Abstract
Background and Objectives:
In recent decades, enterococcal resistance to antimicrobials has greatly increased. Furthermore, these chemicals include several side effects on the patients. Since no reports are available of the bacteriophages’ effects on eukaryotic cells, they can be good solutions for multidrug-resistant bacterial problems. Therefore, the major aim of this study was to isolate bacteriophages from wastewaters on clinical antibiotic-resistant enterococci.
Materials and Methods:
Clinical bacteria were isolated, then enterococcal isolates were identified using different methods. The antibiotic resistance scheme of the enterococcal isolates was assessed. The bacterial isolates were exposed to wastewater samples containing potential bacteriophages. Technically, isolated bacteriophages were studied by electron microscopy.
Results:
Isolated bacteria were verified as Enterococcus faecium. Results showed that bacteriophages could easily be isolated from wastewater sources. The isolated bacteriophages were effective on E. faecium as well as Streptococcus dysgalactiae. Furthermore, these bacteriophages were challenged with five other bacteria (ATCC) with no visible effects. In general, the isolated bacteriophages belonged to the Myoviridae, Siphoviridae, and Inoviridae families.
Conclusion:
Further studies on bacteriophages and their efficacy on enterococcal strains could increase the treatment possibility of enterococcal infections. Due to these bacteriophages’ effects on Streptococcus strains, bacteriophages may be used to treat streptococcal infections as well.
Keywords: Enterococcus, Bacteriophages, Antibiotics, Wastewaters
INTRODUCTION
Enterococcus spp. are Gram-positive gastrointestinal tract colonizers that opportunistically colonize wounds (GIT) and bloodstream, causing life-threatening infections such as bacteremia and endocarditis (1, 2). These bacteria are particularly linked to central-line-associated bloodstream infection (CLABSI), a type of hospital-acquired infection (HAI) caused by the use of central venous catheters. In the United States, enterococci are linked to 18% of all CLABSI (3). The bacteria are parts of the normal intestinal microflora of humans, mammals, and birds. Of all enterococcal species, Enterococcus faecalis and E. faecium are the most commonly identified species in human samples, whereas E. gallinarum and E. casseliflavus are less identified (4). Enterococci are the cause of nosocomial infections, most frequently associated with intra-abdominal, pelvic, catheter, surgical, central nervous system (CNS) and urinary tract (UT) infections and endocarditis (5). It has been reported that enterococci from livestock and companion animals can be transmitted to humans via direct contact (6). Regarding public and animal health, it is important to prevent transmission of multiple-drug-resistant (MDR) enterococcal strains between the animals or from animals to humans (7). A previous study reported that animals treated with antibiotics in intensive care units (ICU) were sources or zoonotic transmitters of MDR Enterococcus species (8). In the US, 35–75% of enterococcal infections are caused by E. faecium; from which, a majority are vancomycin-resistant (9). In addition to potential threats by vancomycin-resistant enterococci (VRE), MDR enterococci naturally serve as reservoirs for horizontal gene transfer (HGT) of antibiotic resistance to other pathogens; as verified by reports of vanA transfer from Enterococcus species to Staphylococcus aureus (10).
Bacteriophages (phages) are viruses capable of infecting and replicating within bacterial cells. They are the most abundant and ubiquitous entities on Earth, playing important roles in microbial physiology, evolution, population dynamics, and therapeutics. Bacteriophages replicate through two primary life cycles or dynamic mechanisms, which are important in their therapeutic uses. Virulent or obligate lytic bacteriophages infect and quickly kill their bacterial hosts, whereas temperate or lysogenic bacteriophages may stably integrate into their host genome or enter a lytic life cycle (11). Phage therapy is described as direct administrations of lytic bacteriophages to patients to lyse bacterial pathogens (12). Recently, phage therapy has widely been interested as an alternative antimicrobial strategy to treat antibiotic-resistant biofilm-forming pathogens. For example, topical phage therapy is now considered a good option in infections of burn wounds primarily caused by Pseudomonas aeruginosa and S. aureus (13). Several benefits are associated with to use of bacteriophages in the treatment of bacterial infections. For example, bacteriophages are bactericidal agents, which can contribute to establishing their effective doses (auto dosing). Moreover, bacteriophages include low-inherent toxicities and can be isolated rapidly (14). Relatively, enterococcal bacteriophages can be isolated from wastewaters (15). Therefore, the major aims of this study were to isolate and identify bacteriophages from municipal wastewaters on clinical antibiotic-resistant Enterococcus strains.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains were isolated from 25 clinical patients referred to Imam Khomeini Hospital, Tehran, Iran, 2019–2020. The current study was approved by the Ethical Committee of Tehran University of Medical Sciences, Tehran, Iran (approval no. IR.TUMS.SPH.REC.1397.139). Relative information of the patients was collected using questionnaires. Blood samples were immediately cultured on Bile Esculin Agar and incubated at 37°C for 24 h. Isolates were verified using morphological, biochemical, and molecular techniques. First, isolates were Gram-stained and studied using a direct light microscope. Then, isolates were biochemically identified using arabinose fermentation test, salt tolerance test, optochin susceptibility test, CAMP test, and PYR test (16). Molecular identification of the isolates was carried out through amplification of the tuf gene using the polymerase chain reaction (PCR) technique. An amplicon of each isolate was sequenced using the partial Sanger sequencing method.
