Abstract
Objective.
To identify risk factors for glycemic failure in youth with type 2 diabetes (T2D).
Methods.
A retrospective review of HbA1c, anthropomorphic measures, medication records, and laboratory studies was performed using registry data from a dedicated pediatric type 2 diabetes clinic. Latent profile analysis (LPA) was performed to model longitudinal trajectory of HbA1c over five years.
Results.
The registry includes 229 youth with T2D, of whom 80% self-identify as Latinx. The odds ratio (OR) for uncontrolled diabetes five years after diagnosis correlated with diagnostic HbA1c, with OR of 2.41 if HbA1c at diagnosis >8.5% (sensitivity 68%, specificity 54%, P=0.015). LPA modeling identified three HbA1c profiles: (A) mean HbA1c <8% throughout the 5 years, (B) persistent elevation of mean HbA1c >9%, and (C) mean HbA1c of 12% at diagnosis, rapid decline to 6.4% by 4–6 months, and increase to 11% by 18 months. Our analysis of medication regimen showed that, amongst patients treated with metformin, the addition of multiple daily injections (MDI) did not improve HbA1c compared to those on basal insulin. Finally, weight loss over the first year after diagnosis correlated with improvement in HbA1c in both subjects prescribed metformin monotherapy, as well as insulin-containing regimen.
Conclusion.
Youth with T2D exhibit distinct HbA1c profiles. Patients with diagnostic HbA1c >8.5% are at high risk for glycemic failure, irrespective of short-term improvement in HbA1c. Weight management has the potential to improve short-term HbA1c outcome in youth with T2D. Additional studies are needed to determine the role of medication adherence on glycemic control.
Keywords: Diabetes Mellitus, Type 2, Metformin, Adolescent, HbA1c, Risk Factors
Introduction
With the burgeoning obesity epidemic, the incidence of youth-onset type 2 diabetes is on the rise1. Multiple studies have established that disease progression is more rapid than adult-onset type 2 diabetes2–5. The median time to glycemic failure occurred just 11.5 months after treatment randomization in the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study6. The rapid loss of glycemic control is partly attributed to accelerated beta-cell failure in youth compared to adults4. In addition, the development of type 2 diabetes in youth is associated with more severe albuminuria and neuropathy scores2. Recent computer-simulated modeling further pointed at a potential 15-year decline in life expectancy in adults with youth-onset type 2 diabetes7. Further, microvascular and macrovascular complications develop sooner in youth-onset type 2 diabetes compared to their counterparts with type 1 diabetes8. Recent outcomes report from the TODAY follow-up study found early-onset and an increased rate of cardiovascular and cerebrovascular events compared to findings from the Diabetes Control and Complications Trial9. As well, study participants experienced accelerated diabetic kidney disease, retinopathy, and neuropathy10, 11. These co-morbidities are expected to result in significant disabilities and exact a staggering economic toll.
Identifying patients who are at risk for poor glycemic control is integral to improving the clinical outcome of youth with type 2 diabetes. Our understanding of this condition comes largely from multi-center intervention and natural history studies, such as TODAY, the Pediatric Diabetes Consortium (PDC), and the SEARCH for Diabetes in Youth study (SEARCH)3, 6, 12–14. These have all shown that youth with type 2 diabetes are largely of low socioeconomic status and have a poor follow-up rate15, 16. The TODAY study also identified risk factors for glycemic failure, including high initial HbA1c, low beta-cell function, and maternal history of type 2 diabetes6. More recently, Candler et. al. reported on the outcome from the British Paediatric Surveillance Unit and identified weight loss as a predictor of improved glycemic control, which was not previously reported in the TODAY study17. It remains unclear if certain risk factors contributing to glycemic control are cohort-specific.
We report here the clinical outcomes of a natural history study from a single pediatric center with a dedicated type 2 diabetes clinic. Unlike other studies, our population is of predominant Latinx descent. Our two aims were to: 1) analyze glycemic trends and associated risk factors to help identify a high-risk cohort for intensive case management and 2) determine the impact of weight reduction and medication regimen on glycemic outcome.
Methods
Data collection.
The Type 2 Diabetes Clinic at Children’s Hospital Los Angeles (CHLA) was founded on the premise that the conventional model for management of type 1 diabetes does not adequately meet the needs of youth with type 2 diabetes. Our comprehensive clinic takes place 1 day per week, and is comprised of physicians, nurse practitioners, nurses, dietitians, social workers, a physical therapist, and a clinical psychologist. In addition to clinic visits, our model includes a free weight-management program in the patients’ community and a peer-group that addresses barriers to diabetes self-care. All patients who attended the clinic starting April 2017 were invited to participate in the T2DM Clinic Registry. Consents and assents were obtained from parents and patients as appropriate (ages 10 to 21 years), granting us both retrospective and prospective access to their clinical records. Study data were collected and managed using REDCap electronic data capture tools hosted at CHLA18, 19. Data entry and analysis were in compliance with regulations set forth by the CHLA institutional review board. We limited our data analysis to subjects who were diagnosed after July 2013 (the inception of electronic medical records for the CHLA outpatient clinics) to October 2019. The use of “Hispanic” or “non-Hispanic white” under demographic description in Table 1 reflects the terminology used by the hospital electronic medical records. We use the term “Latinx” elsewhere in the manuscript.
Table 1.
Patient demographics
| Age (yr) | 16.9±2.5 |
| Duration of T2D (yr)† | 2.6±3.1 |
| Gender | 40% M / 60% F |
| HbA1c at diagnosis (%) | 10.2±2.9 |
| Age (yr) at diagnosis** | |
| Male | 14.3±2.3 |
| Female | 13.2±2.3 |
| Race/Ethnicity | |
| Hispanic | 80% |
| Non-Hispanic White | 4% |
| Black | 4% |
| Asian | 1% |
| Other/Not reported | 11% |
| Family history of type 2 diabetes | 80% |
| Mother | 44% |
| Father | 25% |
| Sibling | 7% |
N=229. All continuous variables reported as mean ± standard deviation, except as noted.
Median ± interquartile range.
P=0004.
Inclusion criteria.
The diagnostic criteria for diabetes were based on those set forth by the American Diabetes Association (ADA): HbA1c ≥6.5%, fasting plasma glucose ≥126 mg/dL, or plasma glucose ≥200 mg/dL on random blood glucose testing or a 2-hour oral glucose tolerance test20. In asymptomatic individuals, two abnormal results were used to diagnose diabetes. Diabetes was classified as type 2 based on the following criteria: absence of type 1 diabetes auto-antibodies (GAD65, ICA512, and anti-insulin), elevated serum levels of c-peptide or insulin, body mass index (BMI) greater than the 85th percentile, and the presence of acanthosis nigricans. We included three subjects who demonstrated weak reactivity to GAD65: 3.7 U/mL (reference <0.5 U/mL), 1.2 U/mL (reference <1 U/mL), and 6 IU/mL (reference <5 IU/mL). The different reference ranges are due to changes over time in contracted laboratories that performed the assays. Two subjects had weak reactivity to ICA512 (levels of 1.1 and 1.2 U/mL, with a reference range <1.0 U/mL). We also included one subject with a positive IAA titer of 4.4 U/mL (reference range <0.4 U/mL) and one with a positive GAD titer of 43 IU/mL (reference 5 IU/mL). These subjects did not require insulin treatment for at least 6 months after diagnosis of diabetes while maintaining their HbA1c <7.0%. Subjects were excluded from this analysis if they were diagnosed with Prader-Willi Syndrome, hypothalamic obesity (i.e., secondary to intracranial neoplasm), secondary diabetes, or genetically-confirmed monogenic diabetes.
