Abstract
Aim: The prevalence of atherosclerotic cardiovascular (CV) disease has risen in Japan due to increasing metabolic risk factors, including dyslipidemia. A positive linear correlation between low-density lipoprotein cholesterol (LDL-C) levels, incidence of CV events, and preventive effects of lipid-lowering therapy (LLT) is well established; however, data in Japan are limited. This analysis evaluated current lipid management practices and risk of recurrent CV events in Japanese post-acute coronary syndrome (ACS) patients.
Methods: EXPLORE-J is a multicenter, 2-year observational study of hospitalized ACS patients in Japan.
Results: At 2-year follow-up ( n =1944, mean age 66 years, 80.3% male), the cumulative incidence of major adverse cardiovascular events (MACE; death associated with myocardial infarction/cerebrovascular accident [CVA] and other CV death, non-fatal ACS, and non-fatal CVA requiring hospitalization during the observation period) was 6.2%; respective incidences of CV death, non-fatal ACS, and CVA were 0.7%, 4.5%, and 1.7%. Statin, intensive statin, and ezetimibe were prescribed for 93.6%, 8.2%, and 3.9% at visit (V)1 (Day[D]1+14), and 92.3%, 10.5%, and 11.6% of patients at V5 (D730±30 days), respectively. Mean LDL-C was reduced from first post-ACS measurement (121.3 mg/dL) to V5 (79.8 mg/dL). A limited number of patients achieved LDL-C <70 mg/dL from V1–V5 (14.4%–34.6%); those with a greater LDL-C reduction by V1 had a lower probability of MACE, indicating the benefits of early LDL-C reduction post ACS.
Conclusions: Guideline-recommended LDL-C target achievement post ACS in Japan is suboptimal, suggesting the need for LLT intensification. Additional analyses by risk stratification of the study population and the benefits of lipid management are planned.
Keywords: Cardiovascular disease, Acute coronary syndrome, Major adverse cardiovascular events, Lipid-lowering therapy, Japan
Introduction
Despite a lower cardiovascular (CV) event rate than in Western countries, similar to other territories, the prevalence of atherosclerotic cardiovascular disease (ASCVD) in Japan, particularly high-risk ASCVD, and the associated healthcare burden have risen in recent years 1) . This partly reflects the aging of the population and partly the growing adoption of a Western lifestyle that has resulted in an increase in metabolic risk factors, including dyslipidemia, a key promoter of atherosclerosis 2) . ASCVD encompasses a spectrum of clinical manifestations of atherosclerosis, including ischemic stroke, peripheral artery disease (PAD), and coronary artery disease (CAD) 3 , 4) . Importantly, patients with ASCVD, particularly those following a recent stroke or acute coronary syndrome (ACS), are at high risk of recurrent ischemic events and CV mortality 5 , 6) .
An abundance of data from global trials of lipid-lowering therapies (LLTs) 7) has demonstrated a positive association between hypercholesterolemia and the incidence of CAD. Conversely, data have demonstrated low-density lipoprotein cholesterol (LDL-C) lowering and reduced CV events with intensive statin therapy 8) ; and, in high-risk patients, additional LLT with ezetimibe 9) and/or a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor 10 , 11) . Results from the ESTABLISH study in Japan showed that when aggressive statin therapy is started early (within 48 hours of an ACS event) and continued for 6 months, this significantly reduced plaque volume, with the percent change in plaque volume significantly associated with percent LDL-C reduction regardless of baseline LDL-C 12) . Early initiation of statin therapy in post-ACS patients also improved long-term clinical outcomes 13) ; however, data from real-world clinical practice in Japan are limited.
The prospective, observational EXPLORE-J study in Japan was conducted to gain insights into the association between LDL-C management and the CV risk of Japanese patients with ACS 14) . At the time of this study (2015/2016), the 2012 Japan Atherosclerosis Society (JAS) guidelines 15) recommended lowering LDL-C to <100 mg/dL for high-risk patients with established CAD, with statins as the first-line treatment option, and combination or alternative therapy with a non-statin LLT (resin, probucol, eicosapentaenoic acid [EPA], and/or ezetimibe). The American College of Cardiology/American Heart Association 16) and the European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) 5 , 17) lipid management guidelines at the time specified a lower LDL-C goal (<70 mg/dL) and at least a 50% LDL-C reduction, using intensive statin therapy and additional LLT with ezetimibe and/or a PCSK9 inhibitor to reduce the risk of CV morbidity and mortality in high-risk patients, including those with ACS. Previously presented results from EXPLORE-J 14) focused on the prevalence of familial hypercholesterolemia 18) as well as the background characteristics of these patients, including comorbidities and metabolic status 19) . Here, we present results from the EXPLORE-J study obtained from investigating the association between the current status of lipid management and the risk of recurrent CV events over 2 years in Japanese post-ACS patients.
Aim
To determine LLT use and risk of recurrent CV events in post-ACS patients in Japan, where the prevalence of atherosclerotic CV disease has increased in recent years yet lipid management practices are not clear.
Methods
Study Design
Details of the study design and methods of this 2-year, multicenter, prospective, observational study of Japanese patients presenting with ACS at 59 sites in Japan have been reported previously 14 , 19) . In brief, 2016 patients enrolled consecutively required hospitalization for ACS during the enrolment period from April 2015 to August 2016 were registered at 59 sites and followed-up for 2 years ( Fig.1 ) .
Fig.1. Study design.
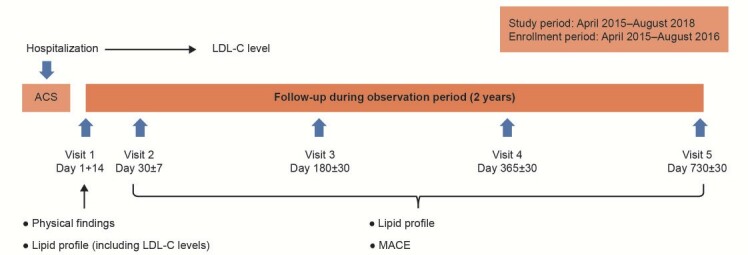
ACS, acute coronary syndrome; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events.
The study was conducted in compliance with the Declaration of Helsinki (amended in October 2013) and the Ethical Guidelines for Medical and Health Research Involving Human Subjects (enacted on December 22, 2014). The study protocol was approved by each participating clinical site’s respective ethical review committee prior to study commencement. All patients provided written informed consent, and patient anonymity is protected.
Study Participants
Study inclusion criteria were as follows: Japanese patients aged ≥ 20 years hospitalized for any ACS (ST-segment-elevation myocardial infarction [STEMI], non-STEMI, or unstable angina [UA]), who provided written informed consent within 7 days of hospitalization. Key exclusion criteria were as follows: chest pain and coronary artery disease associated with prespecified concomitant serious diseases; in-stent thrombosis; enrollment in other investigational studies with interventions that could affect lipid profiles, such as clinical trials with LLTs; and a judgment of being inappropriate for inclusion from the investigators or subinvestigators based on observations of the presence of characteristics that may interfere with the natural course of ACS.
Background Characteristics
Patient background data, including demographic characteristics, physical findings, laboratory data, and medical history, were collected within 14 days of hospitalization (at Visit 1) from electronic case report forms, as described previously 14) . The presence of a comorbidity was determined at the discretion of the attending/reporting physician.
Lipid Profile
The first LDL-C measurement following hospitalization was reported. LDL-C levels (direct and calculated) and other lipid parameters were obtained at Visit 1 (within 14 days of hospitalization for ACS [Day 1+14]), and at subsequent visits, Visit 2 (Day 30±7), Visit 3 (Day 180±30), Visit 4 (Day 365±30), and Visit 5 (Day 730±30). LDL-C levels post ACS were assessed according to the type of LLT (any LLT, statin, intensive statin therapy, PCSK9 inhibitors, fibrates, ezetimibe, or EPA/docosahexaenoic acid [DHA]).
