Abstract
Background:
The exposure–response association between prenatal and postnatal household air pollution (HAP) and infant growth trajectories is unknown.
Objectives:
To evaluate associations between prenatal and postnatal HAP exposure and stove interventions on growth trajectories over the first year of life.
Methods:
The Ghana Randomized Air Pollution and Health Study enrolled pregnant women at gestation from Kintampo, Ghana, and randomized them to liquefied petroleum gas (LPG), improved biomass, or open fire (control) stoves. We quantified HAP exposure by repeated, personal prenatal and postnatal carbon monoxide (CO) and, in a subset, fine particulate matter [PM with an aerodynamic diameter of ()] assessments. Length, weight, mid-upper arm circumference (MUAC) and head circumference (HC) were measured at birth, 3, 6, 9, and 12 months; weight-for-age, length-for-age (LAZ), and weight-for-length (WLZ)-scores were calculated. For each anthropometric measure, we employed latent class growth analysis to generate growth trajectories over the first year of life and assigned each child to a trajectory group. We then employed ordinal logistic regression to determine associations between HAP exposures and growth trajectory assignments. Associations with stove intervention arm were also considered.
Results:
Of the 1,306 live births, 1,144 had valid CO data and anthropometric variables measured at least once. Prenatal HAP exposure increased risk for lower length [CO 1.17, 95% CI: 1.01, 1.35 per 1-ppm increase; 1.07, 95% CI: 1.02, 1.13 increase], lower LAZ -score (CO 1.15, 95% CI: 1.01, 1.32 per 1-ppm increase) and stunting (CO 1.25, 95% CI: 1.08, 1.45) trajectories. Postnatal HAP exposure increased risk for smaller HC (CO 1.09, 95% CI: 1.04, 1.13 per 1-ppm increase), smaller MUAC and lower WLZ-score ( 1.07, 95% CI: 1.00, 1.14 and 1.09, 95% CI: 1.01, 1.19 increase, respectively) trajectories. Infants in the LPG arm had decreased odds of having smaller HC and MUAC trajectories as compared with those in the open fire stove arm ( 0.58, 95% CI: 0.37, 0.92 and 0.45, 95% CI: 0.22, 0.90, respectively).
Discussion:
Higher early life HAP exposure (during pregnancy and through the first year of life) was associated with poorer infant growth trajectories among children in rural Ghana. A cleaner-burning stove intervention may have improved some growth trajectories. https://doi.org/10.1289/EHP8109
Introduction
In low- and middle-income countries (LMICs), household air pollution (HAP) secondary to the burning of solid fuels in combustion-inefficient traditional stoves resulted in deaths and 91.5 million disability adjusted life years (DALYs) in 2019 (World Bank 2020; GBD 2019 Risk Factors Collaborators 2020). In LMICs, on average, an estimated 65% of households cook with solid fuels (World Bank 2020). Women frequently are the primary household cooks and continue to cook while pregnant. Thus, HAP exposures begin in utero and continue across the life course. Studies using questionnaires to characterize HAP exposure suggest an effect of early life HAP on child growth; however, exposure–response relationships have been limited to birth weight (Balakrishnan et al. 2018; Mishra and Retherford 2007; Quinn et al. 2021). A better understanding of how HAP exposure alters early childhood growth and whether clean-burning interventions to reduce HAP can improve growth is imperative.
Children years of age who are stunted, wasted, or underweight account for at least 2.89 million deaths worldwide each year (Black et al. 2013). Children with poor growth in early childhood who survive have far-reaching health consequences including, but not limited to, impaired cognitive, motor and socioemotional development, infectious and chronic disease morbidity, and mortality extending over the life course (Black et al. 2013). These impairments translate at the population-level to reduced economic growth and increased poverty for entire communities. The prenatal and early childhood periods are critical in determining growth and short- and long-term health-related outcomes (Christian et al. 2015). Emerging evidence suggests that environmental factors, including early life air pollution exposure, may increase the risk for poor growth (Sinharoy et al. 2020).
Previous studies of the effect of HAP exposure on infant growth have classified exposure based on the fuels used for cooking or heating, as reported by study participants. The 2015–2016 Indian National Family Health Survey of households with children years of age found that households using unclean cooking fuels had 16% higher odds of having a stunted child, as compared with households using clean cooking fuels (Islam et al. 2021). Additional work from India suggests that infants born in homes with wood or dung as the primary fuel have an increased risk of being underweight and stunted by 6 months of age (Tielsch et al. 2009). A cross-sectional study of Chinese children 6–17 years of age found that children living in households using solid fuels for cooking and heating had lower length-for-age -scores (LAZ) and an increased risk of stunting, with girls reported to be more vulnerable (Liang et al. 2020). A pooled analysis from 11 studies found that solid fuel use, as compared with the use of cleaner fuels, was associated with the increased risk for stunting (Pun et al. 2021). These studies provide important initial evidence for the impact of HAP on early childhood growth. Exposure–response analyses are needed to begin to understand the temporal associations, to elucidate the mechanistic underpinnings, and to characterize the magnitude of exposure reduction necessary to improve health (Clark et al. 2013).
Prenatal cook stove intervention trials to reduce HAP exposure have focused on birth outcomes, and, to our knowledge, these studies have not extended their anthropometric observations through early childhood. The Ruxolitinib for the treatment of Randomised Exposure Study of Pollution Indoors and Respiratory Effects (RESPIRE) study randomized households to a plancha-type stove with chimney ventilation vs. open fire and found infants born to mothers in the intervention arm weighed, on average, more [95% confidence interval (CI): , 204 g] than control infants (Thompson et al. 2011). An ethanol stove intervention in Nigeria similarly suggested improvements in birth weight (mean , 95% CI: , 194) (Alexander et al. 2018). If a prenatally introduced cookstove intervention maintained through early childhood were found to be associated with improvements in early childhood growth trajectories, these findings could have important implications for the timing of deployment of public health interventions.
The Ghana Randomized Air Pollution and Health Study (GRAPHS) was a cluster-randomized cookstove intervention trial to understand the effects of a prenatally introduced cookstove intervention [liquefied petroleum gas (LPG) or improved biomass stove vs. control] on birth outcomes and pneumonia risk over the first year of life. The primary GRAPHS intention-to-treat analyses did not find that a cookstove intervention improved birth weight or reduced incident pneumonia risk in the first year of life (Jack et al. 2021). Secondary exposure–response analyses found that higher prenatal carbon monoxide (CO) exposure was associated with a lower birth weight (, 95% CI: , increase) and length (, 95% CI: , increase) and increased pneumonia risk in the first year of life; these results have been reported separately (Kinney et al. 2021; Quinn et al. 2021). In addition to the prespecified GRAPHS primary and secondary outcomes, we prospectively measured anthropometric variables every 3 months for the first year of life to explore associations between early life HAP exposures, and the cookstove intervention arm, considered separately, and infant growth trajectories. Specifically, we first constructed growth trajectories for weight, length, head circumference (HC), mid-upper arm circumference (MUAC), and weight-for-length, LAZ, and weight-for-age -scores (i.e., WLZ-, LAZ-, and WAZ-scores) without consideration of exposure or study arm. We assigned each child to a growth trajectory for each anthropometric. Then, we explored exposure–response associations between prenatal and postnatal HAP exposures, as indexed by CO and, in a subset, fine particulate matter [PM with an aerodynamic diameter of ()], and growth trajectory assignment. We also examined whether prenatally introduced cookstove interventions maintained through the infant’s first year of life were associated with better growth trajectories. Finally, we explored effect modification by infant sex.
Methods
Study Participants and Cookstove Intervention Details
GRAPHS was a cluster-randomized cookstove intervention study designed to recruit pregnant women from two largely agricultural districts in rural Ghana, Kintampo North Municipality and Kintampo South District (Jack et al. 2015). Thirty-five communities (clusters) were randomized to LPG ( communities, target women), an improved combustion-efficiency biomass stove ( communities, target women), or open fire stove control ( communities, target women). The LPG arm was a priori designed to have fewer women on the basis of an anticipated substantial reduction in exposure, thereby requiring a smaller sample size to show the predicted effect (Jack et al. 2021). Community-based fieldworkers referred pregnant women to trained research midwives for ultrasound confirmation of pregnancy and gestational age (GA) (Boamah et al. 2014). A woman was eligible to participate if she was pregnant with a live, intrauterine singleton fetus; GA at enrollment; the primary household cook; and a nonsmoker. An estimated date of delivery was assigned by ultrasound measurements alone and used to establish GA throughout the study. Study arm assignment was by community of residence at time of enrollment. All pregnant women provided written informed consent. The study was approved by the regional ethics committees at each participating institution and regulatory authorities in Ghana and was registered with ClinicalTrials.gov (NCT01335490).
The stove intervention began immediately following a baseline exposure assessment and was continued until the index infant’s first birthday. Women enrolled in the LPG arm received a two-burner LPG stove and two cylinders. LPG cylinder refill deliveries were made monthly. Households could also request an LPG cylinder refill at the weekly fieldworker visits or at any other time point by contacting the resident fieldworker. Women enrolled in the improved biomass stove arm received two BioLite HomeStove forced-draft stoves (BioLite Inc.), rated Tier 3 for total emissions (Jetter and Ebersviller 2016). Women enrolled in the control arm continued to cook on traditional open fire stoves. All study households were provided with a treated mosquito bed net and health insurance. Households in the improved biomass and control arms received a two-burner LPG stove and two cylinders after the study conclusion. Fieldworkers visited each study household weekly, repairing intervention stoves as needed and encouraging and querying use; weekly visits to control arm households were framed as bed net use and check-in visits.
Exposure Assessment
The GRAPHS exposure assessment approach has been previously described (Chillrud et al. 2021). Briefly, pregnant women underwent four 72-h personal CO exposure assessments using the Lascar EL-CO-USB Data Logger, once prior to stove allocation and three times equally spaced between stove deployment and the estimated date of newborn delivery. Infants then underwent three 72-h personal CO exposure assessments at 1, 3, and 9 months of age. Pregnant women wore the exposure monitor(s) affixed within their breathing zone except during sleep and bathing, at which time the monitors were placed nearby ( away) and off the floor. Mothers were instructed to clip the infant monitor on the outermost layer of clothing (e.g., a swaddle or cloth carrier) in their infant’s breathing zone except during sleep and bathing, at which time they placed the monitors nearby and off the floor.
