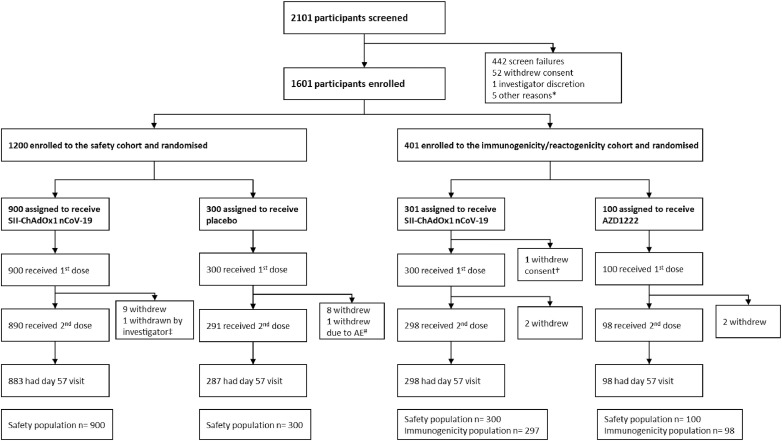
Figure 1.
CONSORT flow chart.
*Participants did not come to site for randomisation visit within window period of 7 days after screening. †1 participant withdrew consent before vaccination. ‡The participant suffered from a neurological serious adverse event and was withdrawn by the investigator. #Participant withdrawn due to adverse event of aggravation of hypertension on day 29 visit. AE=adverse event.