Abstract
Aims: Various pathological processes related to diabetes cause endothelial dysfunction. Eicosanoids derived from arachidonic acid (AA) have roles in vascular regulation. Fibrates have recently been shown to attenuate vascular complications in diabetics. Here we examined the effects of pemafibrate, a selective peroxisome proliferator-activated receptor α modulator, on plasma eicosanoid levels and endothelial function in diabetic mice.
Methods: Diabetes was induced in 7-week-old male wild-type mice by a single injection of streptozotocin (150 mg/kg). Pemafibrate (0.3 mg/kg/day) was administered orally for 3 weeks. Untreated mice received vehicle. Circulating levels of eicosanoids and free fatty acids were measured using both gas and liquid chromatography-mass spectrometry. Endothelium-dependent and endothelium-independent vascular responses to acetylcholine and sodium nitroprusside, respectively, were analyzed.
Results: Pemafibrate reduced both triglyceride and non-high-density lipoprotein-cholesterol levels ( P <0.01), without affecting body weight. It also decreased circulating levels of AA ( P <0.001), thromboxane B 2 ( P <0.001), prostaglandin E 2 , leukotriene B 4 ( P <0.05), and 5-hydroxyeicosatetraenoic acid ( P <0.001), all of which were elevated by the induction of diabetes. In contrast, the plasma levels of 15-deoxy-Δ 12,14 -prostaglandin J 2 , which declined following diabetes induction, remained unaffected by pemafibrate treatment. In diabetic mice, pemafibrate decreased palmitic acid (PA) and stearic acid concentrations ( P <0.05). Diabetes induction impaired endothelial function, whereas pemafibrate ameliorated it ( P <0.001). The results of ex vivo experiments indicated that eicosanoids or PA impaired endothelial function.
Conclusion: Pemafibrate diminished the levels of vasoconstrictive eicosanoids and free fatty acids accompanied by a reduction of triglyceride. These effects may be associated with the improvement of endothelial function by pemafibrate in diabetic mice.
Keywords: Pemafibrate, Eicosanoid, Diabetes, Endothelial function
Abbreviation: AA: arachidonic acid, Ach: acetylcholine, ANOVA: analysis of variance, COX: cyclooxygenase, GC-MS: gas chromatography-mass spectrometry, HDL: high-density lipoprotein, HETE: hydroxyeicosatetraenoic acid, LC-MS/MS: liquid chromatography-tandem mass spectrometry, LOX: lipoxygenase, LTB 4 : leukotriene B 4 , PA: palmitic acid, 15d-PGJ 2 : 15-deoxy-Δ 12,14 -prostaglandin J 2 , PGE 2 : prostaglandin E 2 , PPARα: peroxisome proliferator-activated receptor α, SNP: sodium nitroprusside, SPPARMα: selective peroxisome proliferator-activated receptor α modulator, STZ: streptozotocin, TXB 2: thromboxane B 2
Introduction
Vascular complications associated with atherosclerosis are the most serious consequences in diabetic patients. Endothelial dysfunction is an initiation step of their development 1) . Various diabetes-related pathological processes cause endothelial dysfunction. Eicosanoids derived from arachidonic acid (AA) through the action of cyclooxygenases (COXs) and lipoxygenases (LOXs) play numerous roles in vascular regulation 2 - 4) . Recent studies have shown that diabetic patients have higher enzymatic activity related to the AA cascade and therefore elevated levels of AA-derived eicosanoids 5 - 7) and that these lipid mediators play critical roles in the development of vascular complications in diabetics 8 - 11) .
Previous studies have suggested that fibrates, peroxisome proliferator-activated receptor (PPAR) α agonists, attenuate the development of vascular complications such as endothelial dysfunction, retinopathy, and nephropathy in a diabetic condition 12 - 16) . Fibrates are widely prescribed lipid-lowering drugs for the treatment of hypertriglyceridemia. Plasma triglyceride levels have recently garnered considerable attention as a residual risk for cardiovascular events 17) . Clinical studies have demonstrated that treatment with fibrates reduces cardiovascular events associated with hypertriglyceridemia 18 - 21) . Yet there are several limitations concerning the use of fibrates 22) ; for example, their activity on PPARα is relatively weak, and their efficacy depends on the targeted population. However, previous studies have suggested that fibrates possess pleiotropic effects, including anti-inflammatory effects, in addition to beneficial effects on lipid levels 23) . Pemafibrate, which is a selective PPARα modulator (SPPARMα), which has very high potency and selectivity for PPARα, has recently been introduced into clinical practice 24 , 25) . Both clinical and basic studies have indicated that pemafibrate occupies a substantial role in lowering triglyceride levels and attenuating atherogenesis 26 - 31) .
Nevertheless, the effects of pemafibrate on eicosanoids and endothelial function, especially in diabetes, have not been fully investigated. Therefore, in this study, we examined the effects of pemafibrate on plasma lipid concentrations and AA-derived eicosanoid levels as well as on endothelial function in diabetic mice.
2. Methods
2-1. Animal Model
Male C57BL/6 mice were purchased from Japan SLC, Inc. All mice were fed with normal chow throughout the study. The mice were randomly divided into three groups, namely, non-diabetic control, untreated diabetic, and pemafibrate-treated diabetic. Diabetes was induced in 7-week-old male mice by a single injection of streptozotocin (STZ, 150 mg/kg). Pemafibrate (0.3 mg/kg/day), provided by Kowa Company, Ltd. (Nagoya, Japan), was administered orally to the diabetic mice for 3 weeks; untreated mice received vehicle only. All animal care and experimental procedures conformed to the animal experimentation guidelines of Tokushima University. The protocol was reviewed and approved by the Animal Care and Use Committee of Tokushima University.
2-2. Blood Glucose Levels and Laboratory Data
Glucose levels in tail vein blood were measured using Startstrip XP2 (NIPRO) at three time points: before fasting, 3 days after injection (to confirm a diabetic condition), and at the time of harvest. During harvest, blood was collected from the left ventricle into EDTA-containing tubes. Plasma was separated by centrifugation at 9000 rpm for 15 min at 4℃ and stored at −80℃ until required. The plasma levels of lipids—e.g., total cholesterol, high-density lipoprotein (HDL)-cholesterol, and triglycerides—were measured at SanritsuZelkova (Japan). Non-HDL-cholesterol levels were calculated by subtracting HDL-cholesterol levels from total cholesterol levels.
