Abstract
Invasive meningococcal disease (IMD), caused by Neisseria meningitidis, can have a fatality rate as high as 10%, even with appropriate treatment. In the UK, penicillin is administered to patients in primary care whilst third generation cephalosporins, cefotaxime and ceftriaxone, are administered in secondary care. The first-choice antibiotic for chemoprophylaxis of close contacts is ciprofloxacin, followed by rifampicin. Immunocompromised individuals are often recommended antibiotic chemoprophylaxis and vaccination due to a greater risk of IMD. Resistance to antibiotics among meningococci is relatively rare, however reduced susceptibility and resistance to penicillin are increasing globally. Resistance to third generation cephalosporins is seldom reported, however reduced susceptibility to both cefotaxime and ceftriaxone has been observed. Rifampicin resistance has been reported among meningococci, mainly following prophylaxis, and ciprofloxacin resistance, whilst uncommon, has also been reported across the globe. The Public Health England Meningococcal Reference Unit receives and characterises the majority of isolates from IMD cases in England, Wales and Northern Ireland. This study assessed the distribution of antibiotic resistance to penicillin, rifampicin, ciprofloxacin and cefotaxime among IMD isolates received at the MRU from 2010/11 to 2018/19 (n = 4,122). Out of the 4,122 IMD isolates, 113 were penicillin-resistant, five were ciprofloxacin-resistant, two were rifampicin-resistant, and one was cefotaxime-resistant. Penicillin resistance was due to altered penA alleles whilst rifampicin and ciprofloxacin resistance was due to altered rpoB and gyrA alleles, respectively. Cefotaxime resistance was observed in one isolate which had an altered penA allele containing additional mutations to those harboured by the penicillin-resistant isolates. This study identified several isolates with resistance to antibiotics used for current treatment and prophylaxis of IMD and highlights the need for continued surveillance of resistance among meningococci to ensure continued effective use.
Introduction
Invasive meningococcal disease (IMD), including meningitis and/or septicaemia, is a severe, life-threatening illness caused by the bacterium Neisseria meningitidis (the meningococcus). Six meningococcal serogroups (A, B, C, W, X and Y) are responsible for the majority of IMD cases worldwide [1–3]. Meningococci can be further differentiated into sequence types (STs), with related STs grouped into lineages termed clonal complexes (CCs) [4]. Serogroup B meningococcal disease is the most prevalent in the UK and is the most diverse in terms of clonal complex distribution [5].
IMD is fatal in 5–10% of cases in developed countries, despite prompt commencement of antibiotic treatment [6]. In the UK, patients in primary care with suspected meningococcal disease are administered penicillin, whilst those in secondary care are administered third generation cephalosporins (3GCs), ceftriaxone or cefotaxime [7]. Ciprofloxacin is the chemoprophylactic agent of choice for close contacts of cases and rifampicin is a suitable alternative [8]. Chemoprophylaxis is recommended for close contact of IMD cases and for individuals with deficiencies of the terminal complement pathway, where the risk for IMD development is up to 10,000 fold higher than in the general population [9].
N. meningitidis has remained largely susceptible to the antibiotics used in the treatment and prevention of IMD. Resistance to ciprofloxacin, due to alterations in the gyrA gene (encoding DNA gyrase subunit A), is relatively uncommon, except for in China, where the prevalence of resistance among isolates, in particular of the ST-4821 CC, has led to the withdrawal of its use as a first line prophylactic agent [10, 11]. More recently, ciprofloxacin resistance has been reported in invasive ST-23 CC isolates in the USA [12] and in invasive ST-175 CC isolates in immunocompromised individuals in the UK and Europe [13]. Mutations in the parC gene (encoding DNA topoisomerase IV, subunit A), when accompanied by mutations in the gyrA gene, cause enhanced levels of ciprofloxacin resistance [14].
Primary cases of rifampicin-resistant meningococci due to alterations in the rpoB gene (encoding the beta subunit of RNA polymerase), is uncommon and not associated with particular lineages. It is usually observed following rifampicin prophylaxis [15–17].
Penicillin resistance among meningococci due to the production of beta lactamase is rare, however, has recently been identified among ST-23 CC isolates in the USA [12]. Penicillin resistance and reduced susceptibility due to amino acid substitutions (AASs) (F504L, A510V, I515V, H541N, and I566V) in penA, encoding penicillin binding protein 2 (PBP2), are more frequently observed worldwide [18–21], and several countries have recently reported an increase in penicillin resistant invasive serogroup W ST-11 CC isolates [22, 23].
Meningococcal resistance to 3GCs is rare. A report of cefotaxime and ceftriaxone resistance among serogroup A isolates in India [24] has been met with some scepticism [25]. Reduced susceptibility to cefotaxime has been observed among several invasive isolates in France [26]. The penA gene responsible, penA327, was identical to that causing reduced susceptibility to 3GCs in N. gonorrhoea [27]. The allele, containing only four out of the five aforementioned AASs, also causes penicillin resistance and reduced susceptibly among meningococci [20, 26]. Amino acid substitution A501P in penA has been shown to enhance levels of 3GC resistance among N. gonorrhoea, and there are some concerns regarding the potential selection of this same mutation among meningococci [28]. Surveillance of antibiotic resistance among this species therefore remains of paramount importance.
The Public Health England (PHE) Meningococcal Reference Unit (MRU) offers a complimentary national reference service for confirmation and characterisation of meningococcal isolates in England, Wales and Northern Ireland (E, W and NI). The aim of this study was to characterise isolates from culture-confirmed cases of IMD received at the MRU from July 2010 to June 2019 inclusive (epidemiological years 2010/11 to 2018/19), and to determine the distribution of antibiotic susceptibility.
Materials and methods
The study included 4,122 IMD isolates received by the MRU from July 2010 to June 2019, inclusive.
Serogrouping
Serogrouping was performed using the dot-blot ELISA as previously described [29].
Antibiotic susceptibility testing
Antibiotic susceptibility testing for penicillin, cefotaxime, rifampicin and ciprofloxacin was performed using the Etest (bioMerieux UK Limited, Basingstoke, UK) method on either Mueller Hinton agar plates supplemented with 5% horse blood and 20 mg/L β-nicotinamide adenine dinucleotide (Oxoid Limited, Basingstoke, UK) (isolates received on or after 9th May 2019) or Iso-sensitest blood agar supplemented with 20 mg/L NAD (isolates received prior to 9th May 2019).
MIC values were interpreted according to the European Committee on Antimicrobial Susceptibly Testing (EUCAST; v11.0; 2021-01-01).
Isolates with penicillin MICs ≤0.06 mg/L were categorised as ‘susceptible, standard exposure (S)’ (where there is a high likelihood of therapeutic success using a standard dosing regimen of the agent) and isolates with penicillin MICs >0.25 mg/L were categorised as ‘resistant (R)’ (where there is a high likelihood of therapeutic failure even when there is increased exposure). Isolates with intermediate penicillin MICs were categorised as ‘susceptible, increased exposure (I)’ (where there is a high likelihood of therapeutic success because exposure to the agent is increased by adjusting the dosing regimen or by its concentration at the site of infection) [30].
Beta-lactamase testing
Beta-lactamase testing was performed using Interlactam strips (MAST, UK) following the manufacturer’s instructions.
Genotypic analyses
Draft genomes were available for all isolates on the Meningitis Research Foundation Meningococcus Genome Library (MGL; https://pubmlst.org/bigsdb?db=pubmlst_neisseria_mrfgenomes; accessed 29th June 2020).
Genotypic data were obtained using the BLAST and/or Export Dataset tools [31, 32].
PenA sequencing
Where necessary, a 402 bp fragment (penA) of the NEIS1753 (PBP2) gene (pubmlst.org) was characterised by PCR and Sanger sequencing, as previously described using primers penA1F and penA1R [33].
