Abstract
Background
This phase 2/3 immunobridging study evaluated the safety and immunogenicity of the ChAdOx1 nCoV-19 Coronavirus Vaccine (Recombinant) (SII-ChAdOx1 nCoV-19), manufactured in India at the Serum Institute of India Pvt Ltd (SIIPL), following technology transfer from the AstraZeneca.
Methods
This participant-blind, observer-blind study randomised participants 3:1 to SII-ChAdOx1 nCoV-19 or AZD1222 (ChAdOx1 nCoV-19) (immunogenicity/reactogenicity cohort) and 3:1 to SII-ChAdOx1 nCoV-19 or placebo (safety cohort). The study participants were enrolled from 14 hospitals across India between August 25 and October 31, 2020. Two doses of study products were given 4 weeks apart. The primary objectives were to demonstrate non-inferiority of SII-ChAdOx1 nCoV-19 to AZD1222 in terms of geometric mean titre (GMT) ratio of anti-SARS-CoV-2 spike IgG antibodies 28 days after the second dose (defined as lower limit of 95% CI >0·67) and to determine the incidence of serious adverse events (SAEs) causally related to SII-ChAdOx1 nCoV-19. The anti-spike IgG response was assessed using a multiplexed electrochemiluminescence-based immunoassay. Safety follow-up continued until 6 months after first dose. Trial registration: CTRI/2020/08/027170.
Findings
1601 participants were enrolled: 401 to the immunogenicity/reactogenicity cohort and 1200 to the safety cohort. After two doses, seroconversion rates for anti-spike IgG antibodies were more than 98·0% in both the groups. SII-ChAdOx1 nCoV-19 was non-inferior to AZD1222 (GMT ratio 0·98; 95% CI 0·78–1·23). SAEs were reported in ≤ 2·0% participants across the three groups; none were causally related. A total of 34 SARS-CoV-2 infections were reported; of which 6 occurred more than 2 weeks after the second dose; none were severe.
Interpretation
SII-ChAdOx1 nCoV-19 has a non-inferior immune response compared to AZD1222 and an acceptable safety/reactogenicity profile. Pharmacovigilance should be maintained to detect any safety signals.
Funding
SIIPL funded the contract research organisation and laboratory costs, while the site costs were funded by the Indian Council of Medical Research. The study vaccines were supplied by SIIPL and AstraZeneca.
Keywords: COVID-19, SII-ChAdOx1 nCoV-19, AZD1222, Safety, Immunogenicity
Research in context.
Evidence before this study
There are published data on the safety, immunogenicity and efficacy of AZD1222 (ChAdOx1 nCoV-19). After technology transfer, the vaccine is now manufactured in India from the bulk stage and formulated at the commercial scale as SII-ChAdOx1 nCov-19. We searched PubMed for randomized controlled trials published from database inception until 01 September 2021, with no language restrictions, using the terms “SII-ChAdOx1 nCov-19”, “vaccine”, and “randomized controlled trial”. At the time of the search, there were no peer-reviewed randomized controlled trials available on SII-ChAdOx1 nCov-19.
Added value of this study
We report on the first safety and immunogenicity results of SII-ChAdOx1 nCoV-19 from a phase 2/3 trial in India. SII-ChAdOx1 nCoV-19 has an acceptable safety profile with no serious safety concerns. The vaccine is also non-inferior to AZD1222 (ChAdOx1 nCoV-19) in terms of immune response.
Implications of all the available evidence
The present safety and immunogenicity data successfully bridge SII-ChAdOx1 nCoV-19 to AZD1222. The positive results presented here support widespread regulatory approvals and use of SII-ChAdOx1 nCoV-19. Pharmacovigilance should be maintained to detect any safety signals.
Alt-text: Unlabelled box
1. Introduction
On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a global pandemic [1] that required urgent development of vaccines against the new virus, SARS-CoV-2. Oxford University developed a candidate vaccine, ChAdOx1 nCoV-19 (AZD1222), based on an established platform [2] consisting of the replication-deficient simian adenovirus vector ChAdOx1. AZD1222 has been studied in multiple clinical trials in countries including the UK, Brazil and South Africa; all these studies demonstrated the safety of the vaccine, and showed high immunogenicity and efficacy against COVID-19 [[2], [3], [4], [5]], though the study in South Africa failed to show efficacy of the vaccine against mild to moderate symptomatic disease caused by the B.1.351 (Beta) Variant [6]. The vaccine was first authorised for emergency use in the UK in December 2020 [7] and, as of July 2021, is authorised or approved in more than 165 countries.
After technology transfer from Oxford University/AstraZeneca to Serum Institute of India Pvt Ltd (SIIPL), the ChAdOx1 nCoV-19 vaccine is now also being manufactured at SIIPL from the bulk stage [8] and formulated at the commercial scale. The SIIPL-manufactured product is known as SII-ChAdOx1 nCoV-19 (CovishieldTM). Based on interim results of the immunobridging study reported herein, SII-ChAdOx1 nCoV-19 received Emergency Use Authorisation in India in January 2021 [9] and subsequently received regulatory approval in multiple countries as well as emergency use listing by the WHO [10]. As of July, 2021, more than 400 million doses of SII-ChAdOx1 nCoV-19 have been administered in India alone [11] and more than 65 million doses have been distributed in other countries, including the UK, Canada, Brazil, Ukraine, Saudi Arabia, Egypt and Morocco [Data on file]. Consequently, SII-ChAdOx1 nCoV-19 is one of the most commonly used COVID-19 vaccines in the world [12]. This immunobridging study was conducted in adults in India to assess the safety and immunogenicity of the vaccine, per Indian regulatory requirements and herein results until day 57 visit are presented.
2. Methods
2.1. Study design and participants
This was a phase 2/3, participant-blind, observer-blind, randomised, controlled study in healthy adults in India. The study was approved by the Drugs Controller General of India (DCGI) and the ethics committees of each study site. The study was conducted in accordance with the Good Clinical Practice guidelines of the International Council for Harmonisation and the principles of the Declaration of Helsinki (2013, Fortaleza), as well as the guidelines of the Indian Council of Medical Research (2017) and the New Drugs and Clinical Trial Rules (2019). Written informed consent was provided by each participant before enrolment. Trial registration: CTRI/2020/08/027170.
The study enrolled adults aged 18 years and older. Individuals with controlled comorbid conditions based on clinical judgement of the investigators as per medical history and clinical examination, including diabetes and hypertension, and controlled cardiovascular, respiratory, hepatic, renal and neurological conditions were eligible. Female participants were required to have a negative pregnancy test prior to enrolment and were required to use adequate contraception from 28 days before the first dose through 28 days after the second dose of vaccine. Key exclusion criteria were acute illness, known prior SARS-CoV-2 infection, and an immunosuppressive condition or receipt of immunosuppressive therapy. Full eligibility criteria are listed in the protocol (Appendix 2). Initially, those participants with baseline SARS CoV-2 by reverse transcription polymerase chain reaction (RT PCR) and/or IgG for SARS CoV-2 positive results were excluded. However, considering the real world situation where screening for these tests would not be possible before vaccination, these exclusion criteria were removed and the modified protocol was implemented after regulatory and ethical approvals.
The study participants were enrolled from 14 hospitals across India. The investigators at each hospital were responsible for the conduct of the study as per the approved protocol and they were blinded for study assignments.
