Abstract
Brain metastasis is a serious complication of non-small cell lung cancer (NSCLC) affecting up to 40% of NSCLC patients. A subset of NSCLC tumors has mutations in the epidermal growth factor receptor (EGFR) gene, and determination of tumor EGFR mutation status is essential in guiding treatment decisions, as it directly affects the treatment approach. Patients with EGFR-mutated NSCLC have a higher cumulative incidence of brain metastases, and are especially sensitive to EGFR tyrosine kinase inhibitors (TKIs). Patients with newly diagnosed EGFR-mutated lung cancer presenting to a neurosurgeon with a new diagnosis of brain metastases now have a variety of treatment options available, including whole brain radiation therapy, stereotactic radiosurgery, surgical resection, chemotherapy, and targeted therapeutics such as the EGFR TKIs. In this review, we discuss the impact of EGFR mutation status on brain and leptomeningeal metastasis treatment considerations. Additionally, we present clinical cases of patients treated with EGFR TKIs alone and in combination with other therapies to highlight treatment alternatives.
Keywords: Brain metastasis, Leptomeningeal disease, Epidermal growth factor receptor, Tyrosine kinase inhibitor, Non-small cell lung cancer
ABBREVIATIONS
- CNS
central nervous system
- CT
computed tomography
- EGFR
epidermal growth factor receptor
- LMC
leptomeningeal carcinomatosis
- MRI
magnetic resonance imaging
- NSCLC
non-small cell lung cancer
- PCR
polymerase chain reaction
- SRS
stereotactic radiosurgery
- TKI
tyrosine kinase inhibitor
- WBRT
whole brain radiation therapy
Non-small cell lung cancer (NSCLC) makes up 85% of all lung cancers.1 Brain metastases occur in up to 40% of all patients with NSCLC, marking an acute decline in the quality of life and overall survival.2 Treatment considerations must weigh number, location, size, and associated edema of brain metastases, as well as neurological symptoms, extent of systemic disease, need for tissue or genetic mutation diagnosis, and prior therapies.3 Treatment options include radiation modalities such as whole brain radiation therapy (WBRT) and stereotactic radiosurgery (SRS), surgical resection of large, symptomatic lesions in a subset of patients, and systemic therapies including treatment to decrease brain edema (eg, dexamethasone, bevacizumab), chemotherapy, immunotherapy, and targeted therapeutics in patients whose tumors harbor specific mutations. Outcomes and selection of the treatment options depend in part on the underlying mutations driving tumor progression. A subset of patients with NSCLC have tumors harboring epidermal growth factor receptor (EGFR) mutations, for whom targeted treatment with EGFR tyrosine kinase inhibitors (TKIs) improve progression-free survival (PFS), including those with brain metastases.4-6 TKIs can effectively treat EGFR-mutated brain metastases, and WBRT may be deferred together with the associated neurocognitive side effects. Compared with just a few years ago, patients with newly diagnosed EGFR-mutated lung cancer presenting to a neurosurgeon with a new diagnosis of brain metastases now have a variety of treatment options available.
BACKGROUND ON EGFR MUTATIONS IN LUNG CANCER AND BRAIN METASTASES
To fully stage lung cancer, patients should undergo a contrast-enhanced computed tomography (CT) scan and positron emission tomography CT.7 If biopsy establishes NSCLC larger than a few centimeters, or there is metastatic disease detected, patients should also have central nervous system (CNS) imaging, preferably a magnetic resonance imaging (MRI) with gadolinium contrast. A subset of NSCLC tumors has mutations in the EGFR gene, which can be detected by polymerase chain reaction-based (PCR-based) direct sequencing, or multiplexed PCR testing.7-10 Clinical and pathological characteristics predictive of EGFR-mutated NSCLC include patients of Asian race, adenocarcinoma histology, female sex, lack of prior smoking history, and young age.11 These mutations, which occur in exons 18, 19, and 21 of the EGFR gene, result in a frame deletion or amino acid substitution around the ATP-binding pocket of EGFR tyrosine kinase (Figure 1).2 Deletions in exon 19 and the L858R point mutation in exon 21 account for more than 90% of EGFR mutations.7,12 Determination of the tumor EGFR mutation status is essential in guiding treatment decisions, as it directly affects the treatment approach.11 The cumulative incidence of brain metastases is higher in patients with EGFR mutant NSCLC (39%) vs wild-type NSCLC (28%).13 EGFR mutant NSCLC is exquisitely sensitive to EGFR TKIs, which can also penetrate the CNS.
FIGURE 1.
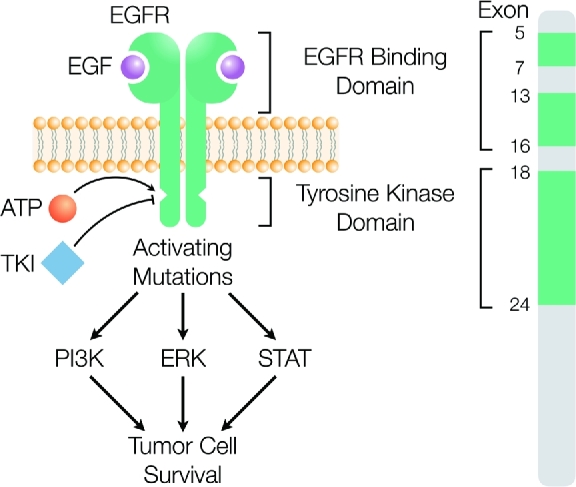
Many patients with brain metastases from NSCLC will have an EGFR mutation, resulting in tumor cell survival. Determination of the tumor mutation status and response or resistance to therapy is essential in guiding treatment decisions.