The Lascar monitors measured CO exposure in parts per million every 10 s. Quality assurance/quality control measures included generating correction factors derived from exposing the monitors to certified span gas ( CO in zero air) every 6 wk, as well as run-time assessment and visual inspection of each deployment, per protocol. CO exposure at each time point was determined by the first 48 h of deployment because battery issues or field pick-up times may have missed a cooking episode on the last day. Deployments lasting were removed from data analysis; 48-h data completeness was high (93.6%). We determined prenatal and postnatal averaging by linear interpolation of CO values between the individual sessions, that is, Sessions 1–4 for prenatal exposure and Sessions 5–7 for postnatal exposure. We assumed that exposures prior to the first prenatal or postnatal session were equal to the first 48-h CO average and that exposures after the last session through the end of the pregnancy or the first year, respectively, were equal to the last 48-h CO average, whereas exposures between successive measurement sessions were estimated using linear interpolation. We then used these values to compute a prenatal average from the beginning of pregnancy to birth and a postnatal average from birth to the first birthday of the child.
A subset of mothers (), selected as a convenience sample, additionally underwent one prenatal and one postnatal personal 72-h exposure assessment using the MicroPEM (RTI International). Prenatal exposure assessments in 351 (92%) were performed after the stove intervention. The MicroPEM was perceived to be too bulky for the infants to wear; therefore, postnatal exposure assessments were performed only in mothers. The MicroPEM provides concurrent real-time particle monitoring based on a light-scattering nephelometer and a Teflon filter (Pall Biotech) for analysis of integrated and an accelerometer for assessing wearing compliance. The MicroPEM was run at a 50% duty cycle (30 s on, 30 s off) and a continuously measured flow rate of 0.4 LPM, within the manufacturers specifications of the dual impactor’s cut point at . Filters were isolated in primary and secondary containers at 4°C after sampling until shipped on ice to Columbia University. Filters were pre- and post-weighed at Columbia University following published methods (Chillrud et al. 2021; Zhang et al. 2017). After baseline adjustment, based on attaching a high-efficiency particulate air filter to the inlet immediately before and after deployment and interpolating the baseline correction between these two values, the net filter weight of was used to adjust the mean nephelometer response during the entire deployment. Similar to CO, deployments lasting (16% of all deployments) were removed from the data analysis, and the mean of the first 48 h of the adjusted nephelometer data was used in subsequent data analysis.
Growth Measurements
Trained fieldworkers measured infant weight, length, HC, and MUAC once within 24 h of birth and at 3, 6, 9, and 12 months of age. We measured weight to the nearest (Tanita digital scale model BD-590; Tanita Corp.) and length to the nearest (Ayrton Infantometer model M-200; Ayrton Corp.). HC was measured using a nonstretchable lasso tape (Child Growth Foundation). MUAC was measured at the upper arm midpoint using the United Nations Children’s Fund MUAC tape.
Using these measurements, we calculated LAZ, WLZ, and WAZ beginning at 3 months of age using the 2006 World Health Organization (WHO) child growth standards (WHO 2006). An infant was considered stunted, wasted, or underweight if their LAZ, WLZ, WAZ, respectively, was below the WHO child growth standard reference median for age and sex (Black et al. 2013).
Covariates
We used directed acyclic graph theory to select covariates for adjustment (Figure S1) (Greenland et al. 1999). The final model was adjusted for child sex, maternal age, ethnicity, and wealth index. Sensitivity models were further adjusted for maternal body mass index (BMI, in milligrams per meter squared), breastfeeding duration, GA at delivery, and birth weight given the associations between maternal size, fetal growth restriction, and preterm birth on childhood growth impairments (Danaei et al. 2016).
At the time of the prenatal screening ultrasound, maternal height and weight were measured and BMI (continuous variable) calculated. Maternal age (continuous variable, in years) and ethnicity (categorical variable, groups 0–5) were determined by questionnaire on enrollment. Ethnicity was queried by asking, “What ethnic group do you belong to?” To generate the ethnicity variables, we collapsed the ethnic categories into the top five responses and the remaining answers into one category, which we label numerically owing to discrimination and privacy concerns. Questionnaires were used to assess a number of household characteristics (Table S1) that were enumerated as counts (e.g., number of livestock) and used to calculate the wealth index, a categorical measure (groups 1–5) of household wealth relative to other enrolled households (Gunnsteinsson et al. 2010). Infant sex (female vs. male) was recorded at birth; GA at birth (continuous variable, in weeks) was derived from date of birth and ultrasound-established estimated date of delivery. We assessed breastfeeding by questionnaire every 3 months by querying, “Are you still breastfeeding your baby?” We categorized the duration of breastfeeding based on the child’s age when the mother last indicated she was still breastfeeding (3, 6, 9, or 12 months).
Statistical Analyses
We constructed latent class growth trajectories for each serially measured growth metric using latent class growth analysis (LCGA) (Berlin et al. 2014; Nagin and Odgers 2010), without consideration of exposure measures or study arm. We included all anthropometric data for all participants, including those without complete data. For each anthropometric variable (length, weight, HC, MUAC) and -score (LAZ, WAZ, and WLZ), we fit models ranging from two to six trajectory classes and then determined the best fitting model (i.e., the number of trajectories). Criteria for model selection, where different models are defined by the number of growth trajectories for a given anthropometric, included a) Akaike information criterion (AIC) and Bayesian information criterion (BIC), where smaller values suggest better fit; b) entropy (range: 0–1), where higher values suggest better class separation; and c) the number of infants assigned to each growth trajectory, where models with adequate sample size per class are preferred (Jung and Wickrama 2008; Nylund et al. 2007). Following model selection, and for each anthropometric, children were assigned to the growth trajectory for which they had the highest probability of correct assignment. These growth trajectory assignments served as the dependent variable for subsequent regression analyses.
Growth trajectory assignments were given a continuous, ordinal number 0–3 if there were four identified trajectories, or 0–2 if there were three identified trajectories, with 0 assigned to the highest/largest trajectory and 3 or 2, respectively, assigned to the lowest/smallest trajectory. We then fit bivariate and multivariable ordinal logistic regression models to assess the odds ratio of HAP exposure on lower/smaller growth trajectory. Our primary exposure of interest was CO. Maternal prenatal and child postnatal CO had low correlation (, ). Secondary analyses considered maternal prenatal and maternal postnatal exposures (). The proportional odds assumption was tested (Harrell 2001) and found to be appropriate (data not shown). In adjusted exposure–response analyses, we included both prenatal and postnatal exposures in the models to determine the relative contribution of each. Multivariable models were adjusted for child sex, wealth index, maternal age, and ethnicity. Sensitivity models were additionally adjusted for maternal BMI at enrollment, breastfeeding duration, GA at delivery, or birth weight. We explored sex-specific associations by first introducing an exposure (CO or )–sex interaction term in the main regression models and second by examining associations between CO exposure and growth in models stratified by infant sex. Finally, we examined linearity of effect by performing analyses by interquartile change in CO for models suggesting an association between CO exposure and odds of lower/smaller trajectory. All analyses included cluster-robust standard errors at the cluster (community) level.
Because mean -scores for children assigned to the lowest/smallest trajectory for WAZ, LAZ, and WLZ were near or below at each time point—consistent with commonly used definitions of underweight, stunting, and wasting—we performed a second set of analyses to estimate associations between average prenatal and postnatal CO exposures and the odds of being assigned to the lowest/smallest trajectory vs. one of the other three trajectories. For convenience, we hereafter refer to these dichotomous outcomes as underweight (lowest WAZ trajectory), stunted (lowest LAZ trajectory), and wasted (lowest WLZ trajectory), although we recognize that not all children assigned to the lowest trajectories would have had at every time point during follow-up. We employed bivariate and multivariable logistic regression to examine whether prenatal and child postnatal CO exposures were associated with risk for stunting, wasting, or underweight, considered separately. Because of sample size limitations, we did not perform similar analyses with exposures.
Following these exposure–response models, we explored whether GRAPHS study arm assignment (improved biomass or LPG vs. control) was independently associated with risk for lower/smaller ordinal growth trajectory assignment. We fit bivariate and multivariable ordinal logistic regression analyses to assess associations between study arm (improved biomass or LPG vs. control) and growth trajectories. Multivariable models were adjusted for child sex, wealth index, maternal age, and ethnicity. Sensitivity models additionally accounted for maternal BMI at enrollment, breastfeeding duration, GA at delivery, or birth weight. We explored sex-specific associations as described above. All analyses included cluster-robust standard errors at the cluster (community) level.
The Stata (version 14; StataCorp) zscore06 module was used to calculate -scores. Mplus (version 7.4; Muthén & Muthén) was used for latent class growth trajectory construction, whereas R (version 3.6.0; R Development Core Team) was used for the regression analysis.
