PURPOSE
The US National Cancer Institute Moonshot initiative calls for improving analysis and reporting of toxicity to inform treatment tolerability. We used existing clinician-reported adverse event (AE) and patient-reported outcome (PRO) questionnaire data from the randomized, double-blind NSABP B-35 clinical trial to explore reasons for anastrozole and tamoxifen discontinuation.
METHODS
Postmenopausal women with ductal carcinoma in situ treated with breast-conserving therapy were randomly assigned to anastrozole or tamoxifen for 5 years. The primary outcome for this analysis was time to treatment discontinuation. AEs were collected every 6 months post–random assignment from all 3,104 participants and summarized using the Toxicity Index (TI). PRO data were collected at baseline and every 6 months from 1,194 participants. Univariate and multivariable analyses of time to treatment discontinuation were performed using Cox regression models with TIs and PROs as time-dependent covariates.
RESULTS
Of 3,046 analyzed participants, 869 (28.5%) discontinued treatment prematurely. In multivariable analysis, when both baseline PROs and on-treatment AEs were considered, thrombosis and arthralgia AEs were associated with discontinuation of both tamoxifen and anastrozole; additional AEs associated with discontinuation varied by drug. In addition, baseline pain interference, hot flashes, and unhappiness were associated with tamoxifen discontinuation (n = 589; overall Harrell's C-statistic 0.686 [95% CI, 0.640 to 0.732]); no baseline PROs were associated with anastrozole discontinuation (n = 589; overall Harrell's C-statistic 0.656 [95% CI, 0.630 to 0.681]). When only baseline PROs were examined, pain interference, hot flashes, and unhappiness were associated with shorter time to discontinuation of tamoxifen; only hot flashes were associated with discontinuation of anastrozole.
CONCLUSION
Analysis of AEs using the TI yielded important insights into reasons for discontinuation of endocrine therapy that was enhanced by the addition of PRO baseline and treatment-emergent symptoms.
INTRODUCTION
Endocrine therapy (ET) plays a central role in the adjuvant treatment of hormone receptor–positive (HR+) invasive breast cancer and for risk reduction in those with high risk of developing breast cancer.1-3 Treatment with either tamoxifen or aromatase inhibitor therapy for 5 years halves the risk of developing either primary breast cancer or disease recurrence.2,3 However, in non–clinical trial settings, more than 50% of patients discontinue ET before 5 years.4-7 In early-stage HR+ breast cancer, adjuvant ET discontinuation before 5 years is associated with increased risks of breast cancer recurrence and mortality.4,8,9
CONTEXT
Key Objective
Maximum-grade adverse event data are widely used to report toxicity of therapies in clinical trials. This secondary analysis of toxicity and patient-reported symptom data from a previously conducted clinical trial was performed using the Toxicity Index (TI), a novel summary measure, to explore reasons for early discontinuation of endocrine therapy (ET).
Knowledge Generated
The TI identified both anticipated and unanticipated toxicities associated with ET discontinuation and yielded greater insights about treatment discontinuation than examining maximum-grade adverse event data alone. The addition of patient-reported symptom data from before treatment initiation and during therapy further enhanced understanding of reasons for treatment discontinuation.
Relevance
These findings may be clinically useful for a priori identification of patients who will have difficulty tolerating a specific ET. In addition, use of the TI can enrich the assessment of treatment tolerability through examination of data from previously conducted clinical trials.
The randomized, double-blind, placebo-controlled NSABP B-35 clinical trial examined risk reduction with anastrozole versus tamoxifen in 3,104 postmenopausal women with HR+ ductal carcinoma in situ.10 A subset of patients completed patient-reported outcome (PRO) questionnaires before random assignment (baseline) and every 6 months during study participation. After a median follow-up of 9 years, breast cancer–free interval improved by 27% in the anastrozole group compared with tamoxifen, primarily in women younger than 60 years. Adverse event (AE) and premature treatment discontinuation rates were similar between groups.10
With funding from the US National Cancer Institute (NCI) Cancer Moonshot research initiative, we designed a secondary analysis of data from the B-35 trial to explore potential reasons for discontinuation of ET, considering both AE data from the entire study sample and data from participants enrolled in the PRO substudy. The primary protocol-directed PRO analysis was previously reported.11 In this newly designed study, we use clinician-reported AEs and PROs to investigate factors associated with discontinuation of tamoxifen and anastrozole.
METHODS
Patients
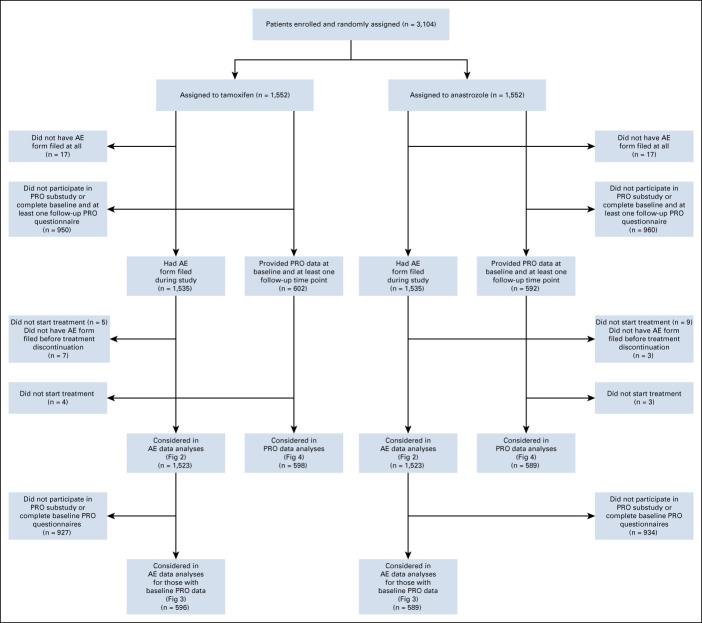
We used available toxicity data from all patients enrolled in the NSABP B-35 clinical trial,10 whose eligibility included postmenopausal status and completion of lumpectomy followed by radiotherapy and no systemic therapy; detailed eligibility criteria were previously reported.10 The Protocol (online only) was institutional review board–approved at all participating sites, and patients signed written informed consent. Patients were randomly assigned 1:1 to tamoxifen 20 mg orally once daily plus an identical placebo for anastrozole, or anastrozole 1 mg orally once daily plus an identical placebo for tamoxifen and treated for 5 years. Random assignment was stratified by age (< 60 v ≥ 60 years). Patients who did not initiate the study intervention (n = 14) and for whom no AE forms were submitted (n = 34) were excluded from this analysis (Fig 1). In addition, if a patient had at least one AE form submitted but none that reported either the time period before treatment discontinuation or the 9 months immediately following treatment discontinuation, that patient was excluded since AEs at the time of treatment discontinuation could not be reliably assessed (n = 10).