2-3. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) and Gas Chromatography-Mass Spectrometry (GC-MS)
The concentrations of lipid mediators and free fatty acids in plasma were measured by LC-MS/MS and GC-MS, respectively, at Kobe University Graduate School of Medicine as previously described 32 , 33) . In this study, AA and AA-derived eicosanoids such as prostaglandin E 2 (PGE 2 ), 15-deoxy-Δ 12,14 -PGJ 2 (15d-PGJ 2 ), thromboxane B 2 (TXB 2 ), leukotriene B 4 (LTB 4 ), and 5-hydroxyeicosatetraenoic acid (5-HETE) were measured in plasma. The system consisted of a Q-Trap 6500 (Sciex) equipped with a Shimadzu LC-30AD HPLC system. A ZORBAX Eclipse Plus C18 column (100 mm×4.6 mm, 3.5 µm; Agilent Technologies) was used with a gradient of methanol/water/acetic acid from 55:45:0.01 to 98:2:0.01 (v/v/v) at a flow rate of 0.4 mL/min. For monitoring and quantifying the levels of eicosanoids, a multiple reaction monitoring (MRM) method was developed with signature ion pairs of Q1 (parent ion)/Q3 (characteristic fragment ion) for each molecule. Identification was conducted according to published criteria by using the LC retention time, specific fragmentation patterns, and at least 6 diagnostic fragmentation ions. Quantification was carried out on the basis of the peak area of the MRM chromatograph, and linear calibration curves were obtained with authentic standards for each compound.
To measure plasma free fatty acid concentrations, nonadecanoic acid (C19:0) was used as an internal standard. After total lipids were extracted by methanol, free fatty acids were converted to methyl esters by derivatization with trimethylsilyl-diazomethane. Fatty acid methyl esters were then analyzed using GC-MS QP2010 Plus (Shimadzu, Kyoto, Japan). The capillary column used for fatty acid separation was SP-2650 (100 m, an inner diameter of 0.25 mm, membrane thickness of 0.20 µm; Sigma-Aldrich). The column temperature was maintained at 140℃ for 5 min and then increased gradually by 4℃/min to 240℃ and held there for 20 min. Afterward, the samples were injected in split mode with a split ratio of 1:5. Each fatty acid methyl ester was detected in the selected ion-monitoring mode. All results were normalized to the peak height of the C19:0 internal standard.
2-4. Vascular Reactivity Assay
At 3 weeks after the initiation of pemafibrate administration, the mice were sacrificed, and the whole aorta was immediately isolated after perfusion with 0.9% sodium chloride solution. After the fat and connective tissue around the aorta were carefully removed, aortic rings of 1.5–2 mm thickness were cut from the thoracic aorta for vascular reactivity analysis as described previously 34) . Briefly, vascular rings handled carefully to avoid damage to the inner surface were mounted on wires in the chambers of a multivessel myograph filled with modified Krebs–Henseleit buffer (118.4 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl 2 , 1.2 mM KH 2 PO 4 , 1.2 mM MgSO 4 , 25 mM NaHCO 3 , and 11.1 mM glucose), which was aerated (95% O 2 , 5% CO 2 ) and warmed (37℃). Changes in isometric tension were recorded on a polygraph (LabChart). The viability of aortic segments was tested with 31.4 mM KCl. To determine the relaxation response, the aortic rings were contracted by adding phenylephrine (10 −9 to 10 −4 M) to submaximal tensions (60% of the maximum relaxation response). After a stable contraction was determined, the rings were exposed to increasing concentrations of acetylcholine (Ach; 10 −9 to 10 −4 M) or sodium nitroprusside (SNP; 10 −9 to 10 −4 M) to obtain a cumulative concentration–response curve. Endothelium-dependent and endothelium-independent vascular reactivity was analyzed in response to Ach and SNP, respectively. In some experiments, aortic segments obtained from non-diabetic wild-type mice were incubated with 5 µM PGE 2 , 20 µM TXB 2 (Cayman Chemical), and 200 µM palmitic acid (PA; Chem Service, Inc.) for 4 h before examining vascular reactivity.
2-5. Western Blotting
Protein was isolated from the liver and separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels as described previously. The following primary antibodies were used: anti-thromboxane synthase, anti-PGE synthase (Abcam), and anti-α-tubulin (MBL). The protein expression level was analyzed with ECL-plus reagent (GE Healthcare) using a luminescent image analyzer (LAS-4000 mini, Fuji Film).
2-6. Statistical Analysis
All results were expressed as mean±standard error of the mean (SEM). Comparison of parameters between two groups was performed with unpaired Student’s t -test. Comparisons of multiple groups were performed using one-way analysis of variance (ANOVA) followed by Scheffe’s post hoc test. Dose–response curves were compared by two-factor repeated-measures ANOVA, followed by Tukey’s post hoc test for between-group comparisons. The correlation between the levels of eicosanoids and triglyceride concentration was examined by univariate analysis. A P value of <0.05 was considered significant.
3. Results
3-1. Effect of Pemafibrate on Lipid Profile
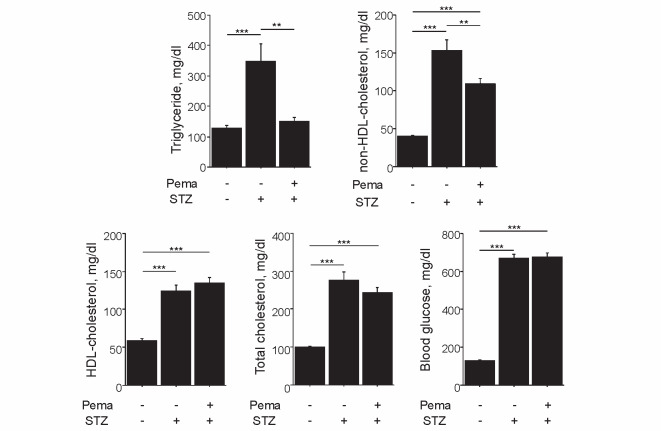
Diabetes induction by STZ injection significantly increased plasma lipid levels, including triglyceride concentrations ( P <0.001), as described previously 34 , 35) . Pemafibrate significantly reduced both triglyceride and non-HDL-cholesterol levels in diabetic mice, without affecting the levels of total cholesterol, HDL-cholesterol, and blood glucose ( Fig.1 ) . Pemafibrate did not affect body weight in diabetic mice (untreated [19.7±2.6 g] vs. treated [19.7±2.1 g]).
