Abstract
This article provides a brief overview of DNA vaccines. First, the basic DNA vaccine design strategies are described, then specific issues related to the industrial production of DNA vaccines are discussed, including the production and purification of DNA products such as plasmid DNA, minicircle DNA, minimalistic, immunologically defined gene expression (MIDGE) and Doggybone™. The use of adjuvants to enhance the immunogenicity of DNA vaccines is then discussed. In addition, different delivery routes and several physical and chemical methods to increase the efficacy of DNA delivery into cells are explained. Recent preclinical and clinical trials of DNA vaccines for COVID-19 are then summarized. Lastly, the advantages and obstacles of DNA vaccines are discussed.
Keywords: : COVID-19, DNA, DNA vaccines, nucleic acid vaccines, SARS-CoV-2, vaccines
Recent advances in biotechnology have revolutionized medicine and offered pioneering solutions to unmet clinical needs. Vaccines are one of the most important medical interventions. There are different types of vaccine platforms for infectious diseases and cancer such as live-attenuated, whole-inactivated, subunit, virus-like particles, viral-vector, mRNA and DNA vaccines [1]. Nucleic acid-based diagnostic, prognostic and therapeutic platforms are promising tools that are replacing older protein-based platforms due to their unique properties such as thermostability, resistance to denaturation and simple storage [2]. It has been documented that a good vaccine platform should be rapid, simply developed, reproducible, thermostable and manufactured with reducing development costs and risks [3]. The DNA platform addresses many of these goals. Wolff et al. were the first to show that the injection of naked plasmid DNA in the mouse muscle results in a local expression of the transgene [4]. This research was a turning point in the use of DNA in vaccine development.
DNA vaccines are DNA vehicles such as bacterial plasmids, minicircle DNA or linear, covalently-closed minimalistic expression constructs including minimalistic, immunologically defined gene expression (MIDGE) DNA and Doggybone™ DNA that contain at least one eukaryotic expression cassette encoding for the antigen of interest. The expression cassettes usually consist of a eukaryotic promoter/enhancer, the antigen gene and a poly(A) signal sequence, which are essential for the expression of the antigen in eukaryotic cells (e.g., muscular cells) [5–7]. DNA vaccines have shown compelling safety and immunogenicity in preclinical studies [8]. Several DNA vaccines are currently licensed for veterinary use in large animals such as horses as well as small animals such as chickens [9,10]. The results from clinical trials of DNA vaccines for West Nile virus (WNV) [11,12], Ebola and Marburg viruses [13,14] and SARS-CoV-2 [15–19] have shown that antibodies are generated in humans a few weeks after immunization. However, there are also many cases of poor immunogenicity in clinical trials. Target antigen and optimization of construct, formulation and delivery methods appear to be key elements in the immunogenicity of DNA vaccines [8]. This review will discuss the design, production, delivery and administration of DNA vaccines, factors that may improve the immunogenicity of DNA vaccines, and summarize the recent preclinical and clinical trials of DNA vaccines for COVID-19.
Design of DNA vaccines
The plasmid DNA vaccines are comprised of a bacterial origin of replication and at least one antibiotic resistance gene as a selectable marker. It was shown that bacterial backbone can reduce gene expression in mammalian cells [20]. The formation of heterochromatin in bacterial sequences spreading into the expression cassette may be one of the reasons for the silencing of transgene expression [21]. In addition, it was found that increasing the A/T sequence composition in plasmid antibiotic resistance genes can increase the stable transcription of backbone genes as well as adjacent expression cassettes in mice [22]. Changes in sequence composition and deletion of bacterial backbone sequences in DNA vaccines may increase antigen expression. Minicircle DNA, MIDGE and Doggybone™ are DNA constructs composed of the gene expression cassette(s) without the bacterial backbone of plasmids [5–7].
Sometimes, we need to express multiple genes in a single DNA vaccine, for example, designing multi-antigen DNA vaccines or expressing a genetic adjuvant combined with the antigen. In this regard, three strategies were used: first, we can use different expression cassettes for each gene with individual promoters for independent expression of multiple transgenes; second, we can use bi-cistronic or multi-cistronic vectors with a single promoter for the expression of multiple genes which are separated by internal ribosome entry site (IRES) elements for independent translation of multiple genes; third, we can use a virus-derived T2A sequence instead of IRES between genes, where, after translation, the corresponding peptide sequence is recognized and cleaved by an endogenous protease [23–27].
The genetic material of a DNA vaccine must first enter the nucleus for subsequent transcription of the encoding genes of antigens or genetic adjuvants. Then, the transcribed mRNA(s) are exported from the nucleus into the cytoplasm for translation. The efficient DNA transfer to the cell nucleus is an important barrier for the expression of transgenes in DNA vaccines, especially for mitotically inactive cells such as antigen-presenting cells (APCs) [27]. Certain DNA sequences such as the simian virus (SV) 40 enhancer have a nuclear localization signal (NLS), and binding of specific transcription factors to this NLS signal in the cytoplasm leads to active nuclear transport of DNA [28]. In addition, insertion of some tissue-specific transcription factor-binding sequences in DNA plasmids may lead to tissue-specific nuclear import of plasmid DNA [29]. Alternatively, some DNA binding proteins such as NFκB (p50) and engineered NLS-tetracycline repressor can be used to form protein–DNA complexes before administration, improving the nuclear localization of DNA in cells [30–32]. In addition, covalent or noncovalent conjugation of a virus-derived NLS peptide to either natural or synthetic polycation DNA condensing agents or directly to DNA could be another strategy to improve the nuclear translocation of exogenous DNA [33–37].
Typically, viral promoters such as the human cytomegalovirus (CMV) promoter which are ubiquitously active are used for gene expression in human cells. However, viral promoters are often inactivated in eukaryotic cells due to hypermethylation [38,39]. For long-term expression of transgenes in human cells, eukaryotic or eukaryotic/viral hybrid promoters are used, which remain active for a long time [40,41]. In addition, cell-type-specific promoters may be used in expression cassettes of DNA vaccines, which can restrict the expression of antigens and genetic adjuvants to APC cells such as dendritic cells (DC), macrophages and B cells. The restriction of gene expression to APCs prevents tolerance induced by regulatory cells such as myeloid-derived suppressor cells (MDSC) and Tumor-associated macrophages (TAM). The promoter of the gene encoding for Fascin-1 is a DC-specific promoter which is highly expressed in activated DC cells as well as in neuronal cells in humans and mice. Immunization of mice with DNA vaccines containing the Fascin-1 promoter activated Th1-biased immune responses and cytotoxic T cells (CTL). However, transcriptional targeting of DC with the fascin-1 promoter also eliminates antigen expression in B cells, which may impair the induction of humoral immune responses [27,42–49]. Alternatively, APCs can be targeted ex vivo, in which the cells are isolated from the body, transfected with DNA vaccine in vitro, and then injected back into the body [50,51].
Production of DNA vaccines
Different DNA constructs, including circular DNA constructs such as plasmid DNA [52] and minicircle DNA [53,54], or linear, covalently-closed minimalistic expression constructs such as MIDGE [55,56] and Doggybone™ [57–59], are produced by different methods.
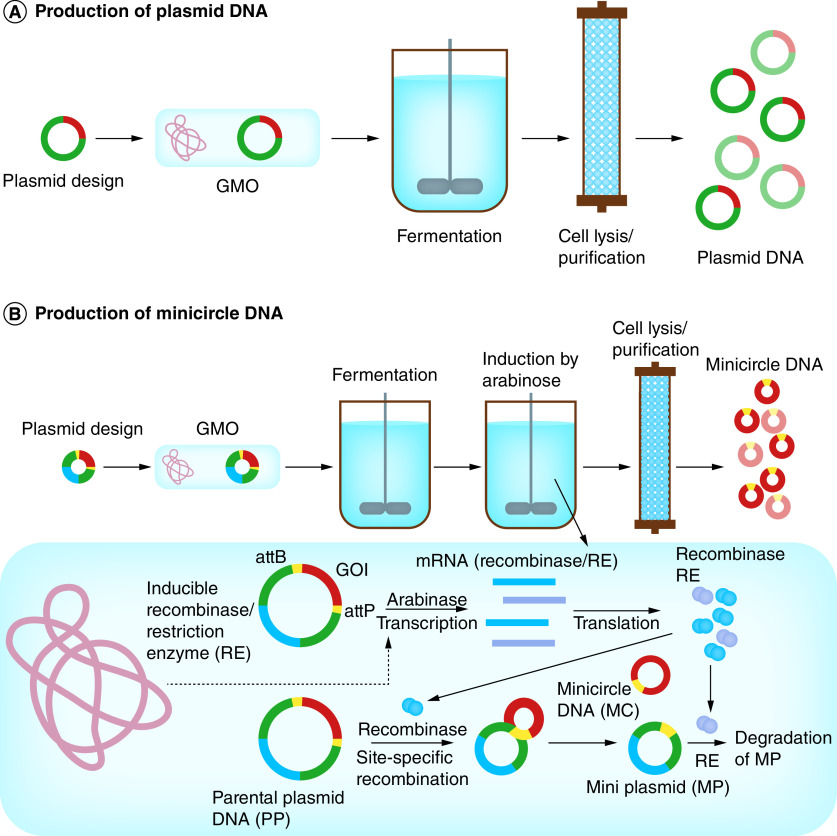
Plasmid DNA is produced through genetically modified bacteria, usually Escherichia coli (Figure 1 A). The good manufacturing process (GMP)-production of plasmid DNA at preclinical and clinical scale requires careful development of optimal and economical commercial processes [60]. Bacterial cells are grown under fermentation conditions usually in defined or minimal cell culture media consisting of chemically defined substances such as glucose or glycerol as carbon sources, salts, vitamins, etc. After fermentation, bacterial cells are harvested by centrifugation or microfiltration. Then chemical, physical or mechanical methods are employed for cell lysis. The lysate generated by cell lysis contains cell debris, plasmid DNA and soluble impurities. Clarification techniques such as cross-flow microfiltration are used to remove solids from the lysate. Then, several steps of purification such as contaminant precipitation, plasmid precipitation, chromatographic purification (anion exchange chromatography, followed by hydrophobic interaction chromatography and sometimes by size exclusion chromatography) are employed for the removal of contaminants (e.g., host proteins, endotoxins, RNA, genomic DNA and linear and open-circular forms of plasmid DNA). The purified plasmid DNA is formulated with excipients and adjuvants and filtered through sterilizing filters such as polyethersulfone (PES) and polyvinylidene difluoride (PVDF) membranes [52].
