Abstract
Due to the infection by the SARS-CoV-2 virus (COVID-19) there were also reported neurological symptoms, being the most frequent and best cited those that affect the cerebrovascular, sensorial, cognitive and motor functions, together with the neurological diffuse symptoms as for examples headache or dizziness. Besides, some of them behave high risk of mortality. Consequently, it is crucial to elucidate the mechanisms of action in brain of SARS-CoV-2 virus in order to create new therapeutic targets to fight against this new disease. Since now the mechanisms of arrival to the brain seems to be related with the following processes: blood brain barrier (BBB) disruption together with nervous or axonal transport of the virus by the trigeminal nerve, the vagus nerve, or the brain-gut-axis. Being two the mechanisms of brain affectation most cited: a direct affectation of the virus in the brain through neuroinvasion and an indirect mechanism of action due to the effects of the systemic infection. Both processes include the triggering of inflammation, hypoxia and the increased likelihood of secondary infections. This topic supposes a major novel challenge for neuroscience. Therefore, the aim of this review is to provide summarized information about the neurological symptomatology and the brain pathogenic mechanisms involved and reported in COVID-19.
Keywords: SARS-CoV-2, COVID-19, Brain, Neurological symptoms, Neuroinvasion, Neuroscience
Graphical abstract
Highlights
-
•
COVID-19 generates cerebrovascular, sensitive, motor, cognitive and diffuse brain disorders.
-
•
The trigeminal and vagus nerve or the gut-brain axis are the entrance of SARS-CoV-2 in brain.
-
•
SARS-CoV-2 affects brain by neuroinvasion and by the consequences of the systemic infection.
-
•
COVID-19 favors BBB disruption, inflammation, hypoxia, and secondary infections.
-
•
The study of the neurological affectation of COVID-19 raises a new challenge for neuroscience.
1. Introduction
The Coronavirus Disease 2019 (COVID-19) is a respiratory illness emerged in December 2019 in Wuhan, situated in the Hubei province of China. This virus has rapidly spread throughout the world, being declared by the World Health Organization (WHO) as a “Public Health Emergency of International Concern” on February 11, 2020. One month later, on March 11, 2020, declared as a “Pandemic of Alarming Levels of Spread and Severity”, with more than 113 countries affected. Genetic and phylogenetic analysis show that this virus shares a strong relationship with the Severe Acute Respiratory Syndrome (SARS) and the Middle East Respiratory Syndrome (MERS), belonging phylogenetically to the Coronaviridae family and being taxonomically known as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (Wu et al., 2020). In consonance, epidemiology data published show that this virus shares similar pathogenesis and symptomatology with the pneumonia induced by SARS-CoV or MERS-CoV (Liu et al., 2020). Besides, although less explored, together with these respiratory symptoms, COVID-19 has been sometimes linked to the development of neurological symptomatology, by their effects in sensorial, cerebrovascular, cognitive or motor function (Abbatecola and Antonelli-Incalzi, 2020). The approach to the study of the effect of this new virus in brain functionality raises a new important challenge for neuroscience research, because the infection in brain can influence not only the general health, but also it can generate risk of comorbidities and mortality. Therefore, the purpose of this review is to summarize the information reported so far in scientific publications, regarding the neurological symptomatology triggered by SARS-CoV-2 infection, as well as to look into the underlying mechanisms that so far could explain the physiopathology of this new virus in brain.
2. Methodology
To address the topic the methodological framework followed was the classical for a state-of-the-art review, whose aim it is to offer current state of the knowledge for future investigation and research in a narrative way (Grant and Booth, 2009). For searching the information, a list with the following words was done: COVID-19, SARS-CoV-2, coronavirus, central nervous system (CNS), brain, neurological symptoms, neurological manifestations, brain effects, neurodegeneration, neurology, neuroscience, cognition, cerebrovascular, neurological infection, neuroinvasion. Then, there were made combinations with these words, containing always the words “COVID-19” or “SAR-CoV2” or “Coronavirus” plus another from the list mentioned above. These combinations were used for searching information in the following sources: PubMed, Embase, Medline, Scopus, Web of Knowledge, Google Scholar and BiblioSalut. The type of articles accepted for being included in this work were indexed articles published in English with the following formats: clinical cases, original articles, meta-analysis reviews, narrative reviews, and comments to editor, with qualitative and quantitative data. For the search the time framework was limited from November 1, 2019 to July 31, 2021. After finding the information an integrative approach was conducted, summarizing the most important information according to the following structure: neurological clinical manifestations of SARS-CoV-2 infection; arrival and affectation of SARS-CoV-2 in brain; future challenges for neuroscience and conclusions. For further information about the review methodology see (Snyder, 2019).
3. Neurological clinical manifestations of SARS-CoV-2 infection
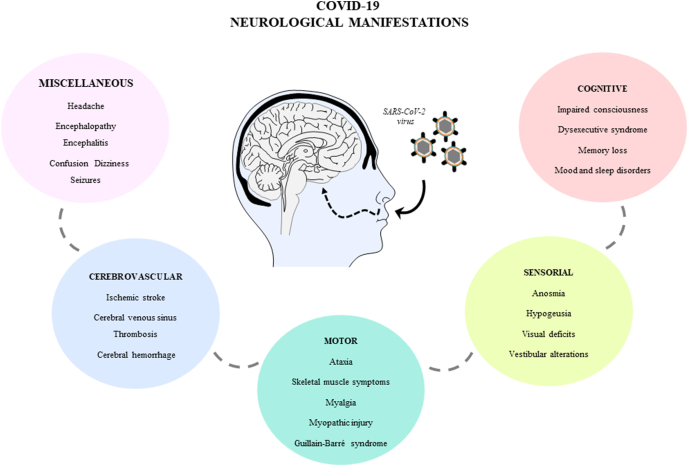
Despite not being in the list of the main diagnostic criteria, in the bibliography were reported considerable neurological clinical manifestations as consequence of the SARS-CoV-2 infection, being currently most of them under study. In order to summarized these neurological symptoms, first there were extracted the most reported and best cited. Second, these were organized in the following categories: neurological diffuse or miscellaneous, cerebrovascular, motor, sensorial, cognitive symptoms (Fig. 1). The information regarding each category is summarized in the following paragraphs.
Fig. 1.
Leading neurological manifestations in COVID-19. Symptoms are classified in miscellaneous, cerebrovascular, motor, sensorial and cognitive.
3.1. Diffuse or miscellaneous neurological symptoms in SARS-CoV-2 infection
Retrospective and prospective case series about patients with COVID-19 reported the presence of diffuse neurological symptoms in levels up to 36,4% of them (L. Mao et al., 2020), being the headache, encephalopathies, encephalitis, confusion, dizziness and seizure the most cited. Following, it is summarized the information about these symptoms: a) Headache: Although there is not a concern about their exact prevalence, it has been pointed to be a frequent symptom in COVID-19 patients. The maximum percentage reported was in a prospective study, which included 130 patients and described 74.6% of presence of headache in these infected patients, having the 24.7% of them severe headache (Caronna et al., 2020). The published frequencies are varied, for example a meta-analysis with 104.751 infected patients from 17 studies indicates a headache rate of 25.2%. These studies are based in samples of patients with moderate severity of COVID-19. However, in severe COVID-19 patients the headache frequency reported was about 27.83%, including 309 COVID-19 cases from 15 studies (Mutiawati et al., 2020). Surprisingly, in relation with this symptom it was also indicated that the duration of the clinical symptoms of COVID-19 in patients with headache is shorter (23.9 ± 11.6 days) than in infected patients without headache (31.2 ± 12.0 days), being significant (p = 0.028) and suggesting that the headache could be an alarm sign, to protect the body against the virus through the activation of the immune system (Caronna et al., 2020). It was also described an increase in mortality concomitant to the onset of the headache in severe infected patients (Shapiro et al., 2021). Physiologically, the headache has migraine-like features, probably reflecting an activation of the trigeminovascular system, direct by the virus or indirect as a consequence of the systemic inflammation. Authors suggest a relationship with the symptom of anosmia frequently cited. Because the virus would act not only in the olfactory epithelium causing loss of smell, but also in the branches of the trigeminal nerve, very close to the nostrils through which the virus could cross brain barrier causing neuroinvasion (Bolay et al., 2020). Therefore, the direct invasion of trigeminal nerve endings in the nasal or oral cavity by the virus seems one of the most reasonable mechanisms underlying headache and anosmia/ageusia connection. Besides, SARS-CoV-2 is a neurotropic virus and viral particles have been observed in the human brain (Ding et al., 2004)(Gu et al., 2005). Therefore, it is highly likely that SARS-CoV-2 may also enter to the nervous system via the cranial nerves (Izquierdo-Dominguez et al., 2020)(Baysal-Kirac and Uysal, 2020). Although the SARS-COV-2 trans-synaptic transfer has not been still proven, the possibility of a trans-synaptic route was documented for other coronaviruses (Mengeling et al., 1972). Entrance from the nasal cavity to the olfactory bulb, then spreading to the brainstem via the piriform cortex with both passive diffusion and axonal transport has been demonstrated (Desforges et al., 2019)(Uygun et al., 2020). Therefore, although there is still matter of study, the neurotropic ability of the virus, could not only affect the trigeminal nerve but also the other olfactive nerves, by their anatomical proximity, together with the loss of sensitive functionality when there is a trigeminal inflammation in presence of nasal congestion symptoms due to the respiratory infection, supporting altogether the relationship between nasal sensitive symptoms and headache. Besides, nasal obstruction and rhinorrhea were frequently reported accompanied by anosmia and ageusia. Accordingly, there were found significant levels of anosmia and ageusia in patients with headache (54.6% vs. 18.2% in headache vs control group; p < 0.0001) (Caronna et al., 2020).
b) Encephalopathy and encephalitis: encephalopathy is defined as an alteration of one or more brain functions (e.g. altered level of consciousness, seizures, confusional state, acute focal deficits) caused by a systemic disease (e.g. anoxia, ischemia, metabolic disorders, etc.), and is typically reversible (Al Mazrouei et al., 2020). Encephalitis is characterized by typically focal brain alterations, with or without meningeal involvement, with different possible causes (infectious, inflammatory, autoimmune, paraneoplastic, etc.), and can be confirmed with histology studies or through the detection of inflammatory cells in the cerebrospinal fluid (CSF) (Blinder and Lewerenz, 2019). The clinical features are fever, headache and symptoms of brain dysfunction. Infectious etiology is common, therefore in patients with COVID-19 there was also observed the presence of this neurological complication with these cited manifestations, being frequently classified insight the category of infectious toxic encephalopathy, also known as acute toxic encephalitis. In this sense, in a Spanish registry of 232 COVID-19 patients, there was found a frequency of 21.9% of encephalopathy. Manifesting mild or moderate confusion (33% of cases) in moderated infected patients and severe encephalopathy or coma (9.8% of cases) in Intensive Care Unit (ICU) patients, being these symptoms detected after 8.02 days of infection (Abenza Abildúa et al., 2021). Brain MRI studies were performed in 47% of these patients, detecting alterations in 7.8% of them. Besides, EEG studies were also performed in 41.3% of these patients, indicating alterations in 61.9% of them (Abenza Abildúa et al., 2021). However, other smallest studies pointed encephalopathies in lower frequencies, as for example in a retrospective study developed in Geneva, it is reported 4,4% (Uginet et al., 2021). The Spanish registry cited before found viral RNA of SARS-CoV-2 in cerebrospinal fluid (CSF) of patients with encephalitis in only one case (Abenza Abildúa et al., 2021) and the study from Geneve did not find in any of the patient (Uginet et al., 2021). In agreement, other studies that identified cases of encephalopathies did not detected the virus in CSF, or detected it with low frequencies 7%, suggesting that viral neuroinvasion by SARS-CoV-2 it is not the exclusive mechanism of action (Helms et al., 2020). This point should be studied with accuracy, because there is not already consensus. In this regard, researchers suggest the hypothesis that the neurological manifestations due to COVID-19 infection are triggered by a combination of direct and an indirect mechanisms (Lucchese, 2020), pointed the disturbed brain homeostasis, the inflammatory state and the vascular dysfunction, the main causes for example of the endotheliitis (Uginet et al., 2021).