Results
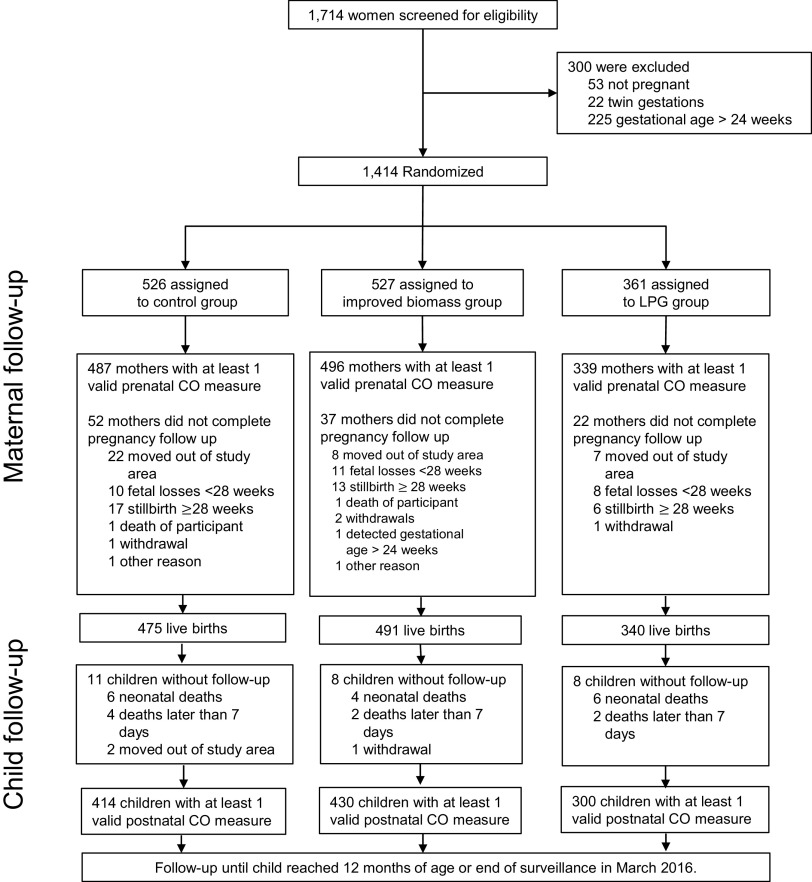
GRAPHS enrolled pregnant women, all of whom received a stove allocation [control, ; improved biomass, ; LPG, (Figure 1)]. On average, pregnant women were enrolled at 16.2 ( 4.3, range: 6–26), 16.1 ( 4.4, range: 6–24), and 14.6 ( 4.2, range: 6–23) wk gestation in the control, improved biomass, and LPG arms, respectively. The time from enrollment to delivery of stove intervention was 14.7 ( 20.6) and 12.7 ( 19.9) d for the improved biomass and LPG arms, respectively (-test ). The GRAPHS cohort resulted in (92.3%) live births. Of these, (87.8%) infants had prenatal (maternal) and postnatal (infant) CO exposure data and at least one anthropometric measurement and were included in these analyses (control, ; improved biomass, , LPG, ).
Figure 1.
CONSORT diagram of mother–infant dyads in the Ghana Randomized Air Pollution and Health Study. All mother–infant dyads with valid maternal prenatal and child postnatal CO had at least one valid anthropometric measure and were included in the growth trajectories construction. In the child follow-up section, the deaths of children at of age did not have any recorded fieldworker follow-up. Note: CO, carbon monoxide; LPG, liquified petroleum gas.
Table 1 describes participant characteristics. Overall, half of the infants were male (, 51%). Persons from a range of ethnic groups—including Akan, Dagarti, Gonja, Konkomba, Mo, and others—were enrolled. Recruitment of individual households with pregnant women from within each community (cluster) assigned to a stove intervention resulted in some variability across arms. For example, differences in wealth index were noted across arms, with 21%, 13%, and 26% of households in control, improved biomass, and LPG arms, respectively, described as “Very Poor” (see Table S1 for baseline economic characteristics used to construct the wealth index). The majority of mothers were breastfeeding at 1 y postpartum [All children, (85%); control, (78%); improved biomass, (84%); LPG, (96%)]. Median average prenatal maternal CO exposure was (IQR: 0.65–1.65) and median average postnatal child CO exposure was (IQR: 0.24–1.02); only prenatal CO was statistically different across study arms (Kruskal-Wallis prenatal , postnatal ). A representative time-series plot of personal CO exposure is shown in Figure S2. For those with maternal prenatal and postnatal exposure assessments (), the median prenatal exposure was (IQR: 43.2–96.4), measured at the median gestation age of 22.7 wk (IQR: 18.4–26.9), and the median postnatal exposure was (IQR: 38.7–79.5), measured at a median child age of 3.5 months (IQR: 2.7–5.2). Estimated prenatal CO and exposures were lower in the LPG arm than in the control or improved biomass stove arms (Table 1), but post-intervention exposure distributions demonstrated significant overlap among all three study arms (Figure S3) (Chillrud et al. 2021). Among those with both CO and exposure measurements, we noted that prenatal CO and , and postnatal CO and , were weakly correlated (prenatal: , ; postnatal: , by Pearson’s correlation). We postulated that changes in cooking behavior of the women over the course of the study (e.g., primary cook during pregnancy with a shift in responsibilities after delivery as the mother focused on the newborn) and different exposures between a woman as cook and an infant child who may be cared for by other relatives during cooking periods may explain these differences.
Table 1.
Ghana Randomized Air Pollution and Health Study (GRAPHS) participant characteristics.
| Characteristic | All children () | Open fire stove, control () | Improved biomass stove () | LPG stove () |
|---|---|---|---|---|
| Categorical variables [ (%)] | ||||
| Child sex | ||||
| Male | 584 (51.0) | 199 (48.1) | 238 (55.3) | 147 (49.0) |
| Wealth index | ||||
| Mean (SD) | 3.02 (1.4) | 3.06 (1.5) | 3.13 (1.3) | 2.81 (1.4) |
| By category: | ||||
| 1 (very poor) | 221 (19.3) | 87 (21.0) | 57 (13.3) | 77 (25.7) |
| 2 | 236 (20.6) | 87 (21.0) | 93 (21.6) | 56 (18.7) |
| 3 | 220 (19.2) | 58 (14.0) | 103 (24) | 59 (19.7) |
| 4 | 230 (20.1) | 77 (18.6) | 91 (21.1) | 62 (20.7) |
| 5 (least poor) | 237 (20.7) | 105 (25.4) | 86 (20.0) | 46 (15.3) |
| Ethnicity | ||||
| 0 | 254 (22.2) | 133 (32.1) | 72 (16.7) | 49 (16.3) |
| 1 | 199 (17.4) | 84 (20.3) | 88 (20.5) | 27 (9.0) |
| 2 | 165 (14.4) | 36 (8.7) | 55 (12.8) | 74 (24.7) |
| 3 | 159 (13.9) | 33 (8.0) | 76 (17.7) | 50 (16.7) |
| 4 | 149 (13.0) | 62 (15.0) | 52 (12.1) | 35 (11.7) |
| 5 | 218 (19.1) | 66 (15.9) | 87 (20.2) | 65 (21.7) |
| Breastfeeding duration (months) (missing data )a | ||||
| 3 | 22 (1.9) | 2 (0.5) | 20 (4.7) | 0 (0.0) |
| 6 | 56 (4.9) | 25 (6.0) | 29 (6.7) | 2 (0.7) |
| 9 | 90 (7.9) | 63 (15.2) | 18 (4.2) | 9 (3.0) |
| 973 (85.0) | 323 (78.0) | 361 (84.0) | 289 (96.3) | |
| Pattern of available growth data (time points) (five time , 3, 6, 9, and 12 months) | ||||
| Five | 880 (76.9) | 306 (73.9) | 320 (74.4) | 254 (84.7) |
| Four | 162 (14.2) | 69 (16.7) | 51 (11.9) | 42 (14) |
| Three | 76 (6.6) | 35 (8.5) | 37 (8.6) | 4 (1.3) |
| Two | 23 (2.0) | 3 (0.7) | 20 (4.7) | 0 (0.0) |
| One | 3 (0.3) | 1 (0.2) | 2 (0.5) | 0 (0.0) |
| Continuous variables {ppm [median (IQR)]} | ||||
| Maternal average prenatal CO exposureb | 1.07 (0.65–1.65) | 1.15 (0.71–1.76) | 1.12 (0.68–1.60) | 0.94 (0.53–1.50) |
| Child average postnatal CO exposureb | 0.53 (0.24–1.02) | 0.54 (0.28–1.13) | 0.55 (0.24–1.01) | 0.48 (0.21–0.91) |
| Maternal prenatal exposure ()c | 67.8 (43.2–96.4) | 75.8 (47.3–101.1) | 71.7 (48.6–101.5) | 42 (29.2–65.9) |
| Maternal postnatal exposure ()c | 54.7 (38.7–79.5) | 57.5 (39.5–88.8) | 60.8 (41.8–79.6) | 48.4 (33.6–58.4) |
| Maternal age at enrollment (y) | 26.9 (22.3–33.6) | 27.3 (21.8–33.7) | 27.7 (23.3–34.2) | 25.9 (21.8–32.2) |
| Maternal BMI at enrollment () | 22.9 (21.1–25) | 22.7 (21.1–25) | 23.1 (21.4–25) | 22.8 (20.9–24.8) |
| Gestational age at delivery (wk) | 39.7 (38.9–40.6) | 39.7 (38.9–40.6) | 39.7 (38.9–40.7) | 39.6 (38.9–40.4) |
Note: BMI, body mass index; CO, carbon monoxide; IQR, interquartile range; LPG, liquified petroleum gas; , fine particulate matter (PM with an aerodynamic diameter of ); SD, standard deviation.
Breastfeeding assessed via questionnaire every 3 months over the infant’s first year of life.
Prenatal maternal and postnatal infant personal CO exposure measured in parts per million at four and three time points, respectively. Prenatal average CO exposures include pre-intervention and post-intervention exposure measurements. All children included in these analyses had prenatal and postnatal CO measurements ().
Prenatal and postnatal maternal personal exposure measured in micrograms per meter cubed, in those with paired prenatal and postnatal data (total ; by study arm: , , ). Of the prenatal assessments, 351 (92%) occurred after the stove intervention began. Prenatal was measured at the median gestational age of 22.7 wk (IQR: 18.4–26.9) and postnatal was measured at the median child age of 3.5 months (IQR: 2.7–5.2).
Latent Class Trajectory Modeling: Identification of the Number of Trajectories
Anthropometric measurement data completeness was high, and 1,042 (91%) of infants had growth measurements performed either four (, 14%) or five (, 77%) times (Table 1). Distribution of anthropometric measurements by child age is shown in Table S2. Table S3 displays the LCGA model fits—specifically AIC, BIC, and entropy—and resulting class sizes for each anthropometric variable. Using AIC, BIC, entropy, and number of infants per class, we determined that the optimal fit for weight, MUAC, WLZ, LAZ, and WAZ was four classes, whereas the optimal fit for length and HC was three classes. Table S4 displays the summary data for probability of assignment to each latent class. The cohort median posterior probabilities across all trajectories ranged from 0.88 (MUAC, 0.88, IQR: 0.72–0.96, range: 0.45–1.00) to 0.97 (WAZ, 0.97, IQR: 0.83–0.99, range: 0.49–1.00).