TREATMENT OPTIONS FOR PATIENTS WITH EGFR-MUTATED LUNG-TO-BRAIN METASTASES
Careful consideration of the patient's functional status, prior exposure to chemotherapy, targeted therapeutics or radiation, associated symptoms, number and size of the lesions, and the mutation profile helps to determine the best treatment option for EGFR-mutated lung-to-brain metastases.14-16 Here, we discuss surgical resection, WBRT, SRS, targeted therapeutics, chemotherapy, and combination therapy. We additionally present 3 clinical cases highlighting these treatment options. The case studies were approved by our home institution's institutional review board. Patient information was retrieved retrospectively from the patient chart and deidentified to protect confidentiality.
Treatment decisions are highly dependent on the individual patient's circumstances, and aggressive treatment of the primary lung cancer is a major factor associated with overall survival.2,17 Table 1 summarizes the prognosis of patients with NSCLC treated with the various treatment options discussed. Additional negative prognostic indicators include metastases at initial NSCLC diagnosis, multiple brain metastases, and uncontrolled primary disease.
TABLE 1.
Survival and Brain Metastasis Treatment. Studies Reporting the Median Survival Time and PFS Depending on the Brain Metastasis Treatment Received. Treatments Included Surgical Resection, WBRT, SRS, EGFR-Targeted TKI, and Chemotherapy
| Study | n | EGFR status | Treatment | Group n | MST (mo) | P | PFS (mo) | P |
|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||||
| Aoyama,23 2006 (1-4 BM) | 132 | Unknown | SRS + WBRT | 65 | 7.5 | .42 | – | |
| SRS | 67 | 8.0 | ||||||
| Brown,26 2016 (1-3 BM) | 213 | Unknown | SRS + WBRT | 102 | 7.4 | .92 | ||
| SRS | 111 | 10.4 | ||||||
| Chang,24 2009 (1-3 BM) | 58 | Unknown | SRS + WBRT | 28 | 5.7 | .003 | – | – |
| SRS | 30 | 15.2 | ||||||
| Lim,29 2015 (1-4 BM) | 98 | Unknown | Chemo | 49 | 15.3 | .418 | 9.4 | .248 |
| SRS + Chemo | 49 | 14.6 | 6.6 | |||||
| Post hoc analysis | ||||||||
| LUX-Lung 3644 (asymptomatic BM) | 81 | Mutated | Afatinib | 48 | 22.4 | .6412 | 8.2 | .0297 |
| Chemo | 33 | 25.0 | 5.4 | |||||
| Retrospective | ||||||||
| Choi,20 2012 (> 2 cm BM) | 97 | Unknown | Surgery +SRS | 97 | 15.6 | – | – | – |
| Lin,2 2015 | 23 874 | Mixed (mutated received TKI) | WBRT | 6.36 | <.0001 | – | – | |
| WBRT + TKI | 12.12 | |||||||
| WBRT + SRS | 17.52 | |||||||
| WBRT + TKI + SRS | 27 | |||||||
| Magnuson,22 2017 | 351 | Mutated | SRS then TKIa | 131 | 46 | <.001 | 23 | .025 |
| WBRT then TKI | 120 | 30 | 24 | |||||
| TKI then SRS or WBRT | 100 | 25 | 17 | |||||
| Prospective | ||||||||
| Barlesi,49 2011 (asymptomatic BM) | 43 | Unknown | Cisplatin/pemetrexed | 7.4 | – | 4.0 | – | |
| Dinglin,51 2013 | 41 | Mixed | WBRT + cisplatin/pemetrexed | 12.6 | – | 10.6 | – | |
| Park,46 2012 | 28 | Exon 19 or 21 mutated | First generation EGFR TKI | 15.9 | – | 6.6 | – | |
| Yamamoto,28 2014 (1-10 BM) | 1194 | Unknown | SRS | <.0001 | – | – | ||
| 1 BM | 455 | 13.9 | ||||||
| 2-4 BM | 531 | 10.8 | ||||||
| 5-10 BM | 208 | 10.8 | ||||||
aPatients received the second treatment at intracranial progression (eg, SRS at presentation then TKI at intracranial progression).
MST, median survival time; BM, brain metastasis.
Surgical Resection
Surgical resection of accessible solitary intracranial lesions is indicated for selected patients with controlled or absent extracranial disease and good performance status (Karnofsky performance status ≥70).16 Resection of a large, symptomatic brain metastasis also has the benefit of rapid relief of the mass effect, histologic confirmation of the diagnosis, genetic testing for targetable mutations, and decreasing brain edema. If only a tissue diagnosis and mutation profile is required, a percutaneous, stereotactic biopsy is a reasonable alternative to an open biopsy or resection. Postoperative radiation to the resection cavity regularly follows surgical resection to reduce the likelihood of local recurrence.17-20 Unlike WBRT, SRS to the resection cavity limits the exposure of normal brain tissue to radiation. This approach is beneficial in EGFR-mutated lung cancer patients who are TKI naïve, as they may have an extended survival. Thus, a lower integral dose to the brain can decrease the risk of developing potential long-term neurocognitive decline.21 As is highlighted in the following case, the adjunct of postoperative SRS allows surgeons to leave a small volume of residual tumor, rather than risk devastating neurological injury.
