Abstract
Background
The link between e-cigarette use and subsequent development of respiratory diseases remains an open question.
Aims and Methods
A subset of a probability sample of U.S. adults from the Population Assessment of Tobacco and Health Study Waves 1 and 2 were selected for biospecimen analysis (n = 4614). Subjects were divided into three mutually exclusive groups at baseline: nonusers (n = 2849), exclusive e-cigarette users (n = 222), and poly e-cigarette/tobacco users (n = 1,543). Geometric mean concentrations of baseline biomarkers from five classes of harmful and potentially harmful constituents were reported. Multivariable linear regressions were conducted to examine the relationship between baseline biomarkers and subsequent respiratory symptoms among user groups.
Results
Baseline exclusive e-cigarette users (33.6%[confidence interval, CI: 26.7% to 41.4%]) and poly e-cigarette/tobacco users (50.8%[CI: 47.4% to 54.2%]) had higher prevalence of subsequent respiratory symptoms than nonusers (21.7%[19.2% to 24.4%]). As compared with nonusers, poly e-cigarette/tobacco users had higher concentrations in clinically relevant biomarkers at baseline than exclusive e-cigarette users. Among poly e-cigarette/tobacco users, baseline nicotine metabolites (TNE2, cotinine), tobacco-specific nitrosamine (NNAL), PAH (1-NAP, 3-FLU), and volatile organic compound (N-Acetyl-S-(2-carboxyethyl)-l-cysteine, N-acetyl-S-(2-cyanoethyl)-l-cysteine) were significantly higher among those reporting subsequent respiratory symptoms than those who did not. Among exclusive e-cigarette users, baseline NNAL was significantly higher among those reporting subsequent respiratory symptoms than those who did not. Within subjects with subsequent respiratory symptoms, NNAL was 2.5 times higher in exclusive e-cigarette users (10.7[6.5 to 17.5]) and 63.4 times higher in poly e-cigarette/tobacco users (199.6[176.7 to 225.4]) than nonusers (3.1[2.4 to 3.9]).
Conclusions
E-cigarette use is associated with higher concentrations of known tobacco-related toxicants and risks of subsequent respiratory symptoms than nonusers. Poly e-cigarette/tobacco users exhibit higher risk than exclusive e-cigarette users.
Implications
This longitudinal study identified positive associations between baseline urinary biomarkers of exposure to tobacco-related toxicants and increased risks of subsequent respiratory symptoms across varying e-cigarette use groups. E-cigarette use is associated with increased exposure to known tobacco-related toxicants, and certain toxicant exposure increases the risk of respiratory symptoms.
Introduction
Tobacco use is the leading cause of preventable death worldwide. Tobacco smoke contains a deadly mix of more than 7000 chemicals, and smoking increases the risk of health problems, including coronary heart disease, stroke, cancer, and many other diseases.1 The tobacco use landscape is changing among U.S. adults. Although combustible cigarettes continue to be the most commonly used tobacco product by U.S. adults, e-cigarette use has been rising steadily in the United States.2 In 2018, an estimated 13.7% (34.2 million) and 3.2% (8.1 million) of adults aged 18 years and older reported smoking cigarettes or using e-cigarettes in the past 30 days, respectively. Furthermore, 19% of current tobacco users (9.3 million adults) reported using two or more tobacco products in 2018.2 E-cigarette makers have been extensively using harm reduction and a healthier alternative of cigarettes as their marketing strategies to promote e-cigarettes, which has led to the initiation of e-cigarette use among never and former smokers with a high prevalence of dual-use of e-cigarette and other tobacco products.3
As of February 18, 2020, a national outbreak of severe respiratory diseases with 2807 E-cigarette, or Vaping, Product-Use-Associated Lung Injury case-patients have been reported with 68 confirmed deaths.4 While this outbreak has been linked to tetrahydrocannabinol-containing e-cigarette products, particularly from informal sources and specifically strongly linked to Vitamin E acetate that is often an additive to tetrahydrocannabinol,5 few studies have examined the relationship between e-cigarette use and other respiratory diseases among U.S. adults.6–9 For instance, Bhatta and Glantz8 analyzed the first 3 Waves of the adult Population Assessment of Tobacco and Health (PATH) Study. They found that current and former e-cigarette use at Wave 1 without respiratory disease were significantly associated with having incident respiratory disease at Wave 2 or 3. However, all of these studies were based on self-reported tobacco use measures, so objective measures of nicotine exposure and toxicants are needed to confirm the association with respiratory diseases.
Biomarkers of exposure to nicotine and harmful constituents can play an important role in assessing the risk of e-cigarette use to the general population, as biomarkers can serve as intermediate endpoints for assessing the health risk of new tobacco products in the absence of long-term epidemiological evidence.10,11 Evidence suggests that e-cigarette aerosol is less toxic than combustible cigarette smoking.3 For instance, a study analyzing the biomarker outcomes found that exclusive e-cigarette users showed 10%–98% significantly lower concentrations of biomarkers of exposure, including tobacco-specific nitrosamines (TSNAs), polycyclic aromatic hydrocarbons (PAHs), most volatile organic compounds (VOCs), and nicotine, compared with exclusive cigarette smokers.12 However, e-cigarette aerosol is not harmless as studies have identified harmful and potentially harmful constituents (HPHCs) in e-cigarettes.13 For instance, compared with exclusive e-cigarette users, never users had 19%–81% significantly lower concentrations of biomarkers of exposure to nicotine, TSNAs, some metals, and some VOCs.12 A national assessment using 2015 Nielsen point of sales data identified that 99% of e-cigarette products sold in the market contain nicotine, which is a highly addictive stimulant to the central nervous system.14 Furthermore, flavored e-cigarette use was reported by over 80% of U.S. adult current e-cigarette users,15,16 and concerns have been raised about the potential toxicity of these flavorings.17 For example, Benzaldehyde, a key ingredient in natural fruit flavor, has been shown to cause irritation of respiratory airways,18 and diacetyl has been linked to serious lung disease.13 However, the potential association between biomarkers of e-cigarette use and subsequent respiratory diseases or symptoms remains unknown.
To better demonstrate this association, this study analyzes biospecimen data to examine the longitudinal associations of e-cigarette use on functionally important respiratory symptoms by linking the PATH biomarker restricted-use files at Wave 1 with PATH adult interview files at Waves 1 and 2. We seek to (1) examine the associations between self-reported e-cigarette use status at baseline (Wave 1) and respiratory symptoms at the 1-year follow-up (Wave 2) and (2) assess the relationship between concentration levels of biomarkers at Wave 1 and self-reported respiratory symptoms at Wave 2 across e-cigarette use groups. We hypothesize that exposure to certain HPHC varies by e-cigarette use status at baseline, which is associated with the subsequent respiratory symptoms.