c) Confusion: signs of confusion were also detected in COVID-19 patients, involving delirium, acute or chronic confusional state, acute brain failure or acute encephalopathy impaired consciousness, disorientation, lack of attention, agitation and other cognitive problems (Soiza and Myint, 2019). One sign of encephalopathy reported was mild or moderate confusion (Abenza Abildúa et al., 2021). The frequencies arrive up to levels of 27% (Pranata et al., 2021) or 33% (Abenza Abildúa et al., 2021). As it is described delirious patients are more likely to have longer hospital stay and to develop in-hospital complications and ICU hospitalization comparing with non-delirious patients that are ambulatory or hospitalized in infectious departments prepared for COVID-19 patients (Cipriani et al., 2020). In some cases delirium may precede symptoms as headache, fever and cough, being the presence of delirium associated with an increased risk of mortality in ICU or not ICU hospitalized older adults with COVID-19 (Pranata et al., 2021).
d) Dizziness: it is also presented in COVID-19 infected patients, although it is not a specific COVID-19 symptom, because its leading cause include, acute labyrinthitis, vestibular neuritis, acute otitis media or secondary to stroke following COVID-19. Furthermore, this symptom is reported as the initial presentation of COVID-19 illness, thereupon during a moderate severity phase, with frequencies from 2.13% (Saniasiaya and Kulasegarah, 2021), arriving also up to a maximum of 17% in a series of 214 infected patients (Mao Li et al., 2020, Li et al., 2020, Li et al., 2020). Therefore, dizziness requires a deep investigation to determine its leading cause. Among the postulated mechanisms causing dizziness are the direct neuroinvasion by the union of the virus with the angiotensin-converting enzyme 2 receptors (ACE2) found in the capillary endothelium of the brain (Baig et al., 2020). ACE2 is expressed in a wide variety of tissues, including the brain and most of the cardiovascular-relevant tissues, and the current consensus is that the distribution of this protein is ubiquitous. In brain ACE2 is widespread throughout different regions, for example present in nuclei involved in the central regulation of cardiovascular functions like the cardio-respiratory neurons of the brainstem, as well as in non-cardiovascular areas such as the motor cortex and raphe. Activation of ACE2 receptor promotes vasodilation, antiproliferation and antihypertrophy. Therefore, it constitutes a target in the treatment of the hypertension and other cardiovascular diseases with impact in brain functionality (Xia and Lazartigues, 2008). Other postulated cause of dizziness is the indirect effect of the hypoxia, hypercoagulopathy, as well as immune-mediated insult during COVID-19 infection (Saniasiaya and Kulasegarah, 2021). These authors emphasize that dizziness should not be taken lightly as it has been proven to be a notable clinical manifestation among COVID-19 patients. Dizziness also occurs in patients with encephalopathy, concomitant with headache (Romero-Sánchez et al., 2020), possibly because the virus affects mechanisms involved in the triggering of all these symptoms, such as the inflammation of the neural nerves, hypoxia, hypercoagulopathy and the general replay of the brain against the pathogen (Saniasiaya and Kulasegarah, 2021).
e) Seizure: is a paroxysmal alteration of the neurologic function caused by the excessive, hypersynchronous discharge of neurons in the brain. The term epileptic seizure is used to distinguish a seizure caused by abnormal neuronal firing from a nonepileptic event, such as a psychogenic seizure (Stafstrom and Carmant, 2015). In COVID-19 the onset of seizures seems to be occasional and reported with low frequencies, beginning from 0.5% (Mao Li et al., 2020) up to 9% in small series (Liu et al., 2020). These studies pointed an indirect effect, arguing that the seizure could be triggered by fever and hyperthermia, which can disrupt the blood brain barrier (BBB) (Nikbakht et al., 2020). Because extreme hyperthermia increases the acute activation of glial cells and the BBB permeability (Kiyatkin and Sharma, 2009). Furthermore, a severe disease course or the advanced disease stages for example in ICU hospitalized patients could result for example in hypoxic encephalopathy, cerebrovascular events and cytokine storm, which may trigger the development of acute seizure (Sohal and Mansur, 2020). Patients who had seizure needed to be hospitalized not necessarily in ICU, but usually for a long in-hospital stay. Besides, they had four times more probability to die than patients without seizures, suggesting that this kind of neurological complication may be an important contributor to the morbidity and mortality associated with COVID-19 (Kuroda, 2021). This aspect is under study principally in the context of epilepsy, because the effect of COVID-19 on individuals with this neurological disease remains unclear.
3.2. Cerebrovascular symptoms in SARS-CoV-2 infection
Acute cerebrovascular symptoms remain one of the most serious neurologic complications observed in COVID-19 patients, being more frequent in severe or ICU hospitalized than in non-severe or not hospitalized patients (Bridwell et al., 2020). Although some cases were reported, fortunately it seems that their onset is infrequent, being published low level of frequency, e.g. 0.2% in a study of 21.483 hospitalized patients with COVID-19 registered in the COVID-19 Cardiovascular Disease registry of the American Heart Association (Leasure et al., 2021), and also 0.2% in a retrospective study with a sample size of 85.645 patients in the USA (Qureshi et al., 2021). In severe patients are also reported low levels 1.3% (Qureshi et al., 2021), up to a maximum of 6% of frequency of acute cerebrovascular diseases (Yaghi et al., 2020). The type of cerebrovascular symptoms published so far are ischemic stroke, intracerebral hemorrhage, and cerebral venous thrombosis; being the stroke one of the most reported, with frequency rates from 2.5% to 6%, with a large majority of them being acute ischemic stroke, causing ICU hospitalization (Leasure et al., 2021). It is suggested that the comorbidities like hypertension, atrial fibrillation, hyperlipidemia, congestive heart failure or diabetes, were present in patients with acute ischemic stroke. In fact, COVID-19 is characterized as a predominantly prothrombotic disease with elevated D-dimer, higher Sequential Organ Failure Assessment (SOFA) score, high fibrinogen levels, but only mildly decreased antithrombin levels and microvascular thrombosis rather than a bleeding diathesis (Gao et al., 2020)(C. Wu et al., 2020)(Yang et al., 2020). Therefore, the plausible mechanism that causes these ischemic processes is the so called "sepsis-induced coagulopathy" (Abbas et al., 2021). Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. For clinical operationalization, organ dysfunction can be represented by an increase in the SOFA score of 2 points or more (Singer et al., 2016). Early phase of sepsis is characterized by hypercoagulability due to tissue factor expression on circulating monocytes and microparticles, increased fibrinogen plasma concentration, platelet activation and subsequent dysfunction, and hypofibrinolysis/fibrinolysis shutdown (Sun, 2006)(Adamzik et al., 2012). Tissue factor expression on circulating monocytes and microparticles is triggered by pathogen toxins and other pathogen-associated molecular patterns via NF-kB activation and subsequent induction of plasminogen activator inhibitor type 1 and proinflammatory cytokines such as interleukin-6 (IL-6) (Mussbacher et al., 2019)(Liu et al., 2017) and neutrophil extracellular traps (NETs). NETs are extracellular webs of chromatin, microbicidal proteins, and oxidant enzymes that are released by neutrophils to contain infections, including cell-free DNA. All these molecules are elevated in severe COVID-19 patients receiving mechanical ventilation in ICU hospitalized, and it strongly correlates with acute phase reactants, including CRP, D-dimer and LDH. These NETs have the potential to propagate inflammation and microvascular thrombosis (Zuo et al., 2020). Whether extracellular RNA derived from SARS-CoV-2 contributes to the hypercoagulability seen in COVID-19 is not still known (Görlinger et al., 2020). Furthermore, other authors affirm that the risk of mortality increase with the occurrence of acute ischemic stroke in patients with COVID-19 (Abbas et al., 2021), especially when comorbidities are present (Qureshi et al., 2021). Intracerebral hemorrhage has been also reported (Agarwal et al., 2020), with emphasis in the elderly and in ICU hospitalized COVID-19 patients, being categorized as a rare manifestation with a high risk of mortality (Abboud et al., 2020; Yanan Li et al., 2020), and described up to rates of 59% of lethality (Abbas et al., 2021). This symptom in some cases is accompanied by severe immune thrombocytopenia following upper respiratory tract infection (Magdi and Rahil, 2019) or multiple microhemorrhages (e.g. intraparenchymal hemorrhage, subarachnoid hemorrhage and subdural hematoma), and finally sometimes accompanied by cognitive declined (Magdi and Rahil, 2019). It is required to elucidate the causes, the risk and the outcome of the cerebrovascular complications through studies using large sample sizes.
3.3. Motor symptoms in SARS-CoV-2 infection
Although the motor symptomatology is less frequent than other neurological symptoms in COVID-19 illness, some symptoms were detected, such as the neuropathic pain (McWilliam et al., 2021), ataxia, skeletal muscular injury and myopathic changes, especially in critical patients associated to the long recovery in-hospital stay or ICU stay (Liu et al., 2020; Mao et al., 2020; Rossi et al., 2021; Villa et al., 2021). The literature also report neurological motor syndromes such as the Guillain-Barre Syndrome (GBS) (Ellul et al., 2020). GBS is a symmetric, ascending flaccid paralysis, often preceded by respiratory or gastrointestinal infections from a virus or bacteria (Bridwell et al., 2020). Isolated case reports and case series have suggested a connection between COVID-19 and the occurrence of GBS. Arguing that probably post-viral deregulation of the immune system generated by COVID-19 triggers the GBS, because the clinical characteristics and disease evolution seem to be similar to those observed in GBS secondary to other etiologies. In the general population, the incidence of GBS is well establishes, being about 1 or 2 in 100.000 individuals per year (Van Den Berg et al., 2014). In COVID-19 positive patients the incidence of GBS it is still not definitively established, but reports found in bibliography refer mostly to different Italian regions. For example, there were reported 1 in 100.000 patients in Friuli Venezia-Giulia (Gigli et al., 2021), or 5 cases in 1.000–1.200 patients in Lombardia (Toscano et al., 2020), or recently 47,9/10.000 in a retrospective multicenter study from hospitals situated in Lombardia and in Veneto (Filosto et al., 2021). Besides, in Spain there is reported 1 in 847 in the Spanish registry ALBACOVID of Castilla La Mancha (Romero-Sánchez et al., 2020). The cited motor symptoms together with the loss of muscle density are associated with higher risk of mortality, especially in ICU hospitalized patients (Rossi et al., 2021). Motor symptoms are usually present in ICU patients because critically ill patients present several additional risk factors for nervous system damage, which affect motor function, as they are direct viral invasion of the nervous system, systemic cytokine storm and severe hypoxemia. Furthermore, the deep sedation and extended muscular paralysis, bed rest for several days even months, and the inability to receive proper physical rehabilitation present frequently in ICU patients add risk factors for the development of motors symptoms. In fact after ICU treatments, COVID-19 patients generally require an extensive rehabilitation program to recover motor functionality and general health state (Deana et al., 2021). Therefore, also this topic needs to be deepened studied and understand.
