Abstract
Now-a-days, plant-based extracts, as a cheap source of growth activators, are being widely used to treat plants grown under extreme climatic conditions. So, a trial was conducted to assess the response of two maize (Zea mays L.) varieties, Sadaf (drought tolerant) and Sultan (drought sensitive) to foliar-applied sugar beet extract (SBE) under varying water-deficit conditions. Different SBE (control, 1%, 2%, 3% & 4%) levels were used in this study, and plants were exposed to water-deficit [(75% and 60% of field capacity (FC)] and control (100% FC) conditions. It was observed that root and shoot dry weights (growth), total soluble proteins, RWC-relative water contents, total phenolics, chlorophyll pigments and leaf area per plant decreased under different water stress regimes. While, proline, malondialdehyde (MDA), RMP-relative membrane permeability, H2O2-hydrogen peroxide and the activities of antioxidant enzymes [CAT-catalase, POD-peroxidase and SOD-superoxide dismutase] were found to be improved in water stress affected maize plants. Exogenous application of varying levels of SBE ameliorated the negative effects of water-deficit stress by enhancing the growth attributes, photosynthetic pigments, RWC, proline, glycinebetaine (GB), activities of POD and CAT enzymes and levels of total phenolics, whereas it reduced the lipid peroxidation in both maize varieties under varying water stress levels. It was noted that 3% and 4% levels of SBE were more effective than the other levels used in enhancing the growth as well as other characteristics of the maize varieties. Overall, the sugar beet extract proved to be beneficial for improving growth and metabolism of maize plants exposed to water stress.
Introduction
Water-deficit stress is one of the most crucial environmental cues for growth and yield outcomes of crops grown either in natural or agricultural systems, because an optimum amount of water is essential for the normal functioning of all metabolic activities taking place within the cells or tissues [1,2]. Water stress induces osmotic stress, overproduces reactive oxygen species (ROS), causes stomatal closure, perturbs carbohydrate assimilation, alters gas exchange characteristics and nutrients’ uptake. All individually or in combination are the major functions which alter the growth and yield production of major crops [3–7]. Owing to stressful conditions, the accumulation of ROS in the cellular organelles, e.g., mitochondria, chloroplasts, and peroxisomes results in membrane damage, ion leakage and inactivation of key enzymes [8–11].
Stress resistant plants can regulate their growth and development by improving the rates of photosynthesis, ion flux, respiration, carbohydrate metabolism, upregulating oxidative defense system, and enhancing the levels of plant growth promoters that usually undergo impairment under stressful environments [12,13]. It is now well evident that some metabolites including total phenolics, GB, carbohydrates and proline help sustain plant growth by improving drought tolerance and neutralizing ROS [14,15]. The scavenging network of ROS which includes enzymatic antioxidants such as catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), and ascorbate peroxidase (APX), and non-enzymatic antioxidants including glutathione, tocopherols, ascorbate (non-enzymatic) antioxidants helps mediate the harmful effects of stressful factors in plants [16–20].
Plant based extracts are natural sources of organic and inorganic compounds that can be used exogenously to improve stress tolerance of plants [21]. Sugar beet (Beta vulgaris L.) extract is rich in flavonoids, phenolics, ascorbic acid, carotenoids and GB [22]. Exogenous application of SBE has been used to neutralize the negative influences of water stress conditions on plants [22,23]. Recently, foliar application of SBE has been shown to enhance growth, photosynthesis, antioxidants and yield of wheat plants subjected to water stress conditions [22]. Glycinebetaine is a well-known osmoprotectant that enables plants to sustain growth under stress conditions. It has multiple roles in the survival of different plants under non-stress and stress conditions [24,25]. Foliar application of glycinebetaine usually increases endogenous GB concentration in GB low- or non-accumulator plant species and improves growth and yield by counteracting the adverse effects of stress conditions [26,27]. It has been reported that GB mostly translocates to young tissues rather than older ones [26,28]. Earlier studies somehow prove the promotive effect of exogenous application of GB on yield, growth as well as different physio-biochemical attributes involved in stress tolerance of many crops such as wheat, sunflower, maize, tobacco, pea, etc. [29–33].
Maize (Zea mays L.) plant generally requires a high amount of water to complete its life cycle. However, when the crop is subjected to water deficit conditions, its growth and reproduction are adversely affected. Thus, for cultivating this crop in water deficit regions, some cost-effective and efficient measures must be undertaken. One of the key means of improving crop stress tolerance is the exogenous application of growth regulator substances, inorganic or organic in nature. As stated earlier, sugar beet extract has been reported to contain a significant amount of natural GB along with a variety of inorganic nutrients and organic substances [34,35]. In a previous study with okra, Habib, Ashraf [34] have shown that the sugar beet extract as a foliar spray ameliorated the negative effects of salinity on this potential vegetable crop. Thus, in the present study, we applied fresh extract of sugar beet to maize plants grown under water-deficit conditions and observed alterations occurring in different physiological and metabolic processes in maize plants.
Materials and methods
To assess the antioxidative defense system in maize (Zea mays L.) plants, an experiment was carried-out under natural environment. So, seeds of selected maize varieties [36] viz. Sadaf (drought tolerant) and Sultan (drought sensitive) were soaked in water before germination. Then eight seeds were planted per plastic pot having 8 kg soil. The properties of sandy-loam experimental soil were as follows: EC, 2.09 dS m-1; pH, 7.45; P, 2.53 mg kg-1; K, 159 mg kg-1; organic matter, 0.92%; saturation, 35%; sand,48.7%; silt, 23.4%; clay, 27.4%; Zn, 0.46 mg kg-1; iron, 2.06 mg kg-1 and copper, 0.17 mg kg-1. Following one week of seed germination, seedlings were uniformly maintained to five seedlings per pot by hand thinning. Plants were subjected to different water stress (100%, 75% and 60% of FC) levels after two weeks of germination. Field capacity was calculated on the basis of moisture contents and the saturation percentage of the soil used. Common beet (Beta vulgaris L.) known as sugar beet was obtained from the local market of Faisalabad, Pakistan. Different concentrations (control, 1%, 2%, 3% & 4%) of SBE were applied as a foliar spray after two weeks of water deficit treatment. Half a liter of each solution concentration was prepared and 4% was used as a stock solution to prepare other concentrations. Tween-20 was mixed to each solution and an aliquot (10 mL) of the solution was applied per plant using a plastic sprayer. Two plants were harvested from each replicate after 14 days of exogenous treatment. The plant samples were washed with deionized water and growth parameters including root and shoot fresh and dry weights and leaf area per plant were determined. The following attributes were also measured on the harvested samples:
Chlorophyll contents
A young top 3rd leaf (500 mg) was extracted in 80% of acetone. To determine chlorophyll contents, optical densities of all extracts were observed using a spectrophotometer at 663 and 645 nm following Arnon [37].
Relative water contents (RWC)
Fresh weights of young leaf samples were measured and dipped in water for an hour to note turgid weights. Then, the leaf samples were dried for appraising dry weights. The RWCs of young leaf samples were calculated according to Barrs and Weatherley [38].
Relative membrane permeability (RMP)
Following the protocol of Yang, Rhodes [39], RMP was measured. A leaf (0.5 g) was taken and dipped in water to record EC0 using an EC meter. Then, EC1 was measured after keeping samples for 24 h at 4°C. Then, EC2 was noted after incubating the samples.
Proline
Sulfo-salicylic acid (3%) was used to grind a fresh leaf (0.5 g), and proline contents were determined following the protocol of Bates, Waldren [40]. To the filtrate, glacial acetic acid (2 mL) and acid ninhydrin (2 mL) were added. The mixtures were kept in a water bath at 100°C for half an hour. After cooling, 4 mL toluene were added and shaken for 30 sec and absorbance recorded at 520 nm. For proline estimation, standard solutions of varying concentrations of proline (10–100 ppm) were used to draw a standard curve.
