Abstract
The Rho family of small GTPases has been implicated in cytoskeletal reorganization and subsequent morphological changes in various cell types. Among them, Rac and Cdc42 have been shown to be involved in neurite outgrowth in neuronal cells. In this study, we examined the role of RhoG, another member of Rho family GTPases, in nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells. Expression of wild-type RhoG in PC12 cells induced neurite outgrowth in the absence of NGF, and the morphology of wild-type RhoG-expressing cells was similar to that of NGF-differentiated cells. Constitutively active RhoG-transfected cells extended short neurites but developed large lamellipodial or filopodial structures at the tips of neurites. RhoG-induced neurite outgrowth was inhibited by coexpression with dominant-negative Rac1 or Cdc42. In addition, expression of constitutively active RhoG elevated endogenous Rac1 and Cdc42 activities. We also found that the NGF-induced neurite outgrowth was enhanced by expression of wild-type RhoG whereas expression of dominant-negative RhoG suppressed the neurite outgrowth. Furthermore, constitutively active Ras-induced neurite outgrowth was also suppressed by dominant-negative RhoG. Taken together, these results suggest that RhoG is a key regulator in NGF-induced neurite outgrowth, acting downstream of Ras and upstream of Rac1 and Cdc42 in PC12 cells.
In the developing nervous system, neurite outgrowth is an essential process underlying the formation of the highly specific pattern of connections between neurons. The outgrowth of neurites toward their proper targets is guided by the growth cone in response to several kinds of environmental cues (22). Growth cones advance through cyclical extension of filopodia and lamellipodia, and their shapes are largely determined by the organization of the actin cytoskeleton (46).
The Rho family of small GTPases has been implicated in the reorganization of the actin cytoskeleton and subsequent morphological changes in various cell types (13, 17). Like other GTPases of the Ras superfamily, they serve as molecular switches by cycling between an inactive GDP-bound state and an active GTP-bound state. Activation of the Rho family proteins requires GDP-GTP exchange catalyzed by various guanine-nucleotide exchange factors (GEFs), and their activation is regulated by GTPase-activating proteins (GAPs), which stimulate the intrinsic GTPase activities of the G proteins. In addition, guanine-nucleotide dissociation inhibitors inhibit the exchange of GDP for GTP and might also serve to regulate the association with membranes (40). Presently, at least 14 mammalian Rho family proteins have been identified: RhoA, -B, and -C, Rac1, -2, and -3, Cdc42, Rnd1, -2, and -3, RhoD, TC10, RhoH/TTF, and RhoG. Among them, the functions of Rho, Rac, and Cdc42 have been extensively characterized. In fibroblasts, the activation of Rho leads to formation of actin stress fibers and assembly of focal adhesions (34), whereas the activation of Rac and Cdc42 induces formation of lamellipodia and filopodia, respectively (32, 35). Recently, there has been an accumulation of evidence for the role of Rho family proteins in the regulation of the cytoskeleton required for neurite extension and retraction. Studies on neuronal cell lines have shown that Rac and Cdc42 are involved in the formation of lamellipodia and filopodia of the growth cone, respectively, and that they are required for the outgrowth of neurites. On the other hand, Rho is required for the collapse of the growth cone and the retraction of neurites (12, 19, 23, 39). Furthermore, Rho family proteins are also involved in axon and dendrite formation in various types of neurons (1, 26, 36, 48), and defects in the regulation of these GTPase activities have been reported to affect the development of the nervous system (21, 27, 28, 52).
Rat pheochromocytoma PC12 cells have been used as a model system for neuronal differentiation and neurite outgrowth. After stimulation with nerve growth factor (NGF), they stop growing and begin to extend neurites. It is well known that the binding of NGF to its tyrosine kinase receptor, Trk, activates a Ras-dependent extracellular signal-regulated kinase pathway which leads to neuronal differentiation (7, 45, 47, 50). Indeed, constitutively active Ras mutants can induce morphological differentiation in PC12 cells (2, 33). Recent studies have shown that Rho family proteins Rac and Cdc42 play critical roles in the regulation of the cytoskeletal changes required for neurite outgrowth in response to NGF in PC12 cells (6, 8, 24). However, the mechanisms involved in the regulation of Rac and Cdc42 activities during neurite outgrowth in PC12 cells have not yet been elucidated.
RhoG was first identified as the product of a growth-stimulated gene from fibroblasts (49). In fibroblasts, constitutively active RhoG produces both Rac1- and Cdc42-dependent morphological and cytoskeletal changes: the formation of membrane ruffles, lamellipodia, filopodia, and microvilli (4, 11). Functions of RhoG in various cell types other than fibroblasts have not yet been examined, although RhoG mRNA expression was detected in a wide variety of tissues (49). Here, we have examined the role of RhoG in neuronal differentiation in PC12 cells. We have shown that RhoG induces neurite outgrowth through the activation of Rac1 and Cdc42. Furthermore, RhoG is involved in the NGF-induced neurite outgrowth acting downstream of Ras.
MATERIALS AND METHODS
Construction of expression plasmids.
Mammalian expression vector pEF-BOS was kindly provided by S. Nagata (Osaka University). Human Rac1 was obtained as described previously (14). cDNA for H-Ras was obtained from Health Science Research Resources Bank (Osaka, Japan). The coding sequence for human RhoG (49) was obtained by reverse transcription-PCR (RT-PCR) from HEK293 cells using primers 5′-CCCGGATCCCAGAGCATCAAGTGCGTGGTG-3′, containing a BamHI site, and 5′-GCCGAATTCCAGGGTCACAAGAGGATGCAG-3′, containing an EcoRI site. The coding sequence for human Cdc42 (43) was obtained by RT-PCR from HL-60 cells using primers 5′-ACAAAATTATTGGATCCCCGCAGACAATTAAGTGTGT-3′, containing a BamHI site, and 5′-CTTTAGTTTGAATTCAACATTGCTTTTAGT-3′, containing an EcoRI site. The PCR products were cloned into the pCR2.1 vector (Invitrogen) and sequenced completely. RhoGV12, RhoGA37, RhoGV12A37, RhoGN17, Rac1V12, Rac1N17, Cdc42V12, Cdc42N17, and RasV12 were generated by PCR-mediated mutagenesis (16). BamHI/EcoRI sites were used to fuse coding sequences for wild-type RhoG and all mutant RhoG proteins in-frame with a sequence in pEF-BOS encoding an initiating methionine followed by the Myc epitope tag sequence (wild-type RhoG, RhoGV12, RhoGA37, RhoGV12A37, RhoGN17, wild-type Rac1, and Rac1V12) or the hemagglutinin (HA) epitope tag sequence (Rac1N17, wild-type Cdc42, Cdc42V12, Cdc42N17, and RasV12) at the NH2 terminus. cDNA for a variant of the Aequorea victoria green fluorescent protein (GFP) was obtained from pEGFP-C1 (Clontech) and inserted into mammalian expression vector pcDNA3 (Invitrogen).
