Objective
The aim of this study is to determine a development of humoral response after COVID-19 vaccination in persons with secondary progressive multiple sclerosis (pwSPMS) on siponimod, compared to healthy controls (HC).Methods: In 13 pwSPMS taking siponimod and 11 HC, testing for SARS-CoV2 antibodies was performed after vaccination against COVID-19.Results: pwSPMS taking siponimod had a significantly lower titer of SARS-CoV2 antibodies compared to healthy controls (19.4 (0-250) vs. 250 (250), p>0.001). Two (15.4%) pwSPMS on siponimod had unmeasurable titers of SARS-CoV2-2 antibodies, while all HC had positive titers.Conclusion: Although the results of this study are limited by a small sample size, results have consistently shown low titers of SARS-CoV-2 IgG after COVID-19 vaccinations in pwSPMS on siponimod.
Keywords: Secondary progressive multiple sclerosis, Siponimod, COVID-19, Vaccines, SARS-CoV-2 IgG antibody
1. Introduction
Compared to relapsing-remitting multiple sclerosis (RRMS), secondary progressive multiple sclerosis (SPMS) is characterized by older age, higher level of disability, and higher occurrence of cardiovascular comorbidities (Cree et al., 2021). This is of particular importance in the light of COVID-19 pandemics, as real-world studies published so far identified age and cardiovascular comorbidities as independent risk factors for poor COVID-19 outcomes, with level of neurological disability and progressive multiple sclerosis (MS) phenotypes additionally increasing this risk (Sormani et al., 2021; Salter et al., 2021; Arrambide et al., 2021; Louapre et al., 2020). More and more data also suggest an attenuated humoral response after SARS-COV-2 infection and after COVID-19 vaccination in people with RRMS, depending on the disease modifying therapies used (Habek et al., 2021; Achiron et al., 2021).
As of recently, Siponimod—a sphingosine 1-phosphate receptor modulator—has been approved for the treatment of the active SPMS (Anon, 2021). As limited data exist on response to vaccination in people with SPMS (pwSPMS) treated with siponimod, the aim of this study is to determine a development of humoral response after COVID-19 vaccination in pwSPMS on siponimod, compared to healthy controls (HC).
2. Methods
In 13 pwSPMS taking siponimod and 11 HC, testing for SARS-CoV2 antibodies was performed after vaccination against COVID-19. Elecsys® Anti-SARSCoV-2 S assay (Roche Diagnostics Int, Rotkreuz, Switzerland) was used per the manufacturer's instructions, (Anon, 2021) using Cobas e 801 analytical unit for immunoassay tests (F. Hoffmann-La Roche Ltd.). Antibody titer ≥0.8 U/mL was considered positive, as recommended by the manufacturer (The WHO International Standard for COVID-19 serological tests conversion factor (BAU/mL) = 1.288; 1 U/mL equals 1.288 BAU/ml (Infantino et al., 2021)). All pwSPMS treated in our center, who were on treatment with siponimod for at least 6 months and who received both doses of COVID-19 vaccine, were invited to participate during their monthly visits to the clinical—administrative visits due to siponimod reimbursement. HC were aged and sex matched healthcare workers who received both doses of COVID-19 vaccine, without any neurological or immune related condition.
The study was approved by the Ethical Committee of the University Hospital Center, Zagreb (02/21 AG).
Statistical analysis was performed with the IBM SPSS v25 software. The data distribution was tested with the Kolmogorov-Smirnov test. The differences between qualitative variables were tested with the Chi-square test. The differences between the quantitative variables were tested with the parametric independent sample t-test and non-parametric Mann-Whitney test. P-values less than 0.05 were considered significant.