Glycinebetaine
A fresh leaf (0.5 g) was homogenized with 0.5% toluene. After filtration, sulfuric acid (1 mL; 2 N) was added to 1 mL of the sample extract. Then, KI3 (0.2 mL) was mixed, cooled for 90 min and 1, 2 dichloroethane (5 mL) along with 2.8 mL of distilled water were added to the reacted sample. The absorbance of the lower layer was recorded at 365 nm Grieve and Grattan [41].
Malondialdehyde (MDA)
Following Cakmak and Horst [42], the lipid peroxidation induced by water deficit conditions in the maize plants was determined. Fresh leaf (0.25 g) was homogenized with TCA (3 mL; 1%). To 1.0 mL of the filtrate, thiobarbituric acid (4 mL; 0.5% in 20% TCA) was added and incubated at 95°C for 50 min. The OD was observed at 532 and 600 nm.
Hydrogen peroxide (H2O2)
A young leaf (0.25 g) was extracted in 0.1% of TCA (5 mL). To 0.5 mL leaf extract, 500 μL potassium phosphate buffer and 1 mL potassium iodide were mixed. The samples were vortexed and hydrogen peroxide contents measured at 390 nm Velikova, Yordanov [43].
Total phenolics
A leaf (100 mg) was ground in 80% of acetone (5 mL) and the mixture was centrifuged. Then, 0.1 mL aliquot, 1 mL Folin-Ciocalteau’s reagent and 2 mL of deionized water were mixed. After shaking, 20% of sodium carbonate (5 mL) was added and maintained final volume up to 10 mL using distilled water. Total phenolics were measured at 750 nm [44].
Ascorbic acid (AsA)
Following Mukherjee and Choudhuri [45], ascorbic acid contents in the maize leaf (0.25 g) were measured by extracting in 10 mL of TCA (6%). One drop of thiourea (10%; used 70% ethanol) along with 2% dinitrophenyl hydrazine (2 mL in 9 N H2SO4) was added to four mL of the sample aliquot. The solution was incubated for 15 min and cooled. The OD was measured at 530 nm after the addition of 80% H2SO4 (5 mL).
Enzymatic antioxidants
Fresh leaf tissue (each 500 mg) was ground in 10 mL phosphate buffer having pH 7.8 and the extract was used for the determination of activities of antioxidant enzymes, i.e., CAT, SOD and POD. The activities of POD and CAT enzymes were measured following Chance and Maehly [46]. However, the activity of superoxide dismutase enzyme was determined according to the protocol of Giannopolitis and Ries [47].
Activity of SOD enzyme
In a cuvette, deionized water (0.4 mL), phosphate buffer (0.25 mL), triton-X (0.1 mL; 0.1%), L-methionine (0.1 mL; 13 mM), nitroblue tetrazolium (0.05 mL), riboflavin (50 μL of 1.3 μM) and enzyme extract (50 μL) were added. The OD of the samples was noted at 560 nm after 15 minutes.
Activity of POD enzyme
The activity of peroxidase (POD) enzyme was determined by preparing a reaction mixture (100 μL of 0.5% H2O2, 100 μL of 0.5% guaiacol, 1.8 mL phosphate buffer and 100 μL enzyme extract). The OD of this mixture was noted at 470 nm for 30 sec. time internal up to 3.0 minutes.
Activity of CAT enzyme
To 100 μL of the extract, H2O2 (1 mL of 5.9 mM) and phosphate buffer (50 mM; 1.9 mL) were added. The absorbance of each treated sample was noted at 240 nm for 180 seconds.
Total soluble proteins
For this, the method of Bradford [48] was followed. To 100 μL aliquot of the sample, 2.0 mL of the Bradford reagent were mixed, and the OD was read at 590 nm.
Statistical analysis
The obtained data were analyzed using a statistical package (Cohort software, Costat V6.303) to work out analysis of variance. LSD (least significance difference) test was used to compare the means of all treatments following Snedecor and Cochran [49].
Results
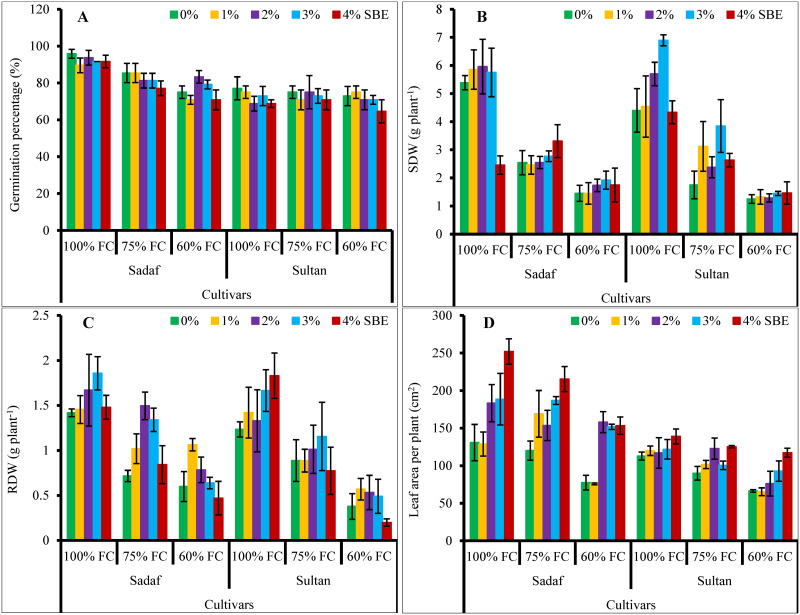
Data for growth characteristics showed that shoot and root dry biomass decreased noticeably (P ≤ 0.001) under different water-deficit conditions. However, exogenously applied SBE at different levels were effective in enhancing the growth attributes of both maize varieties under water-deficit stress (Fig 1). SBE at the concentration of 3% was more effective in enhancing shoot fresh and dry weights, while 4% for leaf area of the water stressed plants. The response of both maize varieties to water stress conditions varied and var. Sadaf was better than var. Sultan at varying water-deficit regimes (Table 1).
Fig 1. Effect of varying levels of sugar beet extract (SBE) on morphological attributes of maize plants subjected to water-deficit stress (n = 3; Mean ± S.E.).
Table 1. Three-way ANOVA of data for morphological and biochemical variables of water stressed plants of two maize varieties subjected to varying (1%, 2%, 3% & 4%) levels of sugar beet extract (SBE).