Family history of type 2 diabetes and race/ethnicity were based on self-report as determined on intake questionnaires. The presence of diabetic ketoacidosis (DKA) was based on chart review.
For the calculation of number of clinic visits per year, we tallied the number of clinic appointments patients attended within 365 days from the initial clinic visit. This analysis was only completed for patients who had been diagnosed with diabetes for > 1 year. For the bivariate analysis of weight with HbA1c change at 1 year, we included all subjects that had a HbA1c measurement within 9 to 15 months from the date of diagnosis. The change in HbA1c was calculated by subtracting the HbA1c at diagnosis from the HbA1c obtained between 9 to 15 months after diagnosis.
We report the body mass index (BMI) as the percentage in excess of the 95th percentile (BMI%/95P) using reference ranges from the Centers for Disease Control and Prevention. This BMI score was used because conventional percentiles and z-scores are mathematically compressed and do not adequately convey quantitative changes in weight in cases of severe obesity21–23. Recent studies support the use of percentage in excess of the 95th percentile (BMI%/95P) in children with severe obesity24, 25. BMI change was expressed as (BMI%/95P at 1 year – BMI%/95P at baseline)/BMI%/95P at baseline.
Medication regimen was identified by prescribed medication documented on clinic departure summaries. The category of “none” describes patients who were not prescribed medications and not patients who did not adhere to prescribed medications. Providers adjusted medications at each clinic visit if deemed appropriate after review of medication adherence, following the recommendations of the ADA and ISPAD guidelines.
Statistical analysis.
Student t-test was used to compare differences in mean age of diagnosis. Spearman correlation was used for bivariate analysis between changes in BMI and HbA1c over the first year after diagnosis. For post-hoc analyses of medication regimen, we utilized the Dwass-Steel-Critchlow-Fligner (DSCF) multiple comparison analysis, which is based on pairwise two-sample Wilcoxon comparisons26. Odds ratio of the relationship between diagnostic HbA1c and 5-year HbA1c outcome was computed by logistic regression, with the 4–6-month HbA1c as a potential effect modifier27. Sensitivity and specificity were calculated using PROC FREQ in SAS 9.328.
For 5-year HbA1c progression data, we accounted for unbalanced contribution of data from different subjects by averaging the repeated measures data for each participant at each time interval. The intervals are defined as follows: < 1 month after diagnosis, at 3-month intervals until 1 year, at 6-month intervals between 1 to 2 years, and yearly thereafter. We considered HbA1c obtained within 30 days (<1 month) after diagnosis as the baseline HbA1c, because patients are sometimes diagnosed first by the referring provider and then seen in our clinic within the next several weeks. Latent profile analysis (LPA) was conducted to model longitudinal trajectory of HbA1c29, 30. LPA enabled identification of heterogeneous patterns of HbA1c change over time. One-way ANOVA was used to examine the effect of fasting c-peptide, HbA1c at diagnosis, clinic attendance, and medication regimen on HbA1c progression and obtain statistical significance31. One-way ANOVA or Kruskal-Wallis test was used for normally (HbA1c at diagnosis) or abnormally (c-peptide level) distributed continuous variables, respectively, to examine the effect of the variables on HbA1c. For analysis using continuous variables, mean/SD was used for variables with normal distribution and median/interquartile range was used for variables with non-normal distribution. Fisher exact test was used to calculate statistical significance for categorical variables.
A 5% level of significance was used for all tests. The statistical analysis was conducted on Prism 8, SAS 9.3 or R32.
Results
Demographics and Clinic Attendance
The demographics of our subjects are presented in Table 1. The mean age of our patient population was 16.9 years, with 60% female. The mean age of diagnosis was 13.2 ± 2.3 years in females, compared to 14.3 ± 2.3 years in males (P=0.0004). The median duration of diabetes was 2.6 years (interquartile range 3.1 years). Eighty percent of our patients self-identify as Latinx, compared to 47.5% of the population in Los Angeles33. Approximately 80% of our patients report a family history of type 2 diabetes. The mean BMI (BMI%/95P) at diagnosis was 115% ± 21% and 123% ± 22% for females and males, respectively. We next determined the incidence of diabetic ketoacidosis (DKA) at the time of diagnosis. We limited our chart review to patients in the clinic registry who attended clinic 1 year (defined as 9 to 15 months) after diagnosis. In this cohort, 9.4% of patients presented with DKA at the time of diagnosis.
Patients with youth-onset type 2 diabetes have been reported to have poor clinic attendance rates. In our registry, 18% of patients did not attend a follow-up visit between 9 to 15 months after their initial visit. For ease of scheduling, we designated appointments as “type 2 diabetes follow-up” slots, protecting the slot from being filled with patients with other types of diabetes. We analyzed if this strategy improved clinic attendance. Clinic attendance rate was calculated by dividing the number of clinic visits attended by the total number of scheduled appointments. Overall clinic attendance rate was unchanged before and after the inception of the Type 2 Diabetes Clinic (65.4% vs. 64.3%, respectively). However, for patients with new-onset diabetes, the number of visits increased by 0.8 in the first year after diagnosis in the new clinic model, compared to the conventional clinic model (4.5 ± 1.4 vs 3.7 ± 1.2, P=0.004).
HbA1c Progression
To determine glycemic excursion over time in our patient cohort, we analyzed the HbA1c over the first 5 years after diagnosis. As shown in Figure 1A, mean HbA1c improved rapidly over the first 6 months after diagnosis (10.2% ± 2.8% at diagnosis, 6.4% ± 1.3% at six months), but rose over the next 18 months, followed by a more gradual increase over the next 3 years. At 5 years, the mean HbA1c was indistinguishable from that at diagnosis. One year after diagnosis (9–15 months), 54% of the participants achieved a HbA1c level <7%, the current recommended HbA1c target (Fig. 1B)20, 34. HbA1c was uncontrolled (>8%) for 27% of the subjects. By 5 years after diagnosis (49–60 months), only 27% of the subjects had a HbA1c level <7%, whereas 60% of the subjects had a HbA1c >8% (Fig. 1C).
Figure 1.