FIG 1.
CONSORT diagram. AE, adverse event; PRO, patient-reported outcome.
Procedures
Clinical evaluation of AEs occurred every 6 months after random assignment for 5 years. At each clinic visit, treatment toxicity was assessed using NCI Common Terminology Criteria for AEs (CTCAE) version 2.0, in a case report form with predesignated grade 2-5 AEs of interest and a write-in section for any additional CTCAE terms.12 Two investigators (P.A.G. and N.L.H.) categorized the additional CTCAE terms into the appropriate standard CTCAE domains.
By Protocol design, the first 1,275 participants enrolled on the parent trial who spoke English, French, or Spanish were included in the PRO substudy and completed questionnaires to assess symptoms and other aspects of health-related quality of life before treatment and every 6 months thereafter for 5 years.11 Instruments used included the Breast Cancer Prevention Trial symptom checklist,13 the Medical Outcomes Study-Short Form 12,14 the vitality scale from the Short Form 36,15 the Medical Outcomes Study Sexual Problems Scale,16 and the Center for Epidemiologic Studies Depression Scale.17 Details about the instruments are provided in the Data Supplement (online only).
For baseline questionnaire data, scales for each instrument were calculated according to author guidelines and included in the analyses. In addition, to examine individual symptom items, three authors (N.L.H., P.A.G., and R.D.H.) reviewed the individual items from all measures and aligned them with PRO-CTCAE version 1.0 items (Data Supplement).18
Study Design and Analysis
The Toxicity Index (TI) is a summary score that considers the frequency and severity of AEs and reflects the impact of multiple toxicities experienced by an individual patient over time (see the Data Supplement for additional details).19-21 Individual CTCAE toxicities reported during study participation starting at 6 months post-treatment initiation were summarized using the TI. In addition, domain-level TIs for 20 standard CTCAE domain levels from individual toxicities within each domain were calculated. TI values for nonreported grade 0 or 1 toxicities were set to zero. Distributions of global TI were compared between drugs using a Wilcoxon rank-sum test. This method contrasts with the standard reporting of a single maximum grade for each AE during a clinical trial.
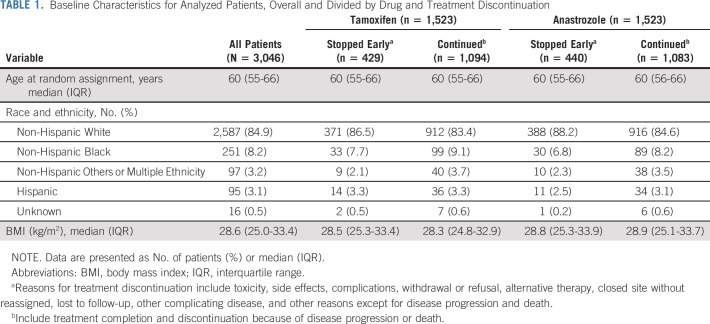
The primary outcome of this secondary analysis was time to treatment discontinuation, defined as time from the date of first treatment to the date the patient discontinued treatment. Study-defined reasons for treatment discontinuation are listed in Table 1. Treatment completion and treatment discontinuation because of disease progression or death were censored (1% with death and 6%-8% with breast cancer recurrence for each drug). Univariate and multivariable analyses of time to treatment discontinuation were performed using a Cox regression model separately for each drug with global TI, domain-level TIs, individual toxicity-level TIs, patient-reported scales or PRO-CTCAE equivalent individual items as time-dependent covariates, and baseline patient-reported scales or PRO-CTCAE equivalent individual items with and without adjustment for baseline demographic or clinical characteristics (age as a continuous variable, race and ethnicity, and body mass index).22 Sensitivity analyses were performed stratified by age (< 60 v ≥ 60 years) as well as subgroup analyses by age (< 60 v ≥ 60 years). In analyses with time-dependent covariates, a hazard at time t depends on the value of a covariate of interest at that time, an estimated effect of the covariate is constant over time, and reported hazard ratios (HRs) represent the exponential of estimated effects of the covariates of interest. Missing data were handled using the last value carried forward approach; numbers of patients with missing data at each time point are listed in the Data Supplement. The proportional hazards assumption was assessed with scaled Schoenfeld residuals.23 Model selection was carried out using a stepwise variable selection procedure on the basis of Akaike Information Criterion.24 The possibility of multicollinearity was assessed by tolerance and the variance inflation factor. Overall performance of multivariable models was measured with Harrell's25 C-statistics. In this secondary analysis, significance was not adjusted for multiple comparisons in analyses of time to treatment discontinuation.
TABLE 1.
Baseline Characteristics for Analyzed Patients, Overall and Divided by Drug and Treatment Discontinuation
Analyses were conducted using R package version 4.0.226 with two-sided tests at a significance level of .05.
