Abstract
Individuals carrying mutations in BRCA1 or p53 genes are predisposed to a variety of cancers, and both tumor suppressor genes have been implicated in DNA damage response pathways. We have analyzed a possible functional link between p53 and BRCA1 genes. Here we show that BRCA1 expression levels are down-regulated in response to p53 induction in cells that undergo either growth arrest, senescence, or apoptosis. Physiological stimuli, such as exposure to DNA-damaging agents, also result in negative regulation of BRCA1 levels in a p53-dependent manner prior to causing cell cycle arrest. Nuclear run-on experiments and luciferase reporter assays demonstrate that the changes in BRCA1 expression are mainly due to transcriptional repression induced by p53. In conclusion, the data show that BRCA1 expression levels are controlled by the presence and activity of wild-type p53 and suggest the existence of an intracellular p53/BRCA1 pathway in the response of cells to stress conditions.
p53, the protein product of a tumor suppressor gene, has been implicated in the control of cell proliferation and tumor progression, as well as in the maintenance of genome integrity in response to DNA-damaging events (1, 28, 29). Deletion or inactivation of the p53 gene is observed in more than half of human cancers (23). p53 is induced in response to suboptimal growth conditions (DNA damage, hypoxia, heat, starvation, etc.) and acts as an “emergency brake” to trigger cells to reversible or irreversible growth arrest or even to apoptosis (8, 15, 20, 38, 54, 62), thus protecting the genome from accumulating an excess of mutations. Once active, p53 binds specifically to p53 response elements and transactivates the expression of genes such as those encoding mdm2, GADD45, p21 (Waf1 or Cip1), bax, PAG608, cyclin G, and IGF-BP3 (1, 26, 28, 29). The p53 protein has also been shown to repress transcription driven from certain viral promoters, as well as from cellular genes. Genes encoding RB, c-fos, MAP4, presenilin, and DNA topoisomerase IIα are among the characterized p53-repressed genes (13, 38, 42, 50, 58).
Given the complexity and importance of the cellular response to p53, its expression must be tightly regulated. The ubiquitin-mediated proteolysis pathway is, at least in part, responsible for maintaining p53 protein levels at a low concentration in normal cells (9). p53 levels and activity are also under tight control by upstream effectors such as the DNA-protein kinase family (60), negative autoregulation (34), phosphorylation (24, 25, 55, 59), and other cellular proteins such as mdm2 and CBP/p300 (17, 30, 32, 49, 57).
BRCA1 is also a tumor suppressor gene. Mutations in the BRCA1 gene account for about 50% of inherited breast cancer cases and 80% of families predisposed to breast and ovarian cancers (5, 39). In fact, there are interesting parallels between p53 and BRCA1. BRCA1, like p53, is a cell cycle-regulated nuclear phosphoprotein (27, 43, 56) and has also been implicated in DNA damage response and repair pathways. In addition, BRCA1 interacts with some of the major proteins involved in eukaryotic double-strand break repair and homologous recombination (46, 47) and participates, directly or indirectly, in transcription-coupled repair of oxidative DNA damage (16, 35, 45). Both p53 and BRCA1 are posttranslationally altered by phosphorylation in response to DNA damage (47, 49, 51, 56). Moreover, BRCA1 acts as a transcription factor, although this property is less well characterized than for p53. In artificial systems, BRCA1 can activate the p21 gene and other genes containing p53-responsive elements (37, 52, 66) or trigger cells to undergo apoptosis (21). BRCA1 is a very complex protein that also seems to be regulated by phosphorylation (47, 56), by its interacting proteins (7, 48, 61, 67), and probably through its complex promoter (63). Nevertheless, little is known about BRCA1 activators and regulators. In addition, p53 coimmunoprecipitates with BRCA1 (6, 37, 53, 66) and BRCA1−/− embryos are partially rescued by null mutation of the p53 gene (19, 31).
In view of these similarities between p53 and BRCA1, we investigated how BRCA1 responds to p53 activation. We show here that exogenously induced p53 causes down-regulation of BRCA1 levels. In response to DNA-damaging agents, both BRCA1 mRNA and protein levels decrease prior to cell cycle arrest in a p53-dependent manner. Finally, we also demonstrate that BRCA1 transcription is repressed by wild-type (wt) p53. These findings indicate that, while present, p53 regulates BRCA1 expression in responding to stress growth conditions.
MATERIALS AND METHODS
Cell culture.
Normal human mammary epithelial cells (hNMEC) were isolated from reduction mammoplasties and were maintained in DFCI-1 complete medium (3) for a few passages (i.e., five or six population doublings). All other cell lines were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum plus antibiotics. For EJ-p53 and EJ-CAT cells, the medium contained 1 μg of tetracycline (tet)/ml. Removal of tet resulted in p53 induction, as previously described (54). Primary mouse mammary epithelial cells (mNMEC) were isolated from mammary glands of 129/Sv (wt p53; Jackson Laboratories) or 129 trp53tmlTyj (p53 knockout; Jackson Laboratories) mice, as previously described (41).
DNA damage treatment.
Cells were plated in 100- or 150-mm-diameter tissue culture dishes and grown to 60% confluence. Mitomycin C (MMC; Sigma) was added to cultures at 10 μg/ml, and cells were harvested after treatment at time points over 24 h. Actinomycin D (Act D; Sigma) was added to cultures at a final concentration of 10 ng/ml, and cells were harvested over 48 h after treatment. For gamma irradiation, cells were exposed to 20 Gy in a mark I137 Cs source irradiator and allowed to recover for up to 12 h. After treatment, cells were collected and processed for Western, Northern, or fluorescence-activated cell sorter (FACS) analyses.
Western and Northern blot analyses.
Samples were adjusted for equal protein loading and separated on 5 (for BRCA1 analysis) or 12% gels under reducing conditions. Western blot transfer onto nitrocellulose was carried out, and blots were probed with antibodies against BRCA1 (Ab17F8; Gene Tex, and MS110 [Ab-1]; Oncogene Science), wt p53 (Ab-6; Oncogene Science), and β-actin (clone AC-15; Sigma). Bands were detected using the ECL chemiluminescence detection method (Amersham) and exposed on X-ray film. For Northern blot analysis, total RNA was isolated after lysis of cells in guanidine-HCl and centrifugation on cesium chloride cushions. Then 20 μg of total RNA per sample was denatured, electrophoresed through a 0.8% agarose-formaldehyde gel, and transferred by capillarity onto a nylon membrane. BRCA1, p21, mdm2, and 36B4 probes were labeled with 32P by using the randomly primed DNA labeling technique. Blots were exposed on X-ray film after being washed. In all cases, the films were scanned (ScanJet IIcs; Hewlett-Packard), analyzed using Adobe Photoshop, and quantified using IPLab gel software.
Nuclear run-on assay.
Nuclear run-on analyses were performed as described previously (2). Briefly, filters containing 300 ng of the purified cDNA inserts were prepared using a slot blot apparatus, and DNA was immobilized on the filters at 80°C for 1 h. Nuclei were isolated from 107 EJ-p53 cells grown in the presence or absence of tet for 24 h. Then, nuclei were frozen in liquid nitrogen. Transcription in isolated nuclei was performed; thawed nuclei were incubated with nucleotides plus 8 μl of 10-mCi/ml [α-32P]UTP (800 Ci/mmol) for 30 min at 30°C and treated with RNase-free DNase I, and proteinase K treatment followed. Synthesized RNA was then extracted with phenol-chloroform and precipitated with ethanol. One milliliter of RNA solution containing 107 cpm was used to hybridize each of the cDNA-immobilized strips for 36 h at 65°C with shaking. Filters were washed two times at 65°C in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 1 h and another time in 2× SSC containing 10 mg of RNase A/ml at 37°C for 1 h. Quantification of the signal was performed on a PhosphorImager (Molecular Diagnostics) using IPLab gel software.