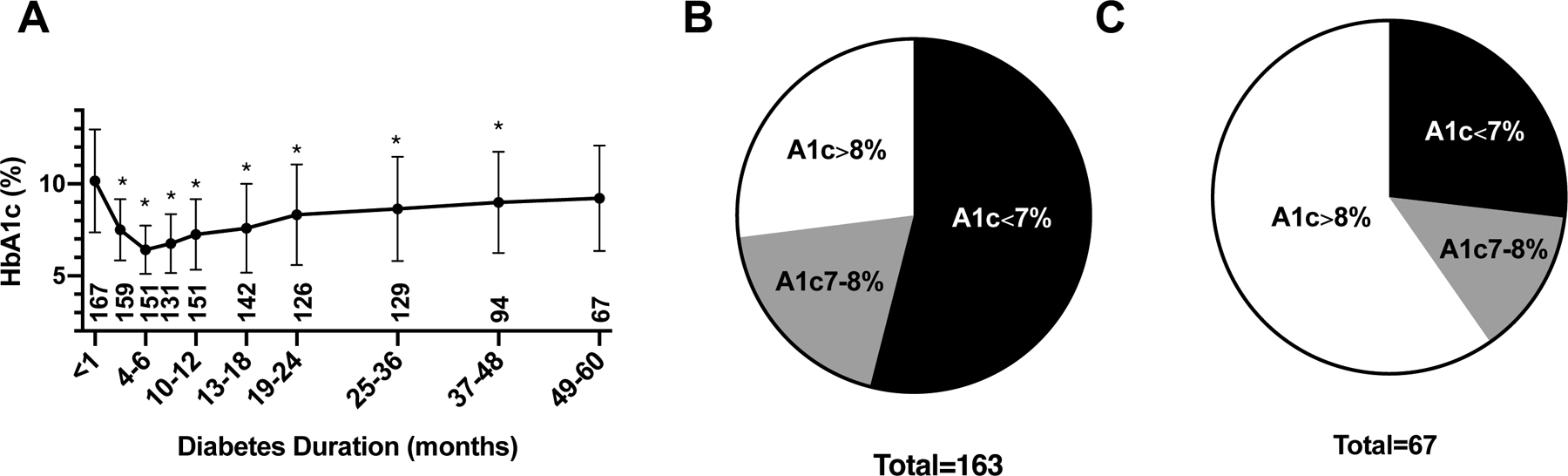
A. HbA1c (mean +/− SD) progression over 5 years. All pairwise comparisons are made to baseline (duration <1 month). * P<0.05. The number below each time point represents sample size. B and C show distribution of HbA1c 1 year (B) and 5 years (C), respectively, after diagnosis.
Previous studies have pointed to HbA1c at diagnosis and its response to metformin as independent prognosticators for durable glycemic control34–36. We sought to examine the utility of combining HbA1c levels at diagnosis and 4–6 months, in estimating the risk of having a HbA1c level >8% by 5 years after diagnosis. As shown in Table 2, the odds ratio of glycemic failure increased as diagnostic HbA1c rose, with an OR of 2.41 at a diagnostic HbA1c >8.5% (P=0.015, sensitivity 67.5%, specificity 53.7%). Although using the 4–6 month HbA1c as a modifier tended to lower the odds ratio, it did not achieve statistical significance (P<0.05) at each of the diagnostic HbA1c levels. This finding indicates that in our cohort, improvement in short-term HbA1c had little impact on the long-term glycemic outcome. Our results here demonstrate that diagnostic HbA1c, but not the short-term treatment response, is the more dominant variable in predicting long-term glycemic control.
Table 2.
Odds ratio of HbA1c > 8% 5 years after diagnosis
| 4-6-mo HbA1c | |||||
|---|---|---|---|---|---|
| Dx HbA1c | -† | <6% | <6.5% | <7% | |
| OR | >7% | 1.64 | 0.98 | 1.01 | 1.26 |
| P-value | 0.198 | 0.961 | 0.976 | 0.567 | |
| OR | >7.5% | 1.84 | 1.13 | 1.25 | 1.40 |
| P-value | 0.099 | 0.785 | 0.589 | 0.392 | |
| OR | >8% | 1.99 | 1.40 | 1.54 | 1.59 |
| P-value | 0.058 | 0.261 | 0.154 | 0.135 | |
| OR | >8.5% | 2.41 | 1.73 | 1.83 | 1.86 |
| P-value | 0.015 | .224 | 0.146 | 0.110 | |
| OR | >9% | 2.68 | 1.99 | 2.06 | 2.11 |
| P-value | 0.006 | 0.126 | 0.083 | 0.054 | |
OR – odds ratio
OR and P-value considering HbA1c at diagnosis as the sole independent variable (excluding the effect of the 4–6 mo HbA1c value)
Latent Profile Analysis of HbA1c progression
Given the heterogeneity of HbA1c levels over time, we sought to examine if there are distinct HbA1c change patterns that contributed to the composite HbA1c curve shown in Figure 2. Latent profile analysis (LPA) was used to identify the heterogeneity of the longitudinal HbA1c trends from all registry subjects with varying durations of diabetes since diagnosis. We found the 4-solution LPA model as the best-fitting model; it has the lowest entropy score and is most clinically meaningful. As shown in Figure 2, four patterns of HbA1c progression were identified. The individual curves represent a fitted model of longitudinal data based on estimated means of HbA1c for each profile at the individual time intervals. Subjects in the “Durable Control” group (67.8%, N=156) had the lowest HbA1c at diagnosis compared to other groups, improved further to a nadir at 6.0% between 4 to 6 months, but then experienced a gradual rise in HbA1c over time. The modeled HbA1c at 60 months in this subgroup remained below 8%. Subjects in the “Transient Response” group (16.7%, N=38) had the highest mean HbA1c at diagnosis. Their HbA1c levels dropped precipitously after treatment initiation and reached a nadir between 4–6 months but began to rise steadily thereafter. In the “Poor Control” group (12.3%, N=28), there was a slight reduction in HbA1c after diagnosis, followed by a rise over time with a modeled HbA1c level >10% by 10 to 12 months after diagnosis. Finally, there were seven subjects in the “other” category that displayed a peculiar HbA1c trend. Further evaluation of the seven subjects showed that several had only one or two follow-up visits, which potentially skewed the HbA1c profile of this cohort. Given the small sample size, little conclusion can be drawn on the clinical significance of this HbA1c profile, and further analysis excluded the “other” group.
Figure 2.