Figure 1. Production of circular DNA constructs.
(A) Production of plasmid DNA. The designed plasmid containing the GOI is transformed to the bacterial host to generate a GMO. After fermentation, bacterial cells are harvested and lysed through chemical, physical or mechanical methods. After the removal of solids from the lysate (clarification), several steps of purification such as chromatographic purification are used for the purification of plasmid DNA. (B) Production of minicircle DNA. After the growth of the genetically modified bacteria containing the parental plasmid, the expression of recombinase and the restriction enzyme is induced by arabinose from the plasmid or bacterial genome. The recombinase initiates the site-specific recombination between its recognition sequences (attB and attP), originating the minicircle DNA and a MP consisting of the bacterial backbone. Then, MP is degraded specifically by the RE. The minicircle DNA is extracted and purified after cell harvest, cell lysis, clarification and several steps of purification.
GOI: Gene of interest; GMO: Genetically modified organism; MP: Mini plasmid; RE: Recombinase.
Minicircle DNA is produced by inducing the intramolecular recombination of a parental plasmid in E. coli. For example, the expression of a recombinase such as ϕC31 integrase and a restriction enzyme (RE) such as I-SceI are induced by an arabinose-inducible gene expression system. The recombinase mediates the site-specific recombination between its recognition sequences (e.g., attB and attP), originating two different circular DNA molecules (i) the minicircle DNA containing the eukaryotic expression cassette and (ii) a mini plasmid (MP) consisting of the bacterial backbone. MP can be degraded specifically by the induced RE. The minicircle DNA can then be extracted, purified and formulated similar to the methods used for DNA plasmids (Figure 1 B) [53,54].
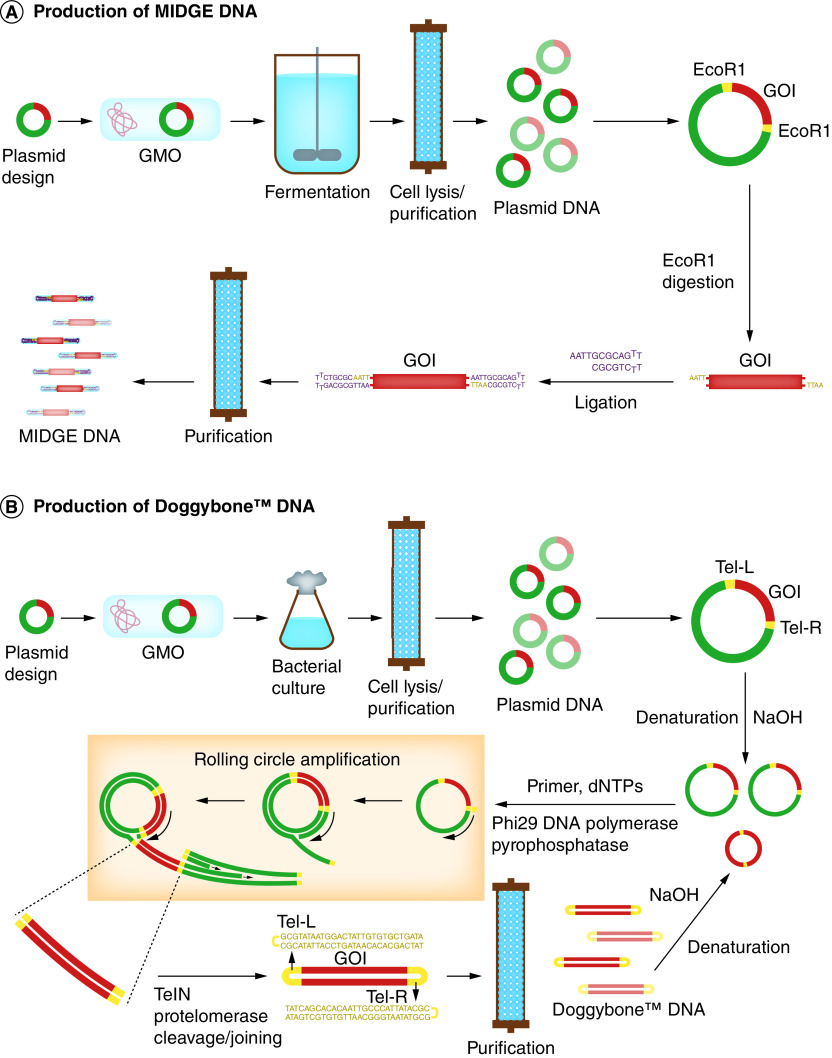
MIDGE vectors are produced by digestion of DNA plasmid using an RE such as EcoRI, and subsequent ligation of the resulting fragments to hairpin oligodeoxynucleotides to generate a covalently closed dumbbell-shaped DNA molecule (Figure 2 A). Unligated fragments including plasmid backbones are digested by T7 DNA polymerase. MIDGE vectors can be further purified by chromatographic purification (e.g., anionic-exchange chromatography) and formulated similar to DNA plasmids [55,56].
Figure 2. Production of linear, covalently-closed minimalistic expression DNA constructs.
(A) Production of MIDGE DNA. The purified parental plasmid containing the GOI flanking with a restriction site (e.g. EcoRI) is digested by the RE. The resulting fragments are ligated to the hairpin oligodeoxynucleotides to generate MIDGE DNA molecules. Then, unligated fragments including plasmid backbones are digested by T7 DNA polymerase and the MIDGE DNA is purified. (B) Production of Doggybone™ DNA. The purified parental plasmid containing the GOI flanking with telomeric ends (Tel-L and Tel-R) is denatured by NaOH and used as a template DNA in a RCA reaction using Phi29 DNA polymerase. The resulting DNA concatemers are cleaved and joined by an enzymatic reaction using TelN protelomerase to generate Doggybone DNA. Doggybone™ DNA molecules are then purified using chromatographic purification. The purified Doggybone DNA may be used as a template DNA for the RCA reaction.
GMO: Genetically modified organism; GOI: Gene of interest; MIDGE: Minimalistic, immunologically defined gene expression; RCA: Rolling circle amplification.
Doggybone™ DNA is produced through an enzymatic process (Figure 2B). The DNA plasmid containing the eukaryotic expression cassette flanking with telomeric ends (Tel-L and Tel-R) is denatured by NaOH and used as a template DNA in a rolling circle amplification (RCA) process. The resulting double-stranded DNA concatemers are cleaved and joined by TelN protelomerase to generate the linear, covalently closed, double-stranded molecules. Doggybone™ DNA molecules are purified using chromatographic purification and formulated similar to DNA plasmids [57–59].
Adjuvants
Vaccine adjuvants may be used for improving the immunogenicity of DNA vaccines by stimulating innate immune responses. Plasmids are usually produced in bacterial hosts and thus have unmethylated CpG motifs, which may have an intrinsic adjuvant effect by stimulating innate immune responses through TLR9 [61]. However, different types of adjuvants have been used with DNA vaccines in exploratory and preclinical studies including classical adjuvants liposomal and nanoparticle adjuvants, and molecular adjuvants [62,63].
When using classical adjuvants such as aluminum salts (alum) in vaccination, in many cases, antigen and adjuvant are mixed before administration, allowing the physical interaction of adjuvant and antigen. This may lead to slower antigen release and longer interaction with immune cells. While several classical adjuvants have been used with DNA vaccines in animal models, no benefits have been found in large animals such as nonhuman primates in comparative studies [61]. The positive effect of such adjuvants in small animals (e.g., mice) has been attributed to the boosting of immune responses elicited by the expressed antigen rather than to the physical interaction of adjuvants and DNA vaccines [61]. In humans, aluminum phosphate adjuvant did not show any significant effect on the immunogenicity of a DNA vaccine for human immunodeficiency virus (HIV) [64].
Different types of adjuvants have been used with DNA vaccines for veterinary applications [65]. Some of these adjuvants have already been licensed for veterinary use. West Nile-Innovator® DNA is a WNV DNA vaccine approved in 2005 for veterinary use in horses, containing a plasmid DNA encoding antigenic proteins of WNV and a lipid-based adjuvant MetaStim™ [66]. West Nile-Innovator DNA vaccine was removed from the market in 2010 [66]. AgriLabs ExactVac is the first commercial DNA vaccine against the H5N1 influenza virus in chickens. This vaccine has been formulated with a lipid/polymer matrix-based adjuvant named ENABL®. It is believed that ENABL enables efficient dispersion of the vaccine micro-particles and more efficient delivery of the vaccine to target cells [67]. However, it has not been reported whether MetaStim and ENABL adjuvants improved the immunogenicity of these DNA vaccines.
Nanoparticles such as liposomes, PLGA and exosomes can boost immune responses even in the empty form. Therefore, nanoparticles may be used as adjuvants in vaccine formulations [68]. Nanoparticles can protect DNA from degradation and therefore can enhance the immune response compared with naked DNA vaccines [69]. Different types of nanoparticles such as cationic liposomes [70], magnesium phosphate nanoparticles [71] and calcium phosphate nanoparticles [72] have been used as adjuvants in DNA vaccine formulations to improve the immunogenicity of DNA vaccines in animal models.
Molecular adjuvants comprise signaling molecules such as toll-like receptor (TLR) agonists, chemokines, cytokines, immune costimulatory molecules and inhibitors of immune-suppressive signaling pathways. The encoding genes for molecular adjuvants could be incorporated into the sequence of DNA vaccines with a eukaryotic expression cassette [62,63].
In recent years, some molecular adjuvants formulated with DNA vaccines have undergone clinical trials in humans, either as immunostimulatory sequences fused to the sequence of the target antigen (e.g. human chemokine CCL20 (MIP3α) and potato virus X coat protein (PVXCP)) [73] or encoded by separate plasmids (e.g. DNA plasmids encoding human cytokines such as IL-2, IL-12, IL-15, IFN-lambda 3 and granulocyte-macrophage colony-stimulating factor (GM-CSF)) [74–77].