Results
Serogroup and clonal complex distribution
Among all isolates, 53% (n = 2,178) were serogroup B, 24% (n = 1,004) were serogroup W, 16% were serogroup Y (n = 651) and 6% were serogroup C (n = 237). The remaining isolates were NG (n = 34), serogroup W/Y (n = 11), serogroup E (n = 4), serogroup X (n = 2) and serogroup A (n = 1). Most of the isolates (n = 3,585; 87%) belonged to eight main clonal complexes (ST-11 CC, n = 1,138; ST-41/44 CC, n = 748; ST-23 CC, n = 585; ST-269 CC, n = 520; ST-213 CC, n = 245; ST-32 CC, n = 191; ST-22 CC, n = 80 and ST-461 CC, n = 78). The remaining isolates belonged to less prevalent CCs (n = 298) or were unassigned to a CC (n = 239).
Distribution of antibiotic susceptibility
More than half of the isolates (63%; n = 2,591) were penicillin-susceptible, standard exposure (PenS; MICs 0.004–0.064 mg/L), 34% (n = 1,418) were penicillin-susceptible, increased exposure (PenI; MICs 0.094–0.25 mg/L) and 3% (n = 113) were penicillin-resistant (PenR; MICs 0.38–0.75 mg/L) (Table 1). The MIC50 for penicillin was 0.064 mg/L and the MIC90 was 0.19 mg/L. The highest proportion of PenR isolates were received in 2018/19 (7%).
Table 1. Number of isolates from culture-confirmed IMD cases by epidemiological year in England Wales and Northern Ireland, from 2010/11 to 2018/19.
| Number of isolates by epidemiological year (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Susceptibility category (MIC) by antibiotic (mg/L) | 2010/11 (n = 500) | 2011/12 (n = 400) | 2012/13 (n = 450) | 2013/14 (n = 403) | 2014/15 (n = 505) | 2015/16 (n = 521) | 2016/17 (n = 501) | 2017/18 (n = 488) | 2018/19 (n = 354) | Total (n = 4,122) |
| Penicillin | ||||||||||
| PenS ≤0.06 mg/L | 336 (67.2) | 277 (69.3) | 277 (61.6) | 247 (61.3) | 349 (69.1) | 272 (52.2) | 335 (66.9) | 313 (64.1) | 185 (52.3) | 2,591 (62.9) |
| PenI 0.094–0.25 mg/L | 157 (31.4) | 123 (30.8) | 164 (36.4) | 148 (36.7) | 150 (29.7) | 223 (42.8) | 150 (29.9) | 159 (32.6) | 144 (40.7) | 1,418 (34.4) |
| PenR >0.25 mg/L | 7 (1.4) | 0 (0.0) | 9 (2.0) | 8 (2.0) | 6 (1.2) | 26 (5.0) | 16 (3.2) | 16 (3.3) | 25 (7.1) | 113 (2.7) |
| Cefotaxime | ||||||||||
| Susceptible ≤0.125 mg/L | 499 | 400 | 450 | 403 | 505 | 521 | 501 | 488 | 354 | 4,121 |
| Resistant >0.125 mg/L | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ciprofloxacin | ||||||||||
| Susceptible ≤0.03 mg/L | 499 | 400 | 450 | 403 | 503 | 521 | 501 | 488 | 352 | 4,117 |
| Resistant >0.03 mg/L | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 5 |
| Rifampicin | ||||||||||
| Susceptible ≤0.25 mg/L | 500 | 400 | 449 | 403 | 505 | 520 | 501 | 488 | 354 | 4,120 |
| Resistant >0.25 mg/L | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
PenS = Penicillin susceptible, standard exposure; PenI = Penicillin-susceptible, increased exposure; PenR = Penicillin-resistant according to EUCAST guidelines. The annual percentage of PenS, I and R isolates is also displayed.
One isolate was cefotaxime-resistant (MIC = 0.25 mg/L) and the remaining isolates were cefotaxime-susceptible (n = 4,121; MICs = <0.002–0.125 mg/L). The MIC50 for cefotaxime was 0.004 mg/L and the MIC90 was 0.008 mg/L.
Five isolates were ciprofloxacin-resistant (MICs = 0.06–0.5 mg/L), and 4,117 isolates were ciprofloxacin-sensitive (MICs = <0.002–0.03 mg/L). The MIC50 for ciprofloxacin was 0.004 mg/L and the MIC90 was 0.008 mg/L.
Two isolates were rifampicin-resistant (MICs = 0.5 and >32 mg/L) and 4,120 isolates were rifampicin-sensitive (MICs = <0.002–0.25 mg/L). The MIC50 for rifampicin was 0.008 mg/L and the MIC90 was 0.023 mg/L.
Only one isolate was resistant to more than one antibiotic (penicillin and cefotaxime; 0.5 mg/L and 0.25 mg/L, respectively; PubMLST ID 20267). Details of all isolates that were resistant to at least one antibiotic are listed in S1 Table.
Penicillin susceptibility
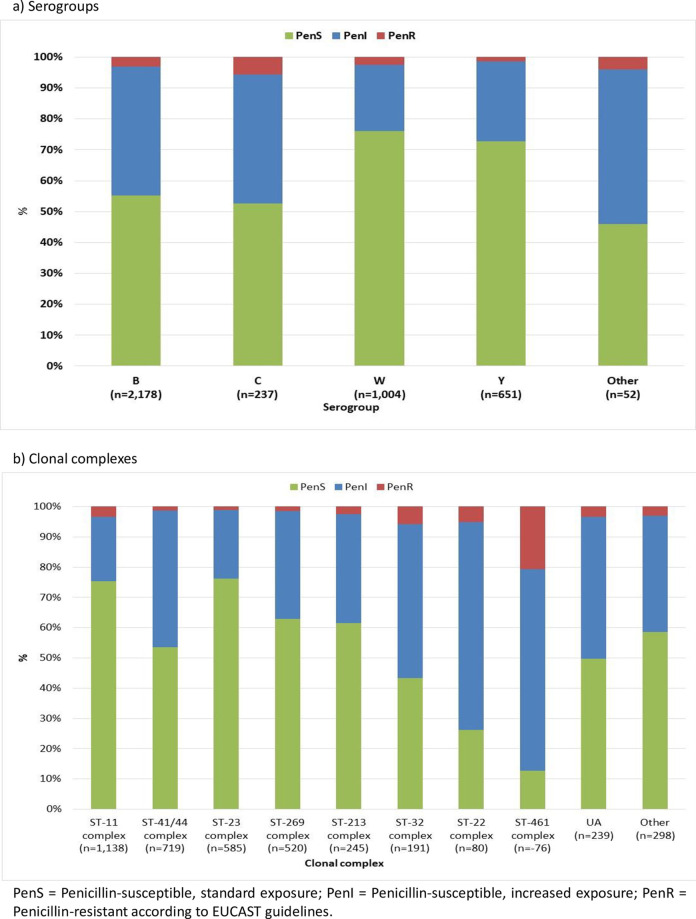
Fifty-five percent of serogroup B isolates were penicillin-susceptible (Fig 1). The proportion of penicillin-susceptible serogroup C, W and Y isolates were 53%, 76% and 73%, respectively. The proportion of serogroup B PenS isolates decreased from 67% in 2010/11 to 38% in 2018/19 (S1 Fig). There was an increase in the proportion of serogroup W PenR isolates over the last four years, with the highest proportion observed in 2018/19 (8%; S1 Fig).
Fig 1. Penicillin susceptibility among serogroups and clonal complexes of IMD isolates in England, Wales and Northern Ireland, 2010/11 to 2018/19.
The most prevalent clonal complex (all serogroups) was ST-11 CC followed by ST-41/44 CC where 75% and 56% of isolates were penicillin-susceptible, respectively (Fig 1). The clonal complex with the lowest proportion of PenS isolates was ST-461 CC (13%), which was also associated with the highest proportion of PenR isolates (21%).