Study Endpoints
The primary endpoint was the incidence of major adverse CV events (MACE) during the period from hospitalization for ACS and the subsequent 2-year observation period. MACE were defined as death associated with myocardial infarction (MI)/cerebrovascular accident (CVA) or other CV death, non-fatal ACS (MI or hospitalization for UA), and non-fatal CVA requiring hospitalization during the observation period.
Secondary endpoints included treatment rate by LLT (based on written prescriptions), including any statin therapy; intensive statin therapy (atorvastatin 20 mg, rosuvastatin ≥ 10 mg, and pitavastatin 4 mg); a PCSK9 inhibitor; fibrates; ezetimibe; or EPA/DHA. Other secondary endpoints included the lipid profile (LDL-C, total cholesterol, high-density lipoprotein cholesterol (HDL-C), non-HDL-C, and triglycerides) from Visits 1 to 5; the incidence of any outcome event (defined as coronary revascularization based on myocardial ischemia, non-coronary revascularization, inpatient treatment due to the occurrence or exacerbation of heart failure, transient ischemic attack [TIA], acute arterial obstruction, central retinal artery occlusion, or other adverse events prolonging or requiring hospitalization) during the period from hospitalization for ACS and the subsequent 2-year observation period; and probability of MACE by LDL-C reduction category from the first measurement after ACS to Visits 1 (Day 1+14 days) and 3 (Day 180±30 days).
Statistical Analysis
The sample size was calculated based on a previous study in Japanese patients with ACS, the Prevention of Atherothrombotic Incidents Following Ischemic Coronary attack (PACIFIC) registry 20) . In the PACIFIC registry, the incidence of MACE was 6.4% at 2 years 21) , hence the planned sample size was 2000 patients with an estimated precision of ±1% in the incidence of MACE with a 95% confidence interval (CI) of 5.3%–7.4%.
Patient demographics and background characteristics are presented as the mean, median, standard deviation, range for continuous data, and number and proportion of subjects in each category for categorial data.
The primary endpoint (the incidence of MACE at 2 years) of the first event after registration was described by Kaplan–Meier curves, and their 95% CI was determined by the Greenwood formula.
Subgroup analyses for the probability of MACE were conducted by baseline LDL-C (<70 vs. ≥ 70 mg/dL) and quartile of absolute and percent LDL-C change; P -values were calculated using the log-rank test. Additionally, Cox proportional hazards models were used to adjust for potential confounding factors. All potential confounders that were used in CREDO-Kyoto risk score were employed. In our data, previous history of both peripheral artery and CAD were highly correlated with hypertension, hence these two risk factors were included instead of hypertension in the Cox model. Albumin was not included in the Cox model because we did not have this data.
LDL-C values from Visits 1 to 5 were calculated using the Friedwald formula. 22) . Further analysis of the secondary endpoints (MACE developed by Visit 2 and between Visits 2 and 5) and treatment rate by LLT were assessed by determining the ratio of subjects on each LLT administered during observation using the full analysis set.
Results
Baseline Characteristics
Of 2016 registered patients at 59 facilities, 1944 were included in this analysis. The most common reason for patient exclusion ( n =72) was failure to obtain informed consent within the stipulated time ( n =62; Supplementary Fig.1 ) 19) .
Supplementary Fig.1.
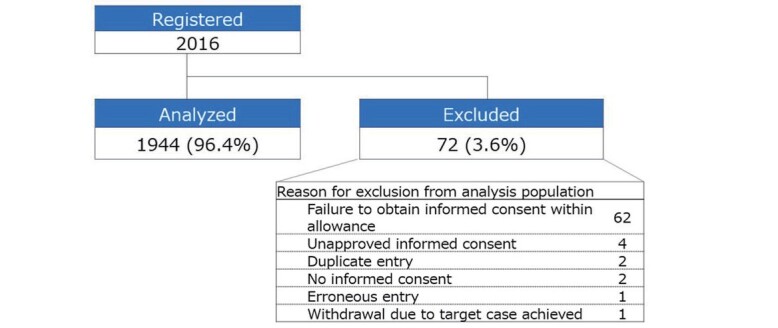
Reasons for patient exclusion
Patients’ baseline mean age was 66.0 years, 80.3% were male, and mean body mass index (BMI) was 24.2 kg/m 2 . Over half the patients presented with STEMI (61.5%) as the index ACS event ( Table 1 ) .
Table 1. Patient baseline characteristics.
| Patients, N | n (%) § | |
|---|---|---|
| Age, years, mean (SD) | 1944 | 66.0 (12.2) |
| Male | 1944 | 1561 (80.3) |
| BMI, kg/m 2 , mean (SD) | 1937 | 24.2 (3.6) |
| ACS type | 1944 | |
| STEMI | 1195 (61.5) | |
| UA | 440 (22.6) | |
| Non-STEMI | 309 (15.9) | |
| eGFR <15 mL/min/1.73 m 2 | 1883 | 41 (2.2) |
| Lipids, mg/dL, mean (SD) # | ||
| LDL-C † | 1827 | 121.3 (40) |
| LDL-C (calculated) | 1767 | 99.4 (31.9) |
| HDL-C | 1831 | 41.1 (11.7) |
| Non-HDL-C | 1788 | 123.8 (35.9) |
| Triglycerides, median (min, max) | 1838 | 109.0 (22, 967) |
| Total cholesterol | 1803 | 165.2 (38.4) |
| History of CV risk factors/comorbidities | 1944 | |
| Dyslipidemia | 1512 (77.8) | |
| Hypertension | 1427 (73.4) | |
| Diabetes mellitus | 679 (34.9) | |
| Smoking | ||
| Current smoker | 739 (38.0) | |
| Previous smoker | 541 (27.8) | |
| None | 664 (34.2) | |
| Coronary artery disease | 355 (18.3) | |
| Cerebrovascular accident | 149 (7.7) | |
| Dialysis | 38 (2.0) | |
| Peripheral artery disease | 37 (1.9) |
§ Unless otherwise specified.
# At visit 1.
† At first measurement after hospitalization.
ACS, acute coronary syndrome; BMI, body mass index; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation; STEMI, ST-segment-elevation myocardial infarction; UA, unstable angina.
Among the reported CV risk factors and comorbidities at baseline, most patients had dyslipidemia (77.8%). Other atherosclerotic risk factors were medical history of hypertension (73.4%) or diabetes mellitus (34.9%), and current smoker (38.0%) or history of smoking (27.8%); a number of patients also had a previous history of CAD, CVA, dialysis, and/or PAD 19) .
Lipid Management Post ACS
Overall, one-third of patients were on an LLT prior to hospitalization for ACS (32.2%), predominantly statin therapy (27.3%); among patients taking any LLT, the proportion on statin therapy was 84.8%. Following hospitalization for ACS, at Visit 1 94.5% of patients were on an LLT, including statin therapy, intensive statin therapy, and ezetimibe, which were prescribed for 93.6%, 8.2% and 3.9% of patients, respectively; at Visit 5 this was 92.3%, 10.5%, and 11.6% of patients, respectively ( Table 2 ) . No patients received PCSK9 inhibitors at Visit 1, and at Visit 5 six patients (0.4%) had received this LLT.