2.2. Randomisation and masking
Participants enrolled to the immunogenicity/reactogenicity cohort were randomly assigned in a 3:1 ratio to receive either SII-ChAdOx1 nCoV-19 or AZD1222. Randomisation was stratified by age to ensure that approximately 25% of participants were ≥60 years of age. Participants enrolled to the safety cohort were randomly assigned in a 3:1 ratio to receive either SII-ChAdOx1 nCoV-19 or placebo. Participants in both cohorts were randomised via an Interactive Web Response system (IWRS). The randomisation scheme was generated by independent personnel at the contract research organisation (CRO) using SAS software version 9.4 by interactive response technology, which linked sequential randomisation numbers to treatment allocation.
In this participant-blind, observer-blind study, vaccine group allocation was concealed from all participants and study personnel involved in endpoint assessments. Designated study personnel involved in obtaining randomisation codes from the IWRS and preparing and administering vaccines were not blinded to participant allocation. These personnel did not perform any study evaluations. Additionally, unblinded team of study monitors and statisticians could access unblinded participant-level data as required. All other study personnel, sponsors, CRO staff, statisticians and laboratory personnel involved in immunological testing were blinded to participant allocation.
2.3. Procedures
Two doses of vaccine or placebo were administered by intramuscular injection in the deltoid muscle at a dosing interval of 4 weeks (+2-week window). Both SII-ChAdOx1 nCoV-19 (SIIPL) and AZD1222 (AstraZeneca, Symbiosis Pharmaceuticals Ltd, UK) consist of the replication-deficient simian adenovirus vector ChAdOx1 containing genetic material encoding the spike protein for SARS-CoV-2. Each 0·5 mL dose contains 5 × 1010 viral particles, plus excipients including histidine, sucrose, sodium chloride, magnesium chloride, polysorbate 80, edetate disodium, ethanol and hydrochloric acid. The vaccines were supplied as ready-to-use liquid formulations in 5 mL vials (SII-ChAdOx1 nCoV-19/CovishieldTM Batch Number: 412003, Date of Manufacture: July 23, 2020; AZD1222 Batch Number: 20482C, Date of Manufacture: July 3, 2020). Placebo (Serum Institute of India Pvt Ltd, India; Batch Number: 421001, Date of Manufacture: July 13, 2020) was formulated similarly to SII-ChAdOx1 nCoV-19/AZD1222 except that it did not contain any viral particles. Study vaccines and placebo were stored and transported at 2–8°C.
At baseline participants were physically examined and medical history was sought for any pre-existing conditions and at follow-up visits scheduled on day 29 (+14 days; day of second dose), day 57 (+14 days) and day 180 (+28 days), they were physically examined and assessed for any adverse events (AEs). Participants were also contacted on day 90 for assessment of safety. In the immunogenicity/reactogenicity cohort, solicited local (injection-site pain, tenderness, redness, warmth, itching, swelling and induration) and systemic (fever, chills, fatigue, malaise, headache, arthralgia, myalgia and nausea) AEs were recorded for 7 days after each vaccine dose using structured diary cards. In both the immunogenicity/reactogenicity and safety cohorts, unsolicited AEs were collected through 28 days after each vaccine dose. Additionally, serious AEs (SAEs) and AEs of special interest (AESIs; based on FDA requirements, including all cases of COVID-19) were collected throughout the study period until the day 180 (+28 days) visit in both cohorts. All AEs were graded for severity using the Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events (corrected version 2.1, July 2017) from the US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases. Investigators followed all AEs for outcomes and assessed causal relationship with study vaccines based on the clinical judgement and the information in the Investigators brochure and literature. Blinding was not removed in such cases unless investigators felt that the product identity was necessary to treat an AE. All SAEs were reported by the investigators to the sponsor, regulatory authorities and ethics committees as per applicable regulatory requirements. Safety was periodically monitored during the study by a Protocol Safety Review Team (PSRT), which included physicians and a biostatistician from the sponsor and CRO. An independent Data Safety Monitoring Board (DSMB), comprising two independent physicians and a biostatistician, was responsible for reviewing safety data during the course of the study.
At any time during the study, if a participant presented with symptoms suggestive of COVID-19 or had contact with a confirmed COVID-19 positive case, a swab from the nose and/or throat was collected and tested for SARS-CoV-2 by RT-PCR test. In addition, all participants were tested for SARS-CoV-2 by RT-PCR at baseline, day 29 and day 180. Second dose was given without waiting for the outcome of the RT-PCR test. Baseline serological evidence of prior COVID-19 infection was assessed based on immunoglobulin G (IgG) antibodies against SARS-CoV-2 prior to vaccination.
Antibody levels were evaluated at baseline, day 29, day 57 and day 180. IgG antibodies against SARS-CoV-2 spike (S) and receptor binding domain (RBD) antigens were assessed using a validated electrochemiluminescence-based multiplexed immunoassay. Neutralising antibodies (NAbs) were assessed using a validated pseudovirus-based microneutralisation (MNT) assay. The MNT assay is a fully quantitative assay fitted with a PPD-developed reference standard. The assay reports the serum neutralising antibody concentration (Ab[C]) per test sample by interpolating the mean of the replicate readout values off the fitted reference standard curve. The interpolated Ab[C] is then dilution-corrected. The final sample Ab[C] is the Ab[C] associated with the lowest dilution with an Ab[C] within the quantifiable range of the assay. The assay is calibrated against the WHO International Reference Standard [13] and results are expressed as relative to the International Standard.
Seroconversion was defined as a 4-fold increase in antibody titres with respect to baseline. Antibody titres that were below the lower limit of quantification (LLOQ) were recorded as 0·5 × LLOQ. Titres that were greater than the upper limit of quantification (ULOQ) were recorded as the ULOQ. A subset of participants was assessed for cell-mediated immune (CMI) responses at baseline, day 29, day 57 and day 180. CMI responses were measured by quantification of antigen-specific T cells using an ex vivo interferon-γ enzyme-linked immunospot (ELISpot) assay. Two hundred and fifty eight overlapping peptides (provided by Jenner Institute Laboratories, University of Oxford) spanning the entire vaccine insert, including the tPA leader sequence were pooled in 12 pools for the CMI assessment (Table S6 Amino acid sequences of SARS-CoV-2 spike peptides used in ELISPOT assays) [2]. Anti-S IgG, anti-RBD IgG and NAbs by MNT assay were performed at PPD Vaccines, Richmond, VA, USA while CMI responses were measured at National AIDS Research Institute, Pune, India.
2.4. Outcomes
The study had two primary outcomes. The first was the comparison of the safety of SII-ChAdOx1 nCoV-19 with placebo in terms of causally related SAEs (as assessed by both investigators and the sponsor) reported through the day 180 study visit. The second was to compare the immunogenicity of SII-ChAdOx1 nCoV-19 with AZD1222 in terms of the ratio of geometric mean titre (GMT) of IgG antibodies against SARS-CoV-2 S protein (anti-S IgG) at 1 month after the second dose of vaccine.
The secondary outcomes were occurrence of solicited AEs for 7 days following each vaccination (only for immunogenicity/reactogenicity cohort), unsolicited AEs for 28 days following each vaccination, SAEs throughout the study duration, proportion with seroconversion and GMTs for anti-S IgG and NAbs at various time points, virologically confirmed symptomatic and asymptomatic cases of COVID-19, severe COVID-19, hospitalizations, intensive care unit (ICU) admissions and deaths associated with COVID-19. Exploratory outcomes were proportion with seroconversion and GMTs for NAbs at day 29 and day 180 and T cell responses and cytokine levels at various time points.