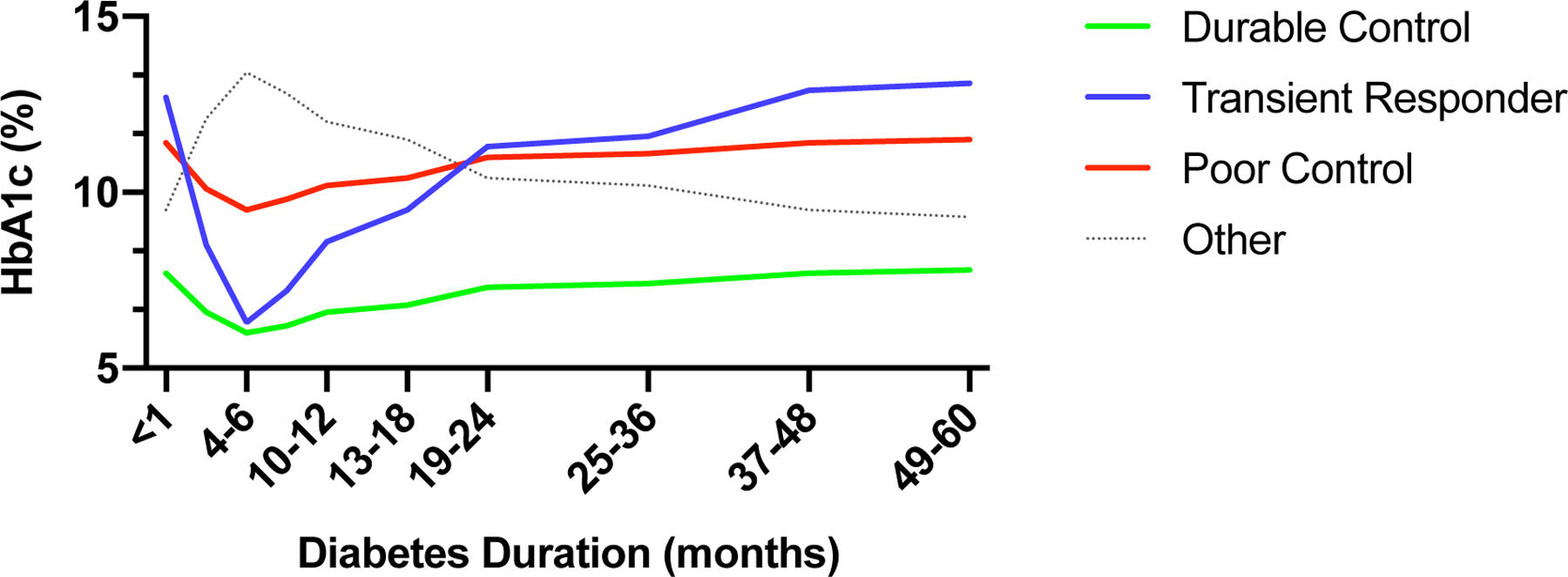
Latent growth-modeling of HbA1c outcome over 5 years.
To identify variables that differed amongst the patients who comprised the three HbA1c profiles, we analyzed the correlation between HbA1c and fasting c-peptide level at diagnosis, medication regimen prescribed at diagnosis, and clinic attendance during the first year after diagnosis. Baseline HbA1c differed amongst the three groups, with subjects in the “Durable Control” group having the lowest HbA1c at diagnosis (Table 3). We also observed a difference in baseline fasting c-peptide level across the three groups. Pairwise analysis showed that the mean c-peptide level for patients in the “Transient Response” group was significantly lower than that in either the “Poor” or “Durable Control” groups.
Table 3.
Comparison amongst the three LPA classes
| Poor Control (1) | Durable Control (2) | Transient Response (3) | Pairwise Two-Sided Multiple Comparison | |||||
|---|---|---|---|---|---|---|---|---|
| n(%) | N(%) or Mean(SD) or Median(IQR) | N(%) or Mean(SD) or Median(IQR) | N(%) or Mean(SD) or Median(IQR) | P-value | 1 vs 2 | 2 vs 3 | 1 vs 3 | |
| HbA1c at diagnosis† | 192 | 11.76 (2.20) | 9.40 (2.77) | 12.30 (2.46) | <0.0001 | 0.0001 | <0.0001 | 0.4576 |
| C-peptide‡ | 143 | 3.63 (3.11) | 3.58 (3.45) | 1.82 (1.46) | 0.0020 | 0.9589 | 0.0024 | 0.0074 |
| Attended follow-up visit at 1 year§ | 183 | 0.2396 | ||||||
| Y | 159 (86.9) | 18 (85.7) | 113 (90.4) | 24 (80.0) | ||||
| N | 24 (13.1) | 3 (14.3) | 12 (9.6) | 6 (20.0 | ||||
| Medication at diagnosis§ | 172 | 21 | 120 | 31 | <0.0001 | <0.0001 | 0.0020 | 0.4792 |
| Metformin only | 1(4.8) | 56(46.7) | 4(12.9) | |||||
| Insulin only | 12(57.1) | 49(40.8) | 19(61.3) | |||||
| Metformin+Insulin | 5(23.8) | 13(10.8) | 7(22.6) | |||||
| Other | 3(1.7) | 2(1.7) | 1(3.2) | |||||
Variable of normal distribution is presented as mean and SD. P-value calculated using one-way ANOVA. The least-square means in generalized linear model was performed for pairwise comparisons.
Variable demonstrating non-normal distribution is presented as median and interquartile range (IQR). P-value calculated using the Kruskal-Wallis test. DSCF procedure was used for pairwise comparisons.
Categorical variables were presented as counts and percentages. P-value for comparison across the three groups was calculated by the Fisher exact test. DSCF procedure was used for pairwise comparisons.
We next sought to examine if the difference in glycemic trends between the “Poor Control” and “Transient Response” groups could be attributable to variance in prescribed medication regimen at the time of diagnosis. A larger percentage of patients in the “Durable Control” group was prescribed metformin monotherapy at diagnosis. There was no difference in the prescribed regimens between patients in the “Poor Control” and the “Transient Response” groups in pairwise comparison. Thus, the lack of glycemic improvement in the “Poor Control” group after diagnosis is likely due to other untested variables rather than differences in medication regimen.
Finally, we compared the three groups in terms of differences in clinic attendance 1 year after diabetes diagnosis. We observed no differences in the number of participants who completed a clinic visit 1 year after diagnosis amongst the three groups (Table 3).
Medication Regimen
To determine if intense insulin regimen (multiple daily injections) improves glycemic outcome, we analyzed the medication regimens prescribed one year after the diagnosis of type 2 diabetes. As shown in Table 4, metformin monotherapy remained the most commonly prescribed medication (59%). Other regimens included multiple daily injections (MDI – 8.6%), metformin with basal insulin (10.5%), and metformin with MDI (9.5%). Of the study participants, 6.7% were no longer prescribed medication. As expected, patients on metformin monotherapy had lower mean HbA1c (6.4%±1.0%) compared to those on regimens including insulin (metformin/MDI 9.8%±1.7%, MDI 9.0%±2.3%, and metformin/basal insulin 9.8%±3.0%). However, the HbA1c was indistinguishable between those prescribed metformin/MDI and metformin/basal insulin. There were six patients in the “Other” category, which included patients on various combinations, including rapid-acting insulin for correction of hyperglycemia, basal insulin, sitagliptin, and metformin; one patient was on glyburide (this patient was switched from glyburide to metformin after MODY testing was found to be negative). Amongst these six patients, four patients were prescribed insulin. The mean HbA1c of this group (8.9%±1.6%) was significantly higher compared to patients on metformin monotherapy (6.4%±1.0%, P=0.03), but comparable to the mean HbA1c of other treatment groups (metformin/MDI 9.8%±1.7%, MDI 9.0%±2.3%, and metformin/basal insulin 9.8%±3.0%). This finding suggests that, in patients with youth-onset type 2 diabetes, an intensive insulin regimen may not be superior to a simplified regimen of once-daily basal insulin in combination with metformin.