| Source of variation | Df | Shoot dry weight | Root dry weight | Chl. a | Chl. b | Total Chl | Chl a/b ratio |
| Water stress (WS) | 2 | 135. 5*** | 9.306*** | 2.185*** | 1.733*** | 6.994*** | 0.566** |
| SBE | 4 | 4. 594** | 0. 437* | 0.403*** | 0.835*** | 2. 275*** | 0.144ns |
| Varieties (Var) | 1 | 0.126ns | 0.822* | 4.137*** | 3.503*** | 15.25*** | 0.423* |
| WS x Var | 2 | 0.442ns | 0. 102ns | 0.766*** | 1.241*** | 3. 957*** | 0.126ns |
| WS x SBE | 8 | 3.573** | 0. 235ns | 0.141*** | 0.205** | 0.426*** | 0.204* |
| Var x SBE | 4 | 1.504ns | 0.115ns | 0.111** | 0.098ns | 0. 199ns | 0.090ns |
| WS x SBE x Var | 8 | 1.844ns | 0. 084ns | 0.186*** | 0.294*** | 0.695*** | 0.168* |
| Error | 90 | 1.114 | 0.163 | 0.030 | 0.062 | 0.082 | 0.077 |
| Df | Leaf area | Relative Water Content | Relative Membrane Permeability | Proline | Glycinebetaine | Malondialdehyde | |
| Water stress (WS) | 2 | 23076.9*** | 1453.4*** | 4197.7*** | 2.097** | 38.53ns | 37.52* |
| Sugar beet extract (SBE) | 4 | 16942.8*** | 1573.9*** | 1453. 5*** | 2.934*** | 437.6*** | 41. 33** |
| Varieties (Var) | 1 | 80272.2*** | 1011.3*** | 403. 3ns | 0.294ns | 289.1*** | 21.67ns |
| WS x Var | 2 | 1197.4ns | 251.8*** | 3291.4*** | 0.285ns | 2115.4*** | 0.495ns |
| WS x SBE | 8 | 865.5ns | 109.7*** | 149.1ns | 0.496ns | 46.96* | 32. 73** |
| Var x SBE | 4 | 4117.9** | 68.47* | 146.7ns | 0.912ns | 131.9*** | 18. 87ns |
| WS x SBE x Var | 8 | 1526.3ns | 31.83ns | 129. 9ns | 0.353ns | 162.3*** | 0.811ns |
| Error | 90 | 908.1 | 22.31 | 107.8 | 0.370 | 18.20 | 10.21 |
| df | Hydrogen peroxide | Total phenolics | Ascorbic acid | Catalase | Peroxidase | Superoxide dismutase | |
| Water stress (WS) | 2 | 269135.6*** | 41.78*** | 71.79*** | 44.07*** | 70.13*** | 481. 54*** |
| Sugar beet extract (SBE) | 4 | 40651.2ns | 15.50*** | 20.22*** | 18.22** | 28.09* | 25.38ns |
| Varieties (Var) | 1 | 277512.9*** | 12.96** | 5.789ns | 105. 8*** | 2546.2*** | 14.35ns |
| WS x Var | 2 | 319739.4*** | 4.084* | 19.45** | 2.724ns | 19.82ns | 43. 58* |
| WS x SBE | 8 | 118127.3***ns | 0.795ns | 1.407ns | 2. 176ns | 24.25** | 5. 126ns |
| Var x SBE | 4 | 26569.1ns | 1.316ns | 4.289ns | 1.056ns | 29.03* | 8.392ns |
| WS x SBE x Var | 8 | 87365. 02**** | 0.886ns | 1.452ns | 0.997ns | 33.73*** | 4.764ns |
| Error | 90 | 23833. 4 | 1.236 | 3.102 | 3. 865 | 8.844 | 12. 06 |
*, 0.05; **, 0.01; ***, 0.001 significance levels; ns, non significant.
Leaf area per plant was found to be significantly suppressed by water stress conditions. However, SBE spray under stress conditions remarkably (P ≤ 0.001, Table 1, Fig 1) enhanced the leaf area per plant in both maize varieties. Sadaf was better in leaf area than var. Sultan. The effects of maize varieties and that of the SBE treatment were significant.
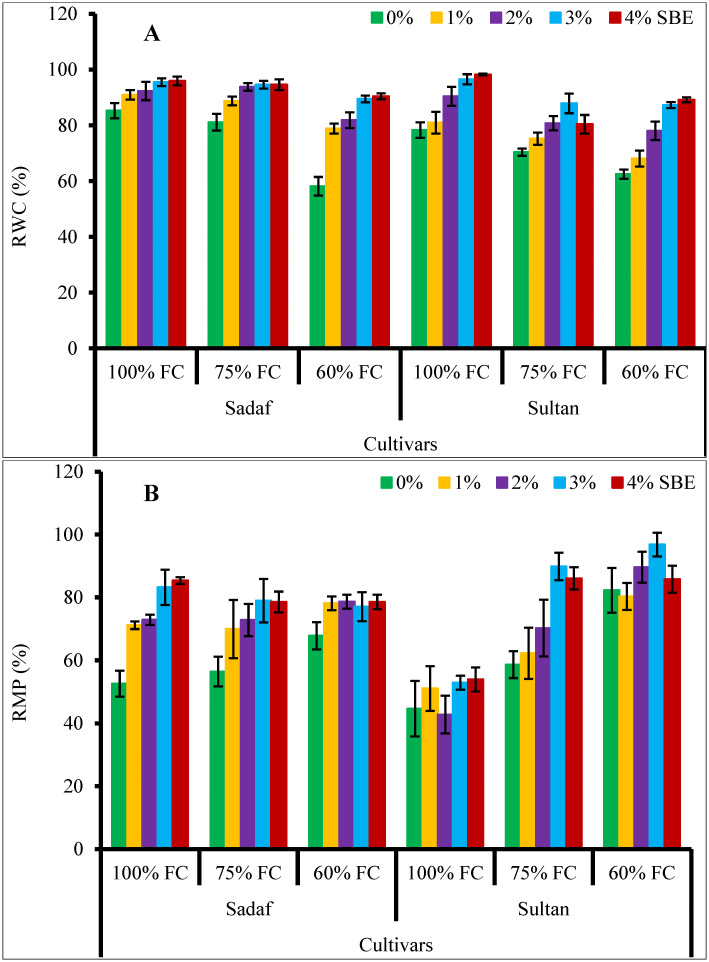
Relative water contents in the maize plants declined significantly (P ≤ 0.001) under water stress conditions. Foliar-applied SBE enhanced the RWC in all maize plants under water stress conditions (Fig 2, Table 1). Sadaf showed higher RWC compared to var. Sultan under varying watering regimes.
Fig 2. Effect of SBE on (A) RWC and (B) RMP of maize plants subjected to water-deficit stress (n = 3; Mean ± S.E.).
Water-deficit stress considerably (P ≤ 0.001, Table 1) increased the RMP in both maize varieties. While, SBE application at different (1% & 4%) concentrations reduced the RMP in var. Sultan under water stress (60% FC) conditions (Fig 2).
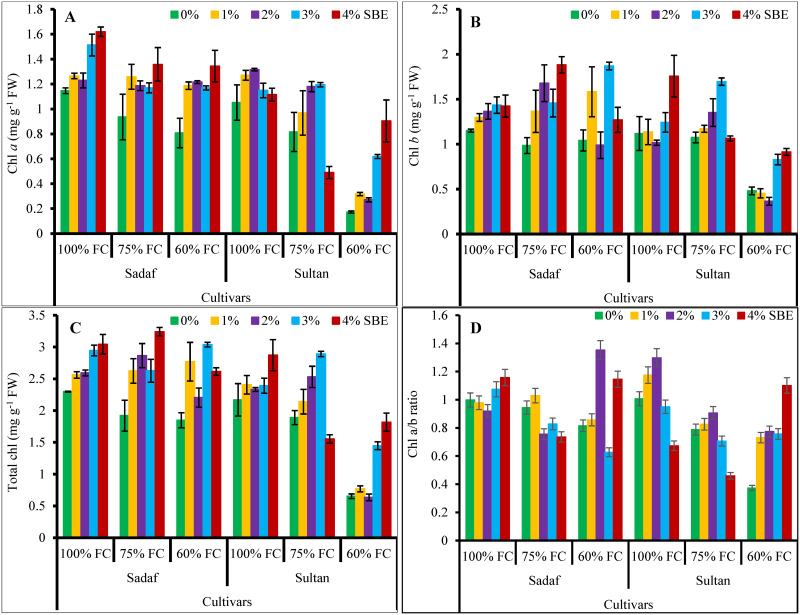
Chlorophyll pigments (a, b, total chlorophyll, a/b ratio) decreased significantly (P ≤ 0.001) under varying watering regimes. Foliar application of SBE at different concentrations improved the chlorophyll a, b and total chlorophyll under water-deficit stress (Table 1, Fig 3). While, no change was noted on chlorophyll a/b ratio. Both maize varieties showed consistency in all these pigment attributes (Fig 3). The interaction of SBE treatment, water stress and varieties was also significant.
Fig 3. Restoration of chlorophyll pigments by supplementation of SBE of maize plants subjected to water-deficit stress (n = 3; Mean ± S.E.).