2.5. Statistical analysis
The planned sample size for the study was n=1600 participants, comprising 400 participants in the immunogenicity/reactogenicity cohort and 1200 participants in the safety cohort. The study was designed to provide a 95% probability of detecting at least one event of a causally related SAE among the 1200 participants receiving SII-ChAdOx1 nCoV-19 if the true frequency of that causally related SAE was 0·25%. The study had at least 90% power to demonstrate non-inferiority of SII-ChAdOx1 nCoV-19 to AZD1222 in terms of immunogenicity, assuming a coefficient of variation of 1·2 and that the proportion of non-evaluable participants in the immunogenicity/reactogenicity cohort was ≤21%. Non-inferiority was to be concluded if the lower limit of the 95% confidence interval (CI) for the GMT ratio for anti-S IgG antibodies between SII-ChAdOx1 nCoV-19 and AZD1222 was greater than 0·67, as per the WHO guidelines for clinical evaluation of vaccines [14]. Additional assessments used a one-sided significance level of 0·025 and GMT ratios of 1 between vaccine groups. Anti-S IgG antibody responses were summarized by age group (≥18 to 59 years, ≥60 years), baseline serostatus and in participants with and without co-morbidity.
Immunogenicity was analysed in the immunogenicity population, which consisted of all participants in the immunogenicity/reactogenicity cohort who received at least one dose of study vaccine, provided at least 1 evaluable post-vaccination serum sample and had baseline data available. Safety was analysed in the safety population, which consisted of all participants who received at least one dose of study vaccine and had safety data available. Demographic baseline characteristics were analysed descriptively by vaccine group. Mean, standard deviation, median, and minimum and maximum values were calculated for quantitative variables, and frequencies and percentages were calculated for categorical variables, including distribution of participants according to gender and age groups.
2.6. Role of the funding source
The funder of the study was involved in study design, data interpretation, and writing of the report, but was not involved in data collection and data analysis. All the authors had access to the data. All authors took the decision to submit the paper for publication
3. Results
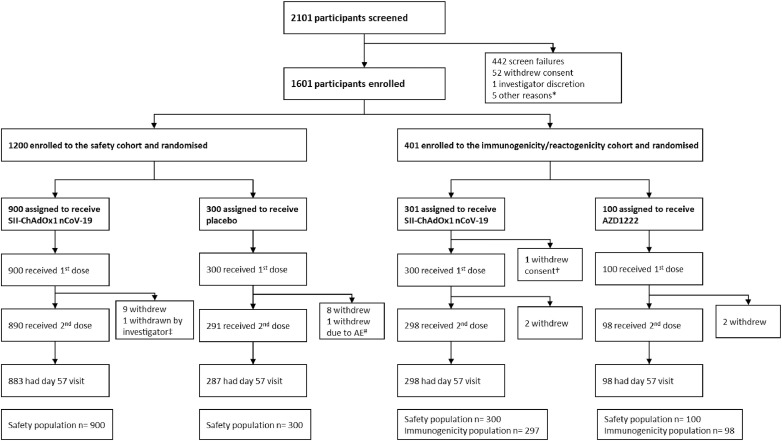
Between August 25 and October 31, 2020, 2101 individuals were screened and 1601 eligible participants were enrolled and randomly assigned, 401 to the immunogenicity/reactogenicity cohort (301 and 100 to receive SII-ChAdOx1 nCoV-19 and AZD1222, respectively), and 1200 to the safety cohort (900 and 300 to receive SII-ChAdOx1 nCoV-19 and placebo, respectively) (Figure 1). Of the 1601 participants who were randomized, 1600 (one participant from immunogenicity/reactogenicity cohort withdrew consent before vaccination) received at least one dose of vaccine/placebo, 21 withdrew consent, one was withdrawn by the investigator due to an unrelated neurological SAE of encephalopathy (SII-ChAdOx1 nCoV-19 group) and one was withdrawn by the investigator due to an unrelated AE of hypertension (placebo group). The remaining 1577 participants received the second dose. The day 57 visit for the last participant was completed on January 15, 2021. All participants completed study follow-up (day 180 visit).
Figure 1.
CONSORT flow chart.
*Participants did not come to site for randomisation visit within window period of 7 days after screening. †1 participant withdrew consent before vaccination. ‡The participant suffered from a neurological serious adverse event and was withdrawn by the investigator. #Participant withdrawn due to adverse event of aggravation of hypertension on day 29 visit. AE=adverse event.
Overall demographic and baseline characteristics in the vaccine/placebo groups resulting from each randomisation did not appear to be substantially different in terms of age, sex, height, weight, and comorbid conditions (Table 1 and supplementary table S3). At baseline, all participants were negative for SARS-CoV-2 by RT-PCR test; 32 (2·0%) were seropositive for SARS-CoV-2 IgG antibodies. At baseline, 389 participants were seronegative for SARS-CoV-2 IgG antibodies in the immunogenicity/reactogenicity cohort (Table 1).
Table 1.
Demographics and baseline characteristics
| Immunogenicity/reactogenicity cohort (N=400) |
Safety cohort (N=1200) |
All SII-ChAdOx1 nCoV-19 (N=1200) | |||
|---|---|---|---|---|---|
| Parameter | SII-ChAdOx1 nCoV-19 (N=300) | AZD1222 (N=100) | SII-ChAdOx1 nCoV-19 (N=900) | Placebo (N=300) | |
| Age, years | 46·4 (15·13) | 46·8 (16·29) | 40·6 (12·22) | 40·2 (13·26) | 42·1 (13·24) |
| [range] | [19–83] | [20–80] | [18–84] | [18–77] | [18–84] |
| Age group | |||||
| 18–59 years | 225 (75·0) | 74 (74·0) | 823 (91·4) | 271 (90·3) | 1048 (87·3) |
| ≥60 years | 75 (25·0) | 26 (26·0) | 77 (8·6) | 29 (9·7) | 152 (12·7) |
| Sex | |||||
| Male | 228 (76·0) | 80 (80·0) | 697 (77·4) | 218 (72·7) | 925 (77·1) |
| Female | 72 (24·0) | 20 (20·0) | 203 (22·6) | 82 (27·3) | 275 (22·9) |
| Height, cm | 167·6 (9·20) | 167·0 (8·47) | 167·1 (9·03) | 165·7 (8·74) | 167·2 (9·07) |
| Weight, kg | 68·58 (12·289) | 69·98 (14·154) | 71·81 (13·565) | 69·66 (13·608) | 71·00 (13·327) |
| Body Mass Index (kg/m2) | 24·40 (3·871) | 25·05 (4·433) | 25·68 (4·197) | 25·29 (4·185) | 25·36 (4·154) |
| Baseline SARS-CoV-2 serostatus | |||||
| Seropositive | 9 (3·0) | 2 (2·0) | 17 (1·9) | 4 (1·3) | 26 (2·2) |
| Seronegative | 291 (97·0) | 98 (98·0) | 883 (98·1) | 296 (98·7) | 1174 (97·8) |
| Comorbidities* | 53 (17·7) | 26 (26·0) | 231 (25·7) | 68 (22·7) | 284 (23·7) |
Data are n (%) or mean (SD), except where stated.