Plasmids, transfections, and enzymatic assays.
Plasmids pGL3-basic vector and pGL3-control vector (pGL3-CV) were obtained from Promega. Constructs pGL3-I through pGL3-V derive from pGL3-basic and correspond to plasmids pGL1, pGL2, pGL5, pGL6, and pGL7 in the paper by Xu et al. (63), respectively. pGL3-I-del was constructed by inserting a 580-bp SmaI fragment from pGL3-I into the pGL3-I vector after removal of the 856-bp HindIII-NruI fragment. pGL3-SV40/TATA-like, pGL3-SV40/mutTATA-like, and pGL3-SV40-ERE/AP1 were constructed by inserting a 50-bp KpnI/BamHI fragment containing a TATA-like sequence within the BRCA1 promoter, a mutated TATA-like sequence, or an ERE/AP1 region, respectively, into pGL3-CV. In all cases, two 38-base ordered oligonucleotides containing complementary 3′ ends were hybridized, filled in with Klenow fragments, and digested with KpnI/BamHI before being introduced into the equally digested pGL3-CV. EJ-p53 cells were grown to 60% confluence in 60-mm-diameter dishes 24 h prior to calcium phosphate transfection. DNA mixtures contained 3 μg of pRSV-LacZ and 5 μg of each of the test plasmids. At 16 h after transfection cells were subjected to a 20% glycerol shock for 1 min, washed three times with phosphate-buffered saline, (PBS) and incubated in a CO2 incubator for 36 to 48 h in fresh media with and without tet. Samples were then collected, and luciferase and β-galactosidase activities were measured. Luciferase assays were performed using a Promega kit by following the manufacturer's instructions. Briefly, cells from 60-mm-diameter dishes were lysed in 250 μl of luciferase lysis buffer containing 1% Triton X-100 as the detergent and incubated at room temperature for 15 min and lysates were transferred to microcentrifuge tubes. After the addition of 100 μl of luciferase assay reagent (which contains luciferin, ATP, and coenzyme A), luciferase activity on 25 μl of each sample was measured by using a luminometer (Monolight 2010; Analytical Luminescence Laboratory). The same lysates (50 μl of each sample) were used to measure the β-galactosidase activity. The substrate o-nitrophenyl-β-d-galactopyranoside (4 mg/ml; Sigma) was added, and reactions were allowed to proceed until a light yellow color developed. Reactions were stopped with 1 M sodium carbonate, and the absorbance at 420 nm was measured. Data obtained for β-galactosidase activities, representing the efficiency of transfection, were used to normalize luciferase measurements, and the transactivation activity of each test construct was calculated relative to the pGL3-basic vector (whose activity was arbitrarily defined as 1). Each construct was analyzed in triplicate and in three independent experiments; thus the relative promoters' activities represent the mean values ± standard deviations.
FACS analyses.
Cells treated with DNA-damaging agents were washed with PBS three times and collected by centrifugation into microcentrifuge tubes. Ice-cold 50% ethanol was then added dropwise over the pellets (106 cells/ml), and these were kept on ice for at least 60 min. After fixation, cells were permeabilized (0.5% Triton X-100 and 230 μg of RNase A/ml in PBS) and propidium iodide was added to 50 μg/ml. Samples were kept in the refrigerator at least 30 min and analyzed by flow cytometry (Becton Dickinson; FACScan) using a 488-nm excitation light and collecting fluorescence above 620 nm. Data were then processed with VERITY ModFit, version 5.2, for Microsoft Windows software for DNA distribution analysis.
RESULTS
Decreased expression of BRCA1 following wt p53 induction.
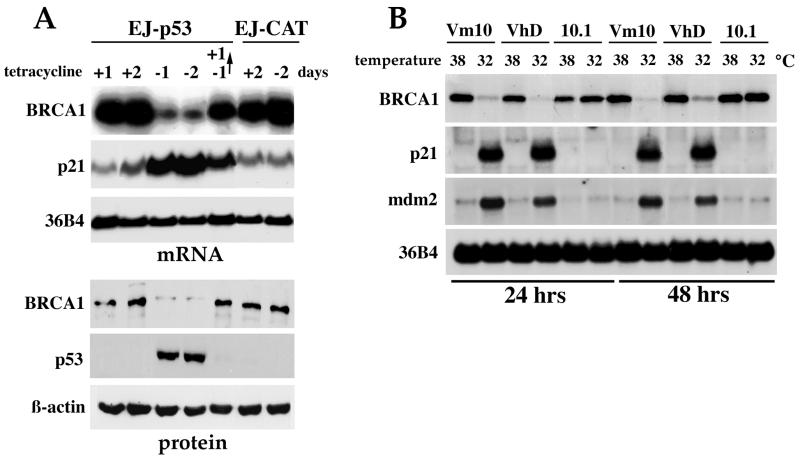
We attempted to determine if expression of wt p53 influenced the level of BRCA1 expression. EJ-p53 cells, a tet-regulatable p53 cell line previously described (54), was used to study the response of BRCA1 to p53 induction. Removal of tet from the culture media of EJ-p53 exponentially growing cells resulted in the expression of wt p53 protein as early as 6 h, with peak levels observed by 48 h (Fig. 1A). Under these conditions, BRCA1 mRNA and protein levels were readily detectable in the presence of tet but decreased markedly to ∼24 and ∼7%, respectively, following wt p53 induction (Fig. 1A). The induction of p53 also caused a slight shift in the BRCA1 protein mobility (Fig. 1A). This reduction of BRCA1 levels following p53 activation was in striking contrast to the induction of expression of well-known p53 target genes, including those encoding p21 (Fig. 1A) and mdm2 (data not shown). As a control, EJ-CAT cells, in which EJ cells were stably transfected with a tet-inducible chloramphenicol acetyltransferase (CAT) vector, did not respond to the tet removal, p53 remained undetectable, p21 was not induced, and BRCA1 levels were unchanged (Fig. 1A). This result indicated that tet itself or the overexpression of the CAT gene had no effect on BRCA1 expression levels and suggested that down-regulation of BRCA1 was specific to the p53 induction. Moreover, p53 expression was reversible, with no p53 detected in EJ-p53 cells within 24 h after tet readdition (Fig. 1A), which indicates that the system is tightly regulated. This absence of p53 after tet readdition was immediately followed by a decrease in p21 levels, and recovery of BRCA1 mRNA and protein to basal levels was also observed (Fig. 1A).
FIG. 1.
Down-regulation of BRCA1 by wt p53 overexpression. (A) Repression of BRCA1 mRNA and protein following p53 induction in a tet-regulated system (EJ-p53). RNA and protein extracts were collected at different time points from EJ-p53 cells grown in the presence (+) or absence (−) of tet. In lane −1→+1, cells were grown in the absence of tet for 1 day to allow expression of p53, and then tet was added for another day before RNA and protein were collected. The Northern blot was consecutively hybridized with probes for BRCA1, p21, and 36B4. Western blotting was performed, and blots were immunoblotted with BRCA1 (antibody Ab17F8; GeneTex), p53, and β-actin antibodies. EJ-CAT cells were used as the negative control. (B) Temperature shift induces decreased BRCA1 mRNA levels in cell lines containing a temperature-sensitive p53 mutant (Vm10 and VhD). The parental murine cell line 10.1, which is null for p53, does not show down-regulation of BRCA1 following the temperature shift.
