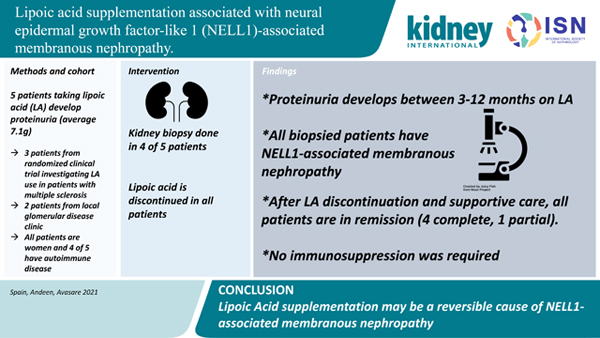
Abstract
Lipoic acid (alpha lipoic acid, thioctic acid) is a popular over-the-counter antioxidant and insulin-mimetic supplement under investigation in a variety of conditions including multiple sclerosis, diabetes, and schizophrenia. Unfortunately, high-grade proteinuria was an unexpected adverse event specific to the treatment arm of our clinical trial investigating lipoic acid supplementation in patients with multiple sclerosis. This observation led to detection of similar patients in our nephrology practice. Here, we describe four biopsy-proven cases of neural epidermal growth factor-like 1 (NELL1)-associated membranous nephropathy following lipoic acid supplementation and a fifth suspected case. Discontinuation of lipoic acid and supportive therapy resulted in remission.
Keywords: membranous nephropathy, albuminuria, nephrotic syndrome, proteinuria, glomerulonephritis, renal biopsy, kidney biopsy, renal pathology
Graphical Abstract
Introduction
Lipoic acid (LA, alpha lipoic acid, thioctic acid) is a popular over-the-counter (OTC) antioxidant and insulin-mimetic supplement under investigation in a variety of conditions including multiple sclerosis (MS), diabetes, and schizophrenia (clinicaltrials.gov). In our 2 clinical trials of LA for MS, we unexpectedly encountered proteinuria in the active treatment arms. There is one previous report of neural epidermal growth factor-like 1 (NELL1)-associated membranous nephropathy (MN) in a kidney transplant recipient taking LA.1 Our data strengthens the case for LA supplementation as a reversible cause of NELL1-associated MN (NELL1-MN).
Methods
Randomized Controlled Trials (RCT) of LA in progressive MS, Patients 1–3
Patient 1 participated in the completed pilot trial (n=51, NCT01188811).2 Patients 2 and 3 participated in the ongoing multi-site Phase IIb trial (n=118, NCT03161028) (protocols available at https://clinicaltrials.gov/). Both 2-year studies enrolled adults with progressive MS and excluded Grade 2 or higher (CTCAE v4 and v5, respectively) baseline laboratory values. The ongoing multi-site trial excluded patients with pre-existing kidney disease and added urine protein to safety laboratory monitoring. Both trials randomized patients 1:1 to racemic LA 1200mg daily versus placebo. Both studies were under DSMB oversight and reported to the FDA under an investigational new drug application.
Oregon Health & Science University (OHSU) Glomerular Disease Clinic, Patients 4–5
Two clinic patients taking LA with anti-phospholipase A2 receptor (PLA2R) antibody-negative MN had biopsies reviewed for secondary MN antigens, prompting re-review of the RCT kidney specimens.
Pathological evaluation
Kidney biopsies had been performed for clinical indication of proteinuria and evaluated by standard renal pathologic techniques: light microscopy with Jones methenamine silver, Periodic acid Schiff, trichrome, and H&E stains, immunofluorescence microscopy on frozen tissue (antibodies against IgG, IgA, IgM, kappa and lambda light chains, C3, C1q, and fibrin/fibrinogen), electron microscopy, and stains for IgG subclasses and glomerular antigens implicated in MN including PLA2R (Sigma HPA012657) and Nell1 (Sigma HPA051535) as available.
Results
Demographic, clinical, and pathologic results are summarized in Table 1, Figures 1 and 2, and Supplementary Figure 1.