Table 4.
Medication regimen 1 year after diagnosis
| Regimen | N(%) | Mean HbA1c(%) | P-value |
|---|---|---|---|
| Metformin/MDI | 10(9.5) | 9.8±1.7 | Reference |
| MDI | 9(8.6) | 9.0±2.3 | 0.8627 |
| Metformin/Basal | 11(10.5) | 9.8±3.0 | 0.9885 |
| Metformin | 62(59.0) | 6.4±1.0 | 0.0001 |
| Other† | 6(5.7) | 8.9±1.6 | 0.7916 |
| Metformin | 62(59.0) | 6.4±1.0 | Reference |
| Other | 6(5.7) | 8.9±1.6 | 0.0324 |
| None | 7(6.7) | 6.2±0.8 | 0.9995 |
DSCF multiple comparison analysis based on pairwise two-sample Wilcoxon comparisons.
P-values for pairwise comparisons of “Other” with MDI and Metformin/Basal were 1.000 and 0.9994, respectively.
Effect of BMI change on HbA1c
We sought to examine changes in BMI in our patients over the first year after diagnosis. As shown in Figure 3, there was no statistically significant change in BMI%/95P in the first year after diabetes diagnosis. To determine if weight reduction improves glycemic control, we next performed correlation analysis in youth with type 2 diabetes. Given the potential confounding effect of insulin on weight gain, we analyzed the correlation in patients who were on metformin monotherapy from those on insulin-containing regimens separately. We correlated the change in HbA1c with change in percentage of BMI%/95P over 1 year. Participants in the metformin monotherapy group had a median reduction of BMI of 2.6% over the first year (change in BMI%/95P relative to the diagnosis BMI%/95P), whereas those in the insulin-containing groups had a median gain in BMI of 1.8%. As shown in Figure 4, we observed a positive correlation between reduction in BMI and lowering of HbA1c in both groups (metformin: P=0.0023, insulin: P=0.0022).
Figure 3.
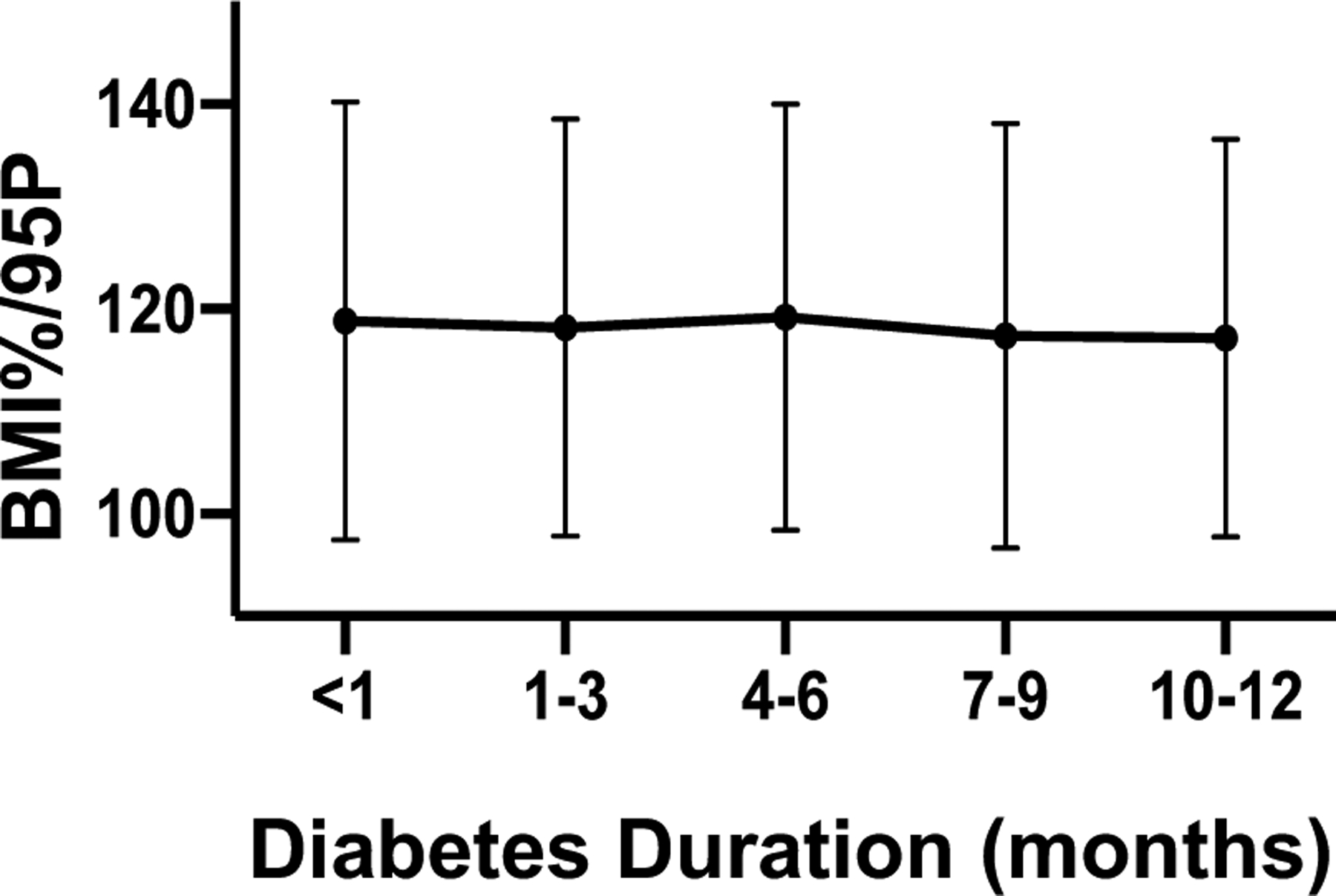
BMI progression over the first year after diagnosis. BMI is expressed as percentage in excess of the 95th percentile.
Figure 4.
