Abstract
Objective
Plaque neovasculature is a major route for lipoprotein and leukocyte ingress into plaques, and has been identified as a risk factor for carotid plaque disruption. Vp, a variable derived from pharmacokinetic modeling of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), correlates with plaque neovasculature density. Because lipid-lowering therapy has been associated with regression of neovasculature in animal models, we sought to determine clinical correlates of carotid plaque neovasculature (as assessed by Vp) in participants on statin therapy for established cardiovascular disease.
Methods
98 participants from an AIM-HIGH sub-study underwent DCE-MRI of their carotid arteries. Expert readers who were blinded to all clinical variables analyzed the MR images to measure carotid plaque Vp in all participants. Associations between Vp and duration of statin therapy and other clinical risk factors were analyzed.
Results
Prior duration of statin treatment at enrollment ranged from <1 year (21%), 1-5 years (40%) and >5 years (39%). In univariate analyses, shorter duration of statin therapy (P=0.01), the presence of metabolic syndrome (P=0.02), and higher body mass index (P=0.01) and lipoprotein(a) (P=0.01) were all significantly associated with higher baseline Vp values. In multivariate analyses, significant associations remained between shorter duration of statin therapy (P=0.004) and lipoprotein(a) (P=0.04).
Conclusions
These are the first human, in vivo findings suggesting a relationship between duration of statin therapy and regression of carotid plaque neovasculature. Future longitudinal studies are warranted both to confirm this finding and to address whether changes in neovasculature may translate into change in risk for plaque disruption.
Keywords: statin, neovasculature, lipoprotein(a), magnetic resonance imaging, atherosclerosis
INTRODUCTION
Over the past two decades, there has been an increasing appreciation for how specific atherosclerotic plaque features increase risk for plaque disruption that result in clinical cardiovascular events.1, 2 Among these plaque features are macrophages3-5 and neovasculature.6 Histological studies in human coronary arteries have documented associations between leukocyte adhesion molecules on neovascular endothelium and plaque inflammatory cell content,7, 8 suggesting that neovasculature may be a major route by which macrophages enter atherosclerotic plaques. In addition, neovasculature has been linked to plaque vulnerability in humans.9 Together, these findings suggest important roles for both neovasculature and macrophages in plaque development and disruption.
Concurrently, efforts have been made to develop non-invasive methods, such as magnetic resonance imaging (MRI), to image plaque components associated with vulnerability. To this aim, several groups also have devoted substantial effort to developing techniques to quantify plaque neovasculature and macrophages. In particular, preoperative, dynamic, contrast-enhanced-magnetic resonance imaging (DCE-MRI) of carotid arteries has been validated against post-operative immunohistochemistry performed on human carotid endarterectomy specimens. These studies have found strong correlations between DCE-MRI-derived markers of carotid plaque neovasculature (fractional blood volume, Vp),10-12 and macrophages (transfer constant, Ktrans).11
Statin therapy is associated with decreased risk for cardiovascular events13, 14 and, in animal models, statin therapy decreases plaque macrophages15, 16 and neovasculature.17-20 Thus, we hypothesized that duration of prior statin therapy, as well as other baseline variables, might be associated with Vp and/or Ktrans values measured at baseline (i.e., prior to randomization) in a sub-study of the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM-HIGH) Trial.21 Analyses were restricted to those participants who were on statins at baseline.
METHODS
Study Design
The objective was to evaluate neovasculature density and its association with clinical and laboratory measures in the AIM-HIGH participants who were on statins at baseline. Participants underwent baseline DCE-MRI scans within 3 months following randomization into statin alone or with statin plus extended-release niacin in the AIM-HIGH Trial. Fractional blood volume (Vp) estimated by kinetic modeling of DCE-MRI, previously found to correlate well with neovasculature content of plaques,11 was the predefined primary endpoint. The transfer constant (Ktrans), another kinetic model parameter that correlates with histological measurements of plaque inflammation,11 was also estimated using DCE-MRI and included in the analysis.
Study Participants
Sub-study participants were recruited from a subset of 21 of 92 AIM-HIGH Trial (NCT00120289) clinical sites in the United States and Canada. Appendices 1 and 2 list the participating clinical and imaging sites and investigators. AIM-HIGH Trial inclusion and exclusion criteria have been published previously.21 Briefly, the primary AIM-HIGH inclusion criteria included: 1) age 45 years or older, 2) documented stable coronary, cerebrovascular/carotid or peripheral arterial disease, and 3) “atherogenic dyslipidemia”, defined as HDL-cholesterol <40mg/dL in men or <50mg/dL in women, triglycerides 150-400 mg/dL; and LDL-cholesterol <180 mg/dL if not taking statin drugs. In total, 94% of AIM-HIGH participants were taking statins at baseline. The duration of statin therapy prior to entering the trial was also recorded as the categories <1 year, 1-5 years and >5 years. This categorization was determined by the AIM-HIGH Trial executive committee prior to data analysis in this study.
Sub-study specific inclusion criteria were: 1) eligible for main AIM-HIGH study, 2) medically able to undergo MRI procedure, 3) willing to provide informed consent for sub-study participation. Sub-study specific exclusion criteria were: 1) history of pacemaker or metallic implants, 2) history of bilateral carotid endarterectomy, or 3) estimated glomerular filtration rate less than 60 mL/min/1.73 m2. This study was approved by the AIM-HIGH Executive Committee and a local institutional review board or research ethics committee at each participating clinical site. Separate signed informed consent was obtained from each participant in this sub-study.
After DCE-MRI scans were obtained, participant scans were excluded from further analysis if image quality was inadequate for analysis based on a previously described 4-point scale22 or there were significant violations of the DCE imaging protocol, including an incorrect time interval, too few time frames, failure to inject contrast agent and improper alignment of images. In addition, participants were excluded if carotid artery thickness was ≤1.0mm, as DCE-MRI measurements are considered unreliable in very thin vessel walls.