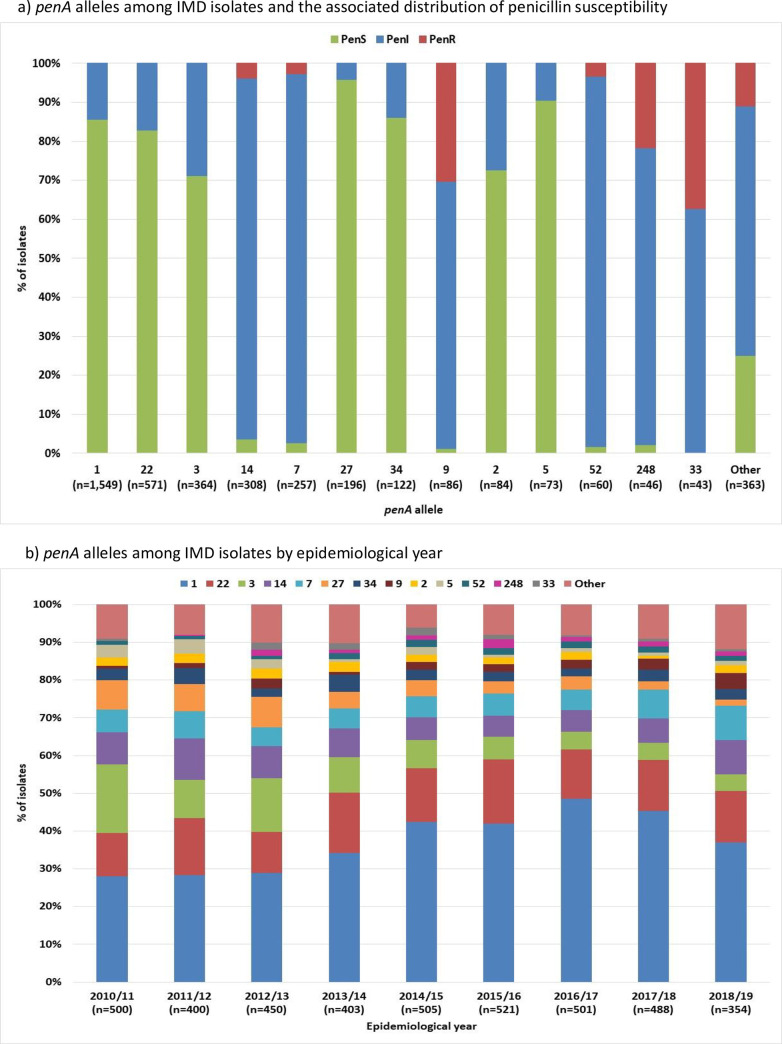
In total, 133 penA alleles were identified. PenA1 was most prevalent (n = 1,549, 38%), followed by penA22 (n = 571, 14%), for which 86% and 83% of isolates were penicillin-susceptible, respectively (Fig 2). PenA33 (n = 43, 1%) was associated with the highest proportion of PenR isolates at 37%. The proportion of isolates with penA3 and penA27 decreased over time and, over the last four years, the proportion of isolates with penA9 increased (Fig 2).
Fig 2. penA alleles among IMD isolates in England, Wales and Northern Ireland 2010/11 to 2018/19.
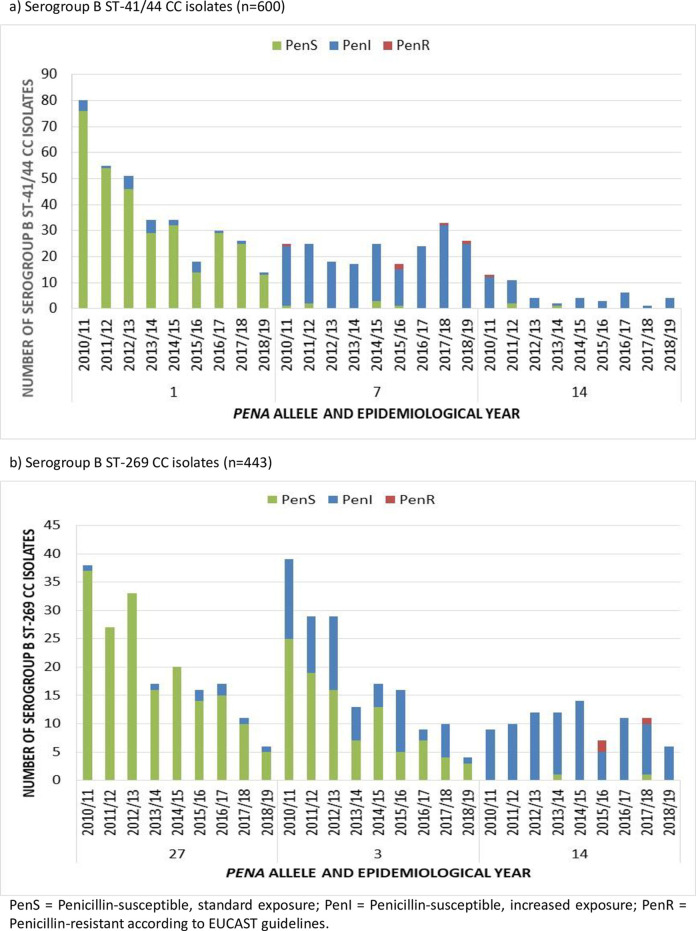
The increase in PenR serogroup W isolates over the last four years (S1 Fig) was due to a rise in the number of serogroup W ST-11 CC isolates harbouring penA9, as reported previously [22]. The decrease of serogroup B PenS isolates overtime (S1 Fig) was largely due to a decrease in serogroup B PenS ST-41/44 CC isolates with penA1 and ST-269 CC isolates with penA3 and penA27 (Fig 3).
Fig 3. Prevalent penA alleles among serogroup B IMD isolates belonging to ST-41/44 and ST-269 clonal complex in England, Wales and Northern Ireland 2010/11 to 2018/19.
Thirty-one penA alleles were identified among the 113 PenR isolates (Table 2). All but three of these had the five AASs associated with penicillin resistance and reduced susceptibility (F504L, A510V, I515V, H541N and I566V). PenA171, found in one PenR isolate, had none of these mutations. PenA209, found in one PenR isolate had three out of the five AASs (F504L, A510V and I515V) and PenA327, found among PenR (n = 1) PenI (n = 3) and PenS (n = 1) isolates, had four out of the five AASs (F504L, A510V, I515V, H541N). All isolates with penA327 (n = 5) also displayed reduced susceptibility to cefotaxime (0.047–0.125 mg/L).
Table 2. PenA alleles among PenR and PenI IMD isolates in England, Wales and Northern Ireland from 2010/11 to 2018/19.