To verify that the observed p53-mediated repression of BRCA1 expression was indeed a general phenomenon and not due to growth arrest triggered by p53 overexpression in one cell type (EJ-p53), another p53-inducible system was used. VhD and Vm10 cell lines are immortalized mouse embryonic fibroblast cells containing a temperature-sensitive p53 mutant (Val-to-Ala mutation in codon 135). These lines express a nonfunctional p53 protein at 37 to 39°C that becomes fully functional at 32°C. VhD cells undergo reversible G1 arrest in a p53-dependent manner at 32°C, while the Vm10 cell line, which expresses tsp53 and the c-myc oncogene, undergoes apoptosis at 32°C (8, 62). In contrast, p53 induction in EJ-p53 triggered cells to undergo irreversible growth arrest or senescence (54). In the two cell lines containing tsp53, the temperature shift from 38 to 32°C caused a marked reduction of BRCA1 mRNA expression within 24 h and was accompanied by the induction of the p53 target genes encoding p21 and mdm2 (Fig. 1B). However, the p53-null parental line of VhD and Vm10 cells (10.1) remained unchanged following the temperature shift: p21 and mdm2 were not induced, and the levels of BRCA1 remained constant (Fig. 1B), similar to the expression levels in VhD and Vm10 cells cultured at 38°C. These results demonstrate that p53-mediated down-regulation of BRCA1 is a generally occurring phenomenon.
Down-regulation of BRCA1 following DNA damage is p53 dependent.
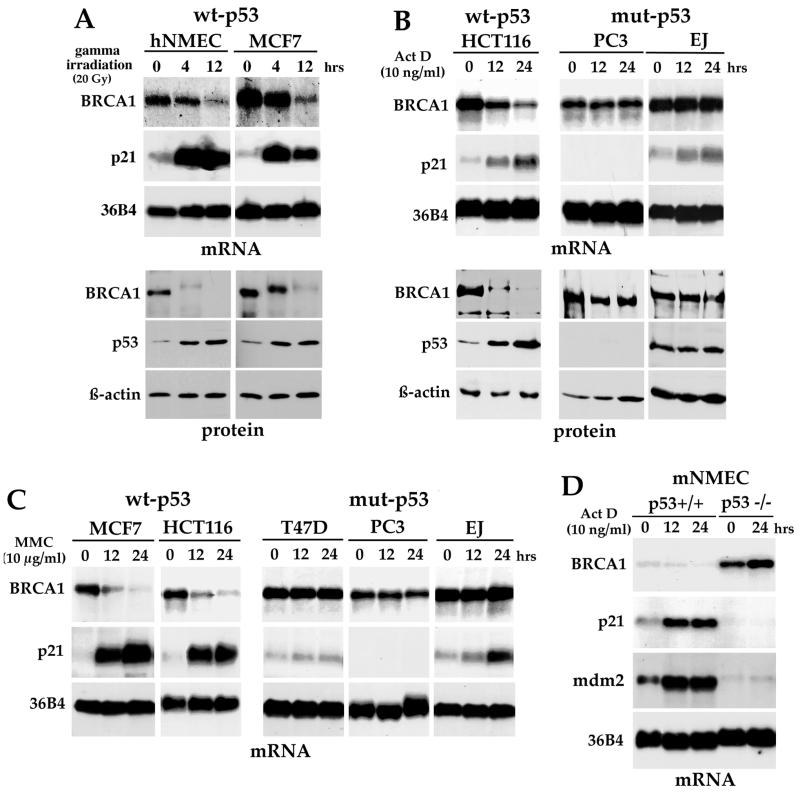
It is now well established that p53 functions to integrate cellular responses to stress such as DNA damage. p53 becomes activated following physiological stimuli such as DNA damage, leading to transcriptional activation of its target genes. Therefore, we analyzed whether BRCA1 expression could be regulated by the accumulation of p53 in response to DNA damage. First, hNMEC and a human breast cancer cell line (MCF7), both containing wt p53, were treated with 20 Gy in a gamma irradiator with a mark I135 Cs source. In both cell types, treatment resulted in a consistent and stepwise decrease in BRCA1 transcript levels, whereas the expression of the 36B4 message remained unchanged (Fig. 2A). The results from several independent Northern experiments indicate that BRCA1 mRNA levels were reduced by approximately 80% as early as 12 h after treatment in both cells. p53 activation was monitored at the protein level, as well as by transactivation of the p53 target gene, the p21 gene (Fig. 2A). Under the same conditions, the expression of the BRCA1 protein was measured by Western blot analysis. A dramatic disappearance of BRCA1 protein expression following p53 induction was observed, while β-actin expression remained constant (Fig. 2A). Of note, prior to its disappearance, the BRCA1 protein in gamma-irradiated cells was found to migrate at a slower rate than that from untreated control cells. This altered BRCA1 gel mobility was probably indicative of BRCA1 posttranslational modifications, such as phosphorylation, as other research groups have already indicated (10, 47, 56). However, irradiated T47D cells, a human breast carcinoma cell line which contains a mutant form of p53, did not show down-regulation of BRCA1 mRNA or protein levels or protein modification (not shown). Therefore, our data indicate that, following physiological induction of p53, the BRCA1 protein is most probably phosphorylated and then both mRNA and, to a further extent, protein levels of BRCA1 are diminished in a p53-dependent manner.
FIG. 2.
Decrease of BRCA1 expression in response to DNA damage requires wt p53. (A) Northern and Western blot analysis of BRCA1 expression following gamma irradiation. hNMEC and MCF7 cells were exposed to 20 Gy of gamma irradiation, and RNA or total proteins were collected at the indicated times following irradiation. After DNA damage exposure, the BRCA1 protein mobility was retarded and then the protein levels decreased. BRCA1 mRNA expression was also reduced. Western blots were immunoblotted with BRCA1 antibody Ab17F8 (GeneTex). (B) Down-regulation of BRCA1 expression in wt p53 cell lines following Act D treatment. A human colon cancer cell line containing wt p53 (HCT116 cells) and two other cell lines containing mutant (mut) p53 (PC3 and EJ) were treated for different time periods with 10 ng of Act D/ml, and then RNA and protein were collected. In this case, Western blots were immunoblotted with BRCA1 Ab1 from Oncogene (clone MS110). (C) Northern blot analysis of BRCA1 in several cell lines following treatment with MMC (10 μg/ml). BRCA1 expression levels were reduced only in the wt p53 cell lines. (D) Enhanced basal levels of BRCA1 in p53−/− cells compared to those in p53+/+ cells. mNMEC from p53 knockout mice or their wt equivalent were isolated and treated with Act D for different time periods; then Northern blot analysis was performed. p53 null cells do not reduce their BRCA1 expression levels in response to Act D treatment.
To further confirm the decrease in BRCA1 expression following DNA damage and to verify that the response was p53 dependent, several cell lines differing in their p53 status were treated with different DNA-damaging agents, such as Act D (Fig. 2B), MMC (Fig. 2C), doxorubicin, and UV irradiation (not shown). Exposure to these agents also resulted in a marked reduction of BRCA1 expression at the mRNA and protein levels in cells containing wt p53 (Fig. 2B and C). We noted further that a slower-migrating BRCA1 band from MCF7 cells appeared as early as 1 h after MMC treatment (data not shown). In contrast, BRCA1 expression levels remained unchanged when p53 mutant cell lines were exposed to DNA-damaging agents (Fig. 2B and C).