A participant was considered as having a comorbidity if they either had a body mass index ≥30 or had one of the following reported terms for medical history: allergic bronchitis, asthma, atrial fibrillation, bronchiectasis, cardiac operation, chronic heart failure, chronic obstructive pulmonary disease, coronary angioplasty, coronary artery bypass, coronary artery disease, diabetes mellitus, dyslipidaemia, essential hypertension, gout, hepatic cirrhosis, hypercholesterolaemia, hyperglycaemia, hyperlipidaemia, hypertension, hyperthyroidism, hypocholesterolaemia, hypothyroidism, ischaemic heart disease, metabolic surgery, myocardial infarction, myocardial ischaemia, nephrectomy, peripheral artery stent insertion, peripheral vascular disease, prostate cancer, renal artery stenosis, seizure, spleen operation, tongue neoplasm malignant stage unspecified, type 1 diabetes mellitus, type 2 diabetes mellitus, valvular heart disease. SD=standard deviation.
3.1. Immunogenicity/efficacy
In the immunogenicity/reactogenicity cohort, baseline anti-S IgG antibody titres were <100 arbitrary units (AU)/mL in both the SII-ChAdOx1 nCoV-19 and AZD1222 groups. One month after the first dose (day 29), GMTs were >6600 AU/mL in both groups, and 1 month after the second dose, GMTs were >28 500 AU/mL in both groups [SII-ChAdOx1 nCoV-19 – GMT 30 245·6 AU/mL (95% CI 26 794·0–34 141·8) and AZD1222 GMT 28 558·3 AU/mL (23 479·3–34 735·8)] (Table 2); there was a >325-fold rise in antibody titres in both groups compared with baseline. The GMTs in the baseline seronegative participants were on the same lines as those of the overall study population (Table 2 and supplementary table S1). Compared with baseline, the post-dose 2 GMTs in the participants with co-morbidity rose >262-fold in SII-ChAdOx1 nCoV-19 group and >295-fold in AZD1222 group while the same in the participants without co-morbidity increased >341-fold in SII-ChAdOx1 nCoV-19 group and >406-fold in AZD1222 group (Supplementary tables S4 and S5). The GMT ratio for anti-S IgG antibodies between SII-ChAdOx1 nCoV-19 and AZD1222 was 0·98 (95% CI 0·78–1·23). The lower limit of the 95% CI was greater than the defined 0·67 threshold, indicating that SII-ChAdOx1 nCoV-19 was non-inferior to AZD1222 (supplementary table S2). In both vaccine groups, ≥98% of participants demonstrated seroconversion after the second dose (p=0.9131) (Table 2).
Table 2.
Summary of GMTs and seroconversion of anti-S and anti-RBD IgG antibodies – immunogenicity analysis population
| Parameter | Age group | n | SII-ChAdOx1 nCoV-19 (N=297) |
n | AZD1222 (N=98) |
||
|---|---|---|---|---|---|---|---|
| Anti-S IgG | Anti-RBD IgG | Anti-S IgG | Anti-RBD IgG | ||||
| Baseline | |||||||
| GMT (95% CI) | Overall | 297 | 95·4 (78·1–116·6) | 170·3 (146·3–198·3) | 98 | 79·4 (58·2–108·4) | 159·8 (125·3–203·7) |
| 18–59 years | 222 | 93·7 (75·3–116·7) | 169·5 (144·1, 199·4) | 72 | 70·7 (53·7–93·1) | 144·3 (117·8, 176·6) | |
| ≥60 years | 75 | 100·8 (63·1–160·8) | 172·6 (119·1, 250·1) | 26 | 109·5 (43·1–278·2) | 212·0 (99·8, 450·1) | |
| 28 days after dose 1 – day 29 (+14) | |||||||
| GMT (95% CI) | Overall | 295 | 10 131·1 (8547·7–12 007·9) | 9786·4 (8122·6–11 791·1) | 98 | 6660·8 (4836·3–9173·7) | 6311·2 (4470·1–8910·6) |
| 18–59 years | 221 | 12 040·3 (10 046·4–14 429·9) | 11929·7 (9801·7–14 519·6) | 72 | 6918·0 (4921·6–9724·3) | 6820·4 (4863·2–9565·3) | |
| ≥60 years | 74 | 6049·7 (4083·1–8963·6) | 5417·1 (3499·0–8386·8) | 26 | 5997·5 (2697·9–13 332·7) | 5090·9 (1968·3–13 167·1) | |
| Seroconversion n (%) [95% CI] | Overall | 295 | 285 (96·6) [93·9–98·4] | 279 (94·6) [91·3–96·9] | 98 | 90 (91·8) [84·5–96·4] | 87 (88·8) [80·8–94·3] |
| 18–59 years | 221 | 216 (97·7) [94·8–99·3] | 211 (95·5) [91·8–97·8] | 72 | 69 (95·8) [88·3–99·1] | 66 (91·7) [82·7–96·9] | |
| ≥60 years | 74 | 69 (93·2) [84·9–97·8] | 68 (91·9) [83·2–97·0] | 26 | 21 (80·8) [60·6–93·4] | 21 (80·8) [60·6–93·4] | |
| 28 days after dose 2 – day 57 (+14) | |||||||
| GMT (95% CI) | Overall | 293 | 30 245·6 (26 794·0–34 141·8) | 38 576·5 (33 928·5–43 861·2) | 95 | 28 558·3 (23 479·3–34 735·8) | 36 648·9 (30 007·6–44 760·2) |
| 18–59 years | 219 | 33 438·2 (29 546·0–37 843·2) | 42 618·2 (37 468·1–48476·1) | 70 | 31 385·9 (25 379·5–38 813·8) | 40 556·5 (32 883·9–50 019·2) | |
| ≥60 years | 74 | 22 474·2 (16 549·5–30 519·9) | 28 725·0 (20 571·2–40 110·8) | 25 | 21 924·1 (13 848·1–34 710·1) | 27 597·2 (16 830·9–45 250·6) | |
| Seroconversion n (%) [95% CI] | Overall | 293 | 287 (98·0) [95·6–99·2] | 284 (96·9) [94·2–98·6] | 95 | 94 (98·9) [94·3–100·0] | 93 (97·9) [92·6–99·7] |
| 18–59 years | 219 | 217 (99·1) [96·7–99·9] | 215 (98·2) [95·4–99·5] | 70 | 70 (100·0) [94·9–100·0] | 69 (98·6) [92·3–100·0] | |
| ≥60 years | 74 | 70 (94·6) [86·7–98·5] | 69 (93·2) [84·9–97·8] | 25 | 24 (96·0) [79·6–99·9] | 24 (96·0) [79·6–99·9] | |
n=number of participants with a non-missing value at the respective visit. For each vaccine, the GMT and 95% CI of anti-S and anti-RBD IgG antibodies were calculated by transforming to the original scale of log 10-transformed mean and its two-sided 95% CI limits at each visit. Seroconversion is defined as a 4-fold increase in titre from baseline. The 95% CIs for each vaccine group were calculated by using the Clopper–Pearson method.
For seroconversion, n in respective intervention column represents number of participants with seroconversion.
CI=confidence interval; GMT=geometric mean titre; IgG=immunoglobulin G; RBD=receptor-binding domain.