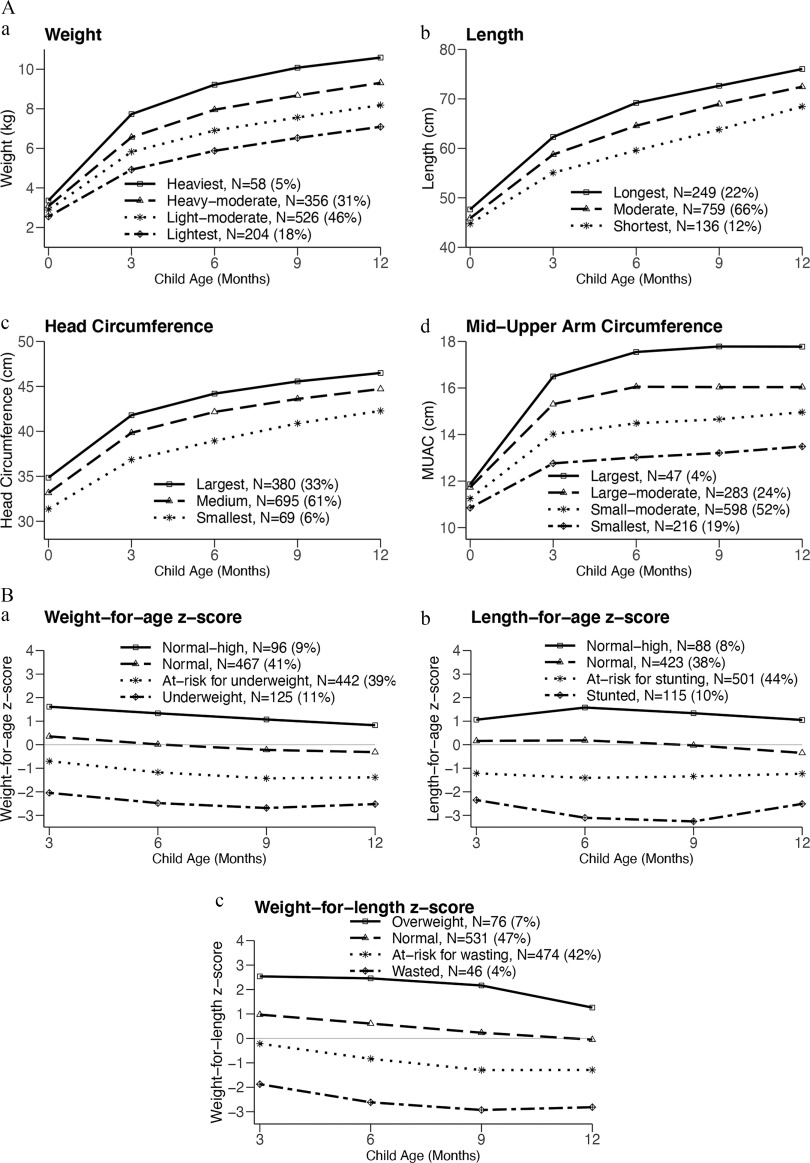
Examination of growth trajectories suggests that, in general, they are distinct at birth without any subsequent crossing of lines or rapid growth/decline periods (Figure 2A,B). However, the trajectories are not parallel with differences in slope observed particularly in the first 6 months of life. The -score trajectories demonstrated that the wasted (, 4%), stunted (, 10%), and underweight (, 11%) trajectories persisted from 3 through 12 months of age (Figure 2B). A higher prevalence of a stunted phenotype compared with a wasted phenotype was observed. Further, a large proportion of children were at risk (-score of ) for wasting (, 42%), stunting (, 45%), and being underweight (, 39%) by 12 months of age. Conversely, no -score trajectory demonstrated a persistently high (-score ) phenotype through 12 months of age.
Figure 2.
(A) Latent class growth trajectories for (a) weight, (b) length, (c) head circumference, and (d) MUAC. Weight, length, head circumference, and MUAC were measured at birth and at 3, 6, 9, and 12 months, and latent class growth analyses were employed to construct trajectories for each measurement. In general, trajectories appear distinct at birth although differences in slope are visualized through 6 months of life. (B) Latent class growth trajectories for (a) WAZ, (b) LAZ, and (c) WLZ calculated from 3 months of age using the 2006 WHO child growth standards. -Score trajectories suggest that trajectories of wasting (), stunting (), and underweight () are largely present at 3 months of age and persist through 12 months of age. Note: LAZ, length-for-age -score; MUAC, mid-upper arm circumference; WAZ, weight-for-age -score; WLZ, weight-for-length -score; WHO, World Health Organization.
Estimated Effect of Prenatal and Postnatal CO on Growth Trajectories
In models that examined associations between prenatal and postnatal CO exposures on growth trajectories, increased prenatal CO was associated with increased odds of lower length (, 95% CI: 1.01, 1.35, per increase in CO) and lower LAZ (, 95% CI: 1.01, 1.32, per increase in CO) trajectories, whereas increased postnatal CO was associated with increased odds of a lower HC trajectory (, 95% CI: 1.04, 1.13, per increase in CO), following adjustment for child sex, maternal age, ethnicity, and wealth index (Table 2; see complete model output in Table S5). Sensitivity models additionally adjusting for maternal BMI [median gestational age at time of 16 wk (IQR: 12.7–19.6)], breastfeeding duration, gestational age at delivery, or birth weight did not substantively change these findings (Table S6). Exploratory sex-stratified analyses suggested that among boys prenatal CO was associated with a risk for a lower length trajectory but was not associated with the outcome in girls ( 0.07), whereas postnatal CO was associated with a risk for a lower length trajectory in girls but was not associated with the outcome in boys ( 0.10; Table S7).
Table 2.
Associations between prenatal and postnatal average carbon monoxide (CO) and growth trajectories.
| Trajectory measurementa | Bivariate model | Multivariable modelb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prenatal | Postnatal | Prenatal | Postnatal | |||||||
| (95% CI) | -Value | (95% CI) | -Value | (95% CI) | p | (95% CI) | -Value | |||
| Weight | 1,144 | 1.03 (0.93, 1.12) | 0.55 | 0.99 (0.93, 1.06) | 0.77 | 1,142 | 1.03 (0.93, 1.14) | 0.59 | 1.01 (0.94, 1.08) | 0.84 |
| Length | 1,144 | 1.16 (1.01, 1.30) | 0.046 | 1.01 (0.94, 1.08) | 0.78 | 1,142 | 1.17 (1.01, 1.35) | 0.04 | 1.03 (0.96, 1.10) | 0.41 |
| Head circumference | 1,144 | 1.04 (0.96, 1.11) | 0.33 | 1.07 (1.03, 1.11) | 1,142 | 1.05 (0.98, 1.12) | 0.19 | 1.09 (1.04, 1.13) | ||
| MUAC | 1,144 | 1.05 (0.96, 1.14) | 0.27 | 1.01 (0.94, 1.07) | 0.84 | 1,142 | 1.07 (0.97, 1.19) | 0.16 | 1.03 (0.97, 1.10) | 0.35 |
| Weight-for-length -score | 1,127 | 0.96 (0.86, 1.06) | 0.40 | 0.97 (0.88, 1.05) | 0.38 | 1,125 | 0.95 (0.85, 1.06) | 0.38 | 0.97 (0.89, 1.06) | 0.49 |
| Length-for-age -score | 1,127 | 1.13 (0.98, 1.27) | 0.10 | 0.97 (0.92, 1.01) | 0.13 | 1,125 | 1.15 (1.01, 1.32) | 0.03 | 0.98 (0.94, 1.02) | 0.25 |
| Weight-for-age -score | 1,130 | 0.99 (0.86, 1.13) | 0.93 | 0.99 (0.91, 1.08) | 0.86 | 1,128 | 1.00 (0.87, 1.14) | 0.94 | 1.00 (0.93, 1.08) | 0.93 |
Note: Per 1-ppm increase in average CO exposure, all effects determined with cluster-robust standard errors. Analyses are ordinal regression where the ORs may be interpreted as the odds of being in a lower/smaller trajectory per 1-ppm increase in average CO exposure. CI, confidence interval; OR, odds ratio; MUAC, mid-upper arm circumference.
All trajectories include four groups except length (3 groups) and head circumference (3 groups).
Multivariable models include both prenatal and postnatal average CO exposure and adjust for child sex, maternal age, ethnicity, and wealth index.
Multivariable logistic regression suggested that prenatal CO exposure increased odds for being in the lowest LAZ trajectory (i.e., stunted) over the first year of life as compared with other trajectories [ 1.25, 95% CI: 1.08, 1.45, per increase in CO (Table 3; see complete model output in Table S8)] and sensitivity models did not substantively change these findings (Table S9). We did not find evidence of an association between prenatal or postnatal CO exposure and odds for being in the lowest WAZ or WLZ trajectories. Sex-stratified analyses suggested that among boys higher prenatal CO exposure was associated with an increased risk for being in the lowest LAZ trajectory (Table S10), whereas no association was observed among girls ( 0.04). Sensitivity models to examine linearity are shown in Figure S4.
Table 3.