Data and Measures
Data
The PATH Study is a longitudinal cohort study of tobacco use behaviors, attitudes, beliefs, and health outcomes among a nationally representative sample of U.S. civilian, non-institutionalized individuals 12-years old and older.19 The Wave 1 data were collected between September 2013 and December 2014, followed by Wave 2 data between October 2014 and October 2015. A four-stage, stratified probability sampling design was used in the PATH Study, which intentionally oversampled adult tobacco users, young adults, and African Americans. The study was conducted by Westat and approved by Westat’s Institutional Review Board. Further details regarding the data collection, study design, and methods can be found in the study user guide.19
All Wave 1 adult respondents (aged 18 and above) were asked to voluntarily provide urine and blood samples. Of those who provided a urine specimen, a stratified probability sample of 11 522 respondents was selected for biomarker analysis to ensure that responders represent a diverse mix of tobacco use groups, including users of multiple tobacco products and never users of any tobacco products. Details of biospecimen collection and laboratory procedures were provided in the biospecimen urine collection procedures. Respondents reported their use of all nicotine-containing products within a 3-day period of biospecimen collection, and nicotine exposure questions were incorporated into the adult interview.20
Measures
Biomarkers at Wave 1
include a variety of biomarkers (n = 55) associated with tobacco exposure, including (1) nicotine metabolites and minor tobacco alkaloids, (2) TSNAs, (3) heavy metals, (4) PAHs, and (5) VOCs. A selected panel of biomarkers (n = 10) with the most relevance to respiratory symptoms are presented in the main text. These biomarkers were selected based on clinical relevance with health outcomes, FDA HPHC classification as respiratory toxicants, International Agency for Research on Cancer (IARC) evaluation of carcinogenicity, and differential concentration level by e-cigarette use status.12,21–24Supplementary material, Table 1 presents the list of 55 tobacco-related biomarkers and their clinical relevance.
Individuals who reported using nicotine replacement therapies in the past 3 days (n = 38) or had creatinine values outside the normal range of 10–370 mg/dL (n = 253) were excluded in this analysis. Biomarker concentrations below the limit of detection were imputed using a common substitution formula (the limit of detection divided by the square root of 2).25
Tobacco Use Status at Wave 1
At baseline, those who reported currently using e-cigarettes some days or every day were classified as current e-cigarette users. Current use (some days or every day) of tobacco products (ie, cigarettes, traditional cigars, cigarillos, filtered cigars, pipe, hookahs, smokeless tobacco, snus, and dissolvable tobacco) was classified as “yes” vs. “no.” Based on the current use of e-cigarettes and tobacco products, we created three mutually exclusive e-cigarette use groups: nonusers, exclusive e-cigarette users, and poly e-cigarette/tobacco users who reported currently using e-cigarettes and one or more type of tobacco product. Those who reported currently using tobacco products but not e-cigarettes were excluded from the study.
Sociodemographics and Other Covariates at Wave 1
Sociodemographic covariates included age (18–24, 25–34, 35–44, 45–54, 55–64, or 65+), sex (male/female), race/ethnicity (non-Hispanic (NH) white, NH black, Hispanic, or other NH classifications), education (less than high school, high school graduate, some college, or Bachelor’s degree or above), income level (<$10 000, $10 000–$24 999, $25 000–$49 999, $50 000–$99 999, $100 000+), region (Northeast, South, Midwest, West), and urbanacity (urban v. not urban).
Baseline Exposure to Secondhand Smoke
was determined by the question, “Not including yourself, does anyone who lives with you now do any of the following? Choose all that apply.” Those who responded “Smoke cigarettes,” “Use smokeless tobacco such as snus, chewing tobacco, snuff, or dip Smoke,” “traditional cigars, cigarillos, or filtered cigars,” and “Use any other form of tobacco” were classified as exposure to SHS. We also created a 3-level variable to measure home rule on smoking a combustible tobacco product (“Not allowed anywhere or at any time,” “allowed in some places or at some times,” and “allowed anywhere and at any time.”) A similar variable on home rule of using noncombustible tobacco products was also created.
Other Substance Use
at Wave 1 includes past 12-month marijuana use (yes/no), past 12-month alcohol use (yes/no), and ever use of other drug (ie, ever misuse of prescription drugs (ie, Ritalin, Adderall, painkillers, sedatives, or tranquilizers) or ever use of cocaine or crack, stimulants like methamphetamine or speed, heroin, inhalants, solvents, or hallucinogens).
Baseline Lung or Respiratory Disease
was determined by the questions “Has a doctor or other health professional ever told you that you had any of the following lung or respiratory conditions? Choose all that apply.” Those who marked “COPD,” “chronic bronchitis,” “emphysema,” “asthma,” or “some other lung or respiratory condition” were coded as having baseline lung or respiratory disease.
Respiratory Symptoms at Wave 2
Respiratory symptoms in the past 12 months were measured by three questions,26 including: “Have you had wheezing or whistling in the chest in the past 12 months?” “In the past 12 months, has your chest sounded wheezy during or after exercise?” “In the past 12 months, have you had a dry cough at night, apart from a cough associated with a cold or chest infection?” We further created a binary variable to measure any respiratory symptoms for those who responded affirmatively to any of these questions.
Statistical Methods
Urinary biomarkers were calculated as a normalized ratio to urinary creatinine concentration in order to control for variations in urine flow rate. Due to the skewness in the distribution, data were transformed using a natural log. Geometric mean and 95% confidence intervals of creatinine corrected biomarker concentration levels were reported. The prevalence of respiratory symptoms was reported by e-cigarette use status. Within each e-cigarette use group, the geometric means of biomarker concentration levels were compared to any respiratory symptom status (yes vs. no). We combined respiratory symptoms to increase the sample size and reduce the Type I error from multiple testing of individual symptoms. A sensitivity analysis was conducted for each respiratory symptom, yielding consistent results.
In univariate analysis, comparisons were performed by sample characteristics and e-cigarette use groups. Multivariable general linear regressions were conducted to examine the relationship between biomarker concentrations at Wave 1 and respiratory symptoms at Wave 2, adjusted by baseline sociodemographics (sex, age, race/ethnicity, and education), self-reported exposure to secondhand smoke (SHS), past 12-month use of marijuana, and lung or respiratory disease.