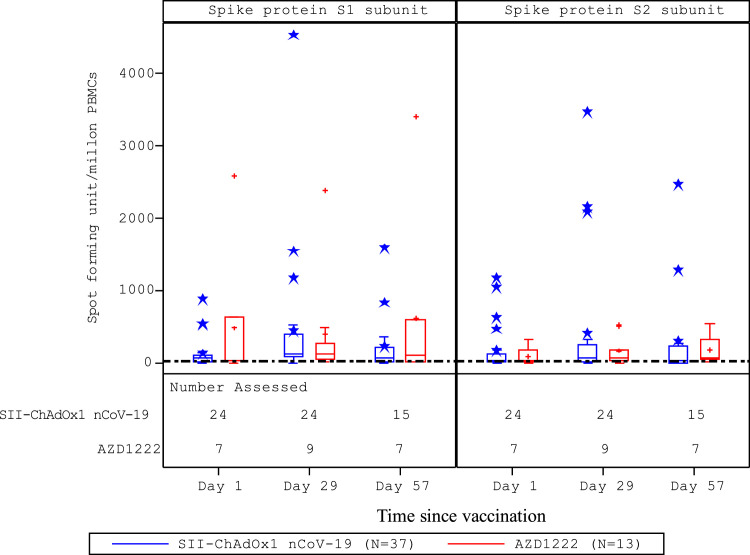
Before vaccination, anti-RBD IgG antibody titres were <171 AU/mL in both vaccine groups. GMTs increased to >6300 AU/mL after the first dose and >36 600 AU/mL after the second dose in both vaccine groups (Table 2). From baseline to 1 month after second dose, there was a >225-fold rise in antibody titres in both groups. After the second dose, >96% (p=0.7334) of participants demonstrated seroconversion, in both vaccine groups (Table 2). Neutralising antibody titres were <6 international units (IU)/mL in both vaccine groups at baseline. These increased to >11 IU/mL after the first dose and >56 IU/mL after the second dose in both groups. The rise in titres from baseline to post-second dose was >12-fold in both groups (Table 3). Seroconversion was demonstrated in >84% (p=0.8498) of participants after the second dose in both groups (Table 3). Interferon-γ ELISpot responses against SARS-CoV-2 S peptides peaked at 28 days after the first dose (Figure 2); there was no increase after the second dose in either group.
Table 3.
Summary of GMTs and seroconversion of neutralising antibodies – immunogenicity analysis population
| Parameter | Age group | n | SII-ChAdOx1 nCoV-19 (N=297) | n | AZD1222 (N=98) |
|---|---|---|---|---|---|
| Baseline | |||||
| GMT (95% CI) | Overall | 297 | 5·2 (4·7–5·8) | 98 | 4·9 (4·1–5·7) |
| 18–59 years | 222 | 5·2 (4·7–5·8) | 72 | 4·4 (3·9–5·0) | |
| ≥60 years | 75 | 5·4 (4·3–6·7) | 26 | 6·3 (3·7–10·5) | |
| 28 days after dose 1 – day 29 (+14) | |||||
| GMT (95% CI) | Overall | 295 | 17·6 (14·8–20·9) | 98 | 11·5 (8·7–15·3) |
| 18–59 years | 221 | 18·9 (15·5–22·9) | 72 | 9·6 (7·2–12·8) | |
| ≥60 years | 74 | 14·2 (9·8–20·6) | 26 | 19·2 (9·3–39·6) | |
| Seroconversion n (%) [95% CI] | Overall | 295 | 135 (45·8) [40·0, 51·6] | 98 | 30 (30·6) [21·7–40·7] |
| 18–59 years | 221 | 113 (51·1) [44·3–57·9] | 72 | 18 (25·0) [15·5–36·6] | |
| ≥60 years | 74 | 22 (29·7) [19·7–41·5] | 26 | 12 (46·2) [26·6–66·6] | |
| 28 days after dose 2 – day 57 (+14) | |||||
| GMT (95% CI) | Overall | 291 | 69·9 (60·8–80·4) | 95 | 56·8 (44·4–72·5) |
| 18–59 years | 218 | 75·9 (65·1–88·4) | 70 | 64·4 (49·5–83·7) | |
| ≥60 years | 73 | 54·8 (39·9–75·3) | 25 | 39·9 (22·2–71·7) | |
| Seroconversion n (%) [95% CI] | Overall | 291 | 249 (85·6) [81·0–89·4] | 95 | 80 (84·2) [75·3–90·9] |
| 18–59 years | 218 | 192 (88·1) [83·0–92·1] | 70 | 62 (88·6) [78·7–94·9] | |
| ≥60 years | 73 | 57 (78·1) [66·9–86·9] | 25 | 18 (72·0) [50·6, 87·9] | |
n's for each group represent the number of participants with a non-missing value at the respective visit. For each study vaccine, the GMT and 95% CI of neutralising antibodies were calculated by transforming to the original scale of log 10-transformed mean and its two-sided 95% CI limits at each visit. Seroconversion was defined as a 4-fold increase in titre from baseline. The 95% CIs for each vaccine group were calculated by using the Clopper–Pearson method.
For seroconversion, n in respective intervention column represents number of participants with seroconversion.
CI=confidence interval; GMT=geometric mean titre.
Figure 2.
Distribution of interferon-γ ELISpot response to peptides spanning the SARS-CoV-2 S1 and S2 spike protein subunits.
Analysis conducted in the CMI analysis population (N=50). The CMI analysis population comprised all participants who received a first dose of study vaccine and had at least one post-vaccination CMI assessment available for outcome/endpoint evaluation. Boxes and horizontal bars denote IQR and median ID50, respectively. Whisker endpoints are equal to the maximum and minimum values below or above the median ± 1.5 x IQR. CMI=cell-mediated immune; ELISpot=enzyme-linked immunospot; ID50=50% infective dose; IQR=interquartile range.
There were 34 cases of SARS-CoV-2 infections reported in the 1600 participants during the study period (22 [1·9%] in the SII-ChAdOx1 nCoV-19 group, 10 [3·4%] in the placebo group and 2 [2·0%] in the AZD1222 group); 18 were reported after the first dose and 16 after the second dose. None were severe, and there were no deaths reported in the study. Of these 34 cases, 6 occurred more than 2 weeks after the second dose and on or before day 57 (+14) and all were in baseline-seronegative participants; 3/1162 (0·3%) were in the SII-ChAdOx1 nCoV-19 group, 0/96 (0%) were in the AZD1222 group and 3/287 (1·0%) were in the placebo group. Four cases were symptomatic COVID-19 (two each in the SII-ChAdOx1 nCoV-19 and placebo groups) and two were asymptomatic (one each in the SII-ChAdOx1 nCoV-19 and placebo groups). Among these four symptomatic COVID-19 cases, one participant each in the SII-ChAdOx1 nCoV-19 (0·1%) and placebo (0·3%) groups was hospitalised for COVID-19 with event onset at 21 and 41 days after second dose, respectively, but these were not severe cases as per the WHO clinical progression scale [15].
3.2. Safety
Among all 1600 participants who received a first dose, a total of 19 SAEs in 19 (1·2%) participants were reported, in 15 of 1200 (1·3%, 95% CI 0·7–2·1) participants who received SII-ChAdOx1 nCoV-19, 2 (0·7%, 95% CI 0·1–2·4) who received placebo and 2 (2·0%, 95% CI 0·2–7·0) who received AZD1222. These included COVID-19 (n=11), fracture/dislocation (n=3), malaria (n=1), megaloblastic anaemia (n=1), cataract (n=1), encephalopathy (n=1) and a vocal cord cyst (n=1). All SAEs resolved without sequelae and none were assessed as related to study vaccine/placebo by the investigators and sponsor. There were no thromboembolic-associated or autoimmune-related SAEs.
