Abstract
Background
Crohn's disease is a remitting and relapsing disorder that can affect the whole gastrointestinal tract. Active disease symptoms include abdominal pain, fatigue, weight loss, and diarrhoea. There is no known cure; however, the disease can be managed, and therefore places a huge financial burden on healthcare systems. Abdominal pain is a common and debilitating symptom of Crohn's and other inflammatory bowel diseases (IBDs), and is multifaceted. Abdominal pain in Crohn's disease could be a symptom of disease relapse or related to medication adverse effects, surgical complications and strictures or adhesions secondary to IBD. In the absence of these factors, around 20 to 50% of people with Crohn's in remission still experience pain.
Objectives
To assess the efficacy and safety of interventions for managing abdominal pain in people with Crohn's disease and IBD (where data on ulcerative colitis and Crohn's disease could not be separated).
Search methods
We searched CENTRAL, MEDLINE, three other databases, and clinical trials registries on 29 April 2021. We also searched the references of trials and systematic reviews for any additional trials.
Selection criteria
All published, unpublished, and ongoing randomised trials that compared interventions for the management of abdominal pain in the setting of Crohn's disease and IBD, with other active interventions or standard therapy, placebo, or no therapy were included. We excluded studies that did not report on any abdominal pain outcomes.
Data collection and analysis
Five review authors independently conducted data extraction and 'Risk of bias' assessment of the included studies. We analysed data using Review Manager 5. We expressed dichotomous and continuous outcomes as risk ratios and mean differences with 95% confidence intervals. We assessed the certainty of the evidence using GRADE methodology.
Main results
We included 14 studies (743 randomised participants).
Five studies evaluated participants with Crohn's disease; seven studies evaluated participants with IBD where the data on ulcerative colitis and Crohn's disease could not be separated; and two studies provided separate results for Crohn's disease participants. Studies considered a range of disease activity states. Two studies provided intervention success definitions, whilst the remaining studies measured pain as a continuous outcome on a rating scale. All studies except one measured pain intensity, whilst three studies measured pain frequency. Withdrawals due to adverse events were directly or indirectly reported in 10 studies.
No conclusions could be drawn about the efficacy of the majority of the interventions on pain intensity, pain frequency, and treatment success, except for the comparison of transcranial direct current stimulation to sham stimulation. The certainty of the evidence was very low in all but one comparison because of imprecision due to sparse data and risk of bias assessed as unclear or high risk.
Two studies compared a low FODMAP diet (n=37) to a sham diet (n=45) in IBD patients. The evidence on pain intensity was of very low certainty (MD ‐12.00, 95% CI ‐114.55 to 90.55). One study reported pain intensity separately for CD participants in the low FODMAP group [n=14, mean(SD)=24 (82.3)] and the sham group [n=12, mean(SD)=32 (69.3)]. The same study also reported pain frequency for IBD participants in the low FODMAP group [n=27, mean(SD)=36 (26)] and sham group [n=25, mean(SD)=38(25)] and CD participants in the low FODMAP group [n=14, mean(SD)=36 (138.4)] and sham group [n=12, mean(SD)=48 (128.2)]. Treatment success was not reported.
One study compared a low FODMAP diet (n=25) to high FODMAP/normal diet (n=25) in IBD patients. The data reported on pain intensity was unclear. Treatment success and pain frequency were not reported.
One study compared medicine‐separated moxibustion combined with acupuncture (n=51) versus wheat bran‐separated moxibustion combined with shallow acupuncture (n=51) in CD patients. The data reported on pain intensity and frequency were unclear. Treatment success was not reported.
One study compared mindfulness with CBT (n=33) versus no treatment (n=33) in IBD patients. The evidence is very uncertain about the effect of this treatment on pain intensity and frequency (MD ‐37.00, 95% CI ‐87.29 to 13.29). Treatment success was not reported.
One study compared soft non‐manipulative osteopathic treatment (n=16) with no treatment besides doctor advice (n=14) in CD patients. The evidence is very uncertain about the effect of this treatment on pain intensity (MD 0.01, 95% CI ‐1.81 to 1.83). Treatment success and pain frequency were not reported.
One study compared stress management (n=15) to self‐directed stress management(n=15) and to standard treatment (n=15) in CD patients. The evidence is very uncertain about the effect of these treatments on pain intensity (MD ‐30.50, 95% CI ‐58.45 to ‐2.55 and MD ‐34.30, 95% CI ‐61.99 to ‐6.61). Treatment success and pain frequency were not reported.
One study compared enteric‐release glyceryl trinitrate (n=34) with placebo (n=36) in CD patients. The data reported on pain intensity was unclear. Treatment success and pain frequency were not reported.
One study compared 100 mg olorinab three times per day (n=8) with 25 mg olorinab three times per day (n=6) in CD patients. Pain intensity was measured as a 30% reduction in weekly average abdominal pain intensity score for the 100mg group (n=5) and the 25mg group (n=6). The evidence is very uncertain about the effect of this treatment on pain intensity (RR 0.66, 95% CI 0.38 to 1.15). Treatment success and pain frequency were not reported.
One study compared relaxation training (n=28) to a waitlist (n=28) in IBD patients. The evidence is very uncertain about the effect of this treatment on pain intensity (MD ‐0.72, 95% CI ‐1.85 to 0.41). Treatment success and pain frequency were not reported.
One study compared web‐based education (n=30) with a book‐based education (n=30) in IBD patients. The evidence is very uncertain about the effect of this treatment on pain intensity (MD ‐0.13, 95% CI ‐1.25 to 0.99). Treatment success and pain frequency were not reported.
One study compared yoga (n=50) with no treatment (n=50) in IBD patients. The data reported on treatment success were unclear. Pain frequency and intensity were not reported.
One study compared transcranial direct current stimulation (n = 10) to sham stimulation (n = 10) in IBD patients. There may be an improvement in pain intensity when transcranial direct current is compared to sham stimulation (MD ‐1.65, 95% CI ‐3.29 to ‐0.01, low‐certainty evidence). Treatment success and pain frequency were not reported.
One study compared a kefir diet (Lactobacillus bacteria) to no intervention in IBD patients and provided separate data for their CD participants. The evidence is very uncertain about the effect of this treatment on pain intensity in IBD (MD 0.62, 95% CI 0.17 to 1.07) and CD (MD ‐1.10, 95% CI ‐1.67 to ‐0.53). Treatment success and pain frequency were not reported.
Reporting of our secondary outcomes was inconsistent.
The most adverse events were reported in the enteric‐release glyceryl trinitrate and olorinab studies. In the enteric‐release glyceryl trinitrate study, the adverse events were higher in the intervention arm. In the olorinab study, more adverse events were observed in the higher dose arm of the intervention. In the studies on non‐drug interventions, adverse events tended to be very low or zero. However, no clear judgements regarding adverse events can be drawn for any interventions due to the low number of events.
Anxiety and depression were measured and reported at the end of intervention in only one study; therefore, no meaningful conclusions can be drawn for this outcome.
Authors' conclusions
We found low certainty evidence that transcranial direct current stimulation may improve pain intensity compared to sham stimulation. We could not reach any conclusions on the efficacy of any other interventions on pain intensity, pain frequency, and treatment success. The certainty of the evidence was very low due to the low numbers of studies and participants in each comparison and clinical heterogeneity amongst the studies.
While no serious or total adverse events were elicited explicitly with any of the treatments studied, the reported events were very low. The certainty of the evidence for all comparisons was very low, so no conclusions can be drawn.
Plain language summary
Treatments for stomach pain in Crohn's disease
What is the aim of this review?
The aim of this Cochrane Review was to find out whether treatments in people with Crohn's disease can improve stomach pain.
We analysed data from 14 studies to answer this question.
Key messages
Based on low‐quality evidence, electrical brain stimulation may improve stomach pain compared to fake brain stimulation.
It is unclear whether there is any difference between a low FODMAP (a group of sugars found in food) diet and a diet that is not low in FODMAP in improving stomach pain.
It is unclear whether there is any difference between a stress management programme, self‐directed stress management, and standard treatment only, in improving stomach pain.
We were unable to draw any conclusions about the safety of any of the interventions.
It is unclear whether any of the treatments for the other comparisons under study are better or worse than another, as the evidence was limited due to the very low numbers of studies and participants and low quality of the reporting.
Further research that addresses the quality issues we have highlighted is needed.
What was studied in the review?
People with Crohn's disease commonly suffer stomach pain whether their disease is active or inactive.
Several types of therapies have been used to try to reduce pain in Crohn's disease, including diets, psychological therapies, alternative therapies, drugs, and exercise therapies.
There is currently no agreement amongst healthcare providers as to which therapy is better.
What are the main results of the review?
We searched for randomised controlled trials (studies in which participants are assigned to one of two or more treatment groups using a random method) comparing any treatment with any other treatment (such as dummy/placebo treatments) in people with Crohn's disease. We found 14 trials including a total of 743 participants who were aged 16 to 80 years old. We made the following conclusions.
• Electrical brain stimulation may be better than fake brain stimulation in improving pain, based on low‐quality evidence • It is unclear whether a low FODMAP diet or a diet that is not low in FODMAP is better in improving pain. • It is unclear whether a stress management programme, self‐directed stress management, or standard treatment only is better in improving pain. • It is unclear whether there is any difference between any of the other therapies in their effects on the management of pain. • It is unclear whether any therapy leads to a difference in major and minor side effects.
How up‐to‐date is this review?
This review is up‐to‐date as of April 2021.
Summary of findings
Background
Description of the condition
Inflammatory Bowel Disease (IBD) is a term for a group of disorders characterised by inflammation of the gut. The two main types of IBD are Crohn's Disease (CD) and Ulcerative Colitis (UC), which have several subtypes based on onset, disease location and clinical behaviour for CD, and extent for UC. There also exists a categorisation for the much rarer condition indeterminate colitis (Satsangi 2006).
CD is a remitting and relapsing disease of the gastrointestinal tract that affects over 3.5 million young people and adults in the USA and Europe (Kaplan 2015), and more than 6.8 million globally (Alatab 2020). Active CD symptoms include abdominal pain, fatigue, weight loss, and diarrhoea. There is no known cure; however, the disease can be managed via lifelong treatment and care, and therefore places a huge financial burden on healthcare systems. The annual care costs of inflammatory bowel disease (IBD) to the National Health Service (NHS) was estimated at over GBP 1000 million in 2010 (RCP 2012). For CD alone, this amounts to over GBP 6000 per patient annually (Ghosh 2015). Treatment of the disease may involve surgical intervention or immunosuppression using thiopurines and anti‐tumour necrosis factor (anti‐TNF) medications (Gjuladin‐Hellon 2019). These interventions aim to induce remission, maintain remission, and manage symptoms (Greenley 2013).
Abdominal pain is a common and debilitating symptom of CD and other IBDs, which is multifaceted, with multiple causes and contributors, such as a symptom of disease relapse, an adverse effect of medication, surgical complication, or due to problems related to CD itself, such as strictures or adhesions secondary to IBD (Srinath 2012). The pain may also vary between adults and children and may also be influenced by disease activity. Even in the absence of the aforementioned factors, around 20% to 50% of people with CD in remission still experience pain (Bielefeldt 2009). This has been attributed to functional abdominal pain disorders (FAPD) such as irritable bowel syndrome (IBS), abdominal migraine, and functional dyspepsia (Odes 2017), although the definition of such disorders involves explicit exclusion of pathology such as IBD. Evidence is lacking to indicate whether there is a specific variant of functional pain coexisting within people with IBD, or a separate pain disorder that can be attributed to IBD pathologic mechanisms. The aetiology and management of abdominal pain in CD may therefore vary in ways that cannot be fully explained. A common suggestion is that the inflammation in the intestinal wall can lead to the perception of abdominal pain (Docherty 2011).
Description of the intervention
Pharmacological interventions
Medication for CD can reduce inflammation and associated pain by inducing remission. Where pain persists in the absence of inflammation, it has been managed with a variety of agents, including pain‐relieving medication such as antispasmodics, non‐steroidal anti‐inflammatory drugs (NSAIDs), laxatives, antidepressants, antiemetic agents, cyclo‐oxygenase‐2 (COX‐2) inhibitors, and psychoactive drugs such as cannabis and opioids (Srinath 2012). Due to the potential adverse effects of some of these drugs, short‐term use is advised.
Non‐pharmacological interventions
Non‐pharmacological interventions used in managing pain may include dietary, psychological, lifestyle advice, and alternative medicine. These interventions are considered by some as less invasive and may be used as adjuvant treatment. Cognitive behavioural therapy (CBT), stress management, and coping skills training are the most commonly used psychological interventions. These therapies can be very heterogeneous, therefore it is key to consider the specific evidence and conceptual alignment of the approach delivered to understand 'what' the therapy was, as well as 'whether' it was effective. Alternative treatments such as acupuncture and transcutaneous electrical nerve stimulation, which have been used in other conditions such as IBS, are increasingly used in people with IBD, albeit based on limited evidence (Srinath 2012). Dietary interventions studied include the avoidance of FODMAP (fermentable oligo‐, di‐, monosaccharides and polyols) and the use of supplements with prebiotic properties; however, the evidence on their effectiveness appears to be weak and conflicting (Norton 2017).
Whilst some interventions such as neurochemicals and acupuncture have mostly been used in conditions such as IBS, others such as COX‐2 inhibitors have been associated with little to no effect in IBD (Paiotti 2012).
How the intervention might work
The mechanism of action of different interventions depends on the nature or cause of the abdominal pain.
Antispasmodics suppress intestinal spasms which cause pain from inflammation or obstruction (Srinath 2012). Pain related to strictures can be improved by following a low‐residue diet, which can pass through with ease, thereby preventing intestinal pain (Srinath 2012). A low FODMAP diet also aims to limit the intake of non‐absorbable nutrients, and has recently gained considerable attention in the management of IBS (Prince 2016).
Psychological techniques such as CBT, mindfulness, and stress management tend to help people with CD change negative behaviours that might be worsening their pain and provide coping mechanisms (Norton 2017). Yoga‐based programmes are thought to work by improving depression and anxiety (Ewais 2019). Other complementary or alternative therapies, such as the use of herbal and dietary supplements, traditional Chinese practices, and mind‐body techniques, have been proposed to have anti‐inflammatory, stress‐reducing, or other therapeutic modes of action (Lin 2018).
Why it is important to do this review
Abdominal pain in people with CD can lead to depressive symptoms, reduced quality of life, and an increase in the use of healthcare facilities (Srinath 2012). Effective pain management is therefore vital.
There are concerns regarding the safety of pharmacological interventions, such as the relative inefficacy of currently available analgesics and their potential toxicity. Opioids can offer short‐term relief; however, they are associated with such problems as narcotic bowel syndrome and other symptoms like constipation (Thapa 2019). Furthermore, there may be concern amongst people with IBD about the stigma of addiction associated with the use of opioids. The use of opioids in chronic pain can lead to people exhibiting withdrawal symptoms that are similar to CD symptoms (Pauly 2017), which can further complicate treatment. There are also concerns about the use of NSAIDs, which can have effects that mimic or potentially exacerbate CD activity (Long 2016).
Pain management has been highlighted as a priority topic for research by IBD patient groups and charities, but is currently not covered in the National Institute for Health and Care Excellence guidelines (NICE 2019), European Crohn's and Colitis Organisation guidelines (ECCO 2010), or Crohn's and Colitis Foundation guidance. Whilst several non‐Cochrane systematic reviews have assessed interventions for pain management in IBD, none has currently assessed the efficacy and safety of these interventions in Crohn's disease. Although this review covers interventions that have been previously assessed in published Cochrane Reviews in the group portfolio (Iheozor‐Ejiofor 2019; Kafil 2018; Limketkai 2019; Timmer 2011), the focus of this review was only on studies that have been conducted for the purpose of providing relief for abdominal pain.
Objectives
To assess the efficacy and safety of interventions for managing abdominal pain in people with Crohn's disease and IBD (where data on ulcerative colitis and Crohn's disease could not be separated).
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished, and ongoing randomised controlled trials (RCTs) that compared interventions for the management of abdominal pain in the setting of CD and IBD, with other active interventions or standard therapy, placebo, or no therapy. We excluded studies that did not report on any abdominal pain outcomes.
Types of participants
Adults and children with Crohn's disease or IBD who are experiencing abdominal pain. If studies included participants with ulcerative colitis as well as those with CD, these studies were included, and separate data sought for analysis. Where separate data could not be obtained, IBD patients were included as a whole.
Types of interventions
Pain‐relieving drugs such as antispasmodics, antidepressants, laxatives, antidiarrhoeal agents, antibiotics, analgesics, antireflux agents, antiemetic agents, antimigraine agents, antihistaminic agents, serotonergic agents, and psychoactive drugs.
Behaviour therapy, e.g. cognitive behavioural therapy (CBT), hypnotherapy.
Lifestyle advice, e.g. advice on physical activity including exercise.
Dietary interventions such as reduced intake of FODMAP; additional fibre intake; decrease in gas‐producing foods; extra fluid intake; lactulose‐, gluten‐, and histamine‐free diet.
Pre‐ and probiotics.
Other alternative therapies, e.g. acupuncture, homeopathy, body‐oriented therapy, musculoskeletal therapy (osteopathy/chiropractic), yoga.
Types of outcome measures
Both dichotomous and continuous outcomes were valid for inclusion.
Primary outcomes
Treatment success as defined by the authors.
Abdominal pain frequency or change in frequency of pain using any validated scale.
Abdominal pain intensity or change in pain intensity using any validated scale.
Withdrawal due to adverse events.
Secondary outcomes
Anxiety/depression using any validated scale.
Adverse events (total number of participants with any event).
Serious adverse events (as defined by the authors within the primary study)
Search methods for identification of studies
Electronic searches
We searched the following sources from the inception of each database to the date of search on 29 April 2021:
the Cochrane Central Register of Controlled Trials (CENTRAL) (via Ovid EBMR) (inception to Issue 03, 2021);
MEDLINE (via Ovid) (1946 to 29 April 2021);
PsycINFO (via Ovid) (1987 to 29 April 2021);
AMED (via Ovid) (Allied and Complementary Medicine) (1985 to 29 April 2021);
CINAHL (via EBSCO) (Cumulative Index to Nursing and Allied Health Literature) (1984 to 29 April 2021).
We also searched the following trial registers on 29 April 2021:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch/).
We placed no restrictions on language of publication. For detailed search strategies, see Appendix 1.
Searching other resources
As complementary search methods, we carefully checked relevant systematic reviews for potentially eligible studies. We also scrutinised the references of included studies. We sought unpublished trials by contacting experts in the field, and scanned the Internet and abstracts submitted to major international congresses from the three years prior to the search to capture any studies presented but not yet published in full.
In the case of foreign language papers, we planned to obtain translations of papers if necessary.
Data collection and analysis
We carried out data collection and analysis according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Selection of studies
Five review authors independently screened the titles and abstracts identified by the literature search, excluding studies that based on title and abstract did not meet our inclusion criteria. We obtained the full reports of studies deemed potentially eligible. Five review authors independently assessed the full texts for inclusion in the review. Any disagreements were resolved by discussion or by consulting another review author if necessary. We recorded the studies excluded at this or subsequent stages, and the main reason for their exclusion, in the 'Characteristics of excluded studies' tables.
Where there were multiple publications for a given study, we collated the reports of the same study so that each study, rather than each report, was the unit of interest in the review; such studies have a single identifier with multiple references.
Studies that had the primary goal of inducing or maintaining remission in IBD were excluded, regardless as to whether they reported pain outcomes. This reflected the fact that any such pain imporvement was as a result of disease state and not an indepedent pain intervention and all such interventions are considered in seperate reviews. Such studies were excluded at the title and abstract screening stage. Studies that did not report any abdominal pain related outcomes, were excluded at the full text screening stage, and only after contact with the authors whenever the reporting of pain related outcomes was unclear.
Data extraction and management
Five review authors independently performed data extraction using piloted data extraction forms. We extracted the following data from the included studies:
trial setting: country and number of trial centres;
methods: study design, total study duration and date;
participant characteristics: age, sociodemographics, ethnicity, diagnostic criteria, pain location, and total number of participants;
eligibility criteria: inclusion and exclusion criteria;
intervention and comparator;
outcomes: outcome definition, unit of measurement, and time of collection;
results: number of participants allocated to each group, missing participants, and sample size;
funding source.
All treatment arms are described in the 'Characteristics of included studies' tables.
Assessment of risk of bias in included studies
Five review authors independently assessed risk of bias in the included studies based on the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We assessed the following 'Risk of bias' domains:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
other bias such as imbalance in participants' baseline characteristics.
We judged the studies to be at low, high, or unclear risk of bias for each domain assessed, based on the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
After data extraction, the five review authors compared the extracted data, discussing and resolving any discrepancies before transfer of data into the 'Characteristics of included studies' tables.
Measures of treatment effect
We expressed treatment effect as risk ratios (RR) with corresponding 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) with 95% CI for continuous outcomes. Where endpoint and change score were both reported, we used endpoint scores for data analysis. However, if the studies assessed the same continuous outcome in different ways, we estimated the treatment effect using the standardised mean difference (SMD) (Cohen 1988).
Unit of analysis issues
The unit of analysis was the participant. For studies comparing more than two intervention groups, we planned to make multiple pair‐wise comparisons between all possible pairs of intervention groups. To avoid double counting, we would divide shared intervention groups evenly amongst the comparisons. For dichotomous outcomes, we planned to divide both the number of events and the total number of participants. For continuous outcomes, we would only divide the total number of participants, and leave the means and standard deviations (SDs) unchanged. We planned to include cross‐over studies for quantitative analysis only if data were separately reported before and after cross‐over, and use only pre‐cross‐over data. We did not anticipate finding any cluster‐RCTs; we would only use study data from such trials if the authors employed appropriate statistical methods in taking the clustering effect into account. We would also exclude cluster‐RCTs in a sensitivity analysis to assess their impact on the results.
Dealing with missing data
We contacted study authors in the case of missing data or studies that did not report data in sufficient detail. We attempted to estimate missing SDs using relevant statistical tools and calculators available in Review Manager 5 if studies reported standard errors (Review Manager 2020). Studies that failed to report measures of variance were judged as at high risk of reporting bias.
Assessment of heterogeneity
We assessed the included studies to determine their homogeneity in terms of participants, intervention, comparator, and outcome. To test for statistical heterogeneity, we employed a Chi² test using a P value of less than 0.1 to give an indication of the presence of heterogeneity. Inconsistency was quantified and represented by the I² statistic. We interpreted the thresholds as follows (Higgins 2021):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%; may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
Most reporting biases were minimised by using an inclusive search strategy. We planned to investigate publication bias using a funnel plot if there were 10 or more studies. The magnitude of publication bias would be determined by visual inspection of the asymmetry of the funnel plot. In addition, we would test funnel plot asymmetry by performing a linear regression of intervention effect estimate against its standard error, weighted by the inverse of the variance of the intervention effect estimate (Egger 1997).
Data synthesis
To summarise the study characteristics, we conducted a narrative synthesis of all the included studies. We then carried out a meta‐analysis if two or more studies assessed similar populations, interventions, and outcomes. We planned to analyse studies of children, adults, and different sub‐intervention types separately. We used Review Manager 5 (Review Manager 2020). We synthesised study data using the random‐effects model if there was statistical heterogeneity (I² > 0%); otherwise, we used the fixed‐effect model. We combined effect estimates of studies that reported data in a similar way in the meta‐analysis. We pooled RRs for dichotomous outcomes, and MDs or SMDs for continuous outcomes, alongside 95% CIs. Where we were unable to carry out a meta‐analysis (e.g. due to lack of uniformity in data reporting), we presented a narrative summary of the included studies.
Subgroup analysis and investigation of heterogeneity
If we identified heterogeneity, we investigated possible causes and addressed them using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We planned to undertake subgroup analyses of potential effect modifiers if sufficient data were available. We identified several potential modifiers of effect:
disease activity (active versus inactive disease);
pain location;
disease location.
Sensitivity analysis
We planned to undertake a sensitivity analysis on the primary outcome of treatment success in order to assess whether the findings of the review were robust to decisions made during the review process. In particular, we planned to exclude studies at high or unclear risk of bias from analyses. Where data analyses included studies with reported and estimated SDs, we excluded studies with estimated SDs to assess whether this affected the findings of the review. We investigated whether the choice of model (fixed‐effect versus random‐effects) affected the results.
Summary of findings and assessment of the certainty of the evidence
We have presented our primary outcomes results in 'Summary of findings' tables. Each comparison and primary outcome was exported to GRADEpro GDT software for quality assessment (GRADEpro GDT). Based on risk of bias, inconsistency, imprecision, indirectness, and publication bias, we graded the quality of the evidence for each outcome as high, moderate, low, or very low. These ratings have been defined as follows:
high: further research is very unlikely to change our confidence in the estimate of effect;
moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate;
low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate;
very low: any estimate of effect is very uncertain.
We justified all decisions to downgrade the quality of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Information on the results of the search, included and excluded studies, and 'Risk of bias' assessment is provided below.
Results of the search
We completed our literature search on 29 April 2021 (Appendix 1), identifying a total of 3654 records through database searching and 13 additional records from alternative sources. After removal of duplicates, 3282 unique records remained. Title and abstract screening revealed 63 records for full‐text review. After assessing all 63 records, we identified 22 records of 14 studies that met the inclusion criteria and were included in the review. We also identified 9 records of 9 ongoing studies and 24 records of 19 studies awaiting classification. We excluded 8 records of 8 studies for various reasons (see Characteristics of excluded studies). The results of the search are presented in a PRISMA flow diagram (Figure 1). There are two fully published RCTs (Bao 2021; Lee 2021) which are under awaiting classification and not included in our results and meta‐analyses, as they were identified during our updated search. They will be included in future updated of this review.
1.
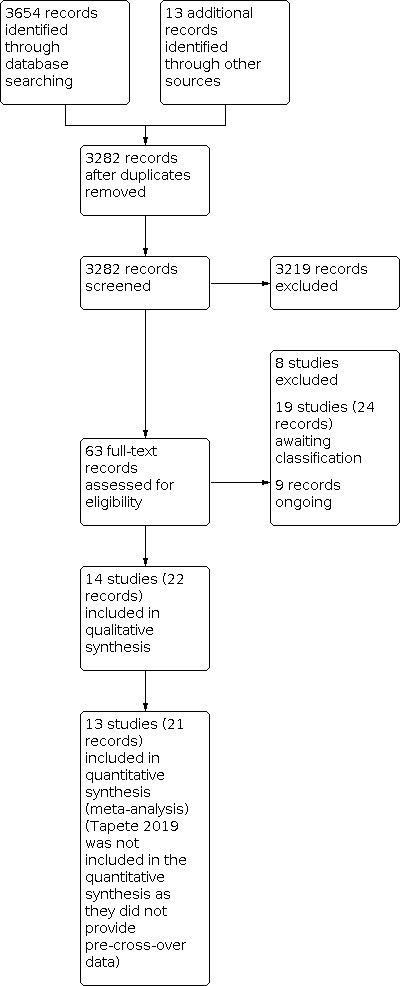
Study flow diagram.
Included studies
Setting
Fourteen RCTs involving a total of 743 participants met our inclusion criteria. One study was conducted in China (Bao 2016), three in the UK (Berrill 2014; Cox 2020; Hawkes 2001), two in Spain (Espi Lopez 2018; Garcia‐Vega 2004), one in the USA (Higgins 2019), two in Turkey (Ozgursoy Uran 2019; Yilmaz 2019), one in India (Sharma 2015), one in Israel (Mizrahi 2012), two in Italy (Tapete 2018; Tapete 2019), and one in Germany (Volz 2016). All of the included studies were conducted in hospitals, medical centres, and gastroenterology units, except Sharma 2015, which was conducted in an institute of medical science, and three studies for which no information was provided about setting (Tapete 2018; Tapete 2019; Yilmaz 2019). Seven studies were single‐centre (Espi Lopez 2018; Garcia‐Vega 2004; Mizrahi 2012; Ozgursoy Uran 2019; Sharma 2015; Volz 2016; Yilmaz 2019); five were multicentre (Bao 2016; Berrill 2014; Cox 2020; Hawkes 2001; Higgins 2019); and two studies did not provide this information (Tapete 2018; Tapete 2019).
Participants
All studies reported age in mean (SD), except for two studies that did not report it at all (Tapete 2018; Tapete 2019); one study that reported mean and range of ages (Yilmaz 2019); and one study that only mentioned their accepted age range for participants (Sharma 2015). The average age of participants ranged from 31.7, in Garcia‐Vega 2004, to 44.9, in Berrill 2014. The lowest accepted age was 16 years (Hawkes 2001; Sharma 2015), and the highest 80 years (Higgins 2019; Volz 2016); 4 studies did not have an upper age limit (Cox 2020; Hawkes 2001; Mizrahi 2012; Ozgursoy Uran 2019). Four studies did not mention age in their inclusion/exclusion criteria (Bao 2016; Garcia‐Vega 2004; Tapete 2018; Tapete 2019).
Five studies examined exclusively CD populations (Bao 2016; Espi Lopez 2018; Garcia‐Vega 2004; Hawkes 2001; Higgins 2019), whilst the remaining studies examined a mix of IBD patients (Berrill 2014; Cox 2020; Mizrahi 2012; Ozgursoy Uran 2019; Sharma 2015; Tapete 2018; Tapete 2019; Volz 2016; Yilmaz 2019). Cox 2020 reported separate CD results. We contacted the authors of the other studies providing mixed IBD results to ask for separate outcome results for their CD participants, and one was able to provide this information (Yilmaz 2019).
Four studies examined participants in an active stage of the disease (Bao 2016; Hawkes 2001; Mizrahi 2012; Volz 2016); six studies participants in an inactive stage of the disease (Berrill 2014; Cox 2020; Garcia‐Vega 2004; Sharma 2015; Tapete 2018; Tapete 2019); one study participants in an inactive or mild stage of the disease (Higgins 2019); one study participants in inactive to moderate stages of the disease (Yilmaz 2019); and one study participants with a mix of inactive and active disease (Ozgursoy Uran 2019). One study did not report on activity of the disease (Espi Lopez 2018).
Nine studies reported disease duration (Bao 2016; Cox 2020; Garcia‐Vega 2004; Hawkes 2001; Higgins 2019; Ozgursoy Uran 2019; Volz 2016; Yilmaz 2019). All of these studies presented disease duration in mean (SD) except Cox 2020, which only provided the mean; Ozgursoy Uran 2019, which reported disease duration in incremental ranges in months; and Yilmaz 2019, which provided the mean and range. Average disease duration ranged from 3 years, in Yilmaz 2019, to 12 years, in Higgins 2019.
Interventions
The following interventions were assessed in the included trials.
Low FODMAP (fermentable oligo‐, di‐, monosaccharides and polyols) diet versus sham diet (Cox 2020; Tapete 2018).
Low FODMAP diet versus high FODMAP/normal diet (Tapete 2019).
Medicine‐separated moxibustion combined with acupuncture versus wheat bran‐separated moxibustion combined with shallow acupuncture (Bao 2016).
Mindfulness with cognitive behavioural therapy (CBT) versus no treatment (both groups received standard medical therapy) (Berrill 2014).
Soft non‐manipulative osteopathic versus no treatment besides doctor advice (Espi Lopez 2018).
Stress management versus self‐directed stress management (it is unclear whether these interventions replaced standard treatment or were added to standard treatment) versus standard treatment (Garcia‐Vega 2004).
Enteric‐release glyceryl trinitrate versus placebo (Hawkes 2001).
100 mg olorinab three times per day versus 25 mg olorinab three times per day (Higgins 2019).
Relaxation training versus waitlist (Mizrahi 2012).
Web‐based education versus standard book‐based education (Ozgursoy Uran 2019).