Table 2. LLT at the time of and post ACS.
| n / N , (%) | Prior | Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 |
|---|---|---|---|---|---|---|
| Any LLT | 626/1944 (32.2) | 1837/1944 (94.5) | 1685/1767 (95.4) | 1685/1774 (95.0) | 1587/1669 (95.1) | 1411/1501 (94.0) |
| Statin | 531/1944 (27.3) | 1819/1944 (93.6) | 1665/1767 (94.2) | 1661/1774 (93.6) | 1556/1669 (93.2) | 1385/1501 (92.3) |
| Intensive statin § | 31/1944 (1.6) | 160/1944 (8.2) | 177/1767 (10.0) | 168/1774 (9.5) | 164/1669 (9.8) | 158/1501 (10.5) |
| PCSK9 inhibitors | 0/1944 (0) | 0/1944 (0) | 0/1767 (0) | 2/1772 (0.1) | 5/1670 (0.3) | 6/1495 (0.4) |
| Fibrates | 35/1944 (1.8) | 14/1944 (0.7) | 15/1768 (0.8) | 15/1775 (0.8) | 15/1670 (0.9) | 21/1499 (1.4) |
| Ezetimibe | 40/1944 (2.1) | 75/1944 (3.9) | 94/1767 (5.3) | 121/1775 (6.8) | 154/1668 (9.2) | 174/1499 (11.6) |
| EPA/DHA | 70/1944 (3.6) | 84/1944 (4.3) | 87/1768 (4.9) | 109/1775 (6.1) | 111/1672 (6.6) | 115/1496 (7.7) |
§ Defined as atorvastatin 20 mg, rosuvastatin ≥ 10 mg, or pitavastatin 4 mg
ACS, acute coronary syndrome; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LLT, lipid-lowering therapy; PCSK9, proprotein convertase subtilisin/kexin type 9.
Lipid Profile up to 2 Years Post ACS
Mean baseline LDL-C was 121.3 mg/dL; other lipid/lipoprotein baseline levels are shown in Table 1 . Mean LDL-C level improved from the first measurement post ACS to Visit 1 (99.4 mg/dL) and V2 (80.9 mg/dL), and remained stable up to Visit 5 (79.8 mg/dL; Fig.2 ). Similarly, the proportion of patients achieving an LDL-C level <70 mg/dL and <100 mg/dL increased from Visit 1 (14.4% and 56.5%, respectively) to Visit 2 (34.3% and 80.0%, respectively), and remained constant up to Visit 5 (34.6% and 82.8%, respectively; Fig.2 ).
Fig.2. Mean (±SD) LDL-C levels over 2 years of follow-up, plus the proportions of patients achieving LDL-C target levels.
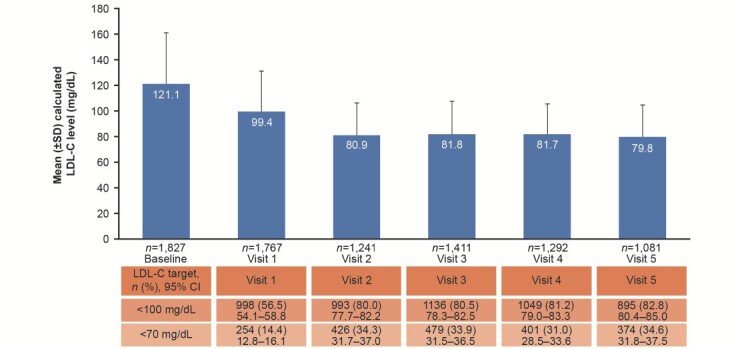
CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation.
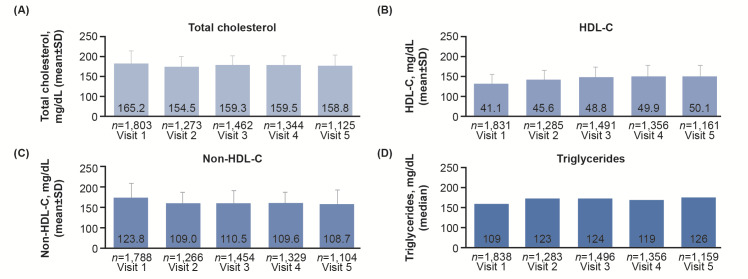
Mean total cholesterol, HDL-C, and non-HDL-C were also improved in a similar manner, with the greatest changes being observed from Visits 1 to 2 and achieved levels remaining stable from Visits 2 to 5 ( Fig.3 ) .
Fig.3. Mean±SD lipid/lipoprotein levels over 2 years of follow-up.
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; SD, standard deviation.
Incidence of MACE Post ACS
Over 2 years post ACS, the incidence of MACE was 6.2% (120/1944) and 133 events were reported ( Table 3 ) . There were 13 CV-related deaths, and the numbers of hospitalizations for ACS and CVA were 85 and 35, respectively. Overall, the incidence of any outcome event over 2 years post ACS was 14.7% ( n =285; 364 events). The most frequent events were coronary revascularization based on myocardial ischemia, inpatient treatment due to the occurrence or exacerbation of heart failure, and non-coronary revascularization, which occurred in 11.3% ( n =219; 250 events), 3.0% ( n =59; 79 events), and 1.2% ( n =23; 28 events) of all patients, respectively. Other events reported were acute arterial obstruction 0.2% ( n =4; four events), TIA 0.1% ( n =1, one event), and other adverse events prolonging or requiring hospitalization 0.1% ( n =2, two events).
Table 3. Incidence of first MACE in post-ACS patients ( N = 1,944) .
| MACE up to Day 730, n (%) | Total number of events | |
|---|---|---|
| Overall MACE | 120 (6.2) | 133 |
| Death associated with MI/CVA, and other CV death | 13 (0.7) | 13 |
| MI | 4 (0.2) | 4 |
| Other CV deaths | 2 (0.1) | 2 |
| CVA | 7 (0.4) | 7 |
| Nonfatal ACS requiring hospitalization | 80 (4.1) | 85 |
| Nonfatal CVA requiring hospitalization | 30 (1.5) | 35 |
MACE were defined as death associated with MI/CVA or other CV death, non-fatal ACS (MI or hospitalization for UA]), and non-fatal CVA requiring hospitalization during the observation period.
ACS, acute coronary syndrome; CV, cardiovascular; CVA, cerebrovascular accident; MACE, major adverse cardiovascular events; MI, myocardial infarction; UA, unstable angina.
At 2-year follow up, the cumulative incidence of MACE was 6.8% (95% CI: 5.7–8.1%; Kaplan–Meier analysis; Fig.4 ). The cumulative incidence of CV death was 0.7% (95% CI: 0.4–1.3%), and that of non-fatal ACS and CVA was 4.5% (95% CI: 3.7–5.6%) and 1.7% (95% CI: 1.2–2.5%), respectively.
Fig.4.Kaplan–Meier curve for the incidence of MACE over 2 years of follow-up.
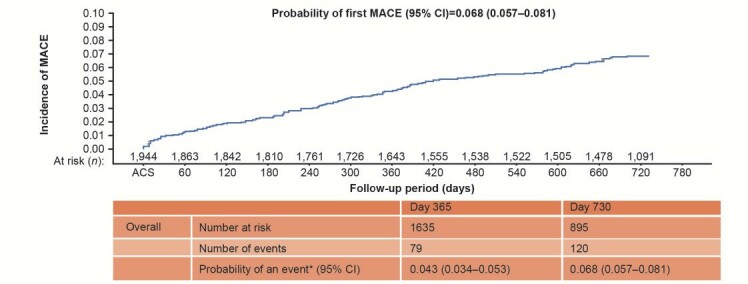
* Kaplan–Meier estimates.
ACS occurrence day was defined as Day 1.
ACS, acute coronary syndrome; CI, confidence interval; MACE, major adverse cardiovascular events.
LDL-C Reduction and Incidence of MACE Post ACS
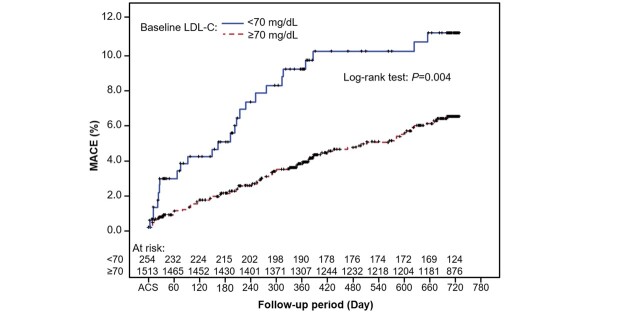
In subgroup analysis by baseline LDL-C, the probability of MACE was higher for patients with baseline LDL-C <70 mg/dL compared with those with LDL-C ≥ 70 mg/dL (log-rank test P =0.004; Supplementary Fig.2 ). The significance did not remain when adjusted for risk factors (age, PAD, diabetes mellitus, glomerular filtration rate, hemoglobin, and previous history of CAD; P =0.6869). No significant difference in the probability of an event was seen between subgroups with baseline LDL-C <100 versus ≥ 100 mg/dL (log-rank test P =0.467).