Delivery & administration routes of DNA vaccines
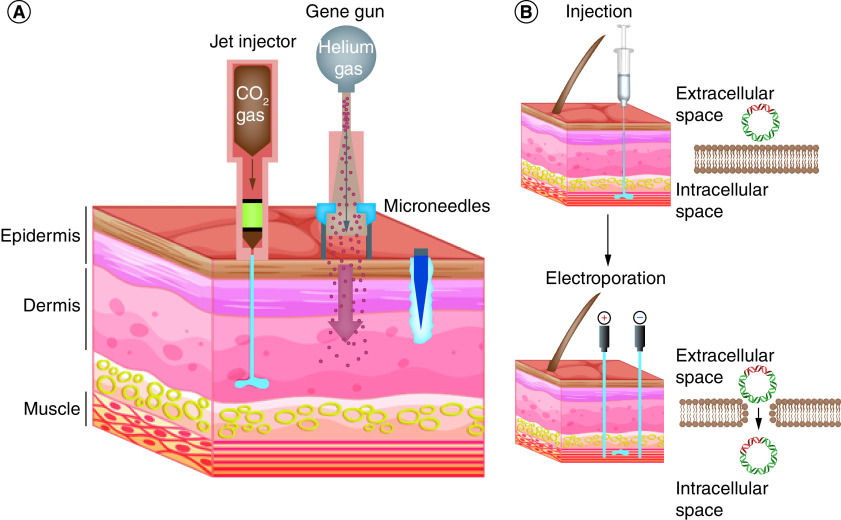
The route of DNA vaccine administration may influence its immunogenicity. Various administration routes such as intramuscular (IM), intradermal (ID), subcutaneous (SC), intravenous (IV), intranodal and intranasal routes have been used to elicit a desired immune response after DNA vaccination. In the first study of DNA vaccines, the vaccine was administrated to mice via the IM route [4]. However, subsequent studies showed that the ID route may increase the expression and immunogenicity of DNA vaccines in mice compared with the IM route [78–80]. Immunization of the skin with DNA vaccines provides DNA for antigen expression in several types of cells including Langerhans cells, dendritic cells and keratinocytes, which are located in the epidermis and the dermis layers, the two main areas of the skin. After maturation, dermal dendritic cells and Langerhans cells can migrate to local lymph nodes and present antigens to T cells, thus starting a variety of immune responses [78]. In addition, SC injection of DNA under high pressure has been reported to evoke greater immune responses in mice than by the IM route [81,82]. Mucosal immunization with DNA vaccines through oral or nasal delivery is another route of delivery for DNA vaccines that generates mucosal as well as systemic immune responses [83,84].
In addition to the administration route, there are multiple physical and chemical methods to increase the efficiency of DNA delivery into cells both in vitro and in vivo and increase its immunogenicity when used as a vaccine [80]. In jet-injector (biojector)-based delivery, usually compressed CO2 gas is used to create a high-pressured stream of medications such as DNA vaccine that can penetrate the skin and elicit higher cellular immunity and antibody responses in humans compared with the conventional syringe and needle vaccine delivery (Figure 3A) [85]. In gene gun delivery, a biolistic system is used that can push DNA-coated microparticles (e.g., DNA-coated gold particles) directly into the skin (Figure 3A). One of the advantages of this method is that lower amounts of DNA are needed to elicit an immune response compared with conventional injection [86]. In the microneedle array-based delivery, over 1000 microneedles (usually 100–1000 μm in length) are used to inject medications such as DNA vaccine with a direct and controlled route to the underlying viable skin layers (Figure 3A) [87,88]. Electroporation is another method for DNA delivery that uses electrical pulses to create transient pores in the cell membrane, thereby increasing DNA uptake (Figure 3B) [89,90]. In humans, in vivo electroporation of DNA for IM delivery elicited a greater magnitude of HIV-specific cellular immunity compared with the traditional syringe and needle IM delivery [91]. The growing number of clinical trials in humans and the corresponding results showed the strong potential of electroporation for DNA vaccination, which combines both efficacy and safety [92]. In addition to these physical methods, liposomes, virosomes, and other synthetic and natural microparticles and nanoparticles such as Fe3O4, polyethyleneimine (PEI), polyamidoamine (PAMAM) and poly(propyl ether imine) (PETIM) dendrimers, chitosan, alginate, dextran, chondroitin sulfate, hyaluronic acid, pullulan, Gelatin, albumin, listeriolysin O (LLO), protamine, epsilon poly-L-lysine, pectin, zein, polyspermine, polyarginine, polydopamine (PDA), polyglutamate (PGA), poly-lactic acid (PLA), poly(lactic-glycolic acid) (PLGA) agarose hydrogel, spermine dextran, cell penetrating peptides (CPPs), poly(beta-amino esters) (PBAE), acrylamide microspheres and protein-based nanoparticles may be employed for DNA vaccine delivery. Such micro- and nanoparticles protect DNA from degradation by nucleases in the body and also increase the cellular uptake of DNA vaccines through endocytosis, thereby improving the immunogenicity of DNA vaccines [80,93–96].
Figure 3. Overview of physical DNA vaccine delivery technologies.
(A) Delivery of DNA vaccines into skin compartments using gene gun, jet injector and microneedles. These physical methods allow for delivery of DNA vaccines into the epidermis, dermis and subcutaneous compartments by providing enhanced efficacy and great safety than conventional needle methods. (B) The use of in vivo electroporation enhances the cellular uptake of injected DNA across the cell membrane.
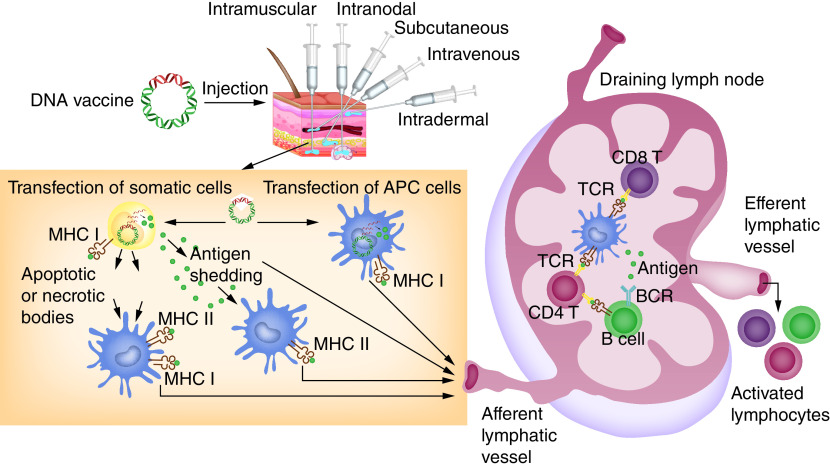
After the DNA vaccine is administrated to the inoculation site using one of several delivery methods, the plasmid translocates to the nucleus of transfected APCs or somatic cells and begins transcription, followed by protein production in cytoplasm and the formation of foreign antigens (Figure 4). After being expressed in APCs and somatic cells, the antigens are taken up by APCs and processed to small peptides which can be displayed on MHC I or II molecules that activate CD8 cytotoxic T lymphocytes (CTLs) and CD4+ T helper (Th) cells, respectively. In addition, B lymphocytes capture antigens released from transfected somatic cells such as myocytes and keratinocytes, which activate humoral immunity (Figure 5) [97,98].
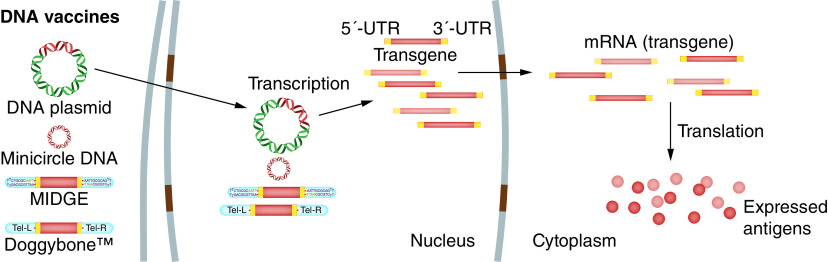
Figure 4. Antigen expression by DNA vaccines.
The DNA construct encoding the transgene (vaccine antigen) translocates to the nucleus, where the transcription to mRNA takes place. Produced mRNAs are then delivered to the cytoplasm to ensure efficient translation of the vaccine antigen.
MIDGE: Minimalistic, immunologically defined gene expression.
Figure 5. Induction of adaptive immune responses by DNA vaccines.
DNA vaccines are administered by multiple routes. The injected DNA enters the somatic cells and/or APCs to produce target antigens, which is the subject of immune surveillance by both MHC class I and class II molecules of APCs. Following the presentation of antigens, activated APCs travel to the draining lymph nodes to present antigenic peptides to CD4 and CD8 T cells. This interaction allows activation and expansion of T cells, as well as antibody-producing B cells. Both humoral and cellular immune responses are induced to trigger a robust response against the target antigens.
APCs: Antigen-presenting cells; BCR: B-cell receptor; MHC: Major histocompatibility complex; TCR: T-cell receptor.
DNA vaccines for COVID-19
The preclinical studies and clinical trials of DNA vaccines for infectious diseases such as influenza virus, HIV, cytomegalovirus (CMV), human hepatitis virus C (HCV), Venezuelan equine encephalitis virus (VEEV), Zika virus (ZIKV), Ebola virus (EBOV) and Middle East respiratory syndrome coronavirus (MERS-CoV) as well as for immunotherapy of viral diseases and cancer have been previously reviewed [3,98]. In this section, we focus on preclinical and clinical trials of DNA vaccines for COVID-19 disease caused by SARS-CoV-2.