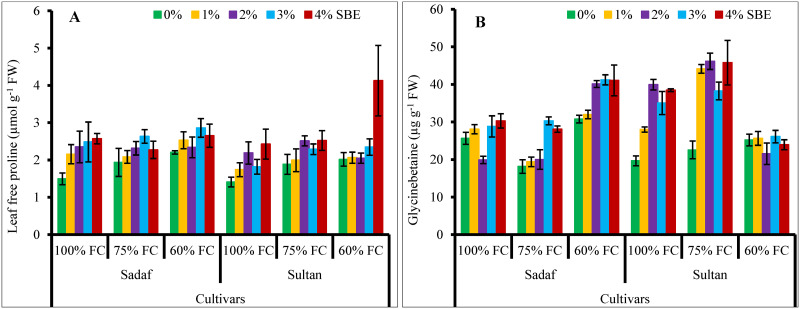
Leaf free proline contents in both maize varieties increased significantly (P ≤ 0.01) under water stress. Foliage spray by SBE appreciably (P ≤ 0.001, Fig 4) enhanced the proline accumulation in both maize varieties under water stress conditions. Both varieties were similar in proline contents under water-deficit conditions.
Fig 4. Application of SBE enhanced (A) leaf free proline and (B) glycinebetaine contents (GB) of maize plants subjected to water-deficit stress (n = 3; Mean ± S.E.).
No change in GB accumulation was noticed in maize plants under water stress. However, foliar-applied SBE noticeably (Table 1) enhanced the GB concentrations in both maize varieties under water stress. High GB contents were observed in var. Sadaf at 60% FC and in var. Sultan at 75% FC. The varieties, SBE treatment and water stress showed a significant interaction (Fig 4).
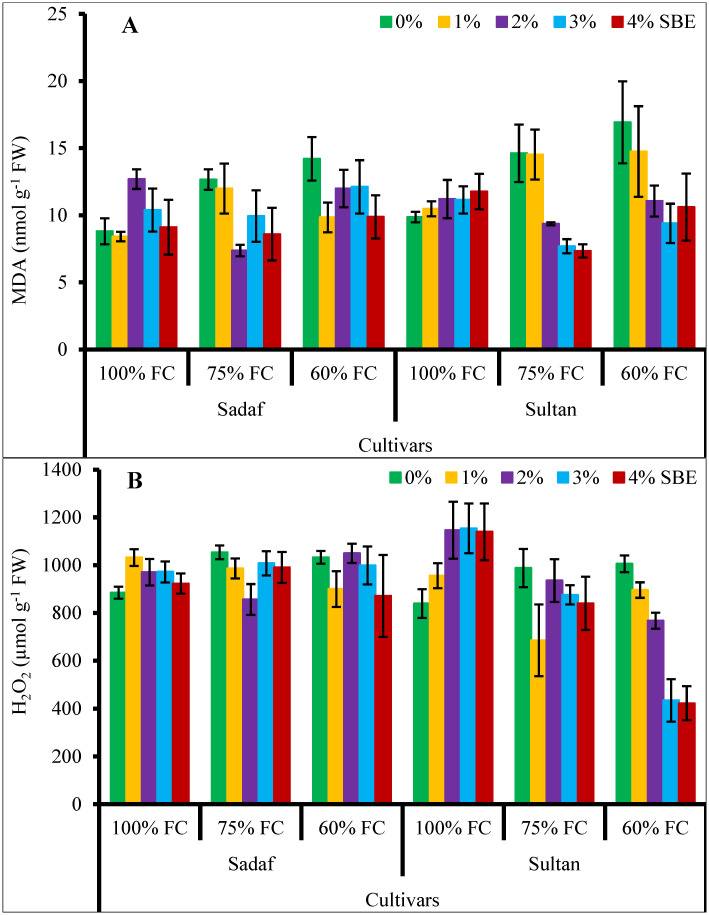
Malondialdehyde (MDA) contents in the maize varieties were found to be improved (P ≤ 0.05) due to water-deficit stress. Exogenously applied SBE considerably (P ≤ 0.01, Fig 2) suppressed the MDA contents in both maize varieties under water stress conditions. Sultan had higher MDA contents than those of Sadaf under water-deficit stress (Table 1, Fig 5).
Fig 5. Sugar beet extract (SBE) regulates accumulation of (A) malondialdehyde (MDA) and (B) hydrogen peroxide (H2O2) content of maize plants subjected to water-deficit stress (n = 3; Mean ± S.E.).
Hydrogen peroxide contents in the maize plants noticeably (P ≤ 0.001) enhanced under different watering regimes. Foliar application of SBE did not affect the H2O2 contents in all maize plants. Both maize varieties responded differentially under water deficit conditions and var. Sadaf accumulated relatively more H2O2 contents under water deficit conditions. The relationship among SBE application, water stress and varieties was significant (Table 1, Fig 5).
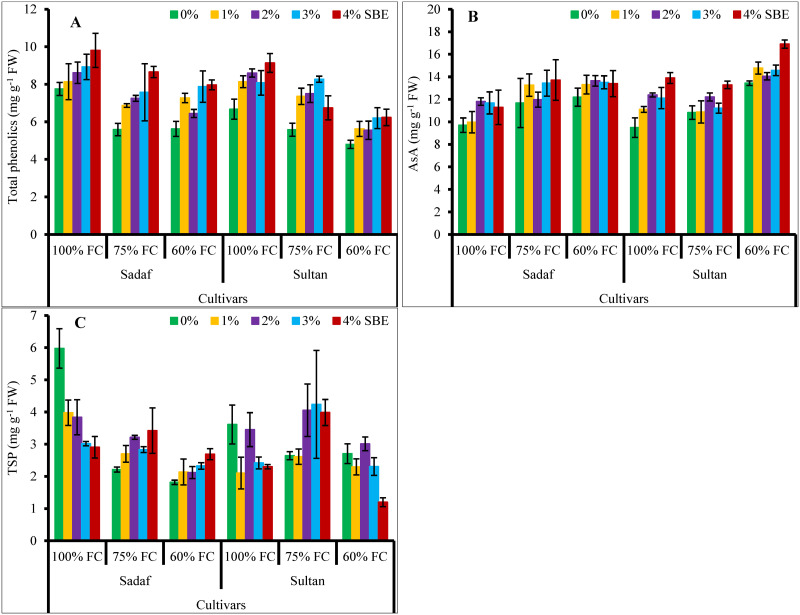
Water deficit conditions considerably (P ≤ 0.001) suppressed the total phenolics of the maize varieties. However, SBE applied as a foliar spray enhanced the total phenolics in the maize plants under water-deficit stress. Variety Sultan showed higher concentration of total phenolics under stress conditions compared with that in the other cultivar (Table 1, Fig 6).
Fig 6. Effect of sugar beet extract (SBE) on (A) total phenolics, (B) ascorbic acid (AsA) and (C) total soluble proteins (TSP) of maize plants subjected to water-deficit stress (n = 3; Mean ± S.E.).
Ascorbic acid (AsA) contents of the maize varieties significantly (P ≤ 0.001) improved under water-deficit stress as well as foliar application of SBE. The interface between varieties and water stress was significant (Fig 6). Both the varieties showed similar AsA contents in response to varying watering regimes.
Water-deficit stress considerably (P ≤ 0.001) decreased the total soluble proteins (TSP) in maize plants (Fig 6). While, SBE at different (1%, 2%, 3% & 4%) concentrations improved TSP in var. Sadaf at 75% and 60% FC. While in var. Sultan, SBE (2%, 3% & 4%) levels enhanced TSP at 75% FC, and 2% SBE level improved TSP at 60% FC. The interaction between water stress and varieties as well as between water stress and SBE was significant. The maize varieties did not differ significantly in total soluble proteins under water-deficit stress.