| penA allele | Total number of isolates | Number of PenS isolates | Number of PenI isolates | Number of PenR isolates | MIC value or range (mg/L) | MIC50 | MIC90 | Number of penicillin resistance associated AASs |
|---|---|---|---|---|---|---|---|---|
| 1 | 1549 | 1327 | 222 | 0 | 0.004–0.25 | 0.047 | 0.094 | 0 |
| 2 | 84 | 61 | 23 | 0 | 0.016–0.25 | 0.064 | 0.125 | 0 |
| 3 | 364 | 259 | 105 | 0 | 0.047–0.25 | 0.064 | 0.094 | 0 |
| 4 | 13 | 11 | 2 | 0 | 0.016–0.094 | 0.047 | 0.094 | 0 |
| 5 | 73 | 66 | 7 | 0 | 0.012–0.19 | 0.047 | 0.064 | 0 |
| 7 | 257 | 7 | 243 | 7 | 0.064–0.38 | 0.19 | 0.25 | 5 |
| 9 | 86 | 1 | 59 | 26 | 0.047–0.75 | 0.25 | 0.38 | 5 |
| 10 | 5 | 0 | 2 | 3 | 0.25–0.38 | 5 | ||
| 11 | 1 | 0 | 0 | 1 | 0.5 | 5 | ||
| 12 | 16 | 0 | 14 | 2 | 0.125–0.5 | 0.19 | 0.38 | 5 |
| 13 | 7 | 0 | 5 | 2 | 0.125–0.38 | 5 | ||
| 14 | 308 | 11 | 285 | 12 | 0.047–0.5 | 0.19 | 0.25 | 5 |
| 16 | 9 | 8 | 1 | 0 | 0.023–0.094 | 0 | ||
| 19 | 22 | 1 | 18 | 3 | 0.047–0.38 | 0.19 | 0.38 | 5 |
| 20 | 6 | 0 | 5 | 1 | 0.125–0.5 | 5 | ||
| 21 | 3 | 0 | 1 | 2 | 0.19–0.38 | 5 | ||
| 22 | 571 | 473 | 98 | 0 | 0.012–0.19 | 0.064 | 0.094 | 0 |
| 25 | 3 | 0 | 2 | 1 | 0.125–0.38 | 5 | ||
| 27 | 196 | 188 | 8 | 0 | 0.008–0.125 | 0.047 | 0.064 | 0 |
| 33 | 43 | 0 | 27 | 16 | 0.094–0.75 | 0.25 | 0.5 | 5 |
| 34 | 122 | 105 | 17 | 0 | 0.012–0.19 | 0.047 | 0.094 | 0 |
| 42 | 11 | 0 | 10 | 1 | 0.125–0.38 | 0.19 | 0.25 | 5 |
| 52 | 60 | 1 | 57 | 2 | 0.064–0.38 | 0.19 | 0.25 | 5 |
| 54 | 1 | 0 | 0 | 1 | 0.38 | 5 | ||
| 62 | 5 | 3 | 2 | 0 | 0.032–0.094 | 0 | ||
| 66 | 5 | 0 | 5 | 0 | 0.094–0.25 | 5 | ||
| 83 | 4 | 2 | 2 | 0 | 0.047–0.125 | 0 | ||
| 90 | 5 | 0 | 5 | 0 | 0.125–0.19 | 5 | ||
| 91 | 4 | 0 | 4 | 0 | 0.125–0.19 | 5 | ||
| 110 | 2 | 0 | 1 | 1 | 0.125–0.38 | 5 | ||
| 119 | 4 | 1 | 3 | 0 | 0.032–0.125 | 0 | ||
| 157 | 15 | 14 | 1 | 0 | 0.032–0.094 | 0.047 | 0.064 | 0 |
| 171 | 1 | 0 | 0 | 1 | 0.38 | 0 | ||
| 179 | 1 | 0 | 0 | 1 | 0.38 | 5 | ||
| 209 | 1 | 0 | 0 | 1 | 0.5 | 3 | ||
| 238 | 5 | 0 | 5 | 0 | 0.094–0.19 | 5 | ||
| 248 | 46 | 1 | 35 | 10 | 0.047–0.38 | 0.25 | 0.38 | 5 |
| 295 | 6 | 0 | 3 | 3 | 0.25–0.5 | 5 | ||
| 327 | 5 | 1 | 3 | 1 | 0.047–0.38 | 4 | ||
| 331 | 1 | 0 | 0 | 1 | 0.5 | 5 | ||
| 342 | 4 | 2 | 2 | 0 | 0.047–0.094 | 0 | ||
| 348 | 16 | 2 | 11 | 3 | 0.047–0.38 | 0.125 | 0.38 | 5 |
| 371 | 5 | 0 | 4 | 1 | 0.125–0.38 | 5 | ||
| 386 | 10 | 1 | 9 | 0 | 0.047–0.125 | 0.125 | 0.125 | 5 |
| 414 | 8 | 0 | 7 | 1 | 0.125–0.38 | 5 | ||
| 419 | 1 | 0 | 0 | 1 | 0.5 | 5 | ||
| 420 | 8 | 0 | 8 | 0 | 0.125–0.25 | 5 | ||
| 435 | 1 | 0 | 0 | 1 | 0.38 | 5 | ||
| 540 | 6 | 0 | 1 | 5 | 0.25–0.38 | 5 | ||
| 593 | 1 | 0 | 0 | 1 | 0.38 | 5 | ||
| 921 | 1 | 0 | 0 | 1 | 0.38 | 5 |
Alleles among n = 3,981 IMD isolates. Alleles among PenI IMD isolates represented by 4+ isolates only. PenS = Penicillin-susceptible, standard exposure; PenI = Penicillin-susceptible, increased exposure; PenR = Penicillin-resistant according to EUCAST guidelines. MIC50 and MIC90 calculated for ≥10 isolates. AASs = amino acid substitutions.
Cefotaxime susceptibility
Seven isolates had cefotaxime MICs ≥ 0.047 mg/L (0.2%), including one resistant isolate (MIC = 0.25 mg/L). These isolates harboured either penA327 (n = 5; MIC = 0.047–0.125 mg/L), penA11 (n = 1; MIC = 0.047 mg/L) or penA419 (n = 1; MIC = 0.25 mg/L). Bar one, these isolates were also PenR (0.38–0.5 mg/L) or PenI (0.125–0.25 mg/L).
Allele penA419, which was also associated with penicillin resistance, was not found in any other isolates on PubMLST (accessed 10th June 2021). In addition to the five AASs associated with penicillin resistance, the allele had substitutions A501T and D511V that were not found among penA alleles harboured by cefotaxime-susceptible PenR isolates received at the MRU.
Isolates with penA alleles harbouring AASs at positions 501 and 511 on PubMLST (n = 56; Table 3) included N. meningitidis (n = 50), N. lactamica (n = 5) and N. polysaccharea (n = 1). All penA alleles amongst these isolates had A501T (n = 17) or A501V (n = 39). Six isolates that had a penA allele with A501T also had an additional AAS at position 511; D511A (penA419; n = 1), D511G (penA910; n = 2, penA836; n = 1) or D511V (penA670; n = 1, penA805; n = 1). Where known, cefotaxime MICs of isolates with penA alleles with A501V only were 0.003–0.012 mg/L (n = 14). Isolates with penA alleles with A501T only had MICs of 0.016–0.125 mg/L (n = 6). Isolates with both A501T and D511 mutations in the penA alleles (penA419, penA910) had MICs of 0.125–0.5 mg/L (n = 3).
Table 3. Isolates with penA alleles harbouring AASs at positions 501 and 511 on PubMLST.