Case 1
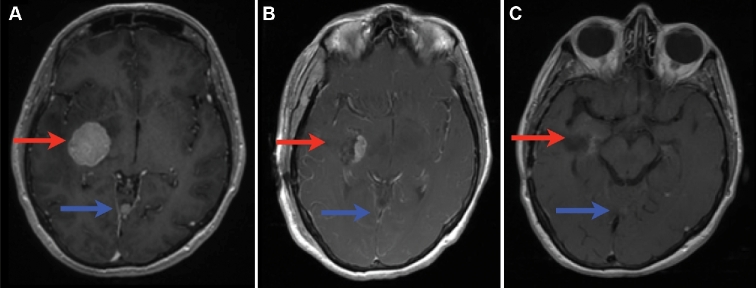
A 49-yr-old never-smoker female presented with a symptomatic 4 cm right medial temporal brain tumor, 2 smaller cerebellar lesions, and a lung mass concerning for metastatic disease from the lung (Figure 2A). The patient underwent a right temporal craniotomy for resection of the large mass. Postoperatively, the patient was neurologically intact following resolution of a transient left upper quadrant field cut. Pathology and genetic testing revealed EGFR L858R-mutated NSCLC. The small residual tumor involving the posterior cerebral artery, and the other intracranial metastases were treated in 1 fraction with SRS at 18 and 20 Gy, respectively (Figure 2B). The patient started erlotinib, with her intracranial lesions showing good response (Figure 2C) and she also responded systemically.
FIGURE 2.
Surgery, SRS, and EGFR-TKI in the treatment of NSCLC brain metastases. Axial T1 with contrast MRI of a 49-yr-old female presenting with brain metastases from EGFR-mutated NSCLC (A) prior to surgical resection, (B) after surgical resection and starting TKI, and (C) 3 mo after SRS and adjuvant TKI. Red arrow shows the initial surgical lesion and the postoperative resection cavity. Blue arrow shows an additional nonsurgical lesion that also shows response to TKI and SRS.
Radiation Therapy
While surgical resection is indicated for solitary symptomatic large brain lesions that are readily accessible, radiotherapy is often preferred for multiple metastases that do not require tissue diagnosis, or in patients who are not surgical candidates. Radiation also prevents progression or recurrence at the site of surgical resection. Radiation therapy can be given to the whole brain or to the lesions only. WBRT remains the standard treatment when focal approaches are not feasible due to numerous intracranial metastases, or leptomeningeal disease.17 SRS has the benefit of delivering focused radiation that minimizes damage to the normal brain.14
WBRT with SRS has demonstrated an improved PFS when compared to WBRT alone.21 In patients with less than 4 brain metastases, SRS boost after WBRT has better local control,22 fewer intracranial relapses, and fewer neurological deaths as compared to those who receive SRS or surgical resection alone.21,23,24 However, the addition of WBRT did not change the duration of functional independence, overall survival, or quality of life.23,25,26 Furthermore, the omission of upfront WBRT in patients treated with SRS have a lower likelihood of learning and memory function decline over time.23,26-28 As many EGFR-mutated NSCLC patients benefit from an extended disease control and survival with targeted therapies, one should consider deferring up front WBRT in TKI-naïve patients if possible to avoid the potential associated long-term cognitive deterioration.
SRS has been established for patients with up to 3 brain metastases at diagnosis or those with stable extracranial disease.2,28 In a trial in which 98 patients with NSCLC and 1 to 3 asymptomatic brain metastases were randomized to SRS plus chemotherapy (n = 49) or chemotherapy alone (n = 49), there was no difference in overall survival, and platinum doublet chemotherapy alone had a 37% intracranial response rate, compared with 57% for SRS plus chemotherapy (P = .011).29 Several trials have since supported the use of SRS alone as initial treatment for up to 10 brain metastases, with tumor volume correlating with survival.28,30,31 The risk of new metastasis occurrence outside the radiated field can be up to 54% within 1 yr.15 Therefore, SRS-treated patients are recommended to adhere to a rigorous radiological follow-up with an MRI every 3 mo, or at the time of symptom onset or systemic disease progression.
In the postoperative setting, an addition of a 2-mm margin to the surgical cavity has demonstrated an excellent overall local control rate of 89% to 100%.20,32 In certain cases, SRS may have comparable or improved results to those of surgical resection in patients with metastases < 2 cm,1,17,21,33 particularly when considering the importance of SRS to the resection cavity to improve local control. In addition, SRS of brain metastases incurs 58.8% of the cost of open surgical resection.33 In a 2017 retrospective multi-institutional study of TKI-naïve patients (n = 351), Magnuson et al34 found that upfront SRS followed by an EGFR TKI at intracranial progression (n = 131) had the longest overall survival (P < .001) as compared to upfront WBRT followed by EGFR TKI (n = 120) or upfront EGFR TKI followed by SRS or WBRT (n = 100) at intracranial disease progression (46, 30, and 25 mo, respectively).34 In patients with a more favorable prognosis—defined by the authors as a disease-specific Graded Prognostic Assessment of 2 to 4—this effect was even more dramatic: median overall survival in patients in the upfront SRS group of 64 vs 32 mo in the upfront EGFR TKI group (P < .001). The following case highlights the rapid response to be expected from combination SRS and EGFR TKI.
