in the pathomechanism of chronic cough regardless of the underlying condition [1, 10]. It is characterised by troublesome coughing in response to low level stimuli, loss of inhibitory cough control and abnormal laryngeal sensation. At the same time it is responsible for limited efficacy of chronic cough treatment [11, 12].
The limited effectiveness of cause-oriented treatment of chronic cough encourages the application of other approaches including a search for treatable chronic cough traits. Identifying different chronic cough endotypes and biomarkers capable of predicting treatment response may facilitate a more specific therapeutic approach resulting in a higher success rate [1]. It has been shown that up to 25–50% of patients with chronic cough are characterised by eosinophilic airway inflammation, which can be diagnosed by analysis of induced sputum [13–15]. The role of eosinophils in the pathomechanism of chronic cough is complex and still not well recognised. In recent trials, the relationship between eosinophils and increased airway sensory nerve density was documented [16, 17]. Moreover, Satia et al. [18] demonstrated heightened cough response to capsaicin when eosinophilic inflammation, caused by allergen exposure, is present. A higher efficacy of chronic cough treatment has been demonstrated in patients with an eosinophilic pattern of airway inflammation (usually in asthma or NAEB) [19]. As availability of sputum induction is limited, it has been suggested that peripheral blood eosinophil count (BEC) could be a good indirect tool to assess eosinophilic airway inflammation [13, 14]. However, previous studies showed a moderate diagnostic accuracy of BEC as a marker for eosinophilic airway inflammation in asthma [20] and only a weak correlation between BEC and sputum eosinophilia was found in patients with COPD [21].
As there is the lack of high-quality data on the role of BEC in the prediction of treatment response in patients with chronic cough, the objective of this study was to find out whether BEC may play this role in predicting treatment response in non-smoking adults with chronic cough and a normal chest radiograph referred to the tertiary cough centre and whether BEC could be a useful marker in patients with chronic cough due to asthma or NAEB.
Materials and methods
General study design
This prospective, single-centre cohort study included all consecutive patients with chronic cough referred to the cough centre in the Department of Internal Medicine, Pulmonary Diseases and Allergy of the Medical University of Warsaw between 2016 and 2019. It was a part of a larger project designed to evaluate the causes of chronic cough and the efficacy of chronic cough treatment which has been running in our institution since 2009. The study protocol was approved by the Institutional Review Board of the Medical University of Warsaw (KB/101/2009) and all enrolled patients signed written informed consent.
Patients
The major inclusion criteria were as follows: 1) cough lasting >8 weeks, 2) age over 18 years, 3) without any antitussive treatment for at least 4 weeks prior to enrolment to the study, 4) non-smoking (for at least 1 year) status and 5) normal or near-normal chest radiograph. The exclusion criteria included: 1) some well-defined pulmonary and extrapulmonary conditions which require specific therapeutic intervention (e.g. interstitial lung diseases, COPD, lung cancer or other malignant diseases, tuberculosis or nontuberculous mycobacteria infections, treatment with angiotensin-converting enzyme inhibitors); and 2) discontinuation of the recommended therapy.
Diagnostic workup
The causes of chronic cough were diagnosed according to the European Respiratory Society, American College of Chest Physicians (ACCP) and British Thoracic Society (BTS) recommendations [22–24]. The routine stepwise diagnostic workup included: a detailed medical history, physical examination and additional investigations, including basic laboratory tests (total and differential blood cell count, total serum IgE level), spirometry, fractional exhaled nitric oxide (FENO), methacholine challenge, induced sputum differential cell count, skin-prick tests and multiple aeroallergens IgE screening assay, imaging studies (chest radiograph, paranasal sinus and chest computed tomography) and ENT specialist consultation as well as 24-hour oesophageal impedance and pH-monitoring (see supplementary Figure 1). In the first step the exclusion of other causes of chronic cough was made (smoking, angiotensin-converting enzyme inhibitor therapy, COPD, lung cancer or interstitial lung disease). The next step included diagnostics of asthma, and in the case of high clinical probability, introduction of anti-asthmatic treatment. In case of no improvement or in the absence of evidence for the diagnosis of asthma, the further diagnostics (GER, UACS and other reasons for cough) and causal treatment were introduced (Supplementary Figures 1 and 2). BEC was measured twice in independent blood samples (Sysmex XN-2000, Kobe, Japan), before the onset of chronic cough treatment, and the higher of the two measurements was considered in further analysis.
Definitions
Chronic cough was defined as cough lasting at least 8 weeks [23]. Blood eosinophilia was defined as BEC ≥300 cells·µL−1, while the cut-off value for sputum eosinophilia was set at 3% [25, 26]. The atopic status of the patient was established based on at least one positive skin-prick test (a mean wheal diameter ≥3 mm) or presence of serum-specific IgE antibody for at least one allergen [27].
Asthma was defined as: 1) the presence of typical symptoms (dyspnoea or cough or wheezing) together with 2) variable airway obstruction (diurnal variation of peak expiratory flow >10% or spirometry with positive reversibility test or provocative concentration (PC20) in methacholine challenge test <16 mg·mL−1) [26, 28]. NAEB was diagnosed in chronic cough patients with elevated sputum eosinophilia and absence of airway hyperresponsiveness [25]. UACS was diagnosed in patients presenting with chronic cough and signs and symptoms of rhinitis or chronic rhinosinusitis confirmed by medical history and ENT examination [29]. Diagnostic criteria for GER were as follows: 1) oesophagitis revealed in gastroscopy, 2) elevated number (>73/24 h) reflux episodes registered in 24-hour pH-impedance monitoring, and 3) typical symptoms of GER disease (e.g. heartburn, regurgitation) and improvement after proton pump inhibitors (PPI) therapy [30–32]. At least one of the above criteria had to be met to diagnose GER. RCC was diagnosed when 6–8 weeks of recommended treatment with good patient adherence did not reduce cough severity. UCC was defined if no reason for cough was found despite thorough diagnostics [8].