To assess the importance of p53 itself in controlling BRCA1 expression in a noncancerous context, we examined BRCA1 levels in mNMEC isolated from p53 knockout mice or their wt equivalent. Northern blot analysis revealed that endogenous BRCA1 expression was very elevated in p53−/− cells compared to that in p53+/+ cells (Fig. 2D). BRCA1 basal expression levels were found to be at least 10-fold higher in the p53 knockout cells. In the presence of p53, BRCA1 mRNA levels were reduced in response to DNA damage exposure, but p53 knockout cells failed to show down-regulation of BRCA1 levels. If anything, exposure of p53 null cells to Act D resulted in a slight induction of BRCA1 mRNA, while p21 and mdm2 remained almost undetectable (Fig. 2D). Altogether, these results show a strong correlation between p53 status and down-regulation of BRCA1 and demonstrate that functional p53 is required for BRCA1 repression to occur following DNA damage.
Down-regulation of BRCA1 occurs prior to cell cycle arrest.
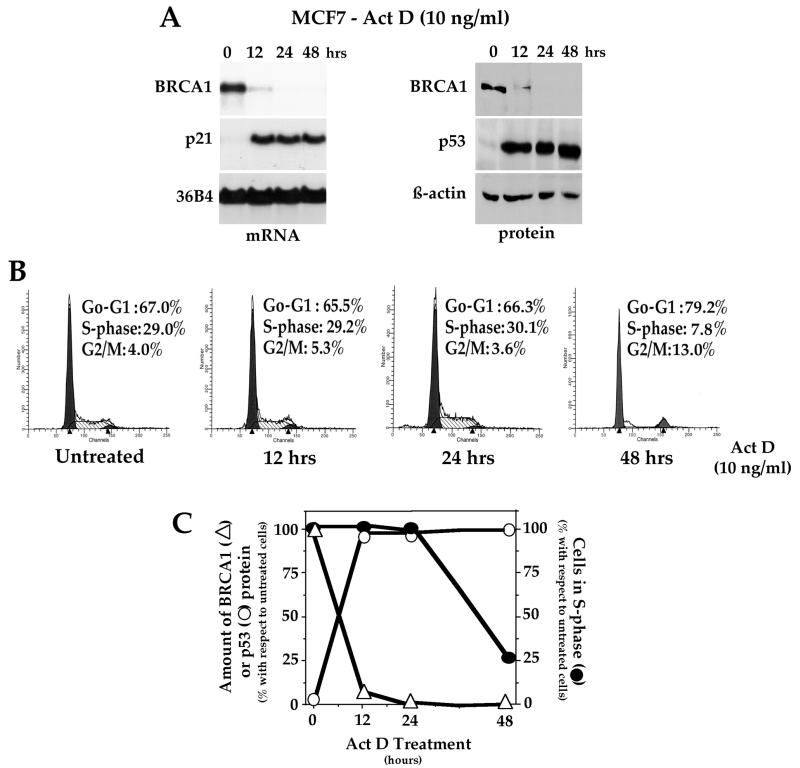
It is well characterized that, following induction and activation of p53, cells undergo arrest or apoptosis. Thus, it was possible that the reduction of BRCA1 might be an indirect effect of cell cycle arrest caused by p53, since BRCA1 is known to be expressed in a cell cycle-dependent manner. To determine if the observed p53-induced BRCA1 repression was indeed specific, we designed experiments to elucidate whether the reduced level of BRCA1 resulted from the arrest at G1 and/or G2/M induced by p53 or occurred prior to it. Thus, MCF7 cells, which contain wt p53, were exposed to a DNA-damaging agent (Act D; 10 ng/ml) and harvested at different times for assessment of BRCA1 mRNA and protein as well as cell cycle analysis by FACS (Fig. 3). For each time point, levels of BRCA1 expression and cell cycle status in Act D-treated cells and untreated control cells were compared. As shown in Fig. 3A, more than 90% of the BRCA1 mRNA and protein disappeared within 12 h after DNA damage, while cells were still progressing through S phase with no signs of arrest. At 24 h, expression of BRCA1 was almost absent, cells were still normally entering into S phase (Fig. 3B), and no apoptotic cells were present (data not shown). Figure 3C summarizes the time course of BRCA1 and p53 expression changes following DNA damage, establishing that down-regulation of BRCA1 expression in response to p53 induction clearly occurs prior to detectable growth arrest or apoptosis.
FIG. 3.
BRCA1 down-regulation in response to DNA damage occurs prior to cell cycle arrest. MCF7 cells were treated with 10 ng of Act D/ml, and then RNA and proteins were prepared and cell cycle analysis was performed at the indicated time points (0, 12, 24, and 48 h). (A) Reduction of BRCA1 expression following DNA damage as shown by Northern and Western blot analysis (Ab1-MS110 [Oncogene] was used to detect BRCA1 protein). (B) Cell cycle analysis of Act D-treated MCF7 cells was performed by FACS. The percentage of cells in each phase of the cell cycle after DNA damage exposure is indicated. No major changes were observed for the first day of treatment. Exposure to Act D for 48 h showed a significant reduction of cells in S phase, but no apoptotic nuclei were observed (data not shown). (C) Summary of the time courses of BRCA1 and p53 expression changes following DNA damage. Data in panels A and B were quantified, and the values obtained for the treated cells with respect to the untreated cells were represented in a graph form. Δ, percentage of BRCA1 protein level; ○, percentage of p53 protein level following Act D treatment; ●, percentage of the cells remaining in S phase after Act D treatment.
Repression of the human BRCA1 gene by wt p53.
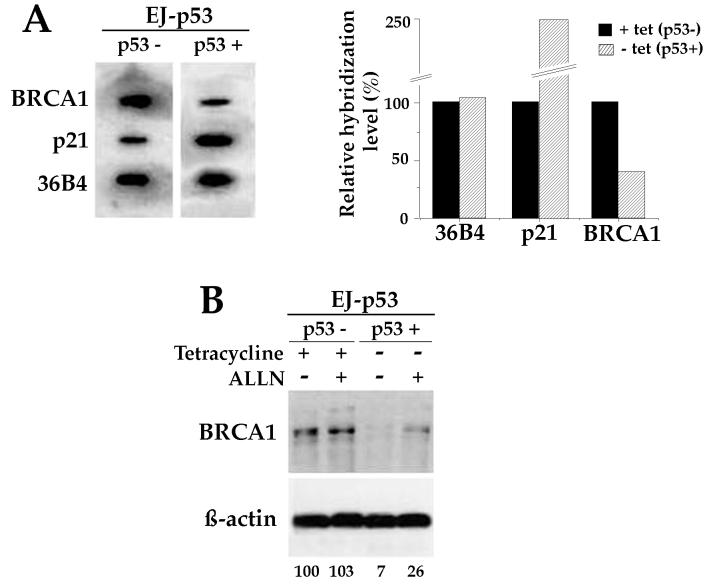
In an effort to determine whether the decrease in BRCA1 mRNA following p53 induction occurred at the transcriptional or posttranscriptional level, nuclear run-on assays were performed. Figure 4A depicts the data from nuclear run-on transcription assays performed on nuclei isolated from EJ-p53 cells maintained with and without tet for 24 h. Following removal of tet the rate of BRCA1 transcription was decreased to ∼40% of the basal level, whereas the rate of transcription from the p21/WAF1 gene, a p53-inducible gene, increased up to ∼250%. 36B4 was used as a control, and its rate of transcription remained unchanged following p53 induction (Fig. 4A). The nuclear run-on experiment indicates that repression of BRCA1 expression following p53 activation occurs mainly via a decrease of transcription from the promoter of the BRCA1 gene.
FIG. 4.