Case 2
A 60-yr-old woman presented with a lung mass and an asymptomatic, solitary, left frontal metastasis. Biopsy of the lung mass confirmed the diagnosis of EGFR-mutated NSCLC. The combination of SRS (20 Gy in 1 fraction) with an EGFR TKI (erlotinib) started the following week achieved a marked decrease in lesion size and surrounding edema at 3-mo follow-up (Figure 3). The patient remained asymptomatic with local control at most recent follow-up, 2 yr after treatment.
FIGURE 3.
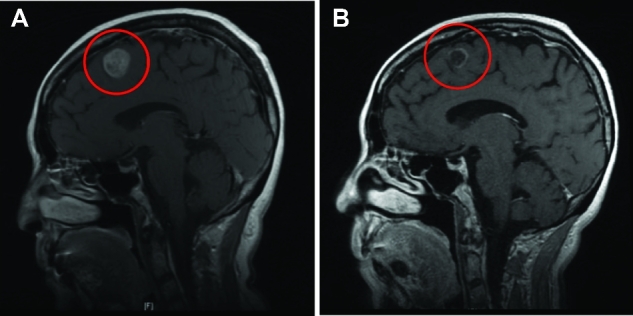
SRS and EGFR-TKI treatment of a solitary NSCLC intracranial metastasis. T1 postcontrast sagittal MRI of a 60-yr-old female with a single left frontal metastasis from EGFR-mutated NSCLC (A) at presentation, and (B) 3-mo follow-up after treatment with SRS followed by erlotinib a week later.
EGFR TKI
Based on evidence from several trials, EGFR TKIs including gefitinib, erlotinib, and afatinib are now considered to be standard first-line therapy for patients with tumors harboring activated EGFR mutations. This is based on clinical trials demonstrating improved PFS vs chemotherapy in the first-line treatment setting.35-40 Two randomized controlled trials have shown that erlotinib significantly increases PFS when compared to chemotherapy.40,41 The EURTAC study (n = 173) reported PFS of 9.7 mo for patients treated with erlotinib (n = 86) as compared to 5.2 mo for those treated with chemotherapy (n = 87, P < .0001).40 The OPTIMAL study (n = 154) found a 13.1-mo PFS in patients treated with erlotinib (n = 82) as compared to 4.6-mo in those treated with carboplatin/gemcitabine (n = 72, P < .0001).41 Similarly, 3 randomized controlled trials have shown that gefitinib significantly increases PFS when compared to chemotherapy, ranging from 9.2 (WJTOG3405 study) to 10.8 mo (NJ002 study).35,42,43
EGFR TKIs are often effective in the treatment of brain metastases in patients with EGFR-mutated NSCLC. A post hoc analysis of the LUX-Lung 3 and 6 revealed a significantly increased PFS (P = .0297) in patients with asymptomatic brain metastases from EGFR-mutated NSCLC treated with afatinib (n = 48, 8.2 mo) as compared to chemotherapy (n = 33, 5.4 mo).44 One-third of patients in each treatment arm had prior WBRT. In a phase II trial of gefitinib in patients with brain metastases from EGFR-mutated lung adenocarcinoma (n = 41), Iuchi et al45 found an 87.8% response rate, with a median PFS of 14.5 mo and median overall survival time of 21.9 mo. In a similar phase II trial, 28 patients with brain metastases from EGFR-mutated (exon 19 or 21) NSCLC received either gefitinib or erlotinib at the treating physician's discretion.46 Patients had not received prior SRS, WBRT, or surgical resection of their brain tumors. The median PFS and overall survival times were 6.6 and 15.9 mo, respectively, with no difference based on EGFR TKI used. The result of these trials supports the use of EGFR TKIs as first-line therapy in patients with EGFR-mutated NSCLC. However, the recent findings of the multi-institutional retrospective study (n = 351), conducted by Magnuson et al,34 suggest that the use of upfront SRS followed by an EGFR TKI at intracranial progression results in longer overall survival than upfront EGFR TKI followed by SRS or WBRT at intracranial progression. In this study, erlotinib was used in 98% (n = 344) of patients who received an EGFR TKI. While no clinical data currently suggest superiority of a specific EGFR TKI, animal data from a new EGFR TKI in development, osimertinib, suggest improved blood-brain barrier penetration with osimertinib as compared to gefitinib, rociletinib, and afatinib.47 Randomized control trials comparing EGFR TKI alternatives and comparing upfront EGFR TKI and upfront SRS are warranted to establish the standard of care. As demonstrated by the following case, TKI-naïve brain metastases can respond well to systemic TKI, and at the time of systemic progression (indicative of TKI-resistance) the prior brain metastases may or may not show interval growth, which can then be treated with SRS.