RESULTS
Patient Characteristics
Of the 3,104 participants randomly assigned to anastrozole or tamoxifen, 1,523 participants treated with each drug were analyzed separately (Fig 1). Baseline characteristics of the analyzed participants are presented in Table 1.
Associations Between TI and Time to Treatment Discontinuation
Of the 3,046 patients available for analysis, 869 (28.5%) discontinued treatment, 429 (28.2%) receiving tamoxifen and 440 (28.9%) receiving anastrozole. Analysis of the global TI as a time-dependent covariate demonstrated that toxicity was associated with treatment discontinuation for both drugs, with HR 1.77 (95% CI, 1.65 to 1.90) for tamoxifen and HR 1.71 (95% CI, 1.60 to 1.83) for anastrozole. In univariate analyses, no demographic or clinical factors were associated with time to treatment discontinuation for either drug (Data Supplement).
There were statistically significant associations between multiple domain-level TIs and time to treatment discontinuation (Data Supplement). On multivariable analysis in tamoxifen-treated patients, higher TI values for blood or bone marrow, general cardiovascular, constitutional symptoms, hepatic, neurology, and pain domains were statistically significantly associated with shorter time to discontinuation (overall Harrell's C-statistic 0.675 [95% CI, 0.656 to 0.693]). For anastrozole-treated patients, higher TI values of TIs of general cardiovascular, constitutional, dermatology, neurology, and pain domains were associated with shorter time to discontinuation (Harrell's C-statistic 0.671 [95% CI, 0.652 to 0.690]).
In the AE-level multivariable analysis of TI, fatigue, arthralgia, myalgia, sensory neuropathy, and cardiac ischemia were each associated with time to discontinuation for both drugs (Fig 2). In tamoxifen-treated patients, additional symptoms associated with time to treatment discontinuation were thrombosis, nausea, transaminitis, dizziness, chest pain, and headache. For anastrozole-treated patients, additional symptoms associated with time to treatment discontinuation included anorexia, cerebrovascular ischemia, pruritus, and bone pain.
FIG 2.
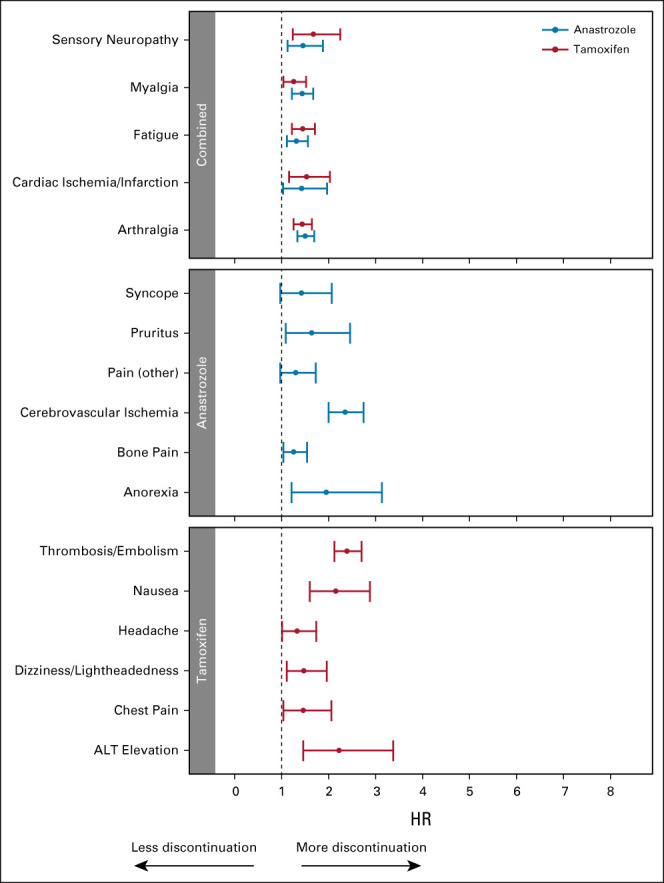
Forest plots demonstrating results of multivariable analyses examining time to off treatment with baseline characteristics and individual AE TI analyzed as time-dependent covariates without patient-reported symptoms. Tamoxifen (red) and anastrozole (blue), individual AE. The overall Harrell's C-statistics for these models are 0.634 (95% CI, 0.619 to 0.649) for tamoxifen (n = 1,523) and 0.649 (95% CI, 0.632 to 0.666) for anastrozole (n = 1,523). HRs > 1 are consistent with greater likelihood of discontinuation. AE, adverse event; HR, hazard ratio; TI, Toxicity Index.
Associations Between TI and Baseline Patient-Reported Symptoms and Time to Treatment Discontinuation
Of the 3,046 analyzed participants with TI data, 1,185 participants had baseline PRO data, 596 treated with tamoxifen and 589 treated with anastrozole (Data Supplement). There were no differences in baseline characteristics between patients who did and did not complete baseline PRO (Data Supplement). In univariate analysis, no demographic or clinical factors were associated with time to treatment discontinuation (data not shown).
Next, we examined associations between time to treatment discontinuation and individual AE-level TIs that occurred during treatment, after incorporating baseline PRO data in the analysis. Since the first AE assessment occurred at 6 months, the baseline PRO data could identify pretreatment patient symptoms affecting subsequent toxicity. There were statistically significant associations between multiple AEs and time to treatment discontinuation for each drug, with different toxicity patterns than the analysis using TI alone (Fig 3, Data Supplement). In tamoxifen-treated patients, on multivariable analysis, worse baseline physical health and depression scores, in addition to multiple AEs, were all significantly associated with shorter time to treatment discontinuation. When individual PRO-CTCAE equivalent symptoms were examined, hot flashes, pain interference with normal work, and unhappiness, in addition to multiple AEs, were all associated with shorter time to tamoxifen discontinuation. For anastrozole-treated patients, no baseline PRO symptoms were statistically significantly associated with time to treatment discontinuation in addition to AE-level TIs.