Associations between prenatal and postnatal average carbon monoxide (CO) and assignment to the lowest -score trajectory.
| Trajectory measurementa | Bivariate model | Multivariable modelb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prenatal | Postnatal | Prenatal | Postnatal | |||||||
| (95% CI) | -Value | (95% CI) | -Value | (95% CI) | -Value | (95% CI) | -Value | |||
| Weight-for-length -score | 1,127 | 1.06 (0.88, 1.28) | 0.55 | 1.02 (0.91, 1.14) | 0.76 | 1,125 | 1.06 (0.87, 1.28) | 0.56 | 1.03 (0.94, 1.13) | 0.47 |
| Length-for-age -score | 1,127 | 1.22 (1.06, 1.41) | 0.95 (0.83, 1.09) | 0.47 | 1,125 | 1.25 (1.08, 1.45) | 0.98 (0.84, 1.14) | 0.75 | ||
| Weight-for-age -score | 1,130 | 0.98 (0.83, 1.15) | 0.76 | 0.99 (0.90, 1.08) | 0.79 | 1,128 | 0.99 (0.84, 1.17) | 0.93 | 1.00 (0.92, 1.10) | 0.92 |
Note: Per 1-ppm increase in average CO exposure, all effects determined with cluster-robust standard errors. The lowest weight-for-length, length-for-age, and weight-for age -score trajectories were at or below at each time point, consistent with commonly used definitions of wasted, stunted, and underweight, respectively. Analyses are logistic regression where ORs may be interpreted as the odds of being in the lowest -score trajectory as compared with others per 1-ppm increase in average CO exposure, respectively. CI, confidence interval; OR, odds ratio.
All trajectories include four groups.
Multivariable models include both prenatal and postnatal average CO exposure and adjust for child sex, maternal age, ethnicity, and wealth index.
Estimated Effect of Prenatal and Postnatal on Growth Trajectories
In models concurrently examining associations between prenatal and postnatal on growth trajectories, increased prenatal exposure was associated with increased odds of a lower length trajectory (, 95% CI: 1.02, 1.13, per increase in ), whereas increased postnatal exposure was associated with increased odds of smaller MUAC (, 95% CI: 1.00, 1.14, per increase in ) and lower WLZ (, 95% CI: 1.01, 1.19, per increase in ) trajectories following adjustment for child sex, maternal age, ethnicity, and wealth index per increase in (Table 4). Sensitivity models did not substantively change these findings (Table S11). Exploratory sex-stratified analyses suggested that among girls higher prenatal exposure was associated with an increased risk for being in a lower length trajectory and a decreased risk of being in a lower WAZ trajectory, whereas no association was observed among boys (length 0.13; WAZ ; Table S12). Further, among girls higher postnatal exposure was associated with an increased risk for being in a lower MUAC trajectory, whereas no association was observed among boys ( 0.10).
Table 4.
Associations between prenatal and postnatal particulate matter () and growth trajectories.
| Trajectorya | Bivariate model | Multivariable modelb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prenatal | Postnatal | Prenatal | Postnatal | |||||||
| (95% CI) | -Value | (95% CI) | -Value | (95% CI) | -Value | (95% CI) | -Value | |||
| Weight | 358 | 0.99 (0.95, 1.04) | 0.70 | 1.01 (0.94, 1.08) | 0.83 | 358 | 0.99 (0.94, 1.05) | 0.78 | 1.02 (0.94, 1.11) | 0.66 |
| Length | 358 | 1.06 (1.01, 1.12) | 0.03 | 1.00 (0.92, 1.08) | 0.97 | 358 | 1.07 (1.02, 1.13) | 0.008 | 0.98 (0.91, 1.05) | 0.53 |
| Head circumference | 358 | 1.02 (0.98, 1.07) | 0.38 | 1.00 (0.95, 1.05) | 0.91 | 358 | 1.03 (0.97, 1.08) | 0.33 | 0.99 (0.93, 1.06) | 0.81 |
| MUAC | 358 | 1.03 (0.98, 1.08) | 0.32 | 1.06 (1.00, 1.12) | 0.06 | 358 | 1.02 (0.97, 1.07) | 0.48 | 1.07 (1.00, 1.14) | 0.046 |
| Weight-for-length -score | 356 | 0.96 (0.92, 1.00) | 0.07 | 1.06 (0.99, 1.14) | 0.09 | 356 | 0.95 (0.91, 1.00) | 0.06 | 1.09 (1.01, 1.19) | 0.03 |
| Length-for-age -score | 356 | 1.03 (0.98, 1.09) | 0.21 | 0.98 (0.92, 1.05) | 0.64 | 356 | 1.04 (1.00, 1.09) | 0.08 | 0.97 (0.91, 1.03) | 0.29 |
| Weight-for-age -score | 356 | 1.00 (0.95, 1.05) | 0.97 | 1.02 (0.95, 1.09) | 0.65 | 356 | 1.00 (0.95, 1.05) | 0.99 | 1.02 (0.95, 1.09) | 0.57 |
Note: Per increase in exposure, all effects determined with cluster-robust standard errors in . Analyses are ordinal regression where ORs may be interpreted as the odds of being in a lower/smaller trajectory per increase in exposure. CI, confidence interval; MUAC, mid-upper arm circumference; , fine particulate matter (PM with an aerodynamic diameter of ).
All trajectories include four groups except length (3 groups) and head circumference (3 groups).
Multivariable models include both prenatal and postnatal exposure and adjust for child sex, maternal age, ethnicity, wealth index.
Estimated Effect of Cookstove Intervention on Growth Trajectories
Unadjusted models demonstrated that children born to mothers randomized to the LPG arm had reduced odds of a smaller MUAC trajectory, as compared with controls (LPG , 95% CI: 0.23, 0.85, ; Table 5). Multivariable models suggested that children born to mothers randomized to the LPG arm had reduced odds of a smaller HC (LPG , 95% CI: 0.37, 0.92, ) and MUAC (LPG , 95% CI: 0.22, 0.90, ) trajectories. Sensitivity models additionally adjusting for maternal BMI, breastfeeding duration, gestational age at delivery, or birth weight did not substantively change these findings (Table S13). Exploratory sex-stratified analyses found that among girls LPG arm assignment was associated with a reduced risk for being in the lower length and HC trajectories, whereas no association was observed among boys (length 0.03; HC ; Table S14). We found no evidence of an association of the improved biomass arm with any growth trajectory as compared with control (Table 5; Tables S13 and S14).
Table 5.
Associations between cookstove intervention arm and anthropometric growth trajectories.
| Trajectory | Participants () | Bivariate model | Multivariable model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Improved biomass | LPG | Improved biomass vs. control (open fire) | LPG vs. control (open fire) | Improved biomass vs. control (open fire) | LPG vs. control (open fire) | |||||
| (95% CI) | -Value | (95% CI) | -Value | (95% CI) | -Value | (95% CI) | -Value | ||||
| Growth trajectories | |||||||||||
| Weight | 414 | 430 | 300 | 1.01 (0.73, 1.40) | 0.93 | 1.02 (0.66, 1.55) | 0.94 | 1.05 (0.74, 1.48) | 0.79 | 0.88 (0.59, 1.33) | 0.55 |
| Length | 414 | 430 | 300 | 0.95 (0.48, 1.89) | 0.88 | 0.89 (0.46, 1.72) | 0.74 | 0.98 (0.50, 1.89) | 0.94 | 0.80 (0.43, 1.48) | 0.48 |
| Head circumference | 414 | 430 | 300 | 0.74 (0.47, 1.17) | 0.20 | 0.68 (0.42, 1.11) | 0.12 | 0.76 (0.48, 1.20) | 0.24 | 0.58 (0.37, 0.92) | 0.02 |
| MUAC | 414 | 430 | 300 | 0.86 (0.52, 1.45) | 0.58 | 0.44 (0.23, 0.85) | 0.02 | 0.92 (0.56, 1.50) | 0.73 | 0.45 (0.22, 0.90) | 0.03 |
| -Score trajectories | |||||||||||
| Weight-for-length -score | 408 | 420 | 299 | 0.97 (0.64, 1.46) | 0.88 | 1.00 (0.59, 1.67) | 0.99 | 0.93 (0.59, 1.45) | 0.74 | 0.90 (0.56, 1.43) | 0.65 |
| Length-for-age -score | 409 | 420 | 298 | 1.19 (0.66, 2.14) | 0.56 | 1.03 (0.61, 1.72) | 0.92 | 1.16 (0.63, 2.12) | 0.64 | 0.97 (0.55, 1.69) | 0.91 |
| Weight-for-age -score | 411 | 420 | 299 | 1.21 (0.87, 1.69) | 0.26 | 1.08 (0.75, 1.56) | 0.67 | 1.16 (0.86, 1.58) | 0.19 | 0.96 (0.70, 1.33) | 0.83 |
Note: Analyses are ordinal regression where ORs may be interpreted as the odds of being in a lower/smaller trajectory as compared with control. Multivariable models adjust for child sex and maternal age, ethnicity, and wealth index. All models include cluster-robust standard errors. All trajectories include four groups except length (3 groups) and head circumference (3 groups). CI, confidence interval; LPG, liquefied petroleum gas; MUAC, mid-upper arm circumference; OR, odds ratio.
Discussion
Early life growth trajectories are important determinants of morbidity and mortality across the life course. GRAPHS was a large, cluster-randomized cookstove intervention study that began the intervention during the prenatal period, included a clean-fuel arm, performed serial prenatal and postnatal personal HAP exposure assessments, and measured anthropometric variables at birth and every 3 months thereafter for the first year of life. Our findings add to evidence from studies that classified HAP exposure on the basis of self-reported use of different types of cooking or heating fuels and provide quantitative evidence for the effect of early life HAP exposure, as indexed by CO and exposure, on infant growth trajectories. Specifically, these data suggest that increasing prenatal exposure impairs length growth and increases risk for stunting, whereas increasing postnatal exposure impairs HC, MUAC, and WLZ growth over the first year of life. Further, this work provides evidence that an LPG cookstove intervention begun during pregnancy may improve HC and MUAC growth trajectories.
These data add to a growing literature supporting an association between HAP exposure and poor growth in infancy. Prior evidence supports an association between prenatal HAP exposure and birth weight (Amegah et al. 2014; Balakrishnan et al. 2018; Quinn et al 2021). For example, Wylie et al. (2017a) estimated that a 1-unit log increase in was associated with a mean decrease in birth weight of . Two cookstove intervention trials have suggested that a prenatally introduced cookstove may improve birth weight (Thompson et al. 2011; Alexander et al. 2018). Evidence beyond birth anthropometrics has been limited to studies employing questionnaires to ascertain HAP exposure, finding that households burning unclean fuels are more likely to have stunted or underweight children. The data provided herein thus extends these observations and finds higher early life CO and exposures are associated with increased risk for poorer length, HC, MUAC, and LAZ growth trajectories and assignment to the lowest LAZ trajectory, consistent with a definition of stunted. A prenatally introduced LPG cookstove, which we previously found to be associated with a 47% reduction (95% CI: 34, 57%) in CO and 32% lower (95% CI: 26, 38%) exposures (Chillrud et al. 2021), was associated with improved MUAC and HC growth trajectories relative to trajectories in control children whose mothers used 3-stone fires throughout pregnancy and the first year of the child’s life.