Supplementary Fig.2.Kaplan–Meier cumulative incidence curve for time to first MACE by subgroup, baseline LDL-C <70 or ≥ 70 mg/dL.
Day is based on ACS occurrence date (Day 1)
ACS, acute coronary syndrome; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular event.
Subgroup analysis by quartiles of change ( Fig.5 ) showed that patients with a greater LDL-C reduction by Visit 1 had a lower probability of MACE, suggesting that an early reduction in LDL-C levels was beneficial in patients following ACS ( Fig.5A ) . Similarly, patients with a sustained reduction in their LDL-C level by Visit 3 had a lower probability of events ( Fig.5C ) . Patient baseline characteristics varied by quartiles of absolute and percent LDL-C change from first measurement to Visit 1 ( Supplementary Tables 1 and 2 ) and first measurement to Visit 3 ( Supplementary Tables 3 and 4 ) , such as age, BMI, type of ACS, lipid levels, and smoking status. The higher the baseline LDL-C, the greater the absolute and percent reduction in LDL-C; however, there was marked difference in incidence of MACE even if there was no significant difference in the achieved LDL-C level.
Fig.5.Probability of first MACE in subgroups by quartile of absolute LDL-C reduction and by quartile of percentage change in LDL-C from first measurement after hospitalization to (A and B) Visit 1 and (C and D) Visit 3.
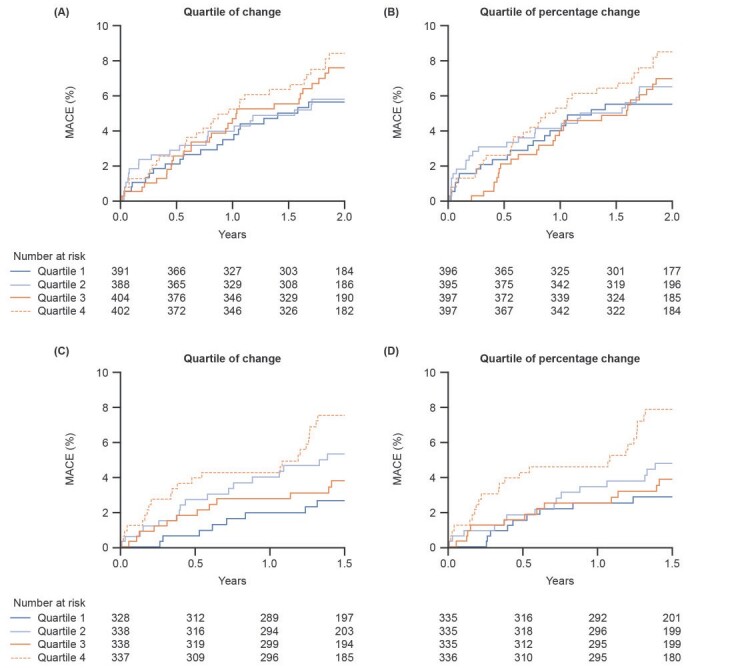
Visit 1 was within 14 days of hospitalization for ACS.
LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events.
Supplementary Table 1. Patient baseline characteristics and lipid profiles, and LLT post ACS and change in LDL-C: subgroup analysis by quartile of absolute LDL-C change from the first measurement after hospitalization to Visit 1 § .
| All patients ( n = 1,585) |
Q1 ( n = 391) |
Q2 ( n = 388) |
Q3 ( n = 404) |
Q4 ( n = 402) |
P -value | |
|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 66.2 (12.2) | 63.7 (12.4) | 65.8 (12.0) | 67.0 (12.0) | 68.1 (11.9) | <0.001 |
| Male, n (%) | 1,268 (80.0) | 306 (78.3) | 315 (81.2) | 324 (80.2) | 323 (80.3) | 0.772 |
| BMI, kg/m 2 , mean (SD) | 24.2 (3.5) | 24.5 (3.4) | 24.2 (3.4) | 24.4 (3.5) | 23.6 (3.8) | <0.001 |
| ACS type, n (%) | <0.001 | |||||
| STEMI | 992 (62.6) | 277 (70.8) | 246 (63.4) | 246 (60.9) | 223 (55.5) | |
| UA | 347 (21.9) | 49 (12.5) | 79 (20.4) | 114 (28.2) | 105 (26.1) | |
| Non-STEMI | 246 (15.5) | 65 (16.6) | 63 (16.2) | 44 (10.9) | 74 (18.4) | |
| eGFR <15 mL/min/1.73 m 2 , n (%) | 27 (1.7) | 2 (0.5) | 5 (1.3) | 10 (2.5) | 10 (2.5) | 0.818 |
| LDL-C, mean (SD), mg/dL, first measurement # | 122.6 (39.5) | 157.8 (35.6) | 125.9 (27.2) | 108.9 (31.3) | 99.0 (35.4) | <0.001 |
| Baseline lipid profile (Visit 1), mg/dL | ||||||
| LDL-C, mean (SD), calculated | 98.9 (31.4) | 93.6 (28.4) | 94.2 (26.2) | 98.4 (30.9) | 109.1 (36.4) | <0.001 |
| HDL-C, mean (SD) | 40.8 (11.6) | 38.8 (11.5) | 40.4 (10.9) | 41.1 (11.2) | 42.8 (12.4) | <0.001 |
| Triglycerides, median (min, max) | 109 (22, 361) | 113 (31, 340) | 107 (31, 361) | 113 (38, 358) | 104 (22, 340) | 0.001 |
| Total cholesterol, mean (SD) | 163.6 (37.0) | 157.2 (34.2) | 158.0 (31.6) | 164.1 (37.1) | 174.5 (41.6) | <0.001 |
| History of CV risk factors/comorbidities, n (%) | ||||||
| Dyslipidemia | 1,240 (78.2) | 326 (83.4) | 283 (72.9) | 321 (79.5) | 310 (77.1) | 0.004 |
| Hypertension | 1,154 (72.8) | 274 (70.1) | 267 (68.8) | 319 (79.0) | 294 (73.1) | 0.006 |
| Diabetes mellitus | 552 (34.8) | 126 (32.2) | 139 (35.8) | 143 (35.4) | 144 (35.8) | 0.666 |
| Smoking | 0.002 | |||||
| Current smoker | 610 (38.5) | 163 (41.7) | 163 (42.0) | 146 (36.1) | 138 (34.3) | |
| Previous smoker | 452 (28.5) | 104 (26.6) | 127 (32.7) | 116 (28.7) | 105 (26.1) | |
| None | 523 (33.0) | 124 (31.7) | 98 (25.3) | 142 (35.1) | 159 (39.6) | |
| Coronary artery disease | 281 (17.7) | 35 (9.0) | 45 (11.6) | 88 (21.8) | 113 (28.1) | |
| Cerebrovascular accident | 126 (7.9) | 30 (7.7) | 23 (5.9) | 40 (9.9) | 33 (8.2) | |
| Dialysis | 24 (1.5) | 1 (0.3) | 3 (0.8) | 10 (2.5) | 10 (2.5) | |
| Peripheral artery disease | 29 (1.8) | 6 (1.5) | 8 (2.1) | 6 (1.5) | 9 (2.2) | |
| LLT at Visit 1, n (%) | ||||||
| Statin | 380 (97.2) | 372 (95.9) | 376 (93.1) | 359 (89.3) | ||
| Intensive statin | 47 (12.0) | 24 (6.2) | 23 (5.7) | 27 (6.7) | ||
| Ezetimibe | 21 (5.4) | 11 (2.8) | 14 (3.5) | 16 (4.0) | ||
| Change in LDL-C from first measurement after hospitalization to Visit 1 | ||||||
| Number | 391 | 388 | 404 | 402 | ||
| mg/dL, mean (SD) | −64.2 (21.5) | −31.7 (6.5) | −10.5 (5.3) | 10.1 (16.1) |
§ Evaluated patients with available and changed LDL-C measurement at Visit 1. Visit 1 was within 14 days of hospitalization for ACS.
