Abstract
Poultry infected with Salmonella mount an immune response initially, however the immune responses eventually disappear leading the bird to be a carrier of Salmonella. The hypothesis of this study is that Salmonella infection induces T regulatory cell numbers and cytokine production and suppress host T cells locally in the gut to escape the host immune responses. An experiment was conducted to comparatively analyze the effect of S. enterica ser. Enteritidis (S. Enteritidis) and S. enterica ser. Heidelberg (S. Heidelberg) infection on CD4+CD25+ T regulatory cell properties in chickens. A total of 144 broiler chicks were randomly distributed into three experimental groups of non-infected control, S. Enteritidis infected and S. Heidelberg infected groups. Chickens were orally inoculated with PBS (control) or 5x106 CFU/mL of either S. Enteritidis or S. Heidelberg at 3 d of age. Each group was replicated in six pens with eight chickens per pen. Chickens infected with S. Enteritidis had 6.2, 5.4, and 3.8 log10 CFU/g, and chickens infected with S. Heidelberg had 7.1, 4.8, and 4.1 log10 CFU/g Salmonella in the cecal contents at 4, 11, and 32 dpi, respectively. Both S. Enteritidis and S. Heidelberg were recovered from the liver and spleen 4 dpi. At 4, 11, and 32 dpi, chickens infected with S. Enteritidis and S. Heidelberg had increased CD4+CD25+ cell numbers as well as IL-10 mRNA transcription of CD4+CD25+ cells compared to that in the control group. CD4+CD25+ cells from S. Enteritidis- and S. Heidelberg-infected chickens and restimulated with 1 μg antigen in vitro, had higher (P < 0.05) IL-10 mRNA transcription than the CD4+CD25+ cells from the non-infected controls Though at 4dpi, chickens infected with S. Enteritidis and S. Heidelberg had a significant (P < 0.05) increase in CD4+CD25- IL-2, IL-1β, and IFNγ mRNA transcription, the CD4+CD25- IL-2, IL-1β, and IFNγ mRNA transcription, were comparable to that in the control group at 11 and 32dpi identifying that the host inflammatory response against Salmonella disappears at 11 dpi. It can be concluded that S. Enteritidis and S. Heidelberg infection at 3 d of age induces a persistent infection through inducing CD4+CD25+ cells and altering the IL-10 mRNA transcription of CD4+CD25+ cell numbers and cytokine production in chickens between 3 to 32 dpi allowing chickens to become asymptomatic carriers of Salmonella after 18 dpi.
Introduction
Among the foodborne pathogens of poultry, Salmonella is the most important foodborne pathogen of concern to the poultry industry [1]. Salmonella infection is caused by S. enterica colonizing poultry intestines at a very early age and the chickens infected with Salmonella after 3 days of age results in persistent infection of poultry with Salmonella [2]. Salmonella is a facultative intracellular bacterium and induces the host innate inflammatory response, characterized by pro-inflammatory cytokines and granulocyte influx [3]. Inflammatory cytokines increase during the early phase of Salmonella infection, and IFNγ is upregulated during a later phase [4]. However, the induced host inflammatory response is ultimately downregulated, leading to Salmonella continued survival and persistence in poultry gut for even up to 10 weeks of age [5]. Though Salmonella infection induces low levels of mucosal IgA and gut-associated T cell response [6] the induced humoral response does not always translate into protective immune responses in infected chickens [7].
One of the pathways through which Salmonella escapes the host immune responses is through inducing T regulatory cell activity, which act to suppress host immune responses directed against commensal bacteria [8]. Gut immune responses facilitate the survival of commensal bacteria by inducing anergy in host immune cells that are directed towards commensal microbes through T regulatory cells. Though Salmonella infection causes severe symptoms in humans, chickens infected with S. enterica do not mount efficient immune responses and are asymptomatic [9], suggesting that S. enterica is a commensal bacteria in the chicken gut [10]. We earlier identified that S. enterica serovar Enteritidis (S. Enteritidis) infection increases T regulatory cell numbers in the chicken gut until 14 dpi [11] to suppress the host immune responses locally in the gut. Identifying if this suppression of host immune responses post-Salmonella infection is a local or systemic effect will identify how to address the persistent infection of Salmonella in poultry.
Recently, several serovars of S. enterica, including Heidelberg, Kentucky, Enteritidis, Typhimurium, Montevideo, Senftenberg, and Thompson [12–16] were involved in causing Salmonella outbreaks in poultry. The multistate outbreak of multidrug-resistant S. enterica serovar Heidelberg (S. Heidelberg) [17] highlights the importance of studying multiple Salmonella serovar infections of chickens. Tight junction proteins like Claudin and Zona occludens are critical components to maintain intestinal barrier function in poultry. S. Typhimurium infection decreases tight junction protein mRNA [18] and thereby allowing the pathogen to cross the intestinal lumen and reach blood circulation [19] and spread to the liver, spleen, ovary, and oviduct [20]. T regulatory cell upregulation has been identified to promote gut integrity in experimental cirrhosis model of mice [21]. Therefore, it will be interesting to identify if Salmonella—induced T regulatory cells can act to maintain the gut integrity in poultry.
Despite the importance of Salmonella as a human pathogen, relatively little is known about how Salmonella manages to escape the poultry immune response especially when the birds come into production at 35 d of age. The study hypothesizes that Salmonella infection induces CD4+CD25+ T regulatory cells and suppresses host T cells locally in the gut to escape the host immune responses. The objective of this study is to comparatively analyze the effect of S. Enteritidis and S. Heidelberg infection on CD4+CD25+ T regulatory cell properties and CD4+CD25- T cells in chickens between 4 to 32 dpi to identify if infection with two different serovars of Salmonella can induce host immune cells properties in an antigen-specific pathway. We also studied CD4+CD25+ T regulatory cell and T cell cytokine profiles (IL-10, TGF-β, IL-1β, LITAF, and IL-2 mRNA) to determine whether the oral infection of Salmonella can translocate the intestinal barrier to reach internal organs like the liver and the spleen and induce systemic effect.
Materials and methods
Ethics statement
All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Georgia (AUP: A2017 07-004-Y3-A0). Researchers involved in the in vivo trial were trained by the University of Georgia on animal care and handling (UGA IACUC 101 course). Chickens were monitored at least once a day for lethargy, loss of body weight, ruffled feathers, diarrhea, and dehydration during the in vivo experiment. Chickens that could not move or refused to eat were immediately humanely euthanized by cervical dislocation. None of the chickens were found dead during this trial no chickens were euthanized for humane reasons. Chickens were euthanized during sampling time points and the last day of the study (day 35).
Chickens and S. Enteritidis and S. Heidelberg infection
Wild type S. Enteritidis (gift from Dr. G. Rajashekara, The Ohio State University) and Wild type S. Heidelberg (gift from Dr. D. Jones, USDA/ARS) were selected on XLT-4 agar for Nalidixic acid resistance at 500 mg L−1 of nalidixic acid (Sigma-Aldrich, St. Louis, MO). The nalidixic acid-resistant colonies were grown at 37°C overnight on Muller-Hinton broth containing 500 mg L−1 nalidixic acid and further used in experimental studies. Inoculum for infection was prepared from 18 to 24 h cultures. The bacterial concentration was determined spectrophotometrically using a standard curve at a reference wavelength of 600 nm. A stock solution (1x109 CFU/mL) was prepared by diluting the culture in sterile phosphate-buffered saline (pH 7.2).