Management, monitoring and outcome assessment
Once any chronic cough trigger was diagnosed, patients received specific treatment. Asthma was treated in accordance with Global Initiative for Asthma (GINA), including the three-step therapy, starting with moderate doses of inhaled corticosteroids (ICS, daily 400 μg of beclometasone dipropionate pMDI, hydrofluoroalkane (HFA), extrafine particle or 800 μg of budesonide pMDI) with long-acting β2-agonist (LABA, 12–24 μg of formoterol daily) and, if not effective, an add-on therapy with leukotriene receptor antagonist (LTRA, montelukast 10 mg daily) and then short-term add-on oral corticosteroids (prednisone 0.5 mg·kg−1 for 10 days) [23, 26, 33]. NAEB was treated with moderate doses of ICS (320 μg of ciclesonide or 800 μg of budesonide daily) [34]. UACS therapy consisted of intranasal corticosteroids (INCS) and add-on oral or intranasal antihistamines (in case of allergic rhinitis) [35, 36]. GER therapy included lifestyle and diet modification and PPI, if not effective – an add-on therapy with prokinetic drugs [30]. RCC was treated with speech therapy or neuromodulators and continuation of treatment of diagnosed cough-associated conditions [8]. UCC was treated with speech therapy or neuromodulators [9]. Patients with more than one cause of chronic cough were treated with therapies for all identified cough reasons [8]. Decisions on the initiation of the treatment were not dependent on BEC.
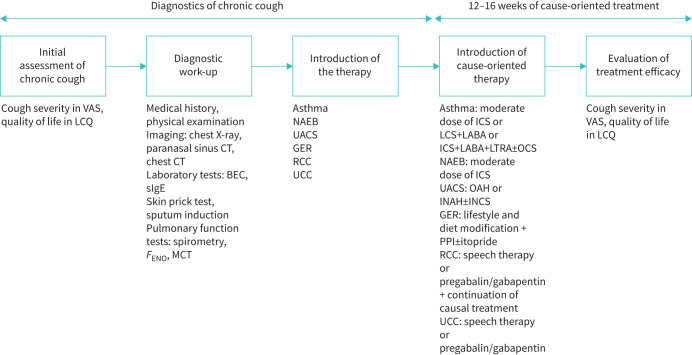
Cough severity was measured with the use of the visual analogue scale (VAS), with a score range from 0 – no cough to 100 mm – the worst possible cough [37]. Cough-related QoL was measured with Leicester Cough Questionnaire (LCQ), which is a 19-item questionnaire, assessing cough-related QoL with a total score ranging from 3 to 21 points, with the lower total LCQ score indicating higher impairment in QoL [23, 38]. Reassessment of cough severity and cough-related QoL was performed between 12 and 16 weeks after the onset of the treatment. A treatment response was defined as a decrease in cough severity by ≥30 mm on VAS and improvement ≥1.3 points in LCQ [39]. The major steps of the study protocol are presented in figure 1.
FIGURE 1.
Study design. VAS: visual analogue scale; LCQ: Leicester Cough Questionnaire; CT: computed tomography; BEC: blood eosinophil count; sIgE: specific immunoglobulin E; FENO: exhaled nitric oxide fraction; MCT: methacholine challenge test; NAEB: non-asthmatic eosinophilic bronchitis; UACS: upper airway cough syndrome; GER: gastro-oesophageal reflux; RCC: refractory chronic cough; UCC: unexplained chronic cough; ICS: inhaled corticosteroids; LABA: long-acting β2-agonist; LTRA: leukotriene receptor antagonist; OCS: oral corticosteroid; OAH: oral antihistamines; INAH: intranasal antihistamines; INCS: intranasal corticosteroids; PPI: proton pump inhibitor.
Statistical analysis
As there have been no previous studies on accuracy of BEC in predicting decrease of chronic cough after therapy, we assumed that accuracy of BEC in predicting response to therapy of chronic cough measured as receiver operating characteristic (ROC) area under the curve (AUC) is 0.6. Thus, the size of the group was calculated for 120 patients based on the study by Hajian-Tilaki [40] assuming 10% marginal error and 80% power of the study. The number of enrolled subjects was planned to be increased by 12 subjects to allow for a 10% dropout rate. Thus, a total number of 132 subjects was the minimum participants required to perform this study.
Data were presented as median and interquartile range or numbers and percentages. The statistical analysis was performed using Statistica 13.1 software package (StatSoft, Tulsa, OK, USA). The correlation analysis between BEC and the reduction in cough severity and LCQ score, FENO and induced sputum eosinophils was made using the Pearson correlation coefficient. Owing to non-normal distribution of BEC, this variable was analysed in Pearson correlation after logarithmic transformation. The ROC curve was constructed to evaluate the value of BEC as the predictor of treatment response. The AUC was calculated to find an optimal BEC cut-off for the prediction of a good response. For further analyses, the patients were classified with low and high BEC, based on the cut-off point estimated by the ROC curve. The differences between these two groups, due to differences in group size, were analysed with non-parametric tests using the Mann–Whitney U-test for continuous and χ2 test for categorical variables. The final evaluation included the subgroup analysis – asthma/NAEB, asthma/NAEB/UACS, UACS, GER and bronchial hyperresponsiveness presence (BHR+) patients with an attempt to determine a cut-off value of BEC for a prediction of treatment response. A p-value lower than 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of patients
163 non-smoking patients with chronic cough were initially enrolled; ultimately, 21 patients were excluded from the final analysis (figure 2). Thus, the proper study group included 142 patients with chronic cough; median age 57.5 years (46–67), predominantly female (103, 72.5%). Patients’ characteristics are presented in table 1. The median BEC was 148.8 cells·µL−1 (99.1–237.8), and 18 (12.7%) patients presented with BEC ≥300 cells·µL−1 (see figure 3). There was a moderate positive correlation between BEC and induced sputum eosinophils and between BEC and FENO (r=0.30, p<0.001 and r=0.25, p=0.008, respectively).
FIGURE 2.
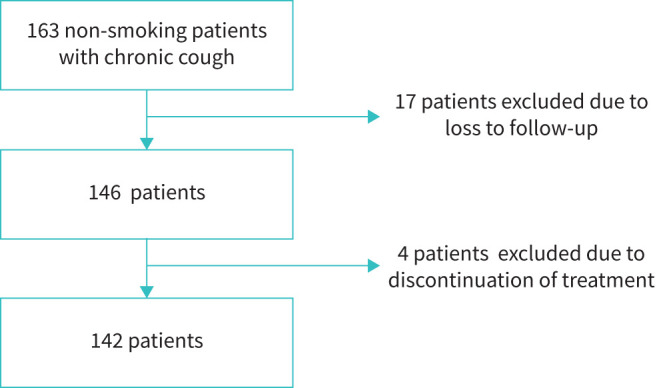
Flowchart of included groups.
TABLE 1.