Yoga intervention versus no treatment (both groups received standard medical therapy) (Sharma 2015).
Transcranial direct current stimulation versus sham stimulation (Volz 2016).
Kefir diet (Lactobacillus bacteria) versus no intervention (Yilmaz 2019).
Outcomes
The length of the interventions ranged from 5 days, in Volz 2016, to 12 months, in Berrill 2014.
Primary outcomes
Treatment success as defined by the authors
Only two studies clearly defined their criteria for treatment success: Sharma 2015, which measured pain as a dichotomous outcome (presence or absence of pain), and Higgins 2019, which defined success as achieving ≥ 30% reduction in weekly average abdominal pain from baseline to week 8. Treatment success was not explicitly mentioned in the remaining studies. Study authors reported pain as a continuous outcome and did not report numbers of responders for their interventions per any definition.
Abdominal pain frequency or change in frequency of pain
Three studies measured pain frequency. Bao 2016 evaluated pain frequency using Traditional Chinese Medicine (TCM) symptom scores on an adapted pain intensity scale of 0 to 3: 0 (none), 1 (light), 2 points (moderate), 3 points (severe). The wording of the adapted scale for pain frequency was not mentioned. Cox 2020 measured pain frequency in days using the Irritable Bowel Syndrome Severity Scoring System (IBS‐SSS) 0‐to‐100 scoring scale, and in days where pain was reported as moderate or severe using the Gastrointestinal Symptom Rating Scale (GSRS). Berrill 2014 used the total IBS‐SSS scores, which measure frequency and severity of abdominal discomfort, severity of abdominal bloating, satisfaction with bowel habit, and impact of symptoms on life in general. Each domain is scored 0 to 100, and an overall score of 0 to 500 is obtained. A higher score is indicative of more severe symptoms.
Abdominal pain intensity or change in pain intensity using any validated scale
All studies except Sharma 2015 measured pain intensity. Five studies used a 0‐to‐100‐millimetre visual analogue scale (VAS), where 0 indicates no pain and 100 (or 10 if measured in centimetres) the worst pain possible (Espi Lopez 2018; Mizrahi 2012; Tapete 2018; Tapete 2019; Volz 2016). One study, Ozgursoy Uran 2019, used the 0‐to‐100‐millimetre VAS to separate participants into groups that experienced no pain, pain from 1 to 5, and pain from 6 to 10. Four studies used scoring scales between 0 and 3. Bao 2016 evaluated pain intensity using TCM symptom scores on a scale of 0 to 3, where 0 = none, 1 = light, 2 = moderate, 3 = severe; Hawkes 2001 used scores that represented the sum of a 7‐day diary card score on a scale of 0 = no pain to 3 = severe pain; Yilmaz 2019 used a symptoms diary where participants rated their pain on a scale of 0 to 3, where 0 = none, 1 = mild, 2 = moderate, and 3 = severe; and Cox 2020 used the IBS‐SSS 0‐to‐100 scale and the GSRS, which measures severity of pain on a 0‐to‐3 scale. Garcia‐Vega 2004 used a symptom diary where participants rated the severity of their daily pain on a scale of 1 to 3 (1 = mild, 2 = moderate, 3 = severe) and devised their own formulas based on diary card scores to calculate their results. Berrill 2014 used the total IBS‐SSS scores, which measure frequency and severity of abdominal discomfort, severity of abdominal bloating, satisfaction with bowel habit, and impact of symptoms on life in general. Each domain is scored 0 to 100, and an overall score of 0 to 500 is obtained. A higher score is indicative of more severe symptoms. Higgins 2019 used a weekly Average Abdominal Pain Score (AAPS), which was the daily pain scores averaged over one week that were larger than 4 on a scale of 0 (no pain) to 10 (worst ever).
Withdrawal due to adverse events
This was reported or could be extracted based on the text in 10 studies (Bao 2016; Berrill 2014; Cox 2020; Espi Lopez 2018; Hawkes 2001; Higgins 2019; Ozgursoy Uran 2019; Sharma 2015; Volz 2016; Yilmaz 2019).
Secondary outcomes
Anxiety/depression
Five studies mentioned having measured anxiety or depression, or both (Berrill 2014; Espi Lopez 2018; Mizrahi 2012; Sharma 2015; Volz 2016). Two of these studies used the Hospital Anxiety and Depression Scale (HADS) (Berrill 2014; Espi Lopez 2018); however, Berrill 2014 only reported baseline scores, and Espi Lopez 2018 did not report any scores. Mizrahi 2012 used Spielberger’s State‐Trait Anxiety Inventory to measure anxiety and the 0‐to‐10‐centimetre VAS to measure depression; Sharma 2015 also used Spielberger’s State‐Trait Anxiety Inventory to measure anxiety. Volz 2016 used the Beck Depression Inventory to measure depression, but only reported baseline scores.
Adverse events (total number of participants with any event)
Six studies reported the total number of participants with adverse events (Bao 2016; Cox 2020; Espi Lopez 2018; Hawkes 2001; Higgins 2019; Sharma 2015). Volz 2016 reported total occurrences of adverse events, but not the number of participants with adverse events.
Serious adverse events (as defined by the authors within the primary study )
The same six studies that reported numbers of participants with adverse events also reported numbers of participants with serious adverse events (Bao 2016; Cox 2020; Espi Lopez 2018; Hawkes 2001; Higgins 2019; Sharma 2015).
Funding sources and conflicts of interest
Eight studies reported their sources of funding (Berrill 2014; Cox 2020; Hawkes 2001; Higgins 2019; Ozgursoy Uran 2019; Sharma 2015; Volz 2016; Yilmaz 2019). Three studies were funded via government grants (Berrill 2014; Sharma 2015; Volz 2016), two by private foundations (Cox 2020; Hawkes 2001), one by an industrial partner (Higgins 2019), and two studies reported having received no funding (Ozgursoy Uran 2019; Yilmaz 2019).
Eight studies made declarations on conflicts of interest (Berrill 2014; Cox 2020; Espi Lopez 2018; Higgins 2019; Ozgursoy Uran 2019; Sharma 2015; Volz 2016; Yilmaz 2019). Six studies declared no conflicts of interest (Berrill 2014; Espi Lopez 2018; Ozgursoy Uran 2019; Sharma 2015; Volz 2016; Yilmaz 2019); one study declared industry connections and ownership of an invention connected to their intervention (Cox 2020); and one study declared that one of the authors is an employee of the industrial partner that provided funding (Higgins 2019).
Excluded studies
We excluded eight studies for various reasons. The reasons for exclusion of each study are presented in the Characteristics of excluded studies table and are summarised below.
Wrong outcomes (3 studies) (ACTRN12617000876392; Engel 2016; Tripp 2017).
Wrong interventions (2 studies) (Forbes 2019; Gearry 2009).
Not RCTs (2 studies) (McCormick 2010; Spagnuolo 2017).
Wrong indication (1 study) (ISRCTN98226923).
Risk of bias in included studies
The results of our ‘Risk of bias’ assessment are presented below (Figure 2; Figure 3). Further details can be found in the ‘Risk of bias’ tables in the Characteristics of included studies tables.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation was described clearly in nine studies, which we rated as at low risk of bias (Bao 2016; Berrill 2014; Cox 2020; Espi Lopez 2018; Hawkes 2001; Ozgursoy Uran 2019; Sharma 2015; Volz 2016; Yilmaz 2019), and insufficiently described in five studies, which were assessed as at unclear risk of bias (Garcia‐Vega 2004; Higgins 2019; Mizrahi 2012; Tapete 2018; Tapete 2019).
Four studies were assessed as at low risk of allocation concealment bias (Berrill 2014; Cox 2020; Espi Lopez 2018; Sharma 2015), as allocation concealment was judged to be adequately described. We rated the other 10 studies as at unclear risk of bias for allocation concealment (Bao 2016; Garcia‐Vega 2004; Hawkes 2001; Higgins 2019; Mizrahi 2012; Ozgursoy Uran 2019; Tapete 2018; Tapete 2019; Volz 2016; Yilmaz 2019), as they provided insufficient or no information.
Blinding
We rated two studies as having a low risk of performance bias since they used as control a placebo that was identical to the intervention product, Hawkes 2001, or a sham procedure that could not be differentiated from the intervention, Volz 2016. We rated Bao 2016 as at unclear risk of performance bias as there was no information about blinding, and we received no response to our enquiries to the author. We assessed the other 11 studies as at high risk of performance bias, as neither participants nor study personnel were blinded to the interventions, or the studies were open‐label (Berrill 2014; Cox 2020; Espi Lopez 2018; Garcia‐Vega 2004; Higgins 2019; Mizrahi 2012; Ozgursoy Uran 2019; Sharma 2015; Tapete 2018; Tapete 2019; Yilmaz 2019); however, Cox 2020 used a sham diet to blind their participants to the intervention, which is not typical in diet RCTs due to the difficulty it entails.
Five studies provided sufficient information about blinding of outcome assessment and were assessed as at low risk of detection bias (Espi Lopez 2018; Garcia‐Vega 2004; Ozgursoy Uran 2019; Volz 2016; Yilmaz 2019). The remaining nine studies provided insufficient information for judgement and were judged to be at unclear risk of detection bias (Bao 2016; Berrill 2014; Cox 2020; Hawkes 2001; Higgins 2019; Mizrahi 2012; Sharma 2015; Tapete 2018; Tapete 2019).
Incomplete outcome data
Ten studies provided sufficient information for judgement and were assessed as at low risk of attrition bias (Bao 2016; Cox 2020; Espi Lopez 2018; Hawkes 2001; Higgins 2019; Mizrahi 2012; Ozgursoy Uran 2019; Sharma 2015; Tapete 2018; Tapete 2019). We assessed three studies as at unclear risk of bias for this domain (Garcia‐Vega 2004; Tapete 2018; Tapete 2019), and one study as at high risk of attrition bias (Berrill 2014).
Selective reporting
Nine studies reported all outcomes they set out to assess and were judged to be at low risk of reporting bias (Bao 2016; Berrill 2014; Cox 2020; Hawkes 2001; Mizrahi 2012; Ozgursoy Uran 2019; Tapete 2018; Volz 2016; Yilmaz 2019). Four studies provided insufficient information in the reports or protocols to permit a judgement as to whether all outcomes had been reported; these studies were assessed as at unclear risk of reporting bias (Garcia‐Vega 2004; Higgins 2019; Sharma 2015; Tapete 2019). We rated the remaining study as at high risk of bias for this domain (Espi Lopez 2018).
Other potential sources of bias
We rated eight studies as at low risk of other potential sources of bias (Berrill 2014; Cox 2020; Espi Lopez 2018; Ozgursoy Uran 2019; Sharma 2015; Tapete 2018; Volz 2016; Yilmaz 2019). We assessed two studies as at unclear risk of bias for this domain, as there were imbalances in the baseline characteristics of participants, and it is unclear how this may have influenced the results (Hawkes 2001; Tapete 2019). We rated four studies as having a high risk of other bias for the following reasons: significant differences in the baseline characteristics of participants that were highly likely to have affected the results (Garcia‐Vega 2004; Mizrahi 2012); the study declared that one or more authors were directly employed by the pharmaceutical companies funding the study (Higgins 2019); bias was detected in the way the efficacy of the studied intervention was presented in their introduction (Bao 2016).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13
Summary of findings 1. Low FODMAP diet compared to sham diet for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.
| Low FODMAP diet compared to sham diet for the management of abdominal pain in Crohn's disease and inflammatory bowel disease | |||||
| Patient or population: people with inflammatory bowel disease Setting: multicentre, 2 gastroenterology clinics in the UK and an unstated setting in Italy Intervention: low FODMAP diet Comparison: sham diet | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with sham diet | Risk with low FODMAP diet | ||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | Note measured |
| Pain frequency IBD (measured in days of pain on the IBS‐SSS questionnaire) | ‐ | MD 2 lower (15.86 lower to 11.86 higher) | ‐ | 52 (1 study) | ⊕⊝⊝⊝ very low a b |
| Pain frequency IBD (measured in days with moderate or severe pain on the GSRS questionnaire) | ‐ | MD 0.4 higher (0.44 lower to 1.24 higher) | ‐ | 52 (1 study) | ⊕⊝⊝⊝ very low a b |
| Pain frequency CD (measured in days of pain on the IBS‐SSS questionnaire) | ‐ | MD 12 lower (114.55 lower to 90.55 higher) | ‐ | 26 (1 study) | ⊕⊝⊝⊝ very low a b |
| Pain intensity IBD (0‐10cm visual analogue scale) | ‐ | MD 8.46 lower (15.76 lower to 1.16 lower) | ‐ | 82 (2 studies) | ⊕⊝⊝⊝ very lowa b |
| Pain intensity IBD (0‐3 point GSRS scale) | ‐ | MD 8 lower (66.27 lower to 50.27 higher) | ‐ | 26 (1 study) | ⊕⊝⊝⊝ very low a b |
| Pain intensity CD (0‐10cm visual analogue scale) | ‐ | MD 0.2 higher (8.67 lower to 9.07 higher) | ‐ | 52 (1 study) | ⊕⊝⊝⊝ very low a b |
| Withdrawal due to adverse events | 4 per 1000 | 7.4 per 1000 (1 to 77) |
RR 1.85 (0.18 to 19.19) | 52 (1 study) | ⊕⊝⊝⊝ very lowa b |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CD: Crohn's disease; CI: confidence interval; FODMAP: fermentable oligo‐, di‐, monosaccharides and polyols; GSRS: Gastrointestinal Symptom Rating Scale; IBD: inflammatory bowel disease; IBS‐SSS: Irritable Bowel Syndrome Severity Scoring System; MD: mean difference; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to risk of bias. bDowngraded two levels due to imprecision from very sparse data.
Summary of findings 2. Medicine‐separated moxibustion combined with acupuncture compared with wheat bran‐separated moxibustion combined with shallow acupuncture for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.
| Medicine‐separated moxibustion combined with acupuncture compared with wheat bran‐separated moxibustion combined with shallow acupuncture for the management of abdominal pain in Crohn's disease and inflammatory bowel disease | |||||
|
Patient or population: people with Crohn's disease Settings: Shanghai Acupuncture Meridian Institute Medical Clinic Acupuncture Inflammatory Bowel Disease Specialist Clinic, Zhongshan Hospital Affiliated Endoscopic Center, and Yueyang Hospital, Shanghai University of Traditional Chinese Medicine Intervention: medicine‐separated moxibustion combined with acupuncture Comparison: wheat bran‐separated moxibustion combined with shallow acupuncture | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with wheat bran‐separated moxibustion combined with shallow acupuncture | Risk with medicine‐separated moxibustion combined with acupuncture | ||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | Not measured |
| Abdominal pain frequency or change in frequency | ‐ | ‐ | ‐ | ‐ | Not measured |
| Abdominal pain intensity or change in intensity | ‐ | ‐ | ‐ | ‐ | Not measured |
| Withdrawals due to adverse events | 0 per 1000 | 0 per 1000 (0 to 0) |
Not estimable | 102 (1 study) |
⊕⊝⊝⊝ very low a b |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels due to imprecision from very sparse data. bDowngraded one level due to risk of bias.
Summary of findings 3. Mindfulness with cognitive behavioural therapy (CBT) compared with no treatment (both groups received standard medical therapy) for the management of abdominal pain in Crohn's Disease and inflammatory bowel disease.
| Mindfulness with cognitive behavioural therapy (CBT) versus no treatment (both groups received standard medical therapy) for the management of abdominal pain in Crohn's Disease and inflammatory bowel disease | |||||
| Patient or population: people with inflammatory bowel disease Setting: multicentre, hospitals in the UK Intervention: mindfulness with CBT Comparison: no treatment | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with standard medical therapy | Risk with mindfulness with CBT + standard medical therapy | ||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain frequency and intensity (measured as part of the 0‐500 point IBS‐SSS questionnaire) | ‐ | MD 37 lower (87.29 lower to 13.29 higher) | ‐ | 66 (1 study) | ⊕⊝⊝⊝ very low a b |
| Withdrawal due to adverse events | 0 per 1000 | 0 per 1000 (0 to 0) |
Not estimable | 66 (1 study) | ⊕⊝⊝⊝ very low a b |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CBT: cognitive behavioural therapy; CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels due to imprecision from very sparse data. bDowngraded one level due to risk of bias.
Summary of findings 4. soft non‐manipulative osteopathic treatment compared to no intervention for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.
| Soft non‐manipulative osteopathic treatment compared to no intervention for the management of abdominal pain in Crohn's disease and inflammatory bowel disease | |||||
| Patient or population: people with Crohn's disease Setting: single centre, hospital in Spain Intervention: soft non‐manipulative osteopathic Comparison: no intervention | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with no intervention | Risk with soft non‐manipulative osteopathic | ||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | Not measured |
| Abdominal pain frequency or change in frequency | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain intensity (0‐10cm visual analogue scale) | ‐ | MD 0.01 higher (1.81 lower to 1.83 higher) | ‐ | 30 (1 study) | ⊕⊝⊝⊝ very low a b |
| Withdrawals due to adverse events | 0 per 1000 | 0 per 1000 (0 to 0) |
Not estimable | 30 (1 study) | ⊕⊝⊝⊝ very low a b |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels due to imprecision from very sparse data. bDowngraded one level due to risk of bias.
Summary of findings 5. Directed stress management (it is unclear whether these interventions replaced standard treatment or were added to standard treatment) versus standard treatment for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.
| Stress management (it is unclear whether these interventions replaced standard treatment or were added to standard treatment) versus standard treatment for the management of abdominal pain in Crohn's disease and inflammatory bowel disease | |||||
| Patient or population: people with Crohn's disease Setting: single centre, Inflammatory Intestinal Disease Unit of Asturias Central Hospital, Spain Intervention: stress management Comparison: no stress management | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with no stress management | Risk with stress management | ||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain frequency or change in pain frequency | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain intensity (author‐derived formula based on a 1‐3 point scale) | ‐ | MD 34.3 lower (61.99 lower to 6.61 lower) | ‐ | 30 (1 study) | ⊕⊝⊝⊝ very lowa b |
| Withdrawals due to adverse events | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels due to imprecision from very sparse data. bDowngraded one level due to risk of bias.
Summary of findings 6. Self‐directed stress management (it is unclear whether these interventions replaced standard treatment or were added to standard treatment) versus standard treatment for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.
| Self‐directed stress management (it is unclear whether these interventions replaced standard treatment or were added to standard treatment) versus standard treatment for the management of abdominal pain in Crohn's disease and inflammatory bowel disease | |||||
| Patient or population: people with Crohn's disease Setting: single centre, Inflammatory Intestinal Disease Unit of Asturias Central Hospital, Spain Intervention: stress management Comparison: no stress management | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with no stress management | Risk with stress management | ||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain frequency or change in pain frequency | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain intensity (author‐derived formula based on a 1‐3 point scale) | ‐ | MD 30.5 lower (58.45 lower to 2.55 lower) | ‐ | 30 (1 study) | ⊕⊝⊝⊝ very lowa b |
| Withdrawals due to adverse events | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels due to imprecision from very sparse data. bDowngraded one level due to risk of bias.
Summary of findings 7. Enteric‐release glyceryl trinitrate compared to placebo for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.
| Enteric‐release glyceryl trinitrate compared to placebo for the management of abdominal pain in Crohn's disease and inflammatory bowel disease | ||||||
| Patient or population: people with Crohn's disease Setting: unstated (centres in the UK) Intervention: enteric‐release glyceryl trinitrate Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with enteric‐release glyceryl trinitrate | |||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Abdominal pain frequency or change in frequency | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Abdominal pain intensity or change in intensity | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Withdrawal due to adverse events | Study population | RR 3.18 (0.94 to 10.76) | 70 (1 study) | ⊕⊝⊝⊝ very low a b | IG: Headache = 1, worsening clinical condition = 4, generalised rash = 1, mood change/irritability = 1, loss of consciousness/memory = 1 CG: Headache = 2, worsening clinical condition = 1 |
|
| 83 per 1000 | 265 per 1000 (78 to 897) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to risk of bias. bDowngraded two levels due to imprecision from very sparse data.
IG: Intervention group
CG: Control group
Summary of findings 8. 100 mg olorinab 3 times/day compared to 25 mg olorinab 3 times/day for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.
| 100 mg olorinab 3 times/day compared to 25 mg olorinab 3 times/day for the management of abdominal pain in Crohn's disease and inflammatory bowel disease | |||||
| Patient or population: people with Crohn's disease Setting: unstated (multicentre, USA) Intervention: 100 mg olorinab 3 times/day Comparison: 25 mg olorinab 3 times/day | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with 25 mg olorinab 3 times/day | Risk with 100 mg olorinab 3 times/day | ||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain frequency or change in pain frequency | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain intensity (30% reduction in weekly AAPS) | Study population | RR 0.66 (0.38 to 1.15) | 14 (1 study) | ⊕⊝⊝⊝ very low a b | |
| 1000 per 1000 | 660 per 1000 (380 to 1000) | ||||
| Withdrawal due to adverse events | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 14 (1 study) | ⊕⊝⊝⊝ very low a b |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to risk of bias. bDowngraded two levels due to imprecision from very sparse data.
Summary of findings 9. Relaxation training compared to waitlist for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.
| Relaxation training compared to waitlist for the management of abdominal pain in Crohn's disease and inflammatory bowel disease | |||||
| Patient or population: people with inflammatory bowel disease Setting: Hadassah Medical Center in Jerusalem Intervention: relaxation training Comparison: waitlist | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with waitlist | Risk with relaxation training | ||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain frequency or change in pain frequency | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain intensity (0‐10cm visual analogue scale) | ‐ | MD 0.72 lower (1.85 lower to 0.41 higher) | ‐ | 56 (1 study) | ⊕⊝⊝⊝ very low a b |
| Withdrawals due to adverse effects | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to risk of bias. bDowngraded two levels due to imprecision from very sparse data.
Summary of findings 10. Web‐based education compared to standard book‐based education for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.
| Web‐based education compared to standard book‐based education for the management of abdominal pain in Crohn's disease and inflammatory bowel disease | |||||
| Patient or population: people with inflammatory bowel disease Setting: single centre, gastroenterology unit in Turkey Intervention: web‐based education Comparison: standard book‐based education | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with standard book‐based education | Risk with web‐based education | ||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | Not measured |
| Abdominal pain frequency or change in frequency | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain intensity (0‐10cm visual analogue scale) | ‐ | MD 0.13 lower (1.25 lower to 0.99 higher) | ‐ | 60 (1 study) | ⊕⊕⊝⊝ very low a b |
| Withdrawals due to adverse events | 0 per 1000 | 0 per 1000 (0 to 0) |
Not estimable | 60 (1 study) | ⊕⊝⊝⊝ very lowa b |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to risk of bias. bDowngraded two levels due to imprecision from very sparse data.
Summary of findings 11. Yoga intervention compared to no treatment (both groups received standard medical therapy) for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.
| Yoga intervention compared to no treatment (both groups received standard medical therapy) for the management of abdominal pain in Crohn's disease and inflammatory bowel disease | |||||
|
Patient or population: people with inflammatory bowel disease Settings: single centre, All India Institute of Medical Science (AIIMS), New Delhi, India Intervention: yoga Comparison: no yoga | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with standard medical therapy | Risk with yoga plus standard medical therapy | ||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | Not measured |
| Abdominal pain frequency or change in frequency | ‐ | ‐ | ‐ | ‐ | Not measured |
| Abdominal pain intensity or change in intensity | ‐ | ‐ | ‐ | ‐ | Not measured |
| Withdrawal due to adverse events | 0 per 1000 | 0 per 1000 (0 to 0) |
Not estimable | 100 (1 study) |
⊕⊝⊝⊝ very low a b |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to risk of bias. bDowngraded two levels due to imprecision from very sparse data.
Summary of findings 12. Transcranial direct current stimulation compared to sham stimulation for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.
| Transcranial direct current stimulation compared to sham stimulation for the management of abdominal pain in Crohn's disease and inflammatory bowel disease | |||||
| Patient or population: people with inflammatory bowel disease Setting: single centre, Medical Department I (Gastroenterology, Infectious Diseases, Rheumatology) of the Charite‐Campus Benjamin Franklin, Germany Intervention: transcranial direct current stimulation Comparison: sham stimulation | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with sham stimulation | Risk with transcranial direct current stimulation | ||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | Not measured |
| Abdominal pain frequency or change in frequency | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain intensity (0‐10cm visual analogue scale) | ‐ | MD 1.65 lower (3.29 lower to 0.01 lower) | ‐ | 20 (1 study) | ⊕⊕⊝⊝ low a |
| Withdrawal due to adverse events | 0 per 1000 | 0 per 1000 (0 to 0) |
Not estimable | 20 (1 study) | ⊕⊕⊝⊝ low a |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; PPT: Pressure Pain Threshold | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels due to imprecision from very sparse data.
Summary of findings 13. Kefir ( Lactobacillus bacteria) compared to no intervention for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.
| Kefir (Lactobacillus bacteria) compared to no intervention for the management of abdominal pain in Crohn's disease and inflammatory bowel disease | |||||
|
Patient or population: people with inflammatory bowel disease Setting: unstated (single centre, Turkey) Intervention: kefir Comparison: no intervention | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with no intervention | Risk with kefir | ||||
| Treatment success as defined by the authors | ‐ | ‐ | ‐ | ‐ | Not measured |
| Abdominal pain frequency or change in frequency | ‐ | ‐ | ‐ | ‐ | Not measured |
| Pain intensity IBD (4‐point rating scale from 0 to 3) |
‐ | MD 0.62 higher (0.17 higher to 1.07 higher) | ‐ | 48 (1 study) |
⊕⊕⊝⊝
very low a b |
| Pain intensity CD (4‐point rating scale from 0 to 3) | ‐ |
MD 1.10 lower (1.67 lower to 0.53 lower) | ‐ | 20 (1 study) |
⊕⊕⊝⊝ very low a b |
| Withdrawals due to adverse events | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 20 (1 study) |
⊕⊝⊝⊝ very low a b |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CD: Crohn's disease; CI: confidence interval; IBD: inflammatory bowel disease; MD: mean difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to risk of bias. bDowngraded two levels due to imprecision from very sparse data.
A summary of the interventions and key outcome definitions and data are presented in Table 14 and Table 15 and explained below.
1. Primary outcome details.
| Comparison | Study ID | Disease type | Disease activity | Length of intervention | Measurement of pain | Number of randomised participants |
| Low FODMAP diet vs sham diet | Cox 2020 | CD/IBD | Inactive | 4 weeks | Pain frequency and intensity: IBS‐SSS for pain rating scale 0 to 100, GSRS rating scale 0 to 3 |
52 (IG: 27; CG: 25) |
| Low FODMAP diet vs sham diet | Tapete 2018 | IBD | Inactive | 6 to 8 weeks | Pain intensity: VAS rating scale 0 to 10 cm |
30 (IG: 10; CG: 20) |
| Low FODMAP diet vs high FODMAP/normal diet | Tapete 2019 | IBD | Inactive | 8 weeks cross‐over (4 weeks x 2) | Pain intensity: VAS rating scale 0 to 10 cm |
50 (IG: 25; CG: 25) |
| Medicine‐separated moxibustion combined with acupuncture vs wheat bran‐separated moxibustion combined with shallow acupuncture | Bao 2016 | CD | Mild to moderate | 12 weeks | Pain frequency and intensity: Traditional Chinese Medicine rating scale 0 to 3 |
102 (IG: 51; CG: 51) |
| Mindfulness with CBT + standard medical therapy vs standard medical therapy | Berrill 2014 | IBD | Inactive | 12 months | Pain frequency and severity of abdominal discomfort, severity of abdominal bloating, satisfaction with bowel habit, and impact of symptoms on life in general. Each domain is scored 0 to 100, and an overall score of 0 to 500 is obtained. | 66 (IG: 33; CG: 33) |
| Soft non‐manipulative osteopathic vs no intervention | Espi Lopez 2018 | CD | Unclear | 30 days | Pain intensity: VAS rating scale 0 to 10 cm |
30 (IG: 16; CG: 14) |
| Stress management vs self‐directed stress management vs standard treatment | Garcia‐Vega 2004 | CD | Inactive | 8 weeks | Pain intensity: rating scale 1 to 3 and author formulas |
45 (IG1: 15; IG2: 15; CG: 15) |
| Enteric‐release glyceryl trinitrate vs placebo | Hawkes 2001 | CD | Moderate to severe | 12 weeks | Pain intensity: rating scale 0 to 3 |
70 (IG: 34; CG: 36) |
| 100 mg olorinab 3 times/day vs 25 mg olorinab 3 times/day | Higgins 2019 | CD | Inactive to mild | 8 weeks | Pain intensity: AAPS of 0‐to‐10 Likert scale |
14 (IG: 8; CG: 6) |
| Relaxation training vs waitlist | Mizrahi 2012 | IBD | Active | 5 weeks | Pain intensity: VAS rating scale 0 to 10 cm |
56 (IG: 28; CG: 28) |
| Web‐based education vs standard book‐based education | Ozgursoy Uran 2019 | IBD | Mix of active and inactive | 8 weeks | Pain intensity: VAS rating scale 0 to 10 cm |
60 (IG: 10; CG: 10) |
| Yoga intervention + standard medical therapy vs standard medical therapy | Sharma 2015 | IBD | Inactive | 8 weeks | Presence or absence of pain | 100 (IG: 50; CG: 50) |
| Transcranial direct current stimulation vs sham stimulation | Volz 2016 | IBD | Active | 5 days | Pain intensity: Pressure Pain Threshold taken with algometer, VAS rating scale 0 to 10 cm |
20 (IG: 10; CG: 10) |
| Kefir diet (Lactobacillus bacteria) vs no intervention | Yilmaz 2019 | CD/IBD | Inactive to moderate | 4 weeks | Pain intensity: rating scale 0 to 3 |
48 (IG: 28; CG: 20) |
AAPS: average abdominal pain score
CBT: cognitive behavioural therapy
CD: Crohn's disease
CG: control group
FODMAP: fermentable oligo‐, di‐, monosaccharides and polyols
IG: intervention group
GSRS: Gastrointestinal Symptom Rating Scale
IBD: inflammatory bowel disease
IBS‐SSS: Irritable Bowel Syndrome Severity Scoring System
VAS: visual analogue scale
2. Primary outcome data.
| Comparison | Study ID | Treatment success end of study data IG/CG | Pain frequency end of study data IG/CG | Pain intenstiy end of study data IG/CG | Withdrawals due to adverse events IG/CG |
| Low FODMAP diet vs sham diet | Cox 2020 | NR | IBS‐SSS all participants mean(SD) IG= 36(26); CG= 38(25) IBS‐SSS CD mean (SD) IG = 36(138.4); CG=48(128.2) GSRS all participants mean(SD) IG= 1.5(1.6); CG= 1.1(1.5) |
IBS‐SSS all participants mean(SD) IG=22(15.6) ; CG=30(15) IBS‐SSS CD mean (SD) IG = 24(82.3); CG= 32(69.3) GSRS all participants mean(SD) IG=0.9(2.6) ; CG= 0.7(22.5) |
IG: 2 CG: 1 |
| Low FODMAP diet vs sham diet | Tapete 2018 | NR | NR | mean(SD): IG=3.3 (1.9) CG= 4.3(2.2) |
NR |
| Low FODMAP diet vs high FODMAP/normal diet | Tapete 2019 | NR | NR | mean(SD): IG=1.1(1.6) CG=3.1(2.3) (unclear if average of both cross‐over arms or from the first or second phase of the cross‐over) |
NR |
| Medicine‐separated moxibustion combined with acupuncture vs wheat bran‐separated moxibustion combined with shallow acupuncture | Bao 2016 | NR | mean (no SD provided) IG=0 CG=0 |
mean (no SD provided) IG=2 CG=1 |
IG=0 CG=0 |
| Mindfulness with CBT + standard medical therapy vs standard medical therapy | Berrill 2014 | NR | Frequency and intensity measured together as part of the IBS‐SSS score mean(SD) IG= 187 (97) CG= 224 (111) |
IG=0 CG=0 |
|
| Soft non‐manipulative osteopathic vs no intervention | Espi Lopez 2018 | NR | NR | mean(SD): IG=2.72(2.66) CG=2.71(2.43) |
IG=0 CG=0 |
| Stress management vs self‐directed stress management vs standard treatment | Garcia‐Vega 2004 | NR | NR | mean(SD): IG=13.3(28) CG=47.6(47) |
NR |
| Enteric‐release glyceryl trinitrate vs placebo | Hawkes 2001 | NR | NR | mean(no SD presented) IG=8.1 CG=8.6 |
IG=9 CG=3 |
| 100 mg olorinab 3 times/day vs 25 mg olorinab 3 times/day | Higgins 2019 | ≥ 30% reduction in weekly AAPS IG: 5 CG: 6 |
NR | 1.5 hours postdose mean(SD could not be calculated from figure) IG=1.9 CG= 1.9 Mean change in AAPS (no SD) IG= ‐4.6 CG= ‐4.6 |
IG=0 CG=0 |
| Relaxation training vs waitlist | Mizrahi 2012 | NR | NR | mean(SD): IG= 2.23(1.83) CG= 2.95(2.44) |
NR |
| Web‐based education vs standard book‐based education | Ozgursoy Uran 2019 | NR | NR | mean(SD): IG= 1.8(2.04) CG= 1.93(2.39) |
IG=0 CG=0 |
| Yoga intervention + standard medical therapy vs standard medical therapy | Sharma 2015 | NR | NR | NR | IG=0 CG=0 |
| Transcranial direct current stimulation vs sham stimulation | Volz 2016 | NR | NR | mean(SD): IG= 2.8(2.3) CG= 4.45(1.3) |
IG=0 CG=0 |
| Kefir diet (Lactobacillus bacteria) vs no intervention | Yilmaz 2019 | NR | NR | CD pain intensity mean(SD) IG= 0.2(0.63) CG= 1.3(0.67) IBD pain intensity mean(SD) IG= 0.9(0.97) CG= 0.28(0.61) |
IG=0 CG=0 |
AAPS: average abdominal pain score
CBT: cognitive behavioural therapy
CD: Crohn's disease
CG: control group
FODMAP: fermentable oligo‐, di‐, monosaccharides and polyols
IG: intervention group
GSRS: Gastrointestinal Symptom Rating Scale
IBD: inflammatory bowel disease
IBS‐SSS: Irritable Bowel Syndrome Severity Scoring System
VAS: visual analogue scale
Low FODMAP diet versus sham diet
Two studies compared a diet low in FODMAP to a sham diet (Cox 2020; Tapete 2018). Both studies included participants with either CD or UC and who were at an inactive stage of their disease. Tapete 2018 (n = 30) included participants with a Crohn's Disease Activity Index (CDAI) score < 150 for CD and a full Mayo score < 3 for UC, whilst in Cox 2020 (n = 52) quiescent IBD was defined by all of the following: physician global assessment, stable medications, no IBD flare in the previous six months, faecal calprotectin < 250 mg/g, and serum C‐reactive protein (CRP) < 10 mg/L. The length of the intervention was four weeks in Cox 2020 and six to eight weeks in Tapete 2018.
Primary outcomes
Treatment success was not reported. Pain in Cox 2020 was measured using the pain subscale of the IBS‐SSS that rates pain on a scale of 0 to 100 and the GSRS pain‐rating scale of 0 to 3, whilst Tapete 2018 used the 0‐to‐100‐millimetre VAS.
Pain frequency
Only Cox 2020 measured pain frequency.
At the end of study, the mean (SD) IBS‐SSS pain frequency in days was 36 (26) days for the 27 CD and UC participants in the low FODMAP group, and 38 (25) days for the 25 participants in the sham diet group. There was no clear difference in days of pain for CD and UC participants when a low FODMAP diet was compared to a sham diet in Cox 2020 (mean difference (MD) −2.00, 95% confidence interval (CI) −15.86 to 11.86). The certainty of evidence was very low due to unclear risk of bias and imprecision (Analysis 1.1; Table 1).
1.1. Analysis.