3. Results
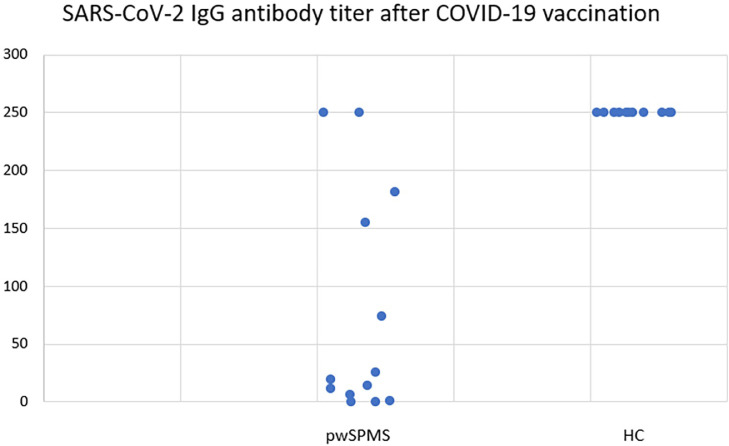
Demographic characteristics, vaccination status, and SARS-CoV2 IgG titer after vaccination are presented in Table 1 . pwSPMS taking siponimod had a significantly lower titer of SARS-CoV2 antibodies compared to healthy controls (19.4 (0–250) vs. 250 (250), p<0.001) (Fig. 1 ). Two (15.4%) pwSPMS on siponimod had unmeasurable titers of SARS-CoV2-2 antibodies, while all HC had positive titers.
Table 1.
Demographic characteristics, vaccination status and SARS-CoV2 IgG titer after vaccination.
| pwSPMS (N = 13) | HC (N = 11) | p value | |
|---|---|---|---|
| Baseline characteristics | |||
| Sex (females) | 5 (38.5%) | 3 (27.3%) | 0.679 |
| Age (years) | 52.8 ± 9.7 | 50.7 ± 9.9 | 0.604 |
| Disease duration (years) | 21.5 ± 9.8 | NA | – |
| EDSS (median, range) | 6.5 (3.0–7.0) | NA | – |
| Treatment duration (months) | 14.5 ± 6 | NA | – |
| Vaccination characteristics | |||
| Type of vaccine Comirnaty® Spikevax® Vaxzevria® |
8 3 2 |
9 0 2 |
0.298 |
| Time between vaccination and blood sampling (days) | 93.5 ± 41.8 | 73.1 ± 42.9 | 0.252 |
| Vaccination response | |||
| SARS-CoV2 IgG titer (U/mL) median (range) | 19.4 (0–250) | 250 (250) | p<0.001 |
Fig. 1.
SARS-CoV-2 IgG antibody titer after COVID-19 vaccination in pwSPMS on siponimod and HC.
4. Discussion
This study has shown blunted humoral response after COVID-19 vaccination in pwSPMS on siponimod.
Siponimod is a sphingosine-1-phosphate (S1P) receptor modulator, which binds with high affinity to both S1P receptors 1 and 5. Its effects on MS are a consequence of reduction of the lymphocyte count, which may lead to compromised immune response function and thus potentially limit the effectiveness of vaccinations. In a randomized, placebo-controlled study, siponimod treatment had no relevant effect on antibody response to Pneumococcal vaccination. However, blunted humoral response was seen after Influenza vaccination (Ufer et al., 2017).
While no data on response to COVID-19 vaccination are available so far for pwSPMS on siponimod, several publications have shown blunted antibody response to COVID-19 vaccinations in fingolimod treated patients (Achiron et al., 2021; Bigaut et al., 2021). In the study by Achiron and colleagues, only 1 out of 26 participants (pwMS treated with fingolimod) had an antibody response that was above the cut-off value for a positive response (Achiron et al., 2021). In another study, pwMS treated with S1P receptor modulator had lower median SARS-CoV2 IgG index, compared to pwMS receiving other or no DMT (Bigaut et al., 2021). Furthermore, one study has shown that Spikevax® vaccine elicits 3.25-higher antibody response compared to Comirnaty® vaccine, suggesting that Spikevax® vaccine may be preferentially considered for patients under fingolimod (Sormani et al., 2021).