Transcriptional repression of BRCA1 by p53. (A) Nuclear run-on analysis using [α-32P]UTP-labeled nuclei from EJ-p53 cells grown in the presence of tet or in the absence of tet for 24 h (left). Filters used for hybridization contained 300 ng of purified cDNA inserts from BRCA1, p21, and 36B4. Quantification of nuclear run-on data relative to those for EJ-p53 cells grown in the presence of tet is shown at the right. BRCA1 transcription is significantly reduced in the presence of p53. (B) BRCA1 protein is destabilized. BRCA1 protein accumulates after inhibition of proteases when p53 is induced. EJ-p53 cells were cultured in the presence or absence of tet and treated with the protease inhibitor ALLN (100 μM) for 24 h. BRCA1 protein was then analyzed from cell extracts by Western blotting using BRCA1 antibody Ab17F8 from GeneTex, and the BRCA1 bands were quantified using IPLab gel software. Data obtained from that quantification, relative to the basal BRCA1 level in the absence of p53, are shown at the bottom.
In addition, we have investigated whether the BRCA1 protein is destabilized in the presence of p53, since a change in protein metabolism might explain why BRCA1 protein disappears to a greater extent than BRCA1 mRNA. For EJ-p53 cells, once p53 is active, BRCA1 protein is down-regulated to ∼7% of the basal level, while mRNA is down-regulated to only ∼24%. So, to further understand BRCA1 protein regulation, we treated EJ-p53 with the protease inhibitor ALLN in the presence or absence of tet. ALLN treatment of EJ-p53 cells partially abrogates p53-induced BRCA1 down-regulation. Figure 4B shows that BRCA1 protein accumulates faster after inhibition of proteases in p53-induced cells and suggests that BRCA1 protein might be degraded faster in a p53 functional system. The data demonstrate that the decrease in BRCA1 following p53 induction occurred mainly at the transcriptional level, although changes in protein stability also might play a role in BRCA1 disappearance.
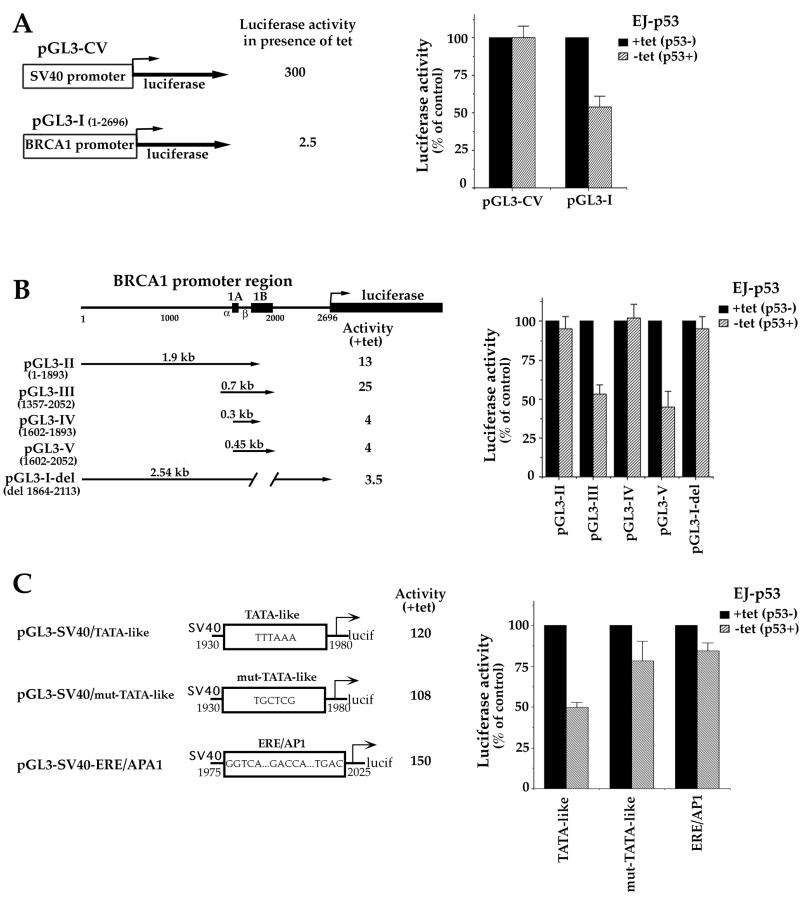
The elucidation of the mechanism whereby p53 down-regulates the expression of the BRCA1 gene was pursued by reporter analysis of the promoter and followed by delineation of those regions necessary to confer transcriptional repression. To do so, a luciferase construct containing the BRCA1 promoter sequence (pGL3-I; Fig. 5A) or a control plasmid containing the simian virus 40 (SV40) promoter (PGL3-CV; Promega) was transfected into EJ-p53 cells in the presence or absence of tet. The luciferase activities shown by these constructs were then measured and compared. As shown in Fig. 5A, induction of p53 decreased the luciferase activity under the control of the BRCA1 promoter by 50% while it had no effect on the luciferase activity of the control vector, pGL3-CV.
FIG. 5.
Analysis of the BRCA1 promoter region(s) necessary to confer p53-mediated BRCA1 transcriptional repression. (A) Transcriptional repression of the BRCA1 promoter by p53. (Left) Schematic diagram of the entire 2.7-kb BRCA1 promoter sequence cloned upstream of the luciferase reporter gene in the pGL3-basic vector (construct pGL3-I). pGL3-CV, which contains the luciferase reporter gene under the control of the SV40 promoter, was drawn for comparison purposes and used as a control. Luciferase activities obtained for each construct in EJ-p53 cells in the presence of tet were calculated relative to that for the pGL3-basic vector (whose activity was arbitrarily defined as 1). (Right) Luciferase activities obtained following transient transfection of the constructs. EJ-p53 cells were transiently transfected with either pGL3-CV or pGL3-I vector and incubated for 36 h in the presence (p53−) or absence (p53+) of tet. The pRSV-lacZ plasmid was cotransfected with each sample and used to normalize changes in the transfection efficiency. The y axis represents the relative luciferase activity after normalization with respect to EJ-p53 cells grown in the presence of tet, with no p53, for each construct. (B) A 160-bp sequence within the BRCA1 gene confers transcriptional repression. (Left) Schematic diagram showing the deletion constructs of the BRCA1 promoter cloned upstream of the luciferase reporter gene in the pGL3-basic vector. Solid boxes, BRCA1 exons 1A and 1B and the luciferase gene; promoters α and β are also marked. pGL3-II to pGL3-V, several fragments of pGL3-I, as indicated by the numbering and arrows; numbering of the constructs was done as described previously (63); pGL3-I-del, construct in which the sequence from bp 1864 to 2113 has been removed from the BRCA1 gene. All mutant promoters were active in EJ-p53 cells grown in the presence of tet. (Right) Relative luciferase activities obtained following transient transfection of those constructs in the presence or absence of tet. Transfection and activity measurements were done as indicated for panel A. Mutant constructs containing the BRCA1 sequence from bp 1893 to 2052 were repressed by p53. (C) Dissection of the bp 1893 to 2052 region of the BRCA1 gene. A 50-bp region including a BRCA1 TATA-like sequence (TTTAAA-containing sequence) under the control of the SV40 promoter causes a 50% decrease in luciferase activity following p53 induction, while its mutation reverses BRCA1 repression by p53.