Comparison 1: Low FODMAP diet versus sham diet, Outcome 1: Pain frequency defined by days of pain by IBS‐SSS scores (0 to 100) for IBD
The mean (SD) days in which pain was rated as moderate or severe on the GSRS during the final week of the diet was 1.5 (1.6) for the low FODMAP group and 1.1 (1.5) for the sham diet group. There was no clear difference in days of pain during the last week of the diet for CD and UC participants when a low FODMAP diet was compared to a sham diet in Cox 2020 (MD 0.40, 95% CI −0.44 to 1.24). The certainty of evidence was very low due to unclear risk of bias and imprecision (Analysis 1.2; Table 1).
1.2. Analysis.

Comparison 1: Low FODMAP diet versus sham diet, Outcome 2: Days with moderate or severe pain by GSRS scores for IBD (0 to 3) for IBD
Cox 2020 also provided separate data for their CD participants. At end of study, the mean (SD) IBS‐SSS pain frequency in days was 36 (138.4) days for the 14 CD participants in the low FODMAP group, and 48 (128.2) days for the 12 participants in the sham diet group. There was no clear difference in days of pain for CD participants when a low FODMAP diet was compared to a sham diet in Cox 2020 (MD −12.00, 95% CI −114.55 to 90.55). The certainty of evidence was very low due to unclear risk of bias and imprecision (Analysis 1.3; Table 1).
1.3. Analysis.

Comparison 1: Low FODMAP diet versus sham diet, Outcome 3: Pain frequency defined by days of pain by IBS‐SSS scores (0 to 100) for CD
We requested separate CD data for the GSRS but were not able to obtain this information from the authors.
Pain intensity
Both studies measured pain intensity, in Cox 2020 on a 0‐to‐100mm VAS and in Tapete 2018 on a 0‐to‐10cm VAS. To combine them for analysis we multiplied the Tapete 2018 results by 10.
When the effects of both studies were analysed together, there was a small difference in intensity of pain for CD and UC participants (MD −8.46, 95% CI −15.76 to −1.16). The certainty of evidence was very low due to high risk of bias and imprecision, so no conclusions could be drawn (Analysis 1.4; Table 1).
1.4. Analysis.

Comparison 1: Low FODMAP diet versus sham diet, Outcome 4: Pain intensity IBD (0 to 100)
In Cox 2020, the mean (SD) pain intensity on the GSRS was 0.9 (2.6) for the low FODMAP group and 0.7 (22.5) for the sham diet group. There was no clear difference in pain intensity for CD and UC participants when a low FODMAP diet was compared to a sham diet on a 4‐point scale in Cox 2020 (MD 0.20, 95% CI −8.67 to 9.07). The certainty of evidence was very low due to unclear risk of bias and imprecision (Analysis 1.5; Table 1).
1.5. Analysis.

Comparison 1: Low FODMAP diet versus sham diet, Outcome 5: Pain intensity IBD (0 to 3)
Cox 2020 also provided separate data for their CD participants. At the end of study, the mean (SD) IBS‐SSS pain intensity was 24 (82.3) for the 14 CD participants in the low FODMAP group and 32 (69.3) for the 12 CD participants in the sham diet group. There was no clear difference in intensity of pain for CD participants when a low FODMAP diet was compared to a sham diet in Cox 2020 (MD −8.00, 95% CI −66.27 to 50.27). The certainty of evidence was very low due to unclear risk of bias and imprecision (Analysis 1.6; Table 1).
1.6. Analysis.

Comparison 1: Low FODMAP diet versus sham diet, Outcome 6: Pain intensity CD (0 to 100)
We requested separate CD data for the GSRS but were not able to obtain this information from the authors.
Withdrawals due to adverse events
Only Cox 2020 reported withdrawals due to adverse events. There were two withdrawals due to adverse events in the low FODMAP group (one IBD relapse, one commencement of antibiotics) and one in the sham diet group (one IBD relapse). There was no clear difference in the effect of the intervention on withdrawals due to adverse events (risk ratio (RR) 1.85, 95% CI 0.18 to 19.19) (Analysis 1.7; Table 1). The certainty of the evidence was very low due to risk of bias and imprecision. We requested data from Tapete 2018 but were unable to obtain this information.
1.7. Analysis.

Comparison 1: Low FODMAP diet versus sham diet, Outcome 7: Withdrawals due to adverse events
Secondary outcomes
Neither Cox 2020 nor Tapete 2018 reported on anxiety/depression. Only Cox 2020 reported adverse events and serious adverse events. There were no serious adverse events, and adverse events were reported in six participants in total. Three of these were the ones mentioned above in withdrawals due to adverse events. The other three were one case of worsening of abdominal pain (sham diet group), and two cases of flu‐like symptoms and sinusitis (one in each group).
One participant each in the intervention and control groups reported flu‐like symptoms and sinusitis, and one participant in the intervention group reported worsening of abdominal pain. One participant each in the intervention and control groups reported IBD relapse.
Low FODMAP diet versus high FODMAP/normal diet
One study (n = 50) compared a diet low in FODMAP to a high FODMAP/normal diet (Tapete 2019). The study included participants with either CD or UC who were at an inactive stage of their disease. Study participants had normal inflammatory parameters (CRP, white blood cells) and were deemed to be in clinical remission (CDAI < 150 for CD and a full Mayo < 2 for UC) but had residual abdominal symptoms (abdominal pain, diarrhoea, bloating). The length of the intervention was eight weeks; the study had a cross‐over design whereby groups alternated interventions after four weeks. We wrote to the authors for pre‐cross‐over data but received no response.
Primary outcomes
Treatment success was not reported. Tapete 2019 measured pain using the 0‐to‐10‐centimetre VAS. Pain frequency and withdrawals due to adverse events were not reported.
Pain intensity
Tapete 2019 reported that at the end of study the mean (SD) pain score was 1.1 (1.6) in the low FODMAP diet group and 3.1 (2.3) in the high FODMAP/normal diet group. However, it is unclear whether these scores were averages of both cross‐over arms, or if they were from the first or second phase of the cross‐over. The cross‐over design of the study precluded any analyses of the results.
Secondary outcomes
Tapete 2019 did not report on anxiety/depression, serious adverse events, or total number of participants with any event.
Medicine‐separated moxibustion combined with acupuncture versus wheat bran‐separated moxibustion combined with shallow acupuncture
One study (n = 102) compared medicine‐separated moxibustion combined with acupuncture to wheat bran‐separated moxibustion combined with shallow acupuncture (Bao 2016). The study included only CD participants who were at a mild to moderate stage of the disease (defined as a CDAI between 151 and 350). The length of the intervention was 12 weeks.
Primary outcomes
Bao 2016 did not report on treatment success. Pain was measured on the TCM 4‐point rating scale from 0 to 3.
Pain frequency
Pain frequency was reduced from an average score of 3 at baseline to 0 at end of study in the medicine‐separated moxibustion group, and from an average score of 1 to 0 in the wheat bran‐separated moxibustion group. The authors did not provide SD values, so we could not conduct any analyses of the results; we requested this information but received no response. It is uncertain whether medicine‐separated moxibustion combined with acupuncture leads to a difference in average pain frequency when compared with wheat bran‐separated moxibustion combined with shallow acupuncture.
Pain intensity
Pain intensity was reduced from an average score of 3 at baseline to 2 at end of study in the medicine‐separated moxibustion group, and remained at an average score of 1 for the wheat bran‐separated moxibustion group. The authors did not provide SD values, so we could not conduct any analyses of the results; we requested this information but received no response. It is uncertain whether medicine‐separated moxibustion combined with acupuncture leads to a difference in average pain intensity when compared with wheat bran‐separated moxibustion combined with shallow acupuncture.
Withdrawals due to adverse events
There were no withdrawals due to adverse events in either the medicine‐separated moxibustion or wheat bran‐separated moxibustion group, so there was no estimable relative effect of the intervention on withdrawals due to adverse events (Analysis 2.1; Table 2).
2.1. Analysis.

Comparison 2: Medicine‐separated moxibustion combined with acupuncture versus wheat bran‐separated moxibustion combined with shallow acupuncture, Outcome 1: Withdrawals due to adverse events
Secondary outcomes
The authors reported that there were no serious adverse events. Two participants experienced any adverse event, one in each treatment group. Anxiety or depression was not reported.
Mindfulness with cognitive behavioural therapy (CBT) versus no treatment (both groups received standard medical therapy)
One study (n = 66) compared mindfulness with CBT to no treatment (Berrill 2014). The study included participants with CD or UC who were at an inactive stage of their disease. Study participants had a diagnosis of UC or CD that was in remission based on a clinical activity index score and a CRP level < 10 mg/L. The length of the intervention was 12 months.
Primary outcomes
Berrill 2014 did not report on treatment success. Pain was measured on the 0‐to‐500 IBS‐SSS, which is the sum of five separate 0‐to‐100 scales that measure five different pain‐related symptoms: pain frequency and severity of abdominal discomfort, severity of abdominal bloating, satisfaction with bowel habit, and impact of symptoms on life in general.
Pain frequency and intensity
At the end of the study, mean (SD) IBS‐SSS score (which includes pain frequency and severity) was 187 (97) for the mindfulness with CBT group and 224 (111) for the no‐treatment group. There was no clear difference in IBS‐SSS symptoms between groups (MD −37.00, 95% CI −87.29 to 13.29). The certainty of evidence was very low due to unclear risk of bias and imprecision (Analysis 3.1; Table 3).
3.1. Analysis.

Comparison 3: Mindfulness with cognitive behavioural therapy (CBT) versus no treatment (both groups received standard medical therapy), Outcome 1: Pain frequency and severity of abdominal discomfort, severity of abdominal bloating, satisfaction with bowel habit, and impact of symptoms on life in general
Withdrawals due to adverse events
There were no withdrawals due to adverse events in either group, so there was no estimable relative effect of the intervention on withdrawals due to adverse events (Analysis 3.2; Table 3).
3.2. Analysis.