The coding sequence for the Cdc42/Rac interactive binding (CRIB) domain of rat αPAK (amino acids 70 to 150) (29) was obtained by RT-PCR from PC12 cells, using primers 5′-AAGGGATTCAAGGAGCGGCCAGAGATTTCT-3′, containing a BamHI site, and 5′-GAAGAATTCTAATCTTAAGCTGACTTATCT-3′, containing a stop codon followed by an EcoRI site. The PCR product was subcloned into the BamHI/EcoRI sites of pGEX-4T-2 (Amersham Pharmacia Biotech) and confirmed by DNA sequencing. The CRIB domain of αPAK was then expressed in Escherichia coli as a fusion protein with glutathione S-transferase (GST-CRIB), purified on glutathione-Sepharose beads, and isolated from the beads with 16 mM reduced glutathione. The purified proteins were dialyzed with 25 mM Tris-HCl (pH 7.5)–1 mM MgCl2–0.2 mM dithiothreitol–5% glycerol and stored at −80°C.
Cell culture and transfection.
PC12 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal bovine serum, 10% horse serum, 4 mM glutamine, 100 U of penicillin/ml, and 0.2 mg of streptomycin/ml under humidified conditions in 95% air and 5% CO2 at 37°C. For transfection, cells were seeded onto poly-d-lysine (Sigma)-coated glass coverslips (circular, 13 mm in diameter) in 24-well plates at a density of 2.5 × 104 cells/well and cultured for 18 h. Then cells were transfected with 0.8 μg of total DNA using Lipofectamine 2000 (Life Technologies Inc.) according to the manufacturer's instructions. Cells were fixed 48 h after transfection. In some experiments, cells were differentiated with 50 ng of NGF (Promega Corporation)/ml in serum-free DMEM after transfection.
Immunofluorescence microscopy.
All steps were carried out at room temperature, and cells were rinsed with phosphate-buffered saline (PBS) between each step. At various times, transfected PC12 cells on coverslips were fixed with 4% paraformaldehyde–PBS for 15 min. After residual formaldehyde had been quenched with 50 mM NH4Cl–PBS for 10 min, cells were permeabilized in 0.2% Triton X-100–PBS for 10 min and incubated with 10% fetal bovine serum in PBS for 30 min to block nonspecific antibody binding. For detection of cells expressing Myc-tagged or HA-tagged small G proteins, cells were incubated with anti-Myc monoclonal antibody 9E10 (0.5 μg/ml) or anti-HA monoclonal antibody 12CA5 (0.4 μg/ml) (Boehringer Mannheim Corp.), respectively, in PBS for 1 h, followed by incubation with a rhodamine-conjugated goat anti-mouse immunoglobulin G (IgG) (Chemicon International Inc.) in PBS (1:500 dilution) for 1 h. For coexpression of Myc-tagged and HA-tagged small G proteins, the expressed Myc-tagged small G proteins were visualized using a rabbit polyclonal anti-Myc antibody (MBL; 1:500 dilution), followed by a fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Chemicon International Inc.; 1:250 dilution). Actin filaments were stained with Alexa 488-conjugated phalloidin (Molecular Probes) in PBS (0.5 U/ml) for 1 h. Cells on coverslips were mounted in 90% glycerol containing 0.1% p-phenylenediamine dihydrochloride in PBS and examined using a Nikon Eclipse TE300 microscope and a Nicon Plan Fluor 40 by 0.60 or 60 by 0.70 objective.
Measurement of endogenous Rac1 and Cdc42 activity.
Measurement of Rac and Cdc42 activity was performed according to the modified method of Benard et al. (3). PC12 cells were seeded in 60-mm-diameter culture dishes at a density of 2 × 106 cells/dish and cultured for 18 h. Then cells were transfected with 4.8 μg of total DNA using Lipofectamine 2000. Thirty-six hours after transfection, cells were serum starved in serum-free DMEM for 12 h and then lysed for 5 min with ice-cold cell lysis buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 2 mM MgCl2, 1% Nonidet P-40, 10% glycerol, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin/ml, 1 μg of leupeptin/ml) containing 4 μg of GST-CRIB. Cell lysates were then centrifuged for 5 min at 10,000 × g at 4°C, and the supernatant was incubated with glutathione-Sepharose beads for 30 min at 4°C. After the beads had been washed with the cell lysis buffer, the bound proteins were eluted in Laemmli sample buffer and separated by sodium dodecyl sulfate (SDS)–12.5% polyacrylamide gel electrophoresis. The separated proteins were electrophoretically transferred onto a polyvinylidene difluoride membrane (Millipore Corporation). The membrane was blocked with 3% low-fat milk in Tris-buffered saline and then incubated with a mouse monoclonal anti-Rac1 antibody (Transduction Laboratories; 1:1,000 dilution) or a rabbit polyclonal anti-Cdc42 antibody (Santa Cruz Biotechnology, Inc.; 1:100 dilution). The Rac1 and Cdc42 antibodies were detected using horseradish peroxidase-conjugated goat anti-mouse (1:3,000 dilution) and anti-rabbit (1:2,000 dilution) IgG antibodies (DAKO), respectively, and an ECL detection kit (Amersham Pharmacia Biotech).
Northern blot analysis.
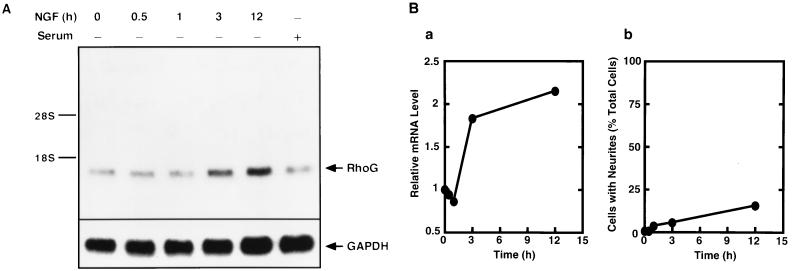
Total RNA from PC12 cells was isolated using an Isogen RNA isolation kit (Nippon-gene, Tokyo, Japan), and 15 μg of total RNA was separated by electrophoresis on a 1.5% agarose gel and transferred onto a nylon membrane (Biodyne; Pall Biosupport Division). The membrane was hybridized at 65°C for 15 h in a mixture containing 6× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate), 0.5% SDS, 5× Denhardt's solution, 100 μg of denatured salmon sperm DNA/ml, and a 32P-labeled probe (2 × 106 cpm/ml) encoding rat RhoG isolated from rat brain by RT-PCR. The membrane was then washed twice in 2× SSC and twice in 2× SSC–1% SDS at 65°C. The membrane was dried and autoradiographed with an X-ray film for 2 days. The same filter was rehybridized with a 32P-labeled probe encoding mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) isolated by RT-PCR (37). The mRNA level of RhoG was normalized to that of GAPDH mRNA by using National Institutes of Health Image software.