To localize the region(s) of the BRCA1 gene mediating the observed negative regulation by p53, a number of deletion constructs spanning the promoter (Fig. 5B) were inserted upstream of the luciferase reporter of pGL3-basic vector (Promega). The constructs were transiently transfected into the EJ-p53 cells in the presence of tet (p53 repressed), and their transactivation activities relative to that of the pGL3-basic vector (whose activity was arbitrarily defined as 1) were calculated. All the constructs were expressed, though they showed different activities. The strongest activity was detected from pGL3-III, while the weakest was from pGL3-I (Fig. 5A and B). In order to study the effect of p53 induction on the activities of BRCA1 deletion mutants, the reporter constructs were transiently transfected into EJ-p53 cells grown in the presence or absence of tet, and their luciferase activities were compared. Those reporters containing a region of 160 bp spanning from bp 1893 to 2052, such as pGL3-III and pGL3-V (Fig. 5B, left) were inhibited up to 60% following p53 induction (Fig. 5B, right). In contrast, constructs containing deletions of bp 1893 to 2052 within the BRCA1 promoter, such as pGL3-II, pGL3-IV, and pGL3-I-del (Fig. 5B, left), failed to show a decrease in luciferase activity upon p53 induction (Fig. 5B, right). A DNA sequence homology search of this 160-bp region with the databases (22) revealed several known transcription factor elements including AP1, ERE, NF-κB, and a TATA-like sequence (TTTAAA), but no consensus p53 binding site. To further analyze the 160-bp region of the β BRCA1 promoter, several constructs, each containing a putative transcription factor binding site, were made upstream of the SV40 luciferase reporter gene of pGL3-CV. The luciferase gene under the control of the 50-bp construct containing the TATA-like sequence showed more than 50% reduction in activity following p53 induction, while a mutated form of this sequence (TTTAAA to TGCTCG) failed to show major inhibition (Fig. 5C). The activities of constructs containing the region including ERE and AP1 (Fig. 5C) and the NF-κB domain (data not shown) remained almost unchanged when tet was removed from the cell media. These data demonstrate that p53 mainly affects BRCA1 transcription and show that BRCA1 repression requires a 160-bp region within the BRCA1 gene, which contains a TTTAAA sequence that allows p53 to repress transcription by a still-unknown mechanism.
DISCUSSION
Eukaryotic cells ensure genetic integrity after sustaining DNA damage or defects in DNA metabolism by regulating the progression through the cell cycle. Under these circumstances, the cellular level of p53 protein increases, causing either cell cycle arrest or apoptosis. In the present study, we show that, as part of the cellular response to sustained DNA damage and/or p53 induction, (i) BRCA1 is first converted to a slower-migrating peptide, which probably corresponds to a phosphorylated form of the protein, then (ii) BRCA1 mRNA and protein levels are down-regulated in a p53-dependent manner, and (iii) this decrease is mainly due to changes in the transcriptional rate of the BRCA1 gene. The results indicate that BRCA1 is negatively regulated by p53 and suggest the existence of an intracellular p53/BRCA1 pathway in responding to stress conditions.
Following exposure of cells to DNA damage, such as gamma irradiation, we observed that the BRCA1 protein was first converted to a slower-migrating form, which likely reflects phosphorylation of the protein, and then the protein levels were significantly reduced in a p53-dependent manner. Several studies have already reported the appearance of a BRCA1 doublet in the first few hours following DNA damage corresponding to the presence of both BRCA1 p220 and a phosphorylated form (47, 56). It is noteworthy that we have not observed this higher-molecular-weight form of BRCA1 or the disappearance of BRCA1 expression in mutant p53-carrying cells, such as T47D, EJ, and PC3 cells, only in cells containing wild-type p53. More-recent reports have indicated that BRCA1 can be phosphorylated on a serine residue (amino acid 1497) by a cyclin-dependent kinase, Cdk2 (44). Furthermore, ATM seems to be required for phosphorylation of BRCA1 in response to radiation ionization (10). Therefore, accumulating evidence suggests that BRCA1 may be a substrate of one or more kinases activated in response to DNA damage.
p53 is known for its ability to act as a transcriptional transactivator by specifically binding to p53 response elements and activating the expression of a number of genes (reviewed in references 1, 28, and 29). However, the p53 protein also has a less-well-characterized activity as a negative regulator of transcription (13, 14, 36, 50, 58). In the present study, we demonstrate that wt p53 induction was followed by a significant reduction of BRCA1 mRNA and protein levels in cells undergoing either apoptosis (Vm10 cells), reversible G1 arrest (VhD cells), or irreversible G1 arrest (EJ-p53). Endogenous BRCA1 gene expression is also decreased following physiological induction of wt p53 by DNA damage. We have also demonstrated, through nuclear run-on experiments, that p53 induction alters the transcription rate of the BRCA1 gene. In addition, constructs containing a 160-bp region within the BRCA1 gene spanning from bp 1893 to 2052, and in particular those containing a TTTAAA sequence within it, showed a reduction in transcriptional activity, as seen in luciferase reporter assays. Our data also suggest that BRCA1 disappearance at the protein level can be explained partially by BRCA1 protein destabilization. Further analyses need to be done in order to determine if the acidic-cysteine proteases known to be involved in BRCA1 protein metabolism are activated by p53. Altogether, these results demonstrate that BRCA1 expression is modulated by p53 and place BRCA1 among the growth control and/or DNA repair-related genes under the regulatory control of wt p53.
Previous reports have indicated that BRCA1 mRNA and protein levels change as the cell cycle progresses, with decreased levels observed in G1 phase (43, 56). Here, we have shown that down-regulation of BRCA1 at the mRNA and protein levels preceded p53-induced cell cycle arrest and apoptosis, implying that BRCA1 modulation is p53 dependent and not a consequence of cell cycle inhibition. It remains to be elucidated whether BRCA1 down-regulation is a requirement for p53-induced cell cycle arrest and apoptosis to occur or if the two phenomena are independent.
There is accumulating evidence suggesting that BRCA1 plays a role in DNA repair pathways (16, 35; reviewed in reference 65). It is then difficult to understand why BRCA1 disappears shortly after the DNA damage has been produced, when apparently it is most needed. In that respect, the BRCA1 gene contains two BRCT domains that could be responsible for the transcriptional activation function of the BRCA1 C-terminal region (33); M. S. Chapman and I. M. Verma, Letter, Nature 382:678–679, 1996. It could be, as for c-fos and other transcription factors (4), that once it is activated BRCA1 targets other genes in the DNA repair chain and then disappears. Thus, one possible scenario is that BRCA1, once phosphorylated, may act synergistically with p53 to activate the p53 pathways of cell cycle arrest and DNA damage response, and then may be repressed and/or degraded in a p53-dependent manner when it is no longer needed. In contrast, in the absence of p53, when DNA damage occurs, we observe that BRCA1 is maintained inside the cells for longer periods of time and postulate that the accumulation of unrepaired damage may later trigger the cells to arrest or apoptosis in a BRCA1-dependent, p53-independent pathway. In this regard, the absence of BRCA1 is also thought to trigger the action of p53 and its target genes in BRCA−/− mice (18, 64). In the present study, we show that BRCA1 phosphorylation and subsequent down-regulation are part of a coordinated response to p53 induction under physiological conditions. While the exact relationship between both tumor suppressor genes remains to be further elucidated, p53 mutations are frequently associated with familial BRCA1-associated tumors (12, 40; T. Crook, S. Crossland, M. R. Compton, P. Osin, and B. A. Gusterson, Letter, Lancet 350:638–639, 1997). An increased incidence of mammary tumors was observed in the BRCA1+/−, p53−/− mice (11). Moreover, the loss of p53 accelerated the formation of mammary tumors in female mice with mammary epithelium-specific inactivation of BRCA1 (64). Altogether, the data suggest that the loss of BRCA1 and p53 genes may be integral to the progression of tumorigenesis in breast cancer.
ACKNOWLEDGMENTS
We thank A. M. Borras, J. R. Garreau, J. Licht, J. Manfredi, C. L. Reimer, and H. Zhang for helpful comments and discussion.
This work was supported by NIH grants CA78356, P50CA68425, P01CA80058, and CA79892.