Case 3
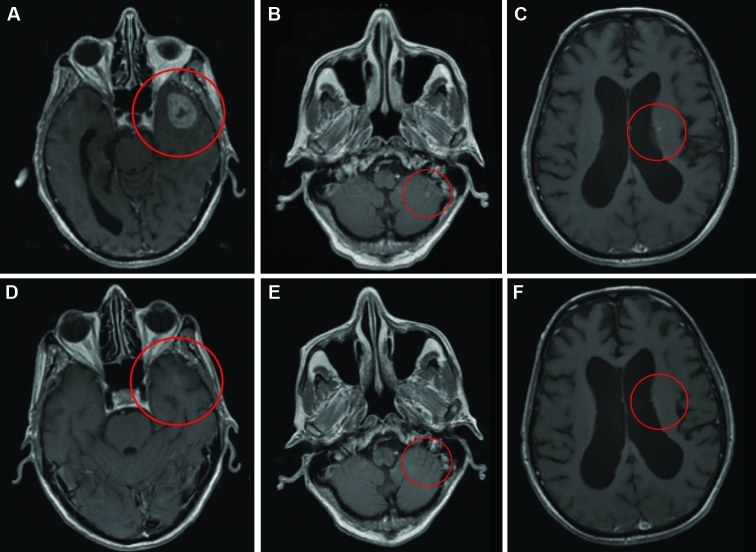
A 79-yr-old male with diffusely metastatic EGFR-mutated NSCLC had evidence of 5 brain metastases at the time of diagnosis. The largest metastasis was 3 cm in the left temporal lobe, and others were subcentimeter in the left frontal vertex, right frontal, left caudate body, and inferior cerebellar vermis. The patient refused WBRT and SRS and was instead treated with erlotinib, which resulted in significant improvement to the left anterior temporal lobe, left frontal vertex, and right frontal region (Figure 4). He was monitored closely with serial follow-up MRI scans showing stable disease. Eleven months later, the patient was found to have progression of his extracranial disease. In preparation for clinical trial enrollment for his extracranial disease, he stopped treatment with erlotinib and received SRS for the stable left anterior temporal lobe lesion. Two months after stopping erlotinib treatment, the patient developed a 2-mm punctate focus of enhancement in the central pons.
FIGURE 4.
Good response to EGFR-TKI alone for treatment of various NSCLC brain metastasis. T1 postcontrast axial MRI of a 79-yr-old male with EGFR-mutated NSCLC and 5 brain metastases at the time of diagnosis prior to (A, B, C) and 3 mo following (D, E, F) systemic treatment with TKI.
RESISTANCE TO EGFR TKIs AND TRANSITION TO CHEMOTHERAPY
Unfortunately, patients with EGFR-mutated NSCLC have disease progression on TKI.6 This can occur by acquiring an EGFR T790M point mutation on exon 20, MET amplification, HER2 amplification, or small cell histologic transformation.48 Although chemotherapy with water-soluble drugs was believed to be ineffective in the treatment of brain metastases due to the blood-brain barrier, the blood-tumor barrier is disrupted by the presence of metastases.14 For patients with EGFR mutant adenocarcinoma, most receive second line platinum-pemetrexed based chemotherapy, which has a cerebral response rate around 40%, similar to that observed for systemic disease responses.49,50 Cisplatin/pemetrexed may be used concurrently with WBRT, though the efficacy and safety of this combination treatment remains uncertain.51 New highly lipid-soluble drugs such as temozolomide in combination with WBRT have been shown to improve neurologic symptoms and radiographic response rates, according to a study conducted by the Hoosier Oncology Group (n = 48).14 Since discontinuation of an EGFR TKI could lead to accelerated primary disease progression, patients with CNS-only progression can be treated with local therapy (surgical resection, radiofrequency ablation, SRS, or conventional radiotherapy to a non-CNS site) with continuation of an EGFR TKI.52
LEPTOMENINGEAL CARCINOMATOSIS
Metastatic spread of tumor cells along the central nervous system leptomeninges (leptomeningeal carcinomatosis, LMC) occurs in 5% of NSCLC patients; if untreated, the median survival of patients is 4 to 6 weeks.53 WBRT may result in longer survival with LMC (median survival 6.4 mo) as compared to systemic chemotherapy (4.7 mo), though it is not always effective54-56 (Table 2). Riess et al57 (n = 30) found that patients who received modern systemic therapy (erlotinib, gefitinib, pemetrexed, bevacizumab, or crizotinib) had a prolonged survival (hazard ratio = 0.24, P = .007) with LMC compared with patients who did not receive these treatments (43% received older chemotherapy regimens and 71% received WBRT for LMC).57 Retrospective studies on the use of erlotinib, gefitinib, and icotinib suggest that they are effective for the treatment of LMC.54-56 Lee et al56 retrospectively compared the efficacy of gefitinib (n = 14) and erlotinib (n = 11) for control of LMC in NSCLC, and found that patients treated with erlotinib showed a better cytologic conversion rate compared to gefitinib (64.3% vs 9.1%, P = .012). All patients in this study also received intrathecal chemotherapy including methotrexate. In a retrospective study of 21 patients with EGFR mutant NSCLC treated with icotinib, 90% of patients reported improvement in dizziness and headache and 100% of patients reported less nausea or vomiting.55 In patients who developed LMC while on icotinib standard therapy, a double dose of icotinib relieved them of their symptoms for more than 4 mo.
TABLE 2.