RESULTS
Expression of RhoG induces neurite outgrowth in PC12 cells.
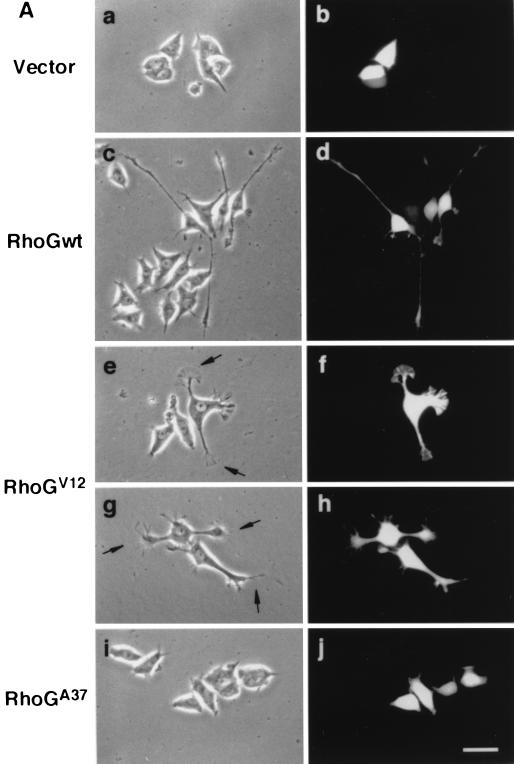
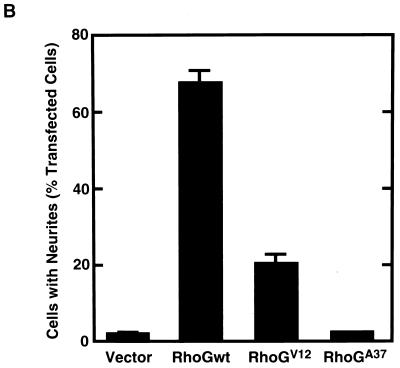
To examine whether RhoG was involved in the regulation of neuronal cell morphology, Myc epitope-tagged wild-type RhoG and various RhoG mutants were transfected into PC12 cells. Transfected cells were identified by cotransfection with GFP, and their morphologies were compared to that of control cells expressing GFP alone. The morphology of undifferentiated PC12 cells was not affected by the expression of GFP alone (Fig. 1A, a and b). However, transfection of wild-type RhoG into PC12 cells dramatically induced neurite outgrowth even in the absence of NGF (Fig. 1A, c and d, and B). Wild-type RhoG-transfected cells extended two or three neurites per cell, and some of them had long neurites (more than 5 cell body diameters in length). This morphological change was similar to that induced by NGF treatment (see Fig. 6A). Cells transfected with the constitutively active RhoG mutant, RhoGV12, also extended neurites (Fig. 1B), but their neurites were clearly distinct from those of wild-type RhoG-transfected cells. RhoGV12-transfected cells had short neurites (about 1 cell body diameter in length) and developed large lamellipodial (Fig. 1A, e and f) or filopodial (Fig. 1A, g and h) structures at the tips of neurites. On the other hand, transfection of RhoGA37, which contains an F37A substitution in the effector region of wild-type RhoG, induced no significant morphological change in PC12 cells (Fig. 1A, i and j). In this experiment, similar expression levels of the wild type and RhoG mutants were detected by immunofluorescence and immunoblotting using an anti-Myc antibody (data not shown).
FIG. 1.
Effect of expression of wild-type RhoG and various RhoG mutants on PC12 cell morphology. (A) PC12 cells were transiently cotransfected with an expression vector encoding GFP and an empty vector (vector; a and b) or an expression vector encoding Myc epitope-tagged wild-type RhoG (RhoGwt; c and d), RhoGV12 (e to h), or RhoGA37 (i and j). At 48 h after transfection, cells were observed under the phase-contrast microscope (a, c, e, g, and i). Transfected cells were identified by the fluorescence of GFP (b, d, f, h, and j). Arrows (e and g), large lamellipodial (e) and filopodial (g) structures at the tips of neurites. The results shown are representative of three independent experiments. Bar, 25 μm. (B) Quantification of neurite outgrowth induced by various RhoG mutants. At 48 h after transfection, cells were stained with an anti-Myc antibody, and positively stained cells were assessed. Cells with neurites were defined as cells that possessed at least one neurite more than 1 cell body diameter in length, and results are percentages of the total number of transfected cells. At least 100 cells were assessed in each experiment, and data are the means ± standard errors of triplicate experiments.
FIG. 6.
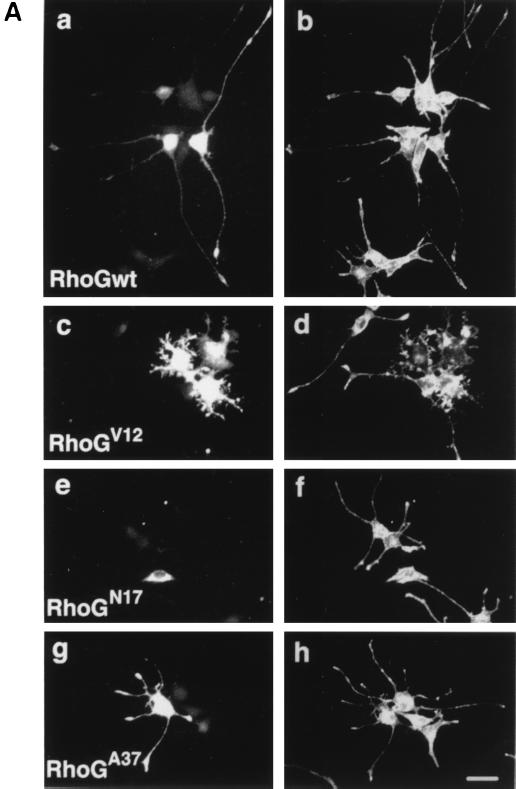
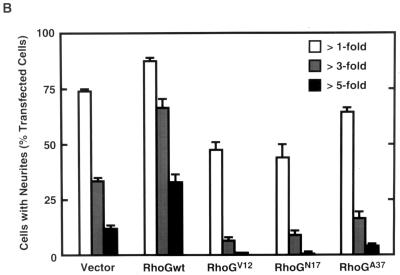
Effect of expression of various RhoG mutants on NGF-induced neurite outgrowth. (A) PC12 cells were transfected with an expression vector encoding Myc-tagged wild-type RhoG (RhoGwt; a and b), RhoGV12 (c and d), RhoGN17 (e and f), or RhoGA37 (g and h) and then treated with 50 ng of NGF/ml for 48 h. Cells were fixed and stained with an anti-Myc antibody (a, c, e, and g) to identify transfected cells. The morphology of the cells was visualized by filamentous actin staining with Alexa 488 phalloidin (b, d, f, and h). The results shown are representative of three independent experiments. Bar, 25 μm. (B) Length distribution of NGF-induced neurites in various RhoG mutant-expressing PC12 cells. PC12 cells were transfected with an empty vector (vector) or vectors encoding various RhoG mutants. At 48 h after transfection, cells were stained with an anti-Myc antibody, and positively stained cells were assessed. In this experiment, a vector encoding GFP was cotransfected to visualize tips of neurites. Cells with neurites exceeding 1-, 3-, or 5 times the length of the cell body were scored as a percentage of the total number of transfected cells. At least 100 cells were assessed in each experiment, and data are the means ± standard errors of triplicate experiments.