Fig.1. Effect of pemafibrate on lipid profile.
Induction of diabetes with STZ significantly elevated plasma lipid concentrations, whereas pemafibrate reduced the levels of triglycerides and non-HDL-cholesterol in diabetic mice. Pemafibrate did not affect the levels of HDL-cholesterol, total cholesterol, and blood glucose ( n =16–18 per group). ** P <0.01 and *** P <0.001. All values are mean±SEM.
3-2. Pemafibrate Reduced Plasma Levels of AA-Derived Eicosanoids
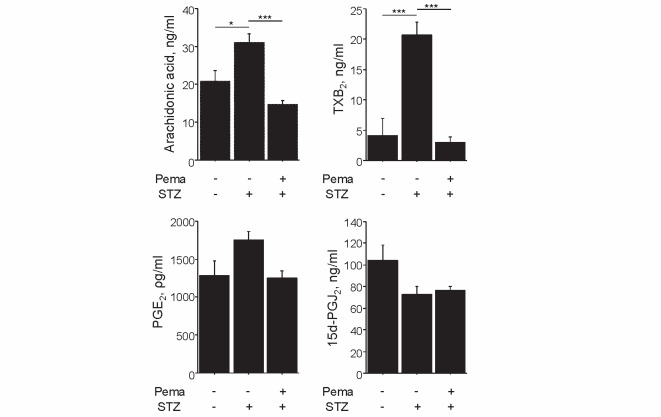
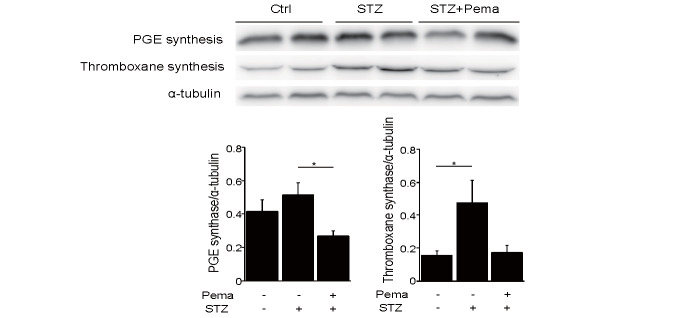
We examined the effects of pemafibrate on eicosanoids derived from AA. Induction of diabetes increased the plasma levels of AA and eicosanoids produced by the COX2 pathway, such as TXB 2 ( P <0.001) and PGE 2 . Treatment with pemafibrate significantly diminished AA and TXB 2 levels ( P <0.001, respectively) and tended to decrease PGE 2 in diabetic mice. In contrast, induction of diabetes reduced 15d-PGJ 2 , which remained unaffected by pemafibrate ( Fig.2 ) . We also explored the effect of pemafibrate on the expression of enzymes synthesizing these vasoconstrictive eicosanoids in the liver. Thromboxane synthase, which was significantly elevated following diabetes induction by STZ ( P <0.05), tended to decline after pemafibrate treatment. Furthermore, the levels of PGE synthase, which tended to increase with STZ-induced diabetes, were significantly lowered by pemafibrate ( P <0.05; Fig.3 ).
Fig.2. Pemafibrate reduced eicosanoids produced by the COX2 pathway.
Induction of diabetes elevated the levels of AA, TXB 2 , and PGE 2 , whereas pemafibrate decreased their expression levels. Induction of diabetes reduced 15d-PGJ 2 levels, which remained unaffected by pemafibrate treatment ( n =5 per group). * P <0.05 and *** P <0.001. All values are mean±SEM.
Fig.3. Pemafibrate reduced the expression of PGE synthase and thromboxane synthase.
Induction of diabetes by STZ significantly promoted the levels of thromboxane synthase, which tended to decline after treatment with pemafibrate. Furthermore, STZ-induced diabetes tended to increase PGE synthase levels, which were significantly lowered by pemafibrate ( n =8 per group). All values are mean±SEM. * P <0.05.
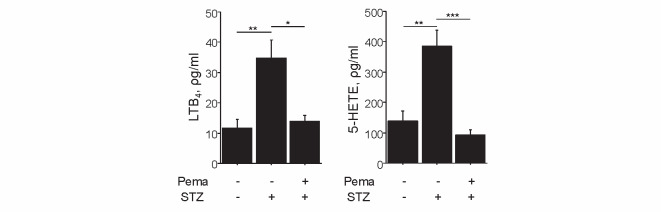
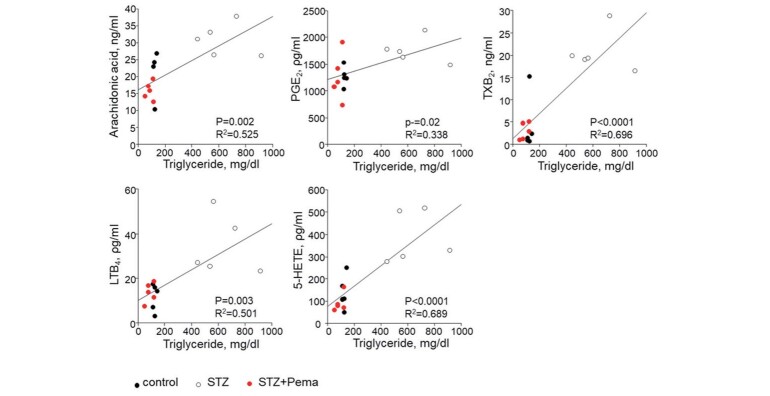
Induction of diabetes also increased the plasma levels of eicosanoids produced by the 5-LOX pathway, such as LTB 4 ( P <0.01) and 5-HETE ( P <0.01), whereas pemafibrate attenuated these levels ( P <0.05 and P <0.001, respectively) in diabetic mice ( Fig.4 ) . Moreover, these eicosanoid levels were found to positively correlate with triglyceride levels ( Fig.5 ) .