A total of 144 broiler chicks (Cobb 500, Cobb hatcheries, Cleveland, GA) were randomly distributed into three experimental groups of non-infected control, S. Enteritidis infected and S. Heidelberg infected groups. All chickens were screened for Salmonella by cloacal swab at 0 d of age. Chickens in the infected groups were orally inoculated with 5x106 CFU/mL in 250 μL of either S. Enteritidis or S. Heidelberg and chickens in the control groups were inoculated with 250 μL of sterile PBS at 3 d of age. Each group was replicated in six pens with eight chickens per pen. At d 7, 14, 21, 28, and 35 d of age (4, 11, 18, 25, and 32 dpi), one bird per pen (n = 6) from each group was randomly selected and killed by cervical dislocation, and samples were collected.
Effect of S. Enteritidis and S. Heidelberg infections on S. Enteritidis and S. Heidelberg load in the ceca, spleen, and liver
On 4, 11, 18, 25, and 32 dpi, cecal content, spleen, and liver were collected aseptically from six chickens (n = 6) per treatment into stomacher bags, placed on ice, and transported to the laboratory. One g of either cecal content or spleen or liver samples were taken in the stomacher bags and macerated with a pestle. The stomacher bags with macerated samples were mixed with 1X (wt./vol.) of buffered peptone water and the bags were stomached for 90 s. A volume of 10 μl of homogenates was either directly plated or serially diluted in 10−1 to 10−5 dilutions using the micro dilutions method as described earlier [22]. From every dilution, a volume of 10μl was spotted in triplicate on XLT-4 agar plates. Plates were then incubated for 48 h at 37.5°C. After incubation, colonies were counted and confirmed by SyBr green qPCR using primers described in Table as described earlier [23]. Enumeration data were recorded as CFU/g and then transformed to log10 CFU/g for statistical analysis.
Effect of S. Enteritidis and S. Heidelberg infection on CD+CD25+ cell percentage in the cecal tonsils and spleen
Spleen and cecal tonsils were teased over a 40 μm cell strainer (Sigma-Aldrich, St. Louis, MO) with approximately 5 ml of RPMI to obtain a single-cell suspension. Single-cell suspensions of spleen and cecal tonsils from six chickens per treatment on 4, 11, 18, 25, and 32 dpi (n = 6) were concentrated for lymphocyte isolation by density centrifugation utilizing Histopaque (1.077 g/mL; Sigma-Aldrich, St. Louis, MO). Anti-chicken CD25 antibody production was earlier described [24]. Anti-chicken CD25 was labeled with PE using the Lighting-link PE kit (Novus Biologicals, LLC Littleton CO). Cells (1 × 106) were incubated with 10 μg/mL PE linked mouse anti-chicken CD25, 1:200 APC-conjugated mouse anti-chicken CD4 (Southern Biotechnology Associates, Birmingham, AL), and 1:200 dilution of unlabeled mouse IgG for 45 min. The unbound primary antibodies were removed by centrifugation. The percentage of CD4+CD25+ cells in different organs was analyzed in a flow cytometer (Guava Eascyte; Millipore, Billerica, MA). A total of 50,000 events were collected. CD4+CD25+ cell percentage was analyzed after gating cells based on forward scatter and side scatter plots for lymphocytes. CD4+ and CD4+CD25+ cell percentage was expressed as a percentage of CD4+ cells.
Effect of S. Enteritidis and S. Heidelberg infection on immune-related mRNA transcription in CD4+CD25+ and CD4+CD25- cells from cecal tonsils and spleen
Single-cell suspension of cecal tonsils and spleen were labeled for CD4 and CD25 as described above and flow-sorted for CD4+CD25+ and CD4+CD25- cells using an iCyt reflection cell sorter, Champaign, IL (~99% pure) after gating on cells based on forward scatter and side scatter plot for lymphocytes. Total RNA from CD4+CD25+ and CD4+CD25- cells at 4, 11, and 32 dpi were extracted and reverse transcribed into cDNA as described earlier [25] and analyzed for the relative expression of IL-10, TGF-β, IL-1β, LITAF, and IL-2 mRNA transcription, after normalizing for β-actin mRNA transcription, using the primers listed in Table 1. Relative mRNA expression was calculated using the 2-ΔΔCt method described earlier [26], where Ct is the threshold cycle.
Table 1. Primers and PCR conditions for RT qPCR.
| Target gene | Sequence (5’-3’) | Annealing Temperature | Reference |
|---|---|---|---|
| IL-10-F | CATGCTGCTGGGCCTGAA | 58.0°C | [27] |
| IL-10-R | CGTCTCCTTGATCTGCTTGATG | ||
| TGF-β4 –F | GACAGCCATCCGCATCTTCT | 58.0°C | [28] |
| TGF-β4 –R | CATACTCCTGGGTCTGGTTGGT | ||
| TLR-4-F | ACCTACCCATCGGACACTTG | 60.0°C | [29] |
| TLR-4-R | TGCCTGAGAGGTCAGGTT | ||
| IL-1β-F | GCATCAAGGGCTACAAGCTC | 58.0°C | [30] |
| IL-1β-R | CAGGCGGTAGAAGATGAAGC | ||
| LITAF-F | ATCCTCACCCCTACCCTGTC | 58.0°C | [31] |
| LITAF-R | GGCGGTCATAGAACAGCACT | ||
| IL-2-F | CTGGGAGAAGTGGTTACTCTGA | 59.0°C | [31] |
| IL-2-R | ACCCGTAAGACTCTTGAGGTTC | ||
| IL-17-F | GCAGATGCTGGATGCCTAAC | 55.5°C | |
| IL-17-R | ATGGAGCCAGTGAGCGTTT | ||
| IFNγ-F | GGCGTGAAGAAGGTGAAAGA | 55.4°C | |
| IFNγ-R | CCTCTGAGACTGGCTCCTTTT | ||
| Claudin-1-F | CATACTCCTGGGTCTGGTTGGT | 55.0°C | [32] |
| Claudin-1-R | GACAGCCATCCGCATCTTCT | ||
| Zona occludens-1-F | TGTAGCCACAGCAAGAGGTG | 55.0°C | [33] |
| Zona occludens-1-R | CTGGAATGGCTCCTTGTGGT | ||
| β-actin–F | ACCGGACTGTTACCAACACC | 57.0°C | |
| β-actin -R | GACTGCTGCTGACACCTTCA | ||
| S. Enteritidis-F | GCCGAGCTTGATGACAAACCTG | 60.0°C | [34] |
| S. Enteritidis-R | GCGCTTCGCTTTTCCAACTGCC | ||
| S. Heidelberg-F | GCCGAGCTTGATGACAAACCTG | 55.0°C | [34] |
| S. Heidelberg-R | GCGCTTCGCTTTTCCAACTGCC |
Effect of S. Enteritidis and S. Heidelberg infections on tight junction protein mRNA transcription in the jejunum and ceca
On 4, 11, and 32 dpi, approximately 1 cm of jejunum or ceca samples were collected in 2 ml of RNAlater (Qiagen, Germantown, MD) Excess RNAlater was removed from tubes, and samples were stored at -80°C until analyzed. Total RNA was extracted from cecal tonsils and reverse transcribed into cDNA [25] and analyzed for the relative expression of Claudin-1 and Zona occludens-1 mRNA transcription, after normalizing for β-actin mRNA transcription, using the primers listed in Table 1. Relative mRNA expression was calculated using the 2-ΔΔCt method described earlier [26], where Ct is the threshold cycle.