FIG 3.
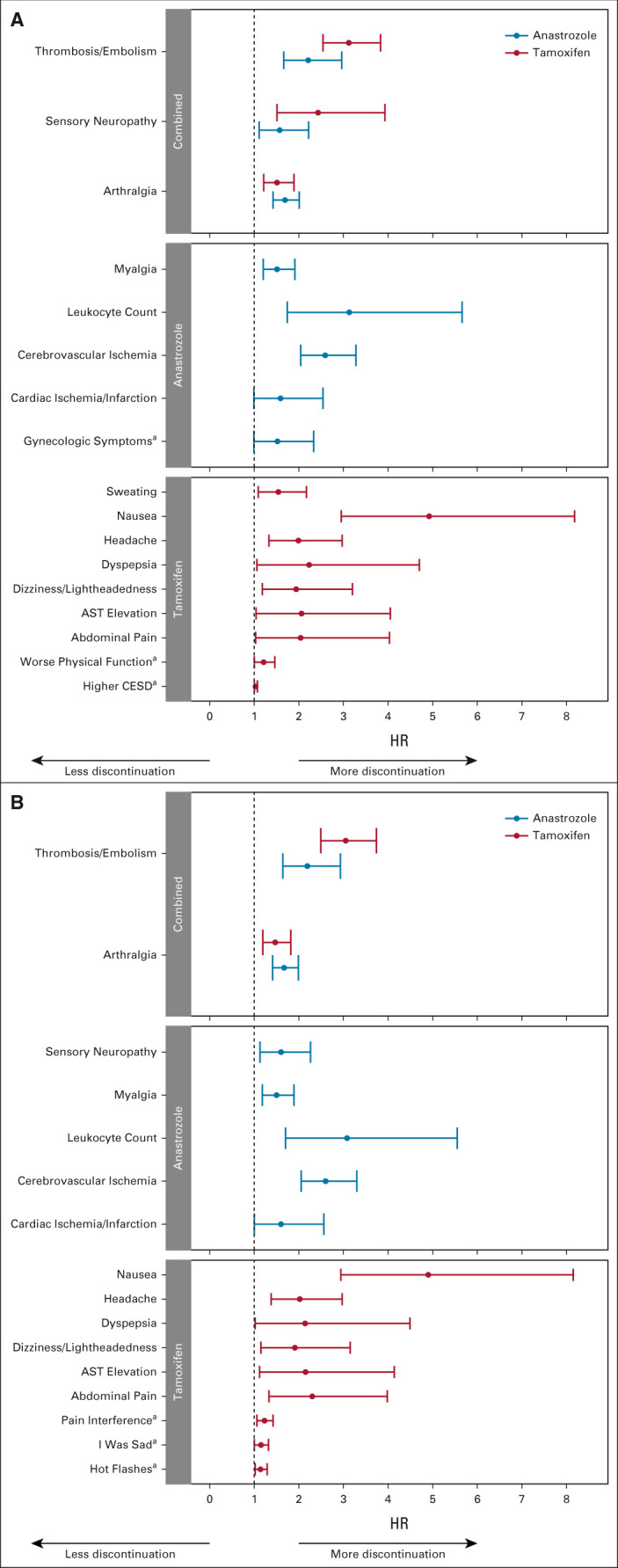
Forest plots demonstrating results of multivariable analyses examining time to off treatment with baseline characteristics and individual AE TI analyzed as time-dependent covariates with baseline patient-reported symptoms. Tamoxifen (red) and anastrozole (blue). (A) Individual AE with patient-reported summary scales. The overall Harrell's C-statistics for the models are 0.696 (95% CI, 0.648 to 0.745) for tamoxifen (n = 534) and 0.678 (95% CI, 0.642 to 0.713) for anastrozole (n = 585). (B) Individual AE with Patient-Reported Outcome-Common Terminology Criteria for Adverse Events equivalent items. The overall Harrell's C-statistics for the models are 0.686 (95% CI, 0.640 to 0.732) for tamoxifen (n = 589) and 0.656 (95% CI, 0.630 to 0.681) for anastrozole (n = 589). HRs > 1 are consistent with greater likelihood of discontinuation. aSignify patient-reported items. AE, adverse event; CESD, Center for Epidemiologic Studies Depression Scale; HR, hazard ratio; TI, Toxicity Index.
Associations Between Patient-Reported Symptoms and Time to Treatment Discontinuation
Of the 1,194 participants who completed both a baseline and at least one follow-up PRO questionnaire, 1,187 reported starting treatment and were considered in our PRO data analyses. Multivariable analysis of time to treatment discontinuation using only baseline PRO symptom data was performed (Fig 4, Data Supplement), and there were similar findings whether age was included as a continuous variable or stratified (< 60 v ≥ 60 years). Worse physical function and depression before treatment initiation were statistically significantly associated with shorter time to off treatment for tamoxifen. For anastrozole-treated patients, no baseline patient-reported scales were associated with time to treatment discontinuation. When individual PRO-CTCAE equivalent symptoms were examined, greater baseline pain interference with normal work, being unhappy, and hot flashes were associated with shorter time to discontinuation of tamoxifen, whereas only more hot flashes at baseline were associated with shorter time to discontinuation of anastrozole.
FIG 4.