Studies Reporting the Median Survival Time of Patients With LMC who Received a variety of Treatments, Including EGFR-TKIs, WBRT, Intrathecal and Systemic Chemotherapy
| Leptomeningeal carcinomatosis | |||||
|---|---|---|---|---|---|
| Study | n | EGFR Status | Treatment | Group n | MST (mo) |
| Xu,54 2015 | 108a | Unknown | SC | 59 | 4.7 |
| SC + WBRT | 32 | 5.2 | |||
| WBRT | 49 | 6.4 | |||
| TKI | 42 | 11.1 | |||
| TKI + SC | 13 | 11.1 | |||
| TKI + WBRT | 19 | 12.3 | |||
| Morris,60 2012 | 125b | Unknown | WBRT | 46 | 3.0 |
| Unknown | IT | 7 | 18 | ||
| Mutated | TKI | 9 | 14 | ||
| Gong,55 2015 | 21 | Mutated | TKI (icotinib) | 21 | 10.1 |
aFor analysis, patients were categorized in all applicable treatment groups (eg, a patient receiving SC + WBRT is included in the “SC,” “WBRT,” and “SC + WBRT” groups), therefore overall sample size is smaller than the sum of the group sizes.
bThe remaining patients received systemic chemotherapy or palliative care, but MST was not reported.
MST, median survival time; IT, intrathecal chemotherapy; SC, systemic chemotherapy.
When standard dosing of an EGFR TKI fails to control LMC, erlotinib administered at a “pulsatile” high dose (1500 mg) once weekly has been reported to be tolerable and control LMC in patients with EGFR sensitive mutations.58 In a phase II clinical trial, the administration of pulsatile high-dose erlotinib (n = 13, a 450 mg dose every 3 d) or gefitinib (n = 29, a 1000 mg dose every 4 d) to patients with drug resistance to conventional erlotinib or gefitinib treatment, respectively, was determined to be safe and efficient.59 Median PFS was 30 mo, with no statistically significant difference between the 2 TKIs.
Time between initial NSCLC diagnosis and presentation with LMC affects prognosis for patients with leptomeningeal disease.57 In a study by Xu et al54 (n = 108), patients presenting with LMC within 12 mo of initial NSCLC diagnosis had a median survival time of 4.9 mo, compared to 7.5 mo in patients presenting with LMC more than 12 mo after initial NSCLC diagnosis.54 Additionally, the presence of parenchymal brain metastases has been found to be a negative prognostic indicator in patients with LM from EGFR-mutated NSCLC. Patients with parenchymal brain metastases had a median survival of 8.1 mo as compared to 11.1 mo in those who did not.55
CONCLUSION
The number, size, symptoms, genetic mutations, and location of brain metastases greatly influence the most appropriate treatment selection. Given the efficacy of targeted therapies in treating both systemic and intracranial metastases in patients with EGFR-mutated NSCLC, radiation and surgical resection of these brain tumors must be carefully tailored to the individual needs of a particular patient. In the case of brain metastases diagnosed at the time of presentation (ie, TKI naïve), patients may live a significantly long period of time, and WBRT could be avoided or delayed depending on response to TKI therapy. Comorbidities factor into a patient's eligibility for surgical resection, and SRS remains a good first-line therapy depending on lesion size, number, and symptoms. Brain metastases in TKI-naïve patients may show response to systemic therapy. Treatment of NSCLC brain metastases requires a complex and often multi-disciplinary approach, with careful consideration of the extent of primary disease, the quantity, size, and associated symptoms of metastases, comorbidities, and EGFR mutation status.
Disclosures
The authors would like to acknowledge funding from the National Institute of Neurological Disorders and Stroke: K08NS901527. The authors have no personal, finanacial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Qin H, Wang C, Jiang Y, Zhang X, Zhang Y, Ruan Z. Patients with single brain metastasis from non-small cell lung cancer equally benefit from stereotactic radiosurgery and surgery: a systematic review. Med Sci Monit. 2015;21:144-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin C-H, Hsu K-H, Chang S-Net al. Increased survival with the combination of stereotactic radiosurgery and gefitinib for non-small cell lung cancer brain metastasis patients: a nationwide study in Taiwan. Radiat Oncol. 2015;10(1):127. doi:10.1186/s13014-015-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sperduto PW, Chao ST, Sneed PKet al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655-661. [DOI] [PubMed] [Google Scholar]
- 4. Gridelli C, De Marinis F, Di Maio M, Cortinovis D, Cappuzzo F, Mok T. Gefitinib as first-line treatment for patients with advanced non-small-cell lung cancer with activating epidermal growth factor receptor mutation: Review of the evidence. Lung Cancer. 2011;71(3):249-257. [DOI] [PubMed] [Google Scholar]
- 5. Guetz G Des, Landre T, Uzzan B, Chouahnia K, Nicolas P, Morere J-F. Is there a survival benefit of first-line epidermal growth factor receptor tyrosine-kinase inhibitor monotherapy versus chemotherapy in patients with advanced non-small-cell lung cancer?: a meta-analysis. Target Oncol. 2015. doi:10.1007/s11523-015-0373-x. [DOI] [PubMed] [Google Scholar]