DCE-MRI Scan Protocol
MRI scans of carotid arteries were performed on 3T MRI scanners (GE HealthCare or Philips Healthcare). The DCE-MRI imaging protocol has been previously described.23 Briefly, the protocol included an axial multi-slice 2D spoiled gradient recalled echo (SGRE) sequence to acquire DCE images. Depending on individual scanner configuration, 4 to 8 contiguous slices were acquired, centered on the bifurcation of the index carotid artery with acquisition parameters: field of view: 160*160mm, matrix 256*256, acquired resolution 0.625*0.625 mm, reconstructed image size 512*512, 2-3 mm slice thickness, repetition time 117-126 ms, echo time 5ms, flip angle 50°. The index artery was selected as the one with more visible plaque. Images were acquired at 18 time points separated by a repetition interval of 18s. Coincident with the third dynamic scan in the sequence, 0.05 mmol/kg of a gadolinium-based contrast agent was injected at a rate of 0.7 ml/s by a power injector. To impose a T1-dependent signal on inflowing blood, a spatial saturation band was used. Other contrast weightings were acquired with a standard multiple-contrast-weighted protocol that included 3D time of flight (TOF), 2D black blood T1-weighted, contrast enhanced 2D black blood T1-weighted, 2D black blood T2-weighted, and 2D black blood proton-density-weighted, and 3D Magnetization Prepared Rapid Gradient Echo (MP-RAGE) imaging, as previously described.24
Image Analysis
Image analysis was conducted by trained reviewers blinded to the corresponding subject's clinical and laboratory data using custom software (CASCADE)25. All images were co-registered using the index carotid bifurcation as a common landmark. Lumen and outer wall boundaries of the carotid artery were first manually traced using the structural multi-contrast images and were then mapped to the DCE-MRI images by an automatic registration algorithm.23 The lumen and wall contours were then further manually adjusted, if needed, to better match the boundaries visible on the DCE-MRI images (illustrated in Figure 1). The contoured DCE-MRI images were processed to generate vasa vasorum (V-V) images as previously described.26 This processing step is fully automated and includes registration and smoothing of the sequence of images, extraction of the arterial input function, and calculation of Vp and Ktrans for each pixel based on the Patlak pharmacokinetic model. The resulting color-coded, parametric V-V images show Vp in red and Ktrans in green (Figure 1). Lastly, aggregated values for Vp and Ktrans were calculated by averaging all pixels within the vessel wall of each participant, except those within 1 mm of the lumen contour. This exclusion minimized any influence from the high intensity lumen signal due to partial volume effects, blurring, and motion. Note that the adventitial layer of the vessel is included within the outer wall boundary, so the average Vp and Ktrans values include both plaque and the adventitia, as in other reports.10-12, 23 Scan-rescan reproducibility of these measurements have been reported.23
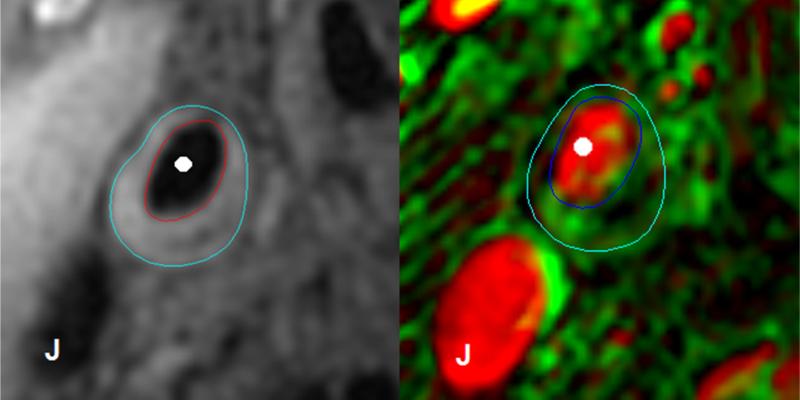
Figure 1. Pre-contrast T1-weighted image of the common carotid artery (left panel) and corresponding vasa vasorum (V-V) image derived from dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) (right panel) of a study subject.
As described in the Image Analysis section of the methods, the lumen and outer wall boundaries were initially drawn using the structural multi-contrast images (red and blue boundaries in the left panel, respectively). These contours were then mapped to the DCE-MRI images and manually adjusted slightly to better fit the image, resulting in the final lumen and outer wall boundaries for DCE-MRI processing (dark and light blue boundaries in the right panel, respectively). The white circles indicate the arterial lumen and the “J” symbols indicate the jugular vein on both images. The Patlak pharmacokinetic model parameters Vp and Ktrans are shown in red and green, respectively, as previously described26. Brighter pixels indicate larger values.
Statistical Methods
Categorical variables were summarized as count (percentage) and continuous variables were summarized as mean ± standard deviation (SD) and median (range). Clinical and laboratory values were compared between the sub-study sample and the remainder of the AIM-HIGH cohort sample using Fisher's exact test and the Mann-Whitney test. Plaque Vp and Ktrans were compared between groups using two-sample t-tests or ANOVA while associations with continuous variables were assessed using Pearson's correlation coefficient. Highly right-skewed variables (triglycerides, lipoprotein(a), total : HDL cholesterol ratio, and apolipoprotein B : A1 ratio) were log-transformed prior to analysis. Multivariate analyses were conducted using linear regression. Variables significantly associated with Vp or Ktrans during univariate analysis were included in a single multivariate model. Backwards elimination was then applied to identify variables independently associated with Vp or Ktrans. A linear trend between plaque Vp and prior duration of statin therapy (a categorical variable) was tested using the linear component from the set of orthogonal polynomial contrasts. Residuals from the univariate and multivariate models were inspected to detect important departures from normality. All statistical calculations were conducted using R (version 2.14.1; The R Foundation for Statistical Computing, Vienna, Austria). Throughout, two-tailed tests were used with statistical significance defined as p<0.05.