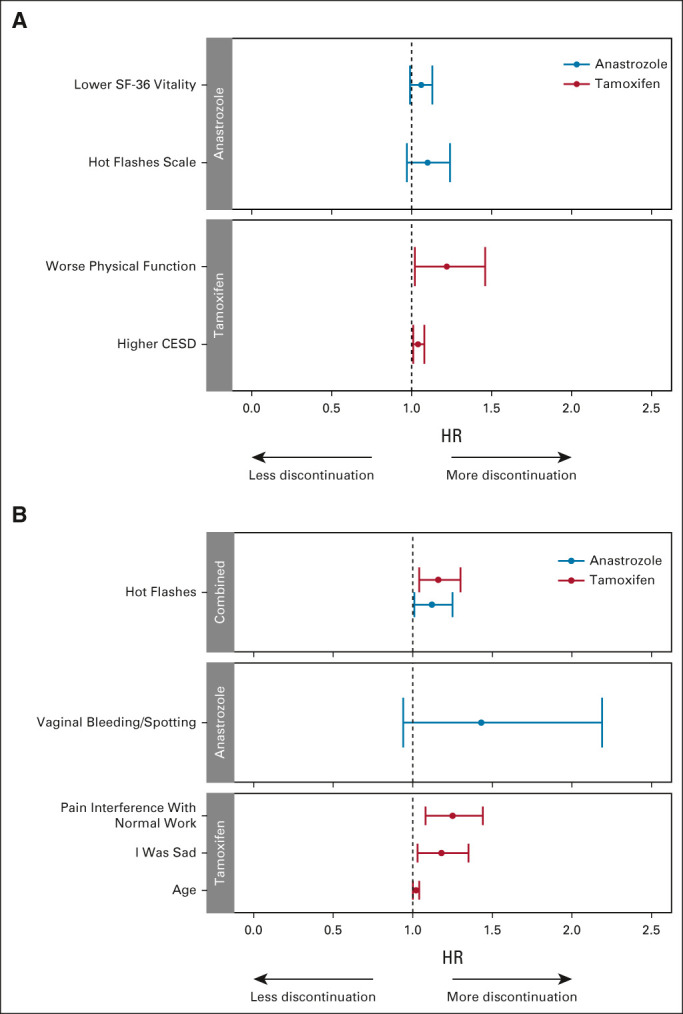
Forest plots demonstrating results of multivariable analyses examining time to off treatment with baseline characteristics and baseline patient-reported symptoms. Tamoxifen (red) and anastrozole (blue). (A) Patient-reported summary scales. The overall Harrell's C-statistics for the models are 0.587 (95% CI, 0.538 to 0.635) for tamoxifen (n = 536) and 0.560 (95% CI, 0.516 to 0.604) for anastrozole (n = 583). (B) Patient-Reported Outcome-Common Terminology Criteria for Adverse Events equivalent items. The overall Harrell's C-statistics for the models are 0.608 (95% CI, 0.562 to 0.653) for tamoxifen (n = 591) and 0.555 (95% CI, 0.513 to 0.597) for anastrozole (n = 582). HRs > 1 are consistent with greater likelihood of discontinuation. CESD, Center for Epidemiologic Studies Depression Scale; HR, hazard ratio; SF-36, Short Form 36.
In sensitivity analyses exploring associations separately by drug and by age, baseline hot flashes were associated with discontinuation of anastrozole in patients ≥ 60 years but not in younger patients (Data Supplement). In contrast, baseline symptoms associated with discontinuation of tamoxifen for patients younger than 60 years were primarily genitourinary, whereas for older patients those were unhappiness, forgetfulness, and pain.
When patient-reported data were examined as time-dependent covariates, worse physical function and depression scores during treatment were statistically significantly associated with shorter time to off treatment for tamoxifen (Figs 5A and 5B, Data Supplement). For anastrozole-treated patients, only worse physical function during treatment was associated with time to treatment discontinuation. When individual PRO-CTCAE equivalent symptoms during treatment were examined, greater pain interference with normal work, forgetfulness, and insomnia were associated with shorter time to discontinuation of tamoxifen, whereas only aches and pains were associated with shorter time to discontinuation of anastrozole.
FIG 5.
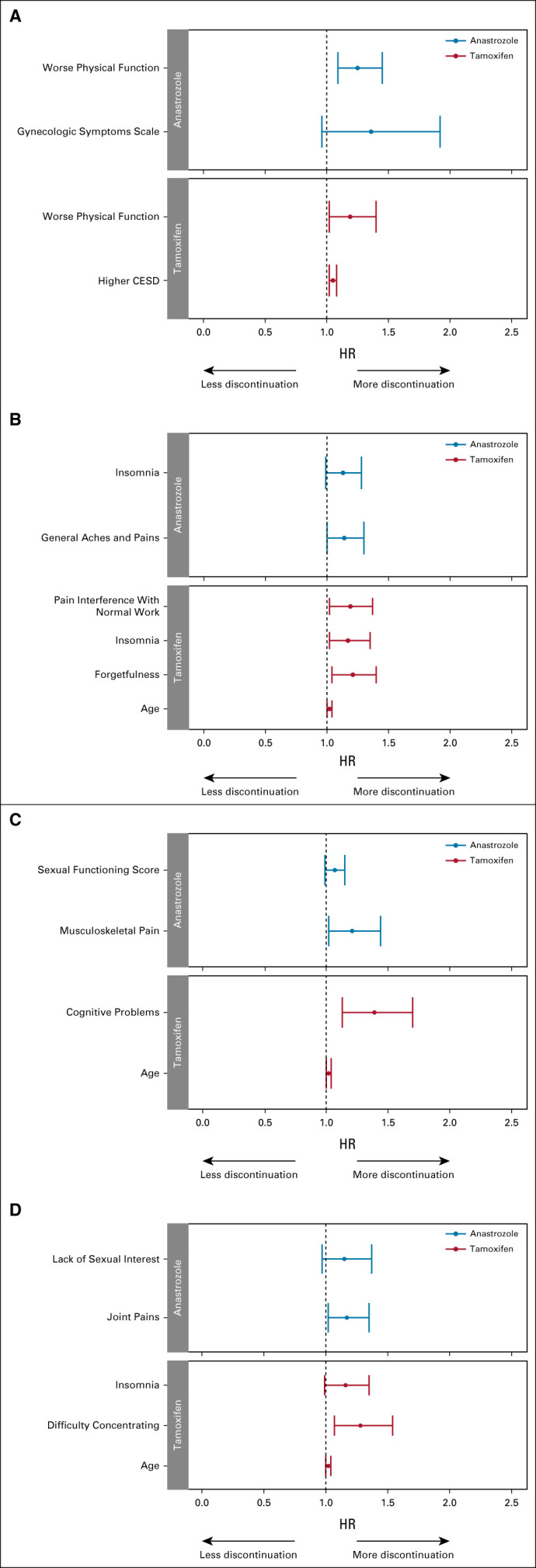
Forest plots demonstrating results of multivariable analyses examining time to off treatment with baseline characteristics and patient-reported symptoms. Tamoxifen (red) and anastrozole (blue). (A) Patient-reported summary scales analyzed as time-dependent covariates. The overall Harrell's C-statistics for the models are 0.618 (95% CI, 0.572 to 0.664) for tamoxifen (n = 591) and 0.573 (95% CI, 0.528 to 0.616) for anastrozole (n = 584). (B) PRO-CTCAE equivalent items analyzed as time-dependent covariates. The overall Harrell's C-statistics for the models are 0.631 (95% CI, 0.586 to 0.676) for tamoxifen (n = 598) and 0.574 (95% CI, 0.530 to 0.617) for anastrozole (n = 589). (C) Change in patient-reported summary scales from baseline analyzed as time-dependent covariates. The overall Harrell's C-statistics for the models are 0.589 (95% CI, 0.544 to 0.634) for tamoxifen (n = 592) and 0.546 (95% CI, 0.505 to 0.586) for anastrozole (n = 524). (D) Change in PRO-CTCAE equivalent items from baseline analyzed as time-dependent covariates. The overall Harrell's C-statistics for the models are 0.607 (95% CI, 0.561 to 0.652) for tamoxifen (n = 589) and 0.548 (95% CI, 0.508 to 0.588) for anastrozole (n = 522). HRs > 1 are consistent with greater likelihood of discontinuation. CESD, Center for Epidemiologic Studies Depression Scale; CTCAE, Common Terminology Criteria for Adverse Events; HR, hazard ratio; PRO, patient-reported outcome.
In contrast, when patient-reported symptoms were examined as change from baseline, only worse cognitive problems in tamoxifen-treated patients and worse musculoskeletal pain in anastrozole-treated patients were statistically significantly associated with shorter time to off treatment (Figs 5C and 5D, Data Supplement). Similar findings were noted when individual PRO-CTCAE equivalent symptoms were examined.