ADDENDUM IN PROOF
W. S. El-Deiry's group has obtained results similar to those presented in this paper, and we thank them for communicating their data before publication (J. Biol. Chem., in press).
REFERENCES
- 1.Agarwal M L, Taylor W R, Chernov M V, Chernova O B, Stark G R. The p53 network. J Biol Chem. 1998;273:1–4. doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Identification of newly transcribed RNA. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 4.10.1–4.10.7. [Google Scholar]
- 3.Band V, Sager R. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proc Natl Acad Sci USA. 1989;86:1249–1253. doi: 10.1073/pnas.86.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brawerman G, Belasco J G. Control of messenger RNA stability. San Diego, Calif: San Diego Academic Press; 1993. [Google Scholar]
- 5.Castilla L H, Couch F J, Erdos M R, Hoskins K F, Calzone K, Garber J E, Boyd J, Lubin M B, Deshano M L, Brody L C, et al. Mutations in the BRCA1 gene in families with early-onset breast and ovarian cancer. Nat Genet. 1994;8:387–391. doi: 10.1038/ng1294-387. [DOI] [PubMed] [Google Scholar]
- 6.Chai Y L, Cui J, Shao N, Shyam E, Reddy P, Rao V N. The second BRCT domain of BRCA1 proteins interacts with p53 and stimulates transcription from the p21WAF1/CIP1 promoter. Oncogene. 1999;18:263–268. doi: 10.1038/sj.onc.1202323. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Silver D P, Walpita D, Cantor S B, Gadzar A F, Tomlinson G, Couch F J, Weber B L, Ashley T, Livingston D M, Scully R. Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol Cell. 1998;2:317–328. doi: 10.1016/s1097-2765(00)80276-2. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Wu X, Lin J, Levine A J. mdm-2 inhibits the G1 arrest and apoptosis functions of the p53 tumor suppressor protein. Mol Cell Biol. 1996;16:2445–2452. doi: 10.1128/mcb.16.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdary D R, Dermody J J, Jha K K, Ozer H L. Accumulation of p53 in a mutant cell line defective in the ubiquitin pathway. Mol Cell Biol. 1994;14:1997–2003. doi: 10.1128/mcb.14.3.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortez D, Wang Y, Qin J, Elledge S J. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 11.Cressman V L, Backlund D C, Avrutskaya A V, Leadon S A, Godfrey V, Koller B H. Growth retardation, DNA repair defects, and lack of spermatogenesis in BRCA1-deficient mice. Mol Cell Biol. 1999;19:7061–7075. doi: 10.1128/mcb.19.10.7061. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 12.Crook T, Brooks L A, Crossland S, Osin P, Barker K T, Waller J, Philip E, Smith P D, Yulug I, Peto J, Parker G, Allday M J, Crompton M R, Gusterson B A. p53 mutation with frequent novel condons but not a mutator phenotype in BRCA1- and BRCA2-associated breast tumours. Oncogene. 1998;17:1681–1689. doi: 10.1038/sj.onc.1202106. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg D, Mechta F, Yaniv M, Oren M. Wild-type p53 can down-modulate the activity of various promoters. Proc Natl Acad Sci USA. 1991;88:9979–9983. doi: 10.1073/pnas.88.22.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopalkrishnan R V, Lam E W F, Kedinger C. The p53 tumor suppressor inhibits transcription of the TATA-less mouse DP1 promoter. J Biol Chem. 1998;273:10972–10978. doi: 10.1074/jbc.273.18.10972. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb E, Oren M. p53 facilitates pRb cleavage in IL-3-deprived cells: novel pro-apoptotic activity of p53. EMBO J. 1998;17:3587–3596. doi: 10.1093/emboj/17.13.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gowen L C, Avrutskaya A V, Latour A M, Koller B H, Leadon S A. BRCA1 required for transcription-coupled repair of oxidative DNA damage. Science. 1998;281:1009–1012. doi: 10.1126/science.281.5379.1009. [DOI] [PubMed] [Google Scholar]
- 17.Gu W, Shi X L, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 18.Hakem R, de la Pompa J L, Sirard C, Mo R, Woo M, Hakem A, Wakeham A, Potter J, Reitmair A, Billia F, Firpo E, Hui C C, Roberts J, Rossant J, Mak T W. The tumor suppressor gene Brca1 is required for embryonic cellular proliferation in the mouse. Cell. 1996;85:1009–1023. doi: 10.1016/s0092-8674(00)81302-1. [DOI] [PubMed] [Google Scholar]
- 19.Hakem R, de la Pompa J L, Elia A, Potter J, Mak T W. Partial rescue of Brca1 (5-6) early embryonic lethality by p53 or p21 null mutation. Nat Genet. 1997;16:298–302. doi: 10.1038/ng0797-298. [DOI] [PubMed] [Google Scholar]
- 20.Hansen R, Oren M. p53; from inductive signal to cellular effect. Curr Opin Genet Dev. 1997;7:46–51. doi: 10.1016/s0959-437x(97)80108-6. [DOI] [PubMed] [Google Scholar]
- 21.Harkin D P, Bean J M, Miklos D, Song Y H, Truong V B, Englert C, Christians F C, Ellisen L W, Maheswaran S, Oliner J D, Haber D A. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97:575–586. doi: 10.1016/s0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- 22.Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel A E, Kel O V, Ignatieva E V, Ananko E A, Podkolodnaya O A, Kolpakov F A, Podkolodny N L, Kolchanov N A. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 1998;26:362–367. doi: 10.1093/nar/26.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollstein M, Rice K, Greenblatt M S, Soussi T, Fuchs R, Sorlie T, Hovig E, Smith-Sorensen B, Montesano R, Harris C C. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994;22:3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 24.Hupp T R, Lane D P. Allosteric activation of latent p53 tetramers. Curr Biol. 1994;4:865–875. doi: 10.1016/s0960-9822(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 25.Hupp T R, Meek D W, Midgley C A, Lane D P. Regulation of the specific DNA binding function of p53. Cell. 1992;71:875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 26.Israeli D, Tessler E, Haupt Y, Elkeles A, Wilder S, Amson R, Telerman A, Oren M. A novel p53-inducible gene, PAG608, encodes a nuclear zinc finger protein whose overexpression promotes apoptosis. EMBO J. 1997;16:4384–4392. doi: 10.1093/emboj/16.14.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Y, Xu X L, Yang M C, Wei F, Ayi T C, Bowcock A M, Baer R. Cell cycle-dependent colocalization of BARD1 and BRCA1 proteins in discrete nuclear domains. Proc Natl Acad Sci USA. 1997;94:12075–12080. doi: 10.1073/pnas.94.22.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 29.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 30.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig T, Chapman D L, Papaioannou V E, Efstratiadis A. Targeted mutations of breast cancer susceptibility gene homologs in mice: lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes Dev. 1997;11:1226–1241. doi: 10.1101/gad.11.10.1226. [DOI] [PubMed] [Google Scholar]