RESULTS
Participant Flow
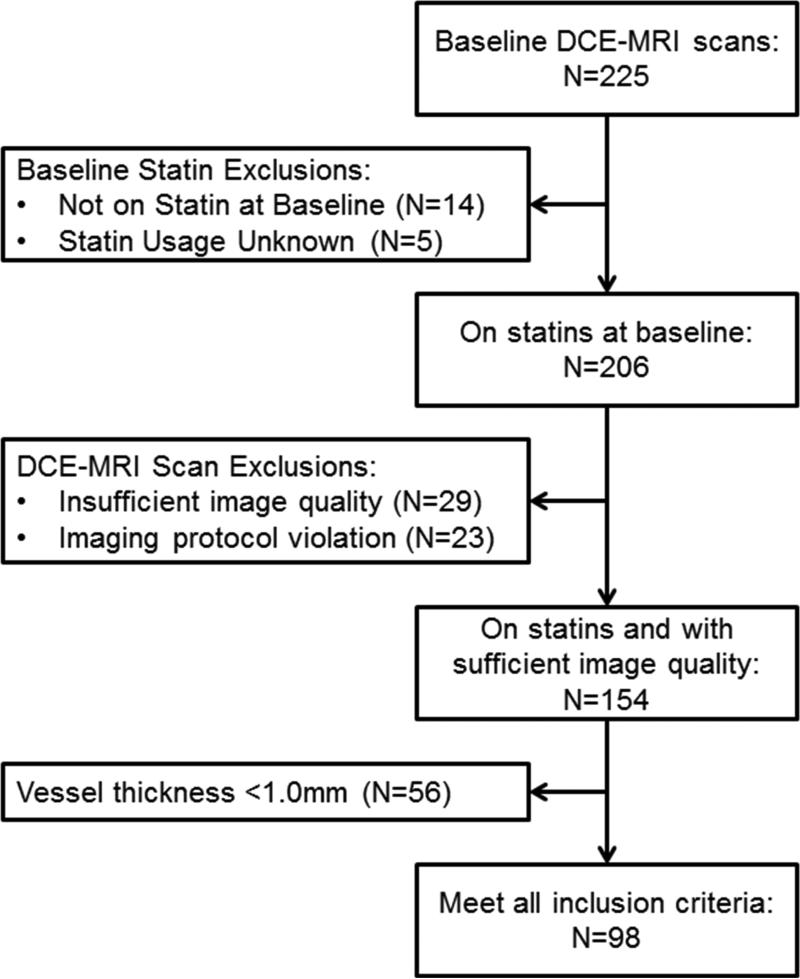
A total of 225 participants from the AIM-HIGH trial met initial sub-study inclusion criteria and underwent baseline DCE-MRI scanning. Of these, 206 were on statin therapy at enrollment. After further excluding participants with insufficient image quality or DCE-MRI protocol violations (N=52) and those who failed to meet the minimum vessel thickness requirement of >1 mm (N=56), there were 98 subjects who met all inclusion/exclusion criteria available for analysis. Participant flow is shown in Figure 2.
Figure 2. Sub-study participant flow.
Flow diagram of participant progress through sub-study exclusion criteria.
Participant Demographics
Baseline demographics, clinical and laboratory variables are summarized in Table 1 of Ref [27]. The age of subjects ranged from 45 to 79 years (median: 62) and 86% of subjects were male. Duration of statin therapy prior to baseline was <1 year for 21% of subjects, 1-5 years for 40% of subjects and >5 years for 39% of subjects. Table 1 of Ref [27] also summarizes the distributions of these variables in the remainder of the AIM-HIGH cohort. Compared to this group, participants in the DCE-MRI sub-study tended to be younger (mean: 62 vs. 64 years, p=0.07), have shorter prior duration of statin therapy (p=0.052), were less likely to have a history of diabetes (22% vs. 34%, p=0.02) and had a lower body mass index (p=0.005), lower triglycerides (p=0.04) and lower ApoA-I (p=0.04).
Table 1.
Univariate analysis results for baseline plaque Vp and Ktrans.