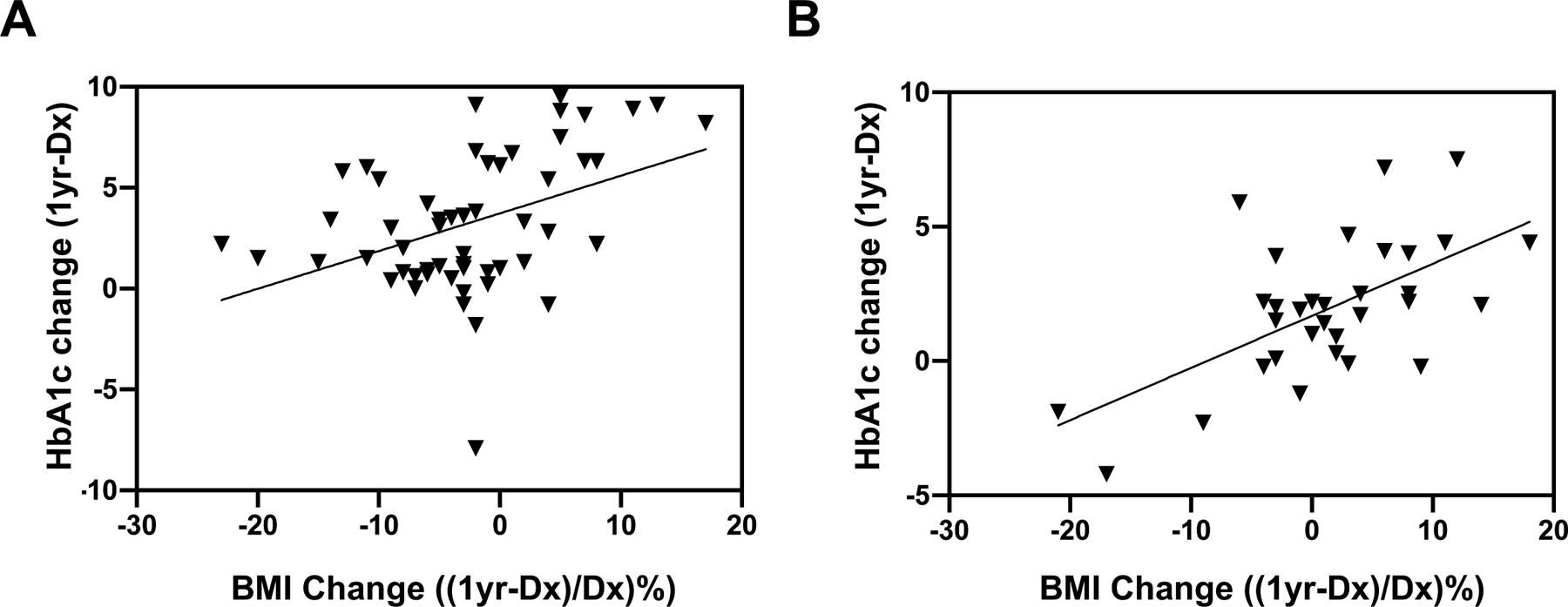
Distribution of changes in HbA1c 1 year after diagnosis as a function of percentage change in BMI (BMI%/95P) in patients on metformin (A) monotherapy and other combinations that include insulin (B). A. Median=−2.6%, R=0.407, P=0.0023. B. Median= 1.8%, R=0.522, P=0.0022.
Discussion
We present here the glycemic outcome of a predominantly Latinx cohort with youth-onset type 2 diabetes. The risk of 5-year glycemic failure heightened if the initial HbA1c was >8.5%. In latent profile analysis, we found that subjects in the cohort with the highest baseline and 5-year HbA1c levels also had the lowest fasting c-peptide levels at diagnosis. In this natural history study, intense insulin regimens did not improve glycemic control compared to patients on a simplified plan. Finally, we showed that weight reduction in the first year after diagnosis correlated with improved HbA1c level.
Our demographic analysis in this largely Latinx cohort identified similar clinical characteristics found in other multi-center studies. Similar to findings from the TODAY study and the Pediatric Diabetes Consortium, there was a very strong family history of type 2 diabetes at the time of diagnosis, as well as a preponderance of females subjects6, 12. The prevalence of DKA at diagnosis in our cohort (9.4%) was also in line with the previously reported rate of 11% in newly diagnosed patients with type 2 diabetes from the Pediatric Diabetes Consortium12. The PDC database showed that 55% of patients were lost to follow-up by 1.3 years after enrollment in the registry16. In our registry, 18% of patients did not attend a follow-up visit between 9 to 15 months after their initial visit. This attendance rate may be an underestimate of the true follow-up rate, however, as it included only patients who consented to be in the registry. Patients who did not consent for inclusion in our registry and did not return for follow-up appointments would not have been included in this analysis. Although the clinic attendance rates (a reflection of no-show rates) did not differ before and after the implementation of the Type 2 Diabetes Clinic, we did observe an improved number of visits attended by patients with new-onset diabetes. We speculate that a plausible explanation for the latter may include increased availability of appointments specifically designated for patients with type 2 diabetes. In addition, the clinic had a designated nurse care manager, a clinic coordinator to assist patients, and culturally sensitive and bilingual diabetes classes, which may help foster the relationship between the patient and the clinical team starting at diagnosis.
Our analysis of HbA1c progression and latent profile analysis identified three major findings. First, rapid HbA1c improvement in the setting of high initial HbA1c does not predict durable glycemic control. As shown in Figure 2, although participants in the “Transient Response” displayed a rapid decline in HbA1c level shortly after diagnosis, their glycemic control 5 years after diagnosis was comparable to that of the “Poor Control” group. This was confirmed in our odds ratio analysis, in which we showed that reduction in HbA1c 4–6 months after diagnosis did not reduce the risk of having uncontrolled glycemia five years after diabetes diagnosis. Second, markers of beta-cell failure (low fasting c-peptide and high initial HbA1c levels) portend poor long-term glycemic control. It is surprising that patients in the “Poor” and “Durable Control” groups had comparable levels of fasting c-peptide. That they had quite disparate glycemic profiles implies other intrinsic differences between these two groups. As this is a natural history study, our assessment of beta-cell function was limited to the use of fasting c-peptide levels measured at diagnosis. It remains plausible that these two groups differed in glucose-stimulated insulin-secretory capacity, and that the c-peptide response mounted by the “Poor Control” group reflected an inadequate response to the degree of hyperglycemia compared to that of patients in the “Durable Control” group. We speculate that short-term achievement of in-target HbA1c may be insufficient in preventing progressive beta-cell function decline. Finally, the HbA1c level attained at 5 years post diagnosis was comparable to the level at diagnosis. This was seen for the entire cohort (Figure 1) as well as all three LPA groups (Figure 2). As medication regimen was adjusted at each clinical encounter if needed, the rise in HbA1c is not due to lack of medication intensification over time. We speculate that the level seen at 5 years may reflect the outcome of progressive beta-cell failures outpacing the clinical efficacy of glucose-lowering medications.
The current guidelines from the International Society for Pediatric and Adolescent Diabetes (ISPAD) recommend the addition of prandial insulin in patients who cannot attain a HbA1c level of 7% despite metformin and basal insulin34. In our cohort, we observed that the mean HbA1c level was indistinguishable among any of the treatment regimens that included insulin. This finding suggests that treatment intensification with prandial insulin and correction dosage (the latter was included in the “other” category) may not be superior to the combination of metformin and basal insulin.