Activation of Rac1 and Cdc42 is required for neurite outgrowth by RhoG.
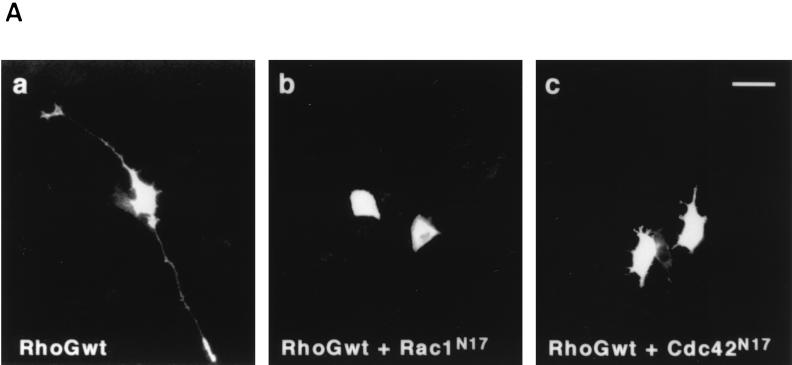
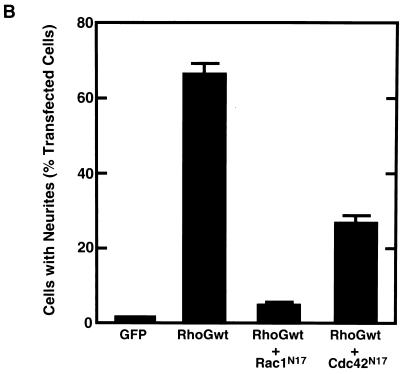
It is known that Rho family GTPases Rac and Cdc42 are required for neurite outgrowth induced by NGF in PC12 cells (6, 8, 24). To examine whether neurite outgrowth induced by wild-type RhoG required activation of Rac and Cdc42, we cotransfected the cells with wild-type RhoG and dominant-negative Rac1 (Rac1N17) or Cdc42 (Cdc42N17). Both Rac1N17 and Cdc42N17 suppressed the wild-type RhoG-induced neurite outgrowth (Fig. 2).
FIG. 2.
Inhibition of RhoG-induced neurite outgrowth by Rac1N17 and Cdc42N17. (A) PC12 cells were cotransfected with an expression vector encoding Myc-tagged wild-type RhoG and an empty vector (a) or a vector encoding HA-tagged Rac1N17 (b) or Cdc42N17 (c). At 48 h after transfection, cells were fixed and stained with an anti-Myc antibody. Expression of HA-tagged Rac1N17 and Cdc42N17 was also detected with an anti-HA antibody (data not shown). The results shown are representative of three independent experiments. Bar, 25 μm. (B) Quantification of the effect of Rac1N17 and Cdc42N17 on RhoG-induced neurite outgrowth. At 48 h after transfection, cells were costained with anti-Myc and anti-HA antibodies, and positively stained cells were assessed as described in the legend to Fig. 1. Cells transfected with GFP were used as a control. Data are the means ± standard errors of triplicate experiments. RhoGwt, wild-type RhoG.
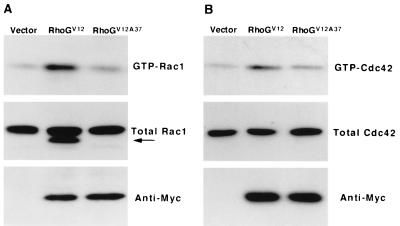
To examine whether the activation of Rac1 and Cdc42 was located in the downstream signaling pathway of RhoG in PC12 cells, we measured the amounts of GTP-bound Rac1 and Cdc42 in RhoGV12-transfected cells using the GST-CRIB domain of αPAK, which specifically binds to Rac and Cdc42 in their active GTP-bound states (3). Transient expression of RhoGV12 in PC12 cells significantly increased the amounts of endogenous GTP-bound Rac1 (2.9-fold) and Cdc42 (3.0-fold), compared to the amount produced by control vector-transfected cells (Fig. 3). Transient expression of wild-type RhoG slightly increased the level of GTP-bound Rac1 (data not shown). On the other hand, expression of RhoGV12A37, which contains an F37A substitution in the effector region of RhoGV12, had little effect on the cellular amount of GTP-bound Rac1 (1.2-fold) or Cdc42 (0.97-fold). Expressed RhoGV12 was not affinity precipitated by GST-CRIB of αPAK (data not shown), consistent with a previous report using a yeast two-hybrid system (11). Previous studies have shown that the activation of Rac1 is located in the downstream signaling pathway of Cdc42 in fibroblasts (32, 38). However, no detectable increase in the amount of GTP-bound Rac1 was induced by the expression of constitutively active Cdc42, Cdc42V12, in PC12 cells, although actin reorganization and morphological change were observed in the Cdc42V12-expressing cells (Fig. 4A, c and d). Therefore, it is unlikely that RhoG regulates Rac1 activity through the activation of Cdc42 in PC12 cells.
FIG. 3.
RhoGV12 activates endogenous Rac1 and Cdc42 in PC12 cells. PC12 cells were transiently transfected with an empty expression vector (vector) or an expression vector encoding RhoGV12 or RhoGV12A37. At 36 h after transfection, cells were serum starved for 12 h. The cell lysates were incubated with GST-CRIB, and the amounts of GTP-bound Rac1 and Cdc42 were determined by immunoblotting using a monoclonal antibody against Rac1 (A, top) and a rabbit polyclonal antibody against Cdc42 (B, top), respectively. Total amounts of Rac1 (A, middle) and Cdc42 (B, middle) in cell lysates and expression of Myc-tagged RhoGV12 and RhoGV12A37 (bottom) are also shown. In this experiment, about 20 to 30% of total cells were transfected with RhoGV12 or RhoGV12A37. The anti-Rac1 antibody used in this experiment is cross-reactive with expressed Myc-tagged RhoGV12 but not with RhoGV12A37 (A, arrow). The results shown are representative of three independent experiments that yielded similar results.