|
Vp
|
Ktrans
|
||||
|---|---|---|---|---|---|
| Variable | r or Mean ± SD | P Value | r or Mean ± SD | P Value | No. |
| Sex | 0.86 | 0.54 | 98 | ||
| Male | 0.075 ± 0.046 | 0.060 ± 0.025 | 84 | ||
| Female | 0.078 ± 0.051 | 0.055 ± 0.024 | 14 | ||
|
| |||||
| Age | −0.24 | 0.020 | 0.05 | 0.63 | 98 |
|
| |||||
| Race | 0.70 | 0.27 | 98 | ||
| White | 0.075 ± 0.047 | 0.060 ± 0.025 | 90 | ||
| Non-white/Other | 0.082 ± 0.040 | 0.050 ± 0.021 | 8 | ||
|
| |||||
| Ethnicity | 0.70 | 0.40 | 97 | ||
| Hispanic or Latino | 0.084 ± 0.045 | 0.048 ± 0.029 | 4 | ||
| Not Hispanic or Latino | 0.075 ± 0.047 | 0.059 ± 0.025 | 93 | ||
|
| |||||
| Tobacco use | 0.51 | 0.21 | 98 | ||
| Current | 0.085 ± 0.047 | 0.067 ± 0.028 | 21 | ||
| Former (quit > 1 year ago) | 0.071 ± 0.046 | 0.058 ± 0.023 | 48 | ||
| Never used | 0.077 ± 0.049 | 0.055 ± 0.024 | 29 | ||
|
| |||||
| Duration of statin therapy | 0.011 | 0.50 | 98 | ||
| <1 year | 0.100 ± 0.043 | 0.056 ± 0.023 | 21 | ||
| 1 - 5 years | 0.076 ± 0.047 | 0.057 ± 0.025 | 39 | ||
| >5 years | 0.062 ± 0.044 | 0.063 ± 0.026 | 38 | ||
|
| |||||
| Prior use of niacin | 0.41 | 0.72 | 98 | ||
| Yes | 0.066 ± 0.022 | 0.057 ± 0.020 | 15 | ||
| No | 0.077 ± 0.050 | 0.059 ± 0.026 | 83 | ||
|
| |||||
| Metabolic syndrome | 0.016 | 0.83 | 98 | ||
| Present | 0.081 ± 0.047 | 0.059 ± 0.025 | 79 | ||
| Absent | 0.053 ± 0.040 | 0.060 ± 0.025 | 19 | ||
|
| |||||
| History of diabetes | 0.49 | 0.70 | 98 | ||
| Yes | 0.070 ± 0.047 | 0.057 ± 0.024 | 22 | ||
| No | 0.077 ± 0.047 | 0.059 ± 0.025 | 76 | ||
|
| |||||
| Body mass index | 0.25 | 0.013 | 0.10 | 0.35 | 98 |
|
| |||||
| Systolic blood pressure | 0.10 | 0.34 | 0.20 | 0.044 | 97 |
|
| |||||
| Diastolic blood pressure | 0.14 | 0.16 | 0.03 | 0.73 | 97 |
|
| |||||
| Total cholesterol | 0.04 | 0.70 | 0.01 | 0.89 | 98 |
|
| |||||
| LDL cholesterol | 0.04 | 0.69 | −0.04 | 0.68 | 98 |
|
| |||||
| Non-HDL cholesterol | 0.06 | 0.58 | 0.01 | 0.96 | 98 |
|
| |||||
| Log(Triglycerides) | 0.04 | 0.70 | 0.08 | 0.41 | 98 |
|
| |||||
| Log(Lipoprotein(a)) | 0.25 | 0.013 | 0.11 | 0.28 | 96 |
|
| |||||
| ApoB | 0.17 | 0.092 | 0.01 | 0.96 | 96 |
|
| |||||
| HDL cholesterol | −0.05 | 0.63 | 0.04 | 0.73 | 98 |
|
| |||||
| ApoA-I | −0.01 | 0.92 | 0.02 | 0.84 | 96 |
|
| |||||
| Log(Total : HDL ratio) | 0.09 | 0.40 | −0.04 | 0.68 | 98 |
|
| |||||
| Log(ApoB : ApoA-I ratio) | 0.17 | 0.094 | −0.03 | 0.75 | 96 |
r = Pearson's correlation coefficient.
Univariate Analyses
At baseline, average plaque Vp was 0.076 ± 0.047 and average plaque Ktrans was 0.059 ± 0.025 min−1. The results of the univariate analyses of the baseline DCE-MRI variables versus clinical and laboratory factors are shown in Table 1. A significant positive association was found between baseline Ktrans and systolic blood pressure (p=0.04), but no other significant associations were found between clinical or laboratory variables and Ktrans.
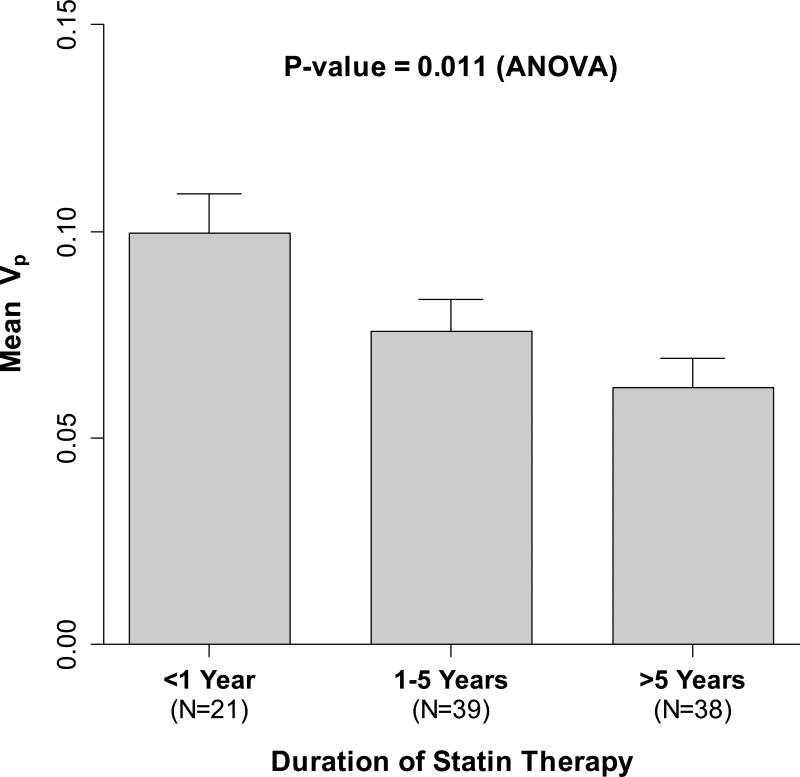
In contrast, significant univariate associations were found between baseline Vp and five demographic, clinical and laboratory variables: younger age, shorter prior duration of statin therapy, presence of metabolic syndrome, higher body mass index and higher lipoprotein(a) (Lp(a)) (Table 2). There were no statistically significant associations of Vp with sex, race/ethnicity, tobacco use, prior niacin use, diabetes, BP, Total-C, NonHDL-C, HDL-C, TGs, apoB, apoA-I, Total:HDL-C ratio, or apoB:apoA-I ratio. Importantly, there also was no significant association of baseline Vp with LDL-C (p=0.7). Figure 3 shows the inverse association of duration of prior statin therapy with baseline plaque Vp.