DISCUSSION
In this secondary analysis of clinician-reported toxicity with AEs and PROs from a large, randomized controlled trial (RCT), TI evaluation of CTCAE data identified both anticipated and unanticipated toxicities associated with treatment discontinuation. Including PROs along with the TI analysis of CTCAE data yielded additional insights. These findings may be clinically useful for a priori identification of patients who will have difficulty tolerating an ET, including both preexisting symptoms that may predispose patients to an increased risk of treatment discontinuation and treatment-emergent symptoms that may require proactive intervention.
The initially reported toxicity analysis from NSABP B-35 demonstrated that absolute rates of maximum-grade AEs and frequency of premature treatment discontinuation were similar for both drugs.10 However, the relationship between these AEs and treatment discontinuation was not previously examined. This secondary analysis of data from NSABP B-35 addresses the goals of the NCI Cancer Moonshot Initiative by identifying new methods of analyzing and reporting cancer treatment toxicity to obtain a more comprehensive understanding of factors associated with treatment tolerability.
In a secondary analysis of data from a rectal cancer clinical trial, our group found that the more comprehensive TI methodology demonstrated statistically significant differences in toxicities between treatment arms that had not been apparent using traditional AE analysis methods that rely solely on examination of maximum-grade AE.19,20 By analyzing the NSABP B-35 data using the TI methodology, we found that although the global TI distribution was similar between drugs, there were important differences in patterns of toxicities that emerged by examining AE domains. Although commonly reported symptoms such as fatigue and arthralgias were associated with treatment discontinuation, less frequent toxicities were also identified. Uncommon toxicities would be unlikely to be identified using traditional CTCAE analysis methods and also would likely be missed through reliance on patient-reported data collection since the less common symptoms would not have been queried.
Another potential advantage of summarizing CTCAE data using the TI methodology is that comprehensive toxicity data are collected from all enrolled patients, which maximizes the sample size and statistical power. In addition, these data are available for most previously conducted trials and could be examined retrospectively to glean more insights into treatment tolerability. However, a limitation of relying on clinician-graded toxicity without direct patient input is that more subtle and subjective findings could be missed. For example, cognitive dysfunction was associated with tamoxifen discontinuation when patient-reported symptoms were examined but wasn't evident when only AEs were analyzed.
Collection of CTCAE data only after therapy initiation fails to address the contribution of baseline symptoms to treatment discontinuation. Treatment-emergent toxicity may not be the sole driver of treatment discontinuation. For postmenopausal women, many symptoms associated with ET are already present before treatment initiation. Therefore, consideration of baseline symptom burden may be particularly relevant as it may directly influence treatment tolerability. It can also be unclear whether the toxicities reported during trial participation were newly developed during treatment versus reflected exacerbation of preexisting symptoms; baseline symptom information can help distinguish these possibilities. Finally, taking the baseline data into account may enable better assessment of which symptoms are likely driving treatment discontinuation and which are less influential.