FIG. 4.
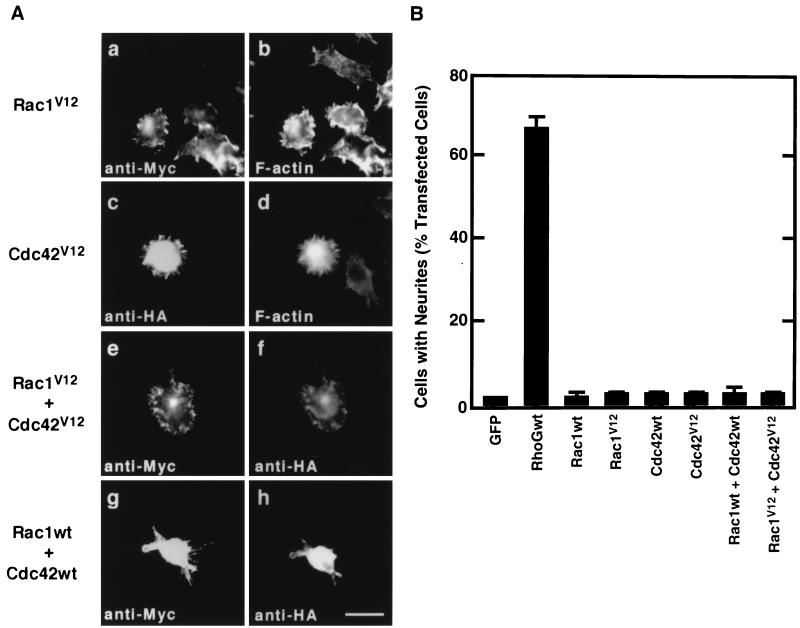
Effects of Rac1 and Cdc42 expression on PC12 cell morphology. (A) PC12 cells were transiently transfected with an expression vector encoding Myc-tagged Rac1V12 (a and b) or HA-tagged Cdc42V12 (c and d) or were cotransfected with Myc-tagged Rac1V12 and HA-tagged Cdc42V12 (e and f) or with Myc-tagged wild-type Rac1 (Rac1wt) and HA-tagged wild-type Cdc42 (Cdc42wt) (g and h). At 48 h after transfection, cells were fixed and stained with anti-Myc (a, e, and g) and anti-HA (c, f, and h) antibodies or with Alexa 488 phalloidin to visualize filamentous actin (b and d). The results shown are representative of three independent experiments. Bar, 25 μm. (B) Quantification of effects of Rac1 and Cdc42 expression on neurite outgrowth. At 48 h after transfection, cells were costained with anti-Myc and anti-HA antibodies, and positively stained cells were assessed as described in the legend to Fig. 1. Cells transfected with GFP were used as a control. Data are the means ± standard errors of triplicate experiments.
We next examined whether expression of Rac1, Cdc42, or both was able to induce neurite outgrowth in PC12 cells. Expression of constitutively active forms of Rac1 and Cdc42 as well as their wild types failed to induce neurite formation in PC12 cells (Fig. 4). Instead, cells expressing Rac1V12 became flattened with ruffles (Fig. 4A, a and b) and Cdc42V12-expressing cells produced large numbers of very short spikes around the cell periphery (Fig. 4A, c and d).
Involvement of RhoG in NGF-induced neurite outgrowth.
To examine whether PC12 cells expressed RhoG, we first tried to detect RhoG mRNA by Northern blot analysis. As shown in Fig. 5, PC12 cells expressed RhoG. RhoG mRNA accumulated in response to NGF, followed by induction of neurites with a lag period of several hours. On the other hand, the level of RhoG mRNA was not significantly altered by serum stimulation, contrary to a previous report that RhoG mRNA was induced by serum stimulation in CCL39 fibroblasts (49). This discrepancy may be due to the use of different types of cells. Therefore, we next examined the role of RhoG in NGF-induced neurite outgrowth.
FIG. 5.
mRNA level of endogenous RhoG in PC12 cells. (A) Total RNA (15 μg) isolated from serum-starved PC12 cells treated with 50 ng of NGF/ml for the indicated times or from cells growing in serum-containing medium was subjected to Northern blot analysis with a cDNA probe for RhoG, as described in Materials and Methods. The same membrane was rehybridized with a GAPDH cDNA probe. Arrows, hybridized bands for RhoG and GAPDH. The positions of 18S and 28S rRNAs are indicated. (B) Quantification of changes of the RhoG mRNA level and the percentages of cells with neurites after NGF stimulation. The level of endogenous RhoG mRNA (a) is normalized to that of GAPDH mRNA and expressed as fold increases over the value for serum-starved cells at 0 min. Cells with neurites (b) were defined as the cells that possessed at least one neurite more than 1 cell body diameter in length. At least 100 cells were assessed in each experiment, and data are the means ± standard errors of triplicate experiments.
After transfection with wild-type RhoG and various RhoG mutants, PC12 cells were treated with NGF, and 48 h later the morphologies of the transfected cells were examined. Expression of wild-type RhoG significantly enhanced the NGF-induced neurite outgrowth (Fig. 6A, a and b): the population of the cells with long neurites (exceeding three or five times the length of the cell body) was two- to threefold greater than that of control cells transfected with an empty vector (Fig. 6B). On the other hand, expression of the dominant-negative RhoG, RhoGN17, inhibited NGF-stimulated neurite outgrowth (Fig. 6A, e and f). The constitutively active RhoG, RhoGV12, abrogated NGF-induced neurite outgrowth, and neurites of cells expressing RhoGV12 were very short and highly branched compared to those of untransfected cells (Fig. 6A, c and d). As the result, both RhoGN17 and RhoGV12 mutants decreased the population of the cells bearing long neurites (Fig. 6B). However, the expression of RhoGV12 or RhoGN17 had no significant effect on the viability of the cells compared to that of control cells (data not shown), indicating that overexpression of RhoG mutants did not have any toxic effect. In this experiment, RhoGN17 could not completely suppress the NGF-induced neurite outgrowth (Fig. 6B). This reason might be that RhoGN17 was not efficiently expressed in PC12 cells and that the expression level of RhoGN17 was very low in a large number of RhoGN17-transfected cells (data not shown). Cells expressing RhoGA37 normally produced neurites in response to NGF (Fig. 6A, g and h), but RhoGA37 exhibited a weak inhibitory effect on the ability of NGF to extend long neurites (Fig. 6B). It is possible that a RhoG protein containing the F37A mutation in the effector loop has no ability to bind to its effector and to act as a competitive inhibitor of endogenous RhoG with its GEFs.
Involvement of RhoG in RasV12-induced neurite outgrowth.