Effect of S. Enteritidis and S. Heidelberg infections on antigen-specific in vitro recall response of cecal tonsil CD4+CD25+ and CD4+CD25-cells
Heat-killed S. Enteritidis and S. Heidelberg antigen were prepared by heating 1 mL of 1.25 X 108 S. Enteritidis at 65°C for 1 h [35]. Whole cecal tonsils were passed over a 0.4 μm cell strainer to obtain a single-cell suspension. Cecal tonsil cells (1 X 108) were stimulated in vitro with 0 or 1 μg of heat-killed S. Enteritidis antigen in 1000 μl of RPMI medium supplemented with 5% fetal bovine serum, 1% penicillin plus streptomycin for 48 h. CD4+CD25+ and CD4+CD25- cells from the 48 h culture were flow sorted as described above. The CD4+CD25+ and CD4+CD25- cells cell purities were at least 90% as determined in a flow cytometer. Total RNA was extracted and analyzed for the relative expression of IL-10, IL-2, IFNγ, and IL-17 mRNA as described above.
Statistical analysis
A one-way ANOVA (JMP software, Cary, NC) was used to examine the effect of different parameters studied on dependent variables. When the effects were significant (P < 0.05), differences between means were analyzed by Tukey’s least-square means comparison.
Results
Effect of S. Enteritidis and S. Heidelberg infections on S. Enteritidis and S. Heidelberg load in the ceca, spleen, and liver
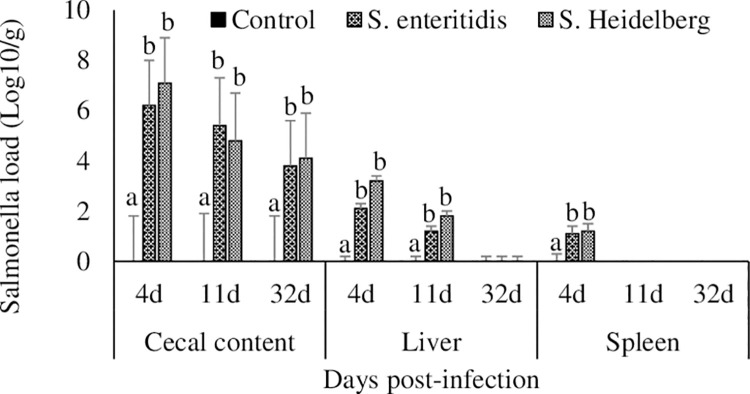
There were no detectable amounts of Salmonella in the cecal content, liver, and spleen of control group chickens at any of the time points studied (Fig 1). Chickens infected with S. Enteritidis had 6.2, 5.4, and 3.8 log10 CFU/g, and chickens infected with S. Heidelberg had 7.1, 4.8, and 4.1 log10 CFU/g Salmonella in the cecal contents at 4, 11, and 32 dpi, respectively. Both S. Enteritidis and S. Heidelberg had crossed the intestinal barrier and were recovered from the liver at 4 and 11 dpi. Both S. Enteritidis and S. Heidelberg were recovered from the spleen at 4 dpi, after which there were no detectable amounts of S. Enteritidis and S. Heidelberg in the spleen.
Fig 1. Effect of S. Enteritidis and S. Heidelberg infections on S. Enteritidis and S. Heidelberg load in the ceca, spleen, and liver.
Chickens (3 d-old) were orally infected with 0 (control) or 5x106 CFU of S. Enteritidis or S. Heidelberg in six replications. At 4, 11, and 32 d post-infection, S. Enteritidis or S. Heidelberg loads in the cecal content, liver, and spleen were estimated by a micro-dilution method (CFU/g) and then log-transformed to log10 CFU/g for statistical analysis. Means ± SEM. Bars without a common superscript differ significantly each measured day post-infection (P < 0.05). n = 6.
Effect of S. Enteritidis and S. Heidelberg infections on CD4+CD25+ cell percentage in the cecal tonsils and spleen
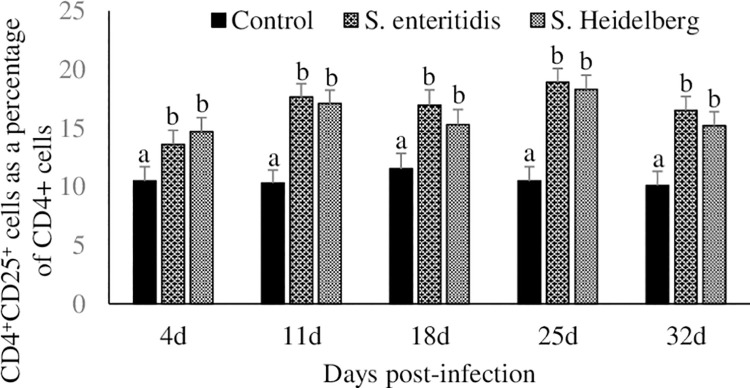
Approximately 10% of the cecal tonsil CD4+ cells were CD4+CD25+ cells in the control group (Fig 2). Chickens infected with S. Enteritidis and S. Heidelberg had a significant (P < 0.05) increase in cecal tonsil CD4+CD25+ cell percentages at 4, 11, 18, 25, and 32 dpi, compared to that in the control group. Chickens in the S. Enteritidis infected groups had further 3.1 to 8.4% and chickens in the S. Heidelberg infected groups had a further 3.8 to 7.8% increase in cecal tonsil CD4+CD25+ cell percentages compared to the control group between 4 to 32 dpi.
Fig 2. Effect of S. Enteritidis and S. Heidelberg infections on CD4+CD25+ cell percentage in the cecal tonsils.
Chickens (3 d-old) were orally infected with 0 (control) or 5x106 CFU of S. Enteritidis or S. Heidelberg in six replications. At 4, 11, 18, 25, and 32 d post-infection, CD4+CD25+ regulatory T cell percentage in cecal tonsils were analyzed by flow cytometry. CD4+CD25+ cell percentage was expressed as a percent of CD4+ cells to facilitate comparison between samples. Means ± SEM. Bars without a common superscript differ significantly each measured day post-infection (P < 0.05). n = 6.
There were no significant differences (P > 0.05) in the spleen CD4+CD25+ cell percentages at any of the time point studied.