Surprisingly, analyses that included baseline B-35 PRO data revealed few preexisting individual symptoms that were associated with premature treatment discontinuation, despite high symptom burden at baseline.11 For both drugs, preexisting hot flashes were associated with shorter time to treatment discontinuation. Interestingly, baseline musculoskeletal symptoms were not associated with time to anastrozole discontinuation, consistent with some but not all published reports.13,27 This may be because no participants had prior chemotherapy exposure, which has been associated with increased risk of aromatase inhibitor–associated arthralgias.28,29 Finally, for tamoxifen, increased unhappiness at baseline was associated with shorter time to discontinuation, although a prior analysis from the NSABP P-1 trial did not demonstrate worsening of depression with tamoxifen chemoprevention.30
Although there are clear benefits from collecting PRO data in clinical trials, including the ability to characterize pretreatment symptoms, there are also limitations. Their use increases participant burden, so the number of symptoms and the number of assessment time points are limited, and often only a subset of enrolled participants complete questionnaires. In addition, investigators must select which symptoms to include at the time of trial design, which can be challenging when investigating new drugs with limited available toxicity information. Last, there can be missing PRO data that is nonrandom because of administrative and patient health status factors.
A key strength of this analysis is the comprehensive assessment of both clinician-assessed toxicity and patient-reported data from this large, double-blind RCT to characterize the patient experience with therapy and associations with treatment discontinuation in a relatively homogeneous patient population. This enabled a focus specifically on the effects of the ET, without potential confounding from prior chemotherapy. In addition, since the trial was double-blinded, participants and clinicians were unaware of treatment allocation.
However, there are some limitations, including the potential lack of generalizability of clinical trial participants to general population patients with DCIS and to those with invasive breast cancer who receive ET after chemotherapy. In addition, the TI cannot be calculated for patients without CTCAE data, which may limit examination of the influence of early-onset toxicity on persistence with therapy. In this study, only 44 (1.4%) participants were excluded because of lack of AE reports. The overall amount of missing AE and PRO data in this 5-year trial was relatively limited. Missing data were handled using the last value carried forward method, a commonly used statistical approach that assumes a patient's toxicity or symptom remained stable over time and does not reflect the uncertainty of what happened. Furthermore, data on symptom management interventions were not collected, which may have mitigated the severity of some symptoms. In addition, only one third of patients were included in the PRO substudy, limiting the statistical power for examining associations between baseline symptoms and premature treatment discontinuation. Finally, adjustment for multiple comparisons was not performed in these exploratory analyses.
In summary, this analysis of clinician-reported AEs using the TI method yielded important insights into reasons for ET discontinuation in patients without prior chemotherapy treatment. Patient-reported symptoms measured before and during treatment further enhanced this understanding. This approach to assessment of treatment tolerability should be considered both when analyzing and interpreting data from previously conducted RCTs and when designing prospective studies examining new treatment interventions for patients with cancer.
ACKNOWLEDGMENT
The authors acknowledge members of the U01 Scientific Advisory Committee (Lari Wenzel, PhD; Claire Snyder, PhD; Michael Brundage, MD; and Elisa Long, PhD) and NRG Oncology statisticians (Hanna Bandos, PhD).
N. Lynn Henry
Research Funding: Pfizer, AbbVie
Open Payments Link: https://openpaymentsdata.cms.gov/physician/27894/summary
Greg Yothers
Employment: Mountainview Pediatrics (I)
Consulting or Advisory Role: Orbus Therapeutics
Patricia A. Ganz
Leadership: Intrinsic LifeSciences (I)
Stock and Other Ownership Interests: Xenon Pharma (I), Intrinsic LifeSciences (I), Silarus Therapeutics (I), Teva, Novartis, Merck, Johnson & Johnson, Pfizer, GlaxoSmithKline, Abbott Laboratories
Consulting or Advisory Role: InformedDNA, Vifor Pharma (I), Ambys Medicines (I), Global Blood Therapeutics (I), GlaxoSmithKline (I), Ionis Pharmaceuticals (I), Akebia Therapeutics (I), Protagonist Therapeutics (I), Regeneron (I), Sierra Oncology (I), Rockwell Medical Technologies Inc (I), Astellas Pharma (I), Gossamer Bio (I), American Regent (I), Disc Medicine (I), Blue Note Therapeutics
Research Funding: Blue Note
Patents, Royalties, Other Intellectual Property: Related to iron metabolism and the anemia of chronic disease (I), Up-to-Date royalties for section editor on survivorship
Travel, Accommodations, Expenses: Intrinsic LifeSciences (I)
No other potential conflicts of interest were reported.
See accompanying editorial on page 3770
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
SUPPORT
Supported in part by the National Cancer Institute of the NIH (1U01CA232859-01; M.A.D., M.L., S.K., R.D.H., G.Y., P.A.G., A.R.); the NIH National Center for Advancing Translational Sciences UCLA CTSI (UL1 TR001881-01) (M.A.D., A.R.); and NCI grants U10CA180868, UG1CA189867, and U10CA180822. This program is supported by funding provided through the Cancer Moonshot.