The TODAY study showed that baseline beta-cell function, and not medication adherence, is the dominant predictor for long-term glycemic outcome37, 38. However, after TODAY subjects met criteria for insulin rescue, only 33% of the subjects achieved a HbA1c improvement of at least 0.5%39. It is unknown, whether medication non-adherence may contribute to the poor efficacy of insulin treatment. We posit that medication non-adherence may also explain the lack of HbA1c improvement in our cohorts prescribed intensive insulin regimens (MDI), compared to basal insulin. It may also contribute to the disparate glycemic profiles between the “Poor Control” and the “Transient Response” groups in our LPA analysis. Presently, there is no validated instrument to assess medication adherence in youth with type 2 diabetes. The development of such an assessment tool would facilitate quantitation of medication adherence, which has the potential to advance our understanding of barriers in diabetes care.
Our results here do not preclude a role for prandial insulin and/or correction dosage for hyperglycemia. The addition of prandial insulin may be necessary for patients with consistent postprandial glycemic excursions, despite the use of basal insulin. For patients and families motivated to adhere to their prescribed regimen, the combination of rapid-acting insulin with basal insulin is expected to improve glycemic control. However, implementing the simplest treatment plan may be more feasible and acceptable for some patients.
The TODAY study demonstrated that intensive lifestyle modifications mitigated weight gain in subjects on metformin monotherapy. However, the collective improvement in BMI did not change the time to glycemic failure6. Our bivariate analysis showed that weight reduction correlated with reduction in HbA1c one year after diagnosis in subjects prescribed metformin as well as insulin-containing regimens. This finding is consistent with findings reported by Candler et al from the British Paediatric Surveillance Unit reporting framework17. Subjects who sustained more than 7% of weight loss (% overweight) in the TODAY study also demonstrated an appreciable improvement in HbA1c40. Our analysis showed that whereas subjects in the metformin monotherapy group experienced a modest reduction in BMI over 1 year (2.6% of baseline BMI), those who received insulin treatment gained 1.8% in BMI. The weight gain observed in the latter group may reflect the lipogenic effect of insulin treatment. In addition, insulin is predominantly prescribed in patients with uncontrolled diabetes, and its use may reverse the catabolic state and promote weight gain. Our findings support the approach of prioritizing weight management as part of the treatment plan for patients with type 2 diabetes. Pediatric trials testing the safety and efficacy of GLP-1 agonists and SGLT-2 inhibitors would expand treatment options that favor weight loss.
A major strength of this natural history study is that the data reflect real-world glycemic trends for youth with type 2 diabetes from a single clinic during the first five years of their disease. As the care was delivered at one single clinic, there was uniformity in clinical delivery. Participants received standard clinical care according to guidelines set forth by the ADA and ISPAD. They did not receive intense follow-up reminders and life-coaching typical of clinical trials. To our knowledge, this is also the first outcomes report of a cohort that is predominantly Latinx. In addition to homogeneity in ethnic background, the subjects also had similar social-economic background, with the great majority of the patients receiving state-supported Medicaid insurance.
Inherent in natural history studies, however, are the following limitations. First, clinical data (such as c-peptide and insulin) may be incomplete due to the lack of uniformity of laboratory tests collected as well as missed appointments. This may limit the power to detect correlations between HbA1c outcome and variables of interest. Limited sample size may have contributed to the lack of statistical significance when we examined the interaction of short-term (4–6 month) HbA1c on the odds ratio of uncontrolled HbA1c 5 years after diagnosis. Second, we were unable to measure beta-cell function (e.g., insulinogenic index) or insulin sensitivity in a natural history study. Although the inverse of insulin has been reported as a surrogate for insulin sensitivity, its utility is limited in cohorts that include patients with progressive beta-cell failure (as suggested by patients with very elevated HbA1c levels)41. Although the great majority of our patients had laboratory findings done in the fasting state, we cannot exclude the possibility that a few patients had c-peptide levels drawn in a non-fasting state, which would artificially elevate the c-peptide level. Finally, our analysis of medication regimen and HbA1c was a cross-sectional analysis performed 1 year after diabetes diagnosis and did not account for changes in medication over that time, which may have obscured changes in glycemic control.
Our results here highlight several areas in youth-onset type 2 diabetes that require additional investigation. Although HbA1c levels at diagnosis may reflect endogenous beta-cell function, there remains a clinical need for a biomarker or tool to quantify beta-cell function. Such a biomarker would allow longitudinal monitoring of beta-cell decline and help guide treatment options. Finally, efforts to improve glycemic outcome need to assess medication adherence. Identifying barriers to medication adherence and addressing the modifiable barriers have the potential to enhance patient engagement, improve glycemic control, and reduce long-term diabetes-related co-morbidities.
Acknowledgments:
REDCap is supported from UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. This work was supported by the Biostatistics Core at Children’s Hospital Los Angeles (CHLA), jointly supported by The Saban Research Institute and the Southern California Clinical and Translational Science Institute. The CHLA Type 2 Diabetes Clinic was funded by the Good Hope Medical Foundation.
Disclosures:
MEG is participating in a clinical trial sponsored by NovoNordisk; is an advisor to AbbVie, Adrenas, Daiichi Sankyo, Eton Pharmaceuticals, Ferring, Neurocrine Biosciences, NovoNordisk, Pfizer, QED, and Spruce Biosciences; serves on data safety monitoring boards for Ascendis, Millendo, and Tolmar; and receives royalties from McGraw Hill and UpToDate.