Effect of S. Enteritidis and S. Heidelberg infections on IL-10, TGF-β, IL-1β, LITAF, and IL-2 mRNA transcription in CD4+CD25+ cells from cecal tonsils and spleen
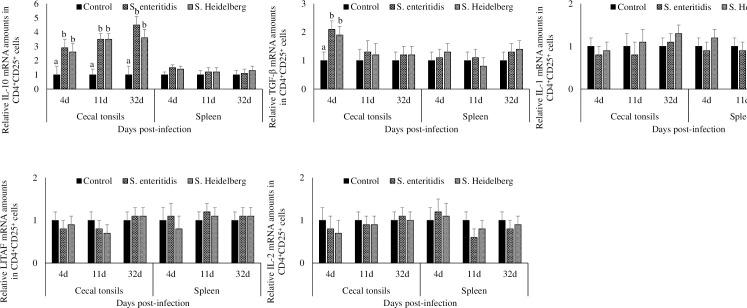
Chickens infected with S. Enteritidis and S. Heidelberg had a significant (P < 0.05) increase in the cecal tonsil CD4+CD25+ cell IL-10 mRNA transcription at 4, 11, and 32 dpi, compared to that in the control group (Fig 3). At 4, 11, and 32 dpi, chickens in the S. Enteritidis infected groups had 2.9-, 3.5-, and 4.5- fold, and chickens in the S. Heidelberg infected groups had 2.6-, 3.5-, and 3.6- fold increase in cecal tonsil CD4+CD25+ cell IL-10 mRNA compared to that in the control group, respectively.
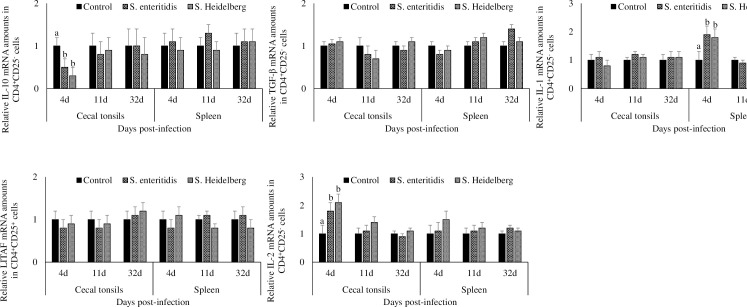
Fig 3. Effect of S. Enteritidis and S. Heidelberg infections on IL-10, TGF-β, IL-1β, LITAF, and IL-2 mRNA amounts in CD4+CD25+ cells from cecal tonsils and spleen.
Chickens (3 d-old) were orally infected with 0 (control) or 5x106 CFU of S. Enteritidis or S. Heidelberg in six replications. At 4, 11, and 32 d post-infection, CD4+CD25+ cells were flow-sorted and analyzed for IL-10, TGF-β, IL-1β, LITAF, and IL-2 mRNA after normalizing for β-actin mRNA. Relative amounts of mRNA were expressed as fold change from the control. Means ± SEM. Bars without a common superscript differ significantly each measured day post-infection (P < 0.05). n = 6.
Chickens infected with S. Enteritidis and S. Heidelberg had comparable splenic CD4+CD25+ cell IL-10 mRNA transcription to that in the control group at all time points studied.
At 4dpi, chickens infected with S. Enteritidis and S. Heidelberg had a significant (P < 0.05) increase of TGF-β mRNA in the cecal tonsil CD4+CD25+ cells compared to that in the control group. At 4 dpi, chickens in the S. Enteritidis infected groups had 2.1- fold, and chickens in the S. Heidelberg infected groups had 1.9- fold increase of TGF-β mRNA in the cecal tonsil CD4+CD25+ cells compared to that in the control group. At 11 and 32 dpi, chickens infected with S. Enteritidis and S. Heidelberg had comparable TGF-β mRNA transcription in the cecal tonsil CD4+CD25+ cells to that in the control group.
There were no significant differences in the cecal tonsil and splenic CD4+CD25+ cell IL-1β, LITAF, and IL-2 mRNA transcription between the treatment groups at any of the time points studied.
Effect of S. Enteritidis and S. Heidelberg infections on antigen-specific in vitro recall response of cecal tonsil CD4+CD25+ and CD4+CD25-cells
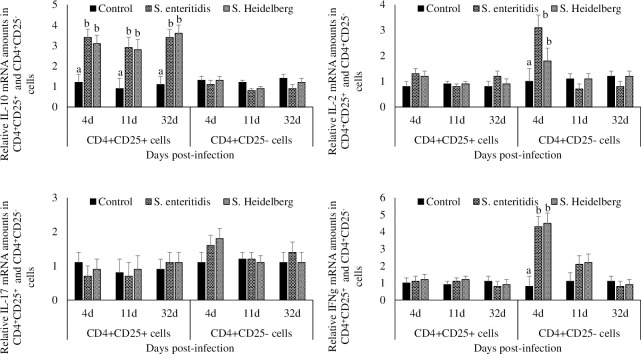
CD4+CD25+ cells from S. Enteritidis- and S. Heidelberg-infected chickens and were re-stimulated with 1 μg antigen in vitro, had higher IL-10 mRNA content than the CD4+CD25+ cells from the non-infected controls (P < 0.05; Fig 4).
Fig 4. Effect of S. Enteritidis and S. Heidelberg infections on antigen-specific in vitro recall response of cecal tonsil CD4+CD25+ and CD4+CD25-cells.
Chickens (3d-old) were orally infected with 0 (control) or 5x106 CFU of S. Enteritidis or S. Heidelberg in six replications. At 4, 11, and 32 d post-infection, 1 X 108 cecal tonsil cells were stimulated in vitro with 0 or 1 μg of heat-killed S. Enteritidis or S. Heidelberg antigens. Cecal tonsil cells were then incubated with killed antigens from either S. Enteritidis or S. Heidelberg for 48h. CD4+CD25+ cells and CD4+CD25- cells were flow-sorted and analyzed for IL-10, IL-2 IL-17 and IFN-γ mRNA after normalizing for β-actin mRNA. Relative amounts of mRNA were expressed as fold change from that in the respective 0 μg antigen treated group. Means ± SEM. Bars without a common superscript differ significantly each measured day post-infection (P < 0.05). n = 6.
CD4+CD25- cells from S. Enteritidis- and S. Heidelberg-infected chickens and stimulated with 1 μg antigen in vitro, had higher IL-2 and IFN-γ mRNA content than the CD4+CD25- cells from the non-infected controls at 4dpi (P < 0.05; Fig 6). There were no significant differences between the IL-2 and IFN-γ mRNA transcription of CD4+CD25- cells collected at 11 and 32dpi.
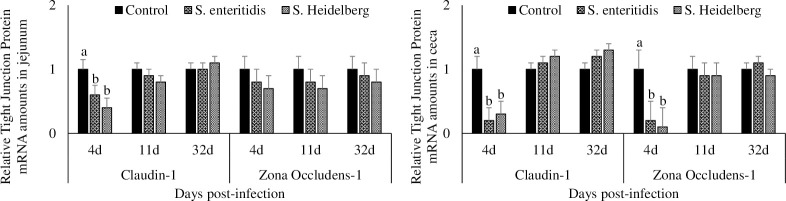
Fig 6. Effect of S. Enteritidis and S. Heidelberg infections on tight junction protein mRNA amounts in the jejunum and ceca.