# At first measurement after hospitalization.
ACS, acute coronary syndrome; BMI, body mass index; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; SD, standard deviation; STEMI, ST-segment- elevation myocardial infarction; UA, unstable angina.
Supplementary Table 2. Patient baseline characteristics and lipid profiles, and LLT post ACS and change in LDL-C: subgroup analysis by quartile of percent LDL-C change from the first measurement after hospitalization to Visit 1 § .
| All patients ( n = 1,585) |
Q1 ( n = 391) |
Q2 ( n = 388) |
Q3 ( n = 404) |
Q4 ( n = 402) |
P -value | |
|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 66.2 (12.2) | 65.2 (12.1) | 65.2 (12.5) | 66.4 (12.0) | 67.9 (11.8) | 0.003 |
| Male, n (%) | 1,268 (80.0) | 310 (78.3) | 317 (80.3) | 324 (81.6) | 317 (79.8) | 0.706 |
| BMI, kg/m 2 , mean (SD) | 24.2 (3.5) | 24.4 (3.4) | 24.2 (3.4) | 24.4 (3.5) | 23.7 (3.8) | 0.007 |
| ACS type, n (%) | <0.001 | |||||
| STEMI | 992 (62.6) | 279 (70.5) | 252 (63.8) | 240 (60.5) | 221 (55.7) | |
| UA | 347 (21.9) | 53 (13.4) | 79 (20.0) | 111 (28.0) | 104 (26.2) | |
| Non-STEMI | 246 (15.5) | 64 (16.2) | 64 (16.2) | 46 (11.6) | 72 (18.1) | |
| eGFR <15 mL/min/1.73 m 2 , n (%) | 27 (1.7) | 4 (1.0) | 4 (1.0) | 9 (2.3) | 10 (2.5) | 0.835 |
| LDL-C, mean (SD), mg/dL, first measurement # | 122.6 (39.5) | 144.0 (37.2) | 131.6 (36.7) | 114.2 (33.0) | 100.8 (36.4) | <0.001 |
| Baseline lipid profile (Visit 1), mg/dL | ||||||
| LDL-C, mean (SD), calculated | 98.9 (31.4) | 82.5 (22.2) | 98.7 (27.8) | 103.3 (29.8) | 111.0 (36.8) | <0.001 |
| HDL-C, mean (SD) | 40.8 (11.6) | 38.5 (11.4) | 40.4 (11.0) | 41.2 (11.1) | 43.0 (12.4) | <0.001 |
| Triglycerides, median (min, max) | 109 (22, 361) | 109 (33, 340) | 108 (31, 361) | 114 (38, 358) | 104 (22, 340) | 0.014 |
| Total cholesterol, mean (SD) | 163.6 (37.0) | 144.9 (27.9) | 163.1 (33.1) | 169.6 (36.0) | 176.7 (42.0) | <0.001 |
| History of CV risk factors/comorbidities, n (%) | ||||||
| Dyslipidemia | 1,240 (78.2) | 309 (78.0) | 302 (77.0) | 319 (80.4) | 308 (77.6) | 0.675 |
| Hypertension | 1,154 (72.8) | 281 (71.0) | 279 (70.6) | 305 (76.8) | 289 (72.8) | 0.182 |
| Diabetes mellitus | 552 (34.8) | 141 (35.6) | 131 (33.2) | 139 (35.0) | 141 (35.5) | 0.879 |
| Smoking | 0.002 | |||||
| Current smoker | 610 (38.5) | 163 (41.2) | 156 (39.5) | 155 (39.0) | 136 (34.3) | |
| Previous smoker | 452 (28.5) | 116 (29.3) | 122 (30.9) | 110 (27.7) | 104 (26.2) | |
| None | 523 (33.0) | 117 (29.5) | 117 (29.6) | 132 (33.2) | 157 (39.5) | |
| Coronary artery disease | 281 (17.7) | 41 (10.4) | 49 (12.4) | 81 (20.4) | 110 (27.7) | |
| Cerebrovascular accident | 126 (7.9) | 29 (7.3) | 29 (7.3) | 36 (9.1) | 32 (8.1) | |
| Dialysis | 24 (1.5) | 2 (0.5) | 3 (0.8) | 9 (2.3) | 10 (2.5) | |
| Peripheral artery disease | 29 (1.8) | 7 (1.8) | 7 (1.8) | 6 (1.5) | 9 (2.3) | |
| LLT at Visit 1 ( n / N ), n (%) | ||||||
| Statin | 385/396 (97.2) | 373/395 (94.4) | 373/397 (94.0) | 356/397 (89.7) | ||
| Intensive statin | 43/396 (10.9) | 27/395 (6.8) | 24/397 (6.0) | 27/397 (6.8) | ||
| Ezetimibe | 18/396 (4.5) | 11/395 (2.8) | 17/397 (4.3) | 16/397 (4.0) | ||
| Change in LDL-C from first measurement after hospitalization to Visit 1 | ||||||
| Number | 396 | 395 | 397 | 397 | ||
| mg/dL, mean (SD) | −42.3 (7.98) | −25.0 (4.27) | −9.3 (4.44) | 13.8 (31.57) |
§ Evaluated patients with available and changed LDL-C measurement at Visit 1.
# At first measurement after hospitalization.
Visit 1 was within 14 days of hospitalization for ACS.
ACS, acute coronary syndrome; BMI, body mass index; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; SD, standard deviation; STEMI, ST-segment- elevation myocardial infarction; UA, unstable angina.
Supplementary Table 3. Patient baseline characteristics and lipid profiles, and LLT post ACS and change in LDL-C: subgroup analysis by quartile of absolute LDL-C change from the first measurement after hospitalization to Visit 3 § .