Despite substantial progress in reducing early childhood mortality, mortality for children years of age remains disproportionately high in sub-Saharan Africa. Stunting alone is responsible for 164,000 deaths and 14.3 million DALYs each year in children years of age (GBD 2019 Risk Factors Collaborators 2020). Causes of faltered early childhood growth are multifactorial, and emerging evidence suggests the importance of early life environmental exposures, including air pollution, beginning prenatally (Zheng et al. 2016). Suboptimal growth, even with mild impairments, increases risk for all-cause mortality and infectious disease mortality, specifically owing to respiratory infections and diarrheal disease (Olofin et al. 2013). Our findings, which are based on a cluster-randomized intervention, extend existing evidence of deleterious effects of HAP on birth weight to include evidence of adverse effects on early childhood growth trajectories, with implications for morbidity and mortality across the life course.
These data suggest that increased prenatal HAP, as indexed by both CO and exposures, was associated with impairments in length growth, whereas increasing postnatal exposure was associated with impairments in WLZ and MUAC trajectories. The underlying mechanisms are unknown. Length is considered a more chronic measure of health, whereas weight is considered a more acute measure of health; however, a plausible underlying link seen in both stunted and wasted children is reduced muscle mass (Briend et al. 2015). HAP exposure may induce inflammation; inflammation may promote insulin resistance, thereby reducing available nutrients for muscle metabolism (Fernández-Real and Ricart 1999; Oluwole et al. 2013). Stunted children often have a history of prior wasting episodes, reflecting the importance of sufficient energy stores to promote length growth (Briend et al. 2015; Pelletier 1994). An attenuated effect of growth hormone on insulin-like growth factor 1 production is seen in wasting and may prevent length growth, resulting in smaller length and LAZ (Freemark 2015). Overall, the finding that prenatal and postnatal HAP exposures are associated with stunting and wasting, respectively, suggests that children with higher early life HAP exposures have a shift in global development toward a frailer phenotype, which in turn is associated with the increased risk for future morbidity and mortality.
Prenatal HAP may alter functioning of the placenta, the maternal–fetal interface, which regulates the in utero environment and plays a central role in programming future development (Saenen et al. 2019). Prenatal HAP exposure may induce thrombotic placental lesions in those highly exposed, which may impair placental functioning, thus contributing to adverse fetal outcomes (Wylie et al. 2017b). Ambient air pollution research in the INfancia y Medio Ambiente Spanish birth cohort demonstrated a 6% decrease in -score for length by 6 months of age per increase in prenatal (first trimester) exposure, mediated partly through placental mitochondrial DNA (mtDNA) content, a biomarker of oxidative stress (Clemente et al. 2017). GRAPHS analyses similarly found that higher prenatal HAP exposure was associated with a lower cord blood mtDNA copy number and shorter cord blood mononuclear cell telomere length, with effects mitigated by the LPG intervention, supporting a role of oxidative stress in prenatal HAP pathogenesis (Kaali et al. 2018, 2021).
Postnatal exposure was negatively associated with MUAC and WLZ trajectories, and the LPG intervention was associated with improvement in MUAC trajectories compared with control. Visualization of the modeled latent class trajectories (Figure 2) suggests that they are distinct at birth, with changes in slope over the first year of life. We thus hypothesize that higher postnatal HAP exposure may worsen MUAC and WLZ growth over the first year of life, suggesting a subacute or acute effect on chronic impairment, as seen in other studies (Garenne et al. 2009; Isanaka et al. 2011). HAP is a major risk factor for infections in early childhood, specifically acute lower respiratory infection (ALRI), and one intervention study found that a cookstove intervention may reduce risk for severe ALRI (GBD 2015 Risk Factors Collaborators 2016; Smith et al. 2011). Therefore, it is plausible that children with higher HAP exposures were more susceptible to repeated infections, resulting in poorer MUAC and WLZ trajectories (Briend et al. 2015). Emerging evidence suggests that inhaled pollutants alter gut microbiome alpha- and beta-diversity and function (Kish et al. 2013; Mutlu et al. 2018), which in turn may modify the lower airway microbiome and immune response, increasing the risk for respiratory disease (Huang and Boushey 2015; Lee-Sarwar et al. 2019). An altered gut microbiome may also increase inflammation and gut permeability (Mutlu et al. 2011), affecting nutrient absorption, with implications for childhood growth. It is also plausible that our LPG intervention indirectly affected health by, for example, reducing time a mother spent gathering fuel and increasing time spent on other activities, such as breastfeeding (96% of LPG mothers breastfed for vs. 84% of improved biomass mothers and 78% of open fire/control mothers; Table 1) or income-generating activities (Williams et al. 2020).
Previously published associations between early life air pollution exposure and head growth suggest prenatal exposure reduces fetal biparietal diameter as measured by ultrasound or HC at birth; however, results are inconsistent (van den Hooven et al. 2012). Although data from LMICs are scarce, an urban Tanzanian cohort did not find an association between prenatal or CO and HC at birth (Wylie et al. 2017a). Similarly, our results did not demonstrate an association between prenatal HAP exposure and HC trajectory but, rather, suggest that postnatal CO increased the risk for reduced HC growth trajectory. The LPG intervention was associated with better HC growth trajectories, as compared with control. The implications of impaired head growth in this population are unknown, but studies of postnatal head growth in at-risk populations, such as preterm infants, suggest head growth may be associated with neurocognitive outcomes (Ghods et al. 2011). Analyses from the Helsinki Birth Cohort Study demonstrated that decreased HC at birth was associated with lower verbal, visuospatial, and arithmetic abilities by 20 years of age (Räikkönen et al. 2009). Supporting evidence from high-income countries suggests a link between air pollution exposure (largely traffic related) and cognition; air pollution exposure during childhood may impair neurodevelopment and academic achievement (Clifford et al. 2016). Higher air pollution exposure has also been associated with white matter hyperintensities in the prefrontal cortex (Calderón-Garcidueñas et al. 2008). Animal mechanistic studies point to a role of neuroinflammation and lipid peroxidation in air pollution-induced cognitive deficits (Block and Calderón-Garcidueñas 2009; Calderón-Garcidueñas et al. 2008). Characterizing neurodevelopmental outcomes in this cohort would allow us to better understand the implications of reduced HC growth and the potential benefit of a clean cookstove intervention.
The LPG intervention was associated with better MUAC and HC trajectories; however, we did not find evidence of association with improved length growth. It is plausible that the sensitive window for programming length was prior to our mid-gestation intervention. First trimester polycyclic aromatic hydrocarbon exposure has been shown to have the largest effect on fetal growth ratio and birth length, as compared with other trimesters (Block and Calderón-Garcidueñas 2009). Further, we note overlap in prenatal exposures by study arm and that all arms had average exposures above the WHO interim guidance of ; a larger prenatal exposure differential or a greater reduction in exposure levels may be required to see health effects. Finally, the effects of intervention on health may have benefited from an effect of the intervention unrelated to exposure (e.g., longer breastfeeding duration) to which weight gain and head growth may be more sensitive.
Potential mechanisms that might explain sex-specific differences in associations between child growth and prenatal or postnatal HAP exposures are not clear. Prior studies have reported differences in associations between prenatal ambient air pollution exposures and fetal growth by sex; however, few have extended these observations to postnatal growth (Rosofsky et al. 2020). Previous analyses of GRAPHS data have suggested stronger associations between prenatal HAP exposures and infant lung function in girls compared with boys, and stronger associations between prenatal HAP exposures and biomarkers of oxidative stress in boys than girls (Kaali et al. 2018; Lee et al. 2019). Our exploratory analyses suggest that sex-related differences in associations between growth outcomes and prenatal or postnatal HAP exposures may differ by pollutant. Specifically, prenatal CO exposure was associated with lower length trajectories in boys but not girls, whereas prenatal exposure was associated with lower length trajectories in girls but not boys. However, an important limitation of these findings is the reduced sample size in the analyses. Further work is needed to confirm these findings.
We note several strengths of our study. We leveraged the GRAPHS cohort, derived from a cluster-randomized stove intervention deployed mid-gestation and maintained through the first year of life, with serially measured prenatal and postnatal personal HAP exposures. Our well-characterized cohort included anthropometric measurements every 3 months beginning at birth, allowing us to construct growth trajectories for a number of important growth metrics. Ultrasound-established gestational dating ensured our cohort of nonsmoking pregnant women received the intervention prior to 24 wk of gestation and provided accurate GAs at delivery for our cohort, which we additionally adjusted for in sensitivity analyses. Questionnaires captured a number of important personal- and household-level covariates.