3.4. Sensorial symptoms in SARS-CoV-2 infection
Perhaps, among the neurological effects, the impact at sensorial level of the SARS-CoV-2 infection has been the most cited one. It is the case of anosmia (the decreased ability to smell) and hypogeusia/ageusia (the decreased ability to taste) (Pallanti, 2020), which are considered in the list of COVID-19's markers of infection by the British Association of Otorhinolaryngology (ENT-UK) (Hopkins, 2020). Bibliography published so far include cohort studies and case reports, being the prevalence of anosmia in COVID-19 patients calculated to be around 44%, and the prevalence of hypogeusia around 43% (Butowt and von Bartheld, 2020). In an international observational study, including 514.459 patients, anosmia accompanied by ageusia were the strongest neurological consistent symptoms (Sudre and Keshet, 2021). Results that are in agreement with other studies, as for example in a study conducted in Ireland, including 46 subjects, it was described that approximately half of patients reported some degree of olfactory (22/46, 48%) or gustatory (25/46, 54%) sense loss. Thirteen patients (28%) reported a complete loss of sense of smell, (8/46, 17%) reported a complete loss of sense of taste while (7/46, 15%) reported total loss of both (Kerr et al., 2020). Besides, a systematic review and meta-analysis including 45 articles with a total of 42.120 COVID-19 patients from 17 different countries, concluded that surprisingly in the case of severe ill patients or in ICU hospitalized patients, the rate of anosmia could be lower (Purja et al., 2021). Therefore, due to the previous information these authors wonder if the anosmia-ageusia could be an early predictor of COVID-19 severity.
Other sensorial manifestations like visual loss (Murchison et al., 2021), central retinal artery occlusion, or post-COVID optic neuritis (Ucar, 2021) have been also reported in case study reports. Therefore, cohort studies are needed to glimpse some clear statement regarding this topic. Besides, there were also mentioned audio-vestibular disorders. Although there is only a very limited amount of literature focusing on this topic and they are mainly case reports. In a meta-analysis is cited sensorineural hearing loss, vertigo and tinnitus as the most reported sensitive symptoms due to COVID-19. Also in other case series are reported the etiopathogenesis of these symptoms as an inner ear disorders related to COVID-19 infection (Fancello et al., 2021). More research is required to assess if these symptoms persist after disease resolution. However, it seems clear that the loss of smell/taste does not depend exclusively on nasal obstruction/rhinorrhea and it can begin even before the typical signs/symptoms of COVID-19 alone or together with headache, thus becoming warning signs even in oligosymptomatic patients and, especially, in the initial stage of the disease, when high viral replication and transmissibility occur. The recovery of smell/taste, when it occurs, usually does so in the first two weeks after the resolution of COVID-19. However, new interpretations appear every week, therefore it is believed that valuing and actively questioning the patient about olfactory/gustatory disorders can help, implemented not only by otorhinolaryngologists, but the entire health team working on the front line of the pandemic control (Costa, 2020).
3.5. Cognitive symptoms in SARS-CoV-2 infection
Impaired consciousness and dysexecutive syndrome have also been found among COVID-19 patients (Hampshire et al., 2021), especially in concomitancy with other systemic pathologies (Jacobs et al., 2021) and in severe or ICU hospitalized patients (Rousseau et al., 2021). A retrospective study of Wuhan reported disturbances of consciousness in hospital admission in nearly one third of the patients from a sample of 113 deceased patients with COVID-19 (Chen et al., 2020). These alterations in consciousness were also found in other similar studies in China (L. Mao et al., 2020). Besides, signs of a dysexecutive syndrome consisting in inattention, disorientation or poorly organized movements in response to commands were found in 36% of infected patients of an observational serie of 58 COVID-19 patients in France (Helms et al., 2020). In severe patients the cognitive impairment seems to be more present, so in a study about COVID-19 adults who survived after being hospitalized in an ICU, it was described that 40.6% of a total of 92 patient presented some cognitive disorder (Rousseau et al., 2021). There is still a little information regarding the nature and prevalence of cognitive problems caused by the SARS-CoV-2 infection in moderated or asymptomatic patients. Therefore, frequency of cognitive decline is not well known, but authors link the cognitive impairment with the so call “long-COVID-19”, defined as an infection by SARSR-CoV-2 not recovered for several weeks or months following the start of symptoms that were suggestive of COVID-19, whether the patient was tested or not (Nabavi, 2020). Long-COVID-19, also is described as a profound fatigue, including cough, breathlessness, muscle and body aches, and chest heaviness or pressure, but also skin rashes, palpitations, fever, headache, diarrhea, pins and needles that persist in time. Besides, cognitive decline could be also due as a consequence of other neurological problems triggered by the infection, as for example cerebrovascular problems, which leads to memory loss, anxiety and sleep disorders. This was described in a cohort of 3.762 patients from 56 countries, where cognitive dysfunction and memory loss were found after 6 month in approximately 88% of the patients (Davis et al., 2021).
4. Arrival and affectation of the SARS-CoV-2 in brain
4.1. Arrival of the SARS-CoV-2 in brain: possible front doors
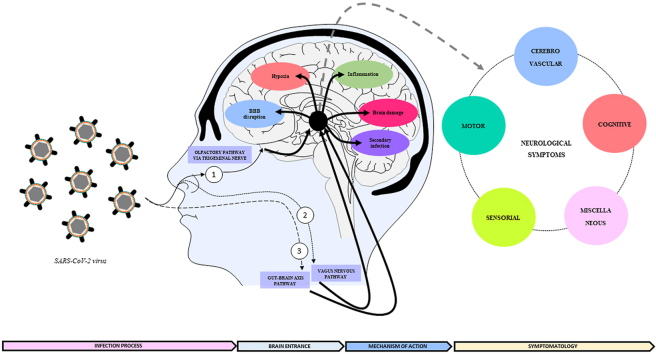
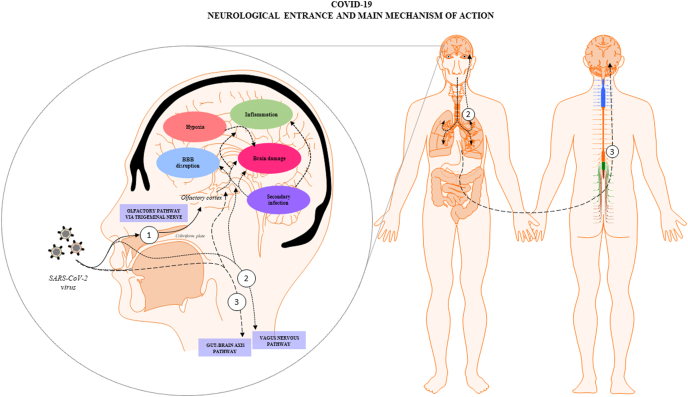
The exact pathogenesis of the SARS-CoV-2 at CNS remains speculative, because it is a new virus whose comprehension requires long time, that has not yet elapsed since the beginning of the pandemic. It is unclear what fraction of the neurological damage is due to the direct CNS invasion, called neuroinvasion, or by the indirect effect caused by the systemic reactions due to the host fight against the infection. Besides, there are mechanisms that have been flagged as to be involved in both cases after the infection. Clinical and laboratory findings glimpsed that the SARS-CoV-2 is a neurotropic virus and can invade nervous tissues, being the coating-mediated endo/exocytosis a possible route to enter and transmit within the brain cells (Yang et al., 2020). The first finding is that, although in low quantities, there were found RNA particles of SARS-CoV-2 in CSF of COVID-19 patients with neurological symptoms (e.g. encephalitis, headache, impaired consciousness), by using genome sequencing or ultrahigh-depth sequencing (Xiang et al., 2020). It was suggested that the difficult to quantify the RNA of the SARS-CoV-2 in the CSF due to their low quantity, could indicate a possible viral clearance preceded (Placantonakis et al., 2020), or that brain symptoms may depend more on the systemic disease severity (Al Saiegh, 2020). On the other hand, supporting the neuroinvasion mechanism there were found high levels of SARS-CoV-2 particles in nervous of COVID-19 died patients (Meinhardt, 2020). It has been hypothesized that the CNS invasion by SARS-CoV-2 may be the result of crossing one of the brain barriers, such as the BBB or the blood-cerebrospinal fluid barrier at choroid plexus, arriving by nerve routes such as the olfactory nerve, trigeminal nerve, gut-brain axis or vagus-nerve (Montalvan et al., 2020). All sustained in the basis that there were identified viral particles in peripheral nerves. Furthermore, there was suggested that once the virus has passed the oropharyngeal tract, it could arrive to the brain coming from sensory and motor nervous endings, by axonal dissemination to brain areas then observed as to be affected by the virus (Conde Cardona et al., 2020; Hu et al., 2020). Having as a result the arriving to the CNS and the consequent possibility of neuronal damage (Spindler and Hsu, 2012)(Fig. 2). The identification of neuropathological findings and viral particles in autopsy of COVID-19 died patients, by using a combination of anatomopathological studies and RT-PCR for detection of SARS-CoV-2 virus supports this theory. These findings included neuronal degeneration of the axon of the trigeminal nerve and ganglion, together with cellular loss and high levels of SARS-CoV-2 viral particles (JM Meinhardt, 2020; Placantonakis et al., 2020). One of the damaged neural tissues found was the nucleus tractus solitarii, leading to microvascular clotting, pulmonary edema, damage of the entorhinal cortex and cytokine storm in COVID-19 affected patients (Anoop and Kavita, 2020). Among the neuronal invasion routes, the olfactory pathway via the trigeminal nerve, the vagus-nerve and the gut-brain axis are the most cited. Therefore, the information about these ways is summarized below:
Fig. 2.
COVID-19 neurological entrance and main mechanisms of action: SARS-CoV-2 viruses could affect brain tissue and generate neurological symptomatology directly by neuroinvasion, arriving to the brain through several pathways such as: (1) the olfactory route via the trigeminal nerve, (2) the vagus nervous in connection with the lungs and (3) the gut-brain axis; or indirectly as a consequence of the effects of the systemic infection, such as hypoxia or hyperthermia. In both cases several mechanisms could be triggered such as BBB disruption, inflammation, and increased risk of secondary infections, among others, all of them being interconnected. The final result is the generation of brain dysfunction and the onset of the mentioned neurological symptomatology in COVID-19 patients.
4.1.1. Neuroinvasion via the olfactory pathway
The olfactory route is the most cited brain front door for the SARS-CoV-2 virus (Fig. 2). Once virus enters in contact with the olfactory epithelium, the cilia, projected from the dendrite of the olfactory sensory neurons, become neurons accessible for being infected for the virus (Wei et al., 2020). In this regard, it was demonstrated in rhesus monkey that SARS-CoV-2 virus invades the CNS primarily via the olfactory bulb. Thereafter, viruses by crossing the brain barriers rapidly spread to functional areas of the CNS, such as the hippocampus, thalamus and medulla oblongata (Jiao, 2021). As we previous mentioned, at cellular level the door lock of SARS-CoV-2 is the ACE2 receptor, which joins with the SARS-CoV-2 spike (S) protein. Besides, the transmembrane protease serine type 2 (TMPRSS2) mediates the priming of the S protein subunits S1 and S2 with the ACE2 receptor, causing the activation of the ACE2 receptors and enhancing the attachment and membrane fusion of the virus with the infected cells (Jaimes et al., 2020; Paniri et al., 2020). The ACE2 receptor is well distributed in brain, being presented in high proportions, especially in precursor cells of oligodendrocytes and astrocytes residing in substantia nigra and cortex, in the endothelial cells of cerebral capillaries, in the brain ventricles, as well as in areas that are directly or indirectly involved in olfactory pathways, including the hypothalamic nuclei, the amygdala, the hippocampus and the frontal cortex (H. Zhang et al., 2020). This fact makes brain more vulnerable to the infection of SARS-CoV-2 virus and supports the neuroinvasive potential of the virus, as well as become it a possible therapeutic target (Xu and Lazartigues, 2020). Furthermore, the analysis of multiple scRNA-seq datasets found a high population of ACE2 receptors in the bipolar neurons of the nasal cavity. This factor can increase the probability of the entrance of viruses in brain by this via (Sungnak et al., 2020). In addition, symptomatology supports this idea, as there have been described olfactory manifestations (e.g. anosmia, hyposmia and augesia) as common symptoms of COVID-19 infection (Xydakis et al., 2020), together with significant amounts of viral particles in the olfactory mucosa underneath the cribriform plate, the olfactory bulb, the trigeminal ganglion, and the medulla oblongata in COVID-19 patients by autopsy (Meinhardt et al., 2021). Besides and regarding this topic, two other reasons may influence in the different rates of smell dysfunction among COVID-19 patients in different populations: genetic variations at the level of the host or genetic variations at the level of the virus. Regarding the host, specifically, there may be genetic polymorphism in the ACE2 or TMPRSS2 host receptors. Therefore, a novel hypothesis is that genetic differences in the prevalence of chemosensory defects due to polymorphisms in ACE2 and TMPRSS2 may result in variations in the binding affinity between the virus and the ACE2 receptor, possibly determining different infectivity and spreading ability of the virus (Butowt and von Bartheld, 2020). Regarding the virus, variants of the SARS-CoV-2 virus may differ in geography, for example, due to mutations in the S protein (Grubaugh et al., 2020)(Korber et al., 2020)(Q. Li et al., 2020) (Phelan et al., 2020)(L. Zhang et al., 2020). The receptor binding domain of the virus S protein (subunit S1) binds with high affinity to the peptidase domain of the entry protein ACE2 and thereby determines viral tropism and infectivity (Shang et al., 2020). Recent studies indicate that SARS-CoV-2 has a significantly lower mutation rate as compared to SARS-CoV-1 virus and that the critical receptor binding domain of the S glycoprotein is particularly well conserved (Jia et al., 2020). Therefore, genetic variability and the mutation rate within the receptor binding domain is of particular interest in the context of population differences in the prevalence of anosmia and ageusia. For example, mutation hot spots were identified in the receptor binding domain, which may affect its binding to the ACE2 receptor (Ou et al., 2020), these mutations were not restricted to one specific geographic area, but were present in Europe, Asia and America (Ou et al., 2020)(van Dorp et al., 2020). Particular mutation identified was the SNP variant, G614, that is located outside the receptor binding domain, and has become the dominant SARS-CoV-2 strain in the pandemic, while the D614 strain was initially dominant in East Asia (Korber et al., 2020). In vitro, the G614 mutation increases viral load, and it is likely but not yet entirely clear whether it is clinically more infectious than D614 (Grubaugh et al., 2020). Taken together, at present it is unclear whether mutations or genetic variability within or near the receptor binding domain of the virus’ spike glycoprotein can increase the likelihood of infection of the olfactory epithelium and thereby influence susceptibility to olfactory deficits (Butowt and von Bartheld, 2020); however, this question requires further attention. The increase in inflammatory products locally released by the virus infection, leads to a local damage and causes olfactory loss, and simultaneously may interfere with the viral spread into the CNS. In this context, olfactory receptors and their genetic determinants could play a role in the SARS-CoV-2 entrance into cells locally, in the CNS and systemically (Gori et al., 2020).
4.1.2. Neuroinvasion via the brain-gut axis
This hypothesis is based first in the established connection among the gastrointestinal tract and the brain through the known gut-brain axis. This is a connection materialized in part by the enteral neuronal network regulated by the vagus and sympathetic nerve (Fig. 2). Second, it is based in the significant presence of ACE2 receptors in the enterocytes of the small intestine and colon (H. Zhang et al., 2020). Furthermore, it is the evidence that the SARS-CoV-2 can cause intestinal dysfunction (e.g. diarrhea, nausea, vomiting and abdominal pain), microbial imbalances in gut microbiota and related immune disorders (Shi et al., 2021; Jiali et al., 2021). Reinforcing all these evidences, there were found virus particles in fecal samples also up to 5 weeks after passed the infection (H. Li et al., 2020; Yongjian et al., 2020). Although the exact number of patients with COVID-19 that shed viral RNA in faeces is not still known. The virus might possibly contaminate sewage, water and food supplies, and possibly contaminate bathroom sites via faecal–aerosol transmission. Consistent with this hypothesis, viral RNA was detected in sewage and on toilet seats, flush buttons and door handles. However, the research on virus titre in faecal fomites and whether the virus titres in faecal fomites are of sufficient concentration and infectivity for subsequent transmission remains unknown. By other hand, SARS-CoV-2 can tolerate human small intestinal fluid but rapidly loses infectivity in gastric fluid within 10 min. It remains unclear whether the virus can survive during food intake or whether it is protected by sputum (Guo et al., 2021). Therefore, the main routes of transmission are reported to be person-to-person, by respiratory droplets and to a lesser degree, by fomites. Although the detection of infectious virus in packaged seafood was reported in China, the possibility of transmission via food and food packaging and whether the virus poses a risk to food safety is still under research. There is not currently evidence about the possibility of being infected of COVID-19 from food. The virus that causes COVID-19 can be killed at temperatures similar to that of other known viruses and bacteria found in food. Although the current evidence suggests that SARS-CoV-2 does not cause foodborne illness, the virus has caused major disruptions to the global food supply chain. Regarding this point there were developed real-time techniques to detect the trace level of the spike-protein for cold-chain food (Zhang et al., 2021), in the search of avoiding that the virus could be reintroduced via cold-chain transportation of contaminated items and might initiate an outbreak. It is known that SARS-CoV-2, like other viruses, cannot multiply in food, therefore, over time, the number of infectious virions is expected to decrease if the virus happens to be present on the surface of a food product. Air disinfection could be considered in food-related environments and two practical methods include room air cleaners such as filters or UV light, as well as upper-room germicidal UV fixtures (Nardell and Nathavitharana, 2020). Much remains to be understood, including which are the viral and the host factors influencing the brain invasion and whether the virus is cleared from the brain subsequent to the acute illness.
4.1.3. Neuroinvasion via the vagus-nerve
In human biopsies, SARS-CoV-2 has been detected by immunohistochemistry in all differentiated cell types of the airway mucosa and the alveolar epithelium (Kong et al., 2020). Being also the ACE2 receptor widely distributed in the endothelia of arteries, veins, arterial smooth muscles, type I and type II, alveolar epithelial cells and lung alveolar epithelial cells (Hamming et al., 2004). In this regard, the lung-originating infection, propagation, and viral multiplication rate have been confirmed and detailed in a transgenic mouse overexpressing the human ACE2 gene. SARS-CoV-2 could interact with the ACE2 receptors for example present in cellular nuclei implicated in the central regulation of cardiovascular function, such as the brainstem, interacting with the vagus nerve (Fig. 2), gaining access to the dorsal vagal complex via blood circulation at the level of the area postrema, due to the lack of the BBB in this neurohemal structure (Hamming et al., 2004). In this regard, the vagus nerve-nucleus of the solitary tract coming from the lungs has been proposed as one important via of transport of the virus towards the brain. It was suggested that the virus attack the vagus nerve by infecting the lung (Rangon et al., 2020). Therefore, the vagus nerve is proposed as another therapeutic target for the brain disturbances in COVID-19 (Mastitskaya et al., 2021).
4.2. Mechanisms of SARS-CoV-2 that cause brain affectation
Although the exact mechanism of damage in brain originated by the SARS-CoV-2 is still unknown, the combination of inflammation, hypoxia status and secondary infections, among others consequences of the infection, are mentioned as the leading causes of the brain injury. Below are explained each of these mechanisms.
4.2.1. Neuroinflammation
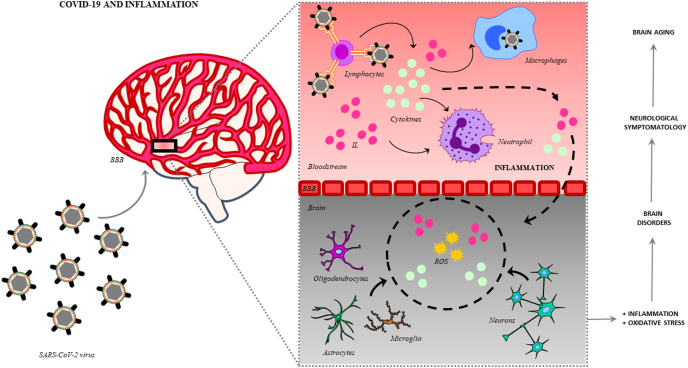
The systemic infection caused by the SARS-CoV-2 activates the immunological system of the host, especially leukocytes, which produce a storm of cytokines, interleukins (IL) and antibodies, and stimulates macrophages and monocytes, activating the immune system and leading to a general state of inflammation (Fig. 3). In brain leukocytes are further activated by the interaction between chemokine receptors expressed on their membranes and chemokines circulating in the brain, together with virus-induced alterations of endothelial adhesion molecules and junctional proteins of the BBB, producing their disruption and favoring viral neuroinvasion. The dysregulation of the BBB, likely caused by a combination of viral and host factors (e.g., secreted viral proteins, inflammatory mediators, antiviral therapies, drugs, aging), has been proposed as a critical component of the virus-induced neuropathology (De Chiara et al., 2012). Once the virus goes through the BBB, moves from the bloodstream to the brain, escaping from the immune system and colonizing the CSF, stimulating the glial cells (astrocytes, oligodendrocytes and microglia) and neurons, generating a process of neuroinflammation (Andriuta et al., 2020). The SARS-CoV-2 viral particles in CSF detected, although in low quantities, reinforce this idea. Besides, this process is characterized by the activation of signaling pathways (e.g. NF-kappaB), which produce the release of more IL and cytokines, generating at the same time an overproduction of reactive oxygen species (ROS), due to the increase in the oxidative state due to the whole inflammatory process (Spindler and Hsu, 2012). Besides, it has been observed that the systemic inflammation generates a decrease in monoamines and neutrophic factors, activating at the same time the microglia, increasing the glutamate and the N-methyl-D-aspartate (NMDA), and favoring excitotoxicity (Boldrini et al., 2021). The most common findings in neurological affected COVID-19 patients, are mild neuropathological changes with pronounced neuroinflammation in the brainstem (Matschke et al., 2020). In this regard, clinical studies about severe and moderate cases of COVID-19 have reported a highlighted increase in plasma levels of interleukins (IL2, IL7, IL10), granulocyte-colony stimulating factor (GSCF), human interferon-inducible protein 10 (IP10), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein-1alpha (MIP1A) and tumor necrosis factor (TNFα), favoring the theory that possibly an exaggerated state of inflammation is produced during the infection. These situations were reported especially in older people, contributing to the lethality of the infection in this group (Butowt and von Bartheld, 2020; Helms et al., 2020; Wang et al., 2020). It was also found an increase of pro-inflammatory cytokines and interleukins (TNFα, IL-6, IL-12p70, IL-10) in CSF in COVID-19 patients associated to severe neurological damage, as for example stroke (Garcia et al., 2021). Considering also that in aging period there is a characteristic imbalance in the regulation of inflammation and oxidative stress, that leads to a state known as inflammaging (Franceschi et al., 2000), the activation of inflammatory mechanisms could cause chronic damage, resulting in alterations of the neuronal function and their viability, triggering neurotoxic pathways and producing molecular hallmarks of neurodegeneration, lack of neutralization of the oxidative stress mechanism, deficient autophagic processes, synaptopathies and neuronal death. Finally, altogether could generate cognitive, motor and sensorial symptomatology. In aging, these effects may act in synergy with the cardiovascular and respiratory comorbidities, aggravating the situation. Perhaps this could be the cause of the important rates of cerebrovascular manifestations in severe elderly affected patients.
Fig. 3.
COVID-19 and inflammation: lymphocytes detect SARS-CoV-2 virus, and through triggering the production of cytokines and interleukins (IL) stimulate the immune system and the virus clearance by the activation of macrophages and neutrophils, generating also an inflammatory state. Among other factors, hyperthermia generated by the infection together with the increased of inflammation favor the disruption of the BBB. These processes become viruses able to move from the bloodstream to the brain tissue, escaping from the immune system and stimulating neural and glial cells (astrocytes, oligodendrocytes, microglial cells). Due to the whole inflammatory process at the same time, it is produced an overproduction of ROS, increasing the oxidative state. Altogether these imbalances produce brain dysfunction and damage, being observed by the onset of neurological symptomatology, generating altogether a risk factor for brain aging.
4.2.2. Hypoxia
The pulmonary affectation caused by the SARS-CoV-2 could induce also brain hypoxia due to the loss of supply of oxygen to the brain. Therefore, hypoxia is another potential mechanism involved in the onset of the neurological symptoms caused by this virus, mainly caused by the disturbs in the gas exchange by the respiratory system. One of the consequences is that hypoxia induces anaerobic metabolism in the mitochondria of neural cells resulting in overproduction of acid lactic. High levels of acid and a decreased in pH, cause intracerebral vasodilation, brain edema, obstruction of cerebral blood flow, hypoxic ischemic encephalopathy and headache. Furthermore, if there is a prolonged hypoxia, intracranial hypertension can appear, inducing BBB disruption, activation of glia cells, oligodendroglial cell injury, demyelination, white matter microhemorrhages and cell injury (Wani AH, 2020). Therefore, in high-risk patients with cardiovascular diseases, hypoxia may also induce the occurrence of neurologic symptoms as acute ischemic stroke, increasing the risk of death (Y. Wu et al., 2020). In this regard, using MRI scan it was described hypoxic brain damage in patients with severe COVID-19 infection hospitalized in the ICU (Kandemirli et al., 2020). Unfortunately, cerebrovascular events were also found in COVID-19 infection, having a multifactorial etiology that should be studied, because it is a factor risk causative of morbidity and mortality. In brain biopsies of some COVID-19 patients affected by cerebrovascular problems there were found signs of thrombotic microangiopathy and endothelial injury, suggesting that endothelial disruption is the primary mechanism of damage. There were also found thrombotic microangiopathy, loss of self-regulation and increased bleeding predisposition in the neuroimaging tests of these patients (Hernandez-Fernandez et al., 2020). In accordance, morphologic alterations such as hypoxic injury with neuronal loss encephalitis, meningitis, petechial bleeding, perivascular hemorrhage, axonal injury/degeneration, white matter clusters of macrophages, perivascular acute disseminated encephalomyelitis-like appearance, focal microscopic areas of necrosis with central loss of white matter and marked axonal injury, astrogliosis, lymphocytic infiltrates and microscopic infarcts, were found by using histological techniques, as it is reported in a meta-analyses that include 662 patients from 58 studies (Meinhardt et al., 2021).
4.2.3. Secondary infections
The brain without the optimal protection due to the deterioration of the BBB, caused by the SARS-CoV-2 virus, becomes vulnerable. In this conditions, it is easier for other pathogens to reach the CNS (Y. Wu et al., 2020). Therefore, neurologic complications could be also due to secondary intracranial infections, that could cause neurological symptoms in COVID-19 patients (Y. Wu et al., 2020), such as encephalitis, possibly with concurrent neurovascular endotheliosis and CNS renin angiotensin system (RAS) dysregulation (Najjar et al., 2020). Recently, another secondary infection reported was the rhino-orbital mucormycosis, which is an angioinvasive disease caused by mold fungi of the genus Rhizopus, Mucor, Rhizomucor, Cunninghamella and Absidia of the Order-Mucorales, Class- Zygomycetes, that usually affects patients with altered immunity, carrying high risk of mortality (Baker, 1957, Tiraboschi et al., 2012). The Rhizopus Oryzae is the most common type and responsible for nearly 60% of mucormycosis cases in humans and also accounts for the 90% of the rhino-orbital-cerebral form (Bennett et al., 2014). Mode of contamination occurs through the inhalation of fungal spores. Globally, the prevalence of mucormycosis varied from 0.005 to 1.7 per million population, while its prevalence is nearly 80 times higher (0.14 per 1000) in India compared to developed countries, in a recent estimate about the years 2019–2020 (Singh et al., 2021). Interestingly, there are case reports of overall, 101 cases of mucormycosis in people with COVID-19, of which 82 cases were from India and 19 from the rest of the world. In this case diabetes mellitus seems to be a factor risk, since it was present in 80% of cases. Also the use of corticosteroids for the treatment of COVID-19 seems to be another factor risk, since it was recorded that 76.3% of patients with mucormycosis and COVID-19 had corticosteroids as treatment (Singh et al., 2021). However, few information is published so far regarding this topic, therefore more comprehensive and detailed studies are required to discover how other virus act in synergy with SARS-CoV-2 invading the neural system.
5. Future challenges for neuroscience
The pandemic caused by the SARS-CoV-2 virus has implied a change in our lifestyle but also a revolution for science. In record time, many scientists around the world have developed different types of vaccines and have also published a great deal of information, describing and exploring this new disease. However, considering that it is a new disease and that from the point of view of science it has elapsed a short period of time since the start of the pandemic, there are so many questions to answer. As well as there is a considerable amount of literature regarding the systemic infection generated by the virus, at neurological level there is less information, despite their importance. Perhaps because neurological symptomatology is not always present and also because the first goal is to combat for example the respiratory disturbs. Therefore, COVID-19 raises a new challenge for neuroscience. From the clinical viewpoint and at symptomatologic level, are needed more retrospective and prospective cohort studies with larger sample size for describing with statistical validity the manifestations relative to neurological disorders including the exact incidence of each symptom, morbidity and mortality. Besides, prospective follow-up studies for answering what is the profile of the patients that could develop neurological symptoms and to know if people who have had neurological symptoms and is recovered from COVID-19, could suffer neurological sequalae at short- and long-term. Find out if there is a brain damage, in severe COVID-19 patients, after they recovery, as for example to study whether sensitization of the trigeminovascular system persists when SARS-CoV-2 infection disappears or what are the motor consequences in severe patients. Besides, it is required to study and identify the determinants or concomitant risk factors for developing neurological symptomatology (e.g., comorbidities, available biomarkers, demographic factors), that may predispose a person with COVID-19 to neurological manifestations.
At a physiopathological level, it is essential to study the mechanisms of action of the SARS-CoV-2 virus during the infection, regarding the molecular host-pathogen interaction. This study should be made taking into consideration in vivo studies with experimental animals, in vitro studies with neural and glial cells, accompanied by clinical data. The main challenge is to unravel whether the neurological effects are due to a direct effect of the virus by their postulated neuroinvasive mechanism or if there are caused by an indirect effect due to the consequences of the systemic infection. This includes to understand in depth the role of inflammation, the molecular interactions once the infection is produced and the role of the blood coagulation or hyperthermia in the neuroinvasion by the disruption of the BBB. Because extreme hyperthermia increases the acute activation of glial cells and BBB permeability (Kiyatkin and Sharma, 2009). In addition, it is also a challenge the study of the relationship between genes and the virus. Recently, there have been published some of the genetic association between COVID-19 and various brain disorders, using a system biology-based network approach, observing 123 brain-related disorders associated with COVID-19 and a brain-disease-gene network with five highly clustered modules, with 35 hub proteins that are also involved in the protein catabolic process, cell cycle, RNA metabolic process, and nuclear transport, demonstrating a greater complexity of COVID-19 infection and glimpsed some therapeutically targets (Prasad et al., 2021). In accordance, other study using network analysis has identified 73 human targets of SARS-CoV-2 associated with brain-related diseases, identifying putative pathogenic genes as for example the Integrin beta-1 (ITGB1). This gene is highly expressed in the brain and codify for ACE2 binding proteins. ITGB1 could be considered as a drug target for the prevention and treatment of CNS symptoms associated with COVID-19 patients. The ITGB1 gene plays a role in many important pathways involve for example in the cell adhesion of molecules, axon guidance, Rap1-signaling pathway, and PI3K-AKT signaling pathways (Khatoon et al., 2020). Furthermore, to explore the temporal expression patterns of ITGB1 in the hippocampus, amygdala, striatum, cerebellar cortex, mediodorsal nucleus of the thalamus, and neocortex, using the Human Brain Transcriptome project (HBT; http://hbatlas.org/pages/hbtd), found that expression appeared to be decreased in early childhood and after that remained stable over adulthood and older age (Khatoon et al., 2020), suggesting a possible reason for the severity of the virus infection in elderly people. Furthermore, a more concrete aspect but not less important is the characterization of the COVID-19 manifestation in patients with neurological diseases such as epilepsy, Alzheimer's disease or Parkinson among others, because it remains unclear. In this regard the study of the relationship between aging and the triggering of COVID-19 in relation with the onset of CNS symptomatology is one of the major goals for neuroscientists, due to the high registered rates of mortality in elderly around the world. The understanding of the disease is vital for the finding of therapeutic targets. It is also crucial to find early predictors of the disease, as for example to know if the neurological symptoms like headache, dizziness or anosmia/augesia could be some of them (Purja et al., 2021). Finally, regarding the effect of vaccines in decreasing neurological consequences caused by COVID-19, there is no already a scientific consensus. Because the specific effect of vaccines on neurological problems caused by COVID-19 must still be studied. However, it is known that the neurological side effects generated by the vaccines are rare. Currently reports of rare neurological complications associated with COVID-19 infection and vaccinations are leading to regulatory, clinical and public health concerns. A publication described the following rare neurological complications in those who received COVID-19 vaccines: risk of acute central nervous system (CNS) demyelinating events, encephalitis meningitis and myelitis, GBS syndrome, Bell's palsy, myasthenic disorders, hemorrhagic stroke and subarachnoid hemorrhage (Farrington et al., 1996). It is also known that the vaccines help to fight against the disease and prevent contagion and therefore reduce the probability of suffering neurological consequences. Vaccines are proving effective at reducing SARS-CoV-2 infections, hospitalizations and deaths (CDC Centers for Disease Control and Prevention, 2021). All approved vaccines have been through randomized clinical trials to test their quality, safety and efficacy. However, the rapid development of the COVID-19 vaccines means that these trials were not large enough to detect very rare adverse events or specific effect for example at neurological level. Interestingly, a recent study found that the risk of neurological complications was higher in people with a positive COVID-19 test than in those who had received COVID vaccines. The risks of adverse neurological events following SARS-CoV-2 infection are much greater than those associated with vaccinations, highlighting the benefits of ongoing vaccination programs (Patone et al., 2021). Therefore, detailed assessments of potential neurological adverse events associated with COVID-19 vaccines and infection are urgently needed. Regarding this topic, The American Academy of Neurology concluded that based on the existing evidence, neurologists should recommend COVID-19 vaccination to their patients. For those patients being treated with immunotherapies, attention should be paid to timing of vaccination with respect to treatment and the potential for an attenuated immune response (Marsh et al., 2021).
6. Conclusion
The clinical manifestations at neurological level of COVID-19 infection could be from very general as headache or dizziness, up to more specific, many of them having a connection. Among the most frequently reported are the following categories of manifestations: the general or miscellaneous as it could be the headache, the cerebrovascular, the motor, the sensorial and the cognitive dysfunctions. The possible infective mechanism of COVID-19 in brain is thought to be caused by a high density of ACE2 receptors in brain and in other visceral tissues, together with the entry of the virus in brain by different front doors, being the most cited the neuronal or axonal transport, by the trigeminal nerve, the vagus nerve or the gastrointestinal nervous pathway. The exact physiopathological mechanism of infection at CNS remains speculative, but seems to be related with a combination of direct processes which includes the neuroinvasion and an indirect effect of the consequences of the systemic infection, both of them triggering neuroinflammation, hypoxia, vulnerability of the BBB, increased predisposition to secondary infections and brain disfunction. In consequence, neuroscience has a new major challenge, which is to deep understand these mechanisms in order to identify new therapeutic target for reducing the comorbidity and mortality risk of the infection. As well as early predictors of the illness. In this sense, it will be crucial the study of molecular networks, including the interaction between the virus and gene expression in combination with the study of symptomatology in COVID-19 patients. The combination of experimental and observational clinical studies, using a translational approach, are fundamental to unravel why this virus affects the brain, as well as to be able to fight against it.
Consent for publication
Not applicable.
Funding
This research received no external funding.
Declaration of competing interest
The authors have no conflicts of interests, financial or otherwise.
Acknowledgements
The authors acknowledge the support of the University Hospital Son Llàtzer.
ABBREVIATIONS
- ACE2
angiotensin-converting enzyme 2
- BBB
blood brain barrier
- CNS
central nervous system
- CSF
cerebrospinal fluid
- GBS
Guillain-Barre Syndrome
- GSCF
granulocyte-colony stimulating factor
- MCP1
monocyte chemoattractant protein 1
- MIP1
macrophage inflammatory protein-1alpha
- MERS
Middle East Respiratory Syndrome
- NETs
neutrophil extracellular traps
- ICU
Intensive Care Unit
- IL2
IL7, IL10 interleukin number
- IP10
human interferon-inducible protein 10
- ITGB1
Integrin beta-1
- RAS
renin angiotensin system
- ROS
reactive oxygen species
- S
spike protein
- SARS-COV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- TMPRSS2
transmembrane protease serine type 2
- TNFα
tumor necrosis factor
- WHO
World Health Organization
References
- Abbas R., El Naamani K., Sweid A., Schaefer J., Al E. Intracranial hemorrhage in COVID-19 patients: a case series. World Neurosurg. 2021:S1878–S1887. doi: 10.1016/j.wneu.2021.07.067. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbatecola A.M., Antonelli-Incalzi R. COVID-19 spiraling of frailty in older Italian patients. J. Nutr. Heal. Aging. 2020 doi: 10.1007/s12603-020-1357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abboud H., Abboud F.Z., Kharbouch H., Arkha Y., El Abbadi N., El Ouahabi A. 2020. COVID-19 and SARS-cov-2 infection: pathophysiology and clinical effects on the nervous system. World neurosurg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abenza Abildúa M.J., Atienza S., Carvalho Monteiro G., Erro Aguirre M.E., Imaz Aguayo L., Freire Álvarez E., García-Azorín D., Gil-Olarte Montesinos I., Lara Lezama L.B., Navarro Pérez M.P., Pérez Sánchez J.R., Romero Delgado F., Serrano Serrano B., Villarreal Vitorica E., Ezpeleta Echávarri D. 2021. Encephalopathy and encephalitis during acute SARS-CoV-2 infection. Spanish Society of Neurology’s COVID-19 Registry. Neurol. English Ed. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamzik M., Görlinger K., Peters J., Hartmann M. Whole blood impedance aggregometry as a biomarker for the diagnosis and prognosis of severe sepsis. Crit. 2012 doi: 10.1186/cc11816. Care 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Vishnu V., Vibha D., Bhatia R., Gupta A., Das A., Srivastava M.V.P. Intracerebral hemorrhage and SARS-CoV-2: association or causation. Ann. Indian Acad. Neurol. 2020;23 doi: 10.4103/aian.AIAN_362_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Mazrouei S.S., Saeed G.A., Al Helali A.A., Ahmed M. COVID-19-associated encephalopathy: neurological manifestation of COVID-19. Radiol. Case Reports 15. 2020. [DOI] [PMC free article] [PubMed]
- Andriuta D., Roger P.A., Thibault W., Toublanc B., Sauzay C., Castelain S., Godefroy O., Brochot E. COVID-19 encephalopathy: detection of antibodies against SARS-CoV-2 in CSF. J. Neurol. 2020. [DOI] [PMC free article] [PubMed]
- Anoop U., Kavita V. Pulmonary edema in COVID19-A neural hypothesis. ACS Chem. Neurosci. 2020 doi: 10.1021/acschemneuro.0c00370. [DOI] [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020 doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Baker R.D. Mucormycosis—a new disease? J. Am. Med. Assoc. 1957;163 doi: 10.1001/jama.1957.02970450007003. [DOI] [PubMed] [Google Scholar]
- Baysal-Kirac L., Uysal H. COVID-19 associate neurological complications. Neurol. Sci. Neurophysiol. 2020 doi: 10.4103/NSN.NSN_28_20. [DOI] [Google Scholar]
- Bennett J.E., Dolin R., Blaser M.J. Mandell, douglas, and bennett's principles and practice of infectious diseases, mandell, douglas, and bennett's principles and practice of infectious diseases. 2014. [DOI]
- Blinder T., Lewerenz J. Cerebrospinal fluid findings in patients with autoimmune encephalitis-a systematic analysis. Front. Neurol. 2019;10 doi: 10.3389/fneur.2019.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolay H., Gül A., Baykan B. COVID-19 is a real headache! Headache 60. 2020. [DOI] [PMC free article] [PubMed]
- Boldrini M., Canoll P.D., Klein R.S. How COVID-19 affects the brain. JAMA psychiatry. 2021. [DOI] [PMC free article] [PubMed]
- Bridwell R., Long B., Gottlieb M. 2020. Neurologic complications of COVID-19. Am. J. Emerg. Med. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R., von Bartheld C.S. Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. 2020:85–94. doi: 10.1177/1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caronna E., Ballvé A., Llauradó A., Gallardo V.J., María Ariton D., Lallana S., Maza S.L., Gadea M.O., Quibus L., Restrepo J.L., Rodrigo-Gisbert M., Vilaseca A., Gonzalez M.H., Gallo M.M., Alpuente A., Torres-Ferrus M., Borrell R.P., Alvarez-Sabin J., Pozo-Rosich P. Headache: a striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia 40. 2020. [DOI] [PMC free article] [PubMed]
- CDC Centers for Disease Control and Prevention . 2021. Science brief: COVID-19 vaccines and vaccination, CDC COVID-19 science briefs. [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020. 30211,30217. [DOI] [PMC free article] [PubMed]
- Cipriani G., Danti S., Nuti A., Carlesi C., Lucetti C., Di Fiorino M. A complication of coronavirus disease 2019: delirium. Acta Neurol. Belgeler. 2020;120 doi: 10.1007/s13760-020-01401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde Cardona G., Quintana Pájaro L.D., Quintero Marzola I.D., Ramos Villegas Y., Moscote Salazar L.R. Neurotropism of SARS-CoV 2: mechanisms and manifestations. J. Neurol. Sci. 2020 doi: 10.1016/j.jns.2020.116824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa K.V.T. da, Carnaúba A.T.L. Smell and taste disorders: warning signs for SARS-CoV-2 infection. Braz. J. Otorhinolaryngol. 2020. [DOI] [PMC free article] [PubMed]
- De Chiara G., Marcocci M.E., Sgarbanti R., Civitelli L., Ripoli C., Piacentini R., Garaci E., Grassi C., Palamara A.T. Infectious agents and neurodegeneration. Mol. Neurobiol. 2012 doi: 10.1007/s12035-012-8320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deana C., Verriello L., Pauletto G., Corradi F., Forfori F., Cammarota G., Bignami E., Vetrugno L., Bove T. Insights into neurological dysfunction of critically ill COVID-19 patients. Trends Anaesth. Crit. Care. 2021 doi: 10.1016/j.tacc.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., Talbot P.J. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019. [DOI] [PMC free article] [PubMed]
- Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis virus transmission pathways. J. Pathol. 2004;203 doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet neurol. 2020. 30221–0. [DOI] [PMC free article] [PubMed]
- Fancello V., Hatzopoulos S., Corazzi V., Bianchini C., Skarżyńska M.B., Pelucchi S., Skarżyński P.H., Ciorba A. SARS-CoV-2 (COVID-19) and audio-vestibular disorders. Int. J. Immunopathol. Pharmacol. 2021;35 doi: 10.1177/20587384211027373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington C.P., Nash J., Miller E. Case series analysis of adverse reactions to vaccines: a comparative evaluation. Am. J. Epidemiol. 1996;143 doi: 10.1093/oxfordjournals.aje.a008695. [DOI] [PubMed] [Google Scholar]
- Filosto M., Cotti Piccinelli S., Gazzina S., Foresti C., Frigeni B., Servalli M.C., Sessa M., Cosentino G., Marchioni E., Ravaglia S., Briani C., Castellani F., Zara G., Bianchi F., Del Carro U., Fazio R., Filippi M., Magni E., Natalini G., Palmerini F., Perotti A.M., Bellomo A., Osio M., Scopelliti G., Carpo M., Rasera A., Squintani G., Doneddu P.E., Bertasi V., Cotelli M.S., Bertolasi L., Fabrizi G.M., Ferrari S., Ranieri F., Caprioli F., Grappa E., Broglio L., De Maria G., Leggio U., Poli L., Rasulo F., Latronico N., Nobile-Orazio E., Padovani A., Uncini A. Guillain-Barre´ Syndrome and COVID-19: an observational multicentre study from two Italian hotspot regions. J. Neurol. Neurosurg. Psychiatry. 2021;92 doi: 10.1136/jnnp-2020-324837. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Gao Y., Li T., Han M., Li X., Wu D., Xu Y., Zhu Y., Liu Y., Wang X., Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020;92 doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M.A., Barreras P.V., Lewis A., Pinilla G., Sokoll L.J., Kickler T., Mostafa H., Caturegli M., Moghekar A., Fitzgerald K.C., Pardo C.A. Cerebrospinal fluid in COVID-19 neurological complications: no cytokine storm or neuroinflammation. medRxiv Prepr. Serv. Heal. Sci. 2021. [DOI] [PMC free article] [PubMed]
- Gigli G., Bax F., Marini A., Pellitteri G., Scalise A., Surcinelli A., Valente M. Guillain-Barré syndrome in the COVID-19 era: just an occasional cluster? J. Neurol. 2021;268:1195–1197. doi: 10.1007/s00415-020-09911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori A., Leone F., Loffredo L., Cinicola B.L., Brindisi G., De Castro G., Spalice A., Duse M., Zicari A.M. COVID-19-Related anosmia: the olfactory pathway hypothesis and early intervention. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlinger K., Dirkmann D., Gandhi A., Simioni P. Covid-19-associated coagulopathy and inflammatory response: what do we know already and what are the knowledge gaps? Anesth. Analg. 2020. [DOI] [PMC free article] [PubMed]
- Grant M., Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Heal. Inf. Librarian. J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Grubaugh N.D., Hanage W.P., Rasmussen A.L. Making sense of mutation: what D614G means for the COVID-19 pandemic remains unclear. Cell 182. 2020. [DOI] [PMC free article] [PubMed]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., Zhuang H., Wu B., Zhong H., Shao H., Fang W., Gao D., Pei F., Li X., He Z., Xu D., Shi X., Anderson V.M., Leong A.S.Y. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202 doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Tao W., Flavell R.A., Zhu S. Potential intestinal infection and faecal–oral transmission of SARS-CoV-2. Nat. Rev. Gastroenterol. Hepatol. 2021. [DOI] [PMC free article] [PubMed]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. For. Pathol. 2004 doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Trender W., Chamberlain S.R., Jolly A.E., Grant J.E., Patrick F., Mazibuko N., Williams S.C., Barnby J.M., Hellyer P., Mehta M.A. Cognitive deficits in people who have recovered from COVID-19. 2021. [DOI] [PMC free article] [PubMed]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/nejmc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Fernandez F., Sandoval Valencia H., Barbella-Aponte R.A., Collado-Jimenez R., Ayo-Martin O., Barrena C., Molina-Nuevo J.D., Garcia-Garcia J., Lozano-Setien E., Alcahut-Rodriguez C., Martinez-Martin A., Sanchez-Lopez A., Segura T. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain 143. 2020. [DOI] [PMC free article] [PubMed]
- Hopkins C. 2020. “Loss of sense of smell as marker of COVID -19 infection.” ENTUK, 2020. [WWW Document] [Google Scholar]
- Hu J., Jolkkonen J., Zhao C. Neurotropism of SARS-CoV-2 and its neuropathological alterations: similarities with other coronaviruses. Neurosci. Biobehav. Rev. 2020 doi: 10.1016/j.neubiorev.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Dominguez A., Rojas-Lechuga M.J., Mullol J., Alobid I. Olfactory dysfunction in the covid-19 outbreak. J Investig. Allergol. Clin. Immunol. 2020;30 doi: 10.18176/jiaci.0567. [DOI] [PubMed] [Google Scholar]
- Jacobs L.M., Gazagnes M.D., Sanoussi S., Collart F., Mesquita M., do C.F. Cognitive impairment in a patient with COVID-19 on hemodialysis: too dangerous to neglect! Hemodial. Int. 2021. [DOI] [PMC free article] [PubMed]
- Jaimes J.A., André N.M., Chappie J.S., Millet J.K., Whittaker G.R. Phylogenetic analysis and structural modeling of SARS-CoV-2 spike protein reveals an evolutionary distinct and proteolytically sensitive activation loop. J. Mol. Biol. 2020 doi: 10.1016/j.jmb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Shen G., Zhang Y., Huang K.S., Ho H.Y., Hor W.S., Yang C.H., Li C., Wang W.L. Analysis of the mutation dynamics of SARS-CoV-2 reveals the spread history and emergence of RBD mutant with lower ACE2 binding affinity. bioRxiv. 2020. [DOI]
- Jiali X., Zifeng W., Zhang M., Shijiang L., Zhou L., Yang C., Cunming L. 2021. The role of the gastrointestinal system in neuroinvasion by SARS-CoV-2. Front. Neurosci. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandemirli S.G., Dogan L., Sarikaya Z.T., Kara S., Akinci C., Kaya D., Kaya Y., Yildirim D., Tuzuner F., Yildirim M.S., Ozluk E., Gucyetmez B., Karaarslan E., Koyluoglu I., Demirel Kaya H.S., Mammadov O., Ozdemir I.K., Afsar N., Yalcinkaya B.C., Rasimoglu S., Guduk D.E., Jima A.K., Ilksoz A., Ersoz V., Eren M.Y., Celtik N., Arslan S., Korkmazer B., Dincer S.S., Gulek E., Dikmen I., Yazici M., Unsal S., Ljama T., Demirel I., Ayyildiz A., Kesimci I., Deveci S.B., Tutuncu M., Kizilkilic O., Telci L., Zengin R., Dincer A., Akinci I.O., Kocer N. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020 doi: 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr C., Hughes G., McKenna L., Bergin C., Prevalence of smell and taste dysfunction in a cohort of CoVID19 outpatients managed through remote consultation from a large urban teaching hospital in Dublin Ireland. Infect. Prev. Pract. 2020;2 doi: 10.1016/j.infpip.2020.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon F., Prasad K., Kumar V. Neurological manifestations of COVID-19: available evidences and a new paradigm. J. Neurovirol. 2020. [DOI] [PMC free article] [PubMed]
- Kiyatkin E.A., Sharma H.S. Permeability of the blood-brain barrier depends on brain temperature. Neuroscience 161. 2009. [DOI] [PMC free article] [PubMed]
- Kong Z., Wang J., Li T., Zhang Z., Jian J. 2019 novel coronavirus pneumonia with onset of dizziness: a case report. Ann. Transl. Med. 2020;8 doi: 10.21037/atm.2020.03.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M., de Silva T.I., Angyal A., Brown R.L., Carrilero L., Green L.R., Groves D.C., Johnson K.J., Keeley A.J., Lindsey B.B., Parsons P.J., Raza M., Rowland-Jones S., Smith N., Tucker R.M., Wang D., Wyles M.D., McDanal C., Perez L.G., Tang H., Moon-Walker A., Whelan S.P., LaBranche C.C., Saphire E.O., Montefiori D.C. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 182. 2020. [DOI] [PMC free article] [PubMed]
- Kuroda N. Epilepsy and COVID-19: updated evidence and narrative review. Epilepsy Behav. 2021 doi: 10.1016/j.yebeh.2021.107785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure A.C., Khan Y.M., Iyer R., Elkind M.S.V., Sansing L.H., Falcone G.J., Sheth K.N. Intracerebral hemorrhage in patients with COVID-19. Stroke 52. 2021. [DOI] [PMC free article] [PubMed]
- Li H., Wang Yangyang, Ji M., Pei F., Zhao Q., Zhou Y., Hong Y., Han S., Wang J., Wang Q., Li Q., Wang Yunshan. Transmission routes analysis of SARS-CoV-2: a systematic review and case report. Front. Cell Dev. Biol. 2020 doi: 10.3389/fcell.2020.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wu J., Nie J., Zhang Li, Hao H., Liu S., Zhao C., Zhang Q., Liu H., Nie L., Qin H., Wang M., Lu Q., Li Xiaoyu, Sun Q., Liu J., Zhang Linqi, Xuguang Li, Huang W., Wang Y. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell 182. 2020. [DOI] [PMC free article] [PubMed]
- Li Y.C., Bai W., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J. Med. Virol. 2020:24–27. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J. Infect. 2020. [DOI] [PMC free article] [PubMed]
- Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchese G. Cerebrospinal fluid findings in COVID-19 indicate autoimmunity. The Lancet Microbe. 2020:6–30147. doi: 10.1016/S2666-5247(20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdi M., Rahil A. Severe immune thrombocytopenia complicated by intracerebral haemorrhage associated with coronavirus infection: a case report and literature review. Eur.. J. Case reports intern. Med. 2019. [DOI] [PMC free article] [PubMed]
- Mao L., Wang M., Chen S., He Q., Chang J., Al E. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. SSRN electron. J. 2020 doi: 10.2139/ssrn.3544840. [DOI] [Google Scholar]
- Marsh E., Kornberg M., Kessler K., Haq I., Patel A., Nath A., Schierman B., Jones L. COVID-19 and vaccination in the setting of neurologic disease: an emerging issue in neurology. Neurology 97. 2021. [DOI] [PMC free article] [PubMed]
- Mastitskaya S., Thompson N., Holder D. Selective vagus nerve stimulation as a therapeutic approach for the treatment of ARDS: a rationale for neuro-immunomodulation in COVID-19 disease. Front. Neurosci. 2021. [DOI] [PMC free article] [PubMed]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., Dottermusch M., Heinemann A., Pfefferle S., Schwabenland M., Sumner Magruder D., Bonn S., Prinz M., Gerloff C., Püschel K., Krasemann S., Aepfelbacher M., Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19 doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam M., Samuel M., Alkufri F. Neuropathic pain post-COVID-19: a case report. BMJ Case Rep. 2021;14(7):e243. doi: 10.1136/bcr-2021-243459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt . 2020. Olfactory transmucosal SARS-CoV-2 invasion as port of Central Nervous System entry in COVID-19 patients. BioRxiv. [DOI] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brünink S., Greuel S., Lehmann M., Hassan O., Aschman T., Schumann E., Chua R.L., Conrad C., Eils R., Stenzel W., Windgassen M., Rößler L., Goebel H.H., Gelderblom H.R., Martin H., Nitsche A., Schulz-Schaeffer W.J., Hakroush S., Winkler M.S., Tampe B., Scheibe F., Körtvélyessy P., Reinhold D., Siegmund B., Kühl A.A., Elezkurtaj S., Horst D., Oesterhelweg L., Tsokos M., Ingold-Heppner B., Stadelmann C., Drosten C., Corman V.M., Radbruch H., Heppner F.L. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24 doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- Mengeling W.L., Boothe A.D., Ritchie A.E. 1972. Characteristics of a coronavirus (strain 67N) of pigs. Am. J. Vet. Res. 33. [PubMed] [Google Scholar]
- Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin. Neurol. Neurosurg. 2020 doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison A.P., Sweid A., Dharia R., Theofanis T.N., Tjoumakaris S.I., Jabbour P.M., Bilyk J.R. Monocular visual loss as the presenting symptom of COVID-19 infection. Clin. Neurol. Neurosurg. 2021;201 doi: 10.1016/j.clineuro.2020.106440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussbacher M., Salzmann M., Brostjan C., Hoesel B., Schoergenhofer C., Datler H., Hohensinner P., Basílio J., Petzelbauer P., Assinger A., Schmid J.A. Cell type specific roles of nf-kb linking inflamation and thrombosis. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutiawati E., Syahrul S., Fahriani M., Fajar J.K., Mamada S.S., Maliga H.A., Samsu N., Ilmawan M., Purnamasari Y., Asmiragani A.A., Ichsan I., Emran T., Bin, Rabaan A.A., Masyeni S., Nainu F., Harapan H. Global prevalence and pathogenesis of headache in COVID-19: a systematic review and meta-analysis. F1000Research 9. 2020. [DOI] [PMC free article] [PubMed]
- Nabavi N. Long covid: how to define it and how to manage it. BMJ 370. 2020. [DOI] [PubMed]
- Najjar S., Najjar A., Chong D.J., Pramanik B.K., Kirsch C., Kuzniecky R.I., Pacia S.V., Azhar S. Central nervous system complications associated with SARS-CoV-2 infection: integrative concepts of pathophysiology and case reports. J. Neuroinflammation. 2020. [DOI] [PMC free article] [PubMed]
- Nardell E.A., Nathavitharana R.R. Airborne spread of SARS-CoV-2 and a potential role for air disinfection. JAMA - J. Am. Med. Assoc. 2020. [DOI] [PubMed]
- Nikbakht F., Mohammadkhanizadeh A., Mohammadi E. How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms. Mult. Scler. Relat. Disord. 2020 doi: 10.1016/j.msard.2020.102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J., Zhou Z., Zhang J., Lan W., Zhao S., Wu J., Seto D., Zhang G., Zhang Q., Sheraton K. RBD mutations from circulating SARS-CoV-2 strains enhance the structure stability and infectivity of the spike protein. bioRxiv. 2020. [DOI]