- 6. Yuan Y, Li X-F, Chen J-Q, Dong C-X, Weng S-S, Huang J-J. Critical appraisal of the role of gefitinib in the management of locally advanced or metastatic non-small cell lung cancer. Onco Targets Ther. 2014;7:841-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westwood M, Joore M, Whiting Pet al. Epidermal growth factor receptor tyrosine kinase (EGFR-TK) mutation testing in adults with locally advanced or metastatic non-small cell lung cancer: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2014;18(32):1-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buttitta F, Felicioni L, Del Grammastro Met al. Effective assessment of egfr mutation status in bronchoalveolar lavage and pleural fluids by next-generation sequencing. Clin Cancer Res. 2013;19(3):691-698. [DOI] [PubMed] [Google Scholar]
- 9. Sequist L V, Heist RS, Shaw ATet al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol. 2011;22(12):2616-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Su J, Zhang X-C, An S-Jet al. Detecting the spectrum of multigene mutations in non-small cell lung cancer by Snapshot assay. Chin J Cancer. 2014;33(7):346-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ellis PM, Coakley N, Feld R, Kuruvilla S, Ung YC. Use of the epidermal growth factor receptor inhibitors gefitinib, erlotinib, afatinib, dacomitinib, and icotinib in the treatment of non-small-cell lung cancer: a systematic review. Curr Oncol. 2015;22(3):e183-e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li B, Sun S-Z, Yang Met al. The correlation between EGFR mutation status and the risk of brain metastasis in patients with lung adenocarcinoma. J Neurooncol. 2015;124(1):79-85. [DOI] [PubMed] [Google Scholar]
- 13. Hsu F, De Caluwe A, Anderson D, Nichol A, Toriumi T, Ho C. EGFR mutation status on brain metastases from non-small cell lung cancer. Lung Cancer. 2016;96:101-107. [DOI] [PubMed] [Google Scholar]
- 14. Taimur S, Edelman MJ. Treatment options for brain metastases in patients with non-small-cell lung cancer. Curr Oncol Rep. 2003;5(4):342-346. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12781078. Accessed June 28, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Kawabe T, Phi JH, Yamamoto M, Kim DG, Barfod BE, Urakawa Y. Treatment of brain metastasis from lung cancer. Prog Neurol Surg. 2012;25:148-155. doi:10.1159/000331188. [DOI] [PubMed] [Google Scholar]
- 16. Tsao MN, Rades D, Wirth Aet al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pollock BE. To remove or not to remove, that is the question? World Neurosurg. 2015;84(1):2-3. [DOI] [PubMed] [Google Scholar]
- 18. Ojerholm E, Lee JYK, Thawani JPet al. Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J Neurosurg. 2014;121(suppl):75-83. doi:10.3171/2014.6.GKS14708. [DOI] [PubMed] [Google Scholar]
- 19. Bougie E, Masson-Côté L, Mathieu D. Comparison between surgical resection and stereotactic radiosurgery in patients with a single brain metastasis from non-small cell lung cancer. World Neurosurg. 2015;83(6):900-906. [DOI] [PubMed] [Google Scholar]
- 20. Choi CYH, Chang SD, Gibbs ICet al. What is the optimal treatment of large brain metastases? An argument for a multidisciplinary approach. Int J Radiat Oncol Biol Phys. 2012;84(3):688-693. [DOI] [PubMed] [Google Scholar]
- 21. Kocher M, Soffietti R, Abacioglu Uet al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29(2):134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427-434. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10487566. Accessed May 26, 2016. [DOI] [PubMed] [Google Scholar]
- 23. Aoyama H, Shirato H, Tago Met al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491. [DOI] [PubMed] [Google Scholar]
- 24. Chang EL, Wefel JS, Hess KRet al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044. [DOI] [PubMed] [Google Scholar]
- 25. Langley RE, Stephens RJ, Nankivell Met al. Interim data from the Medical Research Council QUARTZ Trial: does whole brain radiotherapy affect the survival and quality of life of patients with brain metastases from non-small cell lung cancer? Clin Oncol (R Coll Radiol). 2013;25(3):e23-e30. doi:10.1016/j.clon.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 26. Brown PD, Jaeckle K, Ballman K Vet al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases. JAMA. 2016;316(4):401. doi:10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sneed PK, Suh JH, Goetsch SJet al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002;53(3):519-526. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12062592. Accessed May 26, 2016. [DOI] [PubMed] [Google Scholar]
- 28. Yamamoto M, Serizawa T, Shuto Tet al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387-395. [DOI] [PubMed] [Google Scholar]
- 29. Lim SH, Lee JY, Lee M-Yet al. A randomized phase III trial of stereotactic radiosurgery (SRS) versus observation for patients with asymptomatic cerebral oligo-metastases in non-small-cell lung cancer. Ann Oncol. 2015;26(4):762-768. [DOI] [PubMed] [Google Scholar]
- 30. Shultz DB, Modlin LA, Jayachandran Pet al. Repeat courses of stereotactic radiosurgery (SRS), deferring whole-brain irradiation, for new brain metastases after initial SRS. Int J Radiat Oncol Biol Phys. 2015;92(5):993-999. [DOI] [PubMed] [Google Scholar]
- 31. Soltys SG, Kirkpatrick JP, Laack NN, Kavanagh BD, Breneman JC, Shih HA. Is less, more? the evolving role of radiation therapy for brain metastases. Int J Radiat Oncol. 2015;92(5):963-966. [DOI] [PubMed] [Google Scholar]
- 32. Soltys SG, Adler JR, Lipani JDet al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2008;70(1):187-193. [DOI] [PubMed] [Google Scholar]