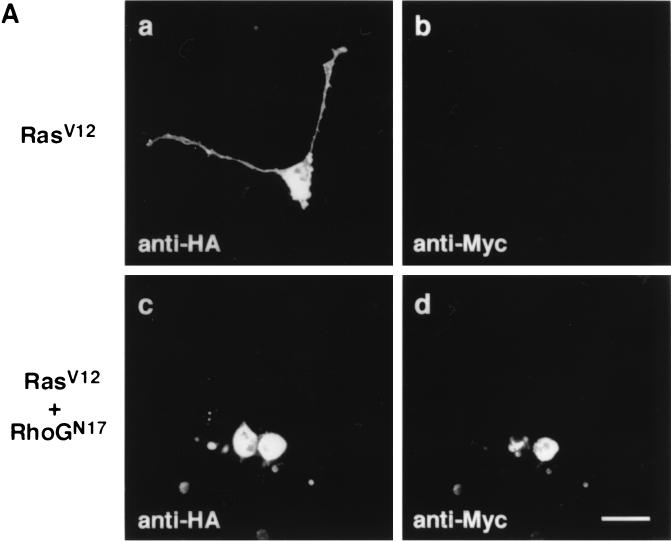
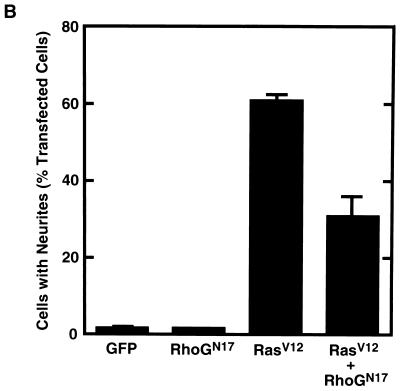
In PC12 cells, the activity of Ras has been known to be required for NGF-induced neurite outgrowth and the expression of constitutively active Ras is sufficient for inducing the outgrowth of neurites (2, 33). Therefore, we next examined whether RhoG was also involved in the Ras-induced neurite outgrowth. Expression of the constitutively active Ras mutant, RasV12, induced neurite outgrowth (Fig. 7A, a and b, and B). When cells were cotransfected with RhoGN17 and RasV12, RhoGN17 suppressed RasV12-induced neurite outgrowth (Fig. 7A, c and d, and Fig. 7B). On the other hand, expression of RhoGN17 alone had no effect on PC12 cell morphology (data not shown) and also had no ability to induce neurite outgrowth (Fig. 7B).
FIG. 7.
Effect of RhoGN17 on RasV12-induced neurite outgrowth. (A) PC12 cells were cotransfected with an expression vector encoding HA-tagged RasV12 and an empty vector (a and b) or a vector encoding Myc-tagged RhoGN17 (c and d). At 48 h after transfection, cells were fixed and costained with anti-HA (a and c) and anti-Myc (b and d) antibodies. The results shown are representative of three independent experiments. Bar, 25 μm. (B) Quantification of the effect of RhoGN17 on RasV12-induced neurite outgrowth. At 48 h after transfection, cells were costained with anti-Myc and anti-HA antibodies, and positively stained cells were assessed as described in the legend to Fig. 1. GFP-transfected cells were used as a control. Data are the means ± standard errors of triplicate experiments.
DISCUSSION
In PC12 cells, NGF induces cell differentiation into the neuronal phenotype through the activation of Ras (2, 33, 45). However, the molecular mechanisms regulating the cytoskeletal changes necessary for neurite outgrowth are still largely obscure. Recent studies have shown that the activity of Rac and Cdc42, members of the Rho family of small GTPases, is required for NGF-induced neurite outgrowth (6, 8, 24). In the present study, we examined the function and signal transduction of another member of the Rho family of small GTPases, RhoG, in PC12 cells. Wild-type RhoG and constitutively active RhoG had the ability to induce neurite outgrowth in PC12 cells, and, furthermore, NGF-induced neurite outgrowth required RhoG. These results demonstrate that RhoG plays a critical role in neurite outgrowth in PC12 cells. The ability of constitutively active RhoG to extend neurites in length was lower than that of wild-type RhoG, while constitutively active RhoG developed large lamellipodial or filopodial structures at the tips of neurites. These structures would enhance cell-substratum adhesion and inhibit the long extension of neurites. Considering that constitutively active RhoG induces the strong activation of Rac1, unlike wild-type RhoG, too much RhoG signal might be deleterious to neurite outgrowth and the modest activation of Rac1 and Cdc42 by wild-type RhoG would lead to long extension of neurites. Consistent with this, wild-type RhoG enhanced NGF-induced neurite outgrowth but constitutively active RhoG abrogated it, suggesting that modest activation of RhoG signaling may be required for neurite extension induced by NGF.
In fibroblasts, expression of constitutively active RhoG caused morphological and cytoskeletal changes that were dependent on Rac1 and Cdc42 activity (4, 11). In the present study, we have shown that RhoG-induced neurite outgrowth was inhibited by dominant-negative Rac1 and Cdc42 and that constitutively active RhoG increased the levels of endogenous GTP-bound forms of Rac1 and Cdc42. On the other hand, the effector loop RhoG mutant, RhoGA37, neither induced neurite outgrowth nor activated Rac1 and Cdc42. These results demonstrate that Rac1 and Cdc42 are located in the downstream signaling pathway of RhoG and that RhoG induces neurite outgrowth through activation of Rac1 and Cdc42. On the other hand, NGF is known to induce neurite outgrowth through the activation of Ras, and a study of N1E-115 neuroblastoma cells indicated that Rac1 and Cdc42 act downstream of Ras during neurite outgrowth (39). We also found that RhoG acted downstream of Ras. Therefore, these results suggest that RhoG links Ras signaling to Rac1 and Cdc42 activation in the process of neurite outgrowth in neuronal cells and that RhoG is a key regulator of the NGF-induced neurite outgrowth acting downstream of Ras and upstream of Rac1 and Cdc42. In contrast to RhoG, which is able to induce neurite outgrowth, a pair consisting of wild-type Rac1 and Cdc42 or constitutively active Rac1 and Cdc42 could not produce neurites from the cells, indicating that activation of both Rac1 and Cdc42 is not sufficient for inducing neurite outgrowth in PC12 cells. A possible explanation for this inconsistency is that neurite outgrowth might require not only an increase in Rac1 and Cdc42 activities but also their appropriate localization to the sites where neurites are formed and extend and that RhoG might function to activate and appropriately localize Rac1 and Cdc42 in contrast to the overexpression of both constitutively active Rac1 and Cdc42, which causes unpolarized morphological changes.