Despite all these studies showing blunted humoral response in patients treated with S1P receptor modulators, one should bear in mind that the majority of pwMS on these therapies make an unremarkable recovery from COVID-19, implying that innate and T-cell responses are functional. Based on this, these patients are likely to benefit from protective or at least partially protective T-cell responses to the vaccine (Giovannoni et al., 2021).
Although the results of this study are limited by a small sample size, results have consistently shown low titers of SARS-CoV-2 IgG after COVID-19 vaccinations in pwSPMS on siponimod. These results may have significant implications for management of pwSPMS in the light of the recent EMA update of 3rd dose mRNA COVID-19 vaccines in people with weakened immune systems (Anon, 2021). As a personal opinion of the authors, in all pwSPMS treated with siponimod, 3rd dose of the Comirnaty® or Spikevax® should be recommended.
Financial & competing interest disclosure
MKS: received consultation and/or speaker fees from: Sanofi Genzyme, Roche.
DR: Reports no conflict of interest.
IL: Reports no conflict of interest.
DŠ: Reports no conflict of interest.
MH: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals.
Funding
No funding was received for this study.
CRediT authorship contribution statement
Magdalena Krbot Skorić: Formal analysis, Investigation, Methodology, Supervision. Dunja Rogić: Formal analysis, Methodology, Supervision. Ivana Lapić: Formal analysis, Methodology, Supervision. Dragana Šegulja: Formal analysis, Methodology, Supervision. Mario Habek: Data curation, Formal analysis, Methodology, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
None.
References
- Anon https://www.ema.europa.eu/en/news/comirnaty-spikevax-ema-recommendations-extra-doses-boosters, accessed on Oct 4, 2021.
- Anon https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html, accessed September 30, 2021.
- Anon ema.europa.eu/en/documents/product-information/mayzent-epar-product-information_en.pdf, accessed September 30, 2021.
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., Dolev M., Menascu S., Flechter S., Falb R., Gurevich M. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrambide G., Llaneza-González M.Á., Costa-Frossard França L., Meca-Lallana V., Díaz E.F., Moreno-Torres I., García-Domínguez J.M., Ortega-Suero G., Ayuso-Peralta L., Gómez-Moreno M., Sotoca-Fernández J.J., Caminero-Rodríguez A.B., Rodríguez de Antonio L.A., Corujo-Suárez M., Otano-Martínez M.A., Pérez-Miralles F.C., Reyes-Garrido V., Ayuso-Blanco T., Balseiro-Gómez J.J., Muñoz-Pasadas M., Pérez-Molina I., Arnal-García C., Domingo-Santos Á., Guijarro-Castro C., Íñiguez-Martínez C., Téllez Lara N., Castellanos-Pinedo F., Castillo-Triviño T., Cerdán-Santacruz D.M., Pérez-Sempere Á., Torres B.S., Álvarez de Arcaya A., Costa-Arpín E., Durán-Ferreras E., Fragoso-Martínez M., González-Platas M., Landete Pascual L., Millán-Pascual J., Oreja-Guevara C., Meca-Lallana J.E. SARS-CoV-2 infection in multiple sclerosis: results of the Spanish neurology society registry. Neurol. Neuroimmunol. Neuroinflamm. 2021;8:e1024. doi: 10.1212/NXI.0000000000001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigaut K., Kremer L., Fleury M., Lanotte L., Collongues N., de Seze J. Impact of disease-modifying treatments on humoral response after COVID-19 vaccination: a mirror of the response after SARS-CoV-2 infection. Rev. Neurol. 2021 doi: 10.1016/j.neurol.2021.05.001. S0035-3787(21)00569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree B.A.C., Arnold D.L., Chataway J., Chitnis T., Fox R.J., Pozo Ramajo A., Murphy N., Lassmann H. Secondary progressive multiple sclerosis: new insights. Neurology. 