Characteristics of the study population (n=142)
| Age years | 57.5 (46.0–67.0) |
| Sex, female n (%) | 103 (72.5) |
| Smoking history, ex-smokers n (%) | 41 (28.9) |
| Smoking history pack-years | 7.5 (5.0–15.0) |
| Cough duration months | 60.0 (36.0–120.0) |
| Serum total IgE concentration IU·mL−1 | 19.0 (8.0–49.0) |
| BMI kg·m−2 | 26.6 (22.4–30.8) |
| FENO ppb | 15.8 (11.6–25.1) |
| FEV1 L | 2.5 (2.1–3.0) |
| FEV1 % predicted | 94.0 (81.0–100.0) |
| VC L | 3.4 (2.7–4.2) |
| VC % predicted | 105.0 (95.0–113.0) |
| FEV1/FVC % | 75.3 (69.7–79.0) |
| FEV1/FVC (percentile) | 28.0 (11.0–52.0) |
| Bronchial hyperresponsiveness n (%)# | 48 (39.3) |
| PC20 mg·mL−1 | 1.4 (0.3–4.0) |
| Induced sputum eosinophil %¶ | 1.0 (0.0–2.0) |
| Induced sputum eosinophil count >3%, n (%)¶ | 17 (13.6) |
| Blood eosinophil count cells·µL−1 | 148.8 (99.1–237.8) |
| Blood eosinophils ≥300 cells·µL−1, n (%) | 18 (12.7) |
| Baseline LCQ score (points) | 11.1 (8.9–14.0) |
| Baseline cough severity score in VAS mm | 55.0 (37.0–76.0) |
| Atopy n (%) | 43 (30.3) |
| NAEB n (%) | 7 (4.9) |
| Asthma n (%) | 67 (47.2) |
| UACS n (%) | 65 (45.8) |
| GER n (%) | 73 (51.4) |
| Baseline RCC/UCC n (%) | 6 (4.2) |
| Only asthma n (%) | 35 (24.6) |
| Only NAEB n (%) | 1 (0.7) |
| Only UACS n (%) | 11 (7.7) |
| Only GER n (%) | 24 (16.9) |
Data are presented as median and interquartile range or numbers and percentages. n: number of patients; IgE: immunoglobulin E; BMI: body mass index; FENO: exhaled nitric oxide fraction; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; VC: vital capacity; PC20: provocative concentration of methacholine causing a 20% fall in FEV1; LCQ: Leicester Cough Questionnaire; VAS: visual analogue scale; NAEB: non-asthmatic eosinophilic bronchitis; UACS: upper airway cough syndrome; GER: gastro-oesophageal reflux; RCC: refractory chronic cough; UCC: unexplained chronic cough. #: methacholine provocation challenge was performed in 122 (85.9%) of patients. ¶: sputum was collected in 125 (88.0%) patients.
FIGURE 3.
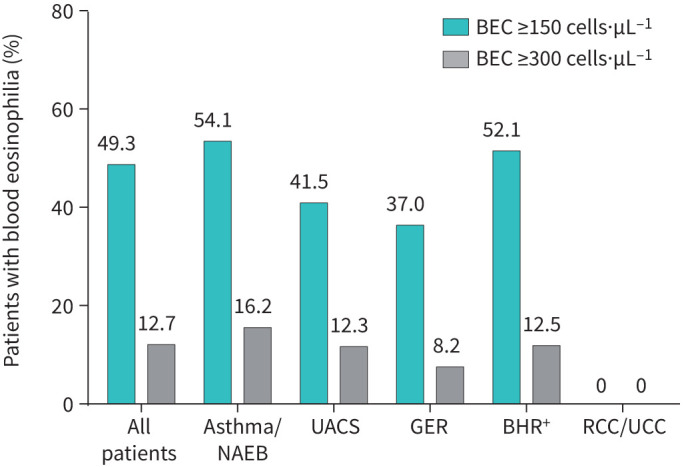
Prevalence of eosinophilia in subgroups with different causes of chronic cough. BEC: blood eosinophil count; NAEB: non-asthmatic eosinophilic bronchitis; UACS: upper airway cough syndrome; GER: gastro-oesophageal reflux; BHR+: presence of bronchial hyperresponsiveness; RCC: refractory chronic cough; UCC: unexplained chronic cough.
BEC and response to therapy in all chronic cough patients
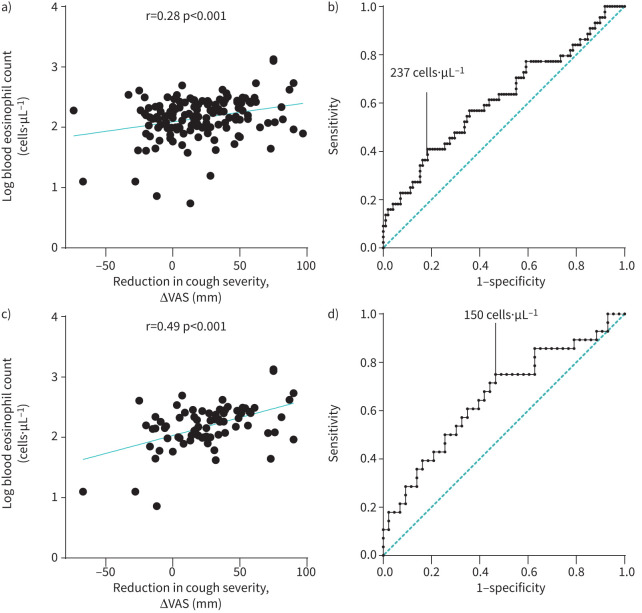
A significant reduction in cough severity following cause-oriented therapy was observed in 44 (31.0%) patients. Higher BEC and FENO were noted in patients who reported improvement (184.8 cells·µL−1 versus 141.8 cells·µL−1, p=0.026 and 18.2 ppb versus 14.7 ppb, p=0.040, respectively). Although no difference was found between the level of BHR (PC20) in patients who responded and in those who did not respond to treatment, the prevalence of BHR was significantly higher in those with observed clinical improvement (55.3% versus 32.1%, p=0.015, table 2). Next, in patients who did not respond to causal treatment, the prevalence of GER was significantly higher (57.1% versus 38.6%, p=0.041, table 2). A weak positive correlation between the decrease in cough severity (dVAS) and BEC was shown (r=0.28, p<0.001, see figure 4a).
TABLE 2.