Fig.4. Pemafibrate reduced eicosanoids produced by the LOX pathway.
Induction of diabetes elevated LTB 4 and 5-HETE levels, whereas pemafibrate reduced their expressions ( n =5 per group). * P <0.05, ** P <0.01, and *** P <0.001. All values are mean±SEM.
Fig.5. Eicosanoid levels positively correlated with triglyceride levels.
The levels of AA and AA-derived eicosanoids positively correlated with triglyceride levels ( n =5 per group).
3-3. Pemafibrate Reduced Plasma Levels of Saturated Free Fatty Acids
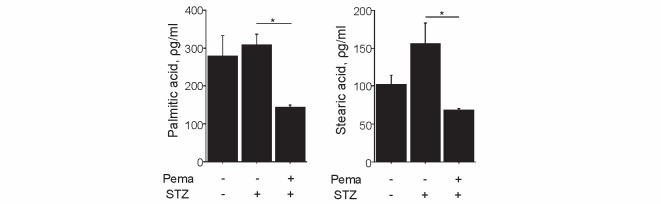
We also examined the effects of pemafibrate on the plasma levels of saturated free fatty acids. Induction of diabetes mildly increased plasma PA and stearic acid levels, which were significantly decreased following treatment with pemafibrate ( P <0.05, respectively), as shown in Fig.6 .
Fig.6. Pemafibrate reduced saturated free fatty acids.
Pemafibrate reduced palmitic acid and stearic acid in diabetic mice ( n =5 per group). * P <0.05. All values are mean±SEM.
3-4. Pemafibrate Ameliorated Endothelial Dysfunction in Diabetic mice
We examined whether pemafibrate ameliorated endothelial dysfunction in diabetic mice. Induction of diabetes impaired endothelium-dependent vasodilation as determined in response to Ach ( P <0.001), whereas pemafibrate ameliorated endothelial dysfunction in diabetic mice ( P <0.001), as compared with vehicle. On the other hand, endothelium-independent vascular reactivity in response to SNP did not differ across the three groups ( Fig.7 ) . These results indicated that pemafibrate ameliorated endothelial dysfunction in diabetic mice.
Fig.7. Pemafibrate attenuated endothelial dysfunction in diabetic mice.
Vascular reactivity to Ach or SNP was determined using aortic rings obtained from our mice. (A) Induction of diabetes impaired endothelium-dependent vasodilation in response to Ach. Pemafibrate ameliorated endothelial dysfunction, as compared with vehicle. (B) Endothelium-independent vascular response did not differ among the three groups ( n =13–18 per group). ** P <0.01 vs. control, † P <0.05 vs. STZ. All values are mean±SEM.
3-5. Eicosanoids and PA Impaired Endothelial Function
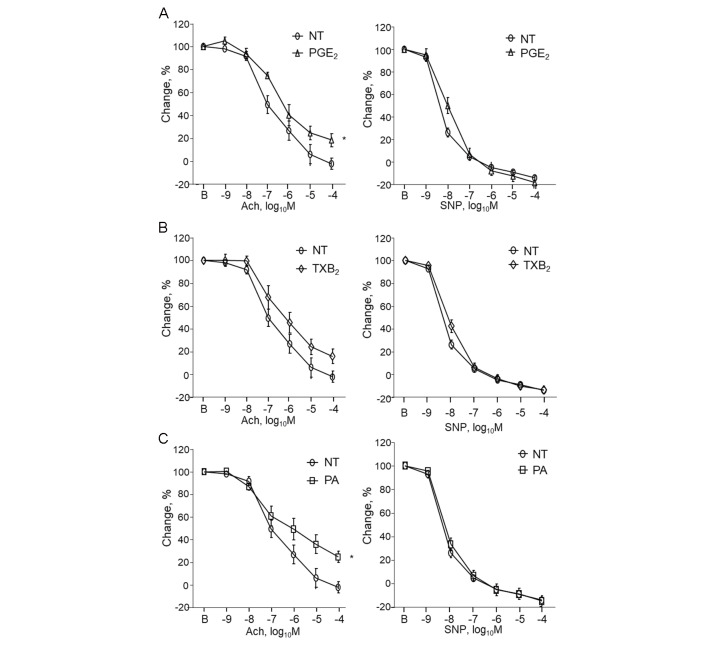
Finally, we examined whether eicosanoids or PA impaired endothelial function. PGE 2 impaired endothelium-dependent vasodilation, as determined by the response to Ach ( P <0.05). TXB 2 tended to impair endothelium-dependent vascular function. In addition, PA resulted in a significant impairment of endothelium-dependent vascular function ( Fig.8 ) . Nonetheless, PGE 2 , TXB 2 , and PA did not affect endothelium-independent vascular function, which was measured in response to SNP ( Fig.8 ) . These results suggested that reduced levels of eicosanoids and PA were associated with improved endothelial function following pemafibrate treatment.
Fig.8. Eicosanoids impaired endothelial function in non-diabetic mice.
(A) Incubation with PGE 2 significantly impaired endothelium-dependent vasodilation, as determined by the response to Ach. (B) TXB 2 tended to impair endothelial function although it did not reach statistical significance. (C) PA significantly impaired endothelial function. Endothelium-independent vascular function, as measured in response to SNP, did not differ across these groups ( n =8–9 per group). * P <0.05 vs. NT (non-treatment). All values are mean±SEM.