Comparison 3: Mindfulness with cognitive behavioural therapy (CBT) versus no treatment (both groups received standard medical therapy), Outcome 2: Withdrawals due to adverse events
Secondary outcomes
Anxiety and depression at the end of study were not presented. Serious adverse events or total number of participants with any adverse event was not reported.
Soft non‐manipulative osteopathic versus no intervention
One study (n = 30) compared soft non‐manipulative osteopathic treatment to no intervention (Espi Lopez 2018). The study included only CD participants, and it was unclear whether participants had active or inactive stage of the disease. The length of the intervention was 30 days.
Primary outcomes
Espi Lopez 2018 did not report on treatment success. Pain was measured on the 0‐to‐10‐centimetre VAS. Pain frequency was not measured.
Pain intensity
The mean (SD) score at end of the study was 2.72 (2.66) for the osteopathic group and 2.71 (2.43) for the no‐intervention group. There was no clear difference in pain intensity in the osteopathic group when compared to the no‐intervention group (MD 0.01, 95% CI −1.81 to 1.83). The certainty of evidence was very low due to high risk of bias and imprecision (Analysis 4.1; Table 4).
4.1. Analysis.

Comparison 4: soft non‐manipulative osteopathic treatment versus no intervention, Outcome 1: Pain intensity
Withdrawals due to adverse events
There were no withdrawals due to adverse events in either group, so there was no estimable relative effect of the intervention on withdrawals due to adverse events (Analysis 4.2; Table 4).
4.2. Analysis.

Comparison 4: soft non‐manipulative osteopathic treatment versus no intervention, Outcome 2: Withdrawals due to adverse events
Secondary outcomes
The authors reported that no adverse events occurred in this study. Anxiety/depression results were not reported.
Directed stress management versus self‐directed stress management (it is unclear whether these interventions replaced standard treatment or were added to standard treatment) versus standard treatment
One study (n = 45) compared directed stress management to standard treatment and self‐directed management to standard treatment (Garcia‐Vega 2004). It was unclear whether the interventional treatments included standard treatment or not. We attempted to contact the author but received no response.
The study included only CD participants who were at an inactive stage of the disease based on the Harvey‐Bradshaw Index. The length of the intervention was eight weeks.
Primary outcomes
Garcia‐Vega 2004 did not report on treatment success. Pain scores were calculated with author‐derived formulas that were based on a 3‐point rating scale from 1 to 3. Pain frequency and withdrawals due to adverse events were not reported in this study.
Pain intensity
At end of the study the mean (SD) pain score was 13.3 (28.8) for the directed stress management group; 17.1 (29) for the self‐directed stress management group; and 47.6 (47) for the standard treatment group. There was a difference in the directed stress management group when compared to the standard treatment group ( MD ‐34.30, 95% CI ‐61.99 to ‐6.61 ) and in the self‐directed stress management group when compared to the standard treatment group ( MD ‐30.50, 95% CI ‐58.45 to ‐2.55 ). The certainty of evidence was very low due to high risk of bias and imprecision, therefore no conclusions could be drawn (Analysis 5.1; Analysis 6.1 Table 5; Table 6).
5.1. Analysis.

Comparison 5: Directed stress management versus standard treatment, Outcome 1: Pain intensity
6.1. Analysis.

Comparison 6: Self‐directed stress management vs conventional therapy, Outcome 1: Pain intensity
Secondary outcomes
Garcia‐Vega 2004 did not report anxiety/depression, serious adverse events, or total number of participants with any adverse event.
Enteric‐release glyceryl trinitrate versus placebo
One study (n = 70) compared enteric‐release glyceryl trinitrate to placebo (Hawkes 2001). The study included only CD participants at a moderate to severe stage of the disease with a CDAI of between 150 and 450. The length of the intervention was 12 weeks.
Primary outcomes
Hawkes 2001 did not report on treatment success. Pain was measured on a 4‐point scale from 0 to 3. Pain frequency was not reported in this study.
Pain intensity
At end of study the mean score was 8.1 for the glyceryl group and 8.6 for the placebo group. No analyses could be conducted with these results as SD values were unobtainable from the authors. It is uncertain whether enteric‐release glyceryl trinitrate leads to any differences in pain intensity compared to placebo.
Withdrawal due to adverse events
Nine participants in the glyceryl group (headache = 1, worsening clinical condition = 4, generalised rash = 1, mood change/irritability = 1, loss of consciousness/memory = 1) and three participants in the placebo group (headache = 2, worsening clinical condition = 1) experienced adverse events that led to withdrawal from the study. There was no clear difference in withdrawals due to adverse events when enteric‐release glyceryl trinitrate was compared to placebo (RR 3.18, 95% CI 0.94 to 10.76). The certainty of the evidence was very low due to unclear risk of bias and high imprecision (Analysis 7.1; Table 7).
7.1. Analysis.

Comparison 7: Enteric‐release glyceryl trinitrate versus placebo, Outcome 1: Withdrawals due to adverse events
Secondary outcomes
The authors of Hawkes 2001 reported that there were no serious adverse events in either group. Nineteen participants in the enteric‐release glyceryl trinitrate group and 13 participants in the placebo group experienced any adverse event. Anxiety/depression was not measured in this study.
100 mg olorinab three times/day versus 25 mg olorinab three times/day
One study (n = 14) compared 100 mg olorinab taken three times per day to 25 mg olorinab taken three times per day (Higgins 2019). The study only included CD participants who were at an inactive or mild stage of the disease with a simple endoscopic score CD < 10 or faecal calprotectin < 500 μg/g. The length of the intervention was eight weeks.
Primary outcomes
Treatment success in Higgins 2019 was defined as a ≥ 30% reduction in weekly Average Abdominal Pain Score (AAPS) from baseline to week 8, as measured on a 0‐to‐10 Likert scale. Pain frequency was not measured in this study.
Pain intensity
At end of study, five of eight participants had achieved a 30% reduction in weekly AAPS in the 100 mg olorinab group, whilst six of six participants had achieved a 30% reduction in the 25 mg olorinab group. There was no clear difference in pain intensity between groups (RR 0.66, 95% CI 0.38 to 1.15). The certainty of the evidence was very low due to high risk of bias and imprecision (Analysis 8.1; Table 8).
8.1. Analysis.

Comparison 8: 100 mg olorinab 3 times/day versus 25 mg olorinab 3 times/day, Outcome 1: Pain intensity
Withdrawals due to adverse events
There were no withdrawals due to adverse events in either group, so there was no estimable relative effect of the intervention on withdrawals due to adverse events (Analysis 8.2; Table 8).
8.2. Analysis.

Comparison 8: 100 mg olorinab 3 times/day versus 25 mg olorinab 3 times/day, Outcome 2: Withdrawals due to adverse events
Secondary outcomes
One participant in the 100 mg olorinab group experienced a serious adverse event, which the authors described as unrelated to the intervention. No serious adverse events were reported in the 25 mg olorinab group.
Six of eight participants in the 100 mg olorinab group and four of six participants in the 25 mg olorinab group experienced any adverse event.
Anxiety/depression was not reported in this study.
Relaxation training versus waitlist
One study (n = 56) compared relaxation training to a waitlist (the people in the waitlist would receive the training after the end of the study) (Mizrahi 2012). The study included CD and UC participants who were at an active stage of the disease according to the "Disease Activity Questionnaire", by meeting one of the following criteria: more than five bowel movements a day, more than one hospitalisation a year over the previous two years, and had either suffered a fistula during the previous year or was using corticosteroids. The length of the intervention was five weeks.
Primary outcomes
Mizrahi 2012 did not report on treatment success. Pain was measured on the 0‐to‐10‐centimetre VAS. Pain frequency and withdrawals due to adverse events were not reported.
Pain intensity
At end of study the mean (SD) score was 2.23 (1.83) for the relaxation training group and 2.95 (2.44) for the waitlist group. There was no clear difference in pain intensity when relaxation training was compared to a waitlist (MD −0.72, 95% CI −1.85 to 0.41). The certainty of the evidence was very low due to high risk of bias and imprecision (Analysis 9.1; Table 9).
9.1. Analysis.

Comparison 9: Relaxation training versus waitlist, Outcome 1: Pain intensity
Secondary outcomes
Mean (SD) anxiety as measured on the Spielberger’s State‐Trait Anxiety Inventory at end of study was 36.67 (10.65) for the relaxation training group and 40.51 (12.57) for the waitlist group.
Mean (SD) depression measured using the 0‐to‐10‐centimetre VAS at end of study was 1.39 (2.23) for the relaxation training group and 1.9 (1.99) for the waitlist group.
Serious adverse events or total number of participants with any adverse event was not reported in this study.
Web‐based education versus standard book‐based education
One study (n = 60) compared web‐based education to standard book‐based education (Ozgursoy Uran 2019). The study included participants with CD or UC who were in either an active or inactive stage of the disease. The length of the intervention was eight weeks.
Primary outcomes
Ozgursoy Uran 2019 did not report on treatment success. Pain intensity was measured on the 0‐to‐10‐centimetre VAS. Pain frequency was not reported in this study.
Pain intensity
At end of study, the mean (SD) score was 1.8 (2.04) for the web‐based education group and 1.93 (2.39) for the standard book‐based education group. There was no clear difference in pain intensity when web‐based education was compared with standard book‐based education (MD −0.13, 95% CI −1.25 to 0.99). The certainty of the evidence was very low due to unclear risk of bias and imprecision (Analysis 10.1; Table 10).
10.1. Analysis.

Comparison 10: Web‐based education versus standard book‐based education, Outcome 1: Pain intensity
Withdrawals due to adverse events
There were no withdrawals due to adverse events in either group, so there was no estimable relative effect of the intervention on withdrawals due to adverse events (Analysis 10.2; Table 10).
10.2. Analysis.

Comparison 10: Web‐based education versus standard book‐based education, Outcome 2: Withdrawals due to adverse events
Secondary outcomes
Ozgursoy Uran 2019 did not report on anxiety/depression, serious adverse events, or total number of participants with any adverse event.
Yoga intervention versus no treatment (both groups received standard medical therapy)
One study (n = 100) compared a yoga intervention to no yoga (Sharma 2015). The study only included participants with CD or UC who were at an inactive stage of the disease with a CDAI score < 150; UC activity was measured on the Truelove and Witts Severity Index. The length of the intervention was eight weeks.
Primary outcomes
Treatment success in this study was measured as a dichotomous outcome of presence or absence of pain; however, results were reported only for the UC participants and not for CD participants. We contacted the authors for the missing data but received no response. Pain frequency or intensity was not reported.
Withdrawals due to adverse events
There were no withdrawals due to adverse events in either group, so there was no estimable relative effect of the intervention on withdrawals due to adverse events (Analysis 11.1; Table 11).
11.1. Analysis.

Comparison 11: Yoga intervention versus no treatment (both groups received standard medical therapy), Outcome 1: Withdrawals due to adverse events
Secondary outcomes
The authors reported that no serious adverse events occurred in either group.
There was one reported total adverse event in the yoga group (bone fracture).
Anxiety level results were presented only for UC and not for CD participants. Depression was not reported.
Transcranial direct current stimulation versus sham stimulation
One study (n = 20) compared transcranial direct current stimulation to sham stimulation (Volz 2016). The study included CD and UC participants who were at an active stage of the disease as measured with either the Simple Clinical Colitis Activity Index for UC participants or Harvey–Bradshaw Index for CD participants. The length of the intervention was five days.
Primary outcomes
Treatment success was not reported. Pain was measured on the 0‐to‐10‐centimetre VAS and mechanically on the Pressure Pain Threshold (PPT) via an algometer on the right and left abdomen. Pain frequency was not reported.
Pain intensity
At the end of study, the mean (SD) VAS score for pain intensity was 2.8 (2.3) for the transcranial stimulation group and 4.45 (1.3) for the sham stimulation group. Transcranial direct current stimulation may improve pain intensity when compared to sham stimulation (MD −1.65, 95% CI −3.29 to −0.01). The certainty of the evidence was low due to high imprecision (Analysis 12.1; Table 12).
12.1. Analysis.

Comparison 12: Transcranial direct current stimulation versus sham stimulation, Outcome 1: Pain intensity (0 to 100)
Withdrawals due to adverse events
There were no withdrawals due to adverse events in either group, so there was no estimable relative effect of the intervention on withdrawals due to adverse events (Analysis 12.2; Table 12).
12.2. Analysis.

Comparison 12: Transcranial direct current stimulation versus sham stimulation, Outcome 2: Withdrawals due to adverse events
Secondary outcomes
The authors reported that no serious adverse events occurred in this study. They reported on total adverse events, but as number of incidents rather than number of participants with adverse events.
Depression was measured at baseline, but no end‐of‐study results were reported. Anxiety was not reported.
Kefir diet (Lactobacillus bacteria) versus no intervention
One study (n = 48) compared a kefir diet with Lactobacillus bacteria to no intervention (Yilmaz 2019). The study included participants with CD or UC whose disease activity ranged from inactive to moderate. The length of the intervention was four weeks.
Primary outcomes
Yilmaz 2019 did not report on treatment success. Pain was measured on a 4‐point rating scale from 0 to 3. Pain frequency was not reported.
Pain intensity
At the end of study, mean (SD) pain score for pain intensity was 0.2 (0.63) for the CD kefir group and 1.3 (0.67) for the no‐intervention group. No clear difference was detected in pain intensity scores when kefir diet was compared to no intervention (MD −1.10, 95% CI −1.67 to −0.53). The certainty of the evidence was very low due to unclear risk of bias and imprecision (Analysis 13.1; Table 13).
13.1. Analysis.

Comparison 13: Kefir versus no intervention, Outcome 1: CD pain intensity (0 to 3)
At the end of study, mean (SD) pain score for pain intensity was 0.9 (0.97) for the IBD kefir group and 0.28 (0.61) for the no‐intervention group. No clear difference was detected in pain intensity scores when kefir diet was compared to no intervention (MD 0.62, 95% CI 0.17 to 1.07). The certainty of the evidence was very low due to unclear risk of bias and imprecision (Analysis 13.2; Table 13).
13.2. Analysis.

Comparison 13: Kefir versus no intervention, Outcome 2: IBD pain intensity (0 to 3)
Withdrawals due to adverse events
There were no withdrawals due to adverse events in either group, so there was no estimable relative effect of the intervention on withdrawals due to adverse events (Analysis 13.3; Table 13).
13.3. Analysis.