Chickens (3 d-old) were orally infected with 0 (control) or 5x106 CFU of S. Enteritidis or S. Heidelberg in six replications. At 4, 11, and 32 d post-infection, jejunal and ceca samples were analyzed for Claudin-1 and Zona occludens-1 mRNA after normalizing for β-actin mRNA. Relative amounts of mRNA were expressed as fold change from the control. Means ± SEM. Bars without a common superscript differ significantly each measured day post-infection (P < 0.05). n = 6.
There were no significant differences between the IL-17 mRNA transcription of CD4+CD25+ cells and CD4+CD25- cells collected at 11 and 32dpi.
Effect of S. Enteritidis and S. Heidelberg infections on IL-10, TGF-β, IL-1β, LITAF, and IL-2 mRNA transcription in CD4+CD25- cells from cecal tonsils and spleen
Chickens infected with S. Enteritidis and S. Heidelberg had a significant decrease in the cecal tonsil CD4+CD25- cell IL-10 mRNA transcription at 4 dpi, compared to that in the control group (P < 0.05; Fig 5). At 4 dpi, chickens in the S. Enteritidis infected groups had 50% and chickens in the S. Heidelberg infected groups had a 70% decrease in cecal tonsil CD4+CD25- cell IL-10 mRNA compared to that in the control group.
Fig 5. Effect of S. Enteritidis and S. Heidelberg infections on IL-10, TGF-β, IL-1β, LITAF, and IL-2 mRNA amounts in CD4+CD25- cells from cecal tonsils and spleen.
Chickens (3 d-old) were orally infected with 0 (control) or 5x106 CFU of S. Enteritidis or S. Heidelberg in six replications. At 4, 11, and 32 d post-infection, CD4+CD25- cells were flow-sorted and analyzed for IL-10, TGF-β, IL-1β, LITAF, and IL-2 mRNA after normalizing for β-actin mRNA. Relative amounts of mRNA were expressed as fold change from the control. Means ± SEM. Bars without a common superscript differ significantly each measured day post-infection (P < 0.05). n = 6.
At 4 dpi, chickens infected with S. Enteritidis and S. Heidelberg had a significant increase in the cecal tonsil CD4+CD25- cell IL-1β mRNA compared to that in the control group (P < 0.05). At 4 dpi, chickens in the S. Enteritidis infected groups had 1.9- fold, and chickens in the S. Heidelberg infected groups had a 1.8- fold increase in cecal tonsil CD4+CD25- cell IL-1β mRNA compared to that in the control group. At 11 and 32 dpi, chickens infected with S. Enteritidis and S. Heidelberg had comparable cecal tonsil CD4+CD25- cell IL-1β mRNA transcription to that in the control group.
At 4dpi, chickens infected with S. Enteritidis and S. Heidelberg had a significant increase in the cecal tonsil CD4+CD25- cell IL-2 mRNA compared to that in the control group (P < 0.05). At 4 dpi, chickens in the S. Enteritidis infected groups had 1.8- fold, and chickens in the S. Heidelberg infected groups had 2.1- fold increase in cecal tonsil CD4+CD25- cell IL-2 mRNA compared to that in the control group. At 11 and 32 dpi, chickens infected with S. Enteritidis and S. Heidelberg had comparable cecal tonsil CD4+CD25- cell IL-2 mRNA transcription to that in the control group.
There were no significant differences between the cecal tonsil and splenic CD4+CD25- cell TGF-β and LITAF mRNA transcription between the treatment groups at any of the time points studied.
Effect of S. Enteritidis and S. Heidelberg infections on tight junction protein mRNA transcription in the jejunum and ceca
At 4 dpi, chickens infected with S. Enteritidis had a significant 40% decrease and chickens infected with S. Heidelberg had a significant 60% decrease in jejunal Claudin-1 mRNA transcription compared to the control group (P < 0.05; Fig 6). At 11 and 32dpi, chickens infected with S. Enteritidis and S. Heidelberg had comparable jejunal Claudin-1 mRNA transcription with that of the control chickens.
At 4 dpi, chickens infected with S. Enteritidis had a 20% decrease, and chickens infected with S. Heidelberg had a 30% decrease in jejunal Zona-occludens-1 mRNA transcription compared to the control group (P > 0.05). At 11 and 32 dpi, chickens infected with S. Enteritidis and S. Heidelberg had comparable jejunal Zona occludens-1 mRNA transcription with that of the control chickens.
At 4 dpi, chickens infected with S. Enteritidis had a significant 80% decrease and chickens infected with S. Heidelberg had a significant 70% decrease in cecal Claudin-1 mRNA transcription compared to the control group (P < 0.05). At 11 and 32dpi, chickens infected with S. Enteritidis and S. Heidelberg had comparable cecal Claudin-1 mRNA transcription with that of the control chickens.
At 4 dpi, chickens infected with S. Enteritidis had a 80% decrease, and chickens infected with S. Heidelberg had a 90% decrease in cecal Zona-occludens-1 mRNA transcription compared to the control group (P < 0.05). At 11 and 32 dpi, chickens infected with S. Enteritidis and S. Heidelberg had comparable cecal Zona occludens-1 mRNA transcription with that of the control chickens.
Discussion
This study aimed to evaluate the properties of chicken regulatory T cells and T cells from a gut lymphoid tissue and spleen during S. Enteritidis- and S. Heidelberg infection. Infecting broiler chickens with S. Enteritidis and S. Heidelberg at 3 d of age caused S. Enteritidis and S. Heidelberg colonization of the gut, resulted in S. Enteritidis- and S. Heidelberg recovery in the liver and spleen, decreased jejunal Claudin-1 and Zona occludens-1 mRNA transcription, increased CD4+CD25- regulatory T cell percentages in the cecal tonsils, increased cecal tonsil CD4+CD25+ regulatory T cell IL-10 mRNA content, decreased cecal tonsil CD4+CD25- T cell IL-10 mRNA content and increased IL-10 mRNA production in CD4+CD25+ restimulated with Salmonella antigen.
It has been observed that chickens infected with S. Typhimurium at 1-week of age have the persistent infection until 6–7 weeks of age and the persistence has been attributed to the poorly developed immune system of chickens at hatch [2]. This study observed that both S. Enteritidis- and S. Heidelberg not only persisted in the chicken gut until 35 d of age but also crossed the intestinal barrier and were recovered from both the spleen and the liver. This study also observed that S. Enteritidis and S. Typhimurium decreases the tight junction protein expression in both the ceca and jejunum. Claudin proteins link adjacent enterocytes and S. Typhimurium downregulates the Zona occludens-1 and Claudin-5 mRNA in the ceca of birds to increase translocation of Salmonella to the systemic circulation [36]. It has been suggested that the host toll-like receptor-flagellate interactions regulate the ability of Salmonella serovars to cross the gut barrier. TLR5-flagellin interactions restrict flagellate serovars like Enteritidis and Typhimurium to the intestine, while permitting non-flagellate Salmonella serovars like S.Gallinarum and S.Pullorum to cross the gut barrier and colonize internal organs [37]. In addition, the Type III secretion system of Salmonella facilitates the host signal transduction pathways to decrease tight junction proteins like Zona occludens-1, occludin, and E-cadherin in MDCK-1 cell lines [38] and facilitate Salmonella invading the internal organs. It has been suggested that S. Typhimurium decreases the tight junction proteins in the jejunum [18] likely contributing to the bacteria translocating from the blood circulation to the internal organs like liver and spleen [19].