| PubMLST ID | Country | Year | Site | Species | Cefotaxime MIC (mg/L) | Penicillin MIC (mg/L) | penA allele | A501 mutation | D511 mutation | Other 5 penicillin resistance-associated mutations*? |
|---|---|---|---|---|---|---|---|---|---|---|
| 20267 | England | 2011 | Blood | Neisseria menigitidis | 0.25 | 0.5 | 419 | A501T | D511A | Yes |
| 72882 | Greece | 2020 | NK | Neisseria menigitidis | 0.125 | 0.5 | 910 | A501T | D511G | Yes |
| 72883 | Greece | 2020 | NK | Neisseria menigitidis | 0.19 | 0.5 | 910 | A501T | D511G | Yes |
| 92874 | China | 2015 | Throat | Neisseria lactamica | NK | NK | 836 | A501T | D511G | Yes |
| 38947 | Italy | 2013 | Throat | Neisseria menigitidis | NK | NK | 670 | A501T | D511V | Yes |
| 41652 | Italy | 2015 | NK | Neisseria polysaccharea | NK | NK | 805 | A501T | D511V | Yes |
| 93629 | Germany | 2016 | Blood | Neisseria menigitidis | 0.047 | 0.25 | 909 | A501T | None | Yes |
| 93630 | Germany | 2016 | Blood | Neisseria menigitidis | 0.047 | 0.5 | 909 | A501T | None | Yes |
| 17230 | Niger | 1961 | CSF | Neisseria menigitidis | NK | 0.016 | 84 | A501T | None | No |
| 15965 | France | 2010 | CSF | Neisseria menigitidis | 0.047 | 0.125 | 341 | A501T | None | Yes |
| 16970 | France | 2005 | CSF | Neisseria menigitidis | 0.125 | 0.38 | 61 | A501T | None | Yes |
| 45332 | France | 2016 | NK | Neisseria menigitidis | 0.016 | 0.38 | 734 | A501T | None | Yes |
| 28074 | England | 2013 | Blood | Neisseria menigitidis | 0.004 | 0.012 | 24 | A501V | None | No |
| 35318 | Scotland | 2011 | CSF | Neisseria menigitidis | 0.004 | 0.015 | 24 | A501V | None | No |
| 35288 | Scotland | 2010 | Blood | Neisseria menigitidis | 0.004 | 0.023 | 411 | A501V | None | No |
| 35437 | England | 2013 | Blood | Neisseria menigitidis | 0.008 | 0.023 | 311 | A501V | None | No |
| 19771 | France | 2011 | Blood | Neisseria menigitidis | 0.06 | 0.023 | 311 | A501V | None | No |
| 20303 | England | 2011 | Blood | Neisseria menigitidis | 0.003 | 0.032 | 311 | A501V | None | No |
| 60835 | England | 2017 | Blood | Neisseria menigitidis | 0.006 | 0.032 | 161 | A501V | None | No |
| 19794 | France | 2011 | Blood | Neisseria menigitidis | 0.008 | 0.032 | 311 | A501V | None | No |
| 38060 | England | 2015 | Blood | Neisseria menigitidis | 0.008 | 0.032 | 311 | A501V | None | No |
| 53225 | England | 2017 | Blood | Neisseria menigitidis | 0.008 | 0.032 | 161 | A501V | None | No |
| 20779 | UK | NK | Blood | Neisseria menigitidis | 0.006 | 0.047 | 24 | A501V | None | No |
| 44717 | Northern Ireland | 2016 | Blood | Neisseria menigitidis | 0.006 | 0.047 | 671 | A501V | None | No |
| 35716 | England | 2014 | Blood | Neisseria menigitidis | 0.012 | 0.047 | 24 | A501V | None | No |
| 20401 | England | 2011 | Blood | Neisseria menigitidis | 0.008 | 0.064 | 24 | A501V | None | No |
| 16899 | France | 2004 | CSF | Neisseria menigitidis | NK | 0.094 | 24 | A501V | None | No |
| 63353 | Brazil | 2016 | CSF | Neisseria menigitidis | NK | NK | 61 | A501T | None | Yes |
| 43592 | England | 2014 | Throat | Neisseria lactamica | NK | NK | 605 | A501T | None | Yes |
| 43618 | England | 2015 | Throat | Neisseria lactamica | NK | NK | 611 | A501T | None | Yes |
| 44105 | England | 2015 | Throat | Neisseria lactamica | NK | NK | 611 | A501T | None | Yes |
| 52723 | England | 2015 | Throat | Neisseria lactamica | NK | NK | 611 | A501T | None | Yes |
| 4193 | England | 1999 | Throat | Neisseria menigitidis | NK | NK | 311 | A501V | None | No |
| 7302 | Poland | 2001 | CSF | Neisseria menigitidis | NK | NK | 161 | A501V | None | No |
| 19907 | Sweden | 2010 | Blood | Neisseria menigitidis | NK | NK | 311 | A501V | None | No |
| 20803 | UK | NK | Throat | Neisseria menigitidis | NK | NK | 503 | A501V | None | No |
| 26051 | Ivory Coast | 1998 | NK | Neisseria menigitidis | NK | NK | 24 | A501V | None | No |
| 27068 | Czech Republic | 2013 | Blood | Neisseria menigitidis | NK | NK | 411 | A501V | None | No |
| 29312 | South Africa | 2006 | NK | Neisseria menigitidis | NK | NK | 519 | A501V | None | No |
| 30357 | Australia | 2005 | NK | Neisseria menigitidis | NK | NK | 311 | A501V | None | No |
| 30691 | Ireland | 2014 | Blood | Neisseria menigitidis | NK | NK | 311 | A501V | None | No |
| 31213 | England | 2013 | Blood | Neisseria menigitidis | NK | NK | 161 | A501V | None | No |
| 38224 | Finland | 2015 | NK | Neisseria menigitidis | NK | NK | 660 | A501V | None | No |
| 39150 | Finland | 2013 | NK | Neisseria menigitidis | NK | NK | 311 | A501V | None | No |
| 40185 | France | 2013 | NK | Neisseria menigitidis | NK | NK | 311 | A501V | None | No |
| 42510 | England | 2016 | Blood | Neisseria menigitidis | NK | NK | 24 | A501V | None | No |
| 46605 | Wales | 2015 | Throat | Neisseria menigitidis | NK | NK | 740 | A501V | None | Yes |
| 47204 | Sweden | NK | NK | Neisseria menigitidis | NK | NK | 311 | A501V | None | No |
| 49424 | Wales | 2015 | Throat | Neisseria menigitidis | NK | NK | 740 | A501V | None | No |
| 49490 | Wales | 2015 | Throat | Neisseria menigitidis | NK | NK | 740 | A501V | None | No |
| 52734 | Finland | 2017 | NK | Neisseria menigitidis | NK | NK | 311 | A501V | None | No |
| 56647 | China | 2012 | Blood | Neisseria menigitidis | NK | NK | 311 | A501V | None | No |
| 56697 | Canada | 2004 | NK | Neisseria menigitidis | NK | NK | 24 | A501V | None | No |
| 57042 | England | 1999 | Throat | Neisseria menigitidis | NK | NK | 671 | A501V | None | No |
| 82544 | Finland | NK | NK | Neisseria menigitidis | NK | NK | 311 | A501V | None | No |
| 88917 | Japan | 2007 | Sputum | Neisseria menigitidis | NK | NK | 311 | A501V | None | No |
NK = Not known.
* F504L, A510V, I515V, H541N, I566V.
Ciprofloxacin susceptibility
Five isolates were resistant to ciprofloxacin (0.06–0.5 mg/L; S1 Table) with five different gyrA alleles; gyrA6 (MIC = 0.5 mg/L), gyrA8 (MIC = 0.18 mg/L), gyrA10 (MIC = 0.094 mg/L), gyrA146 (MIC = 0.19 mg/L) and gyrA296 (MIC = 0.06 mg/L). These alleles were not harboured by any other IMD isolate received at the MRU from 2010/11–2018/19.
The gyrA alleles among the ciprofloxacin resistant isolates had AASs D95N (n = 2; MICs = 0.06–0.094 mg/L) or T91I (n = 3; MICs = 0.18–0.5 mg/L). The ciprofloxacin-resistant isolate with the highest ciprofloxacin MIC value (0.5 mg/L) also harboured a mutation in parC (D86N).
Rifampicin susceptibility
Two isolates (ST-11 CC and ST-41/44 CC, respectively) were rifampicin resistant (MICs = 0.5 and >32 mg/L; S1 Table). These harboured rpoB alleles; rpoB238 and rpoB84, respectively. RpoB238 had AAS D542E and RpoB84 had AAS H552N. There was no evidence that suggested either of the two isolates were from close contacts of index cases.
Discussion
From 2010/11 to 2018/19, antibiotic resistance was uncommon among IMD isolates in E, W and NI. However, resistance to all four antibiotics (penicillin, rifampicin, cefotaxime and ciprofloxacin) was observed.
As has been reported in several other countries [20, 23, 34], the proportion of PenR isolates in E, W & NI has increased over time, thus, highlighting the need for surveillance of penicillin susceptibility in order to ensure its continued effective use as a first-line prophylactic agent.
The recent increase was largely due to PenR serogroup W ST-11 CC isolates that have emerged in a lineage that originated in South America in the early 2000s, and that has since spread to Europe, Australasia and North America [35–37]. Since 2016, Australia and the UK have both observed an increase in PenR isolates within the so-called original UK W:cc11 strain or the South American strain sublineage [22, 23]. Reports of penicillin-resistant meningococcal strains causing IMD in complement deficient individuals receiving chemoprophylaxis [38, 39] make this strain particular cause for concern.
Out of the 113 PenR isolates, three had a penA allele (penA327, penA209 and penA171) that did not contain all five of the AASs commonly associated with penicillin resistance (F504L, A510V, I515V, H541N, I566V).
PenA327, (I566V absent), has been previously reported in penicillin-resistant meningococcal isolates and also those with reduced susceptibility to 3GCs [26]. All IMD isolates with penA327 received at the MRU displayed reduced susceptibility to cefotaxime (MICs 0.047–0.125 mg/L).