Statistical analyses were performed with SAS 9.4 (Cary, NC) using urinary sample weight, 100 replicated weights, and the balanced repeated replication method with Fay’s adjustment = 0.3 to account for the PATH Study’s complex design.27,28 Significance was two tailed with adjustment for multiple comparisons using the Bonferroni method (0.05/number of comparisons) in the main text.
Sensitivity Analysis
Since baseline lung or respiratory disease may be related to biomarker concentrations and subsequent respiratory symptoms, we adjusted for baseline lung or respiratory disease in the multivariable analysis. To further remove this potential confounding effect, we conducted a sensitivity analysis for subjects without a history of lung or respiratory disease at baseline (n = 3655) in Supplementary material.
Results
Descriptive Analyses and E-cigarette Use Status
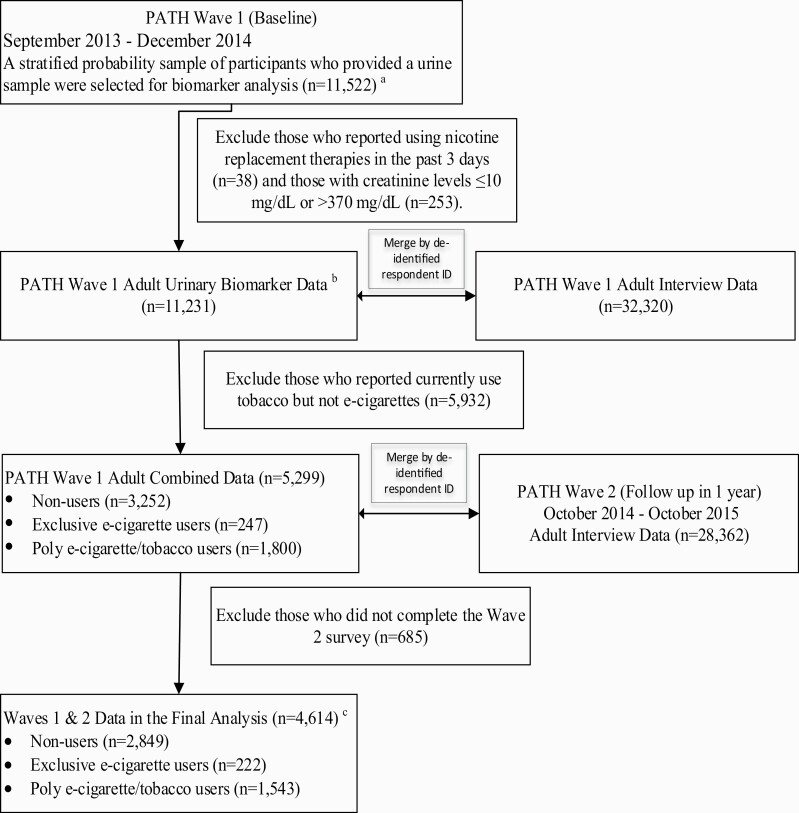
Figure 1 depicts the study design, linkage of biomarker and adult interview data in Waves 1 and 2, and subject selection criteria. In Wave 1, PATH used a stratified probability design to select 11 522 adults who provided a urine sample in biomarker restricted-use files. We excluded participants with nicotine replacement therapy use in the past 3 days (n = 38), creatinine out of normal range (n = 253), use of tobacco but not e-cigarettes (n = 5932) in Wave 1 and those who were lost to follow-up in Wave 2 (n = 685). This resulted in 4614 participants in the final analysis, including 2849 nonusers, 222 exclusive e-cigarette users, and 1543 poly e-cigarette/tobacco users (741 dual users of e-cigarettes and cigarettes, 130 users of e-cigarettes and other noncigarette tobacco products, and 672 users of e-cigarette, cigarettes, and other tobacco products).
Figure 1.
A flow chart of longitudinal study design and subject selection criteria. aWave 1 final person-level urinary specimen sampling weight and 100 replicate weights were applied to produce a nationally representative dataset of U.S. adults with varying tobacco use statuses at Wave 1. bRespondents reported their use of all nicotine-containing products during a 3-day period prior to the time of any biospecimen collection (Nicotine Exposure Questions [NEQs]). NEQs were incorporated into the adult interview. cThe results of the final analysis are presented in the main text. A sensitivity analysis was performed by excluding subjects with a history of respiratory diseases, including COPD, chronic bronchitis, emphysema, asthma, and some other lung or respiratory condition at Wave 1. See Supplementary material.
Sample characteristics and e-cigarette use status at Wave 1 are shown in Table 1. The final analytical sample included 17.3% [95% CI 16.2% to 18.4%] of adults aged 18–24 years old and 27.7% [CI 25.5% to 30.1%] of adults aged 55 years old or above, 58.6% [CI 56.5% to 60.7%] of females, 58.9% [CI 55.9% to 61.9%] of NH whites, 17.9% [CI 16.3% to 19.7%] with less than high school education and 17.7% [CI 15.3% to 20.4%] reported annual income <$10 000. 9.9% [CI 8.9% to 11.1%] reported past 12-month marijuana use, and 27.7% [CI 24.5% to 31.1%] reported exposure to SHS.
Table 1.