Comparison 13: Kefir versus no intervention, Outcome 3: Withdrawals due to adverse events
Secondary outcomes
Yilmaz 2019 did not report on anxiety/depression, serious adverse events, or total number of participants with any adverse event.
Discussion
Summary of main results
This review included a wide range of interventions. Three were forms of diet (Cox 2020; Tapete 2018; Tapete 2019; Yilmaz 2019); three were forms of psychological management (Berrill 2014; Garcia‐Vega 2004; Mizrahi 2012); two were forms of alternative therapies (Bao 2016; Espi Lopez 2018); two were drug interventions (Hawkes 2001; Higgins 2019); one was an educational intervention (Ozgursoy Uran 2019); one was a form of exercise (Sharma 2015); and one was a form of brain stimulation (Volz 2016). Five studies looked exclusively at CD (Bao 2016; Espi Lopez 2018; Garcia‐Vega 2004; Hawkes 2001; Higgins 2019), whilst the remaining studies evaluated participants with both CD and UC; two of these studies provided separate data for their CD population (Cox 2020; Yilmaz 2019). The studies included a range of disease states.
Only two studies defined or reported our primary outcome of treatment success (Higgins 2019; Sharma 2015). In the remaining studies pain was only measured as a continuous outcome by improvement on a rating scale: 0‐to‐100 scales (Berrill 2014; Cox 2020); 0‐to‐10‐centimetre VAS or 0‐to‐10 Likert scale (Espi Lopez 2018; Mizrahi 2012; Ozgursoy Uran 2019; Tapete 2018; Tapete 2019; Volz 2016); 4‐point 0‐to‐3 scale (Bao 2016; Cox 2020; Hawkes 2001; Yilmaz 2019); or a combination of a 3‐point scale and author‐derived formulas (Garcia‐Vega 2004). In all studies a lower rating indicated less pain, whilst a higher rating indicated more pain. One study measured only the absence or presence of pain as a dichotomous outcome (Sharma 2015). Except for this study, all studies measured pain intensity, whilst only three studies measured pain frequency (Bao 2016; Berrill 2014; Cox 2020). Withdrawals due to adverse events were directly or indirectly reported in nine studies (Bao 2016; Berrill 2014; Cox 2020; Espi Lopez 2018; Hawkes 2001; Higgins 2019; Ozgursoy Uran 2019; Sharma 2015; Volz 2016).
This heterogeneity in outcome measures reported and interventions used severely limited our scope for meta‐analysis.
The only low‐certainty evidence found was for the comparison transcranial direct current stimulation versus sham stimulation (Volz 2016); this evidence showed that there may be an improvement in pain intensity for CD and UC participants (no separate CD data were provided), with direct current stimulation when that was measured using visual analogue scales.
In two pooled studies comparing a low FODMAP diet and a sham diet, we found very low‐certainty evidence for a difference between a low FODMAP diet and a sham diet for the outcome pain intensity for CD and UC participants (no separate CD data provided by Tapete 2018) (Cox 2020; Tapete 2018), therefore no conclusions could be drawn about the efficacy of this intervention in improving pain intensity.
In one study comparing stress management to standard treatment and self‐directed stress management to standard treatment (it is unclear whether the stress management interventions replaced standard treatment or were added to standard treatment), we found very low‐certainty evidence for a difference between both stress management and self‐directed stress management in comparison to standard treatment for the outcome pain intensity in CD participants (Garcia‐Vega 2004), therefore no conclusions could be drawn about the efficacy of these interventions in improving pain intensity.
There was no clear difference in pain intensity or frequency between interventions for any other direct comparisons, although the certainty of the evidence was very low due to imprecision from sparse data and risk of bias ratings of unclear and high risk.
Of the studies that reported withdrawals due to adverse events, all reported no withdrawals except for Cox 2020 and Hawkes 2001. Cox 2020 evaluated a low FODMAP diet versus a sham diet, and Hawkes 2001 evaluated a drug intervention comparing enteric‐release glyceryl trinitrate to placebo. The two studies showed no clear effect on withdrawals due to adverse events, although the certainty of the evidence was very low due to unclear risk of bias and high imprecision. Lack of evidence prevented us from drawing any conclusions about the effects of the other interventions on withdrawals due to adverse events.
The reporting of serious adverse events and total number of participants with any adverse event as secondary outcomes was inconsistent. Seven studies reported on serious adverse events and total number of participants with any adverse event (Bao 2016; Cox 2020; Espi Lopez 2018; Hawkes 2001; Higgins 2019; Sharma 2015; Volz 2016). The most adverse events were reported in the two drug interventions (Hawkes 2001; Higgins 2019), whilst adverse events tended to be very low or zero in the non‐drug interventions. However, no conclusions could be drawn regarding adverse events for any of the interventions studied due to the low number of events.
Mizrahi 2012 measured and reported anxiety and depression at end of study. Three studies mentioned measuring anxiety or depression at baseline, but no anxiety or depression end‐of‐study results were presented in Berrill 2014; no anxiety end‐of‐study results for CD participants were reported in Sharma 2015; and no depression end‐of‐study results were reported in Volz 2016. Consequently, no meaningful conclusions could be drawn regarding this outcome.
Overall completeness and applicability of evidence
The included studies not only considered a wide range of interventions, but most considered a mix of disease activity and form of IBD. This is rather distinct from the majority of reviewed studies in the wider Cochrane IBD portfolio, which almost always consider UC and CD separately, or at the very least report results for both groups in a single study. Similarly, the reviews reflect a mixture of stage of CD and IBD. As such, the evidence in this review is very different, with a mixture of both conditions and disease states in all papers, which raises significant issues regarding clinical heterogeneity.
It is also very clear that, despite there being a reasonable number of included studies, the great range of interventions and the small numbers of included participants leave the individual evidence at significant risk of imprecision. This is pervasive across the evidence presented in this review, with no single comparison not at risk of imprecision.
One of the issues experienced when considering the evidence was that, despite our inclusion criteria requiring interventions to be focussed on pain, not all papers provided explicit descriptions of baseline pain. Inclusion criteria could be complex, and therefore pain change scores, as well as clear baseline pain data, are areas which limit the completeness of the evidence for clinical interpretation.
The incidence of IBS amongst IBD patients is higher than in people without IBD (Halpin 2012), and it is very difficult to separate whether IBD patients' continuing pain symptoms are related to their previous disease state. Determining what gastrointestinal symptoms are truly functional symptoms is not possible, which may have affected the overall completeness and applicability of our evidence.
Another issue was the number of participants with pain at baseline, in order to determine what denominator to use for our analyses. All papers explicitly or close to explicitly included participants with gut symptoms or pain, although in same cases there was a lack of clarity. However, considering this issue pragmatically, all pain outcomes studied were continuous, and therefore used the denominator of all participants randomised, as it would not be statistically sound to do otherwise. One exception was the Higgins 2019 trial, which reported a dichotomous pain outcome, but all participants at baseline were experiencing abdominal pain, so again we used the number of all participants randomised as the denominator for our analysis.
We also noted the use of multiple scales to measure pain within the same study (Cox 2020), or proxy or measures, such as the use of a mechanical algometer to measure pain thresholds (Volz 2016). It’s important that future studies consider using the same internationally recognised measures for pain, such as visual analogue scales, in order to limit the heterogeneity that might be caused by using a variety of different methodologies.
Quality of the evidence
There were significant issues related to risk of bias in the studies included in this review. Despite our requests to authors of all included studies, we received no data to change our judgements in these key areas.
No single comparison surpassed 100 participants as a total, which meant that all outcomes were judged to be highly imprecise, and the certainty of the evidence was downgraded twice for this reason.
A number of studies did not clearly describe randomisation, Garcia‐Vega 2004; Higgins 2019; Mizrahi 2012; Tapete 2018; Tapete 2019, or allocation concealment, Bao 2016; Garcia‐Vega 2004; Hawkes 2001; Higgins 2019; Mizrahi 2012; Ozgursoy Uran 2019; Tapete 2018; Tapete 2019; Volz 2016; Yilmaz 2019.
Blinding of participants and personnel was understandably not possible in most of the included studies. However, most studies did not discuss whether their outcome assessors were blinded either.
A number of studies had issues with selective reporting (Espi Lopez 2018; Garcia‐Vega 2004; Higgins 2019; Sharma 2015; Tapete 2019), leading to further issues related to risk of bias and downgrading of the certainty of the evidence. Attrition bias was also an issue, with three studies judged as unclear risk of bias (Garcia‐Vega 2004; Tapete 2018; Tapete 2019), and one as high risk of bias for this domain (Berrill 2014).
Finally, other key sources of bias were present, mainly a potential imbalance in baseline characteristics, which was observed or was unclear in four studies (Garcia‐Vega 2004; Hawkes 2001; Mizrahi 2012; Tapete 2019), and that further impacted the quality of the evidence.
Potential biases in the review process
Clinical heterogeneity is a key area of concern in this review. As previously discussed, the inclusion of studies focusing exclusively on CD patients together with a majority of studies that included CD and UC patients as an indecipherable IBD cohort, as well as the variety of disease states, reflects the evidence as a whole, but ignores the issue of clinical heterogeneity to some extent. It would have been possible to exclude studies that did not differentiate between CD and UC, and this was a discussion point for the team when completing the review. If we decided to exclude these studies from this review, and similarly exclude them from our concomitant UC review, a potentially important body of evidence would have been unaccounted for. We felt that representing this evidence base was vital, together with accepting and transparently presenting the difficulties this led to, related to clinical heterogeneity. On balance, given the extremely limited implications of the current evidence base, we decided to include studies that included CD and UC patients as one IBD cohort and use them for our analysis (we also decided that in our UC review, data exclusively on UC would be included). However, as further data become available, this would not be the approach the team or indeed readers would endorse and is a potential source of bias. As this has not clearly been defined within the original protocol, it must be recognised as a potential source of bias in the review.
Agreements and disagreements with other studies or reviews
This is the first Cochrane Review on this topic.
Considering the international guidelines for IBD, few of the major societies mention the treating of pain in IBD.
The recent British Society of Gastroenterology guidelines do offer recommendations (BSG 2019), citing several of the studies included in this review. They state that psychological interventions may be useful as adjunct therapy, describing this as a weak recommendation with low‐quality evidence, which would be supported by the evidence in this review. They do not define such psychological interventions, and do not comment on any of the other intervention types included in this review.
The current UK National Institute for Health and Care Excellence (NICE) guidelines do not discuss pain relief as a standalone treatment goal (NICE 2019). The American Gastroenterological Association guidelines make no mention of guidelines in this area (AGA 2020).
The European Crohn's and Colitis Organisation guidelines also do not mention therapies in this area (ECCO 2020).
Authors' conclusions
Implications for practice.
Low‐certainty evidence suggests that transcranial direct current stimulation may be effective in improving pain for inflammatory bowel disease patients.
No conclusions could be drawn for any of the other interventions either for primary or secondary efficacy outcomes, as the evidence is of very low certainty due to low numbers of participants for each comparison and clinical heterogeneity amongst studies.
Whilst no serious or total adverse events were specifically elicited with any of the treatments studied, the numbers of reported events were very low, and the certainty of the evidence was very low for all comparisons, so no conclusions can be drawn.
Anxiety and depression were poorly reported, and once again no conclusions can be drawn as to the impact of the included interventions on these outcomes.
Implications for research.
The need for future research is clear. Given the prominence of abdominal pain in people with Crohn's disease, randomised controlled trials that target it as an independent condition and not as part of inducing or maintaining remission are numerous, but the nature of the evidence base leads to much justification for further research. Many of the interventions studied in the trials included in this review are used anecdotally by patients and are available without clinician involvement, therefore clear evidence to inform patients when making treatment decisions is vital.
No high or moderate certainty conclusions could be reached on the efficacy of any of the interventions included in this review. Transcranial direct current stimulation showed low‐certainty efficacy. Other trials that showed some positive results, albeit with very low‐certainty evidence (Cox 2020; Garcia‐Vega 2004), were in participants that had quiescent disease. Irritable bowel syndrome is more common in inflammatory bowel disease patients, and it is possible that all of these interventions are simply treating irritable bowel syndrome. Any of the included interventions could therefore be targets for future research.
Considering the currently ongoing trials identified in this review, they appear to be still very heterogeneous in terms of the range of therapies, with diverse outcome measures and relatively low sample sizes planned, which will limit the impact these studies can have on the evidence base.
We suggest that key stakeholders, including clinicians, those with an understanding of health economics, and most importantly patients, consider which interventions are of interest. All of them are particularly well placed to consider feasibility, acceptability, and tolerability amongst other factors in targeting future research.
Furthermore, researchers can consider addressing risk of bias in their reporting and reporting data by disease type or severity, or both.
The issue of sample size must be highlighted. All of the studies included in this review were very small. We strongly advise the use of indicative odds ratios from this review when performing power calculations. Such accurate calculations are vital to halt the large number of low‐powered studies and include the precision of findings.
History
Protocol first published: Issue 1, 2020
Acknowledgements
Funding for VS and partial funding for MG was provided through a larger National Institute for Health Research (NIHR) Cochrane Programme Grant in the UK.
Maria‐Inti Metzendorf, the Information Specialist of the Cochrane Metabolic and Endocrine Disorders Group, developed the search strategies, which were run by Yuhong Yuan (Information Specialist, Cochrane Gut Group).
The review authors thank peer reviewers Dr Matthew Coates and Dr Laurie Keefer, and editor Dr. Paul Moayyedi, who provided comments to improve the review. We thank Lisa Winer for copy editing the review.
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (Ovid EBMR) |
| 1. exp Pain/ 2. (pain* or headache* or migraine* or fibromyalgia* or neuralgia* or colic*).tw. 3. (discomfort* or ache or aching or aches).tw. 4. or/1‐3 5. Crohn Disease/ 6. Inflammatory Bowel Diseases/ 7. ((Crohn or Crohn*).tw. 8. (inflammatory bowel disease*).tw. 9. (enteritis or ileitis or ileocolitis or colitis).tw. 10. or/5‐9 11. 4 and 10 |
| MEDLINE (Ovid) |
| 1. exp Pain/ 2. (pain* or headache* or migraine* or fibromyalgia* or neuralgia* or colic*).tw. 3. (discomfort* or ache or aching or aches).tw. 4. or/1‐3 5. Crohn Disease/ 6. Inflammatory Bowel Diseases/ 7. (Crohn or Crohn*).tw. 8. (inflammatory bowel disease*).tw. 9. (regional enteritis or regional ileitis or terminal ileitis or granulomatous enteritis or ileocolitis or granulomatous colitis).tw. 10. or/5‐9 11. 4 and 10 [Cochrane Handbook RCT filter ‐ sensitivity max version] 12. randomized controlled trial.pt. 13. controlled clinical trial.pt. 14. randomi?ed.ab. 15. placebo.ab. 16. drug therapy.fs. 17. randomly.ab. 18. trial.ab. 19. groups.ab. 20. or/12‐19 21. exp animals/ not humans/ 22. 20 not 21 23. 11 and 22 [Wong 2006 – systematic reviews filter – sensitivity and specificity best balance version] 24. meta analysis.mp,pt. or review.pt. or search*.tw. 25. 11 and 24 26. 23 or 25 |
| PsycINFO (OvidSP) |
| 1. exp Pain/ 2. Pain Measurement/ 3. Pain Perception/ 4. Pain Management/ 5. (pain* or headache* or migraine* or fibromyalgia* or neuralgia* or colic*).tw. 6. (discomfort* or ache or aching or aches).tw. 7. or/1‐6 8. Crohn Disease/ 9. (Crohn or Crohn*).tw. 10. (inflammatory bowel disease*).tw. 11. (regional enteritis or regional ileitis or terminal ileitis or granulomatous enteritis or ileocolitis or granulomatous colitis).tw. 12. or/8‐11 13. 7 and 12 [Eady 2008 "PsycInfo search strategies" filter – best sensitivity version] 14. control*.tw. OR random*.tw. OR exp Treatment/ 15. 13 and 14 |
| AMED (Ovid) |
| 1. (pain* or headache* or migraine* or fibromyalgia* or neuralgia* or colic*).tw. 2. (discomfort* or ache or aching or aches).tw. 3. or/1‐2 4. (Crohn or Crohn*).tw. 5. (inflammatory bowel disease*).tw. 6. (enteritis or ileitis or ileocolitis or colitis).tw. 7. or/4‐6 8. 3 and 7 |
| CINAHL (EBSCO) |
| S1. MH "Pain+" S2. TI (pain* OR headache* OR migraine* OR fibromyalgia* OR neuralgia* OR colic*) S3. AB (pain* OR headache* OR migraine* OR fibromyalgia* OR neuralgia* OR colic*) S4. TI (discomfort* OR ache OR aching OR aches) S5. AB (discomfort* OR ache OR aching OR aches) S6. S1 OR S2 OR S3 OR S4 OR S5 S7. MH "Crohn Disease" S8. TI (Crohn or Crohn*) S9. AB (Crohn or Crohn*) S10. TI (inflammatory bowel disease*) S11. AB (inflammatory bowel disease*) S12. TI ("regional enteritis" OR "regional ileitis" OR "terminal ileitis" OR "granulomatous enteritis" OR ileocolitis OR "granulomatous colitis") S13. AB ("regional enteritis" OR "regional ileitis" OR "terminal ileitis" OR "granulomatous enteritis" OR ileocolitis OR "granulomatous colitis") S14. S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S15. S6 AND S14 [Wong 2006 "CINAHL therapy studies" filter – best sensitivity version] S16. MH "prognosis+" OR MH "study design+" OR random* S17. S15 AND S16 |
| WHO ICTRP Search Portal (Standard search) |
| pain* AND Crohn* OR headache* AND Crohn* OR migraine* AND Crohn* OR colic* AND Crohn* OR pain* AND inflammatory AND bowel AND disease OR headache* AND Inflammatory AND bowel AND disease OR migraine* AND Inflammatory AND bowel AND disease OR colic* AND Inflammatory AND bowel AND disease OR pain* AND enteritis* OR headache* AND enteritis* OR migraine* AND enteritis* OR colic* AND enteritis* OR pain* AND ileitis* OR headache* AND ileitis* OR migraine* AND ileitis* OR colic* AND ileitis* OR pain* AND ileocolitis* OR headache* AND ileocolitis* OR migraine* AND ileocolitis* OR colic* AND ileocolitis* |
| ClinicalTrials.gov (Advanced search) |
|
Condition/ Disease: (Crohn OR Crohns OR Crohn´s OR “inflammatory bowel disease” OR “regional enteritis” OR “regional ileitis” OR “terminal ileitis” OR “granulomatous enteritis” OR ileocolitis OR “granulomatous colitis”) Other terms: (pain OR pains OR painful OR headache OR headaches OR migraine OR migraines OR fibromyalgia OR neuralgia OR colic OR colics) Study Type: Interventional Studies |
Data and analyses
Comparison 1. Low FODMAP diet versus sham diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain frequency defined by days of pain by IBS‐SSS scores (0 to 100) for IBD | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐15.86, 11.86] |
| 1.2 Days with moderate or severe pain by GSRS scores for IBD (0 to 3) for IBD | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.44, 1.24] |
| 1.3 Pain frequency defined by days of pain by IBS‐SSS scores (0 to 100) for CD | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐12.00 [‐114.55, 90.55] |
| 1.4 Pain intensity IBD (0 to 100) | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐8.46 [‐15.76, ‐1.16] |
| 1.5 Pain intensity IBD (0 to 3) | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐8.67, 9.07] |
| 1.6 Pain intensity CD (0 to 100) | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐8.00 [‐66.27, 50.27] |
| 1.7 Withdrawals due to adverse events | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.18, 19.19] |
Comparison 2. Medicine‐separated moxibustion combined with acupuncture versus wheat bran‐separated moxibustion combined with shallow acupuncture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Withdrawals due to adverse events | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
Comparison 3. Mindfulness with cognitive behavioural therapy (CBT) versus no treatment (both groups received standard medical therapy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pain frequency and severity of abdominal discomfort, severity of abdominal bloating, satisfaction with bowel habit, and impact of symptoms on life in general | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐37.00 [‐87.29, 13.29] |
| 3.2 Withdrawals due to adverse events | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
Comparison 4. soft non‐manipulative osteopathic treatment versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pain intensity | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐1.81, 1.83] |
| 4.2 Withdrawals due to adverse events | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
Comparison 5. Directed stress management versus standard treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Pain intensity | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐34.30 [‐61.99, ‐6.61] |
Comparison 6. Self‐directed stress management vs conventional therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Pain intensity | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐30.50 [‐58.45, ‐2.55] |
Comparison 7. Enteric‐release glyceryl trinitrate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Withdrawals due to adverse events | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 3.18 [0.94, 10.76] |
Comparison 8. 100 mg olorinab 3 times/day versus 25 mg olorinab 3 times/day.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Pain intensity | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.38, 1.15] |
| 8.2 Withdrawals due to adverse events | 1 | 14 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
Comparison 9. Relaxation training versus waitlist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Pain intensity | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.85, 0.41] |
Comparison 10. Web‐based education versus standard book‐based education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Pain intensity | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐1.25, 0.99] |
| 10.2 Withdrawals due to adverse events | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
Comparison 11. Yoga intervention versus no treatment (both groups received standard medical therapy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Withdrawals due to adverse events | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
Comparison 12. Transcranial direct current stimulation versus sham stimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Pain intensity (0 to 100) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐3.29, ‐0.01] |
| 12.2 Withdrawals due to adverse events | 1 | 20 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
Comparison 13. Kefir versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 CD pain intensity (0 to 3) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.67, ‐0.53] |
| 13.2 IBD pain intensity (0 to 3) | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 0.62 [0.17, 1.07] |
| 13.3 Withdrawals due to adverse events | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bao 2016.
| Study characteristics | ||
| Methods | RCT Setting: Shanghai Acupuncture Meridian Institute Medical Clinic Acupuncture Inflammatory Bowel Disease Specialist Clinic, Zhongshan Hospital Affiliated Endoscopic Center, and Yueyang Hospital, Shanghai University of Traditional Chinese Medicine Study period: January 2010 to December 2014 |
|
| Participants |
Inclusion criteria: 1. Patients with confirmed mild or moderate Crohn's disease (CDAI 151 ~ 350); 2. Patients who are not taking any drugs, or taking only salicylic acid drugs, and/or prednisone (dose < 15 mg and at least 1 month); 3. Patients who have not used immunosuppressants, anti‐tumour necrosis factor, or biologic within 3 months before entering the study; 4. Patients who agree and sign the informed consent form Exclusion criteria: 1. Patients during pregnancy or lactation; 2. Patients with serious heart, brain, liver, kidney, and haematopoietic system disease(s); 3. Patients with mental illness; 4. Patients with other serious diseases Age (mean ± SD): IG: 37 (15): CG: 33 (12) Sex (M/F): IG: 31/17; CG: 29/18 Site of disease: NS Use of concurrent medication: NS Disease duration (mean ± SD): IG: 4.7 (3.7) years; CG: 4.8 (4.4) years Disease activity: mild to moderate Number randomised: IG: 51; CG: 51 Number reaching end of study: IG: 48; CG: 47 Number analysed: IG: 48; CG: 47 Postrandomisation exclusion: IG: 3; CG: 4 ("Subjects who received less than 80% (29 times) of treatment units were considered to have dropped out") |
|
| Interventions |
IG: The observation group was treated with medicine‐separated moxibustion combined with acupuncture, both of which were performed simultaneously. CG: The control group was treated with wheat bran‐separated moxibustion combined with shallow acupuncture, both of which were performed simultaneously. |
|
| Outcomes |
Length of intervention: 12 weeks Primary outcomes: Abdominal pain frequency and intensity: Pain (extent, frequency, time) was evaluated using Traditional Chinese Medicine symptom scores which were divided into the following grades according to symptom severity: 0 (none), 1 (light), 2 points (moderate), 3 points (severe). Withdrawal due to adverse events Secondary outcomes: Serious adverse events Total adverse events |
|
| Notes | Funding source: NS Conflict of interest: NS Author contact details: wuhuangan@126.com Study was translated from Chinese by a translator. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. Authors were contacted but provided no response. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned. Authors were contacted but provided no response. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. Authors were contacted but provided no response. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were presented for all participants who completed the study; however, baseline values do not include dropouts. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are reported in the results. |
| Other bias | High risk | There are no declarations of conflicts of interest and funding, but the authors do not present a clear picture of the efficacy of acupuncture in their introduction. Also, the authors mention that there were no significant differences at baseline, but using a t‐test the baseline differences for gender are actually significant. |
Berrill 2014.
| Study characteristics | ||
| Methods | RCT Setting: multicentre, hospitals in the UK Study period: February 2011 to May 2012 The study presents results for the IBD cohort as a whole, does not separate between CD and UC. Where separate data do exist they are presented below. |
|
| Participants |
Inclusion criteria: age 18 to 65 years, diagnosis of UC or CD that was in remission based on a clinical index score and a C‐reactive protein level < 10 mg/L, and the presence of IBS‐type symptoms or a high perceived stress level Exclusion criteria: pregnancy, the presence of ileostomy or colostomy, previous colectomy, change in IBD medication (including use of steroids) within 3 months of study entry, change in psychotropic medication within 3 months of study entry, diagnosis of cognitive impairment, and previous psychological therapy Age (mean ± SD): IG: 44.4 +/‐ 11.7; CG: 45.4 +/‐ 10.6 Sex (M/F): IG: 8/25; CG: 7/26 Site of disease: CD:
UC:
Use of concurrent medication: IG: 5‐ASA 23; immunosuppressants 8; biologics 3 CG: 5‐ASA 22; immunosuppressants 13; biologics 0 Disease duration: NS Disease activity: remission. Clinical remission was assessed using SCCAI for UC and HBI for CD. Biochemical remission was assessed based on faecal calprotectin levels. Number randomised: IG: 33; CG: 33 (UC = 45, CD = 21) Number reaching end of study: IG: 16; CG: 28 Number analysed: ITT IG: 23; ITT CG: 28 PP IG: 16; PP CG: 30 Postrandomisation exclusion: IG: 14; CG: 1 |
|
| Interventions |
IG: multi‐convergent therapy combining mindfulness with cognitive behavioural therapy. The multi‐convergent therapy course consisted of 6 face‐to‐face sessions, each lasting 40 min, taking place over a 16‐week period. CG: no treatment Both groups received standard medical treatment throughout. |
|
| Outcomes |
Length of intervention: 12 months Primary outcomes: Pain frequency and intensity: Improvement on the IBS‐SSS, which includes frequency and severity of abdominal discomfort, severity of abdominal bloating, satisfaction with bowel habit, and impact of symptoms on life in general. Each domain is scored 0 to 100, with an overall score of 0 to 500 obtained. A higher score is indicative of more severe symptoms. Withdrawal due to adverse events Secondary outcomes: Anxiety/depression: measured on the HADS |
|
| Notes | Funding source: National Institute for Social Care and Health Research (NISCHR) Conflict of interest: Authors declare there are no conflicts of interest. Author contact details: jamesberrill1@doctors.org.uk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A blocked randomisation process using random permuted blocks of sizes 4 and 6 (selected at random) was generated by the South East Wales Trials Unit. |
| Allocation concealment (selection bias) | Low risk | The sequences were put into sequentially numbered, sealed, opaque envelopes for use in the clinic. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of who performed the assessments or if they were blinded. We contacted the author for this information but received no response. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High number of participants in the IG discontinued the study. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome is reported on, but most outcomes outlined in the methods were not presented in the results. We contacted the authors but received no response. |