The global vaccine research and development (R&D) for the pandemic COVID-19 is unprecedented in scale and speed. Due to the need for speed, vaccine designers and developers are making a fundamental change in the vaccine production process, which previously took more than 10 years, even compared with the 5-year acceleration time scale for the production of the first Ebola vaccine. This accelerated process requires a new paradigm of vaccine development involving parallel development phases, new regulatory processes and manufacturing capacity scaling [99]. Although DNA vaccines have a new platform technology that was not previously available on the market for human use, they are very promising in the SARS-CoV-2 vaccine race [100,101]. DNA vaccines can be designed and produced within days after obtaining the genome sequence of the pathogen or nucleotide sequence of cancer antigens. Plasmid DNA manufacturing processes allow for scalable manufacture of DNA vaccines, making them ideal for rapid control of newly emerging pathogens, which circumvent the problems of conventional vaccines produced in eggs or eukaryotic cell culture bioreactors [102,103]. However, the novel vaccine technology platforms such as DNA vaccines for a new virus target, and novel development paradigms may increase the risks associated with injecting an approved vaccine, requiring careful evaluation of safety and effectiveness at each step of vaccine development. Scientists have developed specific animal models such as human ACE2 (hACE2) expressing transgenic mice, hamsters and non-human primates to evaluate the protective efficacy of anti-COVID-19 vaccines in preclinical studies [99]. Another suitable animal model involves the intranasal delivery of a viral vector encoding hACE2 to wild-type animals prior to viral challenge [104]. Several preclinical studies and clinical trials have demonstrated the immunogenicity of DNA vaccines against SARS-CoV-2, which are summarized in Tables 1 and 2, respectively. Preclinical experiments demonstrated that DNA vaccines can elicit both humoral and cellular immune responses in animal models (Table 1).
Table 1. Overview of published preclinical in vivo studies of DNA vaccines against SARS-CoV-2.
| Category | Animal model | Delivery route | Dose | Antigen | Immune responses | Ref. |
|---|---|---|---|---|---|---|
| Plasmid DNA | BALB/c mice, C57BL/6 mice, and guinea pigs | IM + EP for mice, ID + EP for guinea pigs | Twice (2.5, 10 or 25 μg) for mice, single (100 μg) for guinea pigs | S protein | H, C, nAb | [103] |
| Plasmid DNA | Rhesus macaques | IM | Twice (5 mg) | Different forms of the S protein: full length, deletion of the cytoplasmic tail, the soluble ectodomain, S1 domain with a foldon trimerization tag, RBD with a foldon trimerization tag, prefusion-stabilized soluble ectodomain with two proline mutations, deletion of the furin cleavage site and a foldon trimerization tag | H, C, nAb | [105] |
| Plasmid DNA | BALB/cJ mice | EP | Twice (60 μg) | Engineered RBD, with four novel glycosylation sites, fused to multimerization platforms | nAb | [106] |
| Plasmid DNA | Syrian hamsters | IM + jet injection | Single (0.2 mg) | S protein | nAb | [107] |
| Plasmid DNA | C57BL/6 and BALB/c mice | (IM or ID) + EP | Twice (days 1 and 14 or 21) | Prefusion-stabilized S protein/alone or combined with plasmid IL-12 | nAb | [108] |
| Plasmid DNA | Rhesus macaques | Needle Free Injection System (NFIS)/Syringe-needle injection (ID) | Thrice (days 1, 28 and 56) | S protein | nAb | [109] |
| Plasmid DNA | C57BL/6 mice | IM | Thrice (weeks 0, 2 and 4) | RBD fused to the amino-terminal region of hepatitis B virus preS1 with a W4P mutation | H, C, nAb | [110] |
| Plasmid DNA | ICR mice | IM + EP | Thrice (weeks 0, 2 and 4) | S protein or S1 subunit or S2 subunit | H, C, nAb | [111] |
| Plasmid DNA+ recombinant protein | Rhesus macaques | IM | Thrice (weeks 0, 2 and 8) | S protein (Plasmid DNA) + S1 subunit (Recombinant protein) | nAb | [112] |
C: Cellular immune response; EP: Electroporation; H: Humoral immune response; ID: Intradermal; IM: Intramuscular; nAb: Neutralizing antibody; RBD: Eeceptor-binding domain; S: Spike protein.
Table 2. Overview of clinical trials of DNA vaccines against COVID-19 based on WHO report: draft landscape and tracker of COVID-19 candidate vaccines – 4 May 2021 (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines).
| Vaccine type (vaccine name)/description | Developer | Participants (n) | Dose/route | Clinical stage, outcome | Trial registration no. | Ref. |
|---|---|---|---|---|---|---|
| Plasmid DNA (INO-4800)/a pGX9501 plasmid that encodes for the full length of the S protein | Inovio Pharmaceuticals/ International Vaccine Institute | 40 healthy adults (18–50 years) | 2× (1 or 2 mg) on day 0 and day 28/ID injection followed by EP using the CELLECTRA® 2000 device | I – (INO-4800 was safe and immunogenic in all of the vaccinated subjects. The vaccine elicited either or both humoral or cellular immune responses) | NCT04336410 | [16,19] |
| Plasmid DNA (INO-4800) | Inovio Pharmaceuticals/ International Vaccine Institute | 160 healthy adults (19 years and older) | 2× (1 or 2 mg)/ID injection followed by EP using the CELLECTRA® 2000 device | I/II | NCT04447781 | |
| Plasmid DNA (INO-4800) | Inovio Pharmaceuticals/ International Vaccine Institute | 640 healthy adult (18–60 years) and elderly (60–85 years) volunteers | 2× (ID + EP) | II | ChiCTR2000040146 | |
| Plasmid DNA (INO-4800) | Inovio Pharmaceuticals/ International Vaccine Institute | 6578 healthy adults (18 years and older) | 1× or 2× (1 mg) on day 0 and day 28/ID injection followed by EP using the CELLECTRA® 2000 device | II/III | NCT04642638 | [18] |
| Plasmid DNA (AG0301-COVID19)/a Plasmid DNA + adjuvant | AnGes + Takara Bio + Osaka University | 30 healthy adults (20 years and older) | 2× (1 or 2 mg)/IM | I/II | NCT04463472 | |
| Plasmid DNA (AG0301-COVID19) | AnGes + Takara Bio + Osaka University | 500 healthy adults (18 years and older) | 2× 2 mg/IM | II/III | NCT04655625 | |
| Plasmid DNA (AG0302-COVID19)/a Plasmid DNA + adjuvant | AnGes + Takara Bio + Osaka University | 30 healthy adults (20 years and older) | 2× or 3× 2 mg/IM | I/II | NCT04527081 | |
| Plasmid DNA (nCov Vaccine/ZyCOV-D) | Zydus Cadila | 1048 (18–55 years of age in Phase I, ≥12 years of age in Phase II) | 3× (1 or 2 mg)/ID injection by needle or PharmaJet® | I/II | CTRI/2020/07/026352 | |
| Plasmid DNA (nCov Vaccine/ZyCOV-D) | Zydus Cadila | 150 healthy subjects (18–60 years) | 2× (3 mg)/ID injection by PharmaJet® | I/II | CTRI/2021/03/032051 | |
| Plasmid DNA (Covigenix VAX-001)/DNA vaccines + proteo-lipid vehicle formulation | Entos Pharmaceuticals Inc. | 72 healthy adults (18–84 years) | 2× IM on day 0 and day 14 | I | NCT04591184 | |
| Plasmid DNA (CORVax 12)/encoding SARS-CoV-2 S protein with or without the combination of IL-12p70 plasmid | Providence Health & Services | 36 healthy adults (18 years and older) | 2× ID followed by EP on day 0 and day 28 | I | NCT04627675 | |
| Plasmid DNA (GLS-5310) | GeneOne Life Science, Inc. | 345 healthy adults (19–65 years) | 2× (0.6 or 1.2 mg)/ID on day 0 + 56 or day 0 + 84 | I/II | NCT04673149 | |
| DNA (GX-19)/DNA vaccine encoding SARS-CoV-2 S protein | Genexine Consortium | 210 healthy adults (18–50 years) | 2× IM injection via EP or PharmaJet® | I/II | NCT04445389 | [17] |
| DNA (GX-19N)/DNA vaccine encoding SARS-CoV-2 S protein antigen including the Nucleocapsid protein (NP) antigen | Genexine Consortium | 170 healthy adults (18–55 years) | 2× IM injection via EP | I/II | NCT04715997 | [17] |
| DNA (COVIGEN) | University of Sydney, Bionet Co., Ltd Technovalia |
150 healthy adults (18–75 years) | 2× (IM or ID) | I | NCT04742842 | |
| Plasmid DNA (COVID-eVax)/DNA vaccine encoding SARS-CoV-2 S protein | Takis + Rottapharm Biotech | 160 healthy adults (18–65 years) | 2× (0.5, 1 or 2 mg) IM injection via EP | I/II | NCT04788459 |
EP: Electroporation; ID: Intradermal; IM: Intramuscular; RBD: Receptor-binding domain; S: Spike.
The spike (S) protein of SARS-CoV-2 is the main antigen used in preclinical and clinical trials of DNA vaccines for COVID-19 (Tables 1 and 2). S protein has two subunits, S1 and S2. S1 subunit has a receptor-binding domain (RBD) that binds to the human ACE2 receptor and attaches virus particles to the host cell membrane, initiating the infection process [113]. The S2 subunit contains a fusion peptide that helps the fusion of viral and host cell membranes during the process of virus entry into the host cell [113]. Different forms of the S protein, including the full-length S protein (either wild-type or a prefusion-stabilized version), RBD, S1 subunit, and S2 subunit are currently used in preclinical and clinical trials of DNA vaccines for COVID-19 (Tables 1 and 2). Nucleocapsid (N) protein is another antigen used in a SARS-CoV-2 candidate DNA vaccine named GX-19N (ClinicalTrials.gov number, NCT04715997) along with the S protein (Table 2). N protein protects the viral genome and is also involved in the release of viral particles from infected cells [113].
The IM and ID routes of administration are the main routes of DNA delivery in both preclinical and clinical trials of DNA vaccines for COVID-19 (Tables 1 and 2). In addition, EP and jet-injection are the main physical methods to improve the efficiency of DNA vaccine delivery in both preclinical and clinical trials of COVID-19 (Tables 1 and 2).