- 32.Momand J, Zambetti G P, Olson D C, George D, Levine A J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 33.Monteiro A N, August A, Hanafusa H. Evidence for a transcriptional activation function of BRCA1 C-terminal region. Proc Natl Acad Sci USA. 1996;93:13595–13599. doi: 10.1073/pnas.93.24.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosner J, Mummenbrauer T, Bauer C, Sczakiel G, Grosse F, Deppert W. Negative feedback regulation of wild-type p53 biosynthesis. EMBO J. 1995;14:4442–4449. doi: 10.1002/j.1460-2075.1995.tb00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moynahan M E, Chiu J W, Koller B H, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 36.Murphy M, Hinman A, Levine A J. Wild-type p53 negatively regulates the expression of a microtubule-associated protein. Genes Dev. 1996;10:2971–2980. doi: 10.1101/gad.10.23.2971. [DOI] [PubMed] [Google Scholar]
- 37.Ouchi T, Monteiro A N, August A, Aaronson S A, Hanafusa H. BRCA1 regulates p53-dependent gene expression. Proc Natl Acad Sci USA. 1998;95:2302–2306. doi: 10.1073/pnas.95.5.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polyak K, Waldman T, He T C, Kinzler K W, Vogelstein B. Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev. 1996;10:1945–1952. doi: 10.1101/gad.10.15.1945. [DOI] [PubMed] [Google Scholar]
- 39.Rahman N, Stratton M R. The genetics of breast cancer susceptibility. Annu Rev Genet. 1998;32:95–121. doi: 10.1146/annurev.genet.32.1.95. [DOI] [PubMed] [Google Scholar]
- 40.Ramus S J, Bobrow L G, Pharoah P D, Finnigan D S, Fishman A, Altaras M, Harrington P A, Gayther S A, Ponder B A, Friedman L S. Increased frequency of TP53 mutations in BRCA1 and BRCA2 ovarian tumours. Genes Chromosomes Cancer. 1999;25:91–96. doi: 10.1002/(sici)1098-2264(199906)25:2<91::aid-gcc3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Reimer C L, Borras A, Kurdistani S K, Garreau J R, Chung M, Aaronson S A, Lee S W. Altered regulation of cyclin G in human breast cancer and its specific localization at replication foci in response to DNA damage in p53+/+ cells. J Biol Chem. 1999;274:11022–11029. doi: 10.1074/jbc.274.16.11022. [DOI] [PubMed] [Google Scholar]
- 42.Roperch J P, Alvaro V, Prieur S, Tuynder M, Nemani M, Lethrosne F, Piouffre L, Gendron M C, Israeli D, Dausset J, Oren M, Amson R, Telerman A. Inhibition of presenilin 1 expression is promoted by p53 and p21WAF-1 and results in apoptosis and tumor suppression. Nat Med. 1998;4:835–838. doi: 10.1038/nm0798-835. [DOI] [PubMed] [Google Scholar]
- 43.Ruffner H, Verma I M. BRCA1 is a cell cycle-regulated nuclear phosphoprotein. Proc Natl Acad Sci USA. 1997;94:7138–7143. doi: 10.1073/pnas.94.14.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruffner H, Jiang W, Craig A G, Hunter T, Verma I M. BRCA1 is phosphorylated at serine 1497 in vivo at a cyclin-dependent kinase 2 phosphorylation site. Mol Cell Biol. 1999;19:4843–4854. doi: 10.1128/mcb.19.7.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scully R, Ganesan S, Vlasakova K, Chen J, Socolovsky M, Livingston D M. Genetic analysis of BRCA1 function in a defined tumor cell line. Mol Cell. 1999;4:1093–1099. doi: 10.1016/s1097-2765(00)80238-5. [DOI] [PubMed] [Google Scholar]
- 46.Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston D M. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 47.Scully R, Chen J, Ochs R L, Keegan K, Hoekstra M, Feunteun J, Livingston D M. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–435. doi: 10.1016/s0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 48.Scully R, Anderson S F, Chao D M, Wei W, Ye L, Young R A, Livingston D M, Parvin J D. BRCA1 is a component of the RNA polymerase II holoenzyme. Proc Natl Acad Sci USA. 1997;94:5605–5610. doi: 10.1073/pnas.94.11.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shieh S Y, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 50.Shiio Y, Yamamoto T, Yamaguchi N. Negative regulation of Rb expression by the p53 gene product. Proc Natl Acad Sci USA. 1992;89:5206–5210. doi: 10.1073/pnas.89.12.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siliciano J D, Canman C E, Taya Y, Sakaguchi K, Appella E, Kastan M B. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Somasundaram K, Zhang H, Zeng Y X, Houvras Y, Peng Y, Wu G S, Licht J D, Weber B L, El-Deiry W S. Arrest of the cell cycle by the tumour-suppressor BRCA1 requires the CDK-inhibitor p21WAF1/CiP1. Nature. 1997;389:187–190. doi: 10.1038/38291. [DOI] [PubMed] [Google Scholar]
- 53.Sturzbecher H W, Donzelmann B, Henning W, Knippschild U, Buchhop S. p53 is linked directly to homologous recombination processes via RAD51/RecA protein interaction. EMBO J. 1996;15:1992–2002. [PMC free article] [PubMed] [Google Scholar]
- 54.Sugrue M M, Shin D Y, Lee S W, Aaronson S A. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc Natl Acad Sci USA. 1997;94:9648–9653. doi: 10.1073/pnas.94.18.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takenaka I, Morin F, Seizinger B R, Kley N. Regulation of the sequence-specific DNA binding function of p53 by protein kinase C and protein phosphatases. J Biol Chem. 1995;270:5405–5411. doi: 10.1074/jbc.270.10.5405. [DOI] [PubMed] [Google Scholar]
- 56.Thomas J E, Smith M, Tonkinson J L, Rubinfeld B, Polakis P. Induction of phosphorylation on BRCA1 during the cell cycle and after DNA damage. Cell Growth Differ. 1997;8:801–809. [PubMed] [Google Scholar]
- 57.Thut C J, Goodrich J A, Tjian R. Repression of p53-mediated transcription by MDM2: a dual mechanism. Genes Dev. 1997;11:1974–1986. doi: 10.1101/gad.11.15.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Q, Zambetti G P, Suttle D P. Inhibition of DNA topoisomerase II alpha gene expression by the p53 tumor suppressor. Mol Cell Biol. 1997;17:389–397. doi: 10.1128/mcb.17.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Prives C. Increased and altered DNA binding of human p53 by S and G2/M but not G1 cyclin-dependent kinases. Nature. 1995;376:88–91. doi: 10.1038/376088a0. [DOI] [PubMed] [Google Scholar]
- 60.Woo R A, McLure K G, Lees-Miller S P, Rancourt D E, Lee P W. DNA-dependent protein kinase acts upstream of p53 in response to DNA damage. Nature. 1998;394:700–704. doi: 10.1038/29343. [DOI] [PubMed] [Google Scholar]
- 61.Wu L C, Wang Z W, Tsan J T, Spillman M A, Phung A, Xu X L, Yang M C, Hwang L Y, Bowcock A M, Baer R. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat Genet. 1996;14:430–440. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]
- 62.Wu X, Bayle J H, Olson D, Levine A J. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 63.Xu C F, Chambers J A, Solomon E. Complex regulation of the BRCA1 gene. J Biol Chem. 1997;272:20994–20997. doi: 10.1074/jbc.272.34.20994. [DOI] [PubMed] [Google Scholar]
- 64.Xu X, Wagner K U, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng C X. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Tombline G, Weber B L. BRCA1, BRCA2, and DNA damage response: collision or collusion? Cell. 1998;92:433–436. doi: 10.1016/s0092-8674(00)80936-8. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H, Somasundaram K, Peng Y, Tian H, Bi D, Weber B L, El-Deiry W S. BRCA1 physically associates with p53 and stimulates its transcriptional activity. Oncogene. 1998;16:1713–1721. doi: 10.1038/sj.onc.1201932. [DOI] [PubMed] [Google Scholar]
- 67.Zhong Q, Chen C F, Li S, Chen Y, Wang C C, Xiao J, Chen P L, Sharp Z D, Lee W H. Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science. 1999;285:747–750. doi: 10.1126/science.285.5428.747. [DOI] [PubMed] [Google Scholar]