Salmonella lipopolysaccharide and flagella are pathogen-associated molecular patterns recognized by the host to initiate an immune response that is expected to clear the pathogen [39]. Upon infection with Salmonella, innate inflammatory response, characterized by pro-inflammatory cytokines and heterophils proliferation happens within hours [40,41]. This article observed that there was an increase in IL-1β and IFNγ mRNA content of splenic CD4+CD25- cells. The observed increase in the IL-1β and IFNγ mRNA of CD4+CD25- cells occurred only in the internal organ but not in the cecal tonsils, suggesting that the systemic host immune response to Salmonella infection differs from the mucosal immune response to Salmonella. IL-17 was not upregulated at any point of the infection with S. Enteritidis and S. Heidelberg. S. Enteritidis has been earlier shown to upregulate IL-17 in the cecal wall at 4 d post-infection, though by 7d post-infection, IL-17 levels are back to normal [42]. This suggests that the effect of S. Enteritidis on IL-17 is time dependent.
The interplay between regulatory T cells and other immune cells determines if the outcome of an infection is a successful infection of the host by a pathogen or the successful clearance of the pathogen by the host [43]. T regulatory cells facilitate the survival of commensal bacteria in mammals [44] and intestinal bacteria have co-evolved within the host to stimulate T regulatory cell activity to survive the host gut immune response [45]. Depleting Tregs in mice alters the balance between Salmonella proliferation and host immune response and results in clearance of Salmonella during a persistent Salmonella infection [46]. In this study, we observed that both the numbers of cecal tonsil CD4+CD25+ cells and the IL-10 mRNA content of the cecal tonsil CD4+CD25+ cells increased in chickens infected with S. Enteritidis- and S. Heidelberg. Since IL-10 is a regulatory cytokine that acts to reverse proinflammatory cytokine actions, increased IL-10 transcription of CD4+CD25+ cells in the gut can be expected to neutralize the increase in pro-inflammatory cytokine produced by other host T cells that will act to clear S. Enteritidis- and S. Heidelberg infection. This study observed an increase in IL-1β transcription in the spleen of birds infected with S. Enteritidis- and S. Heidelberg. Increased T regulatory cell numbers and functions can be expected to dampen the immune response against Salmonella in the gut leading to Salmonella persistence in the chicken gut. Interestingly S. Enteritidis- and S. Heidelberg infection mediated an increase in the suppressive properties of CD4+CD25+ cells only in the gut, but not in the spleen; suggesting that Salmonella-induced host immune suppression is not systemic but only a localized mucosal response. Though Salmonella infection induces macrophage, B and T cell immune response against Salmonella, these immune responses eventually wane [47], leading to persistent Salmonella infection. Regulatory cells are likely involved in suppressing the host immune response leading to persistent infection of the chicken gut, but not the internal organs.
T regulatory cells in mammalians are characterized by the presence of FoxP3 transcription factor, but functional FoxP3 is yet to be identified in chickens [48]. In the absence of FoxP3, chicken CD4+CD25+ have been characterized as T regulatory cells [24]. Because CD25 markers are not unique for T regulatory cells, IL-10 mRNA content and ability to suppress naïve T cells using a naïve T cell proliferation suppression assay of CD4+CD25+ cells are typically used as additional parameters to confirm the T regulatory properties of CD4+CD25+ cells [24,29]. Our earlier study with S. Enteritidis identified that CD4+CD25+ cells from cecal tonsils of S. Enteritidis infected birds had increased IL-10 and suppressed naïve T cells [11]. Though this study analyzed IL-10 mRNA amounts of CD4+CD25+ cells, but did not analyze the naïve T cell proliferation suppression assay of CD4+CD25+ cells, the previous study with CD4+CD25+ cells from S. Enteritidis birds, wherein naïve T cell proliferation study was conducted, suggest that CD4+CD25+ cells are indeed T regulatory cells. It is possible that some of the CD4+CD25+ cells analyzed in this study are activated T cells or other immune cells.
S. Enteritidis- and S. Heidelberg mediated increase in CD4+CD25+ suppressive properties are antigen-specific. Salmonella induces iNOS expression to inhibit the proliferation and differentiation of T cells [49] through T regulatory cells as depletion of T regulatory cells abrogated the S. Typhimurium induced loss of Th1 response [50]. We observed that the Salmonella antigen-induced IL-10 mRNA increase in CD4+CD25+ cells is antigen-specific as CD4+CD25+ cells from the control group when restimulated with Salmonella antigen did not increase IL-10 production. Earlier it has been observed that S. Typhi induces antigen-specific regulatory T cells in humans and migration of antigen-specific regulatory cells to the gut plays an important role in inducing typhoid fever through suppressing antigen-specific T effector cells [51].
This study utilized killed Salmonella antigens to stimulate CD4+CD25+ and CD4+CD25- cells in vitro. Though recall responses evaluate the ability of the antigens to stimulate different components of T cell signaling pathway, the assay is non-specific in that it cannot distinguish the presence of cross-reactive T-cell responses. Earlier it has been shown that cross priming where peptides from exogenous antigens were presented on class I MHC molecules leading to CD8+ T cell stimulation [52]. Another possibility is that the heat-killed antigens used in this study could have stimulated the TLR pathway in a non-antigenic pathway [53] or stimulated the natural killer T cells which can be activated by different bacterial antigens and lipopolysaccharide or flagellin [54].
It can be concluded that S. Enteritidis and S. Heidelberg infection at 3 d of age induces a persistent infection through induction of T regulatory cells. Altering the IL-10 mRNA transcription of T regulatory cells in chickens between 3 to 32 dpi and chickens become asymptomatic carriers of Salmonella after 18 dpi. The upregulation of T regulatory cell IL-10 cytokine production by Salmonella was antigen-specific and the immune response was observed only in the gut where Salmonella had colonized until 32 dpi. Salmonella infection of chickens induced T cell IL-2 and IL-1β transcription at 3 dpi, however there were no differences in the studied cytokine transcription of T cells between the control group and Salmonella infected groups after 11 dpi.
Acknowledgments
We would like to express our gratitude to UGA poultry science research farm personnel for the help with animal trial.
Data Availability
All relevant data are within the manuscript.