We also acknowledge limitations. Given the cost and field logistics of performing serial personal exposure measures in a resource-poor setting, we were unable to measure CO and exposures continuously, although we note that our exposure strategy involved repeated CO measures in both prenatal and postnatal periods. Each observed (personal) short-term measured exposure therefore provides an unbiased estimate of a participant’s exposure during the observed interval, and repeated sampling improves the ability of short-term personal exposures to estimate long-term exposures (McCracken et al. 2009). We also note that pollution sources outside of cooking may have contributed to these exposures. Our primary exposure was CO; was measured only in a subset of participants, limiting our ability to investigate and sex-specific effects. Some maternal characteristics differed by study arm and, although we adjust for these variables in multivariable analyses, findings should be interpreted with caution despite the randomized trial design. We report multiple comparisons and therefore some results may appear significant due to chance. For example, we perform 10 regressions with our primary pollutant CO, and only our results for prenatal CO on stunting and postnatal CO on HC would remain significant after Bonferroni correction. Maternal BMI was measured after enrollment and therefore during pregnancy. Prepregnancy maternal BMI would be a more accurate measure of maternal size; however, this data was not available. We measured anthropometric variables once at each assessment; measurement in duplicate or triplicate may have reduced measurement error. In particular, the larger standard deviations for length and HC measurements may be related to population heterogeneity but more likely suggest measurement error, which may bias findings to the null. We did not measure nutrition intake or enteric infections; however, note that we did adjust for household wealth index, which we conceptualized as an antecedent of nutrition and enteric infections in early life (Akombi et al. 2019). Further, we prospectively and serially assessed breastfeeding practices over the first year of life and additional adjustment for breastfeeding duration did not substantively change our results (Fekadu et al. 2015). Finally, we note that our CO exposure distribution was lower than reported in other HAP studies, possibly owing to the outdoor nature of cooking in our cohort compared with indoor cooking in others, and larger or additional effects may be seen in a more exposed population.
If these results are replicated elsewhere, they would suggest that increased early life HAP may increase risk for poorer growth trajectories over childhood. Primary strategies for reducing child mortality focus on widespread implementation of low-cost technologies, such as immunization, oral rehydration therapy, and antibiotics; however, poor growth and disease have multiple etiologies and an optimal strategy may involve a combination of environmental, health, and nutritional interventions. Future work is needed to confirm these findings and better characterize the future health implications of the identified impaired growth trajectories.
Supplementary Material
Acknowledgments
P.L.K., D.J., K.P.A., A.G.L., and S.O-A. conceived of the study and oversaw implementation. E.B-K., F.B.O., and A.G.L. led data analysis and manuscript writing. P.L.K., S.C., M.M., K.A.A., and D.J. led exposure assessment teams. K.P.A., S.O-A., K.A.A., E.B-K., M.M., S.G., F.B.O., and S.K. oversaw daily field operations. E.B-K., K.A.A., M.M., F.A.O., and A.G.L. oversaw anthropometric measurements and data cleaning. All authors assisted with data interpretation, manuscript preparation and final manuscript review.
The Ghana Randomized Air Pollution and Health Study (GRAPHS) was supported by the National Institutes of Health (NIH) National Institute of Environmental Health Sciences (NIEHS) grants R01 ES019547, P30 ES 009089, and 1S10OD016219, Thrasher Research Fund, and the Clean Cooking Alliance. B.J.W. was additionally supported by NIEHS grant K23 ES021471. C.F.G. was supported by NIEHS grant F31 ES031833. A.G.L. was additionally supported by the National Heart, Lung and Blood Institute grant K23 HL135349. The funding institute and foundations had no role in the study design, data collection, data analysis, or writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Deidentified data will be made available upon request.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIH or the Department of Health and Human Services.
GRAPHS was registered with Clinicaltrials.gov number NCT01335490 (https://clinicaltrials.gov/ct2/show/NCT01335490).
References
- Akombi BJ, Agho KE, Renzaho AM, Hall JJ, Merom DR. 2019. Trends in socioeconomic inequalities in child undernutrition: evidence from Nigeria Demographic and Health Survey (2003–2013). PLoS One 14(2):e0211883, PMID: , 10.1371/journal.pone.0211883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DA, Northcross A, Karrison T, Morhasson-Bello O, Wilson N, Atalabi OM, et al. 2018. Pregnancy outcomes and ethanol cook stove intervention: a randomized-controlled trial in Ibadan, Nigeria. Environ Int 111:152–163, PMID: , 10.1016/j.envint.2017.11.021. [DOI] [PubMed] [Google Scholar]
- Amegah AK, Quansah R, Jaakkola JJ. 2014. Household air pollution from solid fuel use and risk of adverse pregnancy outcomes: a systematic review and meta-analysis of the empirical evidence. PLoS One 9(12):e113920, PMID: , 10.1371/journal.pone.0113920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan K, Ghosh S, Thangavel G, Sambandam S, Mukhopadhyay K, Puttaswamy N, et al. 2018. Exposures to fine particulate matter (PM2.5) and birthweight in a rural-urban, mother-child cohort in Tamil Nadu, India. Environ Res 161:524–531, PMID: , 10.1016/j.envres.2017.11.050. [DOI] [PubMed] [Google Scholar]
- Berlin KS, Parra GR, Williams NA. 2014. An introduction to latent variable mixture modeling (part 2): longitudinal latent class growth analysis and growth mixture models. J Pediatr Psychol 39(2):188–203, PMID: , 10.1093/jpepsy/jst085. [DOI] [PubMed] [Google Scholar]
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. 2013. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382(9890):427–451, PMID: , 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- Block ML, Calderón-Garcidueñas L. 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32(9):506–516, PMID: , 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boamah EA, Asante K, Ae-Ngibise K, Kinney PL, Jack DW, Manu G, et al. 2014. Gestational age assessment in the Ghana Randomized Air Pollution and Health Study (GRAPHS): ultrasound capacity building, fetal biometry protocol development, and ongoing quality control. JMIR Res Protoc 3(4):e77, PMID: , 10.2196/resprot.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briend A, Khara T, Dolan C. 2015. Wasting and stunting—similarities and differences: policy and programmatic implications. Food Nutr Bull 36(1 suppl):S15–S23, PMID: , 10.1177/15648265150361S103. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Mora-Tiscareño A, Ontiveros E, Gómez-Garza G, Barragán-Mejía G, Broadway J, et al. 2008. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn 68(2):117–127, PMID: , 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Chillrud SN, Ae-Ngibise KA, Gould CF, Owusu-Agyei S, Mujtaba M, Manu G, et al. 2021. The effect of clean cooking interventions on mother and child personal exposure to air pollution: results from the Ghana Randomized Air Pollution and Health Study (GRAPHS). J Expo Sci Environ Epidemiol 31(4):683–698, PMID: , 10.1038/s41370-021-00309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, Mullany LC, Hurley KM, Katz J, Black RE. 2015. Nutrition and maternal, neonatal, and child health. Semin Perinatol 39(5):361–372, PMID: , 10.1053/j.semperi.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Clark ML, Peel JL, Balakrishnan K, Breysse PN, Chillrud SN, Naeher LP, et al. 2013. Health and household air pollution from solid fuel use: the need for improved exposure assessment. Environ Health Perspect 121(10):1120–1128, PMID: , 10.1289/ehp.1206429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente DBP, Casas M, Janssen BG, Lertxundi A, Santa-Marina L, Iñiguez C, et al. 2017. Prenatal ambient air pollution exposure, infant growth and placental mitochondrial DNA content in the INMA birth cohort. Environ Res 157:96–102, PMID: , 10.1016/j.envres.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Clifford A, Lang L, Chen R, Anstey KJ, Seaton A. 2016. Exposure to air pollution and cognitive functioning across the life course—a systematic literature review. Environ Res 147:383–398, PMID: , 10.1016/j.envres.2016.01.018. [DOI] [PubMed] [Google Scholar]
- Danaei G, Andrews KG, Sudfeld CR, Fink G, McCoy DC, Peet E, et al. 2016. Risk factors for childhood stunting in 137 developing countries: a comparative risk assessment analysis at global, regional, and country levels. PLoS Med 13(11):e1002164, PMID: , 10.1371/journal.pmed.1002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekadu Y, Mesfin A, Haile D, Stoecker BJ. 2015. Factors associated with nutritional status of infants and young children in Somali Region, Ethiopia: a cross-sectional study. BMC Public Health 15:846, PMID: , 10.1186/s12889-015-2190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Real JM, Ricart W. 1999. Insulin resistance and inflammation in an evolutionary perspective: the contribution of cytokine genotype/phenotype to thriftiness. Diabetologia 42(11):1367–1374, PMID: , 10.1007/s001250051451. [DOI] [PubMed] [Google Scholar]
- Freemark M. 2015. Metabolomics in nutrition research: biomarkers predicting mortality in children with severe acute malnutrition. Food Nutr Bull 36(1 suppl):S88–S92, PMID: , 10.1177/15648265150361S114. [DOI] [PubMed] [Google Scholar]
- Garenne M, Willie D, Maire B, Fontaine O, Eeckels R, Briend A, et al. 2009. Incidence and duration of severe wasting in two African populations. Public Health Nutr 12(11):1974–1982, PMID: , 10.1017/S1368980009004972. [DOI] [PubMed] [Google Scholar]
- GBD 2015 Risk Factors Collaborators. 2016. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053):1659–1724, PMID: , 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Risk Factors Collaborators. 2020. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396(10258):1223–1249, PMID: , 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods E, Kreissl A, Brandstetter S, Fuiko R, Widhalm K. 2011. Head circumference catch-up growth among preterm very low birth weight infants: effect on neurodevelopmental outcome. J Perinat Med 39(5):579–586, PMID: , 10.1515/jpm.2011.049. [DOI] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. 1999. Causal diagrams for epidemiologic research. Epidemiology 10:37–48, PMID: , 10.1097/00001648-199901000-00008. [DOI] [PubMed] [Google Scholar]