PenA209 had only three of the five AASs (F504L, A510V, I515V) alongside V447L and A516G. PenA alleles identified on PubMLST that contained F504L, A510V and I515V without V447L and A516 had lower penicillin MICs (0.047–0.094 mg/L) compared to those that also had V447L and A516 (0.12–0.5 mg/L). This may suggest that penA alleles harbouring the three AASs may not be adequate to confer penicillin resistance alone, however, do when in combination with V447 and A516. Future work is needed to confirm the role that these AASs have in conferring resistance to penicillin among meningococci.
PenA171 contained none of the five AASs commonly associated with conferring reduced susceptibility and resistance to penicillin. It did contain P551S, a mutation which has been observed among gonococcal isolates resistant to penicillin [40], however, other meningococcal isolates with penA171 on the PubMLST database were penicillin-susceptible, suggesting penA171, and the P551S AAS alone, are not responsible for causing penicillin resistance among meningococci. Further work is required to determine the cause of penicillin resistance in this isolate which was beta-lactamase negative, as reflected in the relatively low MIC (0.38 mg/L). Other mechanisms of penicillin resistance have been determined among N. gonorrhoeae, which include mutations in the transcriptional repressor of the efflux pump (mtrCDE) operon, which is encoded by the mtrR gene [41]. The operation of the mtrCDE efflux pump in meningococci appears to differ than in that of N. gonorrhoeae [42], with no evidence to suggest that mutations in the mtrR gene of meningococci can cause penicillin resistance.
Only seven isolates had cefotaxime MICs ≥ 0.047 mg/L, one of which was cefotaxime-resistant (MIC = 0.25 mg/L). Five of the seven isolates harboured penA327, which has previously been associated with reduced susceptibility to cefotaxime and penicillin in meningococci. Four of these were serogroup C isolates belonging to ST-11 CC and one was a serogroup B strain belonging to ST-41/44 CC. The allele has been previously identified among ST-11 CC isolates of serogroups B and C, suggesting successful clonal expansion of this particular strain [26]. The allele is identical to that of an allele identified among gonococcal isolates displaying resistance to ceftriaxone in this species [27].
Apart from penA327, no other penA allele has been associated with reduced susceptibility or resistance to 3GCs among meningococci. High levels of 3GC resistance due to A501P have been observed among gonococcal isolates [43]. Other AASs at this position close to the core of the active site motif of PBP2, A501T and A501V, have been described among gonococcal isolates displaying reduced susceptibility to cefotaxime [44–46]. It is thought that the substitution of methyl side chain alanine (A501) interferes with 3GC binding, or leads to structural modifications causing higher levels of 3GC resistance [43].
The cefotaxime-resistant meningococcal isolate identified in this study harboured penA419 with two unique AASs, A501T and D511V, when compared to cefotaxime-susceptible penicillin-resistant isolates. Among other isolates on PubMLST with penA AASs at positions 501 and 511, isolates harbouring A501V had lower MICs than those with A501T. This suggests that AAS A501V does not confer reduced susceptibility to cefotaxime in meningococci, unlike in gonococci [45], whilst A501T may. When A501T was present in combination with an AAS at 511 (D511V or D511G), cefotaxime MICs among the meningococcal isolates were further enhanced. Amino acid substitutions at D511 are extremely rare. Further work is therefore required to confirm the role that this AAS plays in susceptibility to cefotaxime among meningococci. No meningococcal isolates with AAS A501P were identified, however, it has already been suggested that this mutation may eventually be selected in meningococci [28].
Five isolates were ciprofloxacin-resistant (MICs = 0.06–0.5 mg/L). Each of these harboured AASs in the gyrA gene (D95N or T91I) which have previously been associated with conferring ciprofloxacin resistance among meningococcal isolates worldwide [47–49]. The ciprofloxacin-resistant isolate with the highest MIC (MIC = 0.5 mg/L) also harboured an AAS in its parC allele (D86N). Mutations in parC among meningococcal isolates which also harbour mutations in gyrA have been shown to cause enhanced levels of resistance to ciprofloxacin (MICs of 0.5–1 mg/L).
The five ciprofloxacin-resistant isolates received at the MRU belonged to five different clonal complexes. In China, where ciprofloxacin resistance is prevalent, resistance is commonly seen among isolates belonging to ST-4821 CC, which has also been observed among ciprofloxacin-resistant isolates in other countries [10, 48, 50]. In the USA, a recent study identified ciprofloxacin resistance among several meningococcal isolates belonging to ST-23 CC [12]. Since the ciprofloxacin-resistant isolates in the present study do not seem to be prevalent among a certain clonal complex, it may suggest that these particular strains in the UK have not successfully expanded with the altered gyrA alleles and indicates that levels of ciprofloxacin resistance may continue to remain low within the population. Recently however, ciprofloxacin resistance has been observed among a particular strain of NG meningococci of the ST-175 CC in the UK and Europe, causing IMD in immunocompromised individuals. Occasionally, ciprofloxacin may be recommended to immunocompromised individuals or prescribed as a rescue therapy for when symptoms of IMD occur despite other prophylactic regimes [51–53] and so the presence of this non-groupable ST-175 CC strains in the UK remains a concern.
Only two isolates were resistant to rifampicin which is rare among meningococci considering its widespread use as a prophylactic agent. The majority of meningococcal rifampicin resistance is found among isolates from cases of close contacts following rifampicin prophylaxis [15, 17]. However, rifampicin resistance has also been observed among index cases [54]. There was no evidence to suggest that the two rifampicin-resistant isolates received at the MRU were from close contact cases.
One rifampicin-resistant isolate harboured rpoB238 (MIC = 0.5 mg/L), which had AAS 542E. The other rifampicin-resistant isolate rpoB84 (MIC = >32 mg/L), which had AAS H552N. Both AASs have been previously reported among rifampicin-resistant meningococcal isolates, with mutations at AA position 552 associated with higher levels of resistance than those with mutations at AA position 542 [55]. Studies have also identified the same AASs among multiple isolates with varying levels of rifampicin resistance [54, 56]. This, along with the observed difference in rifampicin MIC values displayed by the two resistant isolates in this study, may indicate that additional unknown mechanisms can cause increased levels of rifampicin resistance among meningococci.
This study identified several IMD isolates with resistance to antibiotics used for current treatment and prophylaxis of IMD. Future genetic engineering work is needed to confirm the role that certain penA mutations have in conferring resistance to penicillin and cefotaxime among meningococci. Further molecular work is also required to determine possible alternative penicillin resistance mechanisms of meningococci. This study also identified emerging threats and the progression of antibiotic resistance among strains over time. Consideration should be given before the mass use of antibiotics in single-dose prophylaxis strategies to control outbreaks of meningococcal disease, particularly where outbreaks frequently occur such as in the African meningitis belt [57]. Sustained surveillance of antibiotic resistance among the meningococcal population is essential to maintain successful treatment regimens for IMD and chemoprophylaxis regimes.
Supporting information
(TIF)
(XLSX)
Acknowledgments
This publication made use of the Neisseria Multi Locus Sequence Typing website (https://pubmlst.org/neisseria/) sited at the University of Oxford (Jolley et al. Wellcome Open Res 2018, 3:124). The development of this site has been funded by the Wellcome Trust and European Union. This publication also made use of the Meningitis Research Foundation Meningococcus Genome Library (http://www.meningitis.org/research/genome) developed by Public Health England, the Wellcome Trust Sanger Institute and the University of Oxford as a collaboration. The project is part funded by Meningitis Research Foundation.