There are currently 11 candidate DNA vaccines in clinical trials for COVID-19 (Table 2). INO-4800 is a pGX9501 plasmid that encodes the full length of the SARS-CoV-2 S protein. In a Phase I clinical trial (ClinicalTrials.gov number, NCT04336410), INO-4800 was injected into 40 healthy adults aged between 18 and 50 years in two doses (1 or 2 mg of plasmid DNA at day 0 and day 28) by ID injection followed by EP using the CELLECTRA® 2000 device [16]. INO-4800 elicited adequate humoral responses against SARS-CoV-2. The vaccine induced binding or neutralizing antibodies in 95% (18/19) of the participants in both dose groups. Neutralizing antibodies were seen in 78% (14/18) and 84% (16/19) of the participants in the 1- and 2-mg dose groups, respectively. The corresponding geometric mean titers (GMTs) were 102.3 (95% CI: [37.4, 280.3]) for the 1-mg dose group and 63.5(95% CI: [39.6, 101.8]) for the 2-mg dose group based on the plaque-reduction neutralization testing (PRNT) assay with live SARS-CoV-2, at day 42 [16]. The range of GMTs overlaps with the PRNT IC50 titers reported from convalescent patients and meets the US FDA recommended (160) and minimal (80) GMT for convalescent plasma use [114]. In addition, good CD4 and CD8 T-cell responses were observed in the trial, especially in the 2 mg-dose group. T-cell responses were activated in 74 and 100% of the 1- and 2-mg dose groups, respectively. However, cellular immune responses were higher in the 2-mg dose group than in the convalescent samples. According to the report, INO-4800 was safe and immunogenic in all of the vaccinated individuals [16]. The antibody response persisted 6 months following the second dose of INO-4800 vaccine, and a booster dose 6–10.5 months following the second dose significantly increased immune responses [19]. In a Phase II clinical trial (ClinicalTrials.gov number, NCT04642638), INO-4800 was evaluated in 401 participants in two doses (1 or 2 mg of plasmid DNA at day 0 and day 28) by ID injection followed by EP using the CELLECTRA® 2000 device. At day 42, the GMT (SD of log10) of neutralizing antibody in the 1- and 2-mg dose groups were 93.6 (0.47) and 150.6 (0.46), respectively. The baseline GMTs for the 1- and 2-mg dose groups were 32.2 (0.38) and 35.8 (0.45), respectively. Based on this clinical trial, humoral and cellular immune responses were higher in the 2-mg dose group compared with the 1-mg dose group and thus INO-4800 2-mg dose was selected for advancement into a Phase III efficacy evaluation [18].
Based on a pseudovirus neutralization assay using sera collected from INO-4800 vaccinated individuals two weeks after administration of a third dose (0.5 mg, 1 mg, or 2 mg; NCT04336410), there was a 2.1- and 6.9-fold reduction of neutralizing activity against SARS-CoV-2 variants B.1.1.7 (first reported in the UK) and B.1.351 (first reported in South Africa), respectively, while there was no difference between P.1 (first reported in Brazil) variant and wild-type (WT). Surprisingly, despite recent studies indicating a reduction in neutralizing activity against SARS-CoV-2 variant P.1, INO-4800 vaccine generated neutralizing antibodies at levels comparable to the WT. INO-4800 cellular immune response was similar against these variants and WT [115].
GX-19 is a DNA vaccine that contains a plasmid DNA encoding SARS-CoV-2 S protein, and GX-19N contains a plasmid DNA encoding SARS-CoV-2 RBD and N protein as well as a plasmid DNA encoding SARS-CoV-2 S protein. In two Phase I trials of GX-19 and GX-19N (NCT04445389 and NCT04715997), GX-19 and GX-19N vaccines were evaluated in 40 and 21 participants, respectively [17]. Two doses (1.5 mg or 3 mg of plasmid DNA for GX-19 and 3 mg for GX-19N at day 0 and day 28) were injected by the IM route followed by EP. GX-19 and GX-19N showed low GMTs of neutralizing antibodies. In GX-19N group, neutralizing antibodies significantly increased after vaccination, but the GMT of neutralizing antibodies on day 57 (37.26) was lower than those from human convalescent serum. However, GX-19 and GX-19N showed significantly enhanced T-cell responses. S-specific T-cell responses were seen in 50% (10/20) of the participants in both dose groups in the GX-19 trial. GX-19N vaccine induced stronger T cell immune responses than GX-19 vaccine and exhibited S- and N-specific T-cell responses. T-cell responses were seen in 75% (15/20) of the participants in the GX-19N trial [17].
nCov Vaccine (ZyCoV-D) is a DNA vaccine that contains a plasmid DNA encoding SARS-CoV-2 S protein. In a Phase I clinical trial, the safety and immunogenicity of ZyCoV-D was evaluated in 126 participants. Three doses of ZyCoV-D (1 mg or 2 mg) were administrated by the ID route via NFIS device 28 days apart. ZyCoV-D was found to be safe and immunogenic in the Phase I trial. However, the GMTs of neutralizing antibodies were low (<40). Based on the ELISPOT assay, ZyCoV-D vaccine induced cellular immune responses when administered ID via NFIS at 2-mg dose. However, there were no significant changes in IFN-γ, IL-2, IL-4, IL-6, IL-10, TNF-alpha and Th-17A cytokines levels compared with baseline [15]. Based on the unpublished results of a Phase III clinical trial, ZyCoV-D has been found to be 67% protective against symptomatic COVID-19 [116]. India's drug regulator has approved ZyCoV-D vaccine for emergency use against COVID-19 [116]. ZyCoV-D is the world's first approved DNA vaccine to be administered in humans [117].
Advantages & obstacles of DNA vaccines
DNA vaccines hold several advantages that can make them a suitable vaccine against newly emerging pathogens such as SARS-CoV-2 virus: i) The manufacturing of DNA vaccines is inexpensive, rapid and scalable. A bacterial culture fermenter is used to provide large-scale plasmid DNA. Initial plasmid construction, cell bank preparation, bacterial fermentation and plasmid purification are completed within 2–4 weeks [60]; ii) DNA vaccines have fast and flexible R&D. They can be rapidly designed and produced for preclinical studies and clinical trials; iii) DNA vaccines express viral and cancer antigens matching better with their native form. DNA vaccines deliver genetic material into a host cell and use the host cell translational machinery to express protein antigens in their native folding and glycosylation pattern, without the problems commonly associated with protein expression and purification issues such as protein solubility in the production of recombinant proteins [100]; IV) DNA vaccines generate foreign intracellular antigens which are presented in the context of MHC class I as well as class II molecules of APCs, eliciting both cellular and humoral immune responses; V) DNA vaccines are relatively stable at ambient temperatures; VI) DNA vaccines can simply be equipped with an additional expression cassette encoding a molecular adjuvant to elicit stronger immune responses; VII) unlike viral vector vaccines, pre-existing immunity to the vaccine backbone is not a problematic factor for DNA vaccines due to the lack of a host immune response to plasmid DNA [118].
However, in the application of DNA vaccines, some obstacles may need to be overcome: i) Once administered, naked DNA vaccines are rapidly degraded by nucleases in the mucosa, skin, and plasma, and only small amounts of injected DNA are taken up by cells, resulting in low efficiencies; ii) different biological barriers including cell membrane, endosomes and nucleus membrane are barriers for the DNA vaccines reaching their target site; iii) after reaching the cell nucleus, low antigen expression is another barrier that may lead to low immunogenicity of DNA vaccines; IV) DNA vaccines may continuously express the target antigen, which may lead to potential tolerance to the pathogen [119].
Improving delivery and antigen expression may improve the performance of DNA vaccines [120]. Delivery systems can increase the delivery of DNA vaccines to reach their target (cell nucleus) and/or protect them from degradation by extracellular nucleases. In the development of DNA vaccines for COVID-19, physical methods (e.g., electroporation and jet-injection) are the most widely used delivery systems for delivering DNA vaccines to the required intracellular location (Table 2). DNA vaccines are stable and therefore usually administered in naked form. But milligram amounts of a DNA vaccine are required to be injected into a human to elicit potent immune responses. This is a barrier to success in industrial production of DNA vaccines as that amount of DNA is expensive to produce. Physical methods such as electroporation, jet-injection and gene gun can reduce the amount of DNA needed for immunization and increase delivery efficiency. But specialized devices are needed for in vivo electroporation, jet-injection and gene gun-mediated delivery of DNA vaccines in research and in the hospital setting. Several types of natural and synthetic nanoparticles may be used for DNA vaccine delivery and protection from degradation. Although DNA plasmids can be integrated into the genome of host cells, the level of integration is relatively low [121]. Six weeks after intramuscular injection of three different DNA plasmids in mice, the level of free plasmids in the treated muscle ranged from 1000 to 4000 copies/μg of host DNA (1 μg of DNA represented ∼150,000 diploid cells). After six months, the free plasmids were still stable in muscle, in the range of 200–800 copies/μg of DNA, and no integration of the DNA plasmid to genomic DNA was observed [122]. Therefore, it is believed that the risk of insertional mutations due to the integration of DNA plasmids following injection is negligible and integration is usually done at rates that are less than the frequency of the spontaneous mutations [97]. In contrary, there are some reports of leukemogenesis driven by viral vector insertional mutagenesis in severe combined immunodeficiency (SCID) gene therapy clinical trials in humans [123,124]. In addition, linearized plasmids have an increased probability of chromosomal integration [125]. Therefore, there is a potential risk of insertional mutagenesis following mechanical shearing of plasmid DNA. The FDA designed guidelines for DNA vaccines to ensure that the frequency of plasmid integration would be lower than the spontaneous mutation rate [126]. Based on these guidelines, we can monitor the biodistribution of plasmids in tissues of vaccinated animals by sensitive q-PCR assays. The integration of plasmids can be assessed by separating high molecular weight genomic DNA from smaller free plasmids. The q-PCR and/or repeat-anchored integration capture (RAIC)-PCR techniques are then used to detect and quantify the integration of plasmid in the genomic DNA. Based on studies on multiple different plasmids, and of the same plasmid with various DNA inserts, FDA proposed that DNA vaccines prepared using a plasmid DNA previously documented to have an acceptable DNA biodistribution/integration profile could waive biodistribution/persistence studies. Integration studies are required for novel plasmids and novel methods of formulation and delivery for plasmids that persist at amounts of higher than 10,000 copies per microgram of host DNA [126]. Recently, the FDA paused the planned Phase II/III trial of the vaccine candidate INO-4800 due to questions about the design and use of the INOVIO delivery machine ‘CELLECTRA® 2000’, which is used to deliver the vaccine directly into the skin [127]. Less than two months later, the FDA allowed INOVIO to move forward with the Phase 2/3 trial (clinical trial identifier: NCT04642638) [127]. Given that free plasmids remain stable in the host cell for months, the question of whether prolonged expression of antigen by DNA vaccines can lead to tolerance requires further investigation.