2021;97:378–388. doi: 10.1212/WNL.0000000000012323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G., Hawkes C.H., Lechner-Scott J., Levy M., Yeh E.A., Baker D. COVID-19 vaccines and multiple sclerosis disease-modifying therapies. Mult. Scler. Relat. Disord. 2021;53 doi: 10.1016/j.msard.2021.103155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habek M., Jakob Brecl G., Bašić Kes V., Rogić D., Barun B., Gabelić T., Emeršič A., Horvat Ledinek A., Grbić N., Lapić I., Šegulja D., Đurić K., Adamec I., Krbot Skorić M. Humoral immune response in convalescent COVID-19 people with multiple sclerosis treated with high-efficacy disease-modifying therapies: a multicenter, case-control study. J. Neuroimmunol. 2021;359 doi: 10.1016/j.jneuroim.2021.577696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantino M., Pieri M., Nuccetelli M., Grossi V., Lari B., Tomassetti F., Calugi G., Pancani S., Benucci M., Casprini P., Manfredi M., Bernardini S. The WHO International Standard for COVID-19 serological tests: towards harmonization of anti-spike assays. Int. Immunopharmacol. 2021;100 doi: 10.1016/j.intimp.2021.108095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., Deschamps R., Créange A., Wahab A., Pelletier J., Heinzlef O., Labauge P., Guilloton L., Ahle G., Goudot M., Bigaut K., Laplaud D.A., Vukusic S., Lubetzki C., De Sèze J., Covisep investigators, Derouiche F., Tourbah A., Mathey G., Théaudin M., Sellal F., Dugay M.H., Zéphir H., Vermersch P., Durand-Dubief F., Françoise R., Androdias-Condemine G., Pique J., Codjia P., Tilikete C., Marcaud V., Lebrun-Frenay C., Cohen M., Ungureanu A., Maillart E., Beigneux Y., Roux T., Corvol J.C., Bordet A., Mathieu Y., Le Breton F., Boulos D.D., Gout O., Guéguen A., Moulignier A., Boudot M., Chardain A., Coulette S., Manchon E., Ayache S.S., Moreau T., Garcia P.Y., Kumaran D., Castelnovo G., Thouvenot E., Taithe F., Poupart J., Kwiatkowski A., Defer G., Derache N., Branger P., Biotti D., Ciron J., Clerc C., Vaillant M., Magy L., Montcuquet A., Kerschen P., Coustans M., Guennoc A.M., Brochet B., Ouallet J.C., Ruet A., Dulau C., Wiertlewski S., Berger E., Buch D., Bourre B., Pallix-Guiot M., Maurousset A., Audoin B., Rico A., Maarouf A., Edan G., Papassin J., Videt D. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77:1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., Halper J., Li D.K.B., Kanellis P., Costello K., Bebo B., Rammohan K., Cutter G.R., Cross A.H. Outcomes and risk factors associated with SARS-CoV-2 infection in a north American registry of patients with multiple sclerosis. JAMA Neurol. 2021 doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M., Trojano M., Zaratin P., Tedeschi G., Comi G., Battaglia M.A., Patti F., Salvetti M., Musc-19 Study Group Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89:780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Inglese M., Schiavetti I., Carmisciano L., Laroni A., Lapucci C., Da Rin G., Serrati C., Gandoglia I., Tassinari T., Perego G., Brichetto G., Gazzola P., Mannironi A., Stromillo M.L., Cordioli C., Landi D., Clerico M., Signoriello E., Frau J., Ferrò M.T., Sapio A.D., Pasquali L., Ulivelli M., Marinelli F., Callari G., Iodice R., Liberatore G., Caleri F., Repice A.M., Cordera S., Battaglia M.A., Salvetti M., Franciotta D., Uccelli A. CovaXiMS study group on behalf of the Italian Covid-19 Alliance in MS. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufer M., Shakeri-Nejad K., Gardin A., Su Z., Paule I., Marbury T.C., Legangneux E. Impact of siponimod on vaccination response in a randomized, placebo-controlled study. Neurol. Neuroimmunol. Neuroinflamm. 2017;4:e398. doi: 10.1212/NXI.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]