Data Availability
Draft genomes are available for all isolates on the Meningitis Research Foundation Meningococcus Genome Library (MGL; https://pubmlst.org/bigsdb?db=pubmlst_neisseria_mrfgenomes; accessed 29th June 2020).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009. Jun;27 Suppl 2:B51–63. doi: 10.1016/j.vaccine.2009.04.063 [DOI] [PubMed] [Google Scholar]
- 2.Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, et al. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: Epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines. 2019. Jan;18(1):15–30. doi: 10.1080/14760584.2019.1557520 [DOI] [PubMed] [Google Scholar]
- 3.Aye AMM, Bai X, Borrow R, Bory S, Carlos J, Caugant DA, et al. Meningococcal disease surveillance in the Asia–Pacific region (2020): The global meningococcal initiative. J Infect. 2020; doi: 10.1016/j.jinf.2020.07.025 [DOI] [PubMed] [Google Scholar]
- 4.Maiden MCJ, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, et al. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A. 1998;95(6):3140–5. doi: 10.1073/pnas.95.6.3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control. Invasive meningococcal disease annual epidemiological report for 2017 [Internet]. Annual epidemiological report on communicable diseases in Europe. 2017. p. 1–10. Available from: http://ecdc.europa.eu/sites/portal/files/documents/AER_for_2017-invasive-meningococcal-disease.pdf 29720073 [Google Scholar]
- 6.Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine. 2019. May;37(21):2768–82. doi: 10.1016/j.vaccine.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence. Meningitis (bacterial) and meningococcal septicaemia in under 16s: recognition, diagnosis and management [Internet]. NICE Clinical guideline [CG102]. 2015. Available from: https://www.nice.org.uk/guidance/CG102/chapter/1-Guidance#pre-hospital-management-of-suspected-bacterial-meningitis-and-meningococcal-septicaemia [Google Scholar]
- 8.Public Health England. Guidance for public health management of meningococcal disease in the UK. Updated August 2019 [Internet]. Publich Health England. 2012. [cited 2021 Mar 26]. p. 18–24. Available from: http://www.hpa.org.uk/webc/hpawebfile/hpaweb_c/1194947389261 [Google Scholar]
- 9.Hellenbrand W, Koch J, Harder T, Bogdan C, Heininger U, Tenenbaum T, et al. Background paper for the update of meningococcal vaccination recommendations in Germany: use of the serogroup B vaccine in persons at increased risk for meningococcal disease. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58(11–12):1314–43. doi: 10.1007/s00103-015-2253-z [DOI] [PubMed] [Google Scholar]
- 10.Zhu B, Fan Y, Xu Z, Xu L, Du P, Gao Y, et al. Genetic diversity and clonal characteristics of ciprofloxacin-resistant meningococcal strains in China. J Med Microbiol. 2014. Nov 1;63:1411–8. doi: 10.1099/jmm.0.078600-0 [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Guo Q, Wang Y, Zou Y, Wang G, Zhang X, et al. Shifts in the antibiotic susceptibility, serogroups, and clonal complexes of Neisseria meningitidis in Shanghai, China: A time trend analysis of the pre-quinolone and quinolone eras. PLoS Med. 2015. Jun 1;12(6):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNamara LA, Potts C, Blain AE, Retchless AC, Reese N, Swint S, et al. Detection of ciprofloxacin-resistant, β-lactamase-producing Neisseria meningitidis serogroup Y isolates—United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):735–9. doi: 10.15585/mmwr.mm6924a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willerton L, Lucidarme J, Campbell H, Caugant DA, Claus H, Jacobsson S, et al. Geographically widespread invasive meningococcal disease caused by a ciprofloxacin resistant non-groupable strain of the ST-175 clonal complex. J Infect [Internet]. 2020;81(4):575–84. Available from: doi: 10.1016/j.jinf.2020.08.030 [DOI] [PubMed] [Google Scholar]
- 14.Chen M, Zhang C, Zhang X, Chen M. Meningococcal quinolone resistance originated from several commensal Neisseria species. Antimicrob Agents Chemother. 2020;64(2):1–12. doi: 10.1128/AAC.01494-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rainbow J, Cebelinski E, Bartkus J, Glennen A, Boxrud D, Lynfield R. Rifampin-resistant meningococcal disease. Emerg Infect Dis [Internet]. 2005;11(6):977–9. Available from: www.cdc.gov/eid doi: 10.3201/eid1106.050143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson S, Fey R, McNulty C. Meningococcal disease in siblings caused by rifampicin sensitive and rifampicin resistant strains. Commun Dis Public Heal. 1999;2(3):215–6. [PubMed] [Google Scholar]
- 17.Taha M, Zarantonelli M, Ruckly C, Giorgini D, Michel Alonso J. Rifampin-resistant Neisseria meningitidis. Emerg Infect Dis [Internet]. 2006;12(5):859–60. Available from: www.cdc.gov/eid doi: 10.3201/eid1205.051296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedberg ST, Fredlund H, Nicolas P, Caugant DA, Olcén P, Unemo M. Antibiotic susceptibility and characteristics of Neisseria meningitidis isolates from the African meningitis belt, 2000 to 2006: Phenotypic and genotypic perspectives. Antimicrob Agents Chemother. 2009 Apr;53(4):1561–6. doi: 10.1128/AAC.00994-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorla MC, Pinhata JMW, Dias UJ, de Moraes C, Lemos AP. Surveillance of antimicrobial resistance in Neisseria meningitidis strains isolated from invasive cases in Brazil from 2009 to 2016. J Med Microbiol. 2018 Jun;67(6):750–6. [DOI] [PubMed] [Google Scholar]
- 20.Vacca P, Fazio C, Neri A, Ambrosio L, Palmieri A, Stefanelli P. Neisseria meningitidis antimicrobial resistance in Italy, 2006 to 2016. Antimicrob Agents Chemother. 2018;62(9):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.du Plessis M, von Gottberg A, Cohen C, de Gouveia L, Klugman KP. Neisseria meningitidis intermediately resistant to penicillin and causing invasive disease in South Africa in 2001 to 2005. J Clin Microbiol [Internet]. 2008 Oct 1;46(10):3208–14. Available from: http://jcm.asm.org/content/46/10/3208.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willerton L, Lucidarme J, Walker A, Lekshmi A, Clark SA, Gray SJ, et al. Increase in penicillin-resistant invasive meningococcal serogroup W ST-11 complex isolates in England. Vaccine [Internet]. 2021; Available from: https://www.sciencedirect.com/science/article/pii/S0264410X2100270X doi: 10.1016/j.vaccine.2021.03.002 [DOI] [PubMed] [Google Scholar]
- 23.Mowlaboccus S, Jolley KA, Bray JE, Pang S, Lee YT, Bew JD, et al. Clonal expansion of new penicillin-resistant clade of Neisseria meningitidis serogroup W clonal complex 11, Australia. Emerg Infect Dis. 2017. Aug 1;23(8):1364–7. doi: 10.3201/eid2308.170259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manchanda V, Bhalla P. Emergence of non-ceftriaxone-susceptible Neisseria meningitidis in India. Vol. 44, Journal of clinical microbiology. 2006. p. 4290–1. doi: 10.1128/JCM.01903-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolas P. Emergence of non-ceftriaxone-susceptible Neisseria meningitidis in India. J Clin Microbiol [Internet]. 2007. Apr 1;45(4):1378 LP– 1378. Available from: http://jcm.asm.org/content/45/4/1378.abstract doi: 10.1128/JCM.02495-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deghmane AE, Hong E, Taha MK. Emergence of meningococci with reduced susceptibility to third-generation cephalosporins. J Antimicrob Chemother. 2017;72(1):95–8. doi: 10.1093/jac/dkw400 [DOI] [PubMed] [Google Scholar]
- 27.Ohnishi M, Saika T, Hoshina S, Iwasaku K, Nakayama S, Watanabe H, et al. Ceftriaxone-resistant Neisseria gonorrhoeae. Vol. 17, Emerging infectious diseases. 2011. p. 148–9. doi: 10.3201/eid1701.100397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zapun A, Morlot C, Taha M-K. Resistance to β-lactams in Neisseria spp due to chromosomally encoded penicillin-binding proteins. Antibiotics. 2016. Sep 28;5(4):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray SJ, Trotter CL, Ramsay ME, Guiver M, Fox AJ, Borrow R, et al. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol. 2006. Jul;55(Pt 7):887–96. [DOI] [PubMed] [Google Scholar]