Conclusion
Over the past few years, many advances have been made in the field of DNA vaccines. The advancements in DNA construction, delivery and administration routes and use of molecular adjuvants have enhanced the immunogenicity of DNA vaccines. It is promising that DNA immunization will revolutionize the vaccine field. The lower cost of manufacturing and storage of DNA vaccines makes them an ideal candidate vaccine for global vaccination, even in low-income countries. This new vaccine platform is now very promising in clinical trials for COVID-19, and scientists are trying to get the first DNA vaccine license for humans.
Future perspective
In the future, we could devise new platforms of DNA vaccines such as minicircle DNA, minimalistic, immunologically defined gene expression, and Doggybone™ for DNA vaccination. The use of these new platforms may lead to more transgene expression in vivo. Successful DNA delivery and the use of adjuvants remain key challenges in DNA vaccines, especially for large animals and humans, that need to be addressed in the future. We need control groups without receiving adjuvants in clinical trials to accurately assess the efficacy of adjuvants in DNA vaccine formulation. The use of nanoparticles or the design of inexpensive efficient devices for DNA vaccine delivery can facilitate the administration of DNA vaccines in the hospital setting. In addition, we need to examine the impact of different platforms, formulations, storage conditions and DNA delivery methods on the risk of insertional mutations due to DNA integration. The data reviewed here indicate that the DNA platform addresses many goals of a good vaccine platform and will be a new class of future vaccines, especially for emerging pathogens such as SARS-Cov-2.
Executive summary.
Design of DNA vaccines
DNA vaccines are DNA constructs that contain at least one eukaryotic expression cassette encoding for the antigen of interest.
Changes in sequence composition and deletion of bacterial backbone sequences in DNA vaccines can increase antigen expression.
Minicircle DNA, minimalistic, immunologically defined gene expression (MIDGE) and Doggybone™ are DNA constructs composed of the gene expression cassette(s) without the bacterial backbone of plasmids.
Individual expression cassettes, bi-cistronic or multi-cistronic vectors and T2A peptide sequence can be used to express multiple genes in a single DNA vaccine.
Nuclear localization signal (NLS) nucleotide sequences, transcription factor-binding proteins, DNA-binding proteins and NLS peptide sequences can be used for enhancing the nuclear localization of DNA vaccines.
For long-term expression of transgenes in human cells, eukaryotic or eukaryotic/viral hybrid promoters can be used in DNA constructs, which remain active for a long time.
Cell-type-specific promoters can be used in expression cassettes, which can restrict the expression of antigens to antigen-presenting cells and prevent tolerance induced by regulatory cells.
Production of DNA vaccines
Plasmid DNA is produced through genetically modified bacteria. After fermentation, bacterial cells are harvested and lysed through chemical, physical or mechanical methods. Then, clarification and purification techniques are used for the purification of plasmid DNA.
Minicircle DNA is produced by inducing the intramolecular recombination of a parental plasmid in bacteria.
MIDGE vectors are produced by digestion of a plasmid using a recombinase, and subsequent ligation of the resulting fragments to hairpin oligodeoxynucleotides to generate a covalently closed dumbbell-shaped DNA molecule.
Doggybone™ DNA is produced through RCA process using a DNA plasmid as template. The resulting DNA concatemers are cleaved and joined by an enzymatic process to generate covalently closed dumbbell-shaped DNA molecules.
Adjuvants
Vaccine adjuvants may be used for improving the immunogenicity of DNA vaccines by stimulating innate immune responses.
Classical adjuvants, nanoparticle adjuvants, and molecular adjuvants have been used with DNA vaccines in exploratory and preclinical studies.
In humans, aluminum phosphate adjuvant did not show any significant effect on the immunogenicity of a DNA vaccine.
In recent years, some molecular adjuvants formulated with DNA vaccines have undergone clinical trials in humans, either as immunostimulatory sequences fused to the sequence of the target antigen or encoded by separate plasmids.
Delivery & administration routes of DNA vaccines
The route of DNA vaccine administration may influence its immunogenicity.
Intramuscular, intradermal, subcutaneous, intravenous, intranodal and intranasal administration routes have been used to elicit a desired immune response after DNA vaccination.
There are multiple physical and chemical methods to increase the efficiency of DNA delivery into cells.
Jet-injection, gene gun, microneedle array and electroporation are physical methods to enhance DNA vaccine delivery into cells.
Nanoparticles can be employed for DNA vaccine delivery.
DNA vaccines for COVID-19
Several preclinical and clinical trials have demonstrated the immunogenicity of DNA vaccines against SARS-CoV-2.
preclinical and clinical trials demonstrated that DNA vaccines can elicit both humoral and cellular immune responses.
The spike protein of SARS-CoV-2 is the main antigen used in preclinical and clinical trials of DNA vaccines for COVID-19.
India's drug regulator has approved ZyCoV-D, the first DNA vaccine against COVID-19 for emergency use.
Author contributions
M Shafaati, F Hazrati, R Mirzaei, S Karampoor, S Kazemi, B Yavari, M Safaei, P Samadi and Y Ahmadyousefi prepared the first draft. Y Ahmadyousefi and R Mirzaei managed the whole manuscript. Y Ahmadyousefi designed the figures and the hypothesis. M Saidijam, M Soleimani and B Amirheidari edited the manuscript. H Mahaki and F Rahbarizadeh participated in the preparation of the first draft and revised the manuscript.
Acknowledgments
This work was supported by Hamadan University of Medical Sciences, Hamadan, Iran.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Jain S, Batra H, Yadav P, Chand S. COVID-19 vaccines currently under preclinical and clinical studies, and associated antiviral immune response. Vaccines 8(4), 649 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmadyousefi Y, Malih S, Mirzaee Y, Saidijam M. Nucleic acid aptamers in diagnosis of colorectal cancer. Biochimie 156, 1–11 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Gary EN, Weiner DB. DNA vaccines: prime time is now. Curr. Opin. Immunol. 65, 21–27 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff JA, Malone RW, Williams P et al. Direct gene transfer into mouse muscle in vivo. Science 247(4949), 1465–1468 (1990). [DOI] [PubMed] [Google Scholar]; •• The first report that showed injection of naked plasmid DNA into mouse muscle results in a local expression of the transgene.
- 5.Stenler S, Blomberg P, Smith CE. Safety and efficacy of DNA vaccines: plasmids vs. minicircles. Hum. Vaccines Immunother. 10(5), 1306–1308 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Fuertes L, Perez-Jimenez E, Vila-Coro A et al. DNA vaccination with linear minimalistic (MIDGE) vectors confers protection against Leishmania major infection in mice. Vaccine 21(3–4), 247–257 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Walters AA, Kinnear E, Shattock RJ et al. Comparative analysis of enzymatically produced novel linear DNA constructs with plasmids for use as DNA vaccines. Gene Ther. 21(7), 645–652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu MA. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines 7(2), 37 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redding L, Weiner DB. DNA vaccines in veterinary use. Expert Rev. Vaccines 8(9), 1251–1276 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee LYY, Izzard L, Hurt AC. A review of DNA vaccines against influenza. Front. Immunol. 9, 1568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin JE, Pierson TC, Hubka S et al. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a Phase I clinical trial. J. Infect. Dis. 196(12), 1732–1740 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledgerwood JE, Pierson TC, Hubka SA et al. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a Phase I clinical trial. J. Infect. Dis. 203(10), 1396–1404 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarwar UN, Costner P, Enama ME et al. Safety and immunogenicity of DNA vaccines encoding Ebolavirus and Marburgvirus wild-type glycoproteins in a Phase I clinical trial. J. Infect. Dis. 211(4), 549–557 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tebas P, Kraynyak KA, Patel A et al. Intradermal SynCon® Ebola GP DNA vaccine is temperature stable and safely demonstrates cellular and humoral immunogenicity advantages in healthy volunteers. J. Infect. Dis. 220(3), 400–410 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Momin T, Kansagra K, Patel H et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): results of an open-label, non-randomized Phase I part of Phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine 38, 101020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The results of a Phase I clinical trial for the ZyCoV-D vaccine.
- 16.Tebas P, Yang S, Boyer JD et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, Phase I clinical trial. EClinicalMedicine 31, 100689 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The results of a Phase I clinical trial for the INO-4800 vaccine.
- 17.Ahn JY, Lee J, Suh YS et al. Safety and immunogenicity of a recombinant DNA COVID-19 vaccine containing the coding regions of the spike and nucleocapsid proteins: preliminary results from an open-label, Phase I trial in healthy adults aged 19–55years. medRxiv (2021) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The results of two Phase I clinical trial for GX-19 and GX-19N vaccines.
- 18.Mammen MP, Tebas P, Agnes J et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of a randomized, blinded, placebo-controlled, Phase 2 clinical trial in adults at high risk of viral exposure. medRxiv (2021) (Epub ahead of print). [Google Scholar]; •• The results of a Phase II clinical trial for the INO-4800 vaccine.