Among the 1200 participants in the safety cohort, 374 (31·2%) reported 631 unsolicited AEs (which, for the safety cohort, could include those AEs that were solicited from participants in the immunogenicity/reactogenicity cohort), including 302 (33·6%) participants reporting 530 events in the SII-ChAdOx1 nCoV-19 group and 72 (24·0%) participants reporting 101 events in the placebo group. A total of 236 (26·2%) participants reported 384 unsolicited AEs in the SII-ChAdOx1 nCoV-19 group and 39 (13·0%) participants reported 50 unsolicited AEs in the placebo group that were assessed as causally related to study vaccine/placebo (Table 4). The causally related unsolicited AEs in the SII-ChAdOx1 nCoV-19 group with frequency >1% were injection-site pain (n=104, 11·6%) and constitutional symptoms including pyrexia (n=73, 8·1%), body-ache (n=39, 4·3%), headache (n=30, 3·3%), myalgia (n=24, 2·7%), malaise (n=20, 2·2%), asthenia (n=18, 2·0%) and fatigue (n=13, 1·4%). After both the doses (Dose 1 as well as Dose 2), 57 participants (6.3%) reported 135 treatment related AEs in the SII-ChAdOx1 nCoV-19 group whereas 5 participants (1.7%) reported 9 treatment related AEs in the placebo group in the safety cohort. Common reactions (≥1%) were injection site pain, pyrexia, body-ache, myalgia, malaise and headache.
Table 4.
Summary of unsolicited AEs, AESI and SAEs occurring after either vaccine dose - safety population
| Number of participants, n (%), [number of events] | Safety cohort |
Immunogenicity/reactogenicity cohort |
||||
|---|---|---|---|---|---|---|
| SII-ChAdOx1 nCoV-19 (N=900) | Placebo (N=300) | Total (N=1200) | SII-ChAdOx1 nCoV-19 (N=300) | AZD1222 (N=100) | Total (N=400) | |
| Any AE | 302 (33·6) [530] | 72 (24·0) [101] | 374 (31·2) [631] | 50 (16·7) [72] | 20 (20·0) [32] | 70 (17·5) [104] |
| Severe | 3 (0·3) [3] | 1 (0·3) [1] | 4 (0·3) [4] | 0 | 0 | 0 |
| Any treatment-related AE | 236 (26·2) [384] | 39 (13·0) [50] | 275 (22·9) [434] | 0 | 0 | 0 |
| Severe | 1 (0·1) [1] | 0 | 1 (0·1) [1] | 0 | 0 | 0 |
| Any AESI | 20 (2·2) [20] | 10 (3·3) [10] | 30 (2·5) [30] | 5 (1·7) [5] | 2 (2·0) [2] | 7 (1·8) [7] |
| Severe | 1 (0·1) [1] | 0 | 1 (0·1) [1] | 0 | 0 | 0 |
| Any SAEs | 12 (1·3) [12] | 2 (0·7) [2] | 14 (1·2) [14] | 3 (1·0) [3] | 2 (2·0) [2] | 5 (1·3) [5] |
| Severe | 2 (0·2) [2] | 1 (0·3) [1] | 3 (0·3) [3] | 1 (0·3) [1] | 0 | 1 (0·3) [1] |
| SAE causality | ||||||
| Related | 0 | 0 | 0 | 0 | 0 | 0 |
| Not related | 12 (1·3) [12] | 2 (0·7) [2] | 14 (1·2) [14] | 3 (1·0) [3] | 2 (2·0) [2] | 5 (1·3) [5] |
After either vaccine dose=post-first dose as well as post-second dose data combined. n=number of participants with event. Percentages are based on the number of participants in the safety population within each treatment group and total at each level of summarisation. If a participant had a multiple event with different severity (or causality), then the participant was counted only once at the worst severity (or causality) for the number of participants (n).
AE=adverse event; AESI=AE of special interest; SAE=serious AE.
Among the 400 participants in the immunogenicity/reactogenicity cohort, 70 (17·5%) participants reported 104 unsolicited AEs, including 50 (16·7%) participants reporting 72 events in the SII-ChAdOx1 nCoV-19 group and 20 (20·0%) participants reporting 32 events in the AZD1222 group (Table 4); none of these events was judged by the investigator or sponsor to be causally related to study vaccine. Additionally, in the immunogenicity/reactogenicity cohort, 164 (41·0%) participants reported 564 solicited AEs after the first dose and 93 (23·5%) participants reported 256 solicited AEs after the second dose (Table 5). All solicited AEs were reported within 1 week of the study vaccinations. A total of 145 (36·3%) participants reported ≥1 local solicited AE after any dose, of which 129 (32·3%) participants reported events of mild severity and 16 (4·0%) reported events of moderate severity. A total of 144 (36·0%) participants reported ≥1 systemic solicited AE after any dose, of which 118 (29·5%) participants reported events of mild severity, 23 (5·8%) reported event of moderate severity and 3 (0·8%) reported severe events. The frequencies of solicited AEs were similar between the SII-ChAdOx1 nCoV-19 and AZD1222 groups. All solicited AEs resolved without sequelae. The frequencies of solicited AEs with SII-ChAdOx1 nCoV-19 and AZD1222 were lower with the second dose (23·2% and 24·5%, respectively) compared with the first dose (38·7% and 48·0%, respectively). After both the doses (Dose 1 as well as Dose 2), 47 participants (15.7%) reported 359 solicited AEs in the SII-ChAdOx1 nCoV-19 group and 17 participants (17.0%) reported 93 solicited AEs in the AZD1222 group. Common reactions (≥1%) were local injection site reactions of pain, warmth, induration, erythema, swelling and pruritus and systemic reactions of myalgia, fatigue, malaise, headache, chills, arthralgia, pyrexia and nausea.
Table 5.
Summary of solicited AEs (immunogenicity/reactogenicity cohort – safety population)
| Number of participants, n (%) [number of events] | SII-ChAdOx1 nCoV-19 (N=300) | AZD1222 (N=100) | SII-ChAdOx1 nCoV-19 (N=298) | AZD1222 (N=98) |
|---|---|---|---|---|
| First dose | Second dose | |||
| Participants with at least one solicited AE | 116 (38·7) [397] | 48 (48·0) [167] | 69 (23·2) [199] | 24 (24·5) [57] |
| Participants with at least one local solicited AE | 82 (27·3) [181] | 36 (36·0) [69] | 51 (17·1) [109] | 17 (17·3) [27] |
| Pain | 75 (25·0) [114] | 33 (33·0) [51] | 46 (15·4) [71] | 11 (11·2) [17] |
| Warmth | 17 (5·7) [17] | 9 (9·0) [9] | 8 (2·7) [8] | 4 (4·1) [4] |
| Induration | 19 (6·3) [19] | 5 (5·0) [5] | 8 (2·7) [8] | 0 |
| Swelling | 14 (4·7) [14] | 1 (1·0) [1] | 8 (2·7) [8] | 1 (1·0) [1] |
| Erythema | 9 (3·0) [9] | 1 (1·0) [1] | 10 (3·4) [10] | 3 (3·1) [3] |
| Pruritus | 8 (2·7) [8] | 2 (2·0) [2] | 4 (1·3) [4] | 2 (2·0) [2] |
| Participants with at least one systemic solicited AE | 85 (28·3) [216] | 39 (39·0) [98] | 37 (12·4) [90] | 14 (14·3) [30] |
| Fatigue | 41 (13·7) [41] | 23 (23·0) [23] | 18 (6·0) [18] | 8 (8·2) [8] |
| Malaise | 38 (12·7) [38] | 18 (18·0) [18] | 20 (6·7) [20] | 5 (5·1) [5] |
| Myalgia | 37 (12·3) [37] | 13 (13·0) [13] | 20 (6·7) [20] | 6 (6·1) [6] |
| Headache | 24 (8·0) [24] | 15 (15·0) [15] | 10 (3·4) [10] | 3 (3·1) [3] |
| Pyrexia | 22 (7·3) [22] | 9 (9·0) [9] | 3 (1·0) [3] | 2 (2·0) [2] |
| Chills | 20 (6·7) [20] | 10 (10·0) [10] | 6 (2·0) [6] | 3 (3·1) [3] |
| Arthralgia | 22 (7·3) [22] | 6 (6·0) [6] | 10 (3·4) [10] | 2 (2·0) [2] |
| Nausea | 12 (4·0) [12] | 4 (4·0) [4] | 3 (1·0) [3] | 1 (1·0) [1] |
AE= adverse event.