Table 2.
Multivariate analysis results for baseline plaque Vp as the outcome variable.
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| Variable | β | (95% CI) | P Value | β | (95% CI) | P Value |
| Duration of statin therapy | 0.056* | 0.004* | ||||
| 1-5 years vs. <1 year | −0.0189 | (−0.045, 0.007) | −0.0238 | (−0.048, 0.000) | ||
| >5 years vs. <1 year | −0.0282 | (−0.057, 0.000) | −0.0365 | (−0.061, −0.012) | ||
|
| ||||||
| Log(lipoprotein(a)), per 1 SD increase | 0.0091 | (0.000, 0.018) | 0.049 | 0.0096 | (0.001, 0.019) | 0.040 |
|
| ||||||
| Age, per 10 year increase | −0.0024 | (−0.014, 0.009) | 0.69 | - | ||
|
| ||||||
| Body mass index, per 1 SD increase | 0.0014 | (−0.001, 0.004) | 0.25 | - | ||
|
| ||||||
| Presence of metabolic syndrome | 0.0149 | (−0.010, 0.040) | 0.25 | - | ||
Model 1: initial model containing all variables significantly associated with Vp in univariate analysis;
Model 2: final model after applying backwards elimination to model 1;
β: regression coefficient;
Trend test.
Figure 3. Plaque Neovasculature (Vp) at Baseline by Duration of Prior Statin Therapy.
Bar heights correspond to mean Vp values and the error bars correspond to one standard error of the mean (SEM). Longer durations of prior statin therapy were associated with significantly lower mean Vp on the baseline DCE-MRI scans.
Multivariate Analysis
Factors significantly associated with Vp during univariate analysis were entered into a multivariate model (Table 2). After applying backwards elimination, only duration of statin therapy (p=0.004) and Lp(a) (p=0.04) remained independently associated with Vp.
DISCUSSION
This study demonstrates that DCE-MRI markers of carotid plaque neovasculature and inflammation can be reliably detected and quantified in a multi-center clinical trial. More importantly, it identifies potential links between specific clinical factors and the extent of carotid plaque neovasculature as represented by Vp. Specifically, the study finds, in patients with established cardiovascular disease, that: a) higher Lp(a) levels are positively associated with more extensive neovasculature, and b) longer duration of statin therapy is associated with less neovasculature.
Neovasculature long has been recognized as a feature of human atherosclerosis,28 and studies in the 1990s demonstrated a strong association between neovasculature and leukocyte and lipoprotein deposition in human coronary atherosclerosis.7, 8 Thus, neovasculature likely serves as a key route for lipoprotein and leukocyte infiltration into human atherosclerotic plaques.6, 29 Moreover, neovasculature has been associated with increased risk for plaque disruption.9, 30 However, if conditions are changed (e.g., marked reduction in plasma lipids) to facilitate atherosclerotic plaque regression,31-33 neovasculature also likely serves as a route by which macrophages might remove cholesterol and other debris from atherosclerotic plaques.34-36 Therefore, the ability to monitor therapy-induced changes in plaque neovasculature may represent a novel window into therapy-induced changes in plaque biology.
It is important to recognize that although these studies were performed in the context of the larger AIM-HIGH Trial, they were obtained very early in the trial, within three months of randomization either to statin or to statin plus extended-release niacin; therefore, it is unlikely that trial-specific therapy modified the major findings. No univariate association was found between prior niacin use with Vp. However, only 15 participants were on niacin prior to study entry, which limits the power of this comparison. It also is important to acknowledge that because AIM-HIGH was performed in patients with documented cardiovascular disease, the baseline rates of statin treatment in study participants was very high. Moreover, the average LDL cholesterol level in this population was only 72 mg/dL and blood pressure was well-controlled. Thus, the trial provided an excellent opportunity to examine potential relationships between a number of clinical, demographic and laboratory variables with DCE-MRI parameters in carotid plaques of patients with established cardiovascular disease who were treated to contemporary risk reduction standards.
It is intriguing that in multivariate analysis, Lp(a) was the only lipid variable found to be significantly associated with the baseline extent of plaque neovasculature. Epidemiologic37, 38 and genetic39, 40 studies have shown strong associations of Lp(a) with risks for cardiovascular diseases, including stroke. Multiple mechanisms by which Lp(a) might increase cardiovascular risk have been proposed, including lipid deposition in the artery wall,41 with subsequent oxidative modification,42 as well as a potential prothrombotic effect related to competitive inhibition of plasminogen by the structurally similar Kringle IV domains of apolipoprotein(a).43 Interestingly, statin therapy alone has minimal to no effect on circulating Lp(a) levels44 and, in contrast to other lipoprotein variables, only Lp(a) retained its on-trial relationship to risk for CV events in both arms of the AIM-HIGH Trial.45, 46
In both univariate and multivariate analyses, the most striking finding of this study was the statistically significant, inverse association of Vp with statin therapy duration. A small study of 28 humans observed a decrease, though not statistically significant, in Vp over 12 months of atorvastatin (0.068 to 0.059, p=0.3).47 However, their measure of Vp was restricted to the region within 0.625 mm (1 pixel) of the outer wall boundary which targets the adventital layer, while the Vp measurement in this study included the vascularity of both plaque and adventitia. Animal studies have demonstrated that hypercholesterolemia increases plaque neovasculature,29 while statin treatment has been shown to decrease neovasculature in hypercholesterolemic pig,17 rabbit19, 20 and apoE-deficient mouse18 models of atherosclerosis. Intriguingly, the decreases in neovasculature seen in these models associated with statins were found to be independent of changes in plasma cholesterol. In our study, we found a strong inverse association between carotid plaque neovasculature, and duration of statin therapy, but no association between neovasculature and any lipid/lipoprotein variable other than Lp(a), the levels of which are unaffected by statins. Thus, the in vivo observations of this human study are consistent with the limited literature on the effects of statin intervention on neovasculature in animal models and humans.