- 33. Cohen-Inbar O, Sheehan JP. Fighting cancer on all fronts: stereotactic radiosurgery and the role for aggressive primary treatment in non-small cell lung cancer patients with one brain metastasis. World Neurosurg. 2015;83(6):1015-1016. [DOI] [PubMed] [Google Scholar]
- 34. Magnuson WJ, Lester-Coll NH, Wu AJet al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor–mutant non-small-cell lung cancer: a retrospective multi- institutional analysis. J Clin Oncol. 2017;35(10):1070-1077. [DOI] [PubMed] [Google Scholar]
- 35. Mok TS, Wu Y-L, Thongprasert Set al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med. 2009;361(10):947-957. Available at: http://dx.doi.org/101056/NEJMoa0810699. Accessed July 12, 2016. [DOI] [PubMed] [Google Scholar]
- 36. Fukuoka M, Wu Y-L, Thongprasert Set al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866-2874. [DOI] [PubMed] [Google Scholar]
- 37. Chen G, Feng J, Zhou Cet al. Quality of life (QoL) analyses from OPTIMAL (CTONG-0802), a phase III, randomised, open-label study of first-line erlotinib versus chemotherapy in patients with advanced EGFR mutation-positive non-small-cell lung cancer (NSCLC). Ann Oncol. 2013;24(6):1615-1622. [DOI] [PubMed] [Google Scholar]
- 38. Sequist L V, Yang JC-H, Yamamoto Net al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334. [DOI] [PubMed] [Google Scholar]
- 39. Moro-Sibilot D, Smit E, de Castro Carpeño Jet al. Non-small cell lung cancer patients with brain metastases treated with first-line platinum-doublet chemotherapy: Analysis from the European FRAME study. Lung Cancer. 2015;90(3):427-432. [DOI] [PubMed] [Google Scholar]
- 40. Rosell R, Carcereny E, Gervais Ret al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. [DOI] [PubMed] [Google Scholar]
- 41. Zhou C, Wu Y-L, Chen Get al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. [DOI] [PubMed] [Google Scholar]
- 42. Mitsudomi T, Morita S, Yatabe Yet al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. 2010;11(2):121-128. [DOI] [PubMed] [Google Scholar]
- 43. Maemondo M, Inoue A, Kobayashi Ket al. Gefitinib or Chemotherapy for Non-Small-Cell Lung Cancer with Mutated EGFR. N Engl J Med. 2010;362(25):2380-2388. Available at: http://dx.doi.org/101056/NEJMoa0909530. Accessed July 12, 2016. [DOI] [PubMed] [Google Scholar]
- 44. Schuler M, Wu Y-L, Hirsh Vet al. First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol. 2016;11(3):380-390. [DOI] [PubMed] [Google Scholar]
- 45. Iuchi T, Shingyoji M, Sakaida Tet al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013;82(2):282-287. [DOI] [PubMed] [Google Scholar]
- 46. Park SJ, Kim HT, Lee DHet al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77(3):556-560. [DOI] [PubMed] [Google Scholar]
- 47. Ballard P, Yates JWT, Yang Zet al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20). Available at: http://clincancerres.aacrjournals.org.laneproxy.stanford.edu/content/22/20/5130.long. Accessed May 21, 2017. [DOI] [PubMed] [Google Scholar]
- 48. Yu HA, Arcila ME, Rekhtman Net al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barlesi F, Gervais R, Lena Het al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol. 2011;22(11):2466-2470. [DOI] [PubMed] [Google Scholar]
- 50. Bailon O, Chouahnia K, Augier Aet al. Upfront association of carboplatin plus pemetrexed in patients with brain metastases of lung adenocarcinoma. Neuro Oncol. 2012;14(4):491-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dinglin X-X, Huang Y, Liu H, Zeng Y-D, Hou X, Chen L-K. Pemetrexed and cisplatin combination with concurrent whole brain radiotherapy in patients with brain metastases of lung adenocarcinoma: a single-arm phase II clinical trial. J Neurooncol. 2013;112(3):461-466. [DOI] [PubMed] [Google Scholar]
- 52. Yu HA, Sima CS, Huang Jet al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8(3):346-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25(2):103-119. [DOI] [PubMed] [Google Scholar]
- 54. Xu Q, Chen X, Qian Det al. Treatment and prognostic analysis of patients with leptomeningeal metastases from non-small cell lung cancer. Thorac cancer. 2015;6(4):407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gong L, Xiong M, Huang Z, Miao L, Fan Y. Icotinib might be effective for the treatment of leptomeningeal carcinomatosis in non-small cell lung cancer with sensitive EGFR mutations. Lung Cancer. 2015;89(3):268-273. [DOI] [PubMed] [Google Scholar]
- 56. Lee E, Keam B, Kim D-Wet al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol. 2013;8(8):1069-1074. [DOI] [PubMed] [Google Scholar]
- 57. Riess JW, Nagpal S, Iv Met al. Prolonged survival of patients with non-small-cell lung cancer with leptomeningeal carcinomatosis in the modern treatment era. Clin Lung Cancer. 2014;15(3):202-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grommes C, Oxnard GR, Kris MGet al. Pulsatile high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13(12):1364-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhu Y, Du Y, Liu H, Ma T, Shen Y, Pan Y. Study of efficacy and safety of pulsatile administration of high-dose gefitinib or erlotinib for advanced non-small cell lung cancer patients with secondary drug resistance: A single center, single arm, phase II clinical trial. Thorac Cancer. 2016;7(6):663-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morris PG, Reiner AS, Szenberg ORet al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7(2):382-385. [DOI] [PubMed] [Google Scholar]