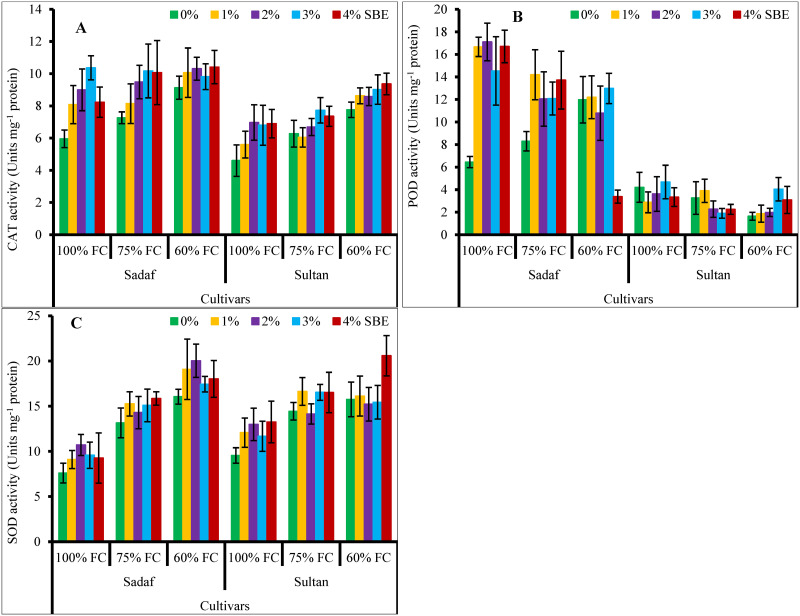
Varying watering regimes considerably (P ≤ 0.001) enhanced the activities of SOD, CAT and POD enzymes (Fig 7). Exogenous application of SBE also enhanced the activities of CAT and POD enzymes, while it did not affect the SOD activity under stress conditions. Variety Sadaf was better in CAT and POD activities than Sultan, whereas they both had a similar trend in SOD activity.
Fig 7. Supplementation of sugar beet extract (SBE) enhanced the activities of (A) CAT, (B) POD and (C) SOD enzymes of maize plants subjected to water-deficit stress (n = 3; Mean ± S.E.).
Discussion
The present study was conducted to evaluate the effects of varying levels of sugar beet extract as a source of glycinebetaine on two maize varieties under water deficit conditions. Sugar beet is an excellent source of GB, carotenoids, AsA, flavonoids, phenolics, betalains etc. [22,23,34]. Overall, GB concentration in sugar beet is comparatively high i.e., 50 mM [47]. It can be applied to the foliage of plants for attaining improved stress tolerance of plants [22]. In the present study, growth characteristics (shoot and root dry weights) of all maize plants were suppressed under water-deficit stress conditions. However, these attributes were enhanced by foliar-applied SBE levels. Impairment in mitosis, and decreased turgor and water flow from xylem to other nearby cells may be the reasons for reduction in maize plants’ growth under water stress [1]. Recently, SBE was applied as a foliar spray to wheat plants under water stress [22]. It was illustrated that SBE ameliorated the drastic effects of water-deficit stress by increasing the growth, antioxidative defense system and nutrient homeostasis in wheat plants under stress conditions.
Chlorophyll pigments of the both maize varieties decreased under water deficit conditions in the current study. While, SBE treatment accelerated the photosynthetic pigments in all maize plants under stress conditions. Chlorophyll pigments decrease due to stomatal closure and reduction in leaf conductance, ultra-structural modifications to thylakoid lamellae, incomplete penetration of sunlight, and its use, inhibition in pigments of photosynthesis [50–52]. Another reason of reduced chlorophyll pigments could have been the production of ROS such as H2O2, O2-, and 1O2 which might have led to lipid peroxidation and oxidative damages. A ROS, 1O2 is predominantly produced at PS II under stressful environment and it can lead to cell death under drought stress conditions [53]. Noman, Ali [22] reported an increase in chlorophyll pigments of wheat plants under water stress conditions which was found to be associated with enhanced plant growth. It was also reported that SBE acted as a bio-stimulant of plant growth under water-deficit stress conditions, since it also includes phenolics, flavonoids, ascorbic acid, etc. which can further reinforce the role of SBE as a growth promoter [21,22].
Cell growth is inhibited due to deficiency of water which leads to reduction in leaf development and leaf area, and hence overall reduced growth. As a result of reduced leaf area, transpiration rate decreases due to less uptake of water from soil. Leaf area determines the light interception and thus it is an important attribute to determine plant growth [54–56]. In the present investigation, leaf area was reduced by water stress conditions, but it was improved by foliar-applied SBE to the maize plants under water-deficit stress. Reduction in leaf area was also reported in Sorghum plants under drought stress by Yadav, Lakshmi [57]. It was reasoned that leaf area reduction might have been due to loss in turgor potential leading to less leaf expansion. In a recent study of pea plants, SBE along with other phytoextracts improved the leaf area of pea plants under salinity stress [35]. It might have been due to the presence of growth promoting compounds like, proline, GB, phenolics, flavonoids and tocopherols in the sugar beet extract [35,58].
Foliar applied SBE at different concentrations improved the RWCs of maize varieties which were reduced by the water-deficit conditions in the current study. Availability of water to soil reduces due to loss in transpiration, and an imbalanced water status leads to reduced plant RWC under drought stress conditions [59]. Reduction in relative water contents was found in many plants such as wheat [60], peanut [61], barley [62], pigeon pea [63], Thymus citriodorus [53], and canola [64] subjected to water deficit conditions. RWC can also be used as a potential indicator to explore better yielding genotypes which can balance cell turgor in response to water stress regimes [61,65].
Relative membrane permeability is considered as a measure of oxidative stress indicator in plant cells [66]. In the current investigation, RMP was found to be improved by water stress, whereas foliar application of SBE at different (1% and 4%) concentrations decreased the RMP in var. Sultan under 60% FC. Relatively drought sensitive cultivar, Sultan, showed high RMP under stress conditions. Similarly, water stress-induced elevation in RMP was also found in chickpea [66] and Sesbania [3] plants producing low biomass under arid conditions.
Osmoprotectants like proline, GB, soluble sugars and amino acids are soluble compounds which accumulate in plant species under stress conditions. These osmolytes help protect enzymes and macromolecules from ROS-induced damage under stress conditions [32,67,68]. In the present study with two maize varieties, proline and GB contents enhanced under water-deficit stress and foliar applied SBE. Likewise, Osman [32] reported that proline contents of pea plants increased which in turn reduced the lipid peroxidation and improved the growth of pea plants under water deficit conditions. Increase in GB and proline contents was also observed in wheat under water stress imposed by withholding of irrigation [69]. Proline and GB maintain stomatal conductance, photosynthesis, water balance and growth of plants through osmotic adjustment under stress conditions. These osmolytes not only help sustain plant life by osmoregulation [70], but also by regulation of enzymes’ activities and levels of proteins, scavenging of ROS and stabilization of membrane integrity [70,71].
Hydrogen peroxide is a highly reactive ROS and enzymes related to the Calvin cycle are more sensitive to it [72]. The MDA contents were improved due to production of ROS under stress conditions which can also be used as a stress indicator of lipid peroxidation [73]. In the maize plants, MDA and H2O2 contents increased under water stress conditions, while exogenously-applied SBE decreased the MDA contents, but in contrast, H2O2 contents remained unaffected under stress conditions. Recently, Avramova, AbdElgawad [74] reported that MDA and H2O2 contents increased in sensitive maize hybrids (EG3, EG4 and EG5) under varying (43% and 34% soil water contents) water regimes. Decrease in MDA and H2O2 contents by SBE might have been due to increase in the activities of SOD and POD enzymes under drought stress [22].
Total phenolics decreased in all maize plants under water stress conditions, while SBE applied at different concentrations enhanced it. Dissimilar to our findings, high accumulation of total phenolics was reported in Aloe vera [75] and rice [76] varieties under water stress.
Ascorbic acid is non-enzymatic antioxidant present in plant cells that can counteract ROS under stress conditions [77]. Ascorbic acid contents in the present study of maize plants increased due water stress and SBE treatment. It was examined in radish [78], tomato [79] and cauliflower [13] plants that AsA contents increased significantly under water stress. Ascorbic acid is involved in the defense of plant cell organelles from the oxidative damage caused due to ROS accumulation under stress conditions. It also acts as a cofactor of several enzymes, is involved in the biosynthesis of hormones, and restoration of antioxidants as well as it controls the division and expansion of cells [80–82].