| Other bias | Low risk | No conflicts of interest and well‐balanced baseline characteristics, despite age differences |
Cox 2020.
| Study characteristics | ||
| Methods | RCT, single‐blind, placebo controlled Setting: multicentre, 2 gastroenterology clinics in the UK Study period: February 2016 to May 2017 The study presents results for the IBD cohort as a whole, does not separate between CD and UC. Where separate data do exist they are presented below. |
|
| Participants |
Inclusion criteria: Eligible patients were 18 years of age, with quiescent CD or UC, experiencing ongoing gut symptoms and naïve to low FODMAP diet. Ongoing gut symptoms were required to meet the Rome III criteria for either diarrhoea predominant (IBS‐D), mixed subtype (IBS‐M), or unsubtyped IBS (IBS‐U), functional bloating, or functional diarrhoea, experiencing abdominal pain, bloating, and/or diarrhoea on 2 days during the baseline screening week and reporting inadequate relief of gastrointestinal symptoms. Exclusion criteria: Patients with dose changes of azathioprine, mercaptopurine, methotrexate, or biologics in the preceding 12 weeks; oral 5‐ASA in the preceding 4 weeks; or antibiotics, probiotics, or prebiotics in the preceding 8 weeks were excluded. Patients with pure perianal CD, a current stoma, previous extensive gastrointestinal resection, or a current stricture were excluded. Patients with established bile acid malabsorption were excluded because gut symptoms relating directly to bile acid malabsorption may not be modifiable by a low FODMAP diet. Patients with constipation‐predominant symptoms were excluded because these symptoms could be exacerbated by a low FODMAP diet. Patients with self‐reported lactose intolerance were included if they continued to experience gut symptoms despite low‐lactose diet. Patients were excluded if they had significant comorbidities, or if they were pregnant or lactating. Age (mean ± SD): IG: 33 (11); CG: 40 (13) Sex (M/F): IG: 10/17; CG: 13/12 Site of disease: IG: CD: ileal 4, colonic 4, ileocolonic 6; UC: proctitis: 6, left‐sided: 4, extensive: 3 CG: CD: ileal 2, colonic 4, ileocolonic 6; UC: proctitis: 3, left‐sided: 7, extensive: 3 Use of concurrent medication: IG: mesalamine 12, thiopurine 9, infliximab 10, adalimumab 2, vedolizumab 0, methotrexate 2 CG: mesalamine 11, thiopurine 12, infliximab 4, adalimumab 4, vedolizumab 1, methotrexate 1 Disease activity: quiescent. Quiescent IBD was defined by all of the following: physician global assessment, stable medications, no IBD flare in the previous 6 months, faecal calprotectin < 250 mg/g, and serum CRP < 10 mg/L. The threshold for faecal calprotectin was chosen according to evidence proposing optimal sensitivity and specificity for detecting endoscopically quiescent disease. Disease duration: IG: 7 years; CG: 11 years Number randomised: IG: 27 (UC: 13, CD: 14) CG: 25 (UC: 13, CD: 12) Number reaching end of study: (PP) IG: 24, IG: 22 Number analysed: (ITT) IG: 27, IG: 25 Postrandomisation exclusion: IG: 3 (1 withdrew consent, 1 antibiotics, 1 steroids); CG: 3 (1 withdrew consent, 1 pregnancy, 1 steroids) |
|
| Interventions |
IG: low FODMAP diet. The diet involves the restriction of dietary fructans, galacto‐oligosaccharides, lactose, fructose in excess of glucose, and polyols, including sorbitol and mannitol. CG: sham diet. The selection of an appropriate control group and difficulties in masking intervention and control are challenging in dietary intervention studies, but for research on dietary advice (which most closely mimics clinical practice), 'sham' dietary advice is considered gold standard. The sham diet in this trial aimed to provide participants in the control group with an exclusion diet of similar intensity and burden to a low FODMAP diet, whilst not affecting nutrient, fibre, or FODMAP intakes. Dietary counselling for both low FODMAP diet and sham diet participants lasted approximately 20 minutes, and both groups received written information. |
|
| Outcomes |
Length of intervention: 4 weeks Primary outcomes: Abdominal pain frequency and intensity: measured in days using the IBS‐SSS and in days where pain was reported as moderate or severe in GSRS at the final week of the diet (As the authors only presented SEM and not SD, we calculated the SD with the formula SD = SEM*√randomised participants for the measurements below) Withdrawal due to adverse events Secondary outcomes: Serious adverse events Total adverse events |
|
| Notes | Funding source: The study was funded by the Kenneth Rainin Foundation (Innovator and Breakthrough awards). The Kenneth Rainin Foundation had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. Conflict of interest: Authors Kevin Whelan and Miranda C Lomer are the co‐inventors of a mobile application to assist patients following the low FODMAP diet. Kevin Whelan has received consultancy fees from Danone, and a research grant from Clasado. The remaining authors have no conflicts to disclose. Author contact details: kevin.whelan@kcl.ac.uk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random allocation sequence was prepared online (www.sealedenvelope.com) by an independent researcher using block randomisation, with a 1:1 ratio of low FODMAP to placebo sham diet. Randomisation was stratified by diagnosis (CD or UC) and faecal calprotectin at screening (100 mg/g and 101 to 249 mg/g). |
| Allocation concealment (selection bias) | Low risk | Allocation sequences were sealed in opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Participants were blinded to diet allocation and informed that both diets would change the types of carbohydrates consumed, but that one was the diet under investigation, whereas the other was a sham diet. The terms 'fermentable carbohydrates,' 'low FODMAP diet,' or the mechanisms of the diet were not mentioned to participants." However, personnel were unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. We contacted the author for this information but received no response. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data are presented for all participants. |
| Selective reporting (reporting bias) | Low risk | Outcomes were presented as per trial registration and methods. |
| Other bias | Low risk | Authors have disclosed conflicts of interest. Baseline characteristics are balanced. |
Espi Lopez 2018.
| Study characteristics | ||
| Methods | RCT, single‐blind Setting: single centre, hospital in Spain Study period: May 2016 to June 2016 |
|
| Participants |
Inclusion criteria: aged between 18 and 62 years, diagnosed with CD at least 1 year before, and presenting abdominal pain and receiving usual treatment with an adequate diet Exclusion criteria: non‐specific inflammatory bowel pain, abdominal tenderness, infection, ischaemia, physical damage, specific immunologic sensitivity, pregnancy, or breastfeeding mothers Age (mean ± SD): IG: 42.56 +/‐ 10.09; CG: 40.14 +/‐ 12.32 Sex (M/F): IG: 8/8; CG: 2/12 Site of disease: NS Use of concurrent medication: NS Disease activity: Data not shown, even though authors mention the data had been measured and used for their analysis. Disease duration: NS Number randomised: IG: 16; CG: 14 Number reaching end of study: IG: 16; CG: 14 Number analysed: IG: 16; CG: 14 Postrandomisation exclusion: IG: 0; CG:0 |
|
| Interventions |
IG: Soft non‐manipulative osteopathic including soft tissue techniques over 30 days. Participants were treated once every 10 days. The sessions lasted 45 minutes and were conducted by a physiotherapist who had extensive experience in manual therapy. The intervention included 6 techniques: frontal‐occipital cranial technique, cranial temporal rotation technique, neuro‐lymphatic reflexes technique, viscerosomatic reflexes technique, myofascial induction technique, visceral technique. Following the intervention, participants remained in supine position with a neutral head and neck position for 10 minutes, in order to obtain relaxation and diminish tension after treatment. CG: The CG only came to the evaluation sessions and received no treatment besides that recommended by the doctor. |
|
| Outcomes |
Length of intervention: 30 days Primary outcomes: Abdominal pain intensity: Pain was assessed with the VAS, where participants marked their level of pain intensity on a 10‐centimetre horizontal line (0 = no pain to 10 = maximum pain) at the time the assessment was carried out. However, it is not clear if the VAS results were reported. Withdrawal due to adverse events Secondary outcomes: Serious adverse events Total adverse events |
|
| Notes | Funding source: NS Conflict of interest: The authors declare that they have no conflicts of interest. Author contact details: gemma.espi@uv.es |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated to 2 different groups through computer software by an external assistant who was blinded to the study objectives. |
| Allocation concealment (selection bias) | Low risk | Participants were randomly allocated to 2 different groups through computer software by an external assistant who was blinded to the study objectives. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not possible for this type of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 1 physiotherapist performed the assessments and was unaware of the groups to which participants belonged. To reduce bias, participants were instructed not to tell the physiotherapist about the treatment they had received. The statistician was also blinded to the goals of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reached study end |
| Selective reporting (reporting bias) | High risk | There is a lack of description of results in both text and table, and what is presented is unclear and different to what was originally planned. |
| Other bias | Low risk | The authors mention no significant difference between groups in pain baseline scores. There is no mention of funding, but authors declare no conflicts of interest. |
Garcia‐Vega 2004.
| Study characteristics | ||
| Methods | RCT Setting: Spain, single centre, Inflammatory Intestinal Disease Unit of Asturias Central Hospital Study period: NS |
|
| Participants |
Inclusion criteria: non‐active stage of the illness (HBI) and receiving pharmacological treatment with sulfasalazine (5‐ASA compounds), no dietary restrictions Exclusion criteria: dietary restrictions, as variability of these was considered to be a potential cause of error in the results Age (mean ± SD): IG: SM: 28.7 +/‐ 6.4 (19 to 52) IG: SDSM: 31 +/‐ 5.7 (22 to 40) CG: 35.3 +/‐ 9.1 (20 to 52) Sex (M/F): IG: SM: 5/10, SDSM: 5/10 CG: 6/9 Site of disease: NS Use of concurrent medication: NS Disease activity: non‐active. All participants had been diagnosed and treated in the Inflammatory Intestinal Disease Unit of Asturias Central Hospital (Spain). The evaluation was done by a physician on the basis of clinical history, physical examination, and radiological and laboratory tests. Disease duration: IG: SM: 5.6 +/‐ 6 (1 to 26 years) (text says 1 to 32 years range) CG: 8.2 +/‐ 5.7 (1 to 21 years) Number randomised: IG: SM: 15, SDSM: 15 CG: 15 Number reaching end of study: IG: SM: 13, SDSM: 14 CG: 13 Number analysed: IG: SM: 15, SDSM: 15 (2 groups) CG: 15 Postrandomisation exclusion: not clear 5 (2 SM, 1 SDSM, 2 CG) |
|
| Interventions |
IG 1: SM: Stress management group. The aim of this treatment was to provide the participant with effective techniques to mitigate the physiological effects of stress and tension, and to modify or improve his/her coping skills.
IG 2: SDSM: Self‐directed stress management group. This condition was similar to stress management, but the participant was his/her own therapist. Participants followed a self‐directed stress management programme according to a written guide on stress management procedures. Treatment of participants in the 2 experimental groups consisted of 8‐week individual sessions led by the same psychologist. The first 2 sessions were dedicated to the psychological evaluation of each participant in all 3 groups, establishment of the baseline symptomatology, and providing the participant basic information about gastrointestinal function. The remaining 6 sessions depended on the treatment group to which the participants had been allocated. It is unclear whether IG group 1 and 2 received conventional therapy as per the control group. There is no mention of any concurrent therapies or what the standard care protocol was. We wrote to the authors for clarity on this matter but received no response. CG: Participants in the control group received conventional medical treatment and did not receive any other special therapy. As mentioned above, participants attended the first and second visit to the therapist, at which they completed the semi‐structured interview and the symptoms self‐monitored in this period were recorded. |
|
| Outcomes |
Length of intervention: 8 weeks of intervention and follow‐up again at 6 and 12 months Primary outcomes: Pain intensity: average intensity symptom (AIS) = sum of the daily symptom intensities/number of symptomatic days Secondary outcomes: None reported |
|
| Notes | Funding source: NS Conflict of interest: NS Author contact details: elenagv@correo.uniovi.es |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors mention that sequence generation was random but do not explain how this was done. We contacted the author for this information but received no response. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. We contacted the author but received no response. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All patients were assessed and treated by the same gastroenterologist and the same psychologist", "The 6 month check‐up was carried out by a gastroenterologist who did not know to which experimental group the patient belonged", "The 12 month check‐up was carried out by the gastroenterologist and the psychologist" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The data presented are not for all randomised participants; the number of dropouts can be inferred, but more details are needed. We contacted the author for this information but received no response. |
| Selective reporting (reporting bias) | Unclear risk | The statistical analysis methods are presented in the results section; indices are presented instead of raw data; and there are no pain intensity results, no protocol, and methods are unclear. We contacted the author for clarification but received no response. |
| Other bias | High risk | There is a considerable baseline difference between the SM and SDSM groups for abdominal pain frequency. |
Hawkes 2001.
| Study characteristics | ||
| Methods | RCT, placebo controlled Setting: unstated (centres in the UK) Study period: November 1998 to November 2000 |
|
| Participants |
Inclusion criteria: Patients with a CDAI of > 150 and < 450 were identified after an initial interview and completion of a diary card for symptoms over 2 weeks. All participants were more than 16 years of age, with CD involving colon proximal to the rectosigmoid junction and/or distal small intestine (verified by colonoscopy, barium, or white cell scan studies). No participants were on maintenance doses of prednisolone greater than 20 mg, and all treatment, including 5‐ASA, azathioprine, and metronidazole, had remained unchanged for at least 6 weeks before entrance in the trial. Exclusion criteria: Previous surgical resection of ileum > 100 cm, the presence of an ileostomy, pouch, or colostomy, and a history of significant cardiac (especially hypertrophic obstructive cardiomyopathy, aortic or mitral valve stenosis, constrictive pericarditis or hypotensive episodes), respiratory, endocrine, renal, hepatic, neurological or psychiatric disease. Other exclusion criteria included significant coexisting gastrointestinal disease, anaemia, glaucoma, total parenteral nutrition, and patients already taking nitrates or calcium antagonists. Pregnancy, breastfeeding, participation in an interventional study within the past 3 months, or any factor likely to result in poor compliance with the study protocol were also reasons for exclusion. Age (mean ± SD): IG: 42.9 (15.1) CG: 35.8 (12.6) Sex (M/F): IG: 12/22 CG: 10/26 Site of disease: IG: small bowel only: 16, large bowel only: 3, both small and large bowel: 14, not known: 1 CG: small bowel only: 20, large bowel only: 2, both small and large bowel: 11, not known: 3 Use of concurrent medication: IG:
CG:
Disease activity: moderate‐severe CDAI of > 150 and < 450 verified by colonoscopy, barium, or white cell scan studies Disease duration IG: 10.9 (11.9) CG: 8 (7) Number randomised: IG: 34; CG: 36 Number reaching end of study: IG: 24; CG: 29 Number analysed: IG: 31; CG: 35 Postrandomisation exclusion: IG: 10 (headache 2, worsening clinical condition 4, generalised rash 1, mood change/irritability 1, loss of consciousness/memory 1, patient request 0, poor compliance 1) CG: 7 (headache 2, worsening clinical condition 1, generalised rash 0, mood change/irritability 0, loss of consciousness/memory 0, patient request 2, poor compliance 2) |
|
| Interventions |
IG: GTN (enteric‐release glyceryl trinitrate)
The study medication contained 3 mg of GTN dispersed in a polyglycolised triglyceride (Gelucire, Gattefosse) wax matrix, designed to produce both a delayed and sustained release of the drug. The matrix was contained in a size 1 hard gelatin capsule coated with Eudragit‐L (Rohm Pharma), an acrylic resin with an optimal dissolution at pH 6.8, which allowed the release of GTN to commence in the distal small bowel (pH 6.8) and continue through to the large bowel (pH 7.2). Those in the active treatment group were given GTN 6 mg twice daily for the first 6 weeks; if this was well tolerated, the dose was increased to 9 mg twice daily for the remaining 6 weeks. CG: placebo. "Identical placebo was used" |
|
| Outcomes |
Length of intervention: 12 weeks Primary outcomes: Abdominal pain intensity: scores represent the sum of the previous 7‐day diary card score: for pain, on a scale of 0 = no pain to 3 = severe pain Withdrawal due to adverse events Secondary outcomes: Serious adverse events Total adverse events |
|
| Notes | Funding source: The trial capsules were produced by MW Encap Ltd, Livingston, UK. Drs Hawkes and Richardson were supported by the Gastrointestinal Foundation Trust. Conflict of interest: NS Author contact details: (email contact is out of date, we could not find any other contact details for authors) drjohnrhodes@cardiff40.freeserve.co.uk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised according to a computer‐generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. Emails to the author went undelivered, and we could not identify any contact details for the other authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors mention that an identical placebo was used to ensure that both the trial investigator and participant remained blind to the treatment allocation; no further details were provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. Emails to the author went undelivered, and we could not identify any contact details for the other authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data are presented for all participants included in ITT. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in methods were reported in results and were appropriate for the trial. |
| Other bias | Unclear risk | The authors mention baseline differences for smokers, which could have affected the results. Emails to the author went undelivered, and we could not identify any contact details for the other authors. |
Higgins 2019.
| Study characteristics | ||
| Methods | RCT, open‐label parallel‐group, phase 2a Setting: unstated (multicentre, USA) Study period: NS |
|
| Participants |
Inclusion criteria: 18 to 80 years of age; diagnosed with CD for 3 months; quiescent to mild inflammation (simple endoscopic score CD < 10 or faecal calprotectin < 500 μg/g); average abdominal pain score 4 over 1 week; if taking concomitant biologic or anti‐inflammatory therapies for CD, must be on a stable dose Exclusion criteria: NS Age (mean ± SD): IG: 36.9 (15.2); 35 (10.8) Sex (M/F): IG: 4/4; CG: 2/4 Site of disease: IG: ileum: 7, colon: 5, rectum: 2, perianal: 2; CG: ileum: 3, colon: 4, rectum: 1, perianal: 1 Use of concurrent medication: IG: on active treatment: 7; CG: on active treatment: 5 Disease activity: quiescent (simple endoscopic score CD < 10 or faecal calprotectin < 500 μg/g) Disease duration (years ± SD): IG: 8.8 (8.9); CG: 15 (6.4) Number randomised: IG: 8; CG: 6 Number reaching end of study: IG: 8; CG: 6 Number analysed: IG: 8; CG: 6 Postrandomisation exclusion: IG: 3 (lost to follow‐up 1, withdrew consent 1, other 1); CG: 0 |
|
| Interventions |
IG: 100 mg olorinab 3 times/day CG: 25 mg olorinab 3 times/day |
|
| Outcomes |
Length of intervention: 8 weeks and follow‐up visit at week 10 Primary outcomes: Treatment success as defined by the authors:Efficacy endpoints included change in AAPS (average abdominal pain score) from baseline week (BL) to weeks 4 and 8, change in AAPS from pre‐dose to 1.5 hours postdose, proportion of clinical responders (≥ 30% reduction in weekly AAPS from BL), pain‐free days, and CD Patient‐Reported Outcome (CD‐PRO) scores. Abdominal pain intensity: measured at 1.5 hours postdose and as change in AAPS from BL to the time of peak concentration during Week 8 Withdrawal due to adverse events Secondary outcomes: Serious adverse events Total adverse events |
|
| Notes | Funding source: Arena Pharmaceuticals, Inc Conflict of interest: 1 of the authors is an employee of Arena Pharma. Medical writing assistance was provided by ApotheCom. Author contact details: bruce.yacyshyn@louisville.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS. Also, both groups using the same medication in different doses, which in a sense negates the control factor. We contacted the author but received no response. |
| Allocation concealment (selection bias) | Unclear risk | NS. We contacted the author for this information but received no response. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS. We contacted the author for this information but received no response. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data presented for all participants but in a confusing way. |
| Selective reporting (reporting bias) | Unclear risk | Data presented as 1 cohort at various points. The reporting of the results and adverse events is unclear and needs clarification. We contacted the author but received no response. |
| Other bias | High risk | An employee of Arena Pharmaceuticals is an author, also there are some differences between groups in disease duration at baseline. |
Mizrahi 2012.
| Study characteristics | ||
| Methods | RCT Setting: Hadassah Medical Center in Jerusalem Study period: NS The study presents results for the IBD cohort as a whole, does not separate between CD and UC. Where separate data do exist they are presented below. |
|
| Participants |
Inclusion criteria: (1) confirmed diagnosis of IBD for at least 6 months prior to recruitment; (2) age over 18 years; (3) suffering from an "active" disease according to the "Disease Activity Questionnaire", by meeting 1 of the following criteria: more than 5 bowel movements a day, more than 1 hospitalisation a year over the previous 2 years, and had either suffered a fistula during the previous year or was using corticosteroids; (4) provided informed consent; (5) fluent in the Hebrew language Exclusion criteria: (1) expected surgery in the following 2 months; (2) diagnosed as suffering from an active psychosis or from active major depression (due to the hazard of psychotic‐symptom‐abreactions this would be contraindicative for relaxation); (3) undergoing psycho‐pharmacotherapy (anti‐anxiety, antidepression, or antipsychotic); (4) already participating in another research study; (5) acquainted with and already practicing relaxation techniques Age (mean ± SD): IG: 35.56 (14.45); CG: 35.57 (12.76) Sex (M/F): IG: 9/9: CG: 13/8 Site of disease: NS Use of concurrent medication: IG: without medication 2, corticosteroids 3, 5‐ASA 13, immunosuppressive drugs 5, alternative treatment 5 CG: without medication 4, corticosteroids 1, 5‐ASA 12, immunosuppressive drugs 8, alternative treatment 10 Disease duration: NS Disease activity: active Number randomised: IG: 28; CG: 28 Number reaching end of study: IG: 18 (CD: 10, UC: 8); CG: 21 (CD: 14, UC: 7) Number analysed: IG: 18; CG: 21 Postrandomisation exclusion: 17 IG: 10 (medical reasons, time constraints, failing to return questionnaires) CG: 7 (failing to return questionnaires) |
|
| Interventions |
IG: Relaxation training. The intervention consisted of 3 individual relaxation‐training sessions at 2‐week intervals. Relaxation training with guided imagery served as the basis for the three 50‐minute treatment sessions. Each treatment session included: (1) a relaxation exercise with guided imagery, (2) a brief review of the relaxation monitoring forms, used to assess difficulties, and (3) a discussion of any problems the participant may have experienced whilst attempting to achieve relaxation. Each participant received an audio disc and was asked to continue to practice at home. Participants were advised to practice at least once a day during the 5‐week period of the study and to record the frequency of home practice in the provided log sheets. CG: Waitlist. The control participants on the waiting list were assessed at baseline and approximately 5 weeks later at the end of the waitlist period. Participants completed symptom‐monitoring diaries both at baseline and at the end of the trial. After completion of the diaries and following analysis of the collected data, the control participants were offered treatment in a group setting. |
|
| Outcomes |
Length of intervention: 5 weeks Primary outcomes: Pain intensity was measured using 10‐centimetre VAS. The time scale referred to the 24 hours prior to assessment. VAS measurements evaluate changes in a person’s subjective perception. The overall scores for each measurement ranged from 0 to 10, with a higher score reflecting a worse state as perceived by the participant. Secondary outcomes: Anxiety/depression |
|
| Notes | Funding source: NS Conflict of interest: NS Author contact details: erang@szmc.org.il |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. We contacted the authors but received no response. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. We contacted the authors but received no response. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. We contacted the authors but received no response. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were presented for all participants who completed the study; however, baseline characteristics do not include the participants who dropped out. |
| Selective reporting (reporting bias) | Low risk | All outcomes were presented in the results. |
| Other bias | High risk | "Statistically significant differences were found on the pain measurements between the groups at pre‐treatment, with the treatment group demonstrating higher levels of pain" |
Ozgursoy Uran 2019.
| Study characteristics | ||
| Methods | RCT Setting: single centre, gastroenterology unit in Turkey Study period: NS The study presents results for the IBD cohort as a whole, does not separate between CD and UC. Where separate data do exist they are presented below. |
|
| Participants |
Inclusion criteria: diagnosed with IBD at least 6 months ago, able to use computer, Internet, and mobile phone, 18 years of age and over Exclusion criteria: patients with advanced comorbid diseases such as cancer, diabetes, chronic obstructive pulmonary disease, hypertension were excluded from the study since symptoms, disease activity, and quality of life would be affected at a different level. Age (mean ± SD): IG: 37.26 (12.99) CG: 41.63 (11.85) Sex (M/F): IG: 17/13 CG: 18/12 Site of disease: NS Use of concurrent medication: NS Disease activity: mix of active and inactive; the Mayo Score was used for UC and the HBI for CD Disease duration: IG: n = 7 (less than 36 months); n = 12 (less than 71 months); n = 11 (greater than 72 months) CG: n = 10 (less than 36 months); n = 6 (less than 71 months); n = 14 (greater than 72 months) Number randomised: IG: 30 (UC: 16, CD: 14); CG: 30 (UC: 16, CD: 14) Number reaching end of study: IG: 30; CG: 30 Number analysed: IG: 30; CG: 30 Postrandomisation exclusion: IG: 0; CG: 0 |
|
| Interventions |
IG: 8 weeks of web‐based education about UC and CD CG: 8 weeks of standard book‐based education about UC and CD The content and scope of the web‐based and standard education programmes carried out with IBD patients were prepared by the researcher to be exactly the same in line with the literature. Definitions, anatomy, and physiology, indications, diagnostic tests, treatment principles, the importance of drug use, nutritional principles, and specific descriptions for special cases such as pregnancy, sexuality, and puberty are included in the content of the education. A website was designed for the web‐based education group, and all information was presented to the user with different interfaces. The standard education group received education via easy‐to‐read, illustrated, colour‐printed books. |
|
| Outcomes |
Length of intervention: 8 weeks Primary outcomes: Pain intensity was measured using VAS scoring system. Participants were asked to rate symptoms experienced in the last 3 months as "0 None" and "10 unendurable". Any reduction in scores was considered a success Withdrawal due to adverse events Secondary outcomes: Serious adverse events |
|
| Notes | Funding source: The authors declare no financial support. Conflicts of interest: The authors declare no conflicts of interest. Author contact details: bernanilgun@gmail.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to author correspondence, randomisation was done using a simple stratified randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. We contacted the authors but received no response. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | According to author correspondence, the analysis was done by a blinded biostatistics specialist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes are presented in the results. |
| Selective reporting (reporting bias) | Low risk | All outcomes are presented in the results. |
| Other bias | Low risk | No conflicts of interest, and baseline characteristics were balanced between groups |
Sharma 2015.
| Study characteristics | ||
| Methods | RCT Setting: single centre, All India Institute of Medical Science (AIIMS), New Delhi, India Study period: 2004 to 2008 |
|
| Participants |