Sample characteristics and e-cigarette use status at Wave 1 (baseline)
| N | Weighted % (95% CI)b | Current E-cigarette use statusa | ||||
|---|---|---|---|---|---|---|
| No use (n = 2849)c | Exclusive E-cigarette use (n = 222)c | Poly e-cigarette/tobacco use (n = 1543)c | p-valued | |||
| Overall | 4614 | 100 | 86.9 (85.7–87.9) | 1.9 (1.5–2.3) | 11.3 (10.3–12.3) | |
| Age | <.0001 | |||||
| 18–24 | 1608 | 17.3 (16.2–18.4) | 84.5 (81.7–86.9) | 1.7 (1.2–2.4) | 13.8 (11.6–16.4) | |
| 25–34 | 955 | 20.2 (18.2–22.4) | 82.4 (79.6–84.9) | 2.4 (1.6–3.6) | 15.2 (12.9–17.8) | |
| 35–54 | 1333 | 34.8 (32.2–37.5) | 86.6 (84.7–88.3) | 1.8 (1.2–2.6) | 11.6 (10.0–13.5) | |
| 55+ | 718 | 27.7 (25.5–30.1) | 91.9 (89.7–93.7) | 1.7 (1.0–2.9) | 6.4 (4.8–8.4) | |
| Sex | <.0001 | |||||
| Male | 2178 | 41.4 (39.3–43.5) | 83.9 (81.9–85.7) | 1.9 (1.4–2.6) | 14.2 (12.5–16.1) | |
| Female | 2436 | 58.6 (56.5–60.7) | 89 (87.6–90.3) | 1.8 (1.4–2.4) | 9.2 (8.0–10.5) | |
| Race/ethnicity | <.0001 | |||||
| NH White | 2669 | 58.9 (55.9–61.9) | 83.9 (82–85.7) | 2.5 (2.0–3.2) | 13.6 (12.0–15.3) | |
| NH Black | 624 | 13.1 (11.3–15.0) | 91.6 (89.2–93.6) | 1.1 (0.6–2.3) | 7.2 (5.4–9.5) | |
| Hispanics | 863 | 18.7 (16.8–20.8) | 91.5 (89.5–93.2) | 0.7 (0.3–1.6) | 7.7 (6.1–9.7) | |
| NH others | 458 | 9.3 (7.8–11.0) | 89.6 (86.4–92) | 1 (0.4–2.5) | 9.4 (7.1–12.4) | |
| Education | <.0001 | |||||
| Less than high school | 1007 | 17.9 (16.3–19.7) | 84.4 (81.5–86.9) | 1.5 (0.9–2.5) | 14.1 (11.8–16.7) | |
| High school graduate | 1149 | 25.3 (22.4–28.4) | 87.4 (85.2–89.3) | 1.8 (1.2–2.8) | 10.8 (9–12.8) | |
| Some college | 1775 | 29.5 (27.2–31.9) | 81.9 (79.6–84.0) | 2.8 (2.1–3.6) | 15.3 (13.4–17.5) | |
| Bachelor’s degree or above | 683 | 27.3 (24.4–30.3) | 93.4 (91.3–95) | 1.1 (0.6–2.2) | 5.5 (4.0–7.5) | |
| Income | <.0001 | |||||
| <$10 000 | 954 | 17.7 (15.3–20.4) | 86.2 (83.3–88.7) | 1.4 (0.8–2.4) | 12.4 (10.1–15.1) | |
| $10 000–$24 999 | 1123 | 20.0 (17.9–22.2) | 83.1 (80.8–85.1) | 2.2 (1.5–3.2) | 14.7 (12.8–16.9) | |
| $25 000–$49 999 | 991 | 22.2 (19.9–24.8) | 84.5 (81.9–86.9) | 2.0 (1.3–3.1) | 13.4 (11.3–15.9) | |
| $50 000–$99,999 | 819 | 24.7 (21.8–27.8) | 88.6 (86.2–90.6) | 2.1 (1.3–3.3) | 9.4 (7.6–11.6) | |
| $100 000+ | 402 | 15.4 (13.0–18.2) | 91.5 (88.3–93.8) | 1.1 (0.5–2.7) | 7.4 (5.2–10.4) | |
| Region | .0105 | |||||
| Northeast | 595 | 17.1 (14.5–20.1) | 91.2 (88.6–93.3) | 1.1 (0.5–2.3) | 7.7 (5.8–10.1) | |
| South | 1067 | 20.1 (18.0–22.2) | 85 (81.7–87.7) | 2.5 (1.7–3.7) | 12.5 (10.1–15.4) | |
| Midwest | 1826 | 38.9 (35.6–42.3) | 86 (83.9–87.9) | 1.9 (1.4–2.7) | 12.1 (10.3–14.0) | |
| West | 1126 | 23.9 (21.1–26.9) | 86.8 (84.1–89) | 1.7 (1.1–2.7) | 11.5 (9.3–14.1) | |
| Urbanicity | .002 | |||||
| Urban | 3639 | 80.7 (76.4–84.3) | 87.6 (86.5–88.7) | 1.8 (1.4–2.3) | 10.5 (9.5–11.6) | |
| Non-urban | 975 | 19.3 (15.7–23.6) | 83.6 (80.5–86.4) | 2.0 (1.3–3.1) | 14.4 (11.9–17.2) | |
| Past 12-month alcohol use | <.0001 | |||||
| No | 1622 | 45.4 (40.9–50.0) | 92.9 (91.5–94.0) | 1.3 (0.8–2.0) | 5.8 (4.8–7.1) | |
| Yes | 2992 | 54.6 (50.0–59.1) | 81.9 (79.9–83.7) | 2.4 (1.9–3.0) | 15.8 (14.1–17.6) | |
| Past 12-month Marijuana use | <.0001 | |||||
| No | 3462 | 90.1 (88.9–91.1) | 89.9 (88.9–90.9) | 1.6 (1.3–2.1) | 8.4 (7.6–9.4) | |
| Yes | 1152 | 9.9 (8.9–11.1) | 59.2 (54.7–63.5) | 4.0 (3.0–5.4) | 36.8 (32.5–41.3) | |
| Ever use of other illicit drug and substance | <.0001 | |||||
| No | 3049 | 81.3 (79.4–83.1) | 91.6 (90.6–92.5) | 1.4 (1.0–1.9) | 7.0 (6.1–7.9) | |
| Yes | 1565 | 18.7 (16.9–20.6) | 66.2 (62.6–69.7) | 3.9 (3.0–5.0) | 29.9 (26.6–33.4) | |
| Exposure to SHS | <.0001 | |||||
| No | 2666 | 72.3 (68.9–75.5) | 91.6 (90.5–92.6) | 1.7 (1.3–2.3) | 6.7 (5.8–7.7) | |
| Yes | 1948 | 27.7 (24.5–31.1) | 74.5 (71.4–77.4) | 2.3 (1.7–3.1) | 23.2 (20.5–26.2) | |
| Home rule for combustible tobacco use | <.0001 | |||||
| Not allowed | 3226 | 82.0 (79.2–84.4) | 90.5 (89.4–91.4) | 1.7 (1.3–2.2) | 7.8 (6.9–8.8) | |
| Partially allowed | 765 | 9.3 (7.8–11.1) | 66.2 (59.8–72) | 3.3 (2.2–4.8) | 30.6 (25–36.7) | |
| Allowed | 606 | 8.7 (7.0–10.7) | 75.5 (69–81.1) | 1.6 (0.8–3.2) | 22.8 (17.8–28.8) | |
| Home rule for noncombustible tobacco use | <.0001 | |||||
| Not allowed | 2509 | 73.9 (71.0–76.7) | 94.5 (93.6–95.4) | 0.7 (0.4–1.1) | 4.8 (4.0–5.7) | |
| Partially allowed | 819 | 11.4 (10.1–12.9) | 71.2 (67.5–74.8) | 4.0 (2.8–5.5) | 24.8 (21.7–28.1) | |
| Allowed | 1253 | 14.7 (12.6–16.9) | 60.3 (54.2–66.1) | 6.4 (5.0–8.3) | 33.3 (28.3–38.6) | |
| Lung or respiratory disease e | ||||||
| No | 3655 | 82.6 (80.2–84.8) | 87.9 (86.7–89.1) | 1.8 (1.4–2.3) | 10.3 (9.3–11.4) | <.0001 |
| Yes | 946 | 17.4 (15.2–19.8) | 81.8 (78.6–84.6) | 2.2 (1.4–3.3) | 16.0 (13.5–18.9) |
aAt Wave 1, subjects were divided into three mutually exclusive e-cigarette user groups (nonusers, exclusive e-cigarette users, and poly e-cigarette/tobacco users) based on the self-report. All analyses applied urinary sample weight, 100 replicated weights, and the balanced repeated replication method with Fay’s adjustment = 0.3 to account for the PATH study’s complex design.