Funding Statement
RKS. 58-6040-8-034 USDA-ARS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. RKS and MK. 2017-05035. USDA-NIFA grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. RKS. Hatch grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gonçalves-Tenório A, Silva BN, Rodrigues V, Cadavez V, Gonzales-Barron U. Prevalence of Pathogens in Poultry Meat: A Meta-Analysis of European Published Surveys. Foods. 2018;7(5):69. doi: 10.3390/foods7050069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beal RK, Wigley P, Powers C, Hulme SD, Barrow PA, Smith AL. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet Immunol Immunopathol. 2004;100(3–4):151–64. Epub 2004/06/23. doi: 10.1016/j.vetimm.2004.04.005 . [DOI] [PubMed] [Google Scholar]
- 3.Berndt A, Wilhelm A, Jugert C, Pieper J, Sachse K, Methner U. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect Immun. 2007;75(12):5993–6007. Epub 2007/08/22. doi: 10.1128/IAI.00695-07 ; PubMed Central PMCID: PMC2168364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rychlik I, Elsheimer-Matulova M, Kyrova K. Gene expression in the chicken caecum in response to infections with non-typhoid Salmonella. Vet Res. 2014;45:119. doi: 10.1186/s13567-014-0119-2 ; PubMed Central PMCID: PMC4256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kogut MH, Swaggerty CL, Byrd JA, Selvaraj R, Arsenault RJ. Chicken-Specific Kinome Array Reveals that Salmonella enterica Serovar Enteritidis Modulates Host Immune Signaling Pathways in the Cecum to Establish a Persistence Infection. Int J Mol Sci. 2016;17(8). Epub 2016/07/30. doi: 10.3390/ijms17081207 ; PubMed Central PMCID: PMC5000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrow PA. ELISAs and the serological analysis of Salmonella infections in poultry: a review. Epidemiology and infection. 1992;109(3):361–9. Epub 1992/12/01. doi: 10.1017/s0950268800050354 ; PubMed Central PMCID: PMC2271946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beal RK, Smith AL. Antibody response to Salmonella: its induction and role in protection against avian enteric salmonellosis. Expert review of anti-infective therapy. 2007;5(5):873–81. Epub 2007/10/05. doi: 10.1586/14787210.5.5.873 . [DOI] [PubMed] [Google Scholar]
- 8.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6(4):353–60. Epub 2005/03/24. ni1181 [pii] doi: 10.1038/ni1181 . [DOI] [PubMed] [Google Scholar]
- 9.Sadeyen JR, Trotereau J, Velge P, Marly J, Beaumont C, Barrow PA, et al. Salmonella carrier state in chicken: comparison of expression of immune response genes between susceptible and resistant animals. Microbes Infect. 2004;6(14):1278–86. Epub 2004/11/24. S1286-4579(04)00259-X [pii] doi: 10.1016/j.micinf.2004.07.005 . [DOI] [PubMed] [Google Scholar]
- 10.Humphrey T. Are happy chickens safer chickens? Poultry welfare and disease susceptibility. Br Poult Sci. 2006;47(4):379–91. Epub 2006/08/15. M15826777L158258 [pii] doi: 10.1080/00071660600829084 [DOI] [PubMed] [Google Scholar]
- 11.Shanmugasundaram R, Kogut MH, Arsenault RJ, Swaggerty CL, Cole K, Reddish JM, et al. Effect of Salmonella infection on cecal tonsil regulatory T cell properties in chickens. Poult Sci. 2015;94(8):1828–35. doi: 10.3382/ps/pev161 . [DOI] [PubMed] [Google Scholar]
- 12.Andino A, Hanning I. Salmonella enterica: survival, colonization, and virulence differences among serovars. TheScientificWorldJournal. 2015;2015:520179. doi: 10.1155/2015/520179 ; PubMed Central PMCID: PMC4310208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakyaraj S, Bhanja SK, Majumdar S, Dash B. Modulation of post-hatch growth and immunity through in ovo supplemented nutrients in broiler chickens. J Sci Food Agric. 2012;92(2):313–20. Epub 2011/07/30. doi: 10.1002/jsfa.4577 . [DOI] [PubMed] [Google Scholar]
- 14.Liljebjelke KA, Hofacre CL, Liu T, White DG, Ayers S, Young S, et al. Vertical and horizontal transmission of salmonella within integrated broiler production system. Foodborne pathogens and disease. 2005;2(1):90–102. doi: 10.1089/fpd.2005.2.90 . [DOI] [PubMed] [Google Scholar]
- 15.Roy P, Dhillon AS, Lauerman LH, Schaberg DM, Bandli D, Johnson S. Results of salmonella isolation from poultry products, poultry, poultry environment, and other characteristics. Avian Dis. 2002;46(1):17–24. doi: 10.1637/0005-2086(2002)046[0017:ROSIFP]2.0.CO;2 . [DOI] [PubMed] [Google Scholar]
- 16.Alali WQ, Thakur S, Berghaus RD, Martin MP, Gebreyes WA. Prevalence and distribution of Salmonella in organic and conventional broiler poultry farms. Foodborne pathogens and disease. 2010;7(11):1363–71. doi: 10.1089/fpd.2010.0566 . [DOI] [PubMed] [Google Scholar]
- 17.Vugia DJ, Samuel M, Farley MM, Marcus R, Shiferaw B, Shallow S, et al. Invasive Salmonella infections in the United States, FoodNet, 1996–1999: incidence, serotype distribution, and outcome. Clin Infect Dis. 2004;38 Suppl 3:S149-56. doi: 10.1086/381581 . [DOI] [PubMed] [Google Scholar]
- 18.Shao Y, Guo Y, Wang Z. beta-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult Sci. 2013;92(7):1764–73. Epub 2013/06/19. doi: 10.3382/ps.2013-03029 . [DOI] [PubMed] [Google Scholar]
- 19.Awad WA, Hess C, Hess M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins. 2017;9(2):60. doi: 10.3390/toxins9020060 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guard J, Gast RK, Guraya R. Colonization of avian reproductive-tract tissues by variant subpopulations of Salmonella enteritidis. Avian Dis. 2010;54(2):857–61. Epub 2010/07/09. doi: 10.1637/9069-091109-Reg.1 . [DOI] [PubMed] [Google Scholar]
- 21.Juanola O, Piñero P, Gómez-Hurtado I, Caparrós E, García-Villalba R, Marín A, et al. Regulatory T Cells Restrict Permeability to Bacterial Antigen Translocation and Preserve Short-Chain Fatty Acids in Experimental Cirrhosis. Hepatology Communications. 2018;2(12):1610–23. doi: 10.1002/hep4.1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortada M, Cosby D, Shanmugasundaram R, Selvaraj R. In vivo and in vitro assessment of commercial probiotic and organic acid feed additives in broilers challenged with Campylobacter coli. Journal of Applied Poultry Research. 2020. [Google Scholar]
- 23.Shanmugasundaram R, Mortada MH, Murugesan RG, Selvaraj RK. In-vitro Characterization and Analysis of Probiotic Species in the Chicken Intestine by Real-time Polymerase Chain Reaction. Poult Sci. 2019;98(11):5840–6. doi: 10.3382/ps/pez188 [DOI] [PubMed] [Google Scholar]
- 24.Shanmugasundaram R, Selvaraj RK. Regulatory T cell properties of chicken CD4+CD25+ cells. J Immunol. 2011;186(4):1997–2002. doi: 10.4049/jimmunol.1002040 [DOI] [PubMed] [Google Scholar]
- 25.Selvaraj RK, Klasing KC. Lutein and Eicosapentaenoic Acid Interact to Modify iNOS mRNA Levels through the PPAR{gamma}/RXR Pathway in Chickens and HD11 Cell Lines. J Nutr. 2006;136(6):1610–6. doi: 10.1093/jn/136.6.1610 [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262 . [DOI] [PubMed] [Google Scholar]