While few associations with plaque Ktrans were found, some trends were consistent with our previous report, including that Ktrans values tended to be higher in current smokers and those with higher systolic blood pressure.11 However, our previously finding of a significant negative correlation between HDL and Ktrans was not seen in the present study. There are important differences between these two studies that limits their comparability. The present study selected patients with dyslipidemia and low HDL-C and this restricted range of HDL-C may have lowered power to detect an association with Ktrans. In addition, plaques in the present study were much less advanced than those in the prior study, which examined carotid endarterectomy patients.
Reproducibility of Vp and Ktrans measurements in the AIM-HIGH trial have recently been reported.23 In that study, Ktrans was found to be more reproducible than Vp overall, but the reproducibility of Vp was better in larger plaques, in particular those with area >25 mm2 (ICC = 0.73). The reproducibility study had a median plaque size of 29 mm2 while the present study had a median size of 36 mm2 (24% higher), so the overall reproducibility of the Vp measurements in this likely better than reported in the reproducibility sub-study. Nonetheless, the reproducibility of the kinetic parameters is generally lower than that for plaque morphology and composition measurements,48 so some associations between the clinical factors and Vp and Ktrans may have been missed due to diminished power.
The present study has some additional limitations that merit discussion. First, although performed in the context of a lipid intervention trial, this study examines associations of variables with neovasculature at baseline; therefore, it is not a randomized trial of statin intervention. Second, while the correlation of Vp with carotid plaque neovasculature has been histologically validated in highly stenotic carotid arteries,10, 11 Vp has not been independently validated in arteries with more moderate disease, as encountered in this study. Third, the study is limited by the need to exclude a relatively large proportion of the potential study participants, due primarily to insufficient image quality or acquisition, as well as to atherosclerotic plaques that were insufficiently large for reliable analyses. This issue was also noted in the AIM-HIGH DCE-MRI reproducibility sub-study.23 That study suggested that improved training for site MR technologists be performed which would allow them to better recognize poor quality and adjust; in addition, stricter quality controls measures could be adopted which monitor DCE-MRI protocol adherence as images are acquired and require repeat scans or retraining after protocol violations. Lastly, black-blood DCE-MRI protocols have recently been developed which may eliminate the need to exclude pixels within 1 mm of the lumen and thereby allow DCE-MRI of smaller, earlier lesions. 49, 50 Thus, it will be interesting to determine whether the findings might be replicated in randomized intervention trials and/or using newer agents or techniques that may potentially be more specific or sensitive markers of plaque neovasculature.
CONCLUSIONS
In summary, this study finds strong associations of shorter duration of statin therapy and higher Lp(a) levels with higher carotid plaque Vp, an MRI-derived marker of plaque neovasculature. The results suggest that this technique may hold promise in evaluating the potential value of neovasculature as a marker of plaque vulnerability, as well as the potential effects of therapies on this key player in both plaque atherogenesis and regression.
HIGHLIGHTS.
Carotid plaque Vp is an imaging-based marker of neovasculature density, previously validated using histology
Vp was measured in 98 subjects with established cardiovascular disease
Longer prior duration of statin therapy was associated with lower Vp
Higher lipoprotein(a) levels was independently associated with higher Vp
ACKNOWLEDGEMENTS
The authors thank the participants and investigators for the AIM-HIGH Trial and its HDL Proteomics and Carotid MRI Sub-studies, as well as the AIM-HIGH Executive Committee for their invaluable contributions to this work.
FUNDING SOURCES
This AIM-HIGH sub-study was supported by National Heart, Lung, and Blood Institute (NHLBI) grants R01-HL089504 (to Dr. O'Brien) and R01-HL088214 (to Dr. Zhao). No commercial entity provided any direct support for this sub-study, nor did any commercial entity have any role in sub-study oversight, design, or data analysis and interpretation.
The parent trial, AIM-HIGH, was supported by NHLBI grants U01-HL-081616 and U01-HL-081649, as well as by an unrestricted grant from Abbott Laboratories (now AbbVie), Chicago, IL, as well as drug donations from Abbott Laboratories and from Merck.