4. Discussion
The present study assessed the safety and immunogenicity of SII-ChAdOx1 nCoV-19 in adults in India and found that it was well tolerated and not associated with vaccine-related SAEs. Additionally, SII-ChAdOx1 nCoV-19 was highly immunogenic, with a GMT ratio for anti-S IgG antibodies that demonstrated statistical non-inferiority to AZD1222. The immunogenicity of AZD1222 has been evaluated in phase 1/2 and phase 3 trials in the UK, South Africa and Brazil [2], [3]. In these studies, the seroconversion rates for anti-S IgG antibody responses were close to 100%, and the anti-S IgG GMTs were approximately 30 000 AU/mL. Seroconversion rates for neutralising antibody responses were greater than 80% after the second dose. Our results for both SII-ChAdOx1 nCoV-19 and AZD1222 were similar. SII-ChAdOx1 nCoV-19 is manufactured at SIIPL Pune, India, using the same ChAdOx1 platform as AZD1222, which is manufactured at a UK facility. Our results successfully bridged SII-ChAdOx1 nCoV-19 to AZD1222.
The safety and reactogenicity of AZD1222 have been extensively demonstrated in clinical trials in the UK, Brazil and South Africa, and no safety-related concerns were identified in these studies [2], [3], [4], [5], [6]. Common reactions to the vaccine included injection-site reactions as well as systemic reactions such as fever, fatigue, malaise, myalgia, asthenia, headache, body-ache and chills. In these trials, most of these reactions were mild in intensity and resolved within 2–3 days. The reactions were also less frequent with the second dose compared to the first dose [2], [3], [4], [5], [6]. The same pattern was also seen in our study.
After receiving regulatory approvals [9,10] millions of doses of SII-ChAdOx1 nCoV-19 as also of AZD1222 have also been distributed across many countries [12]. Very rare events of thrombosis with thrombocytopenia (TTS) and anaphylaxis have been reported from real-world use [16], but overall data supports the continued administration of the vaccine in a two-dose schedule [17].
As seen in our study, previously reported studies found that the immune response to AZD1222 was comparable across age groups (young adults and older adults age group), while reactogenicity was lower in the older adults age group [3], [4], [5]. A study by Kaur et al on post-licensure use of SII-ChAdOx1 nCoV-19 in India has also reported similar trend [18]. The reasons for lower reactogenicity in older adults are not known but may be consistent with the generally lower inflammatory responses seen in this population. Kaur et al hypothesized that pre-existing neutralising antibodies against human adenovirus 5 might be responsible for low reactogenicity in the elderly compared to the young [18].
In the AZD1222 studies [2], [3], [4], [5], [6] and in the present trial, there was a trend for lower reactogenicity after the second dose compared with the first dose. A study on post-licensure use of SII-ChAdOx1 nCoV-19 in India has also reported similar trend [18]. Similar findings are known to occur with the DTwP vaccine, with which systemic reactions (with the exception of fever) may decrease with subsequent doses [19].
The post-dose 2 anti-S IgG and anti-RBD IgG GMTs were similar between young adults and older adults age group in both the vaccine groups. The post-dose 1 GMTs were also similar in both age groups in AZD1222 recipients. However, for SII-ChAdOx1 nCoV-19, the same comparison appeared lower in older adults as compared to the young adults. Since the age group analysis for immune responses was not statistically powered to demonstrate a true difference, this should be viewed with caution.
Seroconversion rates for both IgG and neutralising antibody responses were very high in our study and the AZD1222 clinical trials [[2], [3], [4], [5], [6], [20], [21]]. The MNT assay used in the present study is calibrated against the WHO International Reference Standard, and represents an improvement compared to prior studies that used 50% neutralisation (MN50) titres [2], [3], [5], [20], [21], which may produce variability in the results. It is known that IgG titres correlate with neutralising antibody titres [22], and this was also observed in the present study, although the potential correlation was not evaluated statistically. The effect may be due to anti-S IgG antibodies playing a major role in neutralising the virus. T-cell responses may also have an important role in providing protection against COVID-19. In previous trials of AZD1222, the vaccine showed substantial T-cell immune responses that peaked 2 weeks after the first dose [2], [20], [21]. Similarly, in the present study, T-cell immune responses were seen in both the SII-ChAdOx1 nCoV-19 and AZD1222 groups.
Correlation between serological response and clinical protection has not yet been established for the SARS-CoV-2 virus. Nevertheless, all licensed COVID-19 vaccines have shown a strong humoral immune response as well as substantial efficacy, supporting a link between antibody titres and protection. Real world evidence from India has also shown high protection afforded by SII-ChAdOx1 nCoV-19 against severe COVID-19 and hospitalisation [23], [24], [25]. However, although seroconversion rates for IgG and live virus neutralising antibodies in almost all of the studies have been close to 100%, the efficacy has ranged from 62·1% to 94·1% [26]. In our study, using the validated pseudovirus-based MNT assay, seroconversion rates for neutralising antibodies with SII-ChAdOx1 nCoV-19 and AZD1222 were 85·6% and 84·2%, which are in line with results from the UK study of AZD1222 [2].
There was a slight imbalance in proportion of participants with co-morbidity in the immunogenicity/reactogenicity cohort: 17.7% in SII-ChAdOx1 nCoV-19 group and 26% in AZD1222 group. However, in both with and without co-morbidity participant groups, the fold rise in GMTs with respect to baseline was on a higher side in AZD1222 group as compared to SII-ChAdOx1 nCoV-19 group, thus indicating that the higher proportion of co-morbid participants did not confound the comparison between immune responses of both the groups.
Six cases of COVID-19 occurred more than 2 weeks after the second dose and on or before day 57 (+14) in the study, three of which were in the SII-ChAdOx1 nCoV-19 group. Breakthrough infections, even after receipt of two doses of the vaccine, are not unexpected; this was seen in pooled analysis of trials of AZD1222, in which the vaccine conferred 66·7% (95% CI 57·4, 74·0) protection against symptomatic disease [5]. Importantly, the vaccine has been shown to be highly effective in protecting against severe disease requiring hospitalisation [4], [5], [23], [24], [25].
Our study had a few potential limitations. We excluded previous known SARS-CoV-2 infection cases from the study. Our safety follow up was for 6-months and we did not have longer safety data. However, studies on AZD1222 are collecting data till one year and so far, no delayed safety concern has been reported with the vaccine. We did not age stratify the safety cohort and had a less number of elderly recruited as compared to younger adults. However, consistent with previous clinical studies of AZD12223 and the present study as well, lower reactogenicity was observed in older adults compared with younger age groups and no safety issues were identified in this population. The study did not include participants with uncontrolled comorbid conditions, immunocompromised people, pregnant and lactating women and further studies are needed for these special populations.