Comparison of patients with good and unsatisfactory therapeutic response
| Patients without improvement in cough severity (dVAS <30 mm or LCQ <1.3 points) | Patients with decrease in cough severity (dVAS ≥30 mm and LCQ ≥1.3 points) | p-value | |
| Subjects n (%) | 98 (69.0) | 44 (31.0) | |
| Age years | 56.0 (45.0–67.0) | 61.0 (47.0–67.0) | 0.526 |
| Sex, female n (%) | 69 (70.4) | 34 (77.3) | 0.398 |
| Cough duration months | 60.0 (34.0–120.0) | 60.0 (36.0–120.0) | 0.949 |
| Serum total IgE concentration IU·mL−1 | 16.0 (7.0–45.0) | 25.0 (10.0–52.0) | 0.265 |
| BMI kg·m−2 | 26.9 (23.0–30.8) | 25.7 (22.2–31.0) | 0.241 |
| FENO ppb | 14.7 (10.9–24.6) | 18.2 (15.2–26.7) | 0.040 |
| FEV1 L | 2.5 (2.2–3.2) | 2.4 (1.8–2.7) | 0.099 |
| FEV1 % predicted | 94.0 (81.0–101.0) | 93.0 (82.0–99.0) | 0.647 |
| VC L | 3.5 (2.8–4.5) | 3.2 (2.5–4.0) | 0.234 |
| VC % predicted | 106.0 (96.0–114.0) | 101.5 (93.0–111.0) | 0.211 |
| FEV1/FVC % | 74.9 (70.0–78.6) | 76.5 (68.5–79.0) | 0.956 |
| FEV1/FVC (percentile) | 25.0 (11.0–52.0) | 38.5 (11.0–52.0) | 0.891 |
| Presence of bronchial hyperresponsiveness n (%)# | 27 (32.1) | 21 (55.3) | 0.015 |
| PC20 mg·mL−1# | 1.4 (0.3–3.7) | 1.0 (0.4–4.3) | 0.958 |
| Induced sputum eosinophil %¶ | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 0.630 |
| Induced sputum eosinophil count >3% n (%)¶ | 73 (85.9) | 35 (87.5) | 0.806 |
| Blood eosinophil count cells·µL−1 | 141.8 (97.9–216.5) | 184.8 (118.1–278.8) | 0.026 |
| Blood eosinophils ≥300 cells·µL−1, n (%) | 90 (91.8) | 34 (77.3) | 0.016 |
| Baseline LCQ score (points) | 11.7 (9.6–14.9) | 10.1 (8.1–12.9) | 0.009 |
| Baseline cough severity in VAS mm | 45.5 (30.0–70.0) | 75.0 (57.5–84.5) | <0.001 |
| Post treatment LCQ score (points) | 12.5 (10.0–15.7) | 16.9 (14.0–19.0) | <0.001 |
| Post treatment VAS mm | 40.5 (20.0–68.0) | 15.0 (5.5–27.5) | <0.001 |
| Change in LCQ score (points) | 1.0 (−0.5–3.3) | 5.6 (3.2–7.4) | <0.001 |
| Change in cough severity VAS mm | 4.5 (−10.0–18.0) | 50.0 (40.0–68.0) | <0.001 |
| Atopy n (%) | 29 (29.6) | 14 (31.8) | 0.789 |
| Asthma or NAEB n (%) | 46 (46.9) | 28 (63.6) | 0.065 |
| UACS n (%) | 44 (44.9) | 21 (47.7) | 0.754 |
| GER n (%) | 56 (57.1) | 17 (38.6) | 0.041 |
| Baseline RCC/UCC n (%) | 5 (5.1) | 1 (1.0) | 0.899 |
Data are presented as median and interquartile range or numbers and percentages. n: number of patients; IgE: immunoglobulin E; dVAS: decrease in cough severity; BMI: body mass index; FENO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; VC: vital capacity; PC20: provocative concentration of methacholine causing a 20% fall in FEV1; LCQ: Leicester Cough Questionnaire; VAS: visual analogue scale; NAEB: non-asthmatic eosinophilic bronchitis; UACS: upper airway cough syndrome; GER: gastro-oesophageal reflux; RCC: refractory chronic cough; UCC: unexplained chronic cough. #: methacholine provocation challenge was performed in 122 (85.9%) of patients. ¶: sputum was collected in 125 (88.0%) patients.
FIGURE 4.
a) Correlation between reduction in cough severity and blood eosinophil count in all patients with chronic cough. b) The receiver operating characteristic curve with analysis of blood eosinophil count cut-off for prediction of a treatment response in all patients with chronic cough. c) Correlation between reduction in cough severity and blood eosinophil count in patients with asthma or NAEB. d) The receiver operating characteristic curve with analysis of blood eosinophil count cut-off for prediction of a treatment response in patients with asthma or NAEB. NAEB: non-asthmatic eosinophilic bronchitis; VAS: visual analogue scale.
The area under ROC curve for the performance of BEC in all patients was 0.62 (95% CI 0.51–0.72). The optimal BEC cut-off for the prediction of good treatment response was 237 cells·µL−1 (table 3, figure 4b). Comparison of groups with lower (<237 cells·µL−1) versus higher (≥237 cells·µL−1) BEC showed longer cough duration (69 months (36–138) versus 48 months (24–84), p=0.018) and less severe cough (53 mm (35.5–74) versus 70 mm (44–80), p=0.033) in the low-BEC group but did not reveal any differences in demographic (age, sex, body mass index) and clinical data (cough-related QoL, atopy, induced sputum eosinophil percentage, FENO, BHR severity). Moreover, in the low-BEC group the prevalence of GER was significantly higher (59 (56.7%) versus 14 (36.8%), p=0.036).
TABLE 3.
Diagnostic accuracy of blood eosinophil count in prediction of a good treatment response in patients with chronic cough
| Youden J index | AUC | 95% CI | Cut-off BEC threshold cells·µL−1 | p-value | Sensitivity | Specificity | PPV | NPV | dACC | |
| All patients | 0.23 | 0.62 | 0.51–0.72 | 237 | 0.026 | 0.41 | 0.82 | 0.50 | 0.75 | 0.69 |
| Patients with asthma or NAEB | ||||||||||
| Asthma/NAEB | 0.34 | 0.68 | 0.55–0.81 | 150 | 0.005 | 0.75 | 0.59 | 0.53 | 0.79 | 0.65 |
| Asthma/NAEB/UACS | 0.25 | 0.63 | 0.52–0.74 | 171 | 0.019 | 0.58 | 0.68 | 0.49 | 0.75 | 0.64 |
| ICS/ICS+LABA therapy | 0.37 | 0.69 | 0.56–0.82 | 150 | 0.005 | 0.74 | 0.63 | 0.57 | 0.78 | 0.67 |
| ICS/ICS+LABA/ICS+LABA+LTRA therapy | 0.34 | 0.68 | 0.55–0.81 | 150 | 0.005 | 0.75 | 0.59 | 0.53 | 0.79 | 0.65 |
| Patients with other causes of chronic cough | ||||||||||
| UACS | 0.33 | 0.64 | 0.49–0.80 | 319 | 0.070 | |||||
| GER | 0.17 | 0.53 | 0.37–0.69 | 259 | 0.680 | |||||
| BHR+# | 0.26 | 0.58 | 0.41–0.74 | 150 | 0.378 | |||||
CI: confidence interval; AUC: area under the receiver operating curve; BEC: blood eosinophil count; PPV: positive predictive value; NPV: negative predictive value; dACC: diagnostic accuracy; NAEB: non-asthmatic eosinophilic bronchitis; UACS: upper airway cough syndrome; GER: gastro-oesophageal reflux; BHR+: presence of bronchial hyperresponsiveness; ICS: inhaled corticosteroids; LABA: long-acting β2-agonist; LTRA: leukotriene receptor antagonist. #: methacholine provocation challenge was performed in 122 (85.9%) patients.
BEC and treatment effects in patients with asthma/NAEB
Among patients with asthma or NAEB 28 of 74 (37.8%) individuals showed improvement after treatment. There was a moderate correlation between BEC and dVAS (r=0.49, p<0.001, figure 4c). The analysis of BEC as a surrogate biomarker of eosinophilic airway inflammation in patients with asthma/NAEB showed slightly stronger correlations than in the general chronic cough group (BEC and FENO r=0.29, p=0.02 and BEC and induced sputum eosinophil percentage r=0.36, p=0.003).
The AUC ROC for prediction of favourable response in asthma/NAEB was 0.68 (95% CI 0.55–0.81) with the cut-off for BEC at 150 cells·µL−1 (table 3, figure 4d). The subanalysis of individuals who responded to ICS or ICS+LABA revealed higher accuracy, with AUC ROC 0.69 (95% CI 0.56–0.82) with the cut-off for BEC at 150 cells·µL−1 (table 3). Diagnostic accuracy of BEC in predicting response to anti-cough therapy was similar for all patients, patients with asthma/NAEB and for patients with asthma/NAEB/UACS (table 3).
BEC and treatment effects in patients with other causes of chronic cough
Positive correlations were found for BEC and FENO and BEC and induced sputum eosinophil percentage in the subgroup with UACS (r=0.28, p=0.04; r=0.39, p=0.003, respectively); however, there was no correlation for patients with GER. Moreover, the improvement after treatment (dVAS) moderately correlated with BEC in UACS patients (r=0.31, p=0.01). In contrast, no specific cut-offs were established either for GER, UACS or for BHR+ (table 3).
Discussion
Our study implies that BEC is a poor predictor of clinical response to antitussive treatment in adults with chronic cough. Surprisingly, an elevated baseline BEC was found in only 12.7% of these patients. Using the optimal cut-off level for the entire study group (237 cells·µL−1), BEC was found to have a modestly high specificity (0.82) but low sensitivity (0.41). Even though the subanalysis of patients treated with ICS or ICS+LABA showed a lower cut-off level (150 cells·µL−1) with a higher AUC value, the sensitivity and specificity were still unsatisfactory. Almost a third of our group (31.0%) responded to therapy, and responders were characterised by higher BEC, FENO and more prevalent BHR. Additionally, in non-responders, GER was diagnosed more frequently. Although the results of our study are not encouraging in terms of the application of BEC as a predictor of therapeutic response in unselected patients with chronic cough treated in cough centres, to the best of our knowledge, it is the first study reporting this issue. Thus, we believe this study may fill the gap in knowledge on the treatable traits in the management of chronic cough.
Regardless of the progress in the knowledge on the pathophysiology and management of chronic cough, the therapeutic effects are still disappointing. According to previous studies, cough persists in up to 46% of patients, despite thorough clinical assessment and the application of cause-oriented treatment [8, 41]. The complexity of the diagnostics and specific therapies is further augmented by the coexistence of different causes of chronic cough in the same patient. Previous studies showed co-occurrence of multiple chronic cough causes in up to 72% of patients [41]. Unsatisfactory results of chronic cough treatment may also result from hypersensitivity of the cough reflex, which is a common pathomechanism of RCC. In these patients other treatment modalities should be implemented – primarily speech and language intervention or treatment with neuromodulators. The complexity of chronic cough management requires a multidisciplinary approach and a high level of expertise. This also generates a high economic burden for healthcare systems. In the era of personalised medicine, the need to identify specific biomarkers which could facilitate diagnostics and treatment is emphasised not only in asthma or COPD but also in chronic cough [42]. A therapeutic strategy based on “treatable traits” is currently a recognised paradigm for different respiratory diseases. Treatable traits are phenotypic features or biomarkers that enable the implementation of precision treatment. Eosinophilic airway inflammation is a well-recognised treatable trait in chronic airway diseases [43]. Owing to limited access to markers of eosinophilic airway inflammation, blood eosinophilia is used as a surrogate marker for airway eosinophilic inflammation in both asthma and COPD [21].
The recent ERS and ACCP guidelines on the management of chronic cough highlighted the necessity of the assessment of airway and systemic eosinophilic inflammation [1, 44]. However, to our knowledge the significance of BEC as a predictor of treatment effectiveness in patients with chronic cough has not been previously evaluated. We assumed that it might be useful in the management of patients with chronic cough related to airway eosinophilic inflammation. A T2 inflammatory pattern is certainly responsible for the pathogenesis of some causes of chronic cough, such as asthma, NAEB, allergic rhinitis or chronic rhinosinusitis [15]. On the other hand, the role of BEC in patients with chronic cough associated with other underlying diseases seemed to be uncertain. Furthermore, the coexistence of different cough-associated conditions is common. Therefore, we decided to analyse BEC as a predictor of response to therapy in unselected patients with chronic cough and normal chest radiograph referred to our cough clinic.
Previous studies showed that airway eosinophilia is a frequent feature of chronic cough, accounting for up to 25–50% of patients, with a mean induced sputum eosinophil percentage of 3% [1, 13, 14]. The above data are inconsistent with our results demonstrating sputum eosinophilia in only 13.6% of patients. This may be at least in part attributed to the investigated population, i.e. patients with chronic cough referred for management to a tertiary cough centre. Irrespective of the differences between the prevalence of induced sputum eosinophilia in patients with chronic cough, limited access to induced sputum cytology imposes a search for surrogate biomarkers, including BEC [45]. However, Hastie et al. [46] showed that diagnostic accuracy of BEC in prediction of eosinophilic phenotype of asthma is as low as 0.63. The other accessible and noninvasive tool for the measurement of airway eosinophilic inflammation is FENO. Despite the low prevalence of airway eosinophilic inflammation in this study, significant correlations between BEC and FENO as well as BEC and induced sputum eosinophil count were found for all patients, as well as the subgroups with asthma and UACS but not GER. These findings are consistent with those reported by Sadeghi et al. [47], who showed a moderate correlation between induced sputum eosinophils, FENO and BEC in chronic cough patients. Despite numerous previous studies on the utility of FENO in the prediction of asthma or NAEB in chronic cough patients, no strong evidence for its usefulness as a predictor of response to corticosteroids in chronic cough patients was found [48].
The relationship between blood eosinophils and the efficacy of asthma treatment was confirmed in previous studies [49]. It has been shown that eosinophil-targeted therapy is effective in the asthmatic population with BEC even as low as 150 cells·µL−1, which is consistent with our results [19]. Although the previous studies did not confirm the efficacy of biologic therapies targeting eosinophils in reduction of cough in asthmatics [50], a new randomised trial concerning impact of mepolizumab on chronic cough in asthma is planned (NCT04765722). Besides, the recent cohort trial showed that mepolizumab is effective in reduction of cough in patients with asthma [51].
Although we found a positive correlation between response to therapy and BEC among asthmatics and NAEB patients, the clinical importance of this finding is questionable as this correlation was weak. This weak association between improvement after treatment and BEC could be caused by a low proportion of patients with blood eosinophilia in our study population. As expected, we demonstrated that BEC is not useful in predicting therapeutic response in chronic cough associated with GER. Furthermore, we found that GER was more common in patients who failed to respond to chronic cough therapy. As demonstrated previously, GER may be considered as an unfavourable factor in chronic cough treatment [52]. The low efficacy of GER-related cough therapy is thought to be affected by the lack of available causal treatment options and the coexistence of cough hypersensitivity syndrome [53, 54].
Our finding that the significant reduction in cough severity was achieved in ∼31% of patients suggests low treatment efficacy, and it is consistent with previous studies [41]. Recent reports emphasise the role of cough hypersensitivity syndrome as the important reason for this failure [10]. It has been pointed out that the causes of chronic cough, including asthma, NAEB, GER or UACS, could be only the triggers for cough and treatment of underlying conditions may be insufficient for cough resolution [54]. Thus, irrespective of the identified chronic cough trigger, the management of patients with refractory chronic cough should include speech and language intervention and neuromodulators or new antitussive drugs [8, 55].
We are aware of several limitations in our study. Firstly, this was a single-centre analysis with a limited number of patients. Certainly, the design of this non-controlled study diminishes the power of the results. However, the study design is due to the fact that this analysis was part of a larger real-life study on the causes and efficacy of treatment of chronic cough which has been running since 2009. Thus, confirmation of these results requires the design of a randomised controlled trial. Secondly, blood cell count was evaluated in two blood samples, but only the higher value for each patient was included in the analysis. This seems to be an unresolved issue in different studies with no consensus, to date [56–58]. The third limitation could be a potential selection bias associated with the recruitment of patients in the tertiary cough clinic in a university hospital (a reference centre). This may have increased the proportion of patients with difficult-to-treat chronic cough, characterised with severe, long-lasting chronic cough, with a history of therapy failure, which could also explain the low prevalence of BEC in this group. Next, a cough monitoring system was not available in our cough clinic, so the cough severity assessment was based on VAS and LCQ. Although VAS has not been validated in cough severity assessment and no minimal clinically important difference has been established in chronic cough so far, VAS is commonly used in clinical practice and clinical trials. The 30-mm cut-off point in VAS was set arbitrarily, but the same cut-off has been proposed recently in COUGH-1 and COUGH-2 trials [39]. Lastly, as the study was a non-experimental, real-life, cohort study, the treatment applied to patients differed in some aspects (i.e. different types of inhalers and different doses of drugs), which certainly could affect the treatment efficacy. Despite all these limitations, we believe that the results of this study indicating a low value of BEC as a prognostic marker could fill the gap in knowledge on the treatable traits in the management of unselected patients with chronic cough.
In conclusion, we found that owing to its low accuracy, BEC was a weak predictor of favourable response to therapy in adults with chronic cough treated in the tertiary cough centre. Moreover, an elevated BEC was rather uncommon in the investigated population. Therefore, despite the availability of BEC, its clinical utility in management of chronic cough appears to be limited.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
FIGURE S1 Diagnostic stepwise protocol for patients with chronic cough. CXR, chest X-ray; ACE-I, angiotensin-converting enzyme inhibitors; LC, lung cancer; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; MCT, methacholine challenge test; ENT, otorhinolaryngology; RCC, refractory chronic cough; UACS, upper airway cough syndrome; GER, gastroesophageal reflux, PSG, polysomnography, ECG, electrocardiogram; CC, chronic cough; UCC, unexplained chronic cough. 00432-2021.figureS1 (58.1KB, pdf)
FIGURE S2 General treatment algorithm in patients with chronic cough. ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LTRA, leukotriene receptor antagonist; PPI, proton pump inhibitors; INCS, intranasal corticosteroids.00432-2021.figureS2 (21.6KB, pdf)
Acknowledgements
The authors thank Marta Maskey-Warzechowska, MD, PhD, Katarzyna Mycroft, MD from Department of Internal Medicine, Pulmonary Diseases and Allergy, Medical University of Warsaw, Poland and Aleksandra Szubert-Franczak, MD from Radiology Department 1, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland for language editing and proofreading of the final version of the manuscript.
Footnotes
Provenance: Submitted article, peer reviewed.
This article has supplementary material available from openres.ersjournals.com
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Author contributions: A. Rybka-Fraczek and M. Dabrowska had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analyses; A. Rybka-Fraczek, M. Dabrowska, E.M. Grabczak, K. Bialek-Gosk, K. Klimowicz, O. Truba and R. Krenke contributed to the study concept and design; A. Rybka-Fraczek, M. Dabrowska, E.M. Grabczak, K. Bialek-Gosk, K. Klimowicz, O. Truba, P. Nejman-Gryz and M. Paplinska-Goryca collected, analysed or interpreted the data; A. Rybka-Fraczek, M. Dabrowska and R. Krenke wrote the draft manuscript and did the statistical analyses; and all authors revised the final manuscript for important intellectual content.
Conflict of interest: A. Rybka-Fraczek reports personal fees from Polpharma outside the submitted work.
Conflict of interest: M. Dabrowska reports personal fees from Merck outside the submitted work.
Conflict of interest: E.M. Grabczak reports personal fees from Polpharma and Merck outside the submitted work.
Conflict of interest: K. Bialek-Gosk has nothing to disclose.
Conflict of interest: K. Klimowicz has nothing to disclose.
Conflict of interest: O. Truba has nothing to disclose.
Conflict of interest: P. Nejman-Gryz has nothing to disclose.
Conflict of interest: M. Paplinska-Goryca has nothing to disclose.
Conflict of interest: R. Krenke reports a grant from the National Science Centre, Poland (grant number 2012/05/B/NZ5/01343), during the conduct of the study; and travel expenses and fees for attendance of the European Respiratory Society International Congress (2018 and 2019) from Boehringer Ingelheim, grants, travel expenses and fees for attendance of the 2019 American Thoracic Society (ATS) Conference, and a fee for lectures from Chiesi, grants, travel expenses and fees for attendance of the 2018 ATS Conference, and a fee for lectures from AstraZeneca, and a fee for lectures from Polpharma, outside the submitted work.
References
- 1.Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020; 55: 1901136. doi: 10.1183/13993003.01136-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colak Y, Nordestgaard BG, Laursen LC, et al. Risk factors for chronic cough among 14,669 individuals from the general population. Chest 2017; 152: 563–573. doi: 10.1016/j.chest.2017.05.038 [DOI] [PubMed] [Google Scholar]
- 3.Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015; 45: 1479–1481. doi: 10.1183/09031936.00218714 [DOI] [PubMed] [Google Scholar]
- 4.Calverley PM. Cough in chronic obstructive pulmonary disease: is it important and what are the effects of treatment? Cough 2013; 9: 17. doi: 10.1186/1745-9974-9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madison JM, Irwin RS. Chronic cough and COPD. Chest 2020; 157: 1399–1400. doi: 10.1016/j.chest.2020.02.012 [DOI] [PubMed] [Google Scholar]
- 6.Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003; 361: 449–456. doi: 10.1016/S0140-6736(03)12459-2 [DOI] [PubMed] [Google Scholar]
- 7.Irwin RS, French CL, Chang AB, et al. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest 2018; 153: 196–209. doi: 10.1016/j.chest.2017.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest 2016; 149: 27–44. doi: 10.1378/chest.15-1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson PG, Vertigan AE. Management of chronic refractory cough. BMJ 2015; 351: h5590. doi: 10.1136/bmj.h5590 [DOI] [PubMed] [Google Scholar]
- 10.Morice AH, Millqvist E, Belvisi MG, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 2014; 44: 1132–1148. doi: 10.1183/09031936.00218613 [DOI] [PubMed] [Google Scholar]
- 11.Song WJ, Chung KF. Exploring the clinical relevance of cough hypersensitivity syndrome. Expert Rev Respir Med 2020; 14: 275–284. doi: 10.1080/17476348.2020.1713102 [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Driessen AK, McGovern AE, et al. Peripheral and central mechanisms of cough hypersensitivity. J Thorac Dis 2020; 12: 5179–5193. doi: 10.21037/jtd-2020-icc-007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niimi A, Matsumoto H, Mishima M. Eosinophilic airway disorders associated with chronic cough. Pulm Pharmacol Ther 2009; 22: 114–120. doi: 10.1016/j.pupt.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 14.Carney IK, Gibson PG, Murree-Allen K, et al. A systematic evaluation of mechanisms in chronic cough. Am J Respir Crit Care Med 1997; 156: 211–216. doi: 10.1164/ajrccm.156.1.9605044 [DOI] [PubMed] [Google Scholar]
- 15.Diver S, Russell RJ, Brightling CE. Cough and eosinophilia. J Allergy Clin Immunol Pract 2019; 7: 1740–1747. doi: 10.1016/j.jaip.2019.04.048 [DOI] [PubMed] [Google Scholar]
- 16.Drake MG, Scott GD, Blum ED, et al. Eosinophils increase airway sensory nerve density in mice and in human asthma. Sci Transl Med 2018; 10: eaar8477. doi: 10.1126/scitranslmed.aar8477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro CO, Proskocil BJ, Oppegard LJ, et al. Airway sensory nerve density is increased in chronic cough. Am J Respir Crit Care Med 2021; 203: 348–355. doi: 10.1164/rccm.201912-2347OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satia I, Watson R, Scime T, et al. Allergen challenge increases capsaicin-evoked cough responses in patients with allergic asthma. J Allergy Clin Immunol 2019; 144: 788–795.e1. doi: 10.1016/j.jaci.2018.11.050 [DOI] [PubMed] [Google Scholar]
- 19.Pavord ID, Holliday M, Reddel HK, et al. Predictive value of blood eosinophils and exhaled nitric oxide in adults with mild asthma: a prespecified subgroup analysis of an open-label, parallel-group, randomised controlled trial. Lancet Respir Med 2020; 8: 671–680. doi: 10.1016/S2213-2600(20)30053-9 [DOI] [PubMed] [Google Scholar]
- 20.Korevaar DA, Westerhof GA, Wang J, et al. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: a systematic review and meta-analysis. Lancet Respir Med 2015; 3: 290–300. doi: 10.1016/S2213-2600(15)00050-8 [DOI] [PubMed] [Google Scholar]
- 21.Mycroft K, Krenke R, Gorska K. Eosinophils in COPD-current concepts and clinical implications. J Allergy Clin Immunol Pract 2020; 8: 2565–2574. doi: 10.1016/j.jaip.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 22.Morice AH, McGarvey L, Pavord I. , et al. Recommendations for the management of cough in adults. Thorax 2006; 61: Suppl. 1, i1–24. doi: 10.1136/thx.2006.065144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J 2007; 29: 1256–1276. doi: 10.1183/09031936.00101006 [DOI] [PubMed] [Google Scholar]
- 24.Pratter MR. Overview of common causes of chronic cough: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: 59S–62S. doi: 10.1378/chest.129.1_suppl.59S [DOI] [PubMed] [Google Scholar]
- 25.Spanevello A, Confalonieri M, Sulotto F, et al. Induced sputum cellularity. Reference values and distribution in normal volunteers. Am J Respir Crit Care Med 2000; 162: 1172–1174. doi: 10.1164/ajrccm.162.3.9908057 [DOI] [PubMed] [Google Scholar]
- 26.Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. 2015. Available from: http://ginasthma.org/
- 27.Johansson SG, Hourihane JO, Bousquet J, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2001; 56: 813–824. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x [DOI] [PubMed] [Google Scholar]
- 28.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing – 1999. Am J Respir Crit Care Med 2000; 161: 309–329. doi: 10.1164/ajrccm.161.1.ats11-99 [DOI] [PubMed] [Google Scholar]
- 29.Pratter MR. Chronic upper airway cough syndrome secondary to rhinosinus diseases (previously referred to as postnasal drip syndrome): ACCP evidence-based clinical practice guidelines. Chest 2006; 129: Suppl. 1, 63S–71S. doi: 10.1378/chest.129.1_suppl.63S [DOI] [PubMed] [Google Scholar]
- 30.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101: 1900–1920. quiz 1943. doi: 10.1111/j.1572-0241.2006.00630.x [DOI] [PubMed] [Google Scholar]
- 31.Irwin RS. Chronic cough due to gastroesophageal reflux disease: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: Suppl. 1, 80s–94s. doi: 10.1378/chest.129.1_suppl.80S [DOI] [PubMed] [Google Scholar]
- 32.Shay S, Tutuian R, Sifrim D, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol 2004; 99: 1037–1043. doi: 10.1111/j.1572-0241.2004.04172.x [DOI] [PubMed] [Google Scholar]
- 33.Dicpinigaitis PV. Chronic cough due to asthma: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: Suppl. 1, 75s–79s. doi: 10.1378/chest.129.1_suppl.75S [DOI] [PubMed] [Google Scholar]
- 34.Brightling CE. Chronic cough due to nonasthmatic eosinophilic bronchitis: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: Suppl. 1, 116S–121S. doi: 10.1378/chest.129.1_suppl.116S [DOI] [PubMed] [Google Scholar]
- 35.Brozek JL, Bousquet J, Agache I, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines – 2016 revision. J Allergy Clin Immunol 2017; 140: 950–958. doi: 10.1016/j.jaci.2017.03.050 [DOI] [PubMed] [Google Scholar]
- 36.Hellings PW, Klimek L, Cingi C, et al. Non-allergic rhinitis: Position paper of the European Academy of Allergy and Clinical Immunology. Allergy 2017; 72: 1657–1665. doi: 10.1111/all.13200 [DOI] [PubMed] [Google Scholar]
- 37.Spinou A, Birring SS. An update on measurement and monitoring of cough: what are the important study endpoints? J Thorac Dis 2014; 6: Suppl. 7, S728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003; 58: 339–343. doi: 10.1136/thorax.58.4.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muccino DR, Morice AH, Birring SS, et al. Design and rationale of two phase 3 randomised controlled trials (COUGH-1 and COUGH-2) of gefapixant, a P2X3 receptor antagonist, in refractory or unexplained chronic cough. ERJ Open Res 2020; 6: 00284–2020. doi: 10.1183/23120541.00284-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform 2014; 48: 193–204. doi: 10.1016/j.jbi.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 41.Dabrowska M, Grabczak EM, Arcimowicz M, et al. Chronic cough – assessment of treatment efficacy based on two questionnaires. Arch Med Sci 2014; 10: 962–969. doi: 10.5114/aoms.2014.40642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016; 47: 410–419. doi: 10.1183/13993003.01359-2015 [DOI] [PubMed] [Google Scholar]
- 43.Hiles SA, Gibson PG, Agusti A, et al. Treatable traits that predict health status and treatment response in airway disease. J Allergy Clin Immunol Pract 2021; 9: 1255–1264.e2. doi: 10.1016/j.jaip.2020.09.046 [DOI] [PubMed] [Google Scholar]
- 44.Cote A, Russell RJ, Boulet LP, et al. Managing chronic cough due to asthma and NAEB in adults and adolescents: CHEST guideline and expert panel report. Chest 2020; 158: 68–96. doi: 10.1016/j.chest.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 45.Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. 2021. Available from: http://ginasthma.org/
- 46.Hastie AT, Moore WC, Li H, et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol 2013; 132: 72–80. doi: 10.1016/j.jaci.2013.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadeghi MH, Wright CE, Hart S, et al. Does FeNO predict clinical characteristics in chronic cough? Lung 2018; 196: 59–64. doi: 10.1007/s00408-017-0074-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song WJ, Won HK, Moon SD, et al. Could fractional exhaled nitric oxide test be useful in predicting inhaled corticosteroid responsiveness in chronic cough? A systematic review. J Allergy Clin Immunol Pract 2017; 5: 135–143.e1. doi: 10.1016/j.jaip.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 49.Nair P, O'Byrne PM. Measuring eosinophils to make treatment decisions in asthma. Chest 2016; 150: 485–487. doi: 10.1016/j.chest.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 50.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 2009; 360: 973–984. doi: 10.1056/NEJMoa0808991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faruqi S, Sykes DL, Crooks MG, et al. Objective assessment of cough: an early marker of response to biological therapies in asthma? Lung 2020; 198: 767–770. doi: 10.1007/s00408-020-00391-w [DOI] [PubMed] [Google Scholar]
- 52.Latti AM, Pekkanen J, Koskela HO. Persistence of chronic cough in a community-based population. ERJ Open Res 2020; 6: 00229–02019. doi: 10.1183/23120541.00229-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kahrilas PJ, Altman KW, Chang AB, et al. Chronic cough due to gastroesophageal reflux in adults: CHEST guideline and expert panel report. Chest 2016; 150: 1341–1360. doi: 10.1016/j.chest.2016.08.1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song WJ, Morice AH. Cough hypersensitivity syndrome: a few more steps forward. Allergy Asthma Immunol Res 2017; 9: 394–402. doi: 10.4168/aair.2017.9.5.394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith JA, Kitt MM, Butera P, et al. Gefapixant in two randomised dose-escalation studies in chronic cough. Eur Respir J 2020; 55: 1901615. doi: 10.1183/13993003.01615-2019 [DOI] [PubMed] [Google Scholar]
- 56.Gibson PG. Variability of blood eosinophils as a biomarker in asthma and COPD. Respirology 2018; 23: 12–13. doi: 10.1111/resp.13200 [DOI] [PubMed] [Google Scholar]
- 57.Spector SL, Tan RA. Is a single blood eosinophil count a reliable marker for ‘eosinophilic asthma?’ J Asthma 2012; 49: 807–810. doi: 10.3109/02770903.2012.713428 [DOI] [PubMed] [Google Scholar]
- 58.Katz LE, Gleich GJ, Hartley BF, et al. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc 2014; 11: 531–536. doi: 10.1513/AnnalsATS.201310-354OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
FIGURE S1 Diagnostic stepwise protocol for patients with chronic cough. CXR, chest X-ray; ACE-I, angiotensin-converting enzyme inhibitors; LC, lung cancer; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; MCT, methacholine challenge test; ENT, otorhinolaryngology; RCC, refractory chronic cough; UACS, upper airway cough syndrome; GER, gastroesophageal reflux, PSG, polysomnography, ECG, electrocardiogram; CC, chronic cough; UCC, unexplained chronic cough. 00432-2021.figureS1 (58.1KB, pdf)
FIGURE S2 General treatment algorithm in patients with chronic cough. ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LTRA, leukotriene receptor antagonist; PPI, proton pump inhibitors; INCS, intranasal corticosteroids.00432-2021.figureS2 (21.6KB, pdf)