Enzymatic antioxidants are involved in plant stress tolerance, cell redox balance as well as defense against oxidative damage [74,77,83–86]. In the current study, the activities of SOD, CAT and POD enzymes improved under water-deficit stress. Foliar spray of SBE at different concentrations also improved the activities of CAT and POD enzymes, but no change was observed in SOD activity. In a recent study on wheat plants, the activities of all these key enzymes were also reported to be improved by varying SBE (10%, 20%, 30%, 40% & 50%) levels under water stress (60% FC) conditions. It might have been due to the accumulation of osmoprotectants like GB that can act as an antioxidative compound to counteract ROS [22]. In another study, Shahid, Balal [35] observed that SBE with other phytoextract like that of moringa also enhanced the activities of enzymatic antioxidants in salt-stressed pea plants. These enzymes help eliminate the ROS, and a strong association between growth and antioxidants activities was found in this study.
Conclusion
Overall, water stress (75% and 60% FC) decreased the growth attributes (shoot and root dry weights, leaf area), total soluble proteins, chlorophyll pigments, RWC, and total phenolics, whereas it enhanced proline, MDA, H2O2, RMP, AsA and the activities of CAT, SOD and POD enzymes. Foliar application of SBE enhanced all growth characteristics, RWC, chlorophyll pigments, proline, GB, total phenolics, AsA and the activities of the enzymatic antioxidants (CAT and POD). No change in total soluble proteins, H2O2 and SOD enzyme activity was observed due to the SBE application. However, SBE applied as 3% and 4% was more effective as compared to 1% and 2% SBE in counteracting the adversaries of water stress. Overall, var. Sadaf was better in growth, RWC and the activities of enzymatic antioxidants than var. Sultan.
Supporting information
(PDF)
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Taif University for supporting this work through Researchers Supporting Project number (TURSP—2020/64), Taif University, Taif, Saudi Arabia. Additionally, this manuscript is part of research work conducted by SS and was funded by Higher Education Commission Pakistan through the project Number, HEC project #. NRPU-5599.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors would like to thank the Deanship of Scientific Research at Taif University for supporting this work through Researchers Supporting Project number (TURSP - 2020/64), Taif University, Taif, Saudi Arabia. Additionally, this manuscript is part of research work conducted by SS and was funded by Higher Education Commission Pakistan through the project Number, HEC project #. NRPU-5599.
References
- 1.Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front Plant Sci. 2017;8:1147-. doi: 10.3389/fpls.2017.01147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srivastava N, Kumar G. Influence of water deficit on morphological characteristics of green manure crop (Dhaincha) Sesbania cannabina Poir. Unique Journal Pharmaceutical and Bioogical Sciences. 2014;2(3):15–8. [Google Scholar]
- 3.Ali Z, Ashraf M, Qurainy F, Khan M. Appraising drought tolerance in local accessions of sesbania (Sesbania sesban (l.) merril.) using biomass production, relative membrane permeability and photosynthetic capacity as selection criteria. Pakistan Journal of Botany. 2015;47(3):845–50. [Google Scholar]
- 4.Ashraf M, Harris PJC. Photosynthesis under stressful environments: An overview. Photosynthetica. 2013;51(2):163–90. doi: 10.1007/s11099-013-0021-6 [DOI] [Google Scholar]
- 5.Kosar F, Akram NA, Ashraf M. Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. South African Journal of Botany. 2015;96:71–7. doi: 10.1016/j.sajb.2014.10.015 [DOI] [Google Scholar]
- 6.Begum N, Ahanger MA, Su Y, Lei Y, Mustafa NSA, Ahmad P, et al. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants. 2019;8(12):579. doi: 10.3390/plants8120579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaya C, Ashraf M, Wijaya L, Ahmad P. The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiology and Biochemistry. 2019;143:119–28. doi: 10.1016/j.plaphy.2019.08.024 [DOI] [PubMed] [Google Scholar]
- 8.Ashraf MA, Ashraf M, Ali Q. Response of two genetically diverse wheat cultivars to salt stress at different growth stages: leaf lipid peroxidation and phenolic contents. Pak J Bot. 2010;42(1):559–65. [Google Scholar]
- 9.Caverzan A, Piasecki C, Chavarria G, Stewart CN Jr., Vargas L. Defenses Against ROS in Crops and Weeds: The Effects of Interference and Herbicides. Int J Mol Sci. 2019;20(5):1086. doi: 10.3390/ijms20051086 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noreen Z, Ashraf M. Assessment of variation in antioxidative defense system in salt-treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. Journal of Plant Physiology. 2009;166(16):1764–74. doi: 10.1016/j.jplph.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 11.Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Critical reviews in biotechnology. 2010;30(3):161–75. doi: 10.3109/07388550903524243 [DOI] [PubMed] [Google Scholar]
- 12.Kusaka M, Ohta M, Fujimura T. Contribution of inorganic components to osmotic adjustment and leaf folding for drought tolerance in pearl millet. Physiologia Plantarum. 2005;125(4):474–89. doi: 10.1111/j.1399-3054.2005.00578.x [DOI] [Google Scholar]
- 13.Latif M, Akram NA, Ashraf M. Regulation of some biochemical attributes in drought-stressed cauliflower (Brassica oleracea L.) by seed pre-treatment with ascorbic acid. The Journal of Horticultural Science and Biotechnology. 2016;91(2):129–37. doi: 10.1080/14620316.2015.1117226 [DOI] [Google Scholar]
- 14.Osmolovskaya N, Shumilina J, Kim A, Didio A, Grishina T, Bilova T, et al. Methodology of Drought Stress Research: Experimental Setup and Physiological Characterization. Int J Mol Sci. 2018;19(12):4089. doi: 10.3390/ijms19124089 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farooq A, Bukhari SA, Akram NA, Ashraf M, Wijaya L, Alyemeni MN, et al. Exogenously applied ascorbic acid-mediated changes in osmoprotection and oxidative defense system enhanced water stress tolerance in different cultivars of safflower (Carthamus tinctorious L.). Plants. 2020;9(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdellatif YMR, Ibrahim MTS. Nonenzymatic antioxidants potential in enhancing Hibiscus sabdariffa L. tolerance to oxidative stress. International Journal of Botany. 2018. [Google Scholar]
- 17.Lehtimäki N, Shunmugam S, Jokela J, Wahlsten M, Carmel D, Keränen M, et al. Nodularin uptake and induction of oxidative stress in spinach (Spinachia oleracea). Journal of Plant Physiology. 2011;168(6):594–600. doi: 10.1016/j.jplph.2010.09.013 [DOI] [PubMed] [Google Scholar]
- 18.Møller IM, Jensen PE, Hansson A. Oxidative Modifications to Cellular Components in Plants. Annual Review of Plant Biology. 2007;58(1):459–81. doi: 10.1146/annurev.arplant.58.032806.103946 [DOI] [PubMed] [Google Scholar]
- 19.Ahmad P, Tripathi DK, Deshmukh R, Pratap Singh V, Corpas FJ. Revisiting the role of ROS and RNS in plants under changing environment. 2019. [Google Scholar]
- 20.Kohli SK, Khanna K, Bhardwaj R, Abd_Allah EF, Ahmad P, Corpas FJ. Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants. 2019;8(12):641. doi: 10.3390/antiox8120641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.du Jardin P. Plant biostimulants: Definition, concept, main categories and regulation. Scientia Horticulturae. 2015;196:3–14. doi: 10.1016/j.scienta.2015.09.021 [DOI] [Google Scholar]