4. Discussion
Various pathological processes related to diabetes cause endothelial dysfunction, an initial step in atherosclerosis. Eicosanoids generated from AA have attracted much interest as regulators of vascular homeostasis. In this study, we examined the effects of pemafibrate, a newly developed SPPARMα, on the plasma levels of AA-derived eicosanoids in STZ-induced diabetic mice. Induction of diabetes increased plasma levels of TXB 2 , PGE 2 , LTB 4 , and 5-HETE, all of which potentially impair endothelial function; pemafibrate, however, reduced these levels. In addition, pemafibrate reduced plasma saturated free fatty acid levels in diabetic mice. We also found that pemafibrate ameliorated endothelial dysfunction in diabetic mice. The results of our present study suggested that a reduction in the levels of vasoconstrictive eicosanoids and saturated fatty acids was associated with an improvement in the endothelial function of pemafibrate-treated diabetic mice.
In addition to ameliorating dyslipidemia, fibrates exert various beneficial effects such as anti-inflammatory, antioxidative, and antiapoptotic effects through activating PPARα 15) . Therefore, previous studies have suggested that PPARα agonists have vascular protective effects. Besides, several studies have demonstrated that fibrates attenuate the development of vascular complications (e.g., endothelial dysfunction, retinopathy, and nephropathy) in a diabetic condition 12 - 16) . Endothelial damage interferes with vascular homeostasis and initiates pathological processes related to vascular disease 36) . Thus, impaired endothelial function represents an early hallmark of and a potential therapeutic target for vascular disorders 37) . However, the effect of pemafibrate on endothelial function remains obscure. Hence, we investigated the effects of pemafibrate on endothelial function in diabetic mice and explored the underlying mechanisms.
Eicosanoids derived from AA have various roles in vascular regulation 2 - 4) . In this study, we found that diabetes induction increased the plasma levels of AA, TXB 2 , PGE 2, LTB 4 , and 5-HETE, whereas pemafibrate decreased these levels. The COX2 pathway produces TXB 2 , which is a metabolite of TXA 2 , and PGE 2 in the AA cascade. TXA 2 is one of the major vasoconstrictive eicosanoids. A previous study showed that TXA 2 plays a pivotal role in the development of endothelial dysfunction in diabetic mice 38) . In addition, TXA 2 stimulates platelet activation and leukocyte–endothelial cell interaction, thereby contributing to the initiation and progression of atherogenesis 39) . PGE 2 , too, performs a variety of roles in vascular regulation. It contributes to the production of cytokines and chemokines and induces leukocyte infiltration through an EP receptor. On the other hand, several studies have demonstrated that PGE 2 induces vasodilatation, resulting in increased local blood flow and vascular hyperpermeability 40) . Thus, PGE 2 may have distinct effects on vascular regulation 41) ; however, previous studies have reported that PGE 2 promotes diabetic retinopathy in rats 10) . In our present study, pemafibrate reduced PGE 2 levels, which was associated with the improvement of endothelial function. Besides, the results of our ex vivo experiment revealed that PGE 2 significantly impaired endothelial function in the aortic rings. These findings indicate that PGE 2 has vasoconstrictive effects, at least in our experimental conditions. On the contrary, 15d-PGJ 2 plasma levels, which declined following diabetes induction, were not affected by pemafibrate treatment. 15d-PGJ 2 is a member of the PGJ 2 family and is known as an endogenous PPARγ agonist 42) . Previous studies have proven that PGJ 2 family members have protective effects against vascular disease and may therefore be incorporated into preventive and therapeutic strategies for these conditions 43 - 46) . Both LTB 4 and 5-HETE, which are generated from AA by the 5-LOX pathway, stimulate inflammatory responses by activating neutrophils 47) . In addition, their effects on endothelial cell function have been documented in numerous studies 48) . Previous studies comparing patients with and without diabetes showed an association between diabetes and marked upregulation of 5-LOX activity as well as increased levels of 5-HETE and LTB 4 11) . Furthermore, recent studies have suggested that 5-HETE and LTB 4 can be used as biomarkers of diabetic vascular complications 49 , 50) , with the latter also reported to be associated with the onset of acute coronary syndrome 51) .
Several papers have shown that STZ injection increases the levels of AA and eicosanoids such as PGE 2 and TXA 2 . Diabetes-induced activation of the protein kinase C pathway stimulates phospholipase A2, hence promoting the release of AA 52) . In fact, in this study, induction of diabetes by STZ injection increased AA levels, which were subsequently reduced by pemafibrate in line with the reduction in triglyceride levels. Since triglycerides serve as a source of AA, it can be postulated that the reduction in their levels might have been associated with the decrease in AA 53 , 54) . In the current study, pemafibrate also lowered the levels of AA-derived eicosanoids in line with the reduction in triglyceride levels. These results suggest that triglyceride reduction by pemafibrate may, at least partially, have contributed to the decreased levels of AA and its eicosanoid metabolites. In addition, pemafibrate reduced the expression of thromboxane synthase and PGE synthase, which might have affected eicosanoid levels. Considering these data, various mechanisms were probably involved in the reduction of eicosanoids by pemafibrate treatment. Additionally, the results of our ex vivo experiment demonstrated that PGE 2 and TXB 2 impaired endothelial function. These findings led us to consider post-treatment decreases in the levels of vasoconstrictive eicosanoids, which were elevated following diabetes induction, as one of the possible underlying mechanisms responsible for the amelioration of endothelial function; however, further research is warranted to elucidate underlying mechanisms.
We also observed that pemafibrate decreased the levels of saturated free fatty acids, such as PA and stearic acid, in diabetic mice. In this study, diabetes induction led to a relatively modest increase in PA and stearic acid levels; however, previous studies have reported that elevated plasma free fatty acid concentrations are associated with endothelial dysfunction in diabetic and non-diabetic subjects 55) . In fact, a clinical study reported diminished plasma fatty acid levels after pemafibrate treatment 56) , and the results of the present study demonstrated the impairment of endothelial function by PA. The reduction in these saturated fatty acid levels after treatment with pemafibrate might also have played a role in the improvement of endothelial function in our mice.