- 30.European Committee on Antimicrobial Susceptibility Testing. New definitions of S, I and R from 2019 [Internet]. 2019 [cited 2021 Mar 26]. Available from: https://www.eucast.org/newsiandr/
- 31.Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome open Res. 2018;3:124. doi: 10.12688/wellcomeopenres.14826.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill DMC, Lucidarme J, Gray SJ, Newbold LS, Ure R, Brehony C, et al. Genomic epidemiology of age-associated meningococcal lineages in national surveillance: An observational cohort study. Lancet Infect Dis. 2015. Dec 1;15(12):1420–8. doi: 10.1016/S1473-3099(15)00267-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taha MK, Vázquez JA, Hong E, Bennett DE, Bertrand S, Bukovski S, et al. Target gene sequencing to characterize the penicillin G susceptibility of Neisseria meningitidis. Antimicrob Agents Chemother. 2007. Aug;51(8):2784–92. doi: 10.1128/AAC.00412-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertrand S, Carion F, Wintjens R, Mathys V, Vanhoof R. Evolutionary changes in antimicrobial resistance of invasive Neisseria meningitidis isolates in Belgium from 2000 to 2010: Increasing prevalence of penicillin nonsusceptibility. Antimicrob Agents Chemother. 2012;56(5):2268–72. doi: 10.1128/AAC.06310-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fine A, Layton M, Hakim A, Smith P. Serogroup W-135 meningococcal disease among travelers returning From Saudi Arabia—United States, 2000. Morb Mortal Wkly Rep. 2000;49(16):345–6. [PubMed] [Google Scholar]
- 36.Aguilera JF, Perrocheau A, Meffre C, Hahné S. Outbreak of serogroup W135 meningococcal disease after the Hajj pilgrimage, Europe, 2000. Emerg Infect Dis. 2002;8(8):761–7. doi: 10.3201/eid0808.010422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ladhani SN, Beebeejaun K, Lucidarme J, Campbell H, Gray S, Kaczmarski E, et al. Increase in endemic neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis. 2015. Feb 15;60(4):578–85. doi: 10.1093/cid/ciu881 [DOI] [PubMed] [Google Scholar]
- 38.McNamara LA, Topaz N, Wang X, Hariri S, Fox L, Macneil JR. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. Morb Mortal Wkly Rep. 2017;66(27):734–7. doi: 10.15585/mmwr.mm6627e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh SR, Lucidarme J, Bingham C, Warwicker P, Goodship T, Borrow R, et al. Meningococcal B vaccine failure with a penicillin-resistant strain in a young adult on long-term eculizumab. Pediatrics. 2017. Aug 11;140(3):1–4. [DOI] [PubMed] [Google Scholar]
- 40.Whiley DM, Goire N, Lambert SB, Ray S, Limnios EA, Nissen MD, et al. Reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae is associated with mutations G542S, P551S and P551L in the gonococcal penicillin-binding protein 2. J Antimicrob Chemother. 2010. Aug;65(8):1615–8. doi: 10.1093/jac/dkq187 [DOI] [PubMed] [Google Scholar]
- 41.Maness MJ, Sparling PF. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J Infect Dis [Internet]. 1973. Sep 1;128(3):321–30. Available from: doi: 10.1093/infdis/128.3.321 [DOI] [PubMed] [Google Scholar]
- 42.Rouquette-Loughlin CE, Balthazar JT, Hill SA, Shafer WM. Modulation of the mtrCDE-encoded efflux pump gene complex of Neisseria meningitidis due to a Correia element insertion sequence. Mol Microbiol. 2004;54(3):731–41. doi: 10.1111/j.1365-2958.2004.04299.x [DOI] [PubMed] [Google Scholar]
- 43.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother. 2012. Mar;56(3):1273–80. doi: 10.1128/AAC.05760-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whiley DM, Limnios EA, Ray S, Sloots TP, Tapsall JW. Diversity of penA alterations and subtypes in Neisseria gonorrhoeae strains from Sydney, Australia, that are less susceptible to ceftriaxone. Antimicrob Agents Chemother. 2007. Sep;51(9):3111–6. doi: 10.1128/AAC.00306-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomberg J, Fedarovich A, Vincent LR, Jerse AE, Unemo M, Davies C, et al. Alanine 501 mutations in penicillin-binding protein 2 from Neisseria gonorrhoeae: Structure, mechanism, and effects on cephalosporin resistance and biological fitness. Biochemistry. 2017. Feb 28;56(8):1140–50. doi: 10.1021/acs.biochem.6b01030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomberg J, Unemo M, Davies C, Nicholas RA. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry. 2010. Sep;49(37):8062–70. doi: 10.1021/bi101167x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong E, Thulin Hedberg S, Abad R, Fazio C, Enríquez R, Deghmane AE, et al. Target gene sequencing to define the susceptibility of Neisseria meningitidis to ciprofloxacin. Antimicrob Agents Chemother. 2013. Apr;57(4):1961–4. doi: 10.1128/AAC.02184-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsang RSW, Law DKS, Deng S, Hoang L. Ciprofloxacin-resistant Neisseria meningitidis in Canada: Likely imported strains. Vol. 63, Canadian Journal of Microbiology. Canadian Science Publishing; 2017. p. 265–8. doi: 10.1139/cjm-2016-0716 [DOI] [PubMed] [Google Scholar]
- 49.Skoczyńska A, Alonso J-M, Taha M. Ciprofloxacin resistance in Neisseria meningitidis, France. Emerg Infect Dis. 2008. Sep 26;14(8):1322–3. doi: 10.3201/eid1408.080040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawasaki Y, Matsubara K, Takahashi H, Morita M, Ohnishi M, Hori M, et al. Invasive meningococcal disease due to ciprofloxacin-resistant Neisseria meningitidis sequence type 4821: The first case in Japan. J Infect Chemother. 2018. Apr 1;24(4):305–8. doi: 10.1016/j.jiac.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 51.Struijk GH, Bouts AHM, Rijkers GT, Kuin EAC, Ten Berge IJM, Bemelman FJ. Meningococcal sepsis complicating eculizumab treatment despite prior vaccination. Am J Transplant. 2013;13(3):819–20. doi: 10.1111/ajt.12032 [DOI] [PubMed] [Google Scholar]
- 52.Noone D, Al-Matrafi J, Tinckam K, Zipfel PF, Herzenberg AM, Thorner PS, et al. Antibody mediated rejection associated with complement factor H-related protein 3/1 deficiency successfully treated with Eculizumab. Am J Transplant. 2012;12(9):2546–53. doi: 10.1111/j.1600-6143.2012.04124.x [DOI] [PubMed] [Google Scholar]
- 53.Hawkins K, Hoffman M, Okuyama S, Rowan S. A case of fulminant meningococcemia: It is all in the complement. BMJ Case Rep. 2017;2017:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stefanelli P, Fazio C, La Rosa G, Marianelli C, Muscillo M, Mastrantonio P. Rifampicin resistant meningococci causing invasive disease detection of point mutations in the rpoB gene. J Antimicrob Chemother. 2001;47:219–212. [DOI] [PubMed] [Google Scholar]
- 55.Taha MK, Hedberg ST, Szatanik M, Hong E, Ruckly C, Abad R, et al. Multicenter study for defining the breakpoint for rifampin resistance in Neisseria meningitidis by rpoB sequencing. Antimicrob Agents Chemother. 2010. Sep;54(9):3651–8. doi: 10.1128/AAC.00315-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carter PE, Abadi FJR, Yakubu DE, Pennington TH. Molecular characterization of rifampin-resistant Neisseria meningitidis. Antimicrob Agents Chemother. 1994;38(6):1256–61. doi: 10.1128/AAC.38.6.1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coldiron ME, Assao B, Page A-L, Hitchings MDT, Alcoba G, Ciglenecki I, et al. Single-dose oral ciprofloxacin prophylaxis as a response to a meningococcal meningitis epidemic in the African meningitis belt: A 3-arm, open-label, cluster-randomized trial. PLoS Med [Internet]. 2018. Jun 26;15(6):e1002593–e1002593. Available from: https://pubmed.ncbi.nlm.nih.gov/29944651 doi: 10.1371/journal.pmed.1002593 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(XLSX)
Data Availability Statement
Draft genomes are available for all isolates on the Meningitis Research Foundation Meningococcus Genome Library (MGL; https://pubmlst.org/bigsdb?db=pubmlst_neisseria_mrfgenomes; accessed 29th June 2020).