- 22.Noman A, Ali Q, Naseem J, Javed MT, Kanwal H, Islam W, et al. Sugar beet extract acts as a natural bio-stimulant for physio-biochemical attributes in water stressed wheat (Triticum aestivum L.). Acta Physiologiae Plantarum. 2018;40(6). doi: 10.1007/s11738-018-2681-0 [DOI] [Google Scholar]
- 23.Jamil S, Ali Q, Iqbal M, Javed MT, Iftikhar W, Shahzad F, et al. Modulations in plant water relations and tissue-specific osmoregulation by foliar-applied ascorbic acid and the induction of salt tolerance in maize plants. Brazilian Journal of Botany. 2015;38(3):527–38. doi: 10.1007/s40415-015-0174-6 [DOI] [Google Scholar]
- 24.Kumar V, Shriram V, Hoque TS, Hasan MM, Burritt DJ, Hossain MA. Glycinebetaine-Mediated Abiotic Oxidative-Stress Tolerance in Plants: Physiological and Biochemical Mechanisms. Stress Signaling in Plants: Genomics and Proteomics Perspective, Volume 2: Springer International Publishing; 2017. p. 111–33. [Google Scholar]
- 25.Li D, Zhang T, Wang M, Liu Y, Brestic M, Chen THH, et al. Genetic Engineering of the Biosynthesis of Glycine Betaine Modulates Phosphate Homeostasis by Regulating Phosphate Acquisition in Tomato. Front Plant Sci. 2019;9:1995-. doi: 10.3389/fpls.2018.01995 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Annunziata MG, Ciarmiello LF, Woodrow P, Dell’Aversana E, Carillo P. Spatial and Temporal Profile of Glycine Betaine Accumulation in Plants Under Abiotic Stresses. Front Plant Sci. 2019;10. doi: 10.3389/fpls.2019.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fariduddin Q, Varshney P, Yusuf M, Ali A, Ahmad A. Dissecting the role of glycine betaine in plants under abiotic stress. Plant Stress. 2013;7(1):8–18. [Google Scholar]
- 28.Mitsuya S, Kozaki K, Takabe T. Tissue Localization of the Glycine Betaine Biosynthetic Enzymes in Barley Leaves. Plant Production Science. 2013;16(2):117–22. doi: 10.1626/pps.16.117 [DOI] [Google Scholar]
- 29.Anjum SA, Farooq M, Wang LC, Xue LL, Wang SG, Wang L, et al. Gas exchange and chlorophyll synthesis of maize cultivars are enhanced by exogenously-applied glycinebetaine under drought conditions. Plant, Soil and Environment. 2011;57(No. 7):326–31. doi: 10.17221/41/2011-pse [DOI] [Google Scholar]
- 30.Iqbal N, Ashraf M, Ashraf MY. Glycinebetaine, an osmolyte of interest to improve water stress tolerance in sunflower (Helianthus annuus L.): water relations and yield. South African Journal of Botany. 2008;74(2):274–81. doi: 10.1016/j.sajb.2007.11.016 [DOI] [Google Scholar]
- 31.Mahmood T, Ashraf M, Shahbaz M. Does exogenous application of glycinebetaine as a pre-sowing seed treatment improve growth and regulate some key physiological attributes in wheat plants grown under water deficit conditions. Pak J Bot. 2009;41(3):1291–302. [Google Scholar]
- 32.Osman HS. Enhancing antioxidant–yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Annals of Agricultural Sciences. 2015;60(2):389–402. doi: 10.1016/j.aoas.2015.10.004 [DOI] [Google Scholar]
- 33.Reddy K. Exogenous Application of Glycinebetaine Facilitates Maize (Zea mays L.) Growth under Water Deficit Conditions. American Journal of Experimental Agriculture. 2013;3(1):1–13. doi: 10.9734/ajea/2013/1730 [DOI] [Google Scholar]
- 34.Habib N, Ashraf M, Ali Q, Perveen R. Response of salt stressed okra (Abelmoschus esculentus Moench) plants to foliar-applied glycine betaine and glycine betaine containing sugarbeet extract. South African Journal of Botany. 2012;83:151–8. doi: 10.1016/j.sajb.2012.08.005 [DOI] [Google Scholar]
- 35.Shahid MA, Balal RM, Pervez MA, Abbas T, Aqeel MA, Javaid MM, et al. Foliar spray of phyto-extracts supplemented with silicon: an efficacious strategy to alleviate the salinity-induced deleterious effects in pea (Pisum sativum L.). TURKISH JOURNAL OF BOTANY. 2015;39:408–19. doi: 10.3906/bot-1406-84 [DOI] [Google Scholar]
- 36.Shafiq S, Akram NA, Ashraf M. Assessment of physio-biochemical indicators for drought tolerance in different cultivars of maize (Zea mays L.). Pakistan Journal of Botany. 2019;51(4). doi: 10.30848/pjb2019-4(21) [DOI] [Google Scholar]
- 37.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant physiology. 1949;24(1):1. doi: 10.1104/pp.24.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrs HD, Weatherley PE. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Australian Journal of Biological Sciences. 1962;15(3):413. doi: 10.1071/bi9620413 [DOI] [Google Scholar]
- 39.Yang G, Rhodes D, Joly RJ. Effects of High Temperature on Membrane Stability and Chlorophyll Fluorescence in Glycinebetaine-Deficient and Glycinebetaine-Containing Maize Lines. Functional Plant Biology. 1996;23(4):437. doi: 10.1071/pp9960437 [DOI] [Google Scholar]
- 40.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39(1):205–7. doi: 10.1007/bf00018060 [DOI] [Google Scholar]
- 41.Grieve CM, Grattan SR. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant and Soil. 1983;70(2):303–7. doi: 10.1007/bf02374789 [DOI] [Google Scholar]
- 42.Cakmak I, Horst WJ. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiologia Plantarum. 1991;83(3):463–8. doi: 10.1034/j.1399-3054.1991.830320.x [DOI] [Google Scholar]
- 43.Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant science. 2000;151(1):59–66. [Google Scholar]
- 44.Julkunen-Tiitto R. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. Journal of Agricultural and Food Chemistry. 1985;33(2):213–7. doi: 10.1021/jf00062a013 [DOI] [Google Scholar]
- 45.Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiologia Plantarum. 1983;58(2):166–70. doi: 10.1111/j.1399-3054.1983.tb04162.x [DOI] [Google Scholar]
- 46.Chance B, Maehly AC. Assay of catalases and peroxidases. Methods in Enzymology: Elsevier; 1955. p. 764–75. [DOI] [PubMed] [Google Scholar]
- 47.Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. Plant physiology. 1977;59(2):309–14. doi: 10.1104/pp.59.2.309 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72(1–2):248–54. doi: 10.1006/abio.1976.9999 [DOI] [PubMed] [Google Scholar]
- 49.Snedecor W, Cochran G. Statistical Methods 7th ed ed. Ames, IA: The Iowa State Univ Press; 1980. doi: 10.1152/ajpheart.1980.238.5.H675 [DOI] [Google Scholar]
- 50.Hasan MM-U, Ma F, Prodhan ZH, Li F, Shen H, Chen Y, et al. Molecular and Physio-Biochemical Characterization of Cotton Species for Assessing Drought Stress Tolerance. Int J Mol Sci. 2018;19(9):2636. doi: 10.3390/ijms19092636 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shafei S. Study on water shortage effect at some of physiological traits, yield and yield components in some genotypes of soybean. Farsi: Islamic Azad University; 2005. [Google Scholar]
- 52.Wang GP, Li F, Zhang J, Zhao MR, Hui Z, Wang W. Overaccumulation of glycine betaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica. 2010;48(1):30–41. doi: 10.1007/s11099-010-0006-7 [DOI] [Google Scholar]
- 53.Tátrai ZA, Sanoubar R, Pluhár Z, Mancarella S, Orsini F, Gianquinto G. Morphological and Physiological Plant Responses to Drought Stress in Thymus citriodorus. International Journal of Agronomy. 2016;2016:1–8. doi: 10.1155/2016/4165750 [DOI] [Google Scholar]
- 54.Fathi A, Tari DB. Effect of Drought Stress and its Mechanism in Plants. International Journal of Life Sciences. 2016;10(1):1–6. doi: 10.3126/ijls.v10i1.14509 [DOI] [Google Scholar]