| All patients ( n = 1,411) |
Q1 ( n = 328) |
Q2 ( n = 338) |
Q3 ( n = 338) |
Q4 ( n = 337) |
P -value | |
|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 66.0 (12.1) | 61.4 (12.2) | 65.7 (12.0) | 67.2 (11.6) | 69.5 (11.4) | <0.001 |
| Male, n (%) | 1,135 (80.4) | 272 (82.9) | 274 (81.1) | 269 (79.6) | 260 (77.2) | 0.291 |
| BMI, kg/m 2 , mean (SD) | 24.2 (3.5) | 24.9 (3.4) | 24.4 (3.4) | 23.9 (3.7) | 23.6 (3.3) | <0.001 |
| ACS type, n (%) | 0.007 | |||||
| STEMI | 871 (61.7) | 228 (69.5) | 216 (63.9) | 203 (60.1) | 188 (55.8) | |
| UA | 314 (22.3) | 59 (18.0) | 63 (18.6) | 75 (22.2) | 92 (27.3) | |
| Non-STEMI | 226 (16.0) | 41 (12.5) | 59 (17.5) | 60 (17.8) | 57 (16.9) | |
| eGFR <15 mL/min/1.73 m 2 , n (%) | 23 (1.7) | 0 (0.0) | 3 (0.9) | 5 (1.5) | 15 (4.5) | 0.002 |
| LDL-C, mean (SD), mg/dL, first measurement # | 121.5 (39.0) | 167.6 (33.8) | 125.4 (19.8) | 105.7 (22.2) | 88.7 (25.2) | <0.001 |
| Baseline lipid profile (Visit 1), mg/dL | ||||||
| LDL-C, mean (SD), calculated | 98.8 (30.9) | 116.6 (38.2) | 98.6 (25.7) | 91.5 (24.3) | 87.3 (25.5) | <0.001 |
| HDL-C, mean (SD) | 41.2 (11.6) | 39.8 (11.1) | 40.7 (10.5) | 42.3 (12.9) | 41.9 (11.7) | 0.026 |
| Triglycerides, median (min, max) | 108 (25, 552) | 121 (31, 552) | 106 (39, 337) | 106 (25, 436) | 101 (36, 441) | <0.001 |
| Total cholesterol, mean (SD) | 164.0 (37.2) | 183.8 (45.3) | 162.9 (31.0) | 156.8 (30.9) | 151.0 (31.0) | <0.001 |
| History of CV risk factors/comorbidities, n (%) | ||||||
| Dyslipidemia | 1,109 (78.6) | 286 (87.2) | 251 (74.3) | 251 (74.3) | 267 (79.2) | <0.001 |
| Hypertension | 1,040 (73.7) | 224 (68.3) | 241 (71.3) | 260 (76.9) | 262 (77.7) | 0.014 |
| Diabetes mellitus | 465 (33.0) | 97 (29.6) | 101 (29.9) | 112 (33.1) | 133 (39.5) | 0.022 |
| Smoking | <0.001 | |||||
| Current smoker | 525 (37.2) | 151 (46.0) | 127 (37.6) | 115 (34.0) | 106 (31.5) | |
| Previous smoker | 410 (29.1) | 79 (24.1) | 110 (32.5) | 108 (32.0) | 94 (27.9) | |
| None | 476 (33.7) | 98 (29.9) | 101 (29.9) | 115 (34.0) | 137 (40.7) | |
| Coronary artery disease | 255 (18.1) | 26 (7.9) | 39 (11.5) | 71 (21.0) | 105 (31.2) | |
| Cerebrovascular accident | 100 (7.1) | 20 (6.1) | 20 (5.9) | 21 (6.2) | 31 (9.2) | |
| Dialysis | 20 (1.4) | 0 (0.0) | 4 (1.2) | 4 (1.2) | 11 (3.3) | |
| Peripheral artery disease | 27 (1.9) | 2 (0.6) | 3 (0.9) | 8 (2.4) | 13 (3.9) | |
| LLT at Visit 1 ( n / N ), n (%) | ||||||
| Statin | 321/325 (98.8) | 326/333 (97.9) | 317/331 (95.8) | 295/334 (88.3) | ||
| Intensive statin | 58/325 (17.8) | 18/333 (5.4) | 27/331 (8.2) | 17/334 (5.1) | ||
| Ezetimibe | 38/326 (11.7) | 17/333 (5.1) | 15/331 (4.5) | 14/334 (4.2) | ||
| Change in LDL-C from first measurement after hospitalization to Visit 1 | ||||||
| Number | 328 | 338 | 338 | 337 | ||
| mg/dL, mean (SD) | −93.1 (25.0) | −51.0 (7.7) | −25.6 (7.5) | 9.5 (19.2) |
§ Evaluated patients with available and changed LDL-C measurement at Visit 3.
# At first measurement after hospitalization.
Visit 1 was within 14 days of hospitalization for ACS.
ACS, acute coronary syndrome; BMI, body mass index; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; SD, standard deviation; STEMI, ST-segment- elevation myocardial infarction; UA, unstable angina.
Supplementary Table 4. Patient baseline characteristics and lipid profiles, and LLT post ACS and change in LDL-C: subgroup analysis by quartile of percent LDL-C change from the first measurement after hospitalization to Visit 3 § .
| All patients ( n = 1,411) |
Q1 ( n = 335) |
Q2 ( n = 335) |
Q3 ( n = 335) |
Q4 ( n = 336) |
P -value | |
|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 66.0 (12.1) | 62.3 (12.6) | 65.3 (12.0) | 67.2 (11.6) | 69.2 (11.4) | <0.001 |
| Male, n (%) | 1,135 (80.4) | 281 (83.9) | 279 (83.3) | 262 (78.2) | 253 (75.3) | 0.013 |
| BMI, kg/m 2 , mean (SD) | 24.2 (3.5) | 24.9 (3.4) | 24.5 (3.4) | 23.7 (3.5) | 23.6 (3.3) | <0.001 |
| ACS type, n (%) | 0.007 | |||||
| STEMI | 871 (61.7) | 231 (69.0) | 214 (63.9) | 203 (60.6) | 187 (55.7) | |
| UA | 314 (22.3) | 62 (18.5) | 64 (19.1) | 73 (21.8) | 90 (26.8) | |
| Non-STEMI | 226 (16.0) | 42 (12.5) | 57 (17.0) | 59 (17.6) | 59 (17.6) | |
| eGFR <15 mL/min/1,73 m 2 , n (%) | 23 (1.7) | 0 (0.3) | 2 (0.6) | 5 (1.5) | 15 (4.6) | 0.007 |
| LDL-C, mean (SD), mg/dL, first measurement # | 121.5 (39.0) | 153.1 (38.6) | 129.7 (32.7) | 111.5 (27.7) | 91.9 (26.8) | <0.001 |
| Baseline lipid profile (Visit 1), mg/dL | ||||||
| LDL-C, mean (SD), calculated | 98.8 (30.9) | 107.7 (36.8) | 100.7 (30.8) | 95.4 (25.4) | 89.6 (26.5) | <0.001 |
| HDL-C, mean (SD) | 41.2 (11.6) | 39.6 (10.9) | 40.6 (11.2) | 42.5 (12.6) | 42.0 (11.7) | 0.004 |
| Triglycerides, median (min, max) | 108 (25, 552) | 117 (31, 408) | 106 (35, 552) | 107 (25, 436) | 101 (36, 441) | <0.001 |
| Total cholesterol, mean (SD) | 164.0 (37.2) | 173.3 (41.8) | 165.7 (39.1) | 161.4 (32.0) | 153.7 (31.7) | <0.001 |
| History of CV risk factors/comorbidities, n (%) | ||||||
| Dyslipidemia | 1,109 (78.6) | 275 (82.1) | 262 (78.2) | 249 (74.3) | 269 (80.1) | 0.089 |
| Hypertension | 1,040 (73.7) | 232 (69.3) | 238 (71.0) | 257 (76.7) | 260 (77.4) | 0.036 |
| Diabetes mellitus | 465 (33.0) | 104 (31.0) | 104 (31.0) | 100 (29.9) | 135 (40.2) | 0.015 |
| Smoking | 0.006 | |||||
| Current smoker | 525 (37.2) | 149 (44.5) | 120 (35.8) | 121 (36.1) | 109 (32.4) | |
| Previous smoker | 410 (29.1) | 86 (25.7) | 113 (33.7) | 100 (29.9) | 92 (27.4) | |
| None | 476 (33.7) | 100 (29.9) | 102 (30.4) | 114 (34.0) | 135 (40.2) | |
| Coronary artery disease | 255 (18.1) | 28 (8.4) | 47 (14.0) | 66 (19.7) | 100 (29.8) | |
| Cerebrovascular accident | 100 (7.1) | 18 (5.4) | 21 (6.3) | 21 (6.3) | 32 (9.5) | |
| Dialysis | 20 (1.4) | 1 (0.3) | 2 (0.6) | 5 (1.5) | 11 (3.3) | |
| Peripheral artery disease | 27 (1.9) | 3 (0.9) | 3 (0.9) | 7 (2.1) | 13 (3.9) | |
| LLT at Visit 1 ( n / N ), n (%) | ||||||
| Statin | 329/333 (98.8) | 322/328 (98.2) | 314/329 (95.4) | 294/333 (88.3) | ||
| Intensive statin | 59/333 (17.7) | 18/328 (5.5) | 26/329 (7.9) | 17/333 (5.1) | ||
| Ezetimibe | 37/333 (11.1) | 19/329 (5.8) | 14/329 (4.3) | 14/333 (4.2) | ||
| Change in LDL-C from first measurement after hospitalization to Visit 1 | ||||||
| Number | 335 | 335 | 335 | 336 | ||
| mg/dL, mean (SD) | −57.6 (7.29) | −40.5 (4.30) | −23.8 (5.94) | 12.7 (24.44) |
§ Evaluated patients with available and changed LDL-C measurement at Visit 3.