References
- 1.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med. April 13, 2017 2017;376(15):1419–1429. doi: 10.1056/NEJMoa1610187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Saeed AH, Constantino MI, Molyneaux L, et al. An Inverse Relationship Between Age of Type 2 Diabetes Onset and Complication Risk and Mortality: The Impact of Youth-Onset Type 2 Diabetes. Diabetes Care. May 2016 2016;39(5):823–829. doi: 10.2337/dc15-0991 [DOI] [PubMed] [Google Scholar]
- 3.Jensen ET, Dabelea D. Type 2 Diabetes in Youth: New Lessons from the SEARCH Study. Curr Diab Rep. 05 08, 2018 2018;18(6):36. doi: 10.1007/s11892-018-0997-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadeau KJ, Anderson BJ, Berg EG, et al. Youth-Onset Type 2 Diabetes Consensus Report: Current Status, Challenges, and Priorities. Diabetes Care. September 2016 2016;39(9):1635–1642. doi: 10.2337/dc16-1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA. February 28 2017;317(8):825–835. doi: 10.1001/jama.2017.0686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.TODAY Study Group, Zeitler P, Hirst K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. June 14, 2012 2012;366(24):2247–2256. doi: 10.1056/NEJMoa1109333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. Estimated morbidity and mortality in adolescents and young adults diagnosed with Type 2 diabetes mellitus. Diabet Med. April 2012 2012;29(4):453–463. doi: 10.1111/j.1464-5491.2011.03542.x [DOI] [PubMed] [Google Scholar]
- 8.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. December 2013;36(12):3863–9. doi: 10.2337/dc12-2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levitt Katz LE. Longitudinal Outcomes in Youth with Type 2 Diabetes — The TODAY2 Study — Cardiovascular Complications and Outcomes. In: American Diabetes Association, 79th Scientific Sessions; 2019. [Google Scholar]
- 10.White NH. Longitudinal Outcomes in Youth with Type 2 Diabetes — The TODAY2 Study — Retinopathy and Neuropathy. In: American Diabetes Association, 79th Scientific Sessions; 2019. [Google Scholar]
- 11.Bjornstad P Longitudinal Outcomes in Youth with Type 2 Diabetes — The TODAY2 Study — Renal Complications. In: American Diabetes Association, 79th Scientific Sessions; 2019. [Google Scholar]
- 12.Klingensmith GJ, Connor CG, Ruedy KJ, et al. Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr Diabetes. 06 2016 2016;17(4):266–273. doi: 10.1111/pedi.12281 [DOI] [PubMed] [Google Scholar]
- 13.Nambam B, Silverstein J, Cheng P, et al. A cross-sectional view of the current state of treatment of youth with type 2 diabetes in the USA: enrollment data from the Pediatric Diabetes Consortium Type 2 Diabetes Registry. Pediatr Diabetes. 05 2017 2017;18(3):222–229. doi: 10.1111/pedi.12377 [DOI] [PubMed] [Google Scholar]
- 14.Narasimhan S, Weinstock RS. Youth-onset type 2 diabetes mellitus: lessons learned from the TODAY study. Mayo Clin Proc. June 2014;89(6):806–16. doi: 10.1016/j.mayocp.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. January 2011;96(1):159–67. doi: 10.1210/jc.2010-1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoemaker A, Cheng P, Gal RL, et al. Predictors of Loss to Follow-Up among Children with Type 2 Diabetes. Horm Res in Paediatr. 2017 2017;87(6):377–384. doi: 10.1159/000475595 [DOI] [PubMed] [Google Scholar]
- 17.Candler TP, Mahmoud O, Lynn RM, Majbar AA, Barrett TG, Shield JPH. Treatment adherence and BMI reduction are key predictors of HbA1c 1 year after diagnosis of childhood type 2 diabetes in the United Kingdom. Pediatr Diabetes. 12 2018 2018;19(8):1393–1399. doi: 10.1111/pedi.12761 [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. July 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. April 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association. Children and Adolescents: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019/01/01 2019;42(Supplement 1):S148–S164. doi: 10.2337/dc19-S013 [DOI] [PubMed] [Google Scholar]
- 21.Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr. March 2005;59(3):419–25. doi: 10.1038/sj.ejcn.1602090 [DOI] [PubMed] [Google Scholar]
- 22.Kakinami L, Henderson M, Chiolero A, Cole TJ, Paradis G. Identifying the best body mass index metric to assess adiposity change in children. Arch Dis Child. November 2014 2014;99(11):1020–1024. doi: 10.1136/archdischild-2013-305163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo JG. Using body mass index Z-score among severely obese adolescents: a cautionary note. Int J Pediatr Obes. 2009 2009;4(4):405–410. doi: 10.3109/17477160902957133 [DOI] [PubMed] [Google Scholar]
- 24.Freedman DS, Berenson GS. Tracking of BMI z Scores for Severe Obesity. Pediatrics. September 2017 2017;140(3)doi: 10.1542/peds.2017-1072.2017–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freedman DS, Butte NF, Taveras EM, et al. BMI z-Scores are a poor indicator of adiposity among 2- to 19-year-olds with very high BMIs, NHANES 1999–2000 to 2013–2014. Obesity (Silver Spring). April 2017;25(4):739–746. doi: 10.1002/oby.21782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Critchlow DE, Fligner MA. On Distribution-Free Multiple Comparisons in the One-Way Analysis of Variance. Communications in Statistics -- Theory and Methods. 1991;20:127–139. [Google Scholar]
- 27.Centor RM, Witherspoon JM, Dalton HP, Brody CE, Link K. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1(3):239–46. doi: 10.1177/0272989X8100100304 [DOI] [PubMed] [Google Scholar]
- 28.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3rd Ed. ed. John Wiley & Sons; 2003. [Google Scholar]
- 29.Lazarsfeld PF, Henry NW. Latent structure analysis. Houghton Mifflin; 1968. [Google Scholar]
- 30.Bartholomew D, Knott M, Moustaki I. Latent variable models and factor analysis: A unified approach. 3rd Ed ed. John Wiley & Sons, Ltd.; 2011. [Google Scholar]
- 31.Singh V, Rana RK, Singhal R. Analysis of repeated measurement data in the clinical trials. J Ayurveda Integr Med. April 2013;4(2):77–81. doi: 10.4103/0975-9476.113872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Updated 2019. https://www.R-project.org/ [Google Scholar]
- 33.U.S. Census Bureau. QuickFacts: Los Angeles County, California. 2010. [Google Scholar]
- 34.Zeitler P, Arslanian S, Fu J, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Type 2 diabetes mellitus in youth. Pediatr Diabetes. 10/2018 2018;19:28–46. doi: 10.1111/pedi.12719 [DOI] [PubMed] [Google Scholar]
- 35.Bacha F, Cheng P, Gal RL, et al. Initial Presentation of Type 2 Diabetes in Adolescents Predicts Durability of Successful Treatment with Metformin Monotherapy: Insights from the Pediatric Diabetes Consortium T2D Registry. Horm Res Paediatr. 2018 2018;89(1):47–55. doi: 10.1159/000481687 [DOI] [PubMed] [Google Scholar]
- 36.Zeitler P, Hirst K, Copeland KC, et al. HbA1c After a Short Period of Monotherapy With Metformin Identifies Durable Glycemic Control Among Adolescents With Type 2 Diabetes. Diabetes Care. 12/2015 2015;38(12):2285–2292. doi: 10.2337/dc15-0848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Group TS. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes Care. June 2013;36(6):1749–57. doi: 10.2337/dc12-2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katz LL, Anderson BJ, McKay SV, et al. Correlates of Medication Adherence in the TODAY Cohort of Youth With Type 2 Diabetes. Diabetes Care. November 2016;39(11):1956–1962. doi: 10.2337/dc15-2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bacha F, El Ghormli L, Arslanian S, et al. Predictors of response to insulin therapy in youth with poorly-controlled type 2 diabetes in the TODAY trial. Pediatr Diabetes. November 2019;20(7):871–879. doi: 10.1111/pedi.12906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcus MD, Wilfley DE, El Ghormli L, et al. Weight change in the management of youth-onset type 2 diabetes: the TODAY clinical trial experience. Pediatr Obes. August 2017;12(4):337–345. doi: 10.1111/ijpo.12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. The Journal of Clinical Endocrinology and Metabolism. July 2011 2011;96(7):2136–2145. doi: 10.1210/jc.2010-2813 [DOI] [PMC free article] [PubMed] [Google Scholar]