How does RhoG control the activity of Rac1 and Cdc42? In this study, we demonstrate that RhoG increases cellular GTP-bound forms of Rac1 and Cdc42, suggesting that downstream effectors of RhoG are the regulators of Rac1 and Cdc42 activities. It has of course been shown that RhoG does not directly interact with PAK, POR1, and WASP, the best-known effectors of Rac and Cdc42, to regulate the reorganization of the actin cytoskeleton (11). The activation of Rac1 and Cdc42 is regulated by a variety of proteins, such as GEFs, GAPs, and guanine-nucleotide dissociation inhibitors, and Rac1 and Cdc42 might be downstream targets of RhoG. Until now, potent effectors of RhoG that specifically bind to the GTP-bound form of RhoG have not yet been identified. Our present study focused on the downstream signaling pathway of RhoG, including its effectors, involved in the activation of Rac1 and Cdc42 during neurite outgrowth in PC12 cells. However, we cannot rule out the possibility that activated RhoG directly binds to and titrates some Rac or Cdc42 GAP, leading to an increase in the amount of cellular GTP-bound Rac1 and Cdc42, and that most of the expressed wild-type RhoG might be GTP bound and might also titrate a Rac or Cdc42 GAP, resulting in neurite outgrowth.
Next, how is the activity of RhoG regulated during neurite outgrowth in response to NGF? One good candidate is the Vav family proteins, members of the Dbl family of GEFs for the Rho family GTPases (5). The GDP-GTP exchange activity of Vav family proteins is stimulated by the tyrosine phosphorylation of the GEFs, and their tyrosine phosphorylation actually occurs in response to the activation of tyrosine kinase receptors, including NGF receptor TrkA (30). The Vav family has at least three known members in mammalian cells (Vav, Vav-2, and Vav-3) (15, 20, 31), and, among them, Vav-2 and Vav-3 display GEF activity for RhoG in vitro (31, 42). Unlike Vav, whose expression is restricted mostly to hematopoietic cells, Vav-2 and Vav-3 are ubiquitously expressed, including expression in PC12 cells (31, 41). Therefore, Vav-2 or Vav-3 might be involved in the activation of RhoG to induce neurite outgrowth downstream of NGF signaling pathways in PC12 cells. In addition to the Vav family, Trio, another Dbl family of GEFs, has been shown to preferentially catalyze GDP-GTP exchange on RhoG in vitro, and Trio-induced morphological and cytoskeletal changes in fibroblasts were shown to be suppressed by dominant-negative RhoG (4), suggesting that Trio acts as a GEF for RhoG in vivo. Trio was first identified as a protein associated with the transmembrane tyrosine phosphatase LAR (9), which is involved in the regulation of neural tissue development in mice (51). Although the function of mammalian Trio remains obscure, current evidence suggests that Trio is involved in axonogenesis and growth cone motility (25, 44). Therefore, Trio is another candidate for an activator of RhoG involved in neurite outgrowth in PC12 cells.
Our results suggest that RhoG is a signal transducer from Ras to Rac1 and Cdc42, leading to neurite outgrowth. Ras is a multifunctional regulator of neuronal functions (18). The activity of Ras is required for cell survival as well as morphological changes (10). In contrast to Ras, RhoG, expressed in either its wild-type or constitutively active form, could not protect PC12 cells from apoptosis induced by serum starvation (unpublished observation). Therefore, RhoG does not participate in Ras-mediated cell survival signaling, while RhoG is involved in Ras-mediated morphological changes through activation of Rac1 and Cdc42. On the other hand, we cannot exclude the possibility that dominant-negative RhoG titrates some Rac and Cdc42 GEFs common to all three GTPases. The use of RhoG-specific inhibitors such as the RhoG-binding domain of RhoG-specific effectors would be one of the best approaches to address this issue, although potent effectors of RhoG have not yet been identified.
Finally, Rac1 and Cdc42 have been demonstrated to be involved in axonogenesis, axonal pathfinding, and dendritic formation in various types of neurons (21, 27, 28, 36, 48). A previous study examining the tissue distribution of RhoG mRNA expression demonstrated that RhoG mRNA was significantly expressed in brain (49). Considering that RhoG is able to extend neurites acting upstream of Rac1 and Cdc42 in PC12 cells, it is conceivable that RhoG participates in the regulation of axon and dendrite formation upstream of Rac1 and Cdc42 during nervous system development. Production of a polyclonal antibody against rat RhoG is currently in progress in our laboratory, and we will examine the distribution of RhoG proteins in the rat nervous system in future studies. Further investigations are necessary to understand the role of RhoG in the nervous system.
ACKNOWLEDGMENTS
This work was supported in part by Grants-in-aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan (10470482, 11780579, 12053244) and grants from Uehara Memorial Foundation and Inamori Foundation.
REFERENCES
- 1.Albertinazzi C, Gilardelli D, Paris S, Longhi R, de Curtis I. Overexpression of a neural-specific rho family GTPase, cRac1B, selectively induces enhanced neuritogenesis and neurite branching in primary neurons. J Cell Biol. 1998;142:815–825. doi: 10.1083/jcb.142.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar-Sagi D, Feramisco J R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- 3.Benard V, Bohl B P, Bokoch G M. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 4.Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouviere C, Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J Cell Sci. 2000;113:729–739. doi: 10.1242/jcs.113.4.729. [DOI] [PubMed] [Google Scholar]
- 5.Bustelo X R. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X Q, Tan I, Leung T, Lim L. The myotonic dystrophy kinase-related Cdc42-binding kinase is involved in the regulation of neurite outgrowth in PC12 cells. J Biol Chem. 1999;274:19901–19905. doi: 10.1074/jbc.274.28.19901. [DOI] [PubMed] [Google Scholar]
- 7.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 8.Daniels R H, Hall P S, Bokoch G M. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 1998;17:754–764. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debant A, Serra-Pages C, Seipel K, O'Brien S, Tang M, Park S H, Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci USA. 1996;93:5466–5471. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downward J. Ras signalling and apoptosis. Curr Opin Genet Dev. 1998;8:49–54. doi: 10.1016/s0959-437x(98)80061-0. [DOI] [PubMed] [Google Scholar]
- 11.Gauthier-Rouviere C, Vignal E, Meriane M, Roux P, Montcourier P, Fort P. RhoG GTPase controls a pathway that independently activates Rac1 and Cdc42Hs. Mol Biol Cell. 1998;9:1379–1394. doi: 10.1091/mbc.9.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebbink M F B G, Kranenburg O, Poland M, Horck F P G, Houssa B, Moolenaar W H. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J Cell Biol. 1997;137:1603–1613. doi: 10.1083/jcb.137.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa H, Fujita H, Katoh H, Aoki J, Nakamura K, Ichikawa A, Negishi M. Opposite regulation of transepithelial electrical resistance and paracellular permeability by Rho in Madin-Darby canine kidney cells. J Biol Chem. 1999;274:20982–20988. doi: 10.1074/jbc.274.30.20982. [DOI] [PubMed] [Google Scholar]