- Gunnsteinsson S, Labrique AB, West KP Jr, Christian P, Mehra S, Shamim AA, et al. 2010. Constructing indices of rural living standards in Northwestern Bangladesh. J Health Popul Nutr 28(5):509–519, PMID: , 10.3329/jhpn.v28i5.61600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE. 2001. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer-Verlag. [Google Scholar]
- Huang YJ, Boushey HA. 2015. The microbiome in asthma. J Allergy Clin Immunol 135(1):25–30, PMID: , 10.1016/j.jaci.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isanaka S, Grais RF, Briend A, Checchi F. 2011. Estimates of the duration of untreated acute malnutrition in children from Niger. Am J Epidemiol 173(8):932–940, PMID: , 10.1093/aje/kwq436. [DOI] [PubMed] [Google Scholar]
- Islam S, Rana MJ, Mohanty SK. 2021. Cooking, smoking, and stunting: effect of household air pollution sources on childhood growth in India. Indoor Air 31(1):229–249, PMID: , 10.1111/ina.12730. [DOI] [PubMed] [Google Scholar]
- Jack DW, Ae-Ngibise KA, Gould CF, Boamah-Kaali E, Lee AG, Mujtaba MN, et al. 2021. A cluster randomised trial of cookstove interventions to improve infant health in Ghana. BMJ Glob Health 6(8):e005599, PMID: , 10.1136/bmjgh-2021-005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack DW, Asante KP, Wylie BJ, Chillrud SN, Whyatt RM, Ae-Ngibise KA, et al. 2015. Ghana Randomized Air Pollution and Health Study (GRAPHS: study protocol for a randomized controlled trial. Trials 16:420, PMID: , 10.1186/s13063-015-0930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetter J, Ebersviller S. 2016. Test Report. BioLite HomeStove with Wood Fuel: Air Pollutant Emissions and Fuel Efficiency. EPA/625/R-16/001. Washington, DC: U.S. Environmental Protection Agency. https://cfpub.epa.gov/si/si_public_file_download.cfm?p_download_id=528653&Lab=NRMRL [accessed 8 March 2021]. [Google Scholar]
- Jung T, Wickrama KAS. 2008. An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass 2(1):302–317, 10.1111/j.1751-9004.2007.00054.x. [DOI] [Google Scholar]
- Kaali S, Jack D, Delimini R, Hu L, Burkart K, Opoku-Mensah J, et al. 2018. Prenatal household air pollution alters cord blood mononuclear cell mitochondrial DNA copy number: sex-specific associations. Int J Environ Res Public Health 16(1):26, PMID: , 10.3390/ijerph16010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaali S, Jack D, Opoku-Mensah J, Bloomquist T, Aanaro J, Quinn A, et al. 2021. Prenatal household air pollution exposure, cord blood mononuclear cell telomere length and age four blood pressure: evidence from a Ghanaian pregnancy cohort. Toxics 9(7):169, PMID: , 10.3390/toxics9070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney PL, Asante K-P, Lee AG, Ae-Ngibise KA, Burkart K, Boamah-Kaali E, et al. 2021. Prenatal and postnatal household air pollution exposures and pneumonia risk: evidence from the Ghana Randomized Air Pollution and Health Study. Chest 160(5):1634–1644, PMID: , 10.1016/j.chest.2021.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish L, Hotte N, Kaplan GG, Vincent R, Tso R, Gänzle M, et al. 2013. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS One 8(4):e62220, PMID: , 10.1371/journal.pone.0062220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG, Kaali S, Quinn A, Delimini R, Burkart K, Opoku-Mensah J, et al. 2019. Prenatal household air pollution is associated with impaired infant lung function with sex-specific effects. Evidence from GRAPHS, a cluster randomized cookstove intervention trial. Am J Respir Crit Care Med 199(6):738–746, PMID: , 10.1164/rccm.201804-0694OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Sarwar KA, Kelly RS, Lasky-Su J, Zeiger RS, O’Connor GT, Sandel MT, et al. 2019. Integrative analysis of the intestinal metabolome of childhood asthma. J Allergy Clin Immunol 144(2):442–454, PMID: , 10.1016/j.jaci.2019.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Wang B, Shen G, Cao S, Mcswain B, Qin N, et al. 2020. Association of solid fuel use with risk of stunting in children living in China. Indoor Air 30(2):264–274, PMID: , 10.1111/ina.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JP, Schwartz J, Bruce N, Mittleman M, Ryan LM, Smith KR. 2009. Combining individual- and group-level exposure information: child carbon monoxide in the Guatemala woodstove randomized control trial. Epidemiology 20(1):127–136, PMID: , 10.1097/EDE.0b013e31818ef327. [DOI] [PubMed] [Google Scholar]
- Mishra V, Retherford RD. 2007. Does biofuel smoke contribute to anaemia and stunting in early childhood? Int J Epidemiol 36(1):117–129, PMID: , 10.1093/ije/dyl234. [DOI] [PubMed] [Google Scholar]
- Mutlu EA, Comba IY, Cho T, Engen PA, Yazici C, Soberanes S, et al. 2018. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ Pollut 240:817–830, PMID: , 10.1016/j.envpol.2018.04.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu EA, Engen PA, Soberanes S, Urich D, Forsyth CB, Nigdelioglu R, et al. 2011. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part Fibre Toxicol 8:19, PMID: , 10.1186/1743-8977-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin DS, Odgers CL. 2010. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 6:109–138, PMID: , 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO. 2007. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling 14(4):535–569, 10.1080/10705510701575396. [DOI] [Google Scholar]
- Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, et al. 2013. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS One 8(5):e64636, PMID: , 10.1371/journal.pone.0064636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluwole O, Arinola GO, Ana GR, Wiskel T, Huo D, Olopade OI, et al. 2013. Relationship between household air pollution from biomass smoke exposure, and pulmonary dysfunction, oxidant-antioxidant imbalance and systemic inflammation in rural women and children in Nigeria. Glob J Health Sci 5(4):28–38, PMID: , 10.5539/gjhs.v5n4p28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier DL. 1994. The relationship between child anthropometry and mortality in developing countries: implications for policy, programs and future research. J Nutr 124(10 suppl):2047S–2081S, PMID: , 10.1093/jn/124.suppl_10.2047S. [DOI] [PubMed] [Google Scholar]
- Pun VC, Dowling R, Mehta S. 2021. Ambient and household air pollution on early-life determinants of stunting—a systematic review and meta-analysis. Environ Sci Pollut Res Int 28(21):26404–26412, PMID: , 10.1007/s11356-021-13719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn AK, Adjei IA, Ae-Ngibise KA, Agyei O, Boamah-Kaali EA, Burkart K, et al. 2021. Prenatal household air pollutant exposure is associated with reduced size and gestational age at birth among a cohort of Ghanaian infants. Environ Int 155:106659, PMID: , 10.1016/j.envint.2021.106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räikkönen K, Forsén T, Henriksson M, Kajantie E, Heinonen K, Pesonen A-K, et al. 2009. Growth trajectories and intellectual abilities in young adulthood: the Helsinki Birth Cohort Study. Am J Epidemiol 170(4):447–455, PMID: , 10.1093/aje/kwp132. [DOI] [PubMed] [Google Scholar]
- Rosofsky AS, Fabian MP, Ettinger de Cuba S, Sandel M, Coleman S, Levy JI, et al. 2020. Prenatal ambient particulate matter exposure and longitudinal weight growth trajectories in early childhood. Int J Environ Res Public Health 17(4):1444, PMID: , 10.3390/ijerph17041444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenen ND, Martens DS, Neven KY, Alfano R, Bové H, Janssen BG, et al. 2019. Air pollution-induced placental alterations: an interplay of oxidative stress, epigenetics, and the aging phenotype? Clin Epigenetics 11(1):124, PMID: , 10.1186/s13148-019-0688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinharoy SS, Clasen T, Martorell R. 2020. Air pollution and stunting: a missing link? Lancet Glob Health 8(4):e472–e475, PMID: , 10.1016/S2214-109X(20)30063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, McCracken JP, Weber MW, Hubbard A, Jenny A, Thompson LM, et al. 2011. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet 378(9804):1717–1726, PMID: , 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- Thompson LM, Bruce N, Eskenazi B, Diaz A, Pope D, Smith KR. 2011. Impact of reduced maternal exposures to wood smoke from an introduced chimney stove on newborn birth weight in rural Guatemala. Environ Health Perspect 119(10):1489–1494, PMID: , 10.1289/ehp.1002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielsch JM, Katz J, Thulasiraj RD, Coles CL, Sheeladevi S, Yanik EL, et al. 2009. Exposure to indoor biomass fuel and tobacco smoke and risk of adverse reproductive outcomes, mortality, respiratory morbidity and growth among newborn infants in south India. Int J Epidemiol 38(5):1351–1363, PMID: , 10.1093/ije/dyp286. [DOI] [PubMed] [Google Scholar]
- van den Hooven EH, Pierik FH, de Kluizenaar Y, Willemsen SP, Hofman A, van Ratingen SW, et al. 2012. Air pollution exposure during pregnancy, ultrasound measures of fetal growth, and adverse birth outcomes: a prospective cohort study. Environ Health Perspect 120(1):150–156, PMID: , 10.1289/ehp.1003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2006. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva, Switzerland: World Health Organization, Department of Nutrition for Health and Development. https://apps.who.int/iris/rest/bitstreams/51510/retrieve [accessed 9 November 2021]. [Google Scholar]
- Williams KN, Kephart JL, Fandiño-Del-Rio M, Simkovich SM, Koehler K, Harvey SA, et al. 2020. Exploring the impact of a liquefied petroleum gas intervention on time use in rural Peru: a mixed methods study on perceptions, use, and implications of time savings. Environ Int 145:105932, PMID: , 10.1016/j.envint.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank. 2020. Databank: World Development Indicators. https://databank.worldbank.org/reports.aspx?source=2&series=EG.CFT.ACCS.ZS&country=# [accessed 7 December 7 2020].
- Wylie BJ, Kishashu Y, Matechi E, Zhou Z, Coull B, Abioye AI, et al. 2017a. Maternal exposure to carbon monoxide and fine particulate matter during pregnancy in an urban Tanzanian cohort. Indoor Air 27(1):136–146, PMID: , 10.1111/ina.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie BJ, Matechi E, Kishashu Y, Fawzi W, Premji Z, Coull BA, et al. 2017b. Placental pathology associated with household air pollution in a cohort of pregnant women from Dar Es Salaam, Tanzania. Environ Health Perspect 125(1):134–140, PMID: , 10.1289/EHP256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Chillrud SN, Ji J, Chen Y, Pitiranggon M, Li W, et al. 2017. Comparison of PM2.5 exposure in hazy and non-hazy days in Nanjing, China. Aerosol Air Qual Res 17(9):2235–2246, PMID: , 10.4209/aaqr.2016.07.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Zhang J, Sommer K, Bassig BA, Zhang X, Braun J, et al. 2016. Effects of environmental exposures on fetal and childhood growth trajectories. Ann Glob Health 82(1):41–99, PMID: , 10.1016/j.aogh.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.