bWeighted % and 95% CI were calculated within the column as prevalences of characteristics.
cWeighted % and 95% CI were calculated within the row as prevalences of e-cigarette use status.
dRao Scott chi-square test was performed to compare the distribution of e-cigarette use status by sample characteristics, taking the complex sampling design into account.
eAt Wave 1, baseline lung or respiratory disease was determined by the questions “Has a doctor or other health professional ever told you that you had any of the following lung or respiratory conditions? Choose all that apply.” Those which marked “COPD,” “chronic bronchitis,” “emphysema,” “asthma,” or “some other lung or respiratory condition” were coded as ever having baseline lung or respiratory disease. Baseline lung or respiratory disease was treated as a covariate and adjusted for confounding effects in the main analysis. A sensitivity analysis was further performed by excluding subjects with baseline lung or respiratory disease. See Supplementary material.
Overall, 86.9% [CI 85.7% to 87.9%] of participants were self-reported nonusers, 1.9% [CI 1.5% to 2.3%] were exclusive e-cigarette users, and 11.3% [CI 10.3% to 12.3%] were poly users of e-cigarette and tobacco. Young adults (vs. older adults) and males (vs. females) were more likely to be poly e-cigarette/tobacco users, and NH whites were less likely than adults with other races to be nonusers. Adults with Bachelor’s degree or above (vs. no) or those with income over $100 000 (vs. no) were less likely to report poly use of e-cigarettes and tobacco products. Those who reported past 12-month marijuana use were more likely than non-marijuana users to report exclusive e-cigarette use (4.0% [CI 3.0% to 5.4%] vs. 1.6% [CI 1.3% to 2.1%], p < .0001) and poly-use of e-cigarettes and tobacco (36.8% [CI 32.5% to 41.3%] vs. 8.4% [CI 7.6% to 9.4%], p < .0001). Adults who reported exposure to SHS were less likely to be no current tobacco users than those reporting no exposure (74.5% [CI 71.4% to 77.4%] vs. 91.6% [CI 90.5% to 92.6%], p < .0001).
Self-Reported Respiratory Symptoms at Wave 2
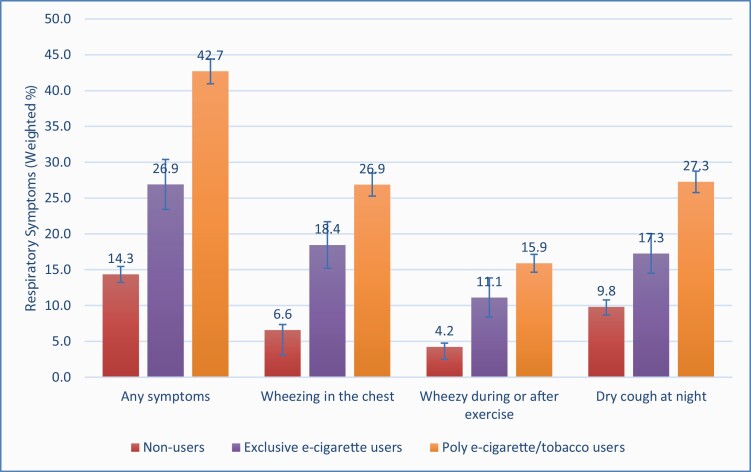
The prevalence of respiratory symptoms at Wave 2 by baseline e-cigarette use status is presented in Figure 2. Adults who reported exclusive e-cigarette use (33.6% [95% CI 26.7% to 41.4%] or poly-use (50.8% [47.4% to 54.2%]) at baseline had a higher prevalence of respiratory symptoms in the past 12 months than those reporting no current tobacco use (21.7% [19.2% to 24.4%]). Significant differences were also observed in all three functionally important respiratory symptoms, including wheezing or whistling in the chest, having chest wheeziness during or after exercise, and having a dry cough at night in the past 12 months.
Figure 2.
Prevalence of functionally important respiratory symptoms at Wave 2a in association with e-cigarette use status at Wave 1b. aAt Wave 2, respiratory symptoms in the past 12 months were measured by three questions, including “Have you had wheezing or whistling in the chest in the past 12 months?” (yes vs. no) “In the past 12 months, has your chest sounded wheezy during or after exercise?” (yes vs. no) “In the past 12 months, have you had a dry cough at night, apart from a cough associated with a cold or chest infection?” (yes vs. no). We further created a binary variable to measure combined respiratory symptoms for those who responded affirmatively to any of these questions. bAt Wave 1, subjects were divided into three mutually exclusive e-cigarette user groups (no use of e-cigarette and tobacco product, exclusive e-cigarette use, and use of e-cigarettes and 1+ type of tobacco product) based on the self-report. cWeighted percentage and standard error are reported to reflect the prevalence of respiratory symptoms during the time of Wave 2.
Association Between Baseline Biomarker and Self-Reported Respiratory Symptoms at Wave 2
The associations between 10 selected biomarkers and respiratory symptoms are shown in Table 2. A complete assessment of all 55 biomarkers is available in Supplementary material, Tables 2–4.
Table 2.
Urinary biomarkersa at Wave 1 in association with functionally important respiratory symptoms at Wave 2
| Urinary biomarkers | Nonusers (n = 2849) | Exclusive E-cigarette users (n = 222) | Poly E-cigarette/tobacco users (n = 1543) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Respiratory symptoms | Respiratory symptoms | Respiratory symptoms | |||||||
| No (2008) | Yes (n = 829) | Adjusted p-valueb | No (n = 153) | Yes (n = 69) | Adjusted p-valueb | No (n = 756) | Yes (n = 781) | Adjusted p-valueb | |
| Geo mean (95% CI) | Geo mean (95% CI) | Geo mean (95% CI) | Geo mean (95% CI) | Geo mean (95% CI) | Geo mean (95% CI) | ||||
| Urinary nicotine metabolites (ng/mg creatinine) | |||||||||
| TNE2c(nmol/mg creatinine) | 0.01 (0.01-0.01) | 0.04 (0.03–0.06)1 | <.0001 | 2.3 (1.2–4.6) | 5.9 (2.3–14.7)2 | 0.017 | 12.5 (9.7–16.2) | 31.1 (26.9–36.0)3 | <.0001 |
| Cotinine | 0.8 (0.6–0.9) | 2.7 (2–3.7)1 | <.0001 | 148.1 (72.9–301.0) | 341.3 (135.3–861.0)2 | 0.0245 | 817.6 (626.1–1067.5) | 2031.4 (1769.0–2332.6)3 | <.0001 |
| TSNAs (pg/mg creatinine) | |||||||||
| NNAL | 1.4 (1.2–1.6) | 3.1 (2.4–3.9)1 | <.0001 | 4.4 (3.5–5.6) | 10.7 (6.5–17.5)2 | 0.0043 | 98.9 (79.7–122.7) | 199.6 (176.7–225.4)3 | 0.0001 |
| N′-Nitrosonornicotine | 2.2 (2.0–2.3) | 2.8 (2.5–3)1 | 0.0053 | 3.3 (2.8–3.8) | 3.6 (2.7–4.8)1 | 0.8939 | 8.0 (6.9–9.4) | 10.2 (9.4–11.2)2 | 0.4374 |
| Heavy metals (ng/mg creatinine) | |||||||||
| Cadmium (UCD) | 0.2 (0.1–0.2) | 0.2 (0.2-0.2)1 | 0.2931 | 0.2 (0.1–0.2) | 0.3 (0.2–0.4)2 | 0.0110 | 0.2 (0.1–0.2) | 0.2 (0.2–0.3)2 | 0.8579 |
| Lead (UPB) | 0.4 (0.3–0.4) | 0.4 (0.3–0.4)1 | 0.5484 | 0.4 (0.3–0.4) | 0.5 (0.4–0.7)2 | 0.0885 | 0.4 (0.4-0.4) | 0.5 (0.4–0.5)2 | 0.5526 |
| PAHs (ng/mg creatinine) | |||||||||
| 1-Naphthol or 1-hydroxynaphthalene (1-NAP) | 1.5 (1.4–1.7) | 2.2 (1.8–2.5)1 | 0.0359 | 1.4 (1–1.9) | 2.5 (1.7–3.6)1 | 0.1526 | 6.9 (5.9–8) | 11.4 (9.9–13)2 | 0.0028 |
| 3-Hydroxyfluorene (3-FLU) | 0.07 (0.07–0.08) | 0.1 (0.09–0.11)1 | 0.0058 | 0.06 (0.05–0.07) | 0.1 (0.08–0.13)1 | 0.0066 | 0.37 (0.33–0.41) | 0.55 (0.5–0.6)2 | 0.0002 |
| VOCs (ng/mg creatinine) | |||||||||
| CEMA) (Acrolein) | 99 (94.4–103.8) | 114.3 (103.9–125.9)1 | 0.0268 | 98.9 (90–108.8) | 123.6(102.3–149.2)1 | 0.0733 | 197.1 (180.4–215.4) | 280 (261.7–299.5)2 | <.0001 |
| CYMA (acrylonitrile) | 1.6 (1.5–1.8) | 3.5 (3–4.1)1 | <.0001 | 3.4 (2.6–4.4) | 7.3 (4.6–11.6)2 | 0.0087 | 58.1 (47.7–70.7) | 122.5 (107.7–139.3)3 | <.0001 |
Geo Mean: geometric mean of concentration.
aAll analyses applied urinary sample weight, 100 replicated weights, and the balanced repeated replication method with Fay’s adjustment = 0.3 to account for the PATH Study’s complex design.
bSeparate analyses were performed for three exclusive e-cigarette user groups (no use of e-cigarette and tobacco product, exclusive e-cigarette use, and use of e-cigarettes and 1+ type of tobacco product) based on the self-report. Multivariable general linear regressions were conducted to examine the relationship between biomarker concentration levels at Wave 1 (outcome variable, log-transformed, and creatinine corrected) and respiratory symptoms at Wave 2, adjusted by baseline sociodemographics (sex, age, race/ethnicity, and education), self-reported exposure to SHS, past 12-month use of marijuana, and lung or respiratory disease. Bold indicates significance at .005 with adjustment for multiple comparisons using the Bonferroni method.
cTNE2: The molar sum of the imputed values of cotinine, and trans-3′-hydroxycotinine, urine.
1,2,3Multivariable general linear regressions were conducted to compare biomarker concentration levels (dependent variables) by e-cigarette use status among subjects with respiratory symptoms. E-cigarette user groups that were significantly different in pairwise contrasts are denoted by nonshared superscript numbers. The analysis was adjusted by baseline sociodemographics (sex, age, race/ethnicity, and education), self-reported exposure to SHS, past 12-month use of marijuana, and lung or respiratory disease.
Among exclusive e-cigarette users, compared with those reporting no respiratory symptoms in the past 12 months, NNAL (a metabolite of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)) was 143% higher among adults reporting respiratory symptoms than those who did not (10.7 [6.5–17.5] vs. 4.4 [3.5–5.6], adjusted p = .0043). Among poly e-cigarette/tobacco users, adults reporting respiratory symptoms in the past 12 months at Wave 2 had significantly higher concentrations in biomarkers of exposure to nicotine (TNE2, cotinine), TSNA (NNAL), PAHs (1-NAP, 3-FLU), and VOCs (N-Acetyl-S-(2-cyanoethyl)-l-cysteine (CYMA) (Acrylonitrile), N-acetyl-S-(2-carboxyethyl)-l-cysteine CEMA (Acrolein)) than those who did not report respiratory symptoms. For instance, NNAL was two times higher among those reporting respiratory symptoms when compared with those who did not (199.6 [176.7–225.4] vs. 98.9 [79.7–122.7], adjusted p = .0001).
Among nonusers, compared with those reporting no respiratory symptoms in the past 12 months, those reporting respiratory symptoms had significantly higher concentrations of biomarkers of exposure to nicotine (TNE2, cotinine), TSNAs (NNAL), and VOC (CYMA (Acrylonitrile)). Overall, the concentration levels are significantly lower in nonusers than exclusive and poly e-cigarette/tobacco users.
Among Subjects with Respiratory Symptoms
(n = 1679), dose–response patterns of increase in clinically relevant biomarker levels were observed across e-cigarette use status. NNAL increased from 3.1 [2.4–3.9] in nonusers to 10.7 [6.5–17.5] in exclusive e-cigarette users, and further increased to 199.6 [176.7–225.4] in poly e-cigarette/tobacco users. Similarly, CYMA increased from 3.5 [3.0–4.1] in nonusers to 7.3 [4.6–11.6] in exclusive e-cigarette users, and further increased to 122.5 [107.7–139.3] in poly e-cigarette/tobacco users.
Sensitivity Analysis
found similar results in the associations between baseline biomarker outcomes and subsequent respiratory symptoms after excluding the subjects with baseline lung or respiratory diseases (Supplementary material, eTables 5–8).
Summary and Discussion
By leveraging nationally representative and large-scale population data, our study identified positive associations between urinary biomarkers of exposure to tobacco-related toxicants and increased risk of functionally important respiratory symptoms after adjusting for confounding effects. These associations existed across several HPHC classes, including nicotine metabolites, TSNAs, PAHs, and VOCs and remained significant after adjusting for the confounding effect of baseline respiratory diseases in the multivariable analyses, suggesting that e-cigarette use may increase the risks of respiratory symptoms.
In our study, the most clinically relevant biomarkers associated with respiratory symptoms are NNAL and CYMA (acrylonitrile). NNAL is a metabolite of a potent lung carcinogen, NNK. NNK is rapidly reduced into NNAL in the body, thus it was not monitored in the assay.29 Based on sufficient evidence in animal studies, NNAL is listed as a class 2B in the IARC carcinogenicity evaluation.23 Our study also shows a positive association between NNAL and increased risks of respiratory symptoms across varying e-cigarette user groups.
Acrylonitrile forms during the heating of glycerol (glycerin) or glycerol-derived fats (eg, triglycerides). Their pyrolysis leads to acrolein, which undergoes ammoxidation to acrylonitrile. Both acrolein and acrylonitrile are respiratory toxicants according to FDA HPHC classification.24 Acrylonitrile is classified as a Class 2B carcinogen by the IARC based on sufficient animal studies.23 Acrylonitrile decomposes by reacting with oxygen and hydroxyl radicals to form formaldehyde, a toxicant that has been identified with the heating of the flavor used in e-cigarettes. As glycerol (“vegetable glycerin”) is a commonly used ingredient in e-liquids, our study provides essential findings bridging e-cigarette use, acrylonitrile biomarkers, and respiratory symptoms in poly e-cigarette/tobacco users.
Use of multiple tobacco products has become common among current users of noncigarette tobacco users.30,31 Some of these users are transitioning from combustible tobacco use to less toxic e-cigarette use, while others may maintain the use of multiple tobacco products, including e-cigarettes.3 This study showed that higher concentrations of TNE2, NNAL, naphthalene, fluorenes, acrolein, acrylonitrile, and 1,3-butadiene were associated with increased risks of subsequent respiratory symptoms among poly e-cigarette/tobacco users. As geometric mean concentrations of urinary nicotine metabolites, TSNAs, PAHs, and VOCs were higher among poly e-cigarette/tobacco users than never tobacco users or exclusive e-cigarette users, our study results are consistent with previous findings that poly tobacco use is associated with increased risk for adverse health effects, including lung or respiratory symptoms.26,31
This study has a number of limitations. First, e-cigarette, tobacco use, and respiratory symptoms are self-reported and they are subject to recall bias. Since tobacco use was measured as current use (some day or every day) and respiratory symptoms were measured as the past 12 months, the difference in timing with respect to the measurement may influence the results. However, given that e-cigarette use was measured at Wave 1 and respiratory symptoms were measured at the 1-year later (Wave 2), the measurement contamination should be minimal. Furthermore, we did not include tobacco use history in the analysis, and some biomarker outcomes with long half-lives (eg, metals) may come from prior combustible tobacco use, passive tobacco exposure or other sources,21 especially for exclusive e-cigarette use. However, most biomarkers analyzed in the study have a short half-life21 and we adjusted a variety of confounders in the multivariable regression model, including exposure to SHS. Second, since the focus of this study is to examine the longitudinal relationship between baseline e-cigarette use and subsequent respiratory symptoms, we did not include the transition in e-cigarette use between Waves 1 and 2 biomarker data in the analysis. Given that continued use of e-cigarette over time is not common,32 future studies should assess the impact of change in e-cigarette use behaviors on biomarker exposure and respiratory symptoms. Furthermore, we only included the first 2 Waves of the PATH Study data given the availability during the analysis. Additional studies to include new Waves of data are needed to assess the long-term health effects. Third, this study did not differentiate the use of various generations of e-cigarette devices as studies have shown that the later-generation of vaping devices like JUUL has high concentrations of nicotine and nicotine salts.33 Therefore, biomarker outcomes may vary by e-cigarette devices and future studies should assess the relationship between biomarkers and respiratory diseases by different e-cigarette devices. Fourth, some biomarker exposures that may be related to e-cigarette use, such as metals (eg, nickel and chromium) and flavorings (eg, formaldehyde), are not included in the PATH biospecimen analysis. Finally, the respiratory symptoms were not measured at Wave 1 of the PATH Study, thus we were not able to adjust for baseline respiratory symptoms in the multivariable analysis. Although our sensitivity analyses excluding subjects with baseline respiratory disease revealed similar findings, it is possible that those with baseline respiratory symptoms have an increased risk of using e-cigarettes. Therefore, the associations observed in this study should not necessarily be interpreted as the biomarkers prospectively predicting the incident symptoms.