- 19.Kraynyak KA, Blackwood E, Agnes J et al. SARS-CoV-2 DNA vaccine INO-4800induces durable immune responses capable of being boosted in a Phase I open-label trial. medRxiv (2021) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z-Y, He C-Y, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol. Ther. 8(3), 495–500 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Maniar LEG, Maniar JM, Chen Z-Y, Lu J, Fire AZ, Kay MA. Minicircle DNA vectors achieve sustained expression reflected by active chromatin and transcriptional level. Mol. Ther. 21(1), 131–138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, Zhang F, Fire AZ, Kay MA. Sequence-modified antibiotic resistance genes provide sustained plasmid-mediated transgene expression in mammals. Mol. Ther. 25(5), 1187–1198 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moeini H, Omar AR, Rahim RA, Yusoff K. Development of a DNA vaccine against chicken anemia virus by using a bicistronic vector expressing VP1 and VP2 proteins of CAV. Comp. Immunol. Microbiol. Infect. Dis. 34(3), 227–236 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Nemattalab M, Shenagari M, Mojtahedi A et al. Design, cloning and expression assay of oipA gene in a bicistronic vector harboring mice IL-18 gene: potential implications for Helicobacter pylori vaccine investigations. J. basic res. med. sci. 4(3), 1–7 (2017). [Google Scholar]
- 25.Meas S, Mekvichitsaeng P, Roshorm YM. Co-expression of self-cleaved multiple proteins derived from porcinereproductive and respiratory syndrome virus by bi-cistronic and tri-cistronic DNA vaccines. Protein Expr. Purif. 177, 105763 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Sekhavati M, Tahmoorespur M, Abbassi-Daloii T et al. Dual promoter vector construction for simultaneous gene expression using spliced overlap extension by polymerase chain reaction (SOE-PCR) technique. Iran. J. Appl. Anim. Sci. 5(4), 853–858 (2015). [Google Scholar]
- 27.Hobernik D, Bros M. DNA vaccines – how far from clinical use? Int. J. Mol. Sci. 19(11), 3605 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dean DA, Dean BS, Muller S, Smith LC. Sequence requirements for plasmid nuclear import. Exp. Cell Res. 253(2), 713 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vacik J, Dean B, Zimmer W, Dean D. Cell-specific nuclear import of plasmid DNA. Gene Ther. 6(6), 1006–1014 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pouton CW, Wagstaff KM, Roth DM, Moseley GW, Jans DA. Targeted delivery to the nucleus. Adv. Drug Deliv. Rev. 59(8), 698–717 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Vaysse L, Harbottle R, Bigger B, Bergau A, Tolmachov O, Coutelle C. Development of a self-assembling nuclear targeting vector system based on the tetracycline repressor protein. J. Biol. Chem. 279(7), 5555–5564 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Mesika A, Grigoreva I, Zohar M, Reich Z. A regulated, NFκB-assisted import of plasmid DNA into mammalian cell nuclei. Mol. Ther. 3(5), 653–657 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Cheraghi R, Nazari M, Alipour M, Majidi A, Hosseinkhani S. Development of a targeted anti-HER2 scFv chimeric peptide for gene delivery into HER2-positive breast cancer cells. Int. J. Pharm. 515(1–2), 632–643 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Park E, Cho H-B, Takimoto K. Effective gene delivery into adipose-derived stem cells: transfection of cells in suspension with the use of a nuclear localization signal peptide-conjugated polyethylenimine. Cytotherapy 17(5), 536–542 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Ludtke JJ, Zhang G, Sebestyén MG, Wolff JA. A nuclear localization signal can enhance both the nuclear transport and expression of 1 kb DNA. J. Cell Sci. 112(12), 2033–2041 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Opanasopit P, Rojanarata T, Apirakaramwong A, Ngawhirunpat T, Ruktanonchai U. Nuclear localization signal peptides enhance transfection efficiency of chitosan/DNA complexes. Int. J. Pharm. 382(1–2), 291–295 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Neves C, Byk G, Scherman D, Wils P. Coupling of a targeting peptide to plasmid DNA by covalent triple helix formation. FEBS Lett. 453(1–2), 41–45 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Duan B, Cheng L, Gao Y et al. Silencing of fat-1 transgene expression in sheep may result from hypermethylation of its driven cytomegalovirus (CMV) promoter. Theriogenology 78(4), 793–802 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Brooks AR, Harkins RN, Wang P, Qian HS, Liu P, Rubanyi GM. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J. Gene Med. 6(4), 395–404 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Siddiqui AA, Phillips T, Charest H et al. Induction of protective immunity against Schistosoma mansoni via DNA priming and boosting with the large subunit of calpain (Sm-p80): adjuvant effects of granulocyte-macrophage colony-stimulating factor and interleukin-4. Infect. Immun. 71(7), 3844–3851 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krinner S, Heitzer A, Asbach B, Wagner R. Interplay of promoter usage and intragenic CpG content: impact on GFP reporter gene expression. Hum. Gene Ther. 26(12), 826–840 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Vanniasinkam T, Reddy S, Ertl H. DNA immunization using a non-viral promoter. Virology 344(2), 412–420 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Ni J, Nolte B, Arnold A, Fournier P, Schirrmacher V. Targeting anti-tumor DNA vaccines to dendritic cells via a short CD11c promoter sequence. Vaccine 27(40), 5480–5487 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Bonkobara M, Zukas PK, Shikano S, Nakamura S, Cruz PD, Ariizumi K. Epidermal Langerhans cell-targeted gene expression by a dectin-2 promoter. J. Immunol. 167(12), 6893–6900 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Ahsan MF, Gore MM. Comparative analysis of macrophage associated vectors for use in genetic vaccine. Genet. Vaccines Ther. 9(1), 1–12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sudowe S, Ludwig-Portugall I, Montermann E, Ross R, Reske-Kunz AB. Transcriptional targeting of dendritic cells in gene gun-mediated DNA immunization favors the induction of type 1 immune responses. Mol. Ther. 8(4), 567–575 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Ross R, Sudowe S, Beisner J et al. Transcriptional targeting of dendritic cells for gene therapy using the promoter of the cytoskeletal protein fascin. Gene Ther. 10(12), 1035–1040 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Mirzaei R, Mohammadzadeh R, Mirzaei H et al. Role of microRNAs in Staphylococcus aureus infection: potential biomarkers and mechanism. IUBMB life 72(9), 1856–1869 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Rasoul M, Rokhsareh M, Mohammad SM, Sajad K, Ahmadreza M. The human immune system against Staphylococcus epidermidis. Crit. Rev. Immunol. 39(3), (2019). [DOI] [PubMed] [Google Scholar]
- 50.Lori F, Kelly LM, Lisziewicz J. APC-targeted immunization for the treatment of HIV-1. Expert Rev. Vaccines 3(Suppl. 1), S189–S198 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Gilboa E. DC-based cancer vaccines. J. Clin. Investig. 117(5), 1195–1203 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xenopoulos A, Pattnaik P. Production and purification of plasmid DNA vaccines: is there scope for further innovation? Expert Rev. Vaccines 13(12), 1537–1551 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Hou XH, Guo XY, Chen Y, He C-Y, Chen Z-Y. Increasing the minicircle DNA purity using an enhanced triplex DNA technology to eliminate DNA contaminants. Mol. Ther. Methods Clin. Dev. 2, 14062 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu K. Vectorology and factor delivery in induced pluripotent stem cell reprogramming. Stem Cells Dev. 23(12), 1301–1315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreno S, Lopez-Fuertes L, Vila-Coro A et al. DNA immunisation with minimalistic expression constructs. Vaccine 22(13–14), 1709–1716 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Schakowski F, Gorschlüter M, Junghans C et al. A novel minimal-size vector (MIDGE) improves transgene expression in colon carcinoma cells and avoids transfection of undesired DNA. Mol. Ther. 3(5), 793–800 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Karbowniczek K, Rothwell P, Extance J et al. Doggybone DNA: an advanced platform for AAV production. Cell Gene Ther. Insights 3, 731–738 (2017). [Google Scholar]
- 58.Karda R, Counsell JR, Karbowniczek K, Caproni LJ, Tite JP, Waddington SN. Production of lentiviral vectors using novel, enzymatically produced, linear DNA. Gene Ther. 26(3), 86–92 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott VL, Patel A, Villarreal DO et al. Novel synthetic plasmid and Doggybone™ DNA vaccines induce neutralizing antibodies and provide protection from lethal influenza challenge in mice. Hum. Vaccines Immunother. 11(8), 1972–1982 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cai Y, Rodriguez S, Hebel H. DNA vaccine manufacture: scale and quality. Expert Rev. Vaccines 8(9), 1277–1291 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Grunwald T, Ulbert S. Improvement of DNA vaccination by adjuvants and sophisticated delivery devices: vaccine-platforms for the battle against infectious diseases. Clin. Exp. Vaccine Res. 4(1), 1–10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li L, Petrovsky N. Molecular adjuvants for DNA vaccines. Curr. Issues Mol. Biol. 22, 17–40 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Scheerlinck J-PY. Genetic adjuvants for DNA vaccines. Vaccine 19(17–19), 2647–2656 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Quirk EK, Brown EL, Leavitt RY et al. Safety profile of the Merck human immunodeficiency virus-1 clade B gag DNA plasmid vaccine with and without adjuvants. Presented at: Open Forum Infect. (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiménez De Oya N, Escribano-Romero E, Blázquez A-B, Martín-Acebes MA, Saiz J-C. Current progress of avian vaccines against West Nile virus. Vaccines 7(4), 126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyce WM, Vickers W, Morrison SA et al. Surveillance for West Nile virus and vaccination of free-ranging island scrub-jays (Aphelocoma insularis) on Santa Cruz Island, California. Vector Borne Zoonotic Dis. 11(8), 1063–1068 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.First DNA Vaccine Licensed for Chickens.(2021). www.prnewswire.com/news-releases/first-dna-vaccine-licensed-for-chickens-300554855.html
- 68.Cappellano G, Abreu H, Casale C, Dianzani U, Chiocchetti A. Nano-microparticle platforms in developing next-generation vaccines. Vaccines 9(6), 606 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eusébio D, Neves AR, Costa D et al. Methods to improve the immunogenicity of plasmid DNA vaccines. Drug Discov. Today (2021). [DOI] [PubMed] [Google Scholar]
- 70.Ishii N, Fukushima J, Kaneko T et al. Cationic liposomes are a strong adjuvant for a DNA vaccine of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 13(16), 1421–1428 (1997). [DOI] [PubMed] [Google Scholar]