In conclusion, our evidence demonstrates that SII-ChAdOx1 nCoV-19, which is now the most commonly used COVID-19 vaccine in the world, has a good safety profile and is highly immunogenic in an adult population in India, with comparable responses to those with AZD1222. These positive results have allowed the use of the vaccine in countries such as the UK and Canada, and support the continued use of SII-ChAdOx1 nCoV-19 worldwide.
5. Contributors
PSK, DK, BG, JV, US, P, NG, and SP contributed to the study design and protocol development. PSK, DK, BG and RV accessed and verified the data. CJB, MT, and SNK led the immunogenicity experiments. CB, SP, and RV contributed to the analysis. PSK, DK, and BG contributed to the manuscript preparation. Manuscript was finalized with considerable inputs from all the authors. AB, MG, PK, BSG, NJG, MT, SL, KS, RM, SM, TSS, KP, DMB, and SRR contributed to data collection. EJK, US and MG contributed to immunogenicity experiments oversight and supervision.
6. Data sharing statement
Individual level participant data will not be made available to others due to privacy concerns. Study protocol is provided in Appendix 2 in Supplementary material.
Declaration of Competing Interest
PSK, CB, AD, MG, US, DK, and BG are employees of SIIPL. JV and EJK are employees of AstraZeneca. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We gratefully acknowledge the contributions of our study participants. We acknowledge the DSMB members Dr Jayaprakash Muliyil, Dr Oommen John and Mr Charudatta Vaman Joglekar, and PPD for clinical monitoring and data management services. We also acknowledge Dr Prakash Bhuyan, MD, PhD, of AstraZeneca Pharmaceuticals LP for advice and critical review of the manuscript. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Stephen Hill and Nicholas White, of Ashfield MedComms, an Ashfield Health company, and funded by AstraZeneca.
Funding
SIIPL funded the contract research organisation and laboratory costs, while the site costs were funded by the Indian Council of Medical Research. The study vaccines were supplied by SIIPL and AstraZeneca.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101218.
Appendix. Supplementary materials
References
- 1.World Health Organization (WHO). WHO Director-General's opening remarks at the media briefing on COVID-19. March 11, 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-COVID-19-11-march-2020 (accessed Jul 5, 2021).
- 2.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AstraZeneca PLC. AstraZeneca's COVID-19 vaccine authorised for emergency supply in the UK. December 30, 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/astrazenecas-COVID-19-vaccine-authorised-in-uk.html (accessed Jul 5, 2021).
- 8.AstraZeneca PLC. AstraZeneca takes next steps towards broad and equitable access to Oxford University's potential COVID-19 vaccine. June 4, 2020. https://www.astrazeneca.com/media-centre/articles/2020/astrazeneca-takes-next-steps-towards-broad-and-equitable-access-to-oxford-universitys-potential-COVID-19-vaccine.html (accessed Jul 5, 2021).
- 9.Ministry of Health and Family Welfare, India. Press statement by the Drugs Controller General of India (DCGI) on Restricted Emergency approval of COVID-19 virus vaccine. January 3, 2021. https://pib.gov.in/PressReleasePage.aspx?PRID=1685761 (accessed Jul 5, 2021).
- 10.World Health Organization (WHO). WHO lists two additional COVID-19 vaccines for emergency use and COVAX roll-out. February 15, 2021. https://www.who.int/news/item/15-02-2021-who-lists-two-additional-COVID-19-vaccines-for-emergency-use-and-covax-roll-out (accessed Jul 5, 2021).
- 11.Ministry of Health and Family Welfare, India. Co-Win – Winning Over COVID_19. https://dashboard.cowin.gov.in/ (accessed Jul 5, 2021).
- 12.Our World in Data. Coronavirus (COVID-19) Vaccinations. https://ourworldindata.org/covid-vaccinations (accessed Jul 5, 2021).
- 13.World Health Organization (WHO) Expert Committee on Biological Standardization. Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody. WHO/BS/2020.2403. December 9–10, 2020. https://cdn.who.int/media/docs/default-source/biologicals/ecbs/bs-2020-2403-sars-cov-2-ab-ik-17-nov-2020_4ef4fdae-e1ce-4ba7-b21a-d725c68b152b.pdf?sfvrsn=662b46ae8&download=true (accessed Jul 28, 2021).
- 14.World Health Organization (WHO). Guidelines on clinical evaluation of vaccines: regulatory expectations. WHO Technical Report Series 1004, Annex 9, 2017. October 21, 2020. https://www.who.int/publications/m/item/WHO-TRS-1004-web-annex-9 (accessed Jul 27, 2021).
- 15.World Health Organization (WHO) Working Group on the Clinical Characterisation and Management of COVID-19 Infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UK Medicines and Healthcare products Regulatory Agency. Summary of Product Characteristics for COVID-19 Vaccine AstraZeneca. Updated June 25, 2021. https://www.gov.uk/government/publications/regulatory-approval-of-COVID-19-vaccine-astrazeneca/information-for-healthcare-professionals-on-COVID-19-vaccine-astrazeneca (accessed Jul 6, 2021).
- 17.Bhuyan P, Medin J, da Silva HG, Yadavalli M, Shankar NK, Mullerova H, Arnold M, Nord M. Very rare thrombosis with thrombocytopenia after second AZD1222 dose: a global safety database analysis. Lancet. 2021 Jul 27 doi: 10.1016/S0140-6736(21)01693-7. S0140-6736(21)01693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur U, Ojhaa B, Pathak BK, et al. A prospective observational safety study on ChAdOx1 nCoV-19 corona virus vaccine (recombinant) use in healthcare workers- first results from India. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO). Information Sheet: Observed rate of vaccine reactions – Diphtheria, Pertussis, Tetanus vaccines. May 2014. https://www.who.int/vaccine_safety/initiative/tools/DTP_vaccine_rates_information_sheet.pdf (accessed Jul 8, 2021).
- 20.Barrett JR, Belij-Rammerstorfer S, Dold C, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27:279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 21.Ewer KJ, Barrett JR, Belij-Rammerstorfer S, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27:270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- 22.Dispinseri S, Secchi M, Pirillo MF, et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Commun. 2021;12:2670. doi: 10.1038/s41467-021-22958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Victor PJ, Mathews KP, Paul H, Mammen JJ, Murugesan M. Protective Effect of COVID-19 Vaccine Among Health Care Workers During the Second Wave of the Pandemic in India. Mayo Clin Proc. 2021;96(9):2493–2494. doi: 10.1016/j.mayocp.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satwik R, Satwik A, Katoch S, Saluja S. ChAdOx1 nCoV-19 effectiveness during an unprecedented surge in SARS COV-2 infections. Eur J Intern Med. 2021 Aug 16 doi: 10.1016/j.ejim.2021.08.005. S0953-6205(21)00271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaur U, Bala S, Mbbs BO, Mbbs SJ, Kansal S, Chakrabarti SS. Occurrence of COVID-19 in priority groups receiving ChAdOx1 nCoV-19 coronavirus vaccine (recombinant): a preliminary analysis from north India. J Med Virol. 2021 Sep 7 doi: 10.1002/jmv.27320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyriakidis NC, López-Cortés A, González EV, et al. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6:28. doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.