Abbreviations
- AIM-HIGH
Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes
- DCE-MRI
dynamic contrast-enhanced magnetic resonance imaging
- Ktrans
transfer constant
- Vp
fractional plasma volume
- SGRE
spoiled gradient recalled echo
- TOF
time of flight
- V-V
vasa vasorum
APPENDIX 1. Participating AIM-HIGH clinical sites and investigators (by AIM-HIGH Site #)
2 - Cardiology Consultants of Philadelphia - Paul Grena, Sharon Budzinski; 9 - Duke University - John Guyton, Shubi Khan; 10 - University of Calgary - Todd Anderson, Bev Madden; 14/30 - University of Pennsylvania /Philadelphia VA - Richard Dunbar, Dalia Roberts, Monica Williams; 15 - University of Southern California - Colletti, Andrea Contreras; 21 - University of Western Ontario - William Kostuk, Cathy Bone; 34 - Health Partners Riverside Clinic - Chhavi Chadha, Maureen Busch; 35 - St. Vincent's Charity Hospital - Laurie Sadler, Mariellen DeSmit, Tania Zalatel; 37 - St. Michael's, University of Toronto - Lawrence Leiter, Leslie Berndl; 43 - University of Maryland - Michael Miller, Abby Roberts; 47 - University of Washington Cardiology - Xue-Qiao Zhao, Kevin O'Brien, Suzanne Peck; 48 - University of Washington NW Lipid Research Center - Robert Knopp, Pathmaja Paramsothy, Alice Dowdy, Barbara Twaddle; 49 - Long Beach VA - Moti Kahyap, Olaf Fallye, Sunil Kakadia; 51 - Puget Sound VA - Kenneth Lehmann, Julie LaGuire; 53 - Vancouver General Hospital - Anthony Fung, Rebecca Fox, Linda Axen; 55 - Wake Forest University Endocrinology - Robin Crouse, Donna Davis; 56 - Wake Forest University Geriatrics - Jamehl Demons, Tricia Wittmer; 58 - Wake Forest University Cardiology - David Herrington, Vickie Wayne, Lynda Doomy; 62 - Baylor College of Medicine - Peter Jones, Terry Techmanski, Diane Tanksley; 67 - Christiana Care Health Services - Edward Goldenberg, Jackie Laucirica; 69 - McGuire VA - Franklin Zieve, Melissa Kimmel; 71 - Cardiovascular Consultants - Chris Geohas, Rose Prasad, Annie Laborin; 72 - Pennsylvania Cardiology Associates - Robert Norris, Maureen Boyle, Julie Yoon; 74 - Mayo Clinic - Stephen Kopecky, Cindy Woltman, Dawn Shelstad; 77 - Johns Hopkins University - Peter Kwiterovich, Kathleen Byrne; 78 - Heart Health Institute - Patrick Ma, Maureen McRae, Donna Louch; 79 - Methodist Hospital - Alan Hoffman, Mary Rangel; 86 - Kelsey Research Foundation - Haroon-Ur Harry Rashid, Stacy Meadows.
APPENDIX 2. Participating AIM-HIGH imaging sites and investigators
BAR - Barrows Neurological Institute - Jim Pipe, Sharmeen Joomun; BAY - Baylor School of Medicine - Joel Morrisett, Karima Ghazzaly; CAL - University of Calgary - Richard Frayne, Brian O'Brien, Frances Raymond; JHU - Johns Hopkins University - Bruce Wasserman, Rena Geckle; MAY - Mayo Clinic - John Huston, Mandie Maroney-Smith; ROB - Robarts Research Institute - Brian Rutt, Cyndi Harper Little; UBC - University of British Columbia - Alex MacKay, Linda Chandler; UOW - University of Washington - Chun Yuan, Baocheng Chu, Niranjan Balu; USC - University of Southern California - Patrick Colletti, Samuel Valencerina; WFU - Wake Forest University - J. Robin Crouse, J. Greg Terry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Outside of the present study, Dr. Yuan reports grants from the NIH and Philips Medical. He also serves as a Member of Radiology Advisory Network, Philips. Mr. Hippe reports grants from GE Healthcare. No other authors have any other potential conflicts of interest to disclose.
ClinicalTrials.gov Identifiers: NCT00880178, NCT01178320 and NCT00120289
REFERENCES
- 1.Falk E, Shah PK, Fuster V. Coronary Plaque Disruption. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 2.Santos-Gallego CG, Picatoste B, Badimón JJ. Pathophysiology of acute coronary syndrome. Curr Atheroscler Rep. 2014;16:401. doi: 10.1007/s11883-014-0401-9. [DOI] [PubMed] [Google Scholar]
- 3.Lendon CL, Davies MJ, Born GV, et al. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis. 1991;87:87–90. doi: 10.1016/0021-9150(91)90235-u. [DOI] [PubMed] [Google Scholar]
- 4.Davies MJ, Richardson PD, Woolf N, et al. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993;69:377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno PR, Falk E, Palacios IF, et al. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 6.Moreno PR, Purushothaman K-R, Sirol M, et al. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien KD, Allen MD, McDonald TO, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien KD, McDonald TO, Chait A, et al. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996;93:672–682. doi: 10.1161/01.cir.93.4.672. [DOI] [PubMed] [Google Scholar]
- 9.Moreno PR, Purushothaman KR, Fuster V, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 10.Kerwin W, Hooker A, Spilker M, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation. 2003;107:851–856. doi: 10.1161/01.cir.0000048145.52309.31. [DOI] [PubMed] [Google Scholar]
- 11.Kerwin WS, O'Brien KD, Ferguson MS, et al. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology. 2006;241:459–468. doi: 10.1148/radiol.2412051336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaens ME, Backes WH, Rozel S, et al. Dynamic contrast-enhanced MR imaging of carotid atherosclerotic plaque: model selection, reproducibility, and validation. Radiology. 2013;266:271–279. doi: 10.1148/radiol.12120499. [DOI] [PubMed] [Google Scholar]
- 13.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 14.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 15.Williams JK, Sukhova GK, Herrington DM, et al. Pravastatin has cholesterol-lowering independent effects on the artery wall of atherosclerotic monkeys. J Am Coll Cardiol. 1998;31:684–691. doi: 10.1016/s0735-1097(97)00537-8. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa M, Rabkin E, Sugiyama S, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 17.Wilson SH. Simvastatin Preserves the Structure of Coronary Adventitial Vasa Vasorum in Experimental Hypercholesterolemia Independent of Lipid Lowering. 2002;105:415–418. doi: 10.1161/hc0402.104119. [DOI] [PubMed] [Google Scholar]