- 71.Bhakta G, Nurcombe V, Maitra A, Shrivastava A. DNA-encapsulated magnesium phosphate nanoparticles elicit both humoral and cellular immune responses in mice. Results Immunol. 4, 46–53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun B, Zhao X, Gu W et al. ATP stabilised and sensitised calcium phosphate nanoparticles as effective adjuvants for a DNA vaccine against cancer. J. Mater. Chem. B 9(36), 7435–7446 (2021). [DOI] [PubMed] [Google Scholar]
- 73.Meleshko A, Petrovskaya N, Savelyeva N, Vashkevich K, Doronina S, Sachivko N. Phase I clinical trial of idiotypic DNA vaccine administered as a complex with polyethylenimine to patients with B-cell lymphoma. Hum. Vaccines Immunother. 13(6), 1398–1403 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin X, Morgan C, Yu X et al. Multiple factors affect immunogenicity of DNA plasmid HIV vaccines in human clinical trials. Vaccine 33(20), 2347–2353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han JW, Sung PS, Hong S-H et al. IFNL3-adjuvanted HCV DNA vaccine reduces regulatory T cell frequency and increases virus-specific T cell responses. J. Hepatol. 73(1), 72–83 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Butterfield LH, Economou JS, Gamblin TC, Geller DA. Alpha fetoprotein DNA prime and adenovirus boost immunization of two hepatocellular cancer patients. J. Transl. Med. 12(1), 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mirzaei R, Attar A, Papizadeh S et al. The emerging role of probiotics as a mitigation strategy against coronavirus disease 2019 (COVID-19). Arch. Virol. 1–22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peachman KK, Rao M, Alving CR. Immunization with DNA through the skin. Methods 31(3), 232–242 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Torres C, Iwasaki A, Barber BH, Robinson HL. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J. Immunol. 158(10), 4529–4532 (1997). [PubMed] [Google Scholar]
- 80.Jorritsma S, Gowans E, Grubor-Bauk B, Wijesundara D. Delivery methods to increase cellular uptake and immunogenicity of DNA vaccines. Vaccine 34(46), 5488–5494 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Matheï C, Van Damme P, Meheus A. Hepatitis B vaccine administration: comparison between jet-gun and syringe and needle. Vaccine 15(4), 402–404 (1997). [DOI] [PubMed] [Google Scholar]
- 82.Phillips AJ. The challenge of gene therapy and DNA delivery. J. Pharm. Pharmacol. 53(9), 1169–1174 (2001). [DOI] [PubMed] [Google Scholar]
- 83.Oh Y-K, Kim J-P, Hwang TS et al. Nasal absorption and biodistribution of plasmid DNA: an alternative route of DNA vaccine delivery. Vaccine 19(31), 4519–4525 (2001). [DOI] [PubMed] [Google Scholar]
- 84.Kaneko H, Bednarek I, Wierzbicki A et al. Oral DNA vaccination promotes mucosal and systemic immune responses to HIV envelope glycoprotein. Virology 267(1), 8–16 (2000). [DOI] [PubMed] [Google Scholar]
- 85.Graham BS, Enama ME, Nason MC et al. DNA vaccine delivered by a needle-free injection device improves potency of priming for antibody and CD8+ T-cell responses after rAd5 boost in a randomized clinical trial. PloS one 8(4), e59340 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gadhave DU, Gaikwad PS, Pimpodkar NV, Udugade SB. DNA vaccines: a hope full ray in Immunology. Asian J. Pharm. Sci. 5(2), 126–131 (2015). [Google Scholar]
- 87.Birchall JC. Microneedle array technology: the time is right but is the science ready? Expert Rev. Med. Devices 3(1), 1–4 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Prausnitz MR. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 56(5), 581–587 (2004). [DOI] [PubMed] [Google Scholar]
- 89.Widera G, Austin M, Rabussay D et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 164(9), 4635–4640 (2000). [DOI] [PubMed] [Google Scholar]
- 90.Maruyama H, Ataka K, Higuchi N, Sakamoto F, Gejyo F, Miyazaki J. Skin-targeted gene transfer using in vivo electroporation. Gene Ther. 8(23), 1808–1812 (2001). [DOI] [PubMed] [Google Scholar]
- 91.Vasan S, Hurley A, Schlesinger SJ et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PloS ONE 6(5), e19252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lambricht L, Lopes A, Kos S, Sersa G, Préat V, Vandermeulen G. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin. Drug Deliv. 13(2), 295–310 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Zhang M, Hong Y, Chen W, Wang C. Polymers for DNA vaccine delivery. ACS Biomater. Sci. Eng. 3(2), 108–125 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Fruk L, Franck CO, Fanslau L, Popov AB, Tyagi P. Biopolymer-based carriers for DNA vaccine design. Angew. Chem. 133(24), 13333–13351 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shah MaA, He N, Li Z, Ali Z, Zhang L. Nanoparticles for DNA vaccine delivery. J. Biomed. Nanotechnol 10(9), 2332–2349 (2014). [DOI] [PubMed] [Google Scholar]
- 96.Farris E, Brown DM, Ramer-Tait AE, Pannier AK. Micro-and nanoparticulates for DNA vaccine delivery. Exp. Biol. Med. 241(9), 919–929 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat. Rev. Genet. 9(10), 776–788 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu L, Mp M, Bagarazzi M. Clinical use of DNA vaccines. Handbook of Electroporation 25, 1933–1952 (2017). [Google Scholar]
- 99.Le TT, Andreadakis Z, Kumar A et al. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 19(5), 305–306 (2020). [DOI] [PubMed] [Google Scholar]
- 100.Ye T, Zhong Z, García-Sastre A, Schotsaert M, De Geest BG. Current status of COVID-19 (pre) clinical vaccine development. Angew. Chem. Int. 59(43), 18885–18897 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mirzaei R, Mohammadzadeh R, Mahdavi F et al. Overview of the current promising approaches for the development of an effective severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. Int. Immunopharmacol. 106928 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brito LA, Kommareddy S, Maione D et al. Self-amplifying mRNA vaccines. In: Advances in Genetics. Elsevier, 179–233 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Smith TR, Patel A, Ramos S et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 11(1), 1–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hassan AO, Case JB, Winkler ES et al. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell 182(3), 744–753 e744 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu J, Tostanoski LH, Peter L et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 369(6505), 806–811 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo Y, He W, Mou H et al. An engineered receptor-binding domain improves the immunogenicity of multivalent SARS-CoV-2 vaccines. Mbio 12(3), e00930–21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brocato RL, Kwilas SA, Kim RK et al. Protective efficacy of a SARS-CoV-2 DNA Vaccine in wild-type and immunosuppressed Syrian hamsters. NPJ vaccines 6(1), 1–7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jensen S, Twitty C, Paustian C et al. Preliminary evaluation of a novel coronavirus vaccine (CORVax) using electroporation of plasmid DNA encoding a stabilized prefusion SARS-CoV-2 spike protein alone or with transfection of plasmid IL-12. J. ImmunoTher. Cancer 8, A296–A296 (2020). [Google Scholar]
- 109.Yadav P, Kumar S, Agarwal K et al. Assessment of immunogenicity and protective efficacy of ZyCoV-D DNA vaccine candidates in Rhesus macaques against SARS-CoV-2 infection. BioRxiv (2021) (Epub ahead of print). [Google Scholar]
- 110.Jeong H, Choi Y-M, Seo H, Kim B-J. A novel DNA vaccine against SARS-CoV-2 encoding a chimeric protein of its receptor-binding domain (RBD) fused to the amino-terminal region of hepatitis B virus preS1 with a W4P mutation. Front. Immunol. 12, 637654 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prompetchara E, Ketloy C, Tharakhet K et al. DNA vaccine candidate encoding SARS-CoV-2 spike proteins elicited potent humoral and Th1 cell-mediated immune responses in mice. PLoS One 16(3), e0248007 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Y, Bi Y, Xiao H et al. A novel DNA and protein combination COVID-19 vaccine formulation provides full protection against SARS-CoV-2 in rhesus macaques. Emerging Microbes & Infections 10(1), 342–355 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Silveira MM, Moreira GMSG, Mendonça M. DNA vaccines against COVID-19: perspectives and challenges. Life Sci. 118919 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee WT, Girardin RC, Dupuis AP et al. Neutralizing antibody responses in COVID-19 convalescent sera. J. Infect. Dis. 223(1), 47–55 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Andrade VM, Christensen-Quick A, Agnes J et al. INO-4800 DNA vaccine induces neutralizing antibodies and T cell Activity against global SARS-CoV-2 variants. bioRxiv (2021) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mallapaty S. India's DNA COVID vaccine is a world first – more are coming. Nature 597(7875), 161–162 (2021). [DOI] [PubMed] [Google Scholar]
- 117.World's first COVID-19 DNA vaccine. (2021). https://dbtindia.gov.in/sites/default/files/World%27s%20First%20DNA%20Vaccine_The%20Scientific%20Journey.pdf
- 118.Dupuy LC, Schmaljohn CS. DNA vaccines for biodefense. Expert Rev. Vaccines 8(12), 1739–1754 (2009). [DOI] [PubMed] [Google Scholar]
- 119.Niezold T, Storcksdieck Genannt Bonsmann M, Maaske A et al. DNA vaccines encoding DEC 205-targeted antigens: immunity or tolerance? Immunology 145(4), 519–533 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams JA, Carnes AE, Hodgson CP. Plasmid DNA vaccine vector design: impact on efficacy, safety and upstream production. Biotechnol. Adv. 27(4), 353–370 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Z, Troilo P, Wang X et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 11(8), 711–721 (2004). [DOI] [PubMed] [Google Scholar]
- 122.Ledwith BJ, Manam S, Troilo PJ et al. Plasmid DNA vaccines: investigation of integration into host cellular DNA following intramuscular injection in mice. Intervirology 43(4–6), 258–272 (2000). [DOI] [PubMed] [Google Scholar]
- 123.Hacein-Bey-Abina S, Garrigue A, Wang GP et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 118(9), 3132–3142 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Howe SJ, Mansour MR, Schwarzwaelder K et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Investig. 118(9), (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brinster RL, Chen HY, Trumbauer ME, Yagle MK, Palmiter RD. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc. Natl Acad. Sci. USA 82(13), 4438–4442 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klinman DM, Klaschik S, Tross D, Shirota H, Steinhagen F. FDA guidance on prophylactic DNA vaccines: analysis and recommendations. Vaccine 28(16), 2801–2805 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.INOVIO announces initiation of Phase 2 segment of its Phase 2/3 clinical trial for its COVID-19 DNA vaccine candidate, INO-4800; trial will be funded by the U.S. Department of Defense.(2021). https://ir.inovio.com/news-releases/news-releases-details/2020/INOVIO-Announces-Initiation-of-Phase-2-Segment-of-its-Phase-23-Clinical-Trial-for-its-COVID-19-DNA-Vaccine-Candidate-INO-4800-Trial-Will-Be-Funded-by-the-U.S.-Department-of-Defense/default.aspx