One of the major limitations of the present study was that we used STZ to induce diabetes. Thus, it is unclear whether our results can be generalized to type 2 diabetes, which represents the most common type of diabetes in humans. Second, the levels of lipids, including triglycerides, in STZ-induced diabetic mice were higher than those in non-diabetic mice. Other studies have shown elevated lipid levels in STZ-induced diabetic mice 34 , 35) . Decreased peripheral lipolysis and/or increased hepatic lipogenesis are suggested as the underlying mechanisms. The activation of PPARα reduced triglyceride levels through various mechanisms, including the promotion of LPL activity 57) . However, STZ-induced diabetic mice have some unique lipid metabolisms. Therefore, further research is required to clarify the mechanism by which pemafibrate reduced triglyceride levels in this study. Moreover, it is recommended that additional studies using different animal models be conducted to confirm the effects of pemafibrate on AA-derived eicosanoids and endothelial function, especially in a diabetic condition.
In summary, pemafibrate decreased the levels of vasoconstrictive eicosanoids, which were elevated following diabetes induction. This might be associated with the amelioration of endothelial dysfunction in pemafibrate-treated diabetic mice. The results of our study suggest that pemafibrate may prevent cardiovascular complications in patients with diabetes mellitus.
Acknowledgements
The authors would like to thank Shintaro Okamoto and Etsuko Uematsu (Tokushima University) for their technical assistance.
Competing Interests
The Department of Cardio-Diabetes Medicine, Tokushima University Graduate School is supported in part by unrestricted research grants from Boehringer Ingelheim. The authors declare that they have no conflict of interest.
Sources of Funding
This work was partially supported by JSPS Kakenhi Grants (Number 19K08584 to D.F. and Number 19H03654 to M.S.), Bristol-Myers Squibb Research Grants (D.F.), Takeda Science Foundation (M.S.), and the Vehicle Racing Commemorative Foundation (M.S.). The funders had no role in the study design, data collection and analysis, or preparation of the manuscript.
References
- 1).Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S and Fadini GP. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care, 2011; 34 Suppl 2: S285-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Yuhki K, Kojima F, Kashiwagi H, Kawabe J, Fujino T, Narumiya S and Ushikubi F. Roles of prostanoids in the pathogenesis of cardiovascular diseases: Novel insights from knockout mouse studies. Pharmacol Ther, 2011; 129: 195-205 [DOI] [PubMed] [Google Scholar]
- 3).Sellers MM and Stallone JN. Sympathy for the devil: the role of thromboxane in the regulation of vascular tone and blood pressure. Am J Physiol Heart Circ Physiol, 2008; 294: H1978-1986 [DOI] [PubMed] [Google Scholar]
- 4).Feletou M, Huang Y and Vanhoutte PM. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol, 2011; 164: 894-912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Bagi Z, Erdei N, Papp Z, Edes I and Koller A. Up-regulation of vascular cyclooxygenase-2 in diabetes mellitus. Pharmacol Rep, 2006; 58 Suppl: 52-56 [PubMed] [Google Scholar]
- 6).Chen SS, Jenkins AJ and Majewski H. Elevated plasma prostaglandins and acetylated histone in monocytes in Type 1 diabetes patients. Diabet Med, 2009; 26: 182-186 [DOI] [PubMed] [Google Scholar]
- 7).Hatley ME, Srinivasan S, Reilly KB, Bolick DT and Hedrick CC. Increased production of 12/15 lipoxygenase eicosanoids accelerates monocyte/endothelial interactions in diabetic db/db mice. J Biol Chem, 2003; 278: 25369-25375 [DOI] [PubMed] [Google Scholar]
- 8).Capra V, Back M, Angiolillo DJ, Cattaneo M and Sakariassen KS. Impact of vascular thromboxane prostanoid receptor activation on hemostasis, thrombosis, oxidative stress, and inflammation. J Thromb Haemost, 2014; 12: 126-137 [DOI] [PubMed] [Google Scholar]
- 9).Waldman M, Peterson SJ, Arad M and Hochhauser E. The role of 20-HETE in cardiovascular diseases and its risk factors. Prostaglandins Other Lipid Mediat, 2016; 125: 108-117 [DOI] [PubMed] [Google Scholar]
- 10).Wang M, Wang Y, Xie T, Zhan P, Zou J, Nie X, Shao J, Zhuang M, Tan C, Tan J, Dai Y, Sun J, Li J, Li Y, Shi Q, Leng J, Wang X and Yao Y. Prostaglandin E2/EP2 receptor signalling pathway promotes diabetic retinopathy in a rat model of diabetes. Diabetologia, 2019; 62: 335-348 [DOI] [PubMed] [Google Scholar]
- 11).Issan Y, Hochhauser E, Guo A, Gotlinger KH, Kornowski R, Leshem-Lev D, Lev E, Porat E, Snir E, Thompson CI, Abraham NG and Laniado-Schwartzman M. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins Other Lipid Mediat, 2013; 100-101: 15-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Ansquer JC, Foucher C, Aubonnet P and Le Malicot K. Fibrates and microvascular complications in diabetes--insight from the FIELD study. Curr Pharm Des, 2009; 15: 537-552 [DOI] [PubMed] [Google Scholar]
- 13).Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi YA, Sullivan D, Hunt D, Colman P, d’Emden M, Whiting M, Ehnholm C, Laakso M and investigators Fs. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet, 2005; 366: 1849-1861 [DOI] [PubMed] [Google Scholar]
- 14).Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d’Emden MC, Crimet DC, O’Connell RL, Colman PG and investigators Fs. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet, 2007; 370: 1687-1697 [DOI] [PubMed] [Google Scholar]