Table 1:
Clinicopathologic features of patients taking lipoic acid with confirmed and suspected NELL1-associated membranous nephropathy
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Clinical findings at presentation | |||||
| Age, sex, race | 56F, Asian, non-Hispanic | 74F, White, non-Hispanic | 66F, White, non-Hispanic | 41F, White, non-Hispanic | 71F, White, non-Hispanic |
| Setting of proteinuria | Pilot Phase 2 RCT (NCT01188811) | Multi-site Phase IIb RCT (NCT03161028) | Multi-site Phase IIb RCT (NCT03161028) | Outpatient glomerular disease clinic | Outpatient glomerular disease clinic |
| Reason for taking LA | Secondary progressive MS, no treatment | Secondary progressive MS, no treatment | Primary progressive MS, no treatment | Arthritis, weight loss, metabolic syndrome | Joint pain, general wellness |
| Co-morbid conditions | HTN | Asthma, basal cell carcinoma, melanoma, osteoporosis | COPD, depression, HTN, migraine | Asthma, Celiac disease | HTN, smoldering multiple myeloma, transient VWF deficiency |
| LA dose | 1200mg LA daily | 1200mg LA daily | 1200mg LA daily | 600 mg LA daily | 1200mg LA daily |
| Exposure to LA | Onset 6 months, biopsy 9 months | Onset 3 months, biopsy 9 months | 12 months | 6 months (inconsistent use) | Over 3 years |
| Serologies (ANA and PLA2R Antibody) | ANA positive (1:320, speckled), PLA2R not tested | ANA not tested, PLA2R negative | ANA not tested, PLA2R not tested | ANA negative, PLA2R negative | ANA negative, PLA2R negative |
| Creatinine at peak proteinuria (mg/dL) | 0.6 | 0.6 | 0.7 | 0.74 | 0.67 |
| Proteinuria | 19 g/g PCR | 2.1 g/g ACR | 4.4 g/g ACR | 5.5g/g PCR | 4.5 g/g PCR |
| Reason for biopsy | Nephrotic syndrome with leg edema | Asymptomatic proteinuria | Nephrotic range proteinuria | Nephrotic syndrome | Nephrotic syndrome in setting of smoldering myeloma |
| Kidney biopsy | |||||
| Light microscopy | Membranous | Membranous | No biopsy performed | Membranous | Membranous |
| Immunofluorescence (IF) | Segmental capillary wall deposits, IgG1 dominant | Segmental capillary wall deposits, polyclonal IgG | Segmental capillary wall deposits of polyclonal IgG, by paraffin IF | Segmental capillary wall deposits, IgG1 dominant, PLA2R-negative | |
| Electron microscopy | Segmentally distributed subepithelial deposits | Segmentally distributed subepithelial deposits with rare basement membrane spikes | Not available | Segmentally distributed subepithelial and focal mesangial deposits | |
| Glomerular antigen | NELL1+ | NELL1+ | NELL1+ | NELL1+ | |
| Clinical outcome | |||||
| Intervention | LA cessation; furosemide, lisinopril | LA cessation; losartan, spironolactone | LA cessation alone | LA cessation; furosemide, losartan | LA cessation; furosemide, losartan |
| Creatinine at time of proteinuria resolution (mg/dL) | 0.6 | 0.7 | 0.7 | 0.78 | 0.60 |
| Proteinuria at last follow-up | Resolution proteinuria at 7 months off LA | Resolution proteinuria at 12 months off LA | Resolution proteinuria at 3 months off LA | Partial remission (0.66g/g Cr) at 7 months off LA; Monitoring ongoing | Partial remission (0.66g/d) at 8 months, Complete resolution at 11 months off LA |
ACR denotes urine albumin to creatinine ratio, ANA anti-nuclear antigen, COPD chronic obstructive pulmonary disease, dsDNA double stranded DNA, g gram, HTN hypertension, IgG immunoglobulin G, LA lipoic acid, MS multiple sclerosis, NELL1 neural epidermal growth factor-like 1 protein, anti-phospholipase A2 receptor PLA2R, PCR urine protein creatinine ratio, and VWF von Willebrand Factor
Figure 1.