Inclusion criteria: Only patients between 16 and 60 years of age who were in the clinical remission phase of the disease were included in the study. UC and CD activity was assessed using the criteria of Truelove and Witts (1955) and the CDAI (Best and colleagues, 1976), respectively. The inclusion criteria for UC patients in the remission phase were (a) 1 or 2 stools a day without blood, (b) no fever, (c) no tachycardia, (d) haemoglobin normal or returning towards normal, and (e) ESR normal or returning towards normal. Patients with a CDAI score < 150 were considered to be in remission. Exclusion criteria: (a) IBD patients with other chronic diseases such as diabetes mellitus, hypertension, or cardiovascular disease, (b) any condition known to affect the cardiovascular autonomic functions such as chronic alcoholism or smoking, (c) patients who have undergone any surgical intervention for IBD, (d) pregnant women, (e) patients on any drug regimen affecting autonomic functions, (f) patients on psychiatric medication, and (g) patients who had practiced yoga within at least 1 year preceding the study Age (mean ± SD): NS Sex (M/F): NS Site of disease: NS Use of concurrent medication: NS "There were no significant differences between the medication used by the yoga and control groups" Disease activity: clinical remission phase The diagnosis of UC was established on the basis of clinical evidence of large bowel diarrhoea, haematochezia and tenesmus; endoscopic evidence of diffuse pattern of involvement of the gastrointestinal mucosa characterised by loss of vascular pattern, erythema, friability, or ulcerations; and histological evidence. The diagnosis of CD was established on the basis of the presence of characteristic clinical manifestations (chronic diarrhoea, haematochezia, abdominal pain, and intestinal obstructive manifestations), endoscopic features (skip lesion, asymmetrical involvement, deep ulcers, ileocaecal valve involvement, and terminal ileum involvement), together with histological evidence (acute or chronic colitis, presence of inflammation extending beyond muscularis mucosa, lymphoid follicles, and noncaseating granulomas). The involvement of the small intestine was assessed by barium meal follow‐through, small bowel enema, and/or retrograde ileoscopy. Disease duration: NS Number randomised: IG: 50 (UC: 30, CD: 20) CG: 50 (UC: 30, CD: 20) Number reaching end of study: IG: UC: 25, CD: 19 CG: UC: 26, CD: 17 Number analysed: IG: 44 (UC: 25, CD: 19) CG: 43 (UC: 26, CD: 17) Postrandomisation exclusion: IG: UC: 5 (relocation to another city 1, pregnancy 2, increased disease activity 1, lost contact 1) CD: 1 (bone fracture) CG: UC: 4 (increased disease activity 2, relocation to another city 1, started alternative therapy 1), CD: 3 (lost contact 1, busy schedule 1, increased disease activity 1) |
|
| Interventions |
IG: yoga intervention. This was comprised of physical postures, pranayama (controlled breathing), and meditation. The supervised yoga intervention (1 week for 1 hour daily) was given under the guidance of a certified yoga trainer. Due to feasibility reasons, the supervised yoga training was provided for 1 week (each session for 1 hour) followed by a daily practice at home continuously over 2 months (1 hour daily). All participants continued standard medical treatment. A single yoga session was offered individually to participants during the follow‐up visits. During the home practice sessions, participants listened to the audio recording for relaxation; an instruction manual on different postures was also provided to all participants. CG: no treatment. The standard pharmacological treatment was used by all participants for maintenance of disease remission in both groups. All participants were treated with maintenance doses of mesalamines and azathioprine, along with multivitamins and calcium supplements. Telephone support was provided to both groups to motivate a high degree of compliance. |
|
| Outcomes |
Length of intervention: 8 weeks (outcomes recorded at baseline, 1 month, 2 months) Primary outcomes: Treatment success as defined by the authors: change from presence to absence of pain Participants were given a symptom diary at the beginning of the study in which they were asked to record the presence or absence of clinical symptoms. In accordance with the wide range of proposals for indices of clinical activity of IBD, the following symptoms were considered: blood, tenesmus, intestinal colic, perianal pain, arthralgia, and anorexia. Participants were asked to fill the self‐report form once per day before going to bed. Withdrawal due to adverse events Secondary outcomes: Serious adverse events Total adverse events |
|
| Notes | Funding source: Central Council for Research in Yoga and Naturopathy (CCRYN), New Delhi, India Conflict of interest: The authors declare that they have no competing interests. Author contact details: purnimareceives@gmail.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group assignment was determined by a randomisation scheme devised from computer‐generated random number tables. The tables were prepared by other researchers who were not involved in the study. |
| Allocation concealment (selection bias) | Low risk | The randomisation schedule was concealed in sequentially numbered, sealed, opaque envelopes. Participants were randomised by the research assistant. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. We contacted the author but received no response. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported for all participants reaching end of study. |
| Selective reporting (reporting bias) | Unclear risk | No pain results for CD reported. All other outcomes mentioned in the methods were reported. We contacted the author but received no response. |
| Other bias | Low risk | No conflicts of interest, no differences at baseline |
Tapete 2018.
| Study characteristics | ||
| Methods | RCT Setting: NS, Italy Study period: 2017 The study presents results for the IBD cohort as a whole, does not separate between CD and UC. Where separate data do exist they are presented below. |
|
| Participants |
Inclusion criteria: clinical remission (CDAI < 150 for CD and full Mayo < 3 for UC) with normal values of ESR, CRP, white blood cells and neutrophil count, but with residual intestinal symptoms (abdominal pain, bloating, and abnormal stool consistency ‐ Bristol stool classification > 5) Exclusion criteria: NS Age (mean ± SD): NS Sex (M/F): NS Site of disease: NS Use of concurrent medication: NS Disease activity: inactive Disease duration: NS Number randomised: IG: 10; CG: 20 Number reaching end of study: NS Number analysed: NS Postrandomisation exclusion: NS |
|
| Interventions |
IG: low FODMAP diet CG: sham control diet |
|
| Outcomes |
Length of intervention: 6 to 8 weeks Primary outcomes: Abdominal pain intensity measured on a 0‐to‐10‐centimetre VAS. Secondary outcomes: None reported |
|
| Notes | Funding source: NS Conflict of interest: NS Author contact details: g.tapete@studenti.unipi.it |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS. We contacted the author but received no response. |
| Allocation concealment (selection bias) | Unclear risk | NS. We contacted the author but received no response. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS. We contacted the author but received no response. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | More information is needed. We contacted the author but received no response. |
| Selective reporting (reporting bias) | Low risk | The outcomes mentioned in the methods are reported in the results. |
| Other bias | Low risk | No apparent sources of bias; conflicts of interest are not clear, but not downgraded |
Tapete 2019.
| Study characteristics | ||
| Methods | RCT, cross‐over Setting: NS, Italy Study period: NR The study presents results for the IBD cohort as a whole, does not separate between CD and UC. Where separate data do exist they are presented below. |
|
| Participants |
Inclusion criteria: UC or CD with normal inflammatory parameters (CRP, white blood cells) and no clinical activity (CDAI < 150 for CD and a full Mayo < 2 for UC) but with residual abdominal symptoms (abdominal pain, diarrhoea, bloating) Exclusion criteria: patients with abdominal abscess, fistula, intestinal active bleeding, or extra‐intestinal manifestations Age (mean ± SD): total cohort: 43.9 (17) years total Sex (M/F): total cohort: 28/22 total Site of disease: NS Use of concurrent medication: All participants were being treated with intravenous biologic therapy (infliximab or vedolizumab). Disease activity: inactive Disease duration: NS Number randomised: NS per IG/CG, 50 in total (CD: 25, UC: 25) Number reaching end of study: NS per IG/CG, 47 in total Number analysed: NS per IG/CG, 47 total Postrandomisation exclusion: 3 |
|
| Interventions |
IG: low FODMAP diet CG: high FODMAP/normal diet |
|
| Outcomes |
Length of intervention: 8 weeks cross‐over (4 weeks per arm) Primary outcomes: Abdominal pain intensity was measured on a 0‐to‐10‐centimetre VAS at the beginning, at 4 weeks (end of first cross‐over arm), and at 8 weeks (end of second cross‐over arm); it was unclear if this measured pain frequency or intensity or what range was used. Secondary outcomes: None reported |
|
| Notes | Funding source: NS Conflict of interest: NS Author contact details: g.tapete@studenti.unipi.it |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NS. We contacted the author but received no response. |
| Allocation concealment (selection bias) | Unclear risk | NS. We contacted the author but received no response. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS. We contacted the author but received no response. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Cohort presented as a whole, more information is needed. We contacted the author but received no response. |
| Selective reporting (reporting bias) | Unclear risk | The outcomes mentioned in methods are reported in the results, but there are no pre‐cross‐over data. We contacted the author for this information but received no response. |
| Other bias | Unclear risk | There is a difference in baseline pain levels, and conflicts of interest are not clear. We contacted the author but received no response. |
Volz 2016.
| Study characteristics | ||
| Methods | RCT, sham‐controlled Setting: single centre, Medical Department I (Gastroenterology, Infectious Diseases, Rheumatology) of the Charite‐Campus Benjamin Franklin, Germany Study period: January 2014 to December 2016 The study presents results for the IBD cohort as a whole, does not separate between CD and UC. Where separate data do exist they are presented below. |
|
| Participants |
Inclusion criteria: (1) age between 18 and 80 years; (2) patients with a diagnosis of IBD (verified by medical report, discharge letter, and outpatient centre records); (3) patients with chronic abdominal pain, defined as duration of 3 months in the past 6 months; (4) patients with a pain intensity of 3/10 on the VAS Exclusion criteria: (1) had additional severe or untreated internal, neurological, or psychiatric disorders; (2) had ongoing drug/substance abuse; (3) or were pregnant and/or breastfeeding. Due to ethical considerations, participants were allowed to stay on anti‐inflammatory drugs and acute pain medication. Age (mean ± SD): IG: 40.6 (12.5) CG: 34.4 (13.2) Sex (M/F): IG: 3/7 CG: 4/6 Site of disease: NS Use of concurrent medication: IG: other anti‐inflammatory drugs 8, pain medication ‐ regular intake 7 CG: other anti‐inflammatory drugs 8, pain medication ‐ regular intake 7 Disease activity: active SCCAI or HBI (not clear which applies to which group): 7.3 (3.3) for IG and 9 (3.6) for CG Disease duration: IG: 10 (8.9) CG: 7 (4.7) Number randomised: IG: 10 (CD: 7, UC: 3); CG: 10 (CD: 7, UC: 3) Number reaching end of study: IG: 10; CG: 10 Number analysed: IG: 10; CG: 10 Postrandomisation exclusion: IG: 0; CG: 0 |
|
| Interventions |
IG: Transcranial direct current stimulation was applied over 5 consecutive days. It was administered through saline‐soaked surface sponge electrodes (35 cm²) and delivered by a battery‐driven constant‐current stimulator (TCT Research Limited, Hong Kong, China). Participants received anodal stimulation for 20 minutes. CG: Sham stimulation. In sham stimulation, the current flow was ramped down after 30 seconds. A constant current of 2 mA in intensity was applied. |
|
| Outcomes |
Length of intervention: 5 days of intervention plus follow‐up after 1 week Primary outcomes: Pain intensity was measured with VAS pain scores. IG average pain in the last 6 months, mean VAS (0 to 10) (SD): 5.8 (1.43) IG average pain in the last 6 months, mean VAS (0 to 10) (SD): 6.3 (1.3) IG baseline: VAS, (0 to 10) (SD): 4.85 (1.7) CG baseline: VAS, (0 to 10) (SD): 4.55 (1.6) IG end of study: VAS, (0 to 10) (SD): 2.8 (2.3) IG VAS 1‐week follow‐up: difference of −1.44 (2.7) from baseline CG end of study: VAS, (0 to 10) (SD): 4.45 (1.3) CG VAS 1‐week follow‐up: difference of +0.28 (2.2) from baseline Withdrawal due to adverse events: IG: 0; CG: 0 Secondary outcomes: Total adverse events |
|
| Notes | Funding source: This study has been supported by the grant “Patienten orientier te Forschung bei CED 2014” of the “Deutsche Morbus Crohn/Colitis ulcerosa Vereinigung e.V.” (DCCV e.V.) commissioned to Magdalena Sarah Pruss geb. Volz. Conflict of interest: The authors have no conflicts of interest to declare. Author contact details: magdalena.pruess@charite.de |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the unblinded researcher (AF) in blocks of 4 generated from a computer‐based random allocation. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. We contacted the author but received no response. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the participant and the researcher were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both the participant and the assessor (who was the researcher) were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full data are provided for every participant. |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported clearly. |
| Other bias | Low risk | No conflicts of interest, and no significant differences at baseline |
Yilmaz 2019.
| Study characteristics | ||
| Methods | RCT Setting: unstated (single centre, Turkey) Study period: May 2015 to December 2016 |
|
| Participants |
Inclusion criteria: Patients with IBD participated in the study. CDAI was used for CD, and Truelove‐Witts scoring systems were used for UC for disease assessment scores (10‐11). Patients with CD whose CDAI score was < 450 were admitted to the study. Patients with UC whose Truelove‐Witts score was severe were not admitted to the study. Participants also had to be > 18 years old. Exclusion criteria: Patients with alcohol consumption > 20 g/day, allergies or intolerance to milk, antibiotic treatment within the last 1 month, column or bowel operation history up to 3 months before the start of the study, and the presence of active infection within 1 month prior to the start of the study or during the study were excluded. In addition, if a participant requested to leave on his/her own will, or if kefir was not consumed continuously for 2 weeks, the trial protocol was assessed and was not approved. Age (mean (range)): IG CD: 33 (24 to 65) CG CD: 42 (21 to 66) IG UC: 33 (19 to 68) CG UC: 43.5 (29 to 76) Sex (M/F): IG CD: 4/6 CG CD: 6/4 IG UC: 9/6 CG UC: 4/6 Site of disease: IG CD: colon: 1; ileum: 6; colon + ileum: 3 CG CD: colon: 0; ileum: 10; colon + ileum: 0 IG UC: colon: 15; ileum: 0; colon + ileum: 0 CG UC: colon: 10; ileum: 0; colon + ileum: 0 Use of concurrent medication: NS Disease activity: inactive to moderate Disease duration: IG CD: 2 (1 to 9) years CG CD: 2 (1 to 10) years IG UC: 4 (1 to 12) years CG UC: NS Number randomised: IG: 28 CG: 20 Number reaching end of study: IG CD: 10 CG CD: 10 IG UC: 15 CG UC: 10 Number analysed: IG CD: 10 CG CD: 10 IG UC: 15 CG UC: 10 Postrandomisation exclusion: IG: 3 (did not want to drink kefir); CG: 0 |
|
| Interventions |
IG: 400 mL/day kefir was administered twice a day to participants for 4 weeks, which contains a total of 2.0 × 10¹⁰ colony‐forming units/mL viable Lactobacillus bacteria. CG: no placebo or other intervention |
|
| Outcomes |
Length of intervention: 4 weeks Primary outcomes: Pain intensity: Participants were asked to fill out the symptoms diary, which included questionnaires about bowel habits. Abdominal pain was rated on a 4‐point scale: 0 = none, 1 = mild, 2 = moderate, 3 = severe. The results were received after contact with the author Secondary outcomes: None reported |
|
| Notes | Funding source: The authors declared that this study received no financial support. Conflict of interest: The authors have no conflicts of interest to declare. Author contact details: ilkayilmaz001@hotmail.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We contacted the author, who responded that randomisation was determined via a computer. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. We requested further information from the author but did not receive a response. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | We contacted the author, who responded that the outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data are presented for all completers. |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported in the results, and scores were provided to us by the author. |
| Other bias | Low risk | The authors report no conflicts of interest, and the baseline characteristics appear to be reasonably balanced, although this is not mentioned in the text. |
5‐ASA: 5‐aminosalicylic acid
CD: Crohn’s disease
CDAI: Crohn’s Disease Activity Index
CG: control group
CRP: C‐reactive protein
ESR: erythrocyte sedimentation rate
FODMAP: fermentable oligo‐, di‐, monosaccharides and polyols
GSRS: Gastrointestinal Symptom Rating Scale
HADS: Hospital Anxiety and Depression Scale
HBI: Harvey‐Bradshaw index
IBD: inflammatory bowel disease
IBS: irritable bowel syndrome
IBS‐SSS: Irritable Bowel Syndrome Severity Scoring System
IG: intervention group
ITT: intention‐to‐treat
NS: not stated
PP: per protocol
RCT: randomised controlled trial
SCCAI: Simple Clinical Colitis Activity Index
SD: standard deviation
SEM: standard error of the mean
SS: sum of squares
UC: ulcerative colitis
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12617000876392 | Wrong outcomes |
| Engel 2016 | Wrong outcomes |
| Forbes 2019 | Wrong study design |
| Gearry 2009 | Wrong study design |
| ISRCTN98226923 | Wrong indication |
| McCormick 2010 | Not an RCT |
| Spagnuolo 2017 | Not an RCT. According to the author no randomisation was performed; consecutive patients were enrolled and, alternating one by one, patients were placed in the experimental and control arm. |
| Tripp 2017 | Wrong outcomes |
RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Bao 2021.
| Methods | Study design: RCT Setting: China |
| Participants | 66 CD patients |
| Interventions | IG: acupuncture with moxibustion CG: sham‐acupuncture with sham‐moxibustion |
| Outcomes | The score of the hospital anxiety‐depression scale (HADS) and the score of intestinal core symptoms (degree of abdominal pain and frequency of diarrhea) were observed in the patients of the two groups. The concentration of plasma indoleamine 2,3‐dioxygenase 1 (IDO1) and the ratios of Kyn/Trp, QuinA/Kyn, KynA/Kyn and KynA/QuinA were compared between the two groups. |
| Notes | This study was identified on our updated search and will be included in the next update of this review |
IRCT20120415009475N5.
| Methods | Study design: RCT, triple‐blind, placebo controlled Setting: Iran |
| Participants | 60 children |
| Interventions | IG: 2 capsules of 250 mg Saccharomyces boulardii per day for 2 months CG: 2 placebo capsules per day for 2 months |
| Outcomes | Start date: 6 March 2018 Estimated completion date: 23 September 2018 Outcomes:
|
| Notes | Funding: Shahid Beheshti University of Medical Sciences Contact: mirrahimi@sbmu.ac.ir We received no response from the author and were therefore unable to determine whether this study meets our inclusion criteria. |
IRCT20200219046553N1.
| Methods | Study design: RCT Setting: Iran |
| Participants | 50 IBD patients 18‐60 years old |
| Interventions | IG: mindfulness‐based cognitive therapy in addition to their usual medical treatment CG: usual medical treatment |
| Outcomes | Quality of Life, Depression, Anxiety, Stress, Severity of pain, pain catastrophizing, Dispositional mindfulness and Disease activity |
| Notes | This study was identified on our updated search and will be included in the next update of this review |
JPRN‐UMIN000012635.
| Methods | Study design: RCT Setting: Japan |
| Participants | 30 participants aged between 16 and 70 years old |
| Interventions | IG: oral administration of Daikenchuto 15 g/day for 24 weeks in addition to ordinary treatment for Crohn's disease CG: ordinary treatment for Crohn's disease |
| Outcomes | Date of first enrolment: 25 December 2013 Estimated completion date: NS Outcomes: Primary
Secondary
|
| Notes | Funding: Kurume University, Tsumura & Co. Contact: nina@med.kurume‐u.ac.jp We received no response from the author and were therefore unable to determine whether this study meets our inclusion criteria. |
Lee 2021.
| Methods | Study design: RCT Setting: USA |
| Participants | 40 CD participants |
| Interventions | IG: hypnosis CG: wait‐list |
| Outcomes | The primary outcome was patient‐ and parent‐reported QoL; secondary outcomes were patient‐reported abdominal pain, depression, anxiety, and sleep; school absences; and disease activity by Pediatric Crohn's Disease Activity Index |
| Notes | This study was identified on our updated search and will be included in the next update of this review |
Leiby 2014.
| Methods | Study design: RCT Setting: USA |
| Participants | 12 patients 11 to 17 years old |
| Interventions | IG: 12 weeks of yoga at 3 months of diagnosis + standard therapy CG: yoga at 6 months of diagnosis + standard therapy |
| Outcomes | Starting date: NS Estimated completion date: NS Outcomes: HRQoL (PedsQL total score) and self‐efficacy using questionnaires |
| Notes | Funding: NS Contact: 001(973)‐971‐5676 001(908)‐522‐8714 We received no response from the author and were therefore unable to determine whether this study meets our inclusion criteria. |
NCT00940576.
| Methods | Study design: RCT, cross‐over Setting: Germany |
| Participants | 15 children and 2 adults |
| Interventions | Oral intake of 250 mL per day mare's milk first, then 250 mL per day placebo |
| Outcomes | Starting date: 16 July 2009 Last update: 25 May 2015 Outcomes:
|
| Notes | Funding: University of Jena Contact: gerhard.jahreis@uni‐jena.de We received no response from the author and were therefore unable to determine whether this study meets our inclusion criteria. |
NCT02559037.
| Methods | Study design: RCT Setting: China |
| Participants | 66 participants 16 to 70 years old |
| Interventions | IG: acupuncture and moxibustion CG: sham acupuncture and moxibustion |
| Outcomes | Starting date: 1 April 2015 Estimated completion date: 30 November 2019 Outcomes: Primary: the proportion of participants with clinical remission [ Time Frame: Week 12 ] Defined as CDAI < 150 and decrease > 70 Secondary: the proportion of participants with clinical remission [ Time Frame: Week 24, 36, and 48 ] Defined as CDAI < 150 and decrease > 70 |
| Notes | Funding: Shanghai Institute of Acupuncture, Moxibustion and Meridian Contact: wuhuangan@126.com We received no response from the author and were therefore unable to determine whether this study meets our inclusion criteria. |
NCT02649075.
| Methods | Study design: RCT Setting: multicentre |
| Participants | 7 adults |
| Interventions | IG: serum‐derived bovine immunoglobulin (SBI) 10 g twice daily compared to matching placebo for 12 weeks followed by a 12‐week open‐label extension SBI 10 g twice daily CG: placebo |
| Outcomes | Starting date: February 2016 Estimated completion date: August 2017 Outcomes: Primary
Secondary
|
| Notes | Funding: Entera Health, Inc Contact: Audrey.Shaw@enterahealth.com Our email went undelivered, and our further attempts to identify contact information were unsuccessful, therefore we were unable to determine whether this study meets our inclusion criteria. |
NCT02963246.
| Methods | Study design: RCT Setting: Spain |
| Participants | 60 adults |
| Interventions | IG: mindfulness intervention (12 months) CG: treatment‐as‐usual |
| Outcomes | Starting date: 5 May 2017 Actual completion date: 14 March 2018 Outcomes: Primary
Secondary
|
| Notes | Funding: Cardenal Herrera University Contact: jose.soria@uchceu.es We received no response from the author and were therefore unable to determine whether this study meets our inclusion criteria. |
NCT02974322.
| Methods | Study design: RCT Setting: NS |
| Participants | 0 |
| Interventions | IG: GED‐0301 1 x 160 mg tablet once daily and GED‐0301 4 x 40 mg tablets once daily CG: placebo once daily |
| Outcomes | Starting date: 1 December 2017 Estimated completion date: abandoned Outcomes: Primary
Secondary
|
| Notes | Funding: Celgene Contact: support@vivli.org This study was never begun. No patients were enrolled (or even screened), so there are no results to share. |
NCT03155945.
| Methods | Study design: RCT Setting: NS |
| Participants | 14 adults |
| Interventions | IG: APD371 low‐dose treatment CG: APD371 high‐dose treatment |
| Outcomes | Starting date: 18 May 2018 Actual completion date: 5 September 2018 Outcomes: Primary
Secondary
|
| Notes | Funding: Arena Pharmaceuticals Contact: ct.gov@arenapharm.com The sponsor is unable to provide any information beyond what is already in the public domain. Insufficient information to determine whether this study meets our inclusion criteria. |
NCT03467620.
| Methods | Study design: RCT Setting: NS |
| Participants | 0 |
| Interventions | IG: 25 mg capsule of cannabidiol (CBD) per day for 12 weeks CG: 1 placebo capsule per day for 12 weeks |
| Outcomes | Starting date: July 2018 Estimated completion date: withdrawn (inadequate funding) Outcomes: Primary
Secondary
|
| Notes | Funding: University of Illinois at Chicago Contact: kgeary3@uic.edu |
Neeb 2019.
| Methods | Study design: phase III, placebo‐controlled RCT Setting: Germany |
| Participants | 36 adults |
| Interventions | IG: transcranial direct current stimulation for 5 days CG: placebo |
| Outcomes | Outcomes:
|
| Notes | Funding: This study has been supported by the grant “Patientenorientierte Forschung bei CED 2014” of the “Deutsche Morbus Crohn /Colitis ulcerosa Vereinigung e.V.” (DCCV e.V.) commissioned to Magdalena Sarah Prüss. Contact: magdalena.pruess@charite.de We received no response from the author and were therefore unable to determine whether this study meets our inclusion criteria. |
NTR3414.
| Methods | Study design: RCT Setting: the Netherlands |
| Participants | 80 participants > 11 years old |
| Interventions | IG: 6 sessions of gut‐directed hypnotherapy CG: 6 sessions of standard medical treatment with supportive therapy |
| Outcomes | Starting date: 1 March 2012 Estimated completion date: 1 September 2013 Outcomes: Primary: the number of participants with > 50% reduction in IBS‐SSS pain score Secondary: the effects of therapy on total IBS‐SSS score, adequate relief, HRQoL, IBD disease activity, health utility index, depression, anxiety, somatisation, abdominal pain‐related cognitions, absence of school or work, use of healthcare resources and additional costs, use of IBD medication, colonic sensitivity to distension, faecal protease activity and microbiota and the ability of participant's faecal supernatant to induce colonic hypersensitivity to distension in rats by colonic infusion |
| Notes | Funding: ZonMw, The Netherlands Organisation for Health Research and Development Contact: d.r.hoekman@amsterdamumc.nl The authors are in the process of publishing data from this trial, with publication expected in 2020. Unfortunately, they were unable to provide any reports prior to publication. We could not determine whether this study meets our inclusion criteria. |
Schoultz 2013.
| Methods | Study design: RCT, exploratory Setting: Scotland |
| Participants | 40 adults |
| Interventions | IG: 16 h of structured group training over an 8‐week period CG: 16 h of structured group training over an 8‐week period, 6 months later than the intervention group |
| Outcomes | Primary outcomes: recruitment, completion/retention rates and adherence and adaptation to the mindfulness‐based cognitive therapy manual for IBD patients Secondary outcome: to assess the feasibility of collecting reliable and valid data on proposed outcome measures such as quality of life, anxiety, depression, disease activity, and mindful awareness |
| Notes | Funding: This project is funded by University of Stirling, NHS Highland, and Crohn’s and Colitis UK. Contact: ms84@stir.ac.uk We received no response from the author and were therefore unable to determine whether this study meets our inclusion criteria. |
Schoultz 2015.
| Methods | Study design: RCT, pilot study Setting: Scotland |
| Participants | 44 adults |
| Interventions | IG: 16 hours of structured group training over 8 consecutive weeks plus guided home practice and follow‐up sessions. CG: The waitlist group received a leaflet entitled ‘Staying well with IBD’. |
| Outcomes | The key objectives were to assess patient eligibility and recruitment/dropout rate; to calculate initial estimates of parameters to the proposed outcome measures (depression, anxiety, disease activity, dispositional mindfulness and quality of life); and to estimate sample size for a future large RCT. |
| Notes | Funding: This project is funded by University of Stirling, NHS Highland, and Crohn’s and Colitis UK. Contact: ms84@stir.ac.uk We received no response from the author and were therefore unable to determine whether this study meets our inclusion criteria. |
Takakura 2020.
| Methods | Study design: RCT |
| Participants | 33 IBD participants |
| Interventions | IG: proactive pain protocol CG: standard‐of‐care reactive pain regimen (as‐needed acetaminophen and opioids) |
| Outcomes | Outcomes included daily pain (assessed by numeric rating scores, 0‐10), average daily morphine milligram equivalents (MME), length of stay (LOS), need for surgery during admission, and 30‐day readmission rates |
| Notes | This study was identified on our updated search and will be included in the next update of this review |
CDAI: Crohn’s Disease Activity Index
CG: control group
CRP: C‐reactive protein
HRQoL: health‐related quality of life
IBDQ: Inflammatory Bowel Disease Questionnaire
IBD: inflammatory bowel disease
IBS‐SSS: Irritable Bowel Syndrome Severity Scoring System
IG: intervention group
MRI: magnetic resonance imaging
mRNA: messenger ribonucleic acid
NS: not stated
PCDAI: Pediatric Crohn's Disease Activity Index
PedsQL: Pediatric Quality of Life Inventory
RCT: randomised controlled trial
SF‐36: 36‐item Short Form Health Survey
VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
ISRCTN71618461.
| Study name | A supported online self‐management for symptoms of fatigue, pain and urgency/incontinence in people with inflammatory bowel disease: the IBD‐BOOST trial |
| Methods | RCT, multicentre |
| Participants | 680 adults 18 and above |
| Interventions | IG: facilitator‐supported online self‐management CG: care as usual |
| Outcomes | Primary: UK Inflammatory Bowel Disease Questionnaire (UK‐IBDQ) and global rating of symptom relief at 6 months after randomisation Secondary:
|
| Starting date | 1 October 2019 |
| Contact information | christine.norton@kcl.ac.uk jonathan.syred@kcl.ac.uk |
| Notes | Sponsor: London North West University Healthcare NHS Trust |
NCT03301311.
| Study name | Personalized research on diet in ulcerative colitis and Crohn's disease (PRODUCE) |
| Methods | RCT, cross‐over |
| Participants | 54 participants 7 to 18 years old |
| Interventions | IG: specific carbohydrate diet CG: modified specific carbohydrate diet |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 10 April 2018 |
| Contact information | Heather C Kaplan, MD; Lisa Opipari‐Arrigan, MD |
| Notes | Sponsor: Children's Hospital Medical Center, Cincinnati |
NCT03348852.
| Study name | Association between functional changes in the brain and the perception of pain in patients with inflammatory bowel diseases (IBD) ‐ measured with functional magnetic resonance imaging |
| Methods | RCT |
| Participants | 84 adults 18 to 80 years old |