- 18.Bot I, Jukema JW, Lankhuizen IM, et al. Atorvastatin inhibits plaque development and adventitial neovascularization in ApoE deficient mice independent of plasma cholesterol levels. Atherosclerosis. 2011;214:295–300. doi: 10.1016/j.atherosclerosis.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Vucic E, Calcagno C, Dickson SD, et al. Regression of inflammation in atherosclerosis by the LXR agonist R211945: a noninvasive assessment and comparison with atorvastatin. JACC Cardiovasc Imaging. 2012;5:819–828. doi: 10.1016/j.jcmg.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian J, Hu S, Sun Y, et al. Vasa vasorum and plaque progression, and responses to atorvastatin in a rabbit model of atherosclerosis: contrast-enhanced ultrasound imaging and intravascular ultrasound study. Heart. 2013;99:48–54. doi: 10.1136/heartjnl-2012-302775. [DOI] [PubMed] [Google Scholar]
- 21.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 22.Underhill HR, Yarnykh VL, Hatsukami TS, et al. Carotid plaque morphology and composition: initial comparison between 1.5- and 3.0-T magnetic field strengths. Radiology. 2008;248:550–560. doi: 10.1148/radiol.2482071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Sun J, Kerwin WS, et al. Scan-rescan reproducibility of quantitative assessment of inflammatory carotid atherosclerotic plaque using dynamic contrast-enhanced 3T CMR in a multi-center study. J Cardiovasc Magn Reson. 2014;16:51. doi: 10.1186/s12968-014-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X-Q, Hatsukami TS, Hippe DS, et al. Clinical Factors Associated With High-Risk Carotid Plaque Features as Assessed by Magnetic Resonance Imaging in Patients With Established Vascular Disease (from the AIM-HIGH Study) Am J Cardiol. 2014 doi: 10.1016/j.amjcard.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerwin W, Xu D, Liu F, et al. Magnetic resonance imaging of carotid atherosclerosis: plaque analysis. Top Magn Reson Imaging. 2007;18:371–378. doi: 10.1097/rmr.0b013e3181598d9d. [DOI] [PubMed] [Google Scholar]
- 26.Kerwin W, Oikawa M, Yuan C, et al. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn Reson Med. 2008;59:507–514. doi: 10.1002/mrm.21532. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien K, Hippe D, Chen H, et al. Summary of Clinical and Laboratory Data from those on Statin Therapy at Enrollment in the AIM-HIGH Trial. Data in Brief. doi: 10.1016/j.dib.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barger AC, Beeuwkes R, Lainey LL, et al. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 29.Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovasc Res. 2007;75:649–658. doi: 10.1016/j.cardiores.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purushothaman K-R, Purushothaman M, Muntner P, et al. Inflammation, neovascularization and intra-plaque hemorrhage are associated with increased reparative collagen content: implication for plaque progression in diabetic atherosclerosis. Vasc Med. 2011;16:103–108. doi: 10.1177/1358863X11402249. [DOI] [PubMed] [Google Scholar]
- 31.Brown G, Albers JJ, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 32.Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–2087. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 33.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 34.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 35.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno PR, Purushothaman M, Purushothaman K-R. Plaque neovascularization: defense mechanisms, betrayal, or a war in progress. Ann N Y Acad Sci. 2012;1254:7–17. doi: 10.1111/j.1749-6632.2012.06497.x. [DOI] [PubMed] [Google Scholar]
- 37.Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamon-Fava S, Marcovina SM, Albers JJ, et al. Lipoprotein(a) levels, apo(a) isoform size, and coronary heart disease risk in the Framingham Offspring Study. J Lipid Res. 2011;52:1181–1187. doi: 10.1194/jlr.M012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, et al. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 40.Trégouët D-A, König IR, Erdmann J, et al. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet. 2009;41:283–285. doi: 10.1038/ng.314. [DOI] [PubMed] [Google Scholar]
- 41.Rath M, Niendorf A, Reblin T, et al. Detection and quantification of lipoprotein(a) in the arterial wall of 107 coronary bypass patients. Arteriosclerosis. 1989;9:579–592. doi: 10.1161/01.atv.9.5.579. [DOI] [PubMed] [Google Scholar]
- 42.Tsimikas S, Brilakis ES, Miller ER, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 43.Marcovina SM, Albers JJ, Gabel B, et al. Effect of the number of apolipoprotein(a) kringle 4 domains on immunochemical measurements of lipoprotein(a) Clin Chem. 1995;41:246–255. [PubMed] [Google Scholar]
- 44.Deshmukh HA, Colhoun HM, Johnson T, et al. Genome-wide association study of genetic determinants of LDL-c response to atorvastatin therapy: importance of Lp(a) J Lipid Res. 2012;53:1000–1011. doi: 10.1194/jlr.P021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albers JJ, Slee A, O'Brien KD, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes) J Am Coll Cardiol. 2013;62:1575–1579. doi: 10.1016/j.jacc.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guyton JR, Slee AE, Anderson T, et al. Relationship of lipoproteins to cardiovascular events: the AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes) J Am Coll Cardiol. 2013;62:1580–1584. doi: 10.1016/j.jacc.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong L, Kerwin WS, Chen H, et al. Carotid artery atherosclerosis: effect of intensive lipid therapy on the vasa vasorum--evaluation by using dynamic contrast-enhanced MR imaging. Radiology. 2011;260:224–231. doi: 10.1148/radiol.11101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J, Zhao X-Q, Balu N, et al. Carotid magnetic resonance imaging for monitoring atherosclerotic plaque progression: a multicenter reproducibility study. Int J Cardiovasc Imaging. 2014 doi: 10.1007/s10554-014-0532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calcagno C, Robson PM, Ramachandran S, et al. SHILO, a novel dual imaging approach for simultaneous HI-/LOw temporal (Low-/Hi-spatial) resolution imaging for vascular dynamic contrast enhanced cardiovascular magnetic resonance: numerical simulations and feasibility in the carotid arteries. J Cardiovasc Magn Reson. 2013;15:42. doi: 10.1186/1532-429X-15-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H, Ricks J, Rosenfeld M, et al. Progression of experimental lesions of atherosclerosis: assessment by kinetic modeling of black-blood dynamic contrast-enhanced MRI. Magn Reson Med. 2013;69:1712–1720. doi: 10.1002/mrm.24415. [DOI] [PubMed] [Google Scholar]