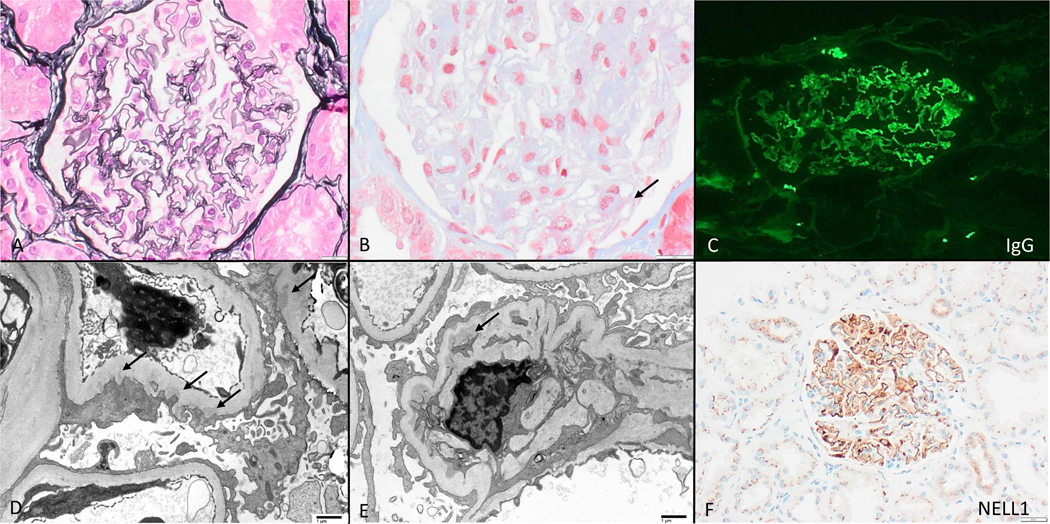
NELL1- associated membranous nephropathy in the setting of lipoic acid supplementation, with nearly-normal appearing glomeruli by light microscopy (A, Jones 400x) with subtle fuschinophilic subepithelial immune deposits (B, arrow, trichrome stain 600x). All cases had segmental capillary wall deposits of polyclonal IgG (C) which was often IgG1 dominant, and associated irregularly distributed subepithelial immune deposits by electron microscopy (D, arrows, direct magnification 2900x); mesangial deposits were present in 1 case (E, arrow, direct magnification 2900x). Glomerular deposits reacted with NELL 1 (F, 200x).
Figure 2.
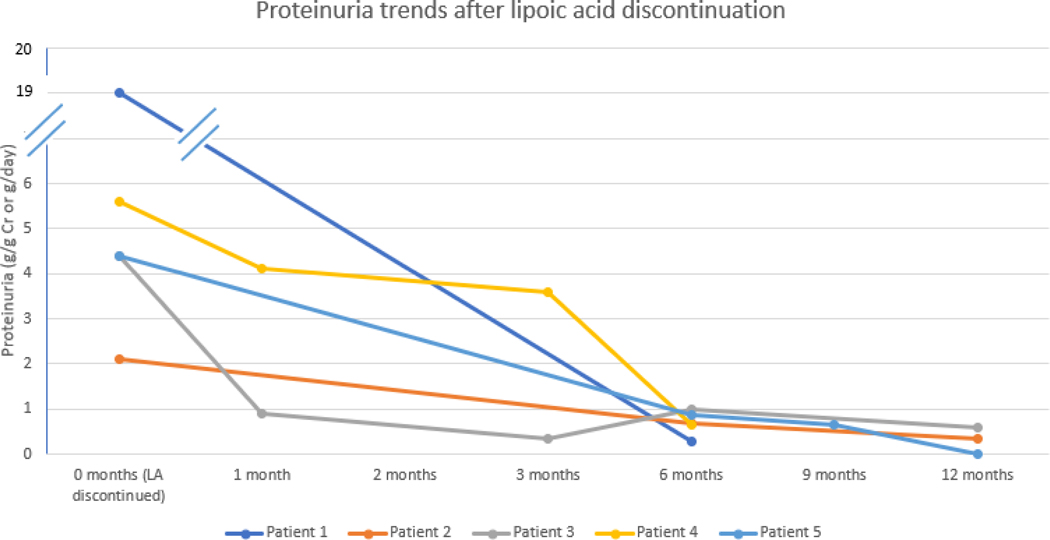
Proteinuria trends after lipoic acid discontinuation. Actual month of resolution noted at closest month category on the figure.
Patient 1: A 56- year old woman with untreated secondary progressive MS and no history of kidney disease in the active LA treatment arm developed leg swelling at study month 6. Laboratory monitoring at months 0, 3 and 6 demonstrated normal kidney function, but did not test urine protein. At month 9, an outside nephrologist discovered proteinuria (19g/g urine protein creatinine ratio, PCR). Kidney biopsy revealed secondary MN. Retrospective staining for NELL1 was positive. Workup for infectious, autoimmune, and neoplastic causes was negative. LA was discontinued, and supportive care with furosemide and lisinopril started. By seven months, proteinuria had resolved, and the patient remains in complete remission.
Patient 2: A 74-year old woman with untreated secondary progressive MS in the LA treatment arm developed proteinuria at month 3, worsening to albumin creatinine ratio (ACR) 2.1g/g Cr at month 9. Kidney biopsy revealed MN, and retrospective staining confirmed NELL1. Serum PLA2R antibody testing was negative. ACR declined to 0.69g/g Cr within 6 months and 0.35g/g Cr by 12 months after LA cessation and starting losartan and spironolactone. No active causes of secondary MN were identified. Subsequently, the study Data Safety Monitoring Board (DSMB) added a nephrologist (R. Avasare), began quantifying proteinuria with ACR, and increased frequency of proteinuria monitoring to every 3 months.
Patient 3: A 66-year old woman with untreated primary progressive MS taking active LA developed albuminuria (4.4g/g Cr) at month 12. No other causes for proteinuria were identified. LA was stopped; however, kidney biopsy was cancelled due to rapidly improving ACR at 1 (0.9g/g Cr) and 3 (0.34g/g Cr) months off LA.
Patient 4: A 41-year old woman without MS presented with nephrotic syndrome (proteinuria 5.6g/day) and easy bruising. Medical history included asthma and Celiac disease. The patient reported inconsistent use of LA 600mg for approximately 6 months prior to experiencing lower extremity edema. Kidney biopsy revealed NELL1-positive MN, and serum PLA2R Ab was negative. After LA discontinuation, proteinuria improved to 3.6g/24 hours after 3 months and 0.66g/g at 7 months. Serum albumin trended up from 2.1 g/dL to 3.5g/dL. The patient remains in active follow-up.
Patient 5: A 71-year-old woman with smoldering myeloma presented with nephrotic syndrome and proteinuria. Kidney biopsy revealed secondary MN without light chain restriction, and serum PLA2R antibody was negative. Urine protein-creatinine ratio (2.6g/g Cr to 4.5g/g Cr) and hypoalbuminemia (2.4 to 3.3mg/dL) continued despite losartan 100mg daily and varying doses of furosemide for three years after kidney biopsy. Knowledge of patients 1–4 lead to recognition of concurrent use of LA 1200mg daily for > 3 years, and retrospective testing showed NELL1-positivity. Within eight months of LA discontinuation, proteinuria declined to 660 mg/day and serum albumin rose to 4.0mg/dL. Proteinuria completely resolved at 11 months off LA.
Pathological Descriptions
Kidney biopsies (Figure 1) from biopsied patients (1, 2, 4, and 5) were relatively similar and showed subtle features of MN by light microscopy (Fig1A), with segmental immune deposits seen along the glomerular basement membrane (Fig1B). None had mesangial or endocapillary hypercellularity, crescents, or segmental sclerosis. All had well-preserved parenchyma with no or minimal global glomerulosclerosis or tubular atrophy and interstitial fibrosis. Table 1 and Figs 1C, 1D, and 1E describe immunofluorescence and electron microscopy findings. All 4 biopsies had NELL1+ glomerular immune deposits (Fig 1F, Supplementary Fig 1).
Discussion
We present 3 MS clinical trial patients and 2 patients from glomerular disease clinic taking LA who developed high-grade proteinuria that decreased after stopping LA. Onset of proteinuria was within 3–12 months of LA supplementation. All 4 biopsied patients had NELL1-MN. All patients are in remission - 4 complete, 1 partial - and none required immunosuppression. Previously, only one case report described NELL1-MN in a kidney allograft occurring after LA and dimercaptopropane sulfonate use with partial remittance of proteinuria after medication discontinuation.1 The delay between initiation of LA and development of proteinuria is interesting and may be attributable to delayed recognition of proteinuria due to lack of testing in the pilot trial and quarterly study visits in the ongoing RCT. Other intriguing possibilities include auto-antibody development occurring over months as seen in methyldopa-induced hemolytic anemia and procainamide-induced lupus, the latter which may be dependent on acetylator status.3,4 Furthermore, we suspect that clinical improvement likely lags behind immunologic remission as seen in PLA2R-antibody positive MN.5,6 The remission rate in our patients is notably higher than spontaneous remission in all-comers with primary MN (~33%).7 However, as described by Hoxha et al, it is important to acknowledge that PLA2R and THS7DA-negative patients may have higher rates of spontaneous remission with supportive care alone over a period of 24-months.8
Since 2009, several target antigens causing secondary MN have been discovered, including PLA2R,9 THSD7A,10 Exostosin 1/2,11 NELL112,13 and others.14,15 After initial description of NELL1-MN by Sethi et al,13 Caza et al12 described their slightly male-predominant patients with NELL1-MN, highlighting an association between NELL1 and malignancy (33% of cohort). Wang et al describe their female-predominant NELL1 patients, none with malignancy, most of whom went into remission with immunosuppressive therapy.16 The high rate of NELL1-positive MN in that Chinese series (comprising 35% of PLA2R-negative and THSD7A-negative MN) compared with US-based cohorts raises the possibility that NELL1-positive MN may be associated with certain genetic backgrounds or exposures. None of these NELL1 cohorts had detailed information on medications or supplements and development of MN. Since one of our patients had smoldering multiple myeloma, we considered the possibility of malignancy-associated NELL1-MN. However, the reversal of proteinuria with removal of LA rather than treatment of plasma cell neoplasm provides evidence against this possibility. All published NELL1-MN cohorts demonstrate segmental or incomplete glomerular distribution of immune deposits, predominantly with IgG1 staining, like patient biopsies described here.
To our knowledge, this is the first series demonstrating an association between LA and NELL1-MN. The occurrence of 2 biopsied and 1 suspected MN cases from approximately 83 participants to date taking active LA in the combined prior and ongoing current RCT far exceeds the expected rate of all MN in the general population of about 10/million/year.17 A recent systemic safety evaluation using pooled data from 71 studies and 2558 LA -treated subjects failed to reveal renal disorders generally, or MN specifically.18 Our own literature review found 2 concerning reports. A study in diabetic neuropathy testing LA 600mg daily in 460 randomized subjects for up to 4 years reported greater “urinary system disorders” in the LA cohort than placebo (22.1% vs 15.1%, p=0.071).19 An 18-month RCT for macular geographic atrophy (n=53) noted 3 occurrences of nephropathy in the LA 1200mg arm only.18 In addition to Case 1 above and previously reported case of worsening creatinine,2 the pilot RCT recorded 14 renal events in the LA group and 13 in the placebo, the majority being urinary tract infections for both groups (unpublished data). Notably, most LA trials had shorter durations (median 12 weeks), often lacked urine protein testing, and provided few AE details.
The cases of MN initially raised concern for LA contamination or toxicity. The occurrence of MN in patients taking LA from different sources (Pure Encapsulations®, Sudbury, MA, and Healthy Origins®, Pittsburg, PA) lessened concern for manufacturing issues. As only trace amounts of LA are absorbed from food, LA toxicity from excess intake is unlikely. LA occurs naturally in the R-conformation. Manufacturing LA produces an equal mixture (racemic) of R- and S- enantiomers which are absorbed differentially and potentially have differing biological effects (19).
Patient-specific determinants of developing MN in the setting of LA use deserve further investigation. Discovery of NELL1-MN in both MS and non-MS patients decreases concern of these events being MS-specific, although 4 of 5 had autoimmune diseases. LA has been described as a trigger for insulin autoimmune syndrome through development of antibodies to insulin that disappear after LA cessation.20,21 Limitations of this report include a lack of serum testing for circulating NELL-1 antibodies and inability to share all safety data due to the ongoing status of the current RCT. Prospective studies examining serum NELL1-antibodies, clinical, and mechanistic features of LA-associated MN are warranted.
Up to 30% of the general adult and 80% of autoimmune disease populations take OTC supplements,22 the adverse events of which may not surface without the scrutiny of trials requiring FDA oversight and careful monitoring as occurred here. LA use should be interrogated in patients with new-onset proteinuria and/or biopsy-proven NELL1-MN, regardless of malignancy status; if present, our observations suggest that LA discontinuation and supportive care lead to disease remission.
Supplementary Material
Supplementary Figure 1. Immunohistochemistry demonstrating positive NELL1 staining of glomerular deposits for patients 1, 2 and 5 (200x).
Translational Statement.
We describe 5 patients who developed proteinuria while taking alpha-lipoic acid, 4 who underwent kidney biopsy that showed NELL1-positive membranous nephropathy, all who went into remission after discontinuing LA. This highlights the importance of reviewing over-the-counter supplements, with a particular focus on lipoic acid for patients with proteinuria. Importantly, LA use may be a reversible cause of NELL1-associated MN. Future studies may elucidate the mechanisms of lipoic acid-associated MN.
Acknowledgements
Support for this study came from the Department of Veterans Affairs (I01 RX002682-01; B7493-W), the National Multiple Sclerosis Society (R-1705-27628), and the Multiple Sclerosis Society of Canada (1073-A-4).
Financial disclosures: R. Spain has research grant funding from Department of Veterans Affairs, National MS Society, and Multiple Sclerosis Society of Canada. A. Solomon receives consulting fees from EMD Serono, Genentech, Biogen, Alexion, Celgene, Greenwich Biosciences. He does non-promotional speaking for EMD Serono. He conducts contracted research for Sanofi, Biogen, Novartis, Alexion, and Genentech. R. Avasare is the nephrology consultant on the Data Safety and Monitoring Board for the Lipoic acid in Progressive Multiple Sclerosis study (NCT03161028).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rebecca I Spain, Portland VA Medical Center, Neurology Division L226; 3181 SW Sam Jackson Park Road, Portland OR 97239.
Nicole K. Andeen, Oregon Health & Science University, Department of Pathology and Laboratory Medicine, Portland, OR.
Pamela C. Gibson, The Robert Larner, M.D. College of Medicine at the University of Vermont, Anatomical and Clinical Pathology, Burlington, VT.
Mary Samuels, Oregon Health & Science University, Oregon Clinical and Translational Research Institute, Portland, OR.
Cynthia D. Morris, Oregon Health & Science University, Oregon Clinical and Translational Research Institute, Portland, OR.
Andrew J Solomon, The Robert Larner, M.D. College of Medicine at the University of Vermont,Department of Neurological Sciences, Burlington, VT.
Richard Solomon, The Robert Larner, M.D. College of Medicine at the University of Vermont, Division of Nephrology and Hypertension, Burlington, VT.
Carin Waslo, Research Division, Portland VA Medical Center, Portland OR.
Rupali S. Avasare, Oregon Health & Science University, Division of Nephrology and Hypertension, Department of Medicine, Portland, OR.
References
- 1.Munch J, Kruger BM, Weimann A, et al. Posttransplant nephrotic syndrome resulting from NELL1-positive membranous nephropathy. Am J Transplant. 2021;21(9):3175–3179. [DOI] [PubMed] [Google Scholar]
- 2.Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: A randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frohlich ED. Methyldopa. Mechanisms and treatment 25 years later. Arch Intern Med. 1980;140(7):954–959. [DOI] [PubMed] [Google Scholar]
- 4.Woosley RL, Drayer DE, Reidenberg MM, Nies AS, Carr K, Oates JA. Effect of acetylator phenotype on the rate at which procainamide induces antinuclear antibodies and the lupus syndrome. N Engl J Med. 1978;298(21):1157–1159. [DOI] [PubMed] [Google Scholar]
- 5.Cravedi P, Ruggenenti P, Remuzzi G. Circulating anti-PLA2R autoantibodies to monitor immunological activity in membranous nephropathy. J Am Soc Nephrol. 2011;22(8):1400–1402. [DOI] [PubMed] [Google Scholar]
- 6.Beck LH Jr., Fervenza FC, Beck DM, et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22(8):1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polanco N, Gutierrez E, Covarsi A, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21(4):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoxha E, Harendza S, Pinnschmidt HO, et al. Spontaneous remission of proteinuria is a frequent event in phospholipase A2 receptor antibody-negative patients with membranous nephropathy. Nephrol Dial Transplant. 2015;30(11):1862–1869. [DOI] [PubMed] [Google Scholar]
- 9.Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364(7):689–690. [DOI] [PubMed] [Google Scholar]
- 10.Tomas NM, Beck LH Jr., Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371(24):2277–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi S, Madden BJ, Debiec H, et al. Exostosin 1/Exostosin 2-Associated Membranous Nephropathy. J Am Soc Nephrol. 2019;30(6):1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caza TN, Hassen SI, Dvanajscak Z, et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021;99(4):967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethi S, Debiec H, Madden B, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020;97(1):163–174. [DOI] [PubMed] [Google Scholar]
- 14.Al-Rabadi LF, Caza T, Trivin-Avillach C, et al. Serine Protease HTRA1 as a Novel Target Antigen in Primary Membranous Nephropathy. J Am Soc Nephrol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sethi S, Madden B, Debiec H, et al. Protocadherin 7-Associated Membranous Nephropathy. J Am Soc Nephrol. 2021;32(5):1249–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang G, Sun L, Dong H, et al. Neural Epidermal Growth Factor-Like 1 Protein-Positive Membranous Nephropathy in Chinese Patients. Clin J Am Soc Nephrol. 2021;16(5):727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofstra JM, Wetzels JF. Introduction of a cyclophosphamide-based treatment strategy and the risk of ESRD in patients with idiopathic membranous nephropathy: a nationwide survey in the Netherlands. Nephrol Dial Transplant. 2008;23(11):3534–3538. [DOI] [PubMed] [Google Scholar]
- 18.Fogacci F, Rizzo M, Krogager C, et al. Safety Evaluation of alpha-Lipoic Acid Supplementation: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Clinical Studies. Antioxidants (Basel) (2020) 9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with alpha-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34(9):2054–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida Y, Ohara T, Okuno Y, et al. Alpha-lipoic acid and insulin autoimmune syndrome. Diabetes Care. 2007;30(9):2240–2241. [DOI] [PubMed] [Google Scholar]
- 21.Moffa S, Improta I, Rocchetti S, Mezza T, Giaccari A. Potential cause-effect relationship between insulin autoimmune syndrome and alpha lipoic acid: Two case reports. Nutrition. 2019;57:1–4. [DOI] [PubMed] [Google Scholar]
- 22.Silbermann E, Senders A, Wooliscroft L, et al. Cross-sectional survey of complementary and alternative medicine used in Oregon and Southwest Washington to treat multiple sclerosis: A 17-Year update. Mult Scler Relat Disord. 2020;41:102041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Immunohistochemistry demonstrating positive NELL1 staining of glomerular deposits for patients 1, 2 and 5 (200x).