- 15).Usui-Ouchi A, Ouchi Y and Ebihara N. The peroxisome proliferator-activated receptor pan-agonist bezafibrate suppresses microvascular inflammatory responses of retinal endothelial cells and vascular endothelial growth factor production in retinal pigmented epithelial cells. Int Immunopharmacol, 2017; 52: 70-76 [DOI] [PubMed] [Google Scholar]
- 16).Xu N, Wang Q, Jiang S, Wang Q, Hu W, Zhou S, Zhao L, Xie L, Chen J, Wellstein A and Lai EY. Fenofibrate improves vascular endothelial function and contractility in diabetic mice. Redox Biol, 2019; 20: 87-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Averna M, Stroes E and lipid alterations beyond LDLewg. How to assess and manage cardiovascular risk associated with lipid alterations beyond LDL. Atheroscler Suppl, 2017; 26: 16-24 [DOI] [PubMed] [Google Scholar]
- 18).Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Grobbee DE, Cass A, Chalmers J and Perkovic V. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet, 2010; 375: 1875-1884 [DOI] [PubMed] [Google Scholar]
- 19).Wang D, Liu B, Tao W, Hao Z and Liu M. Fibrates for secondary prevention of cardiovascular disease and stroke. Cochrane Database Syst Rev, 2015: CD009580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Yamaguchi T, Shirai K, Nagayama D, Nakamura S, Oka R, Tanaka S, Watanabe Y, Imamura H, Sato Y, Kawana H, Ohira M, Saiki A, Shimizu N and Tatsuno I. Bezafibrate Ameliorates Arterial Stiffness Assessed by Cardio-Ankle Vascular Index in Hypertriglyceridemic Patients with Type 2 Diabetes Mellitus. J Atheroscler Thromb, 2019; 26: 659-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Arai H, Yamashita S, Yokote K, Araki E, Suganami H, Ishibashi S and Group KS. Efficacy and Safety of Pemafibrate Versus Fenofibrate in Patients with High Triglyceride and Low HDL Cholesterol Levels: A Multicenter, Placebo-Controlled, Double-Blind, Randomized Trial. J Atheroscler Thromb, 2018; 25: 521-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Fruchart JC. Selective peroxisome proliferator-activated receptor alpha modulators (SPPARMalpha): the next generation of peroxisome proliferator-activated receptor alpha-agonists. Cardiovasc Diabetol, 2013; 12: 82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Zandbergen F and Plutzky J. PPARalpha in atherosclerosis and inflammation. Biochim Biophys Acta, 2007; 1771: 972-982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Fruchart JC. Pemafibrate (K-877), a novel selective peroxisome proliferator-activated receptor alpha modulator for management of atherogenic dyslipidaemia. Cardiovasc Diabetol, 2017; 16: 124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Yamashita S, Masuda D and Matsuzawa Y. Clinical Applications of a Novel Selective PPARalpha Modulator, Pemafibrate, in Dyslipidemia and Metabolic Diseases. J Atheroscler Thromb, 2019; 26: 389-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Yamashita S, Arai H, Yokote K, Araki E, Matsushita M, Nojima T, Suganami H and Ishibashi S. Efficacy and Safety of Pemafibrate, a Novel Selective Peroxisome Proliferator-Activated Receptor alpha Modulator (SPPARMalpha): Pooled Analysis of Phase 2 and 3 Studies in Dyslipidemic Patients with or without Statin Combination. Int J Mol Sci, 2019; 20: 5537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Yokote K, Yamashita S, Arai H, Araki E, Suganami H, Ishibashi S and on Behalf of the K-877 Study Group. Long-Term Efficacy and Safety of Pemafibrate, a Novel Selective Peroxisome Proliferator-Activated Receptor-alpha Modulator (SPPARMalpha), in Dyslipidemic Patients with Renal Impairment. Int J Mol Sci, 2019; 20: 706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Hennuyer N, Duplan I, Paquet C, Vanhoutte J, Woitrain E, Touche V, Colin S, Vallez E, Lestavel S, Lefebvre P and Staels B. The novel selective PPARalpha modulator (SPPARMalpha) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis, 2016; 249: 200-208 [DOI] [PubMed] [Google Scholar]
- 29).Araki E, Yamashita S, Arai H, Yokote K, Satoh J, Inoguchi T, Nakamura J, Maegawa H, Yoshioka N, Tanizawa Y, Watada H, Suganami H and Ishibashi S. Effects of Pemafibrate, a Novel Selective PPARalpha Modulator, on Lipid and Glucose Metabolism in Patients With Type 2 Diabetes and Hypertriglyceridemia: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial. Diabetes Care, 2018; 41: 538-546 [DOI] [PubMed] [Google Scholar]
- 30).Ishibashi S, Arai H, Yokote K, Araki E, Suganami H, Yamashita S and on behalf of the K-877 Study Group. Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor alpha modulator, in patients with dyslipidemia: Results from a 24-week, randomized, double blind, active-controlled, phase 3 trial. J Clin Lipidol, 2018; 12: 173-184 [DOI] [PubMed] [Google Scholar]
- 31).Maki T, Maeda Y, Sonoda N, Makimura H, Kimura S, Maeno S, Takayanagi R and Inoguchi T. Renoprotective effect of a novel selective PPARalpha modulator K-877 in db/db mice: A role of diacylglycerol-protein kinase C-NAD(P)H oxidase pathway. Metabolism, 2017; 71: 33-45 [DOI] [PubMed] [Google Scholar]
- 32).Tsuda S, Shinohara M, Oshita T, Nagao M, Tanaka N, Mori T, Hara T, Irino Y, Toh R, Ishida T and Hirata KI. Novel mechanism of regulation of the 5-lipoxygenase/leukotriene B4 pathway by high-density lipoprotein in macrophages. Sci Rep, 2017; 7: 12989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Mori K, Ishida T, Yasuda T, Hasokawa M, Monguchi T, Sasaki M, Kondo K, Nakajima H, Shinohara M, Shinke T, Irino Y, Toh R, Nishimura K and Hirata K. Serum Trans-Fatty Acid Concentration Is Elevated in Young Patients With Coronary Artery Disease in Japan. Circ J, 2015; 79: 2017-2025 [DOI] [PubMed] [Google Scholar]