# At first measurement after hospitalization.
Visit 1 was within 14 days of hospitalization for ACS.
ACS, acute coronary syndrome; BMI, body mass index; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; SD, standard deviation; STEMI, ST-segment- elevation myocardial infarction; UA, unstable angina.
Discussion
This analysis of data from the EXPLORE-J registry showed that lipid management in post-ACS patients in Japan is suboptimal, resulting in an unfavorable CV event rate in current clinical practice. Although most patients (94.5%) received LLT following hospitalization for ACS, despite the high-risk status of the study population, a very limited proportion received intensive statin therapy or ezetimibe (8.2% and 3.9%, respectively). At 2-year follow-up, the proportion was only marginally greater (10.5% and 11.6%), while 65.4% of patients had inadequately controlled LDL-C levels of ≥ 70 mg/dL at this time.
At 2 years post ACS, the incidence of MACE in this analysis was 6.2%, which is similar to the 2-year incidence of MACE (6.4%) in Japanese patients with ACS in the PACIFIC registry 20 , 21) . Notably, the incidence of MACE was lower among patients with greater absolute LDL-C reduction in our analysis. Randomized controlled trials of LLTs have demonstrated a positive association between further LDL-C lowering, even to below the current recommended targets, and reduced CV events 7 - 11 , 23) ; however, our results need to be interpreted carefully. Baseline LDL-C levels, not treated LDL-C levels, were used in our analysis. Notably, for patients with baseline LDL-C <70 mg/dL there was no significant reduction in the risk of MACE. This result may indicate that there is some difficulty in targeting LDL-C levels with LLT. Several further explanations could be proposed. One is that lower baseline LDL-C levels can reflect factors of an unfavorable prognosis such as poor nutrition, frailty, advanced age, and other comorbidities such as heart failure. Patient data regarding heart failure, ejection failure, and albumin levels would clarify findings; however, this data was not available and further investigation is required. It may be that lower LDL-C at baseline was related to a larger LDL-C reduction due to a larger infarct size, hence a higher MACE incidence can be explained by a more severe event. The event rate is a likely reflection of background high-risk characteristics rather than the prognostic reflection of LDL-C levels. Another explanation is that this population may be undertreated with non-LDL-C-lowering therapies for CV risk reduction because of their low baseline LDL-C levels.
In post-ACS patients, early 24) and sustained 11) reductions in LDL-C levels are beneficial. Most recently, the ESC/EAS updated their guidelines for the management of dyslipidemias. The 2019 update recommends even lower LDL-C levels (<55 mg/dL) in very high-risk patients including those with ACS, largely based on the results from the Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab (ODYSSEY OUTCOMES) trial in 18,924 patients with recent ACS (within 1–12 months) 11) . The target LDL-C in this trial was 25–50 mg/dL. The ESC/EAS guidelines also recommend reducing LDL-C levels by 50% from baseline; in ODYSSEY OUTCOMES LDL-C reductions of >50% were observed early with PCSK9 inhibitor therapy (alirocumab) and were generally sustained during a median 2.8 years of follow-up, reducing the risk of MACE 11) and CV mortality 25) . In our study, subgroup analysis showed that patients with a greater early (by Visit 1) LDL-C reduction had a lower probability of MACE; this was also seen for patients whose LDL-C reduction was maintained at Visit 3. Additionally, a greater reduction in LDL-C levels is associated with better outcomes at early and late stages. This supports the need for intensification of LLT, with initial intensification of statin therapy to maximal tolerated dose, as suggested in the 2019 Japanese Circulation Society (JCS) guidelines for Japanese patients 4) .
The 2012 JAS guidelines for the prevention of ASCVD 15) , recommending an LDL-C target of <100 mg/dL for secondary prevention in high-risk patients with established CAD, were revised in 2017 26) (during the follow-up period of our study) to a lower target of <70 mg/dL. The 2019 JCS guidelines reinforced intensive LDL-C lowering and recommended a maximum tolerated dose of intensive statin therapy for all post-ACS patients 4) . Nevertheless, our study results show that, in a real-life clinical practice setting in Japan, more than half of post-ACS LLT-treated patients were not at guideline-recommended LDL-C target at 2 years post ACS, and the use of high-intensity statins and ezetimibe was very low despite the suboptimal LDL-C target achievement. The lack of alignment with guideline revisions may reflect the difficulty in achieving a ‘treat-to-target’ strategy, which in general has a tendency to lead to inadequate treatment and can be an obstacle in daily clinical work. In our study, LLT titration to achieve target LDL-C levels was suboptimal. This may have been due to concerns regarding adverse events associated with the use of higher-dose statins, and/or lack of awareness regarding urgency to treat. In fact, patients in the fourth quartile of LDL-C change had lower baseline LDL-C and BMI than patients in the other quartiles, suggesting that baseline LDL-C is one of the key determinants in drug titration decisions. Emerging evidence and new guidelines support the implementation of more aggressive residual risk management with higher dose statin therapy followed by PCSK9 inhibitors (which are associated with a very favorable safety profile), with or without ezetimibe, to achieve target LDL-C and CV event reduction according to patients’ baseline risk.
It cannot be discounted that the higher CV event rate seen in our study in patients with residual elevated LDL-C may be attributed in part to confounding factors such as heart failure, or an individual’s muscle mass or nutritional state. Further limitations of this analysis include the observational nature of the study that the data was accessed from, the small sample size and number of events, the fact that the subanalyses did not have formal power calculations, and the study duration (2 years).
Conclusion
Lipid management in post-ACS patients in Japan is suboptimal, and patients remain at considerable risk of recurrent ischemic events. Intensification of LDL-C management with greater use of high-intensity statin therapy, and use of additional LLT with ezetimibe and/or a PCSK9 inhibitor, is needed to reduce the risk of CV morbidity and mortality in high-risk patients in Japan, including those with ACS. To further characterize and quantify the patient population and the benefit from lipid management, an additional analysis of EXPLORE-J by risk stratification of the patient population is planned.
Acknowledgements
The authors thank the patients, their families, and all investigators involved in the study; Tamio Teramoto of Teikyo University (Tokyo, Japan), Shun Ishibashi of Jichi Medical University (Tochigi, Japan), Kotaro Yokote of Chiba University (Chiba, Japan), Tomonori Okamura of Keio University (Tokyo, Japan), and Hiroyuki Daida of Juntendo University (Tokyo, Japan) for collaboration and advice regarding planning of the study; Mebix (Tokyo, Japan) for assistance with study implementation/operation; BML (Tokyo, Japan) for PCSK9-related and genome-related analysis; CTD (Tokyo, Japan) for consulting; and Shizuya Yamashita of Rinku General Medical Center (Osaka, Japan) and Toru Yoshizumi of Kawasaki Hospital (Hyogo, Japan) for advice on aspects of methodology. The authors would also like to thank Yoshiharu Takagi and Yukiko Morimoto of Sanofi for providing support with statistical analysis; and Yasunori Nakahigashi and Makiko Usami of Sanofi for assistance with study implementation/operation. Medical writing assistance and editorial support, under the direction of the authors, were provided by Nila Bhana, MSc, of Prime Global (Knutsford, United Kingdom) according to Good Publication Practice guidelines (http://annals.org/aim/article/2424869/good-publication-practice-communicating-company-sponsored-medical-research-gpp3) and funded by Sanofi. The authors were involved in the study design as well as the collection, analysis, and interpretation of data. All authors had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Sources of Funding
This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Disclosures
Masato Nakamura and Junya Ako have received honoraria from Daiichi Sankyo, Kowa, Bayer, Amgen Astellas Biopharma, and Sanofi.
Hidenori Arai has received honoraria from Sanofi, Daiichi Sankyo, MSD, Kowa, and Pfizer.