- 55.Koester RP, Skoneczka JA, Cary TR, Diers BW, Ainsworth EA. Historical gains in soybean (Glycine max Merr.) seed yield are driven by linear increases in light interception, energy conversion, and partitioning efficiencies. J Exp Bot. 2014;65(12):3311–21. Epub 2014/04/30. doi: 10.1093/jxb/eru187 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weraduwage SM, Chen J, Anozie FC, Morales A, Weise SE, Sharkey TD. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front Plant Sci. 2015;6:167-. doi: 10.3389/fpls.2015.00167 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yadav S, Lakshmi NJ, Maheswari M, Vanaja M, Venkateswarlu B. Influence of water deficit at vegetative, anthesis and grain filling stages on water relation and grain yield in sorghum. Indian Journal of Plant Physiology. 2005;10(1):20. [Google Scholar]
- 58.Sultana S, Akhtar N, Asif HM. Phytochemical screening and antipyretic effects of hydro-methanol extract of Melia azedarach leaves in rabbits. Bangladesh Journal of Pharmacology. 2013;8(2). doi: 10.3329/bjp.v8i2.14708 [DOI] [Google Scholar]
- 59.Bolat I, Dikilitas M, Ercisli S, Ikinci A, Tonkaz T. The effect of water stress on some morphological, physiological, and biochemical characteristics and bud success on apple and quince rootstocks. ScientificWorldJournal. 2014;2014:769732-. doi: 10.1155/2014/769732 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keyvan S. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J Anim Plant Sci. 2010;8(3):1051–60. [Google Scholar]
- 61.Shivakrishna P, Ashok Reddy K, Manohar Rao D. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J Biol Sci. 2018;25(2):285–9. Epub 2017/04/25. doi: 10.1016/j.sjbs.2017.04.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan S, Liu W-J, Zhang N-H, Wang M-B, Liang H-G, Hui Lin H. Effects of water stress on major photosystem II gene expression and protein metabolism in barley leaves. Physiologia Plantarum. 2005;125(4):464–73. doi: 10.1111/j.1399-3054.2005.00577.x [DOI] [Google Scholar]
- 63.Maluventhen V, Pichamuthu M, Thirupathi K, Kumariah M. Effect of polyethylene glycol and mannitol on somatic embryogenesis of pigeonpea, Cajanus cajan (L.) Millsp. African Journal of Biotechnology. 2012;11(45):10340–9. doi: 10.5897/ajb.12.368 [DOI] [Google Scholar]
- 64.Akram NA, Iqbal M, Muhammad A, Ashraf M, Al-Qurainy F, Shafiq S. Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma. 2018;255(1):163–74. doi: 10.1007/s00709-017-1140-x [DOI] [PubMed] [Google Scholar]
- 65.Bayoumi T, Eid MH, Metwali E. Application of physiological and biochemical indices as a screening technique for drought tolerance in wheat genotypes. African Journal of Biotechnology. 2008;7(14). [Google Scholar]
- 66.Gunes A, Inal A, Adak MS, Bagci EG, Cicek N, Eraslan F. Effect of drought stress implemented at pre- or post-anthesis stage on some physiological parameters as screening criteria in chickpea cultivars. Russian Journal of Plant Physiology. 2008;55(1):59–67. doi: 10.1134/s102144370801007x [DOI] [Google Scholar]
- 67.Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, et al. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules. 2019;9(7):285. doi: 10.3390/biom9070285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh M, Kumar J, Singh S, Singh VP, Prasad SM. Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Reviews in Environmental Science and Bio/Technology. 2015;14(3):407–26. doi: 10.1007/s11157-015-9372-8 [DOI] [Google Scholar]
- 69.Gupta N, Thind SK, Bains NS. Glycine betaine application modifies biochemical attributes of osmotic adjustment in drought stressed wheat. Plant Growth Regulation. 2014;72(3):221–8. doi: 10.1007/s10725-013-9853-0 [DOI] [Google Scholar]
- 70.Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany. 2007;59(2):206–16. doi: 10.1016/j.envexpbot.2005.12.006 [DOI] [Google Scholar]
- 71.Basu S, Ramegowda V, Kumar A, Pereira A. Plant adaptation to drought stress. F1000Res. 2016;5:F1000 Faculty Rev-554. doi: 10.12688/f1000research.7678.1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta DK, Palma JM, Corpas FJ. Reactive oxygen species and oxidative damage in plants under stress: Springer; 2015. [Google Scholar]
- 73.Fayez KA, Bazaid SA. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. Journal of the Saudi Society of Agricultural Sciences. 2014;13(1):45–55. doi: 10.1016/j.jssas.2013.01.001 [DOI] [Google Scholar]
- 74.Avramova V, AbdElgawad H, Vasileva I, Petrova AS, Holek A, Mariën J, et al. High Antioxidant Activity Facilitates Maintenance of Cell Division in Leaves of Drought Tolerant Maize Hybrids. Front Plant Sci. 2017;8:84-. doi: 10.3389/fpls.2017.00084 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Habibi G. Effects of mild and severe drought stress on the biomass, phenolic compounds production and photochemical activity of Aloe vera (L.) Burm.f. Acta agriculturae Slovenica. 2018;111(2):463. doi: 10.14720/aas.2018.111.2.19 [DOI] [Google Scholar]
- 76.Quan N, Anh L, Khang D, Tuyen P, Toan N, Minh T, et al. Involvement of Secondary Metabolites in Response to Drought Stress of Rice (Oryza sativa L.). Agriculture. 2016;6(2):23. doi: 10.3390/agriculture6020023 [DOI] [Google Scholar]
- 77.Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. Journal of Botany. 2012;2012:1–26. doi: 10.1155/2012/217037 [DOI] [Google Scholar]
- 78.Shafiq S, Akram NA, Ashraf M. Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Scientia Horticulturae. 2015;185:68–75. doi: 10.1016/j.scienta.2015.01.010 [DOI] [Google Scholar]
- 79.Ghorbanli M, Gafarabad M, Amirkian T, ALLAHVERDI MB. Investigation of proline, total protein, chlorophyll, ascorbate and dehydroascorbate changes under drought stress in Akria and Mobil tomato cultivars. Iran J Plant Physiol. 2013;3:651–8. [Google Scholar]
- 80.Akram NA, Shafiq F, Ashraf M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front Plant Sci. 2017;8. doi: 10.3389/fpls.2017.00613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lisko KA, Aboobucker SI, Torres R, Lorence A. Engineering Elevated Vitamin C in Plants to Improve their Nutritional Content, Growth, and Tolerance to Abiotic Stress. Phytochemicals—Biosynthesis, Function and Application: Springer International Publishing; 2014. p. 109–28. [Google Scholar]
- 82.Naz H, Akram NA, Ashraf M. Impact of ascorbic acid on growth and some physiological attributes of cucumber (Cucumis sativus) plants under water-deficit conditions. Pak J Bot. 2016;48(3):877–83. [Google Scholar]
- 83.Caverzan A, Casassola A, Patussi Brammer S. Reactive Oxygen Species and Antioxidant Enzymes Involved in Plant Tolerance to Stress. Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives: InTech; 2016. [Google Scholar]
- 84.Xie X, He Z, Chen N, Tang Z, Wang Q, Cai Y. The Roles of Environmental Factors in Regulation of Oxidative Stress in Plant. Biomed Res Int. 2019;2019:9732325-. doi: 10.1155/2019/9732325 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kosar F, Akram NA, Ashraf M, Ahmad A, Alyemeni MN, Ahmad P. Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiologia Plantarum. 2020. [DOI] [PubMed] [Google Scholar]
- 86.Raja V, Qadir SU, Alyemeni MN, Ahmad P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech. 2020;10(5):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper.