Abstract
Background
Sudden Sensorineural Hearing Loss (SSNHL) is a relatively rare symptom after immunisation from commonly used vaccines such as rabies, hepatitis B, measles and H1N1 and it could be an occasional symptom of COVID-19, even in mild manifestations of the disease.
Case report
We describe the case of a 57-year-old patient that reported SSNHL and tinnitus in his right ear 2 days after the inoculation of the Oxford-AstraZeneca (VAXZEVRIA) Covid-19 vaccine. The patient almost fully recovered after therapy and was referred to a long term follow-up program.
Conclusion
The current report underlines the importance to consider SSNHL as a possible rare adverse effect of the Covid-19 vaccine and take in account this possibility to promptly proceed with diagnosis and treatment of suspect cases.
Keywords: COVID-19, Vaccine, Adverse reaction, SSNHL
1. Introduction
SSNHL is defined as a HL greater than 30 dB in at least three consecutive frequencies that has developed within 3 days. The pathophysiology of SSNHL remains unknown in most patients. Although several aetiological hypotheses have been proposed, the most likely causative sources seem to be cochlear injury from impaired vascular perfusion or viral infection. SSNHL has a prevalence of one in 10.000–15.000 persons, and it mostly afflicts people between 40 and 54 years old [1]. Disorders of cochlear blood flow due to alteration in plasma viscosity, cellular and platelet aggregability, red blood cell deformability and endothelial function have been reported in patients affected by SSNHL. Neurological manifestations of viral infections are well known [2]. In SSNHL, the viral action can directly damage cochlear nerves and/or cochlear structures or induce responses resulting from the cross-reaction of the inner ear antigens. Animal studies have reported viral induction of HL due to direct inner ear involvement or indirectly via cerebrospinal fluid. Since February 2020 a growing body of literature reports a possible association between Covid-19 and audiological symptoms [[3], [4], [5]]. Ricciardiello et al., noted that SSNHL could be a possible symptom of COVID-19, even in mild manifestations of the disease [6]. Furthermore, cases of SSNHL after immunisation with commonly used vaccines such as rabies, hepatitis B, measles and, more recently, H1N1 have been largely reported in the literature [7]. It is postulated that the viral antigen–antibody complex results in a hypersensitivity reaction, which damages the inner ear due to localised inflammation [1]. We present the case of sudden onset of HL and tinnitus two days after the inoculation of Covid-19 vaccine.
2. Case report
A 57-year-old patient reported the sudden onset of HL and tinnitus in right ear 2 days after the inoculation of the first shot of AstraZeneca (VAXZEVRIA) Covid-19 vaccine. He received the first shot on March 14, 2021, started complaining about tinnitus and Hl on March 16, 2021 and he was admitted at the outpatient Ear Nose and Throat (ENT) Department of “S. Giuseppe Hospital”, located in Empoli, Italy, on March 31, 2021. The patient received an RT-PCR OP/NP swab test, resulted negative for Sars-CoV2 the same day. The patient was a healthy male, without any previous disease, with no history of otological pathology or occupational exposure to noise, and he did not used ototoxic drug during the last year. He had no regular medications and no known drug allergies. There was no focal neurologic sign, and the patient has no history of migraine. Otoscopic examination showed normal external auditory canals and tympanic membranes.
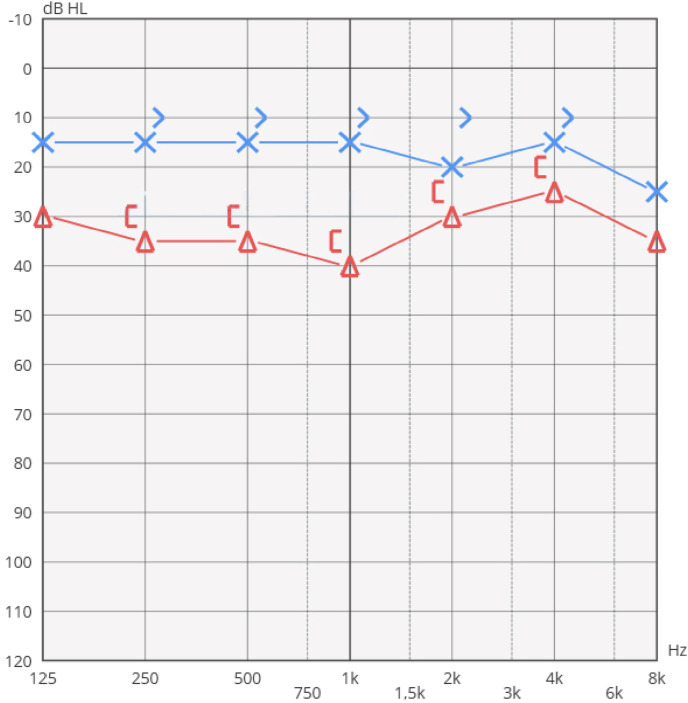
Pure tone audiometry (PTA) demonstrated normal hearing threshold in the left ear and a mild HL in the right ear (Fig. 1 ). Normal (type A) tympanograms were detected in both ears, stapedial reflexes were elicitable in both ears.
Fig. 1.
PTA performed before treatment.
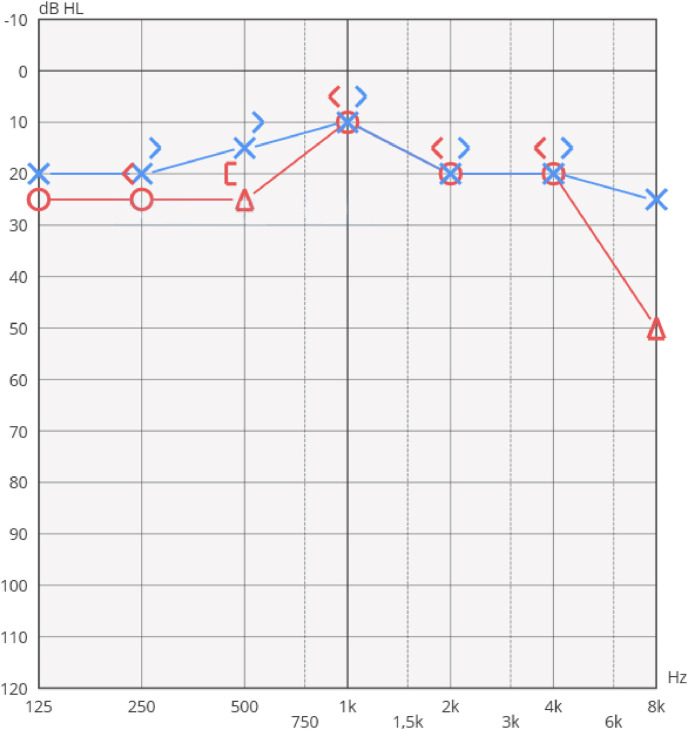
An MRI scan of the brain showed normal intracranial appearances, no abnormalities were seen at the internal auditory canals and cerebellopontine angle. An autoimmune screening (ANA, ENA, ANCA, anti-MPO) was performed, all values resulted within normal range. The patient completed a 12-day cycle of therapy with methylprednisolone according to the following scheme: 40mg intravenous (iv) for 4 days; 20 mg iv for 2 days; 16mg oral administrated for 3 days, and 8 mg oral administrated for 3 days. He received a B-vitamin complex integration of 400mcg/day for 2 Months and a 500mcg/day integration of folic acid. On April 13, the patient underwent a further audiological evaluation: the PTA showed a clear improvement of right ear's hearing threshold, the interaural gap was almost totally closed (Fig. 2 ).
Fig. 2.
PTA performed after treatment.
On April 23, this case was reported to the National Pharmacovigilance Network (RNF) of Italian Medicine Agency (AIFA), marked as non-severe reaction with partial recovery after treatment.
On May 18, the patient underwent a full blood count and inflammatory markers screening: all values were within normal range, except for slightly increased Fibrinogen (622 mg/dL, normal range 200–400). After two months of treatment, the patient was still suffering from tinnitus in right ear, while the PTA remained unchanged.
The patient received a final diagnosis of slight right sided SSNHL and was referred to a long-term follow-up protocol.
3. Discussion
Adenoviral vectors have been widely explored as vaccine agents for a range of infectious diseases because they are highly immunogenic, inducing a strong innate immune response.
The AstraZeneca vaccine is based on a SARS COV-2 spike protein with an adenovirus vaccine vector modified so that it cannot be reproduced in the human body and the genetic code transmits instructions to produce Coronavirus Spike protein, allowing the adenovirus to produce this protein after vaccination. The inflammatory response will lead to the formation of antibodies against the spike proteins [8].
Preliminary findings of data from the Vaccine Adverse Events Reporting System (VAERS), maintained by the US Centres for Disease Control and Prevention, in the early phase of societal COVID-19 vaccination using two messenger RNA vaccines suggest that no association exists between inoculation with a SARS-CoV-2 mRNA vaccine and incident SSNHL. Forty cases of confirmed SSHL out of more than 86 Millions vaccinations produces an estimated 0.3% incidence of SSHL following the COVID-19 vaccination, which not exceed that of the general population [9].
During the clinical trial of another adenovirus-based Covid-19 Vaccine (Covid-19 Vaccine Janssen) six cases of tinnitus were reported in the Ad26.COV2.S group and none in the placebo group. All these cases were labelled as non-serious. No typical temporal pattern was found, and all participants had risk factors and medical conditions that could lead to tinnitus, giving a more plausible explication of the symptom. None of these events have reported further complications such as hearing loss [10].
According to the AIFA, about 10% of the AstraZeneca Vaxzevria reported reactions concerns disorders occurring between the second and the seventh day after vaccination. Approximately 82% of reports were rated as non-serious [11].
The temporal relationship between the administration of the vaccine and the onset of HL, led us to report the event and to hypothesize the causal relationship, however this remains a simple hypothesis, given the difficulty of univocally associating an event, mild and reversible, to vaccination based only on the temporal criterion.
This should not lead to underestimate the possibility of observing similar possible adverse reactions, which although rare are capable of significantly altering the quality of life if not treated promptly.
4. Conclusion
The analysis of the reports on possible side effects carried out during this study showed that SSNHL is a very rare event, reported as a non-serious reaction, its incidence in the vaccinated subpopulation is not higher than that of the non-vaccinated subpopulation. These considerations, in addition to the concrete difficulty in attributing the etiopathological cause with sufficient certainty to the vaccine, requires us not to have any prejudice against vaccination, the only method capable of reducing the impact of the disease on the population.
Based on a scrupulous study of the effectiveness of the vaccination campaign, we can affirm that the vaccine remains the best weapon to face the COVID-19 emergency.
The purpose of this case, beyond the description of the uncommon clinical course, is to raise awareness of the possibility of encountering this type of pathology and be ready to promptly treat it because “time is hearing” when dealing with SSNHL.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee of the University of Catanzaro “Magna Graecia” and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee of the University of Catanzaro “Magna Graecia” and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chen X., Fu Y.Y., Zhang T.Y. Role of viral infection in sudden hearing loss. J Int Med Res. 2019 Jul;47(7):2865–2872. doi: 10.1177/0300060519847860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen B.E., Durstenfeld A., Roehm P.C. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. 2014 Jul 29;18 doi: 10.1177/2331216514541361. 2331216514541361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viola P., Ralli M., Pisani D., Malanga D., Sculco D., et al. Tinnitus and equilibrium disorders in COVID-19 patients: preliminary results. Eur Arch Oto-Rhino-Laryngol. 2020 Oct 23:1–6. doi: 10.1007/s00405-020-06440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spadera L., Viola P., Pisani D., Scarpa A., Malanga D., et al. Sudden olfactory loss as an early marker of COVID-19: a nationwide Italian survey. Eur Arch Oto-Rhino-Laryngol. 2021 Jan;278(1):247–255. doi: 10.1007/s00405-020-06252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilic O., Kalcioglu M.T., Cag Y., Tuysuz O., Pektas E., et al. Could sudden sensorineural hearing loss be the sole manifestation of COVID-19? An investigation into SARS-COV-2 in the etiology of sudden sensorineural hearing loss. Int J Infect Dis. 2020 Aug;97:208–211. doi: 10.1016/j.ijid.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricciardiello F., Pisani D., Viola P., Cristiano E., Scarpa A., et al. Sudden sensorineural hearing loss in mild covid-19: case series and analysis of the literature. Audiol. Res. 2021;11:313–326. doi: 10.3390/audiolres11030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asatryan A., Pool V., Chen R.T., Kohl K.S., Davis R.L., Iskander J.K., VAERS team Live attenuated measles and mumps viral strain-containing vaccines and hearing loss: vaccine Adverse Event Reporting System (VAERS), United States, 1990--2003. Vaccine. 2008 Feb 26;26(9):1166–1172. doi: 10.1016/j.vaccine.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 8.Stachler R.J., Chandrasekhar S.S., Archer S.M., Rosenfeld R.M., Schwartz S.R., et al. American Academy of Otolaryngology-Head and Neck Surgery. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012 Mar;146(3 Suppl):S1–35. doi: 10.1177/0194599812436449. [DOI] [PubMed] [Google Scholar]
- 9.Formeister E.J., Chien W., Agrawal Y., Carey J.P., Stewart C.M., et al. Preliminary analysis of association between COVID-19 vaccination and sudden hearing loss using US centers for disease Control and prevention vaccine adverse events reporting System data. JAMA Otolaryngol Head Neck Surg. 2021 Jul 1;147(7):674–676. doi: 10.1001/jamaoto.2021.0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://www.janssenmd.com/janssen-covid19-vaccine/safety/adverse-events/tinnitus/janssen-covid19-vaccine-adverse-event-tinnitus
- 11.https://www.aifa.gov.it/documents/20142/1315190/Rapporto_sorveglianza_vaccini_COVID-19_6.pdf