Despite these limitations, this study demonstrates e-cigarette use is associated with increased exposure to known tobacco-related toxicants, and certain toxicant exposures were associated with increased risks of subsequent respiratory symptoms. Measuring exposure biomarkers can provide objective assessments of health risks related to the use of e-cigarettes and multiple tobacco products.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Funding
Research of HD was partly supported by grant number [R03CA228909] from the National Cancer Institute and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Author contributions
HD conceptualized the study, acquired the data, and performed the analyses. HD and AK interpreted the results, drafted the manuscript, critically reviewed, and revised the manuscript. Both authors approved the manuscript. HD had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults – United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(45):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Academies of Sciences Engineering and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: The National Academies Press; 2018. [PubMed] [Google Scholar]
- 4. The Centers for Disease Control and Prevention. Outbreak of Lung Injury Associated with E-Cigarette Use, or Vaping, Updated on February 11, 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Accessed February 21, 2020.
- 5. Blount BC, Karwowski MP, Shields PG, et al. ; Lung Injury Response Laboratory Working Group . Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie Z, Ossip DJ, Rahman I, Li D. Use of electronic cigarettes and self-reported chronic obstructive pulmonary disease diagnosis in adults. Nicotine Tob Res. 2020;22(7):1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li D, Sundar IK, McIntosh S, et al. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: cross-sectional results from the Population Assessment of Tobacco and Health (PATH) Study, Wave 2. Tob Control. 2020;29(2):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhatta DN, Glantz SA. Association of E-Cigarette use with respiratory disease among adults: a longitudinal analysis. Am J Prev Med. 2020;58(2):182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wills TA, Pagano I, Williams RJ, Tam EK. E-cigarette use and respiratory disorder in an adult sample. Drug Alcohol Depend. 2019;194:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang CM, Cheng YC, Cho TM, et al. Biomarkers of potential harm: summary of an FDA-sponsored public workshop. Nicotine Tob Res. 2019;21(1):3–13. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Washington, DC: The National Academies Press; 2001. . [PubMed] [Google Scholar]
- 12. Goniewicz ML, Smith DM, Edwards KC, et al. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw Open. 2018;1(8):e185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. U.S. Department of Health and Human Services. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016. [Google Scholar]
- 14. Marynak KL, Gammon DG, Rogers T, Coats EM, Singh T, King BA. Sales of nicotine-containing electronic cigarette products: United States, 2015. Am J Public Health. 2017;107(5):702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rostron BL, Cheng YC, Gardner LD, Ambrose BK. Prevalence and reasons for use of flavored cigars and ENDS among US youth and adults: estimates from Wave 4 of the PATH study, 2016-2017. Am J Health Behav. 2020;44(1):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schneller LM, Bansal-Travers M, Goniewicz ML, McIntosh S, Ossip D, O’Connor RJ. Use of flavored electronic cigarette refill liquids among adults and youth in the US-Results from Wave 2 of the Population Assessment of Tobacco and Health Study (2014–2015). PLoS One. 2018;13(8):e0202744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dinakar C, O’Connor GT. The health effects of electronic cigarettes. N Engl J Med. 2016;375(14):1372–1381. [DOI] [PubMed] [Google Scholar]
- 18. Kosmider L, Sobczak A, Prokopowicz A, et al. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax. 2016;71(4):376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United States Department of Health and Human Services. National Institutes of Health. National Institute on Drug Abuse, and United States Department of Health and Human Services. Food and Drug Administration. Center for Tobacco Products. Population Assessment of Tobacco and Health (PATH) Study [United States] Public-Use Files. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]; 2017. [Google Scholar]
- 20. United States Department of Health and Human Services. National Institutes of Health. National Institute on Drug Abuse, and United States Department of Health and Human Services. Food and Drug Administration. Center for Tobacco Products. Population Assessment of Tobacco and Health (PATH) Study [United States] Biomarker Restricted-Use Files. Inter-university Consortium for Political and Social Research [distributor]; 2020 . [Google Scholar]
- 21. Chang CM, Edwards SH, Arab A, Del Valle-Pinero AY, Yang L, Hatsukami DK. Biomarkers of tobacco exposure: summary of an FDA-sponsored public workshop. Cancer Epidemiol Biomarkers Prev. 2017;26(3):291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang CM, Rostron BL, Chang JT, et al. Biomarkers of exposure among U.S. adult cigar smokers: Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013–2014). Cancer Epidemiol Biomarkers Prev. 2019;28(5):943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon: IARC; 2004: 1–1452. [PMC free article] [PubMed] [Google Scholar]
- 24. US Food and Drug Administration. Harmful and potentially harmful constituents in tobacco products and tobacco smoke; established list. Fed Regist. 2012;77(64):20034–20037. [Google Scholar]
- 25. Hornung RW, Reed LDJAO. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- 26.Li D, Sundar IK, McIntosh S et al. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: cross-sectional results from the Population Assessment of Tobacco and Health (PATH) study, Wave 2. Tob. Control.2020;29(2):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCarthy PJ. Pseudoreplication: further evaluation and application of the balanced half-sample technique. Vital Health Stat. 1969;2(2):1–24. [PubMed] [Google Scholar]
- 28. Judkins DR. Fay’s method for variance estimation. J. Off Stat. 1990;6(3):223–239. [Google Scholar]
- 29. Inter-university Consortium for Political and Social Research. Population Assessment of Tobacco and Health (PATH) Study [United States] Biomarker Restricted-Use Files Urinary Tobacco-Specific Nitrosamines (TSNA) Laboratory Procedure Manual, version 9. Ann Arbor, MI. [Google Scholar]
- 30. Syamlal G, Jamal A, Mazurek JM. Combustible tobacco and smokeless tobacco use among working adults – United States, 2012 to 2014. J Occup Environ Med. 2016;58(12):1185–1189. [DOI] [PubMed] [Google Scholar]
- 31. Sung HY, Wang Y, Yao T, Lightwood J, Max W. Polytobacco use of cigarettes, cigars, chewing tobacco, and snuff among US adults. Nicotine Tob Res. 2016;18(5):817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stanton CA, Sharma E, Edwards KC, et al. Longitudinal transitions of exclusive and polytobacco electronic nicotine delivery systems (ENDS) use among youth, young adults and adults in the USA: Findings from the PATH Study Waves 1–3 (2013–2016). Tob Control. 2020;29(suppl 3):s147–s154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Romberg AR, Miller Lo EJ, Cuccia AF, et al. Patterns of nicotine concentrations in electronic cigarettes sold in the United States, 2013-2018. Drug Alcohol Depend. 2019;203:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.