- 34).Pham PT, Fukuda D, Yagi S, Kusunose K, Yamada H, Soeki T, Shimabukuro M and Sata M. Rivaroxaban, a specific FXa inhibitor, improved endothelium-dependent relaxation of aortic segments in diabetic mice. Sci Rep, 2019; 9: 11206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Willecke F, Scerbo D, Nagareddy P, Obunike JC, Barrett TJ, Abdillahi ML, Trent CM, Huggins LA, Fisher EA, Drosatos K and Goldberg IJ. Lipolysis, and not hepatic lipogenesis, is the primary modulator of triglyceride levels in streptozotocin-induced diabetic mice. Arterioscler Thromb Vasc Biol, 2015; 35: 102-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Bonetti PO, Lerman LO and Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol, 2003; 23: 168-175 [DOI] [PubMed] [Google Scholar]
- 37).Davignon J and Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation, 2004; 109: III27-32 [DOI] [PubMed] [Google Scholar]
- 38).Xie X, Sun W, Wang J, Li X, Liu X and Liu N. Activation of thromboxane A2 receptors mediates endothelial dysfunction in diabetic mice. Clin Exp Hypertens, 2017; 39: 312-318 [DOI] [PubMed] [Google Scholar]
- 39).Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, Arai H, Oida H, Yurugi-Kobayashi T, Yamashita JK, Katagiri H, Majima M, Yokode M, Kita T and Narumiya S. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest, 2004; 114: 784-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Omori K, Kida T, Hori M, Ozaki H and Murata T. Multiple roles of the PGE2 -EP receptor signal in vascular permeability. Br J Pharmacol, 2014; 171: 4879-4889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Frolov A, Yang L, Dong H, Hammock BD and Crofford LJ. Anti-inflammatory properties of prostaglandin E2: deletion of microsomal prostaglandin E synthase-1 exacerbates non-immune inflammatory arthritis in mice. Prostaglandins Leukot Essent Fatty Acids, 2013; 89: 351-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM and Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell, 1995; 83: 803-812 [DOI] [PubMed] [Google Scholar]
- 43).Sasaguri T and Miwa Y. Prostaglandin J2 family and the cardiovascular system. Curr Vasc Pharmacol, 2004; 2: 103-114 [DOI] [PubMed] [Google Scholar]
- 44).Eligini S, Banfi C, Brambilla M, Camera M, Barbieri SS, Poma F, Tremoli E and Colli S. 15-deoxy-delta12,14-Prostaglandin J2 inhibits tissue factor expression in human macrophages and endothelial cells: evidence for ERK1/2 signaling pathway blockade. Thromb Haemost, 2002; 88: 524-532 [PubMed] [Google Scholar]
- 45).Imaizumi T, Kumagai M, Hatakeyama M, Tamo W, Yamashita K, Tanji K, Yoshida H and Satoh K. 15-Deoxy-delta 12,14-prostaglandin J2 inhibits the expression of granulocyte-macrophage colony-stimulating factor in endothelial cells stimulated with lipopolysaccharide. Prostaglandins Other Lipid Mediat, 2003; 71: 293-299 [DOI] [PubMed] [Google Scholar]
- 46).Imaizumi T, Matsumiya T, Tamo W, Shibata T, Fujimoto K, Kumagai M, Yoshida H, Cui XF, Tanji K, Hatakeyama M, Wakabayashi K and Satoh K. 15-Deoxy-D12,14-prostaglandin J2 inhibits CX3CL1/fractalkine expression in human endothelial cells. Immunol Cell Biol, 2002; 80: 531-536 [DOI] [PubMed] [Google Scholar]
- 47).Mehta J, Dinerman J, Mehta P, Saldeen TG, Lawson D, Donnelly WH and Wallin R. Neutrophil function in ischemic heart disease. Circulation, 1989; 79: 549-556 [DOI] [PubMed] [Google Scholar]
- 48).Osher E, Weisinger G, Limor R, Tordjman K and Stern N. The 5 lipoxygenase system in the vasculature: emerging role in health and disease. Mol Cell Endocrinol, 2006; 252: 201-206 [DOI] [PubMed] [Google Scholar]
- 49).Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL and Kern TS. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes, 2008; 57: 1387-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Schwartzman ML, Iserovich P, Gotlinger K, Bellner L, Dunn MW, Sartore M, Grazia Pertile M, Leonardi A, Sathe S, Beaton A, Trieu L and Sack R. Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes, 2010; 59: 1780-1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Seo KW, Lee SJ, Kim CE, Yun MR, Park HM, Yun JW, Bae SS and Kim CD. Participation of 5-lipoxygenase-derived LTB(4) in 4-hydroxynonenal-enhanced MMP-2 production in vascular smooth muscle cells. Atherosclerosis, 2010; 208: 56-61 [DOI] [PubMed] [Google Scholar]
- 52).DeRubertis FR and Craven PA. Eicosanoids in the pathogenesis of the functional and structural alterations of the kidney in diabetes. Am J Kidney Dis, 1993; 22: 727-735 [DOI] [PubMed] [Google Scholar]
- 53).Kopf T, Schaefer HL, Troetzmueller M, Koefeler H, Broenstrup M, Konovalova T and Schmitz G. Influence of fenofibrate treatment on triacylglycerides, diacylglycerides and fatty acids in fructose fed rats. PLoS One, 2014; 9: e106849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Yamazaki T, Wakabayashi M, Ikeda E, Tanaka S, Sakamoto T, Mitsumoto A, Kudo N and Kawashima Y. Induction of 1-acylglycerophosphocholine acyltransferase genes by fibrates in the liver of rats. Biol Pharm Bull, 2012; 35: 1509-1515 [DOI] [PubMed] [Google Scholar]
- 55).Ghosh A, Gao L, Thakur A, Siu PM and Lai CWK. Role of free fatty acids in endothelial dysfunction. J Biomed Sci, 2017; 24: 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Seko Y, Yamaguchi K, Umemura A, Yano K, Takahashi A, Okishio S, Kataoka S, Okuda K, Moriguchi M, Okanoue T and Itoh Y. Effect of pemafibrate on fatty acid levels and liver enzymes in non-alcoholic fatty liver disease patients with dyslipidemia: A single-arm, pilot study. Hepatol Res, 2020; 50: 1328-1336 [DOI] [PubMed] [Google Scholar]
- 57).Schoonjans K, Staels B and Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res, 1996; 37: 907-925 [PubMed] [Google Scholar]