- Pallanti S. 2020. Importance of SARs-Cov-2 anosmia: from phenomenology to neurobiology. Compr Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniri A., Hosseini M.M., Akhavan-Niaki H. First comprehensive computational analysis of functional consequences of TMPRSS2 SNPs in susceptibility to SARS-CoV-2 among different populations. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1767690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patone M., Handunnetthi L., Saatci D., Pan J., Katikireddi S.V., Razvi S., Hunt D., Mei X.W., Dixon S., Zaccardi F., Khunti K., Watkinson P., Coupland C.A.C., Doidge J., Harrison D.A., Ravanan R., Sheikh A., Robertson C., Hippisley-Cox J. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 2021;2021 doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan J., Deelder W., Ward D., Campino S., Hibberd M.L., Clark T.G. Controlling the SARS-CoV-2 outbreak, insights from large scale whole genome sequences generated across the world. bioRxiv. 2020. [DOI]
- Placantonakis D.G., Aguero-Rosenfeld M., Flaifel A., Colavito J., Inglima K., Zagzag D., Snuderl M., Louie E., Frontera J.A., Lewis A. SARS-CoV-2 is not detected in the cerebrospinal fluid of encephalopathic COVID-19 patients. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.587384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Huang I., Lim M.A., Yonas E., Vania R., Kuswardhani R.A.T. Delirium and mortality in coronavirus disease 2019 (COVID-19) A Systematic Review and Meta-analysis. Arch. Gerontol. Geriatr. 2021;95 doi: 10.1016/j.archger.2021.104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K., AlOmar S.Y., Alqahtani S.A.M., Malik M.Z., Kumar V. Brain disease network analysis to elucidate the neurological manifestations of COVID-19. Mol. Neurobiol. 2021;58 doi: 10.1007/s12035-020-02266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purja S., Shin H., Lee J., Kim E. Is loss of smell an early predictor of COVID-19 severity: a systematic review and meta-analysis. Arch Pharm. Res. (Seoul) 2021;44:725–740. doi: 10.1007/s12272-021-01344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A.I., Baskett W.I., Huang W., Shyu D., Myers D., Raju M., Lobanova I., Suri M.F.K., Naqvi S.H., French B.R., Siddiq F., Gomez C.R., Shyu C.R. Acute ischemic stroke and COVID-19: an analysis of 27 676 patients. Stroke. 2021. [DOI] [PMC free article] [PubMed]
- Rangon C.-M., Krantic S., Moyse E., Fougère B. The vagal autonomic pathway of COVID-19 at the crossroad of Alzheimer's disease and aging: a review of knowledge. J. Alzheimer's Dis. 2020 doi: 10.3233/adr-200273. Reports 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., González E., Redondo-Peñas I., Perona-Moratalla A.B., Del Valle-Pérez J.A., Gracia-Gil J., Rojas-Bartolomé L., Feria-Vilar I., Monteagudo M., Palao M., Palazón-García E., Alcahut-Rodríguez C., Sopelana-Garay D., Moreno Y., Ahmad J., Segura T. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology 95. 2020. [DOI] [PMC free article] [PubMed]
- Rossi A.P., Gottin L., Donadello K., Schweiger V., Brandimarte P., Zamboni G.A., Florio A., Boetti R., Pavan G., Zamboni M., Polati E. Intermuscular adipose tissue as a risk factor for mortality and muscle injury in critically ill patients affected by COVID-19. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.651167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau A., Minguet P., Colson C., Kellens I., Chaabane S., Delanaye P., Cavalier E., Chase J., Lambermont B., Misset B. Post-intensive care syndrome after a critical COVID-19: cohort study from a Belgian follow-up clinic. Ann. Intensive Care. 2021;29 doi: 10.1186/s13613-021-00910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saniasiaya J., Kulasegarah J. Dizziness and COVID-19. Ear, nose throat J. 2021. [DOI] [PMC free article] [PubMed]
- Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature 581. 2020. [DOI] [PMC free article] [PubMed]
- Shapiro R.E., Gallardo V.J., Caronna E., Pozo-Rosich P. 2021. The impact of headache disorders on COVID-19 survival: a world population-based analysis. medRxiv. [Google Scholar]
- Shi Y., Li Z., Yang C., Liu C. The role of gut-brain axis in SARA-CoV-2 neuroinvasion: culprit or innocent bystander? Brain. Behav. Immun. 2021. [DOI] [PMC free article] [PubMed]
- Singer M., Deutschman C.S., Seymour C., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., Poll T., Der, Vincent J.L., Angus D.C. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA - J. Am. Med. Assoc. 2016. [DOI] [PMC free article] [PubMed]
- Singh A., Singh R., Joshi S., Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab. Syndr. 2021;15:102146. doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H. Literature review as a research methodology: an overview and guidelines. J. Bus. Res. 2019;104:333–339. [Google Scholar]
- Sohal S., Mansur M. COVID-19 presenting with seizures. IDCases 20. 2020. [DOI] [PMC free article] [PubMed]
- Soiza R.L., Myint P.K. The scottish intercollegiate guidelines network (Sign) 157: guidelines on risk reduction and management of delirium. Med. 2019;55 doi: 10.3390/medicina55080491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K.R., Hsu T.H. Viral disruption of the blood-brain barrier. Trends Microbiol. 2012. [DOI] [PMC free article] [PubMed]
- Stafstrom C.E., Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb. Perspect. Biol. 2015;7 doi: 10.1101/cshperspect.a022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre C., Keshet A. Anosmia, ageusia, and other COVID-19-like symptoms in association with a positive SARS-CoV-2 test, across six national digital surveillance platforms: an observational study. Lancet Digit Heal. 2021:S2589–S7500. doi: 10.1016/S2589-7500(21)00115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H. The interaction between pathogens and the host coagulation system. Physiology. 2006 doi: 10.1152/physiol.00059.2005. [DOI] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L., Banovich N.E., Barbry P., Brazma A., Collin J., Desai T.J., Duong T.E., Eickelberg O., Falk C., Farzan M., Glass I., Gupta R.K., Haniffa M., Horvath P., Hubner N., Hung D., Kaminski N., Krasnow M., Kropski J.A., Kuhnemund M., Lako M., Lee H., Leroy S., Linnarson S., Lundeberg J., Meyer K.B., Miao Z., Misharin A.V., Nawijn M.C., Nikolic M.Z., Noseda M., Ordovas-Montanes J., Oudit G.Y., Pe’er D., Powell J., Quake S., Rajagopal J., Tata P.R., Rawlins E.L., Regev A., Reyfman P.A., Rozenblatt-Rosen O., Saeb-Parsy K., Samakovlis C., Schiller H.B., Schultze J.L., Seibold M.A., Seidman C.E., Seidman J.G., Shalek A.K., Shepherd D., Spence J., Spira A., Sun X., Teichmann S.A., Theis F.J., Tsankov A.M., Vallier L., van den Berge M., Whitsett J., Xavier R., Xu Y., Zaragosi L.E., Zerti D., Zhang H., Zhang K., Rojas M., Figueiredo F. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020 doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraboschi I, Bravo M, Fernández N, Stecher D, Melero M, Lasala M, et al. Mucormycosis. An emergent mycosis. Medicina (B. Aires). 2012;72 [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., Franciotta D., Baldanti F., Daturi R., Postorino P., Cavallini A., Micieli G. Guillain–barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020;382 doi: 10.1056/nejmc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucar F., C S. 2021. Central retinal artery occlusion in a patient who contracted COVID-19 and review of similar cases. BMJ Case Rep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uginet M., Breville G., Assal F., Lövblad K.O., Vargas M.I., Pugin J., Serratrice J., Herrmann F.R., Lalive P.H., Allali G. COVID-19 encephalopathy: clinical and neurobiological features. J. Med. Virol. 2021;93 doi: 10.1002/jmv.26973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygun Ö., Ertaş M., Ekizoğlu E., Bolay H., Özge A., Kocasoy Orhan E., Çağatay A.A., Baykan B. Headache characteristics in COVID-19 pandemic-a survey study. J. Headache Pain. 2020;21 doi: 10.1186/s10194-020-01188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp L., Richard D., Tan C.C.S., Shaw L.P., Acman M., Balloux F. No evidence for increased transmissibility from recurrent mutations in SARS-CoV-2. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-19818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa D., Ardolino G., Borellini L., Cogiamanian F., Vergari M., Savojardo V., Peyvandi F., Barbieri S. Subclinical myopathic changes in COVID-19. Neurol. Sci. 2021;25:1–7. doi: 10.1007/s10072-021-05469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Ming L., Guangshuo O. COVID-19, cilia, and smell. FEBS J. 2020. [DOI] [PMC free article] [PubMed]
- Wu C., Chen X., Cai Y., Xia J., Zhou Xing, Xu S., Huang H., Zhang L., Zhou Xia, Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou Xin, Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA intern. Med. 2020;180 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J. Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang P., Xu X., Gao L., Wang H., Xiong H., Li R. 2020. First case of 2019 novel coronavirus disease with Encephalitis. ChinaXiv. [Google Scholar]
- Xu J., Lazartigues E. Expression of ACE2 in human neurons supports the neuro-invasive potential of COVID-19 virus. Cell. Mol. Neurobiologia. 2020 doi: 10.1007/s10571-020-00915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H., Geisthoff U.W., Bauer C., Hautefort C., Herman P., Manley G.T., Lyon D.M., Hopkins C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect. Dis. 2020. 30293–0. [DOI] [PMC free article] [PubMed]
- Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E., Humbert K., Henninger N., Trivedi T., Lillemoe K., Alam S., Sanger M., Kim S., Scher E., Dehkharghani S., Wachs M., Tanweer O., Volpicelli F., Bosworth B., Lord A., Frontera J. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020. [DOI] [PMC free article] [PubMed]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020. 30079–5. [DOI] [PMC free article] [PubMed]
- Yongjian W., Cheng G., Lantian T., Zhongsi H., Al E. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20. 30083–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Li H.B., Lyu J.R., Lei X.M., Li W., Wu G., Lyu J., Dai Z.M. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int. J. Infect. Dis. 2020. [DOI] [PMC free article] [PubMed]
- Zhang J., Fang X., Mao Y., Qi H., Wu J., Liu X., You F., Zhao W., Chen Y., Zheng L. Real-time, selective, and low-cost detection of trace level SARS-CoV-2 spike-protein for cold-chain food quarantine. npj Sci. Food 5. 2021. [DOI] [PMC free article] [PubMed]
- Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., Woods R.J., Kanthi Y., Knight J.S. Neutrophil extracellular traps in COVID-19. JCI Insight 5. 2020. [DOI] [PMC free article] [PubMed]