Atsushi Hirayama has received honoraria from Bayer, Daiichi Sankyo, Astellas Amgen Biopharma, Astellas, Sanofi, MSD, and Eisai; clinical research funding from Bayer and Daiichi Sankyo; and scholarship grants from MSD, Sanofi, Astellas Amgen Biopharma, and Daiichi Sankyo.
Atsushi Nohara has received honoraria from Sanofi.
Yoshitaka Murakami has no conflicts of interest to declare.
Asuka Ozaki is an employee of Sanofi.
Mariko Harada-Shiba has received honoraria from Amgen Astellas Biopharma, Astellas, Sanofi, MSD, Kowa, and Aegerion; and scholarship grants from Aegerion, Astellas, Kaneka Medics, Takeda, and Recordati.
References
- 1).Davis KL, Meyers J, Zhao Z, McCollam PL and Murakami M: High-Risk Atherosclerotic Cardiovascular Disease in a Real-World Employed Japanese Population: Prevalence, Cardiovascular Event Rates, and Costs. J Atheroscler Thromb, 2015; 22: 1287-1304 [DOI] [PubMed] [Google Scholar]
- 2).Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Gotoh S, Fukuhara M, Ikeda F, Shikata K, Yoshida D, Yonemoto K, Kamouchi M, Kitazono T and Kiyohara Y: Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961-2009). Circulation, 2013; 128: 1198-1205 [DOI] [PubMed] [Google Scholar]
- 3).Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Jr., Sperling L, Virani SS and Yeboah J: 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol, 2019; 73: 3168-3209 [Google Scholar]
- 4).Kimura K, Kimura T, Ishihara M, Nakagawa Y, Nakao K, Miyauchi K, Sakamoto T, Tsujita K, Hagiwara N, Miyazaki S, Ako J, Arai H, Ishii H, Origuchi H, Shimizu W, Takemura H, Tahara Y, Morino Y, Iino K, Itoh T, Iwanaga Y, Uchida K, Endo H, Kongoji K, Sakamoto K, Shiomi H, Shimohama T, Suzuki A, Takahashi J, Takeuchi I, Tanaka A, Tamura T, Nakashima T, Noguchi T, Fukamachi D, Mizuno T, Yamaguchi J, Yodogawa K, Kosuge M, Kohsaka S, Yoshino H, Yasuda S, Shimokawa H, Hirayama A, Akasaka T, Haze K, Ogawa H, Tsutsui H and Yamazaki T: JCS 2018 Guideline on Diagnosis and Treatment of Acute Coronary Syndrome. Circ J, 2019; 83: 1085-1196 [DOI] [PubMed] [Google Scholar]
- 5).Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT and ESC Scientific Document Group: 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J, 2016; 37: 2999-3058 [Google Scholar]
- 6).Rossini R, Capodanno D, Lettieri C, Musumeci G, Limbruno U, Molfese M, Spatari V, Calabria P, Romano M, Tarantini G, Gavazzi A and Angiolillo DJ: Long-term outcomes of patients with acute coronary syndrome and nonobstructive coronary artery disease. Am J Cardiol, 2013; 112: 150-155 [DOI] [PubMed] [Google Scholar]
- 7).Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E and Sabatine MS: Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA, 2016; 316: 1289-1297 [DOI] [PubMed] [Google Scholar]
- 8).Cholesterol Treatment Trialists Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J and Collins R: Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet, 2010; 376: 1670-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM and IMPROVE-IT Investigators: Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med, 2015; 372: 2387-2397 [DOI] [PubMed] [Google Scholar]
- 10).Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR and Fourier Steering Committee Investigators: Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med, 2017; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]
- 11).Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM and ODYSSEY OUTCOMES Committees Investigators: Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med, 2018; 379: 2097-2107 [Google Scholar]
- 12).Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H and Daida H: Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation, 2004; 110: 1061-1068 [DOI] [PubMed] [Google Scholar]
- 13).Dohi T, Miyauchi K, Okazaki S, Yokoyama T, Yanagisawa N, Tamura H, Kojima T, Yokoyama K, Kurata T and Daida H: Early intensive statin treatment for six months improves long-term clinical outcomes in patients with acute coronary syndrome (Extended-ESTABLISH trial): a follow-up study. Atherosclerosis, 2010; 210: 497-502 [DOI] [PubMed] [Google Scholar]
- 14).Nakamura M, Uno K, Hirayama A, Ako J, Nohara A, Arai H and Harada-Shiba M: Exploration into lipid management and persistent risk in patients hospitalised for acute coronary syndrome in Japan (EXPLORE-J): protocol for a prospective observational study. BMJ Open, 2017; 7: e014427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K and Japan Atherosclerosis S: Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. J Atheroscler Thromb, 2013; 20: 517-523 [DOI] [PubMed] [Google Scholar]
- 16).Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Lloyd-Jones DM, Blum CB, McBride P, Eckel RH, Schwartz JS, Goldberg AC, Shero ST, Gordon D, Smith SC, Jr., Levy D, Watson K and Wilson PW: 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol, 2014; 63: 2889-2934 [DOI] [PubMed] [Google Scholar]
- 17).Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs RH, Kjekshus JK, Perrone Filardi P, Riccardi G, Storey RF, David W and Clinical Practice Guidelines Committee of the Spanish Society of Cardiology: [ESC/EAS Guidelines for the management of dyslipidaemias]. Rev Esp Cardiol, 2011; 64: 1168 e1161-1168 e1160 [DOI] [PubMed] [Google Scholar]
- 18).Harada-Shiba M, Ako J, Arai H, Hirayama A, Murakami Y, Nohara A, Ozaki A, Uno K and Nakamura M: Prevalence of familial hypercholesterolemia in patients with acute coronary syndrome in Japan: Results of the EXPLORE-J study. Atherosclerosis, 2018; 277: 362-368 [DOI] [PubMed] [Google Scholar]
- 19).Nakamura M, Ako J, Arai H, Hirayama A, Murakami Y, Nohara A, Uno K, Ozaki A and Harada-Shiba M: Investigation into lipid management in acute coronary syndrome patients from the EXPLORE-J study. J Atheroscler Thromb, 2019; 26: 559-572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Miyauchi K, Morino Y, Tsukahara K, Origasa H, Daida H and PACIFIC Steering Committee Members: The PACIFIC (Prevention of AtherothrombotiC Incidents Following Ischemic Coronary attack) Registry: Rationale and design of a 2-year study in patients initially hospitalised with acute coronary syndrome in Japan. Cardiovasc Drugs Ther, 2010; 24: 77-83 [DOI] [PubMed] [Google Scholar]
- 21).Daida H, Miyauchi K, Ogawa H, Yokoi H, Matsumoto M, Kitakaze M, Kimura T, Matsubara T, Ikari Y, Kimura K, Tsukahara K, Origasa H, Morino Y, Tsutsui H, Kobayashi M and Isshiki T: Management and two-year long-term clinical outcome of acute coronary syndrome in Japan: prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ J, 2013; 77: 934-943 [DOI] [PubMed] [Google Scholar]
- 22).Friedewald WT, Levy RI and Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972; 18: 499-502 [PubMed] [Google Scholar]
- 23).Ray KK, Ginsberg HN, Davidson MH, Pordy R, Bessac L, Minini P, Eckel RH and Cannon CP: Reductions in atherogenic lipids and major cardiovascular events: A pooled analysis of 10 ODYSSEY trials comparing alirocumab with control. Circulation, 2016; 134: 1931-1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S and Stern T: Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA, 2001; 285: 1711-1718 [DOI] [PubMed] [Google Scholar]
- 25).Steg PG, Szarek M, Bhatt DL, Bittner VA, Bregeault MF, Dalby AJ, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Ostadal P, Parkhomenko A, Pordy R, Roe MT, Tricoci P, Vogel R, White HD, Zeiher AM and Schwartz GG: Effect of Alirocumab on Mortality After Acute Coronary Syndromes. Circulation, 2019; 140: 103-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S and Committee for Epidemiology and Clinical Management of Atherosclerosis: Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]