AUTHOR CONTRIBUTIONS
Conception and design: N. Lynn Henry, André Rogatko, Patricia A. Ganz
Financial support: André Rogatko, Patricia A. Ganz
Administrative support: Greg Yothers, André Rogatko
Collection and assembly of data: Reena S. Cecchini, Greg Yothers
Data analysis and interpretation: N. Lynn Henry, Sungjin Kim, Ron D. Hays, Marcio A. Diniz, Michael Luu, Greg Yothers, André Rogatko, Patricia A. Ganz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Toxicity Index, Patient-Reported Outcomes, and Early Discontinuation of Endocrine Therapy for Breast Cancer Risk Reduction in NRG Oncology/NSABP B-35
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
N. Lynn Henry
Research Funding: Pfizer, AbbVie
Open Payments Link: https://openpaymentsdata.cms.gov/physician/27894/summary
Greg Yothers
Employment: Mountainview Pediatrics (I)
Consulting or Advisory Role: Orbus Therapeutics
Patricia A. Ganz
Leadership: Intrinsic LifeSciences (I)
Stock and Other Ownership Interests: Xenon Pharma (I), Intrinsic LifeSciences (I), Silarus Therapeutics (I), Teva, Novartis, Merck, Johnson & Johnson, Pfizer, GlaxoSmithKline, Abbott Laboratories
Consulting or Advisory Role: InformedDNA, Vifor Pharma (I), Ambys Medicines (I), Global Blood Therapeutics (I), GlaxoSmithKline (I), Ionis Pharmaceuticals (I), Akebia Therapeutics (I), Protagonist Therapeutics (I), Regeneron (I), Sierra Oncology (I), Rockwell Medical Technologies Inc (I), Astellas Pharma (I), Gossamer Bio (I), American Regent (I), Disc Medicine (I), Blue Note Therapeutics
Research Funding: Blue Note
Patents, Royalties, Other Intellectual Property: Related to iron metabolism and the anemia of chronic disease (I), Up-to-Date royalties for section editor on survivorship
Travel, Accommodations, Expenses: Intrinsic LifeSciences (I)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dowsett M, Cuzick J, Ingle J, et al. : Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28:509-518, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Visvanathan K, Fabian CJ, Bantug E, et al. : Use of endocrine therapy for breast cancer risk reduction: ASCO Clinical Practice Guideline update. J Clin Oncol 37:3152-3165, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Burstein HJ, Lacchetti C, Anderson H, et al. : Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO Clinical Practice Guideline focused update. J Clin Oncol 37:423-438, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. : Treatment adherence and its impact on disease-free survival in the Breast International Group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol 34:2452-2459, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadji P, Ziller V, Kyvernitakis J, et al. : Persistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: A retrospective database analysis. Breast Cancer Res Treat 138:185-191, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Nekhlyudov L, Li L, Ross-Degnan D, et al. : Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat 130:681-689, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Chlebowski RT, Geller ML: Adherence to endocrine therapy for breast cancer. Oncology 71:1-9, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Hershman DL, Shao T, Kushi LH, et al. : Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126:529-537, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barron TI, Cahir C, Sharp L, et al. : A nested case-control study of adjuvant hormonal therapy persistence and compliance, and early breast cancer recurrence in women with stage I-III breast cancer. Br J Cancer 109:1513-1521, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolese RG, Cecchini RS, Julian TB, et al. : Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): A randomised, double-blind, phase 3 clinical trial. Lancet 387:849-856, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz PA, Cecchini RS, Julian TB, et al. : Patient-reported outcomes with anastrozole versus tamoxifen for postmenopausal patients with ductal carcinoma in situ treated with lumpectomy plus radiotherapy (NSABP B-35): A randomised, double-blind, phase 3 clinical trial. Lancet 387:857-865, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NCI Cancer Therapy Evaluation Program : CTC Version 2.0. 1999. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf [Google Scholar]
- 13.Ganz PA, Day R, Ware JE, Jr, et al. : Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst 87:1372-1382, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Turner-Bowker D, Hogue SJ: Short form 12 health survey (SF-12), in Michalos AC. (ed): Encyclopedia of Quality of Life and Well-Being Research. Dordrecht, the Netherlands, Springer, 2014 [Google Scholar]
- 15.Stewart AL, Hays RD, Ware JE: Health perceptions, energy/fatigue, and health distress measures, in Stewart AL, Ware JE. (eds): Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC, Duke University Press, 1992, pp 143-172 [Google Scholar]
- 16.Sherbourne CD, Stewart AL, Ware JE: Social functioning: Sexual problems measures, in Stewart AL, Ware JE. (eds): Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC, Duke University Press, 1992, pp 194-204 [Google Scholar]
- 17.Radloff LS: The CES-D scale: A self-report depression scale for research in the general population. J Appl Psychol Meas 1:385-401, 1977 [Google Scholar]
- 18.Kluetz PG, Chingos DT, Basch EM, et al. : Patient-reported outcomes in cancer clinical trials: Measuring symptomatic adverse events with the National Cancer Institute's patient-reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Ed Book 35:67-73, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Rogatko A, Babb JS, Wang H, et al. : Patient characteristics compete with dose as predictors of acute treatment toxicity in early phase clinical trials. Clin Cancer Res 10:4645-4651, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Gresham G, Diniz MA, Razaee ZS, et al. : Evaluating treatment tolerability in cancer clinical trials using the toxicity index. J Natl Cancer Inst 112:1266-1274, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razaee ZS, Amini AA, Diniz MA, et al. : On the properties of the toxicity index and its statistical efficiency. Stat Med 40:1535-1552, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox DR: Regression models and life tables. J R Stat Soc B34:187-220, 1972 [Google Scholar]
- 23.Grambsch P, Therneau T: Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81:515-526, 1994 [Google Scholar]
- 24.Yamashita T, Yamashita K, Kamimura R: A stepwise AIC method for variable selection in linear regression. Commun Stat Theory Methods 36:2395-2403, 2007 [Google Scholar]
- 25.Harrell FE, Jr: Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York, NY,Springer, 2001 [Google Scholar]
- 26.R Core Team : R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2020 [Google Scholar]
- 27.Mao JJ, Stricker C, Bruner D, et al. : Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer 115:3631-3639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crew KD, Greenlee H, Capodice J, et al. : Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25:3877-3883, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Sestak I, Cuzick J, Sapunar F, et al. : Risk factors for joint symptoms in patients enrolled in the ATAC trial: A retrospective, exploratory analysis. Lancet Oncol 9:866-872, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Day R, Ganz PA, Costantino JP: Tamoxifen and depression: More evidence from the National Surgical Adjuvant Breast and Bowel Project's breast cancer prevention (P-1) randomized study. J Natl Cancer Inst 93:1615-1623, 2001 [DOI] [PubMed] [Google Scholar]