- 27.Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, et al. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J Immunol. 2004;173(4):2675–82. doi: 10.4049/jimmunol.173.4.2675 [DOI] [PubMed] [Google Scholar]
- 28.Arsenault RJ, Kogut MH. Gut Health: The New Paradigm in Food Animal Production: Frontiers Media SA; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shanmugasundaram R, Selvaraj RK. In vitro lipopolysaccharide treatment alters regulatory T cell properties in chickens. Vet Immunol Immunopathol. 2011;144(3–4):476–81. doi: 10.1016/j.vetimm.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 30.Selvaraj RK, Shanmugasundaram R, Klasing KC. Effects of dietary lutein and PUFA on PPAR and RXR isomer expression in chickens during an inflammatory response. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2010;157(3):198–203. [DOI] [PubMed] [Google Scholar]
- 31.Markazi A, Luoma A, Shanmugasundaram R, Mohnl M, Raj Murugesan G, Selvaraj R. Effects of drinking water synbiotic supplementation in laying hens challenged with Salmonella. Poult Sci. 2018;97(10):3510–8. doi: 10.3382/ps/pey234 [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Cheng Y, Li X, Yang W, Wen C, Zhuang S, et al. Effects of threonine supplementation on the growth performance, immunity, oxidative status, intestinal integrity, and barrier function of broilers at the early age. 2017;96(2):405–13. [DOI] [PubMed] [Google Scholar]
- 33.Oxford JH, Selvaraj RKJJoAPR. Effects of Glutamine Supplementation on Broiler Performance and Intestinal Immune Parameters During an Experimental Coccidiosis Infection. 2019;28(4):1279–87. [Google Scholar]
- 34.Park SH, Ricke SC. Development of multiplex PCR assay for simultaneous detection of Salmonella genus, Salmonella subspecies I, Salm. Enteritidis, Salm. Heidelberg and Salm. Typhimurium. J Appl Microbiol. 2015;118(1):152–60. Epub 2014/11/02. doi: 10.1111/jam.12678 . [DOI] [PubMed] [Google Scholar]
- 35.McSorley SJ, Cookson BT, Jenkins MK. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol. 2000;164(2):986–93. doi: 10.4049/jimmunol.164.2.986 . [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Li L, Lv Y, Chen Q, Feng J, Zhao X. Lactobacillus plantarum Restores Intestinal Permeability Disrupted by Salmonella Infection in Newly-hatched Chicks. Sci Rep-Uk. 2018;8(1):2229. doi: 10.1038/s41598-018-20752-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrow PA, Jones MA, Smith AL, Wigley P. The long view: Salmonella—the last forty years. Avian Pathol. 2012;41(5):413–20. doi: 10.1080/03079457.2012.718071 . [DOI] [PubMed] [Google Scholar]
- 38.Tafazoli F, Magnusson KE, Zheng L. Disruption of epithelial barrier integrity by Salmonella enterica serovar typhimurium requires geranylgeranylated proteins. Infect Immun. 2003;71(2):872–81. Epub 2003/01/24. doi: 10.1128/IAI.71.2.872-881.2003 ; PubMed Central PMCID: PMC145360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciraci C, Lamont SJ. Avian-specific TLRs and downstream effector responses to CpG-induction in chicken macrophages. Dev Comp Immunol. 2011;35(3):392–8. doi: 10.1016/j.dci.2010.11.012 . [DOI] [PubMed] [Google Scholar]
- 40.Kogut M, Rothwell L, Kaiser P. Differential effects of age on chicken heterophil functional activation by recombinant chicken interleukin-2. Dev Comp Immunol. 2002;26(9):817–30. Epub 2002/10/16. doi: 10.1016/s0145-305x(02)00040-x [pii]. . [DOI] [PubMed] [Google Scholar]
- 41.Kogut MH, Chiang HI, Swaggerty CL, Pevzner IY, Zhou H. Gene Expression Analysis of Toll-Like Receptor Pathways in Heterophils from Genetic Chicken Lines that Differ in Their Susceptibility to Salmonella enteritidis. Frontiers in genetics. 2012;3:121. doi: 10.3389/fgene.2012.00121 ; PubMed Central PMCID: PMC3389315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F, et al. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect Immun. 2011;79(7):2755–63. Epub 2011/05/11. doi: 10.1128/IAI.01375-10 ; PubMed Central PMCID: PMC3191970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Gowans EJ, Chougnet C, Plebanski M, Dittmer U. Natural Regulatory T Cells and Persistent Viral Infection. J Virol. 2008;82(1):21–30. doi: 10.1128/JVI.01768-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34(5):794–806. doi: 10.1016/j.immuni.2011.03.021 . [DOI] [PubMed] [Google Scholar]
- 45.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5. doi: 10.1038/nature12726 ; PubMed Central PMCID: PMC3869884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johanns TM, Ertelt JM, Rowe JH, Way SS. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 2010;6(8):e1001043. Epub 2010/08/18. doi: 10.1371/journal.ppat.1001043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kremer CJ, O’Meara KM, Layton SL, Hargis BM, Cole K. Evaluation of recombinant Salmonella expressing the flagellar protein fliC for persistence and enhanced antibody response in commercial turkeys. Poult Sci. 2011;90(4):752–8. Epub 2011/03/17. 90/4/752 [pii] doi: 10.3382/ps.2010-01076 . [DOI] [PubMed] [Google Scholar]
- 48.Denyer MP, Pinheiro DY, Garden OA, Shepherd AJ. Missed, Not Missing: Phylogenomic Evidence for the Existence of Avian FoxP3. PloS one. 2016;11(3):e0150988–e. doi: 10.1371/journal.pone.0150988 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheminay C, Mohlenbrink A, Hensel M. Intracellular Salmonella inhibit antigen presentation by dendritic cells. J Immunol. 2005;174(5):2892–9. Epub 2005/02/25. doi: 10.4049/jimmunol.174.5.2892 . [DOI] [PubMed] [Google Scholar]
- 50.Clay SL, Bravo-Blas A, Wall DM, MacLeod MKL, Milling SWF. Regulatory T cells control the dynamic and site-specific polarization of total CD4 T cells following Salmonella infection. Mucosal Immunol. 2020;13(6):946–57. Epub 2020/05/28. doi: 10.1038/s41385-020-0299-1 ; PubMed Central PMCID: PMC7567643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McArthur MA, Fresnay S, Magder LS, Darton TC, Jones C, Waddington CS, et al. Activation of Salmonella Typhi-specific regulatory T cells in typhoid disease in a wild-type S. Typhi challenge model. PLoS Pathog. 2015;11(5):e1004914. Epub 2015/05/23. doi: 10.1371/journal.ppat.1004914 ; PubMed Central PMCID: PMC4441490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maecker HT, Ghanekar SA, Suni MA, He X-S, Picker LJ, Maino VC. Factors Affecting the Efficiency of CD8+ T Cell Cross-Priming with Exogenous Antigens. The Journal of Immunology. 2001;166(12):7268–75. doi: 10.4049/jimmunol.166.12.7268 [DOI] [PubMed] [Google Scholar]
- 53.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunological reviews. 2011;239(1):178–96. doi: 10.1111/j.1600-065X.2010.00978.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim S, Lalani S, Parekh VV, Vincent TL, Wu L, Van Kaer L. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. The Journal of Clinical Investigation. 2008;118(6):2301–15. doi: 10.1172/JCI33071 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.