- 15.Henske E P, Short M P, Jozwaik S, Bovey C M, Ramlakhan S, Hains J L, Kwiatkowski D J. Identification of VAV2 on 9q34 and its exclusion as the tuberous sclerosis gene TSC1. Ann Hum Genet. 1995;59:25–37. doi: 10.1111/j.1469-1809.1995.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 16.Ito W, Ishiguro H, Kurosawa K. A general method for introducing a series of mutations into cloned DNA using the polymerase chain reaction. Gene. 1991;102:67–70. doi: 10.1016/0378-1119(91)90539-n. [DOI] [PubMed] [Google Scholar]
- 17.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan D R, Stephens R M. Neurotrophin signal transduction by the Trk receptor. J Neurobiol. 1994;25:1404–1417. doi: 10.1002/neu.480251108. [DOI] [PubMed] [Google Scholar]
- 19.Katoh H, Aoki J, Ichikawa A, Negishi M. p160 RhoA-binding kinase ROKα induces neurite retraction. J Biol Chem. 1998;273:2489–2492. doi: 10.1074/jbc.273.5.2489. [DOI] [PubMed] [Google Scholar]
- 20.Katzav S, Martin-Zanca D, Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989;8:2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann N, Wills Z P, van Vactor D. Drosophila Rac1 controls motor axon guidance. Development. 1998;125:453–461. doi: 10.1242/dev.125.3.453. [DOI] [PubMed] [Google Scholar]
- 22.Keynes R, Cook G M W. Axon guidance molecules. Cell. 1995;83:161–169. doi: 10.1016/0092-8674(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 23.Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamoureux P, Altun-Gultekin Z F, Lin C, Wagner J A, Heidemann S R. Rac is required for growth cone function but not neurite assembly. J Cell Sci. 1997;110:635–641. doi: 10.1242/jcs.110.5.635. [DOI] [PubMed] [Google Scholar]
- 25.Lanier L M, Gertler F B. From Abl to actin: Abl tyrosine kinase and associated proteins in growth cone motility. Curr Opin Neurobiol. 2000;10:80–87. doi: 10.1016/s0959-4388(99)00058-6. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo L, Liao Y J, Jan L Y, Jan Y N. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 28.Luo L, Hensch T K, Ackerman L, Barbel S, Jan L Y. Differential effects of the Rac GTPases on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- 29.Manser E, Leung T, Salihuddin H, Zhao Z S, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 30.Melamed I, Patel H, Brodie C, Gelfand E W. Activation of Vav and Ras through the nerve growth factor and B cell receptors by different kinases. Cell Immunol. 1999;191:83–89. doi: 10.1006/cimm.1998.1402. [DOI] [PubMed] [Google Scholar]
- 31.Movilla N, Bustelo X R. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncogenes. Mol Cell Biol. 1999;19:7870–7885. doi: 10.1128/mcb.19.11.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobes C D, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 33.Noda M, Ko M, Ogura A, Lim D G, Amano T, Takano T, Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985;318:73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- 34.Ridley A J, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 35.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 36.Ruchhoeft M L, Ohnuma S, McNeill L, Holt C E, Harris W A. The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J Neurosci. 1999;19:8454–8463. doi: 10.1523/JNEUROSCI.19-19-08454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabath D E, Broome H E, Prystowsky M B. Glyceraldehyde-3-phosphate dehydrogenase mRNA is a major interleukin 2-induced transcript in a cloned T-helper lymphocyte. Gene. 1990;91:185–191. doi: 10.1016/0378-1119(90)90087-8. [DOI] [PubMed] [Google Scholar]
- 38.Sander E E, ten Klooster J P, van Delft S, van der Kammen R A, Collard J G. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1021. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarner S, Kozma R, Ahmed S, Lim L. Phosphatidylinositol 3-kinase, Cdc42, and Rac1 act downstream of Ras in integrin-dependent neurite outgrowth in N1E-115 neuroblastoma cells. Mol Cell Biol. 2000;20:158–172. doi: 10.1128/mcb.20.1.158-172.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki T, Takai Y. The Rho small G protein family-Rho GDI system as a temporal and spatial determinant for cytoskeletal control. Biochem Biophys Res Commun. 1998;245:641–645. doi: 10.1006/bbrc.1998.8253. [DOI] [PubMed] [Google Scholar]
- 41.Schuebel K E, Bustelo X R, Nielsen D A, Song B-J, Barbacid M, Goldman D, Lee I J. Isolation and characterization of murine vav2, a member of the vav family of proto-oncogenes. Oncogene. 1996;13:363–371. [PubMed] [Google Scholar]
- 42.Schuebel K E, Movilla N, Rosa J L, Bustelo X R. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 1998;17:6608–6621. doi: 10.1093/emboj/17.22.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shinjo K, Koland J G, Hart M J, Narasimhan V, Johnson D I, Evans T, Cerione R A. Molecular cloning of the gene for the human placental GTP-binding protein Gp (G25K): identification of this GTP-binding protein as the human homolog of the yeast cell-division-cycle protein CDC42. Proc Natl Acad Sci USA. 1990;87:9853–9857. doi: 10.1073/pnas.87.24.9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steven R, Kubiseski T J, Zheng H, Kulkarni S, Mancillas J, Ruiz Morales A, Hogue C W, Pawson T, Culotti J. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell. 1998;92:785–795. doi: 10.1016/s0092-8674(00)81406-3. [DOI] [PubMed] [Google Scholar]
- 45.Szeberenyi J, Cai H, Cooper G M. Effect of a dominant inhibitory Ha-ras mutation on neuronal differentiation of PC12 cells. Mol Cell Biol. 1990;10:5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka E, Sabry J. Making the connection: cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:171–176. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 47.Thomas S M, DeMarco M, D'Arcangelo G, Halegoua S, Brugge J S. Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell. 1992;68:1031–1040. doi: 10.1016/0092-8674(92)90075-n. [DOI] [PubMed] [Google Scholar]
- 48.Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 49.Vincent S, Jeanteur P, Fort P. Growth-regulated expression of rhoG, a new member of the ras homolog gene family. Mol Cell Biol. 1992;12:3138–3148. doi: 10.1128/mcb.12.7.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood K W, Sarnecki C, Roberts T M, Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992;68:1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- 51.Yeo T T, Yang T, Massa S M, Zhang J S, Honkaniemi J, Butcher L L, Longo F M. Deficient LAR expression decreases basal forebrain cholinergic neuronal size and hippocampal cholinergic innervation. J Neurosci Res. 1997;47:348–360. doi: 10.1002/(sici)1097-4547(19970201)47:3<348::aid-jnr13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 52.Zipkin I D, Kindt R M, Kenyon C J. Role of a new Rho family member in cell migration and axon guidance in C. elegans. Cell. 1997;90:883–894. doi: 10.1016/s0092-8674(00)80353-0. [DOI] [PubMed] [Google Scholar]