| Interventions | IG: transcranial direct current stimulation CG: sham transcranial direct current stimulation |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 24 January 2017 |
| Contact information | magdalena.pruess@charite.de |
| Notes | Sponsor: Charite University, Berlin, Germany |
NCT03422861.
| Study name | Nabilone use for acute pain in inflammatory bowel disease patients |
| Methods | RCT |
| Participants | 80 adults 25 to 65 years old |
| Interventions | IG: nabilone treatment CG: placebo treatment |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | April 2020 |
| Contact information | Naveed.Siddiqui@uhn.ca zeev.friedman@sinaihealthsystem.ca |
| Notes | Sponsor: Samuel Lunenfeld Research Institute, Mount Sinai Hospital |
NCT03667586.
| Study name | Group cognitive behavioral therapy for IBD patients |
| Methods | RCT |
| Participants | 130 adults 18 to 80 years old |
| Interventions | IG: cognitive behavioural psychotherapy sessions for 6 months CG: regular brief follow‐ups by the gastroenterologists and the nurse of the research team |
| Outcomes | Primary outcome measures:
Secondary outcomes measures:
|
| Starting date | 21 February 2019 |
| Contact information | mariakalogeropoulou@yahoo.com chtriantos@hotmail.com We received no response from the author and were therefore unable to determine whether this study meets our inclusion criteria. |
| Notes | Sponsor: University Hospital of Patras |
NCT03798405.
| Study name | Reactive vs. Proactive Pain Control in IBD (PAIN‐Sparing) |
| Methods | RCT |
| Participants | 166 adults 18 years of age and older |
| Interventions | IG: proactive analgesic inpatient narcotic‐sparing CG: reactive traditional prescribing habits |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 1 January 2019 |
| Contact information | gil.melmed@cshs.org |
| Notes | Sponsor: Cedars‐Sinai Medical Center |
NCT03809195.
| Study name | Clinical hypnosis in pediatric Crohn's disease (HypnoCrohns) |
| Methods | RCT |
| Participants | 40 participants 12 to 18 years old |
| Interventions | IG: clinical hypnosis CG: waitlist control |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 14 February 2019 |
| Contact information | amanda.d.lee@vumc.org lynn.walker@vumc.org |
| Notes | Sponsor: Vanderbilt University Medical Center |
NCT03825900.
| Study name | Transcranial direct current stimulation and the interaction between chronic pain and the intestinal epithelial barrier in patients with chronic inflammatory bowel diseases (IBD) |
| Methods | RCT |
| Participants | 84 adults 18 to 80 years old |
| Interventions | IG: active transcranial direct current stimulation CG: sham transcranial direct current stimulation |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 1 June 2018 |
| Contact information | magdalena.pruess@charite.de |
| Notes | Sponsor: Charite University, Berlin, Germany |
NCT04173273.
| Study name | A study evaluating the efficacy and safety of etrasimod in the treatment of patients with moderately to severely active Crohn's disease (CULTIVATE) |
| Methods | RCT, multicentre |
| Participants | 225 adults 18 to 80 years old |
| Interventions | IGa: etrasimod dose A taken by mouth, once daily IGb: etrasimod dose B taken by mouth, once daily CG: matching placebo taken by mouth, once daily |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 12 February 2020 |
| Contact information | ct.gov@arenapharm.com |
| Notes | Sponsor: Arena Pharmaceuticals |
CG: control group
CRP: C‐reactive protein
ESR: erythrocyte sedimentation rate
fMRI: functional magnetic resonance imaging
HBI: Harvey‐Bradshaw Index
IBD: inflammatory bowel disease
IBS‐SSS: Irritable Bowel Syndrome ‐ Severity Scoring System
IG: intervention group
MRI: magnetic resonance imaging
RCT: randomised controlled trial
SCCAI: Simple Clinical Colitis Activity Index
SD: standard deviation
VAS: visual analogue scale
Differences between protocol and review
We planned to undertake subgroup analyses of potential effect modifiers if sufficient data were available; however, this was not the case, therefore we did not carry out any of these analyses.
We also planned to conduct sensitivity analyses, which we did not carry out due to the fact that there were only one to two studies for each included intervention.
We set out to include studies focussed only on Crohn's disease, but it became apparent that many studies included participants with both Crohn's disease and ulcerative colitis. As we are completing a concomitant review looking at pain in ulcerative colitis, a decision was made by the editorial team that any studies that could not provide separate data for Crohn's disease and ulcerative colitis would be included in this review, leaving studies that included data only on participants with ulcerative colitis for inclusion in the other review. This has been updated in the Methods section of this review.
Contributions of authors
VS: contributed the majority of the writing and response to peer review and editing comments; carried out the statistical analysis and narrative synthesis and contributed to Abstract, full‐text screening, and data extraction, and signed off on the final review.
M Gordon: conceived the review question; secured funding; developed, contributed to writing and editing, data extraction, and analysis; made an intellectual contribution to, advised on, approved the final version prior to submission; and is a guarantor of the review.
AKA: developed, made an intellectual contribution to, advised on, and approved the final version of the review prior to submission and signed off on the final review.
M Gasparetto: contributed to Abstract, full‐text screening, and data extraction, and signed off on the final review.
MS: contributed to Abstract, full‐text screening, and data extraction, and signed off on the final review.
JV: contributed to Abstract, full‐text screening, and data extraction; provided consultation on patient perspective on behalf of Crohn's and Colitis UK; and signed off on the final review.
TD: contributed to Abstract, full‐text screening, and data extraction, and signed off on the final review.
Sources of support
Internal sources
-
NIHR, UK
This review was supported by an NIHR Evidence Synthesis grant
External sources
No sources of support provided
Declarations of interest
VS: None.
M Gordon: Since August 2016, I have received travel fees to attend international scientific and training meetings from pharma companies. These grants included no honoraria, inducement, advisory role, or any other relationship and were restricted to the travel‐ and meeting‐related costs of attending such meetings. These include: DDW May 2017, World Congress of Gastroenterology October 2017, DDW May 2018, Advances in IBD December 2018, and DDW May 2019. The companies include: Biogaia (2017‐19), Ferring (2018), Allergan (2017), Synergy (bankrupt ‐ 2018), and Tillots (2017‐19). None of these companies has had any involvement in any works completed by me, and I have never received payment for any other activities from them. From August 2019 onwards, I have made a personal undertaking to receive no further funds from any pharmaceutical or formula company in any form for travel or other related activities. This is to lift the limitations such funding has on my ability to act as a first and corresponding author on reviews, in line with Cochrane policies on such matters, and is reported in line with these policies. These current declarations will expire over the next three years, and this statement will be updated regularly to reflect this.
AKA: None.
M Gasparetto: Since 2015, I have received travel fees to attend international scientific and training meetings from pharma companies. These grants included no honoraria, inducement, advisory role, or any other relationship and were restricted to the travel‐ and meeting‐related costs of attending such meetings. These include ESPGHAN 2017 and ESPGHAN 2019, when I was sponsored by Nutricia. None of these companies has had any involvement in any works completed by me, and I have never received payment for any other activities from them.
MS: None.
JV: None.
TD: None
New
References
References to studies included in this review
Bao 2016 {published data only}
- Bao C, Wu L, Wu H, Liu H, Zhao J, Zeng X, et al. Active Crohn's disease treated with acupuncture and moxibustion: a randomized controlled trial. Chinese Acupuncture & Moxibustion 2016;36(7):683-8. [DOI] [PubMed] [Google Scholar]
Berrill 2014 {published data only}
- Berrill JW, Sadlier M, Hood K, Green JT. Mindfulness-based therapy for inflammatory bowel disease patients with functional abdominal symptoms or high perceived stress levels. Journal of Crohn's & Colitis 2014;8(9):945-55. [DOI] [PubMed] [Google Scholar]
Cox 2020 {published data only}
- Cox SR, Lindsay JO, Fromentin S, Stagg AJ, McCarthy NE, Galleron N, et al. Effects of low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology 2020;158:176-88. [DOI] [PubMed] [Google Scholar]
- ISRCTN17061468. Dietary Interventions in inflammatory bowel disease (IBD). www.isrctn.com/ISRCTN17061468 (first received 20 January 2016).
Espi Lopez 2018 {published data only}
- Espí-López GV, Inglés M, Soliva-Cazabán I, Serra-Añó P. Effect of the soft-tissue techniques in the quality of life in patients with Crohn's disease: a randomized controlled trial. Medicine 2018;97(51):e13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia‐Vega 2004 {published data only}
- Garcia-Vega E, Fernandez-Rodriguez C. A stress management programme for Crohn's disease. Behaviour Research and Therapy 2004;42(4):367-83. [DOI] [PubMed] [Google Scholar]
Hawkes 2001 {published data only}
- Hawkes ND, Richardson C, Ch'Ng CL, Green JT, Evans BK, Williams J. Enteric-release glyceryl trinitrate in active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Alimentary Pharmacology & Therapeutics 2001;15(12):1867-73. [DOI] [PubMed] [Google Scholar]
Higgins 2019 {published data only}
- Higgins P, Ginsburg D, Gilder K, Gilder K, Walsh B, English B, et al. Safety and efficacy of olorinab, a peripherally restricted, highly-selective, cannabinoid receptor 2 agonist in a phase 2A study in chronic abdominal pain associated with Crohn's disease. Journal of Crohn's and Colitis 2019;13:S318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom B, Ruckle J, Turner S, Stauber K, Brierley SM, English BA. Olorinab, a peripherally restricted, highly selective agonist of the cannabinoid receptor type 2 for the management of visceral pain in inflammatory bowel disease (IBD) - preclinical and early clinical development. Inflammatory Bowel Diseases 2019;25 Suppl 1:S318. [Google Scholar]
- Yacyshyn B, Ginsberg DC, Gilder K, Walsh B, English B, Turner SA, et al. Safety and efficacy of olorinab, a peripherally restricted, highly selective, cannabinoid receptor 2 agonist in a phase 2A study in chronic abdominal pain associated with Crohn's disease. Gastroenterology 2019;156(6):S665. [Google Scholar]
Mizrahi 2012 {published data only}
- Mizrahi MC, Reicher-Atir R, Levy S, Haramati S, Wengrower D, Israeli E, et al. Effects of guided imagery with relaxation training on anxiety and quality of life among patients with inflammatory bowel disease. Psychology & Health 2012;27(12):1463-79. [DOI] [PubMed] [Google Scholar]
Ozgursoy Uran 2019 {published data only}
- Ozgursoy Uran BN, Yildirim Y, Senuzun Aykar F, Unsal B. The effect of web-based education on disease activity, symptom management, and quality of life on patients with inflammatory bowel disease. Journal of Crohn's and Colitis 2018;12(Suppl 1):S582. [Google Scholar]
- Ozgursoy Uran BN, Yildirim Y, Senuzun Aykar F, Unsal B. The effect of web-based education on disease activity, symptom management and quality of life in patients with inflammatory bowel disease: randomized-controlled study. Medical Science 2019;23(98):418-31. [Google Scholar]
Sharma 2015 {published data only}
- Sharma P, Poojary G, Dwivedi SN, Deepak KK. Effect of yoga-based intervention in patients with inflammatory bowel disease. International Journal of Yoga Therapy 2015;25(1):101-12. [DOI] [PubMed] [Google Scholar]
Tapete 2018 {published data only}
- Tapete G, De Bortoli N, Ceccarelli L, Mumolo MG, Vinci E, Albano E, et al. Low-FODMAPs diet improves intestinal symptoms in IBD patients with disease remission: randomized case-control study. Digestive and Liver Disease 2018;50(2):e195. [Google Scholar]
Tapete 2019 {published data only}
- Tapete G, Ceccarelli L, De Bortoli N, Bertani L, Albano E, Baiano Svizzero G. Low- versus high- FODMAPs diet in controlling residual abdominal symptoms in inflammatory bowel disease patients: a randomised controlled study with crossing over. Digestive and Liver Disease 2019;51:e219. [Google Scholar]
Volz 2016 {published data only}
- DRKS00005852. Transcranial direct current stimulation to reduce chronic pain in patients with chronic inflammatory bowel diseases. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00005852 (first received 18 December 2015).
- NCT02048137. Transcranial direct current stimulation to reduce chronic pain in patients with chronic inflammatory bowel diseases (tDCS in IBD). clinicaltrials.gov/ct2/show/NCT02048137 (first received 29 January 2014).
- Pruess MS, Farmer A, Siegmund B. Inflammatory bowel disease-induced chronic abdominal pain can be ameliorated by transcranial direct current stimulation. Journal of Crohn's and Colitis 2016;10:S289-90. [Google Scholar]
- Volz MS, Farmer A, Siegmund B. Reduction of chronic abdominal pain in patients with inflammatory bowel disease through transcranial direct current stimulation: a randomized controlled trial. Pain 2016;157(2):429-37. [DOI] [PubMed] [Google Scholar]
Yilmaz 2019 {published data only}
- Yilmaz I, Dolar ME, Ozpinar H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: a randomized controlled trial. Turkish Journal of Gastroenterology 2019;30(3):242-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12617000876392 {published data only}
- ACTRN12617000876392. IBD mindfulness - mindfulness for youth with Inflammatory Bowel Disease (IBD) and depression trial [A pilot randomised-controlled trial (RCT) of mindfulness-based cognitive therapy (MBCT) for youth with Inflammatory Bowel Disease (IBD) and Depression]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373115&isReview=true (first received 12 June 2017).
Engel 2016 {published data only}
- Engel MA, Stracke B. Improvement of health-related quality of life in patients with chronic inflammatory bowel disease after 4 weeks of additive treatment with menthacarin - a randomized, placebo-controlled trial. United European Gastroenterology Journal 2016;4(5):A618. [Google Scholar]
Forbes 2019 {published data only}
- Forbes L. Online Mindfulness Intervention for Inflammatory Bowel Disease Patients: Adherence and Efficacy. Charlotte (NC): The University of North Carolina, 2018. [Google Scholar]
Gearry 2009 {published data only}
- Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease - a pilot study. Journal of Crohn's & Colitis 2009;3(1):8-14. [DOI] [PubMed] [Google Scholar]
ISRCTN98226923 {published data only}
- ISRCTN98226923. Fermentable dietary carbohydrates as triggers of functional gut symptoms in patients with inflammatory bowel disease. www.isrctn.com/ISRCTN98226923 (first received 20 January 2014).
McCormick 2010 {published data only}
- McCormick M, Reed-Knight B, Lewis JD, Gold BD, Blount RL. Coping skills for reducing pain and somatic symptoms in adolescents with IBD. Inflammatory Bowel Diseases 2010;16(12):2148-57. [DOI] [PubMed] [Google Scholar]
Spagnuolo 2017 {published data only}
- Spagnuolo R, Cosco C, Mancina RM, Ruggiero G, Garieri P, Cosco V, et al. Beta-glucan, inositol and digestive enzymes improve quality of life of patients with inflammatory bowel disease and irritable bowel syndrome. European Review for Medical and Pharmacological Sciences 2017;21(Suppl 2):102-7. [PubMed] [Google Scholar]
- Spagnuolo R, Ruggiero G, Cosco C, Cosco V, Garieri P, Gidaro A, et al. Beta-glucan, inositol and digestive enzymes in patients with inflammatory bowel disease associated with irritable bowel syndrome. Digestive and Liver Disease 2016;48 (Suppl):e162. [PubMed] [Google Scholar]
Tripp 2017 {published data only}
- Tripp DA, Verreault P, Gates J, Ropeleski M, Beyak M. Development and piloting of a cognitive-behavioral self management program for patients with inflammatory bowel disease: an early stage update. Gastroenterology 2017;152(5):S799. [Google Scholar]
References to studies awaiting assessment
Bao 2021 {published data only}
- Bao CH, Zhong J, Liu HR, Gu YP, Wu P, Gu K, et al. Effect of acupuncture-moxibustion on negative emotions and plasma tryptophan metabolism in patients with Crohn's disease at active stage. Zhongguo Zhen Jiu 2021;41(1):17-22. [DOI] [PubMed] [Google Scholar]
IRCT20120415009475N5 {published data only}
- IRCT20120415009475N5. Evaluation of the effects of lyophilized Saccharomyces Boulardii capsules on quality of life and clinical outcomes in children with inflammatory bowel disease (IBD). www.irct.ir/trial/32179 (first received 10 October 2018).
IRCT20200219046553N1 {published data only}
- IRCT20200219046553N1. Effect of mindfulness-based cognitive therapy on quality of Life , emotional symptom , dimensions of pain, dispositional mindfulness and disease activity in patients with inflammatory bowel disease. https://en.irct.ir/trial/46526 (registration date 3 April 2020).
JPRN‐UMIN000012635 {published data only}
- JPRN-UMIN000012635. Exploratory study evaluating efficacy, safety and mechanism of Daikenchuto on abdominal pain or bloating accompanied by Crohn disease. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014778 (first received 24 December 2013).
Lee 2021 {published data only}
- Lee A, Moulton D, Acra S, Walker L, Mckernan L, Russell A. P039 Clinical hypnosis in pediatric Crohn's Disease: a randomized controlled trial. Gastroenterology 2020;158(3):S100-1. [DOI] [PubMed] [Google Scholar]
- Lee A, Moulton D, Mckernan L, Russell A, Slaughter JC, Acra S et al. Clinical Hypnosis in Pediatric Crohn's Disease: A Randomized Controlled Pilot Study. Journal of pediatric gastroenterology and nutrition 2021;72(3):e63-e70. [DOI] [PubMed] [Google Scholar]
- Lee AD, Moulton DE, Mckernan LC, Russell A, Acra S, et al. 1165 Clinical hypnosis in pediatric Crohn's Disease: a randomised controlled trial. Gastroenterology 158;6:S236. [DOI] [PubMed] [Google Scholar]
Leiby 2014 {published data only}
- Leiby A, Langseder A, Bustami R, Vazirani M, Marchell M, Ferrara M, et al. A randomized, controlled trial of yoga in pediatric inflammatory bowel disease: preliminary findings. American Journal of Gastroenterology 2014;109:S594. [Google Scholar]
NCT00940576 {published data only}
- NCT00940576. Dietetic efficacy of mare's milk for patients with chronic inflammatory bowel diseases. clinicaltrials.gov/ct2/show/NCT00940576 (first received 16 July 2009).
NCT02559037 {published data only}
- NCT02559037. Acupuncture treatment for active Crohn's disease. clinicaltrials.gov/ct2/show/NCT02559037 (first received 24 September 2015).
NCT02649075 {published data only}
- NCT02649075. To evaluate SBI in the dietary management of mild to moderate Crohn's disease. clinicaltrials.gov/ct2/show/NCT02649075 (first received 4 October 2016).
NCT02963246 {published data only}
- NCT02963246. Mindfulness therapy in inflammatory bowel disease (mindfulness). clinicaltrials.gov/ct2/show/NCT02963246 (first received 15 November 2016).
NCT02974322 {published data only}
- NCT02974322. A study of efficacy and safety of Mongersen (GED-0301) for the treatment of adult and adolescent subjects with active Crohn's disease. clinicaltrials.gov/ct2/show/NCT02974322 (first received 28 November 2016).
NCT03155945 {published data only}
- NCT03155945. Tolerability, pharmacokinetics, and efficacy of APD371 in subjects with Crohn's disease experiencing abdominal pain. clinicaltrials.gov/ct2/show/NCT03155945 (first received 16 May 2017).
NCT03467620 {published data only}
- NCT03467620. Cannabidiol usage as an adjunct therapy for Crohn's disease. clinicaltrials.gov/ct2/show/NCT03467620 (first received 16 March 2018).
Neeb 2019 {published data only}
- NCT02433470. Association of functional changes in the brain and the perception of pain in patients with chronic inflammatory bowel diseases (IBD). clinicaltrials.gov/ct2/show/NCT02433470 (first received 5 May 2015).
- Neeb L, Bayer A, Bayer KE, Farmer A, Fiebach JB, Siegmund B, et al. Transcranial direct current stimulation in inflammatory bowel disease patients modifies resting-state functional connectivity: a RCT. Brain Stimulation 2019;12:978-80. [DOI] [PubMed] [Google Scholar]
NTR3414 {published data only}
- NTR3414. Hypnotherapy for IBD patients with IBS-like symptoms [Hypnotherapy for adolescents and adults with Inflammatory bowel disease and symptoms compatible with Irritable bowel syndrome: a randomized controlled trial]. www.trialregister.nl/trial/3261 (first received 2 May 2012).
Schoultz 2013 {published data only}
- ISRCTN27934462. Can mindfulness-based cognitive therapy (a group self-help program) improve the quality of life for inflammatory bowel disease patients? www.isrctn.com/ISRCTN27934462 (first received 2 August 2013).
- Schoultz M, Atherton IM, Hubbard G, Watson AJM. The use of mindfulness-based cognitive therapy for improving quality of life for inflammatory bowel disease patients: study protocol for a pilot randomised controlled trial with embedded process evaluation. Trials 2013;14:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoultz 2015 {published data only}
- ISRCTN27934462. Can mindfulness-based cognitive therapy (a group self-help program) improve the quality of life for inflammatory bowel disease patients? www.isrctn.com/ISRCTN27934462 (first received 2 August 2013).
- Schoultz M, Atherton I, Watson A. Mindfulness-based cognitive therapy for inflammatory bowel disease patients: findings from an exploratory pilot randomised controlled trial. Trials 2015;16:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takakura 2020 {published data only}
- Takakura W, Berry SK, Patel D, Govalan R, Ghafari A, Bresee C, et al. Opiate Use in Hospitalized Patients With IBD Can Be Significantly Reduced Using a Proactive Pain Protocol: Results of a Randomized Controlled Trial. Official journal of the American College of Gastroenterology 2020;115:S403. [Google Scholar]
References to ongoing studies
ISRCTN71618461 {published data only}
- ISRCTN71618461. A supported online self-management for symptoms of fatigue, pain and urgency/incontinence in people with inflammatory bowel disease: the IBD-BOOST trial. https://www.isrctn.com/ISRCTN71618461?q=71618461&filters=&sort=&offset=1&totalResults=1&page=1&pageSize=10&searchType=basic-search (first received 2 September 2019). [DOI: 10.1186/ISRCTN71618461] [DOI]
NCT03301311 {published data only}
- NCT03301311. Personalized research on diet in ulcerative colitis and Crohn's disease (PRODUCE). clinicaltrials.gov/ct2/show/NCT03301311 (first received 4 October 2017).
NCT03348852 {published data only}
- NCT03348852. Association between functional changes in the brain and the perception of pain in patients with inflammatory bowel diseases (IBD) - measured with functional magnetic resonance imaging. clinicaltrials.gov/ct2/show/NCT03348852 (first received 21 November 2017).
NCT03422861 {published data only}
- NCT03422861. Nabilone use for acute pain in inflammatory bowel disease patients. clinicaltrials.gov/ct2/show/NCT03422861 (first received 6 February 2018).
NCT03667586 {published data only}
- NCT03667586. Group cognitive behavioral therapy for IBD patients. clinicaltrials.gov/ct2/show/NCT03667586 (first received 12 September 2018).
NCT03798405 {published data only}
- NCT03798405. Reactive vs. proactive pain control in IBD (PAIN-Sparing). clinicaltrials.gov/ct2/show/NCT03798405 (first received 10 January 2019).
NCT03809195 {published data only}
- NCT03809195. Clinical hypnosis in pediatric Crohn's disease (HypnoCrohns). clinicaltrials.gov/ct2/show/NCT03809195 (first received 18 January 2019).
NCT03825900 {published data only}
- NCT03825900. Transcranial direct current stimulation and the interaction between chronic pain and the intestinal epithelial barrier in patients with chronic inflammatory bowel diseases (IBD). clinicaltrials.gov/ct2/show/NCT03825900 (first received 1 February 2019).
NCT04173273 {published data only}
- NCT04173273. A study evaluating the efficacy and safety of etrasimod in the treatment of patients with moderately to severely active Crohn's disease (CULTIVATE). clinicaltrials.gov/ct2/show/NCT04173273 (first received 21 November 2019).
Additional references
AGA 2020
- American Gastroenterological Association. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti–TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn's disease. www.gastrojournal.org/article/S0016-5085(13)01521-7/fulltext (accessed 27 April 2020).
Alatab 2020
- Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterology & Hepatology 2020;5(1):17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bielefeldt 2009
- Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflammatory Bowel Diseases 2009;15(5):778-88. [DOI: 10.1002/ibd.20848] [DOI] [PMC free article] [PubMed] [Google Scholar]
BSG 2019
- British Society of Gastroenterology. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. www.bsg.org.uk/wp-content/uploads/2019/12/BSG-IBD-Guidelines-2019.pdf (accessed 27 April 2019).
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
Docherty 2011
- Docherty MJ, Jones RCW 3rd, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterology & Hepatology 2011;7(9):592. [PMC free article] [PubMed] [Google Scholar]
ECCO 2010
- Dignass A, Van Assche G, Lindsay JO, Lemman M, Söderholm J, Colombel JF, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. Journal of Crohn's and Colitis 2010;4(1):28-62. [DOI: 10.1016/j.crohns.2009.12.002] [DOI] [PubMed] [Google Scholar]
ECCO 2020
Egger 1997
- Egger M, Smith GD, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629. [DOI: 10.1136/bmj.315.7109.629] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ewais 2019
- Ewais T, Begun J, Kenny M, Rickett K, Hay K, Ajilchi B, et al. A systematic review and meta-analysis of mindfulness based interventions and yoga in inflammatory bowel disease. Journal of Psychosomatic Research 2019;116:44-53. [DOI] [PubMed] [Google Scholar]
Ghosh 2015
- Ghosh N, Premchand P. A UK cost of care model for inflammatory bowel disease. Frontline Gastroenterology 2015;6(3):169-74. [DOI: 10.1136/flgastro-2014-100514] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gjuladin‐Hellon 2019
- Gjuladin-Hellon T, Gordon M, Iheozor-Ejiofor Z, Akobeng AK. Oral 5-aminosalicylic acid for maintenance of surgically-induced remission in Crohn's disease. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD008414. [DOI: 10.1002/14651858.CD008414.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 6 December 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Greenley 2013
- Greenley RN, Kunz JH, Schurman JV, Swanson E. Abdominal pain and health related quality of life in pediatric inflammatory bowel disease. Journal of Pediatric Psychology 2013;38(1):63-71. [DOI: 10.1093/jpepsy/jss097] [DOI] [PubMed] [Google Scholar]
Halpin 2012
- Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. American Journal of Gastroenterology 2012;107(10):1474-82. [DOI] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane, 2021. [Google Scholar]
Iheozor‐Ejiofor 2019
- Iheozor-Ejiofor Z, Gordon M, Clegg A, Freeman SC, Gjuladin-Hellon T, MacDonald JK, et al. Interventions for maintenance of surgically induced remission in Crohn’s disease: a network meta-analysis. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD013210. [DOI: 10.1002/14651858.CD013210.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kafil 2018
- Kafil TS, Nguyen TM, MacDonald JK, Chande N. Cannabis for the treatment of Crohn's disease. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD012853. [DOI: 10.1002/14651858.CD012853.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplan 2015
- Kaplan G. The global burden of IBD: from 2015 to 2025. Nature Reviews. Gastroenterology & Hepatology 2015;12:720-7. [DOI] [PubMed] [Google Scholar]
Limketkai 2019
- Limketkai BN, Iheozor-Ejiofor Z, Gjuladin-Hellon T, Parian A, Matarese LE, Bracewell K, et al. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database of Systematic Reviews 2019, Issue 2. Art. No: CD012839. [DOI: 10.1002/14651858.CD012839.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2018
- Lin SC, Cheifetz AS. The use of complementary and alternative medicine in patients with inflammatory bowel disease. Gastroenterology and Hepatology 2018;14(7):415-25. [PMC free article] [PubMed] [Google Scholar]
Long 2016
- Long MD, Kappelman MD, Martin CF, Chen W, Anton K, Sandler RS. Role of non-steroidal anti-inflammatory drugs in exacerbations of inflammatory bowel disease. Journal of Clinical Gastroenterology 2016;50(2):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Care Excellence. Crohn's disease: management. www.nice.org.uk/guidance/ng129 (accessed 1 July 2019).
Norton 2017
- Norton C, Czuber-Dochan W, Artom M, Sweeney L, Hart A. Systematic review: interventions for abdominal pain management in inflammatory bowel disease. Alimentary Pharmacology & Therapeutics 2017;46:115-25. [DOI] [PubMed] [Google Scholar]
Odes 2017
- Odes S, Friger M, Sergienko R, Schwartz D, Sarid O, Slonim-Nevo V, et al. Simple pain measures reveal psycho-social pathology in patients with Crohn's disease. World Journal of Gastroenterology 2017;23(6):1076-89. [DOI: 10.3748/wjg.v23.i6.1076] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paiotti 2012
- Paiotti APR, Marchi P, Miszputen SJ, Oshima CTF, Franco M, Ribeiro DA. The role of nonsteroidal antiinflammatory drugs and cyclooxygenase-2 inhibitors on experimental colitis. In Vivo 2012;26(3):381-93. [PubMed] [Google Scholar]
Pauly 2017
- Pauly NJ, Michailidis L, Kindred MG, Flomenhoft D, Lofwall MR, Walsh SL, et al. Predictors of chronic opioid use in newly diagnosed Crohn's disease. Inflammatory Bowel Diseases 2017;23(6):1004-10. [DOI: 10.1097/MIB.0000000000001087] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prince 2016
- Prince AC, Myers CE, Joyce T, Irving P, Lomer M, Whelan K. Fermentable carbohydrate restriction (low FODMAP diet) in clinical practice improves functional gastrointestinal symptoms in patients with inflammatory bowel disease. Inflammatory Bowel Diseases 2016;22(5):1129-36. [DOI] [PubMed] [Google Scholar]
RCP 2012
- Royal College of Physicians. Report of the results for the national clinical audit of adult inflammatory bowel disease inpatient care in the UK. https://www.rcplondon.ac.uk › file › download (accessed 5 May 2021).
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Satsangi 2006
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55(6):749-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Srinath 2012
- Srinath AI, Walter C, Newara MC, Szigethy EM. Pain management in patients with inflammatory bowel disease: insights for the clinician. Therapeutic Advances in Gastroenterology 2012;5(5):339-57. [DOI: 10.1177/1756283X12446158] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thapa 2019
- Thapa N, Kappus M, Hurt R, Diamond S. Implications of the opioid epidemic for the clinical gastroenterology practice. Current Gastroenterology Reports 2019;21(9):44. [DOI] [PubMed] [Google Scholar]
Timmer 2011
- Timmer A, Preiss JC, Motschall E, Rücker G, Jantschek G, Moser G. Psychological interventions for treatment of inflammatory bowel disease. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No: CD006913. [DOI: 10.1002/14651858.CD006913.pub2] [DOI] [PubMed] [Google Scholar]