Bacterial tuf gene partial sequencing.
To sequence the bacterial partial genome, a colony of the bacteria was dissolved in sterile distilled water using a sterile microtube. The microtube was incubated at 90°C for 30 min to extract the genome (17). After centrifugation of the solution, the concentration of the extracted DNA was measured and the 260/280 and 260/230 nm ratios were calculated using NanoDrop One (Thermo Fisher Scientific, USA). In addition, the extracted bacterial genome was exclusively amplified using polymerase chain reaction (PCR) amplification of the genus-specific tuf gene (amplicon size of 112 bp) (17). The tuf gene-specific primers included Ent1: 5′-TACTGACAAACCATTCATGATG-3′ and Ent2: 5′-AACTTCGTCACCAACGCGAAC-3. Briefly, 25 μl from the extracted genome was amplified using a thermal cycler (Bioer Life ECO, China). The initial denaturation was 95°C for 5 min followed by 30 cycles of denaturation at 95°C for 30 min, annealing at 54°C for 30 min and elongation at 72°C for 45 min. Final elongation was carried out at 72°C for 7 min. Furthermore, the genome was quality assessed using agarose gel electrophoresis. The bacterial genome was partially sequenced for tuf gene (Kawsar Biotech, Iran), which is one of the most conserved genes in the bacterial genome, and it has been sequenced. The results were annotated in DNA Data Bank of Japan (DDBJ) under the accession numbers LC580430 and LC580431.
Bacterial antimicrobial susceptibility assessment.
The antimicrobial susceptibility patterns of the isolates were assessed using the Kirby-Bauer method and the following antimicrobials, which were mostly used for the treatment of enterococcal infections in Hospitals. These antibiotics, including ceftriaxone (30 μg),cefoxitin(30μg),clindamycin(2μg),erythromycin (15 μg), linezolid (30 μg) and vancomycin (30 μg).
Detection of bacteriophages.
Briefly, a colony of the bacterial sample was inoculated into 5 ml of trypticase soy broth (TSB) liquid media and incubated at 37°C overnight. Then, various wastewater samples were collected for the bacteriophage isolation and screened in the Microbiology Laboratory, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.Two of the collected wastewater samples included bacteriophages that were capable to lyse enterococcal bacteria. Wastewater samples were centrifuged at 700 g for 10 min. After centrifugation, the supernatant was filtered using 0.45-μm syringe filters. The bacterial samples in TSB were mixed with filtered sewage samples and 5 ml of brain heart infusion (BHI) broth media and incubated at 37°C overnight. A colony of the bacterial sample was inoculated into TSB media and incubated at 37°C overnight. The incubated culture was centrifuged at 700 g for 10 min. The supernatant was filtered through 0.45-μm syringe filters; then, 300 μl of chloroform were added to the filtrate with agitation and set for 10–15 min. This was centrifuged at 1,600 g for 5 min and the supernatant was filtered through 0.22-μm syringe filters. Then, 4.5 ml of 0.75% trypticase soy agar (TSA), Which is a two-layer agar that facilitates the movement of bacteriophages, along with 360 μl of the filtered wastewater sample and 160 μl of overnight bacteria were poured into a sterile microtube and set for 10 min with agitation. This was mixed well with top agar and poured onto the plates. After 10 min of setting at room temperature, plates were incubated at 37°C overnight (15). After overnight incubation, plates were studied for the presence of the bacteriophage plaques. The highlighted steps were used for 25 enterococci strains isolated from the clinical samples.
Plaque purification and propagation.
To purify bacteriophages, a plaque was selected from the plate and transferred into saline magnesium (SM) buffer containing microtube using Pasteur pipettes. The microtube was centrifuged at 1,306g for 20 min to precipitate debris and the liquid was filtered using syringe filters. The liquid was added to the TSB media and after 24 h, 360 μl of TSB media and 160 μl of the bacteria in the logarithmic phase were added to 0.75% TSA top agar (15). After 24 h, the previous steps were repeated three to five times to purify bacteriophages. Plaques achieved from the final purification steps were stored at 4°C after centrifugation and filtration in SM buffer.
Bacteriophage titration.
To prepare bacteriophage samples for TEM imagingand whole-genome sequencing, bacteriophages were first diluted. For the titration of bacteriophages, nine microtubes were prepared for 100 to 10−8 serial dilutions. First, 900 μl of SM buffer was added to each microtube. 100 μl from the bacteriophage samples were transferred into the microtube of 100 dilutions. Then, 100 μl from this solution was transferred into the microtube of 10−1 dilution. This method was repeated to prepare all dilutions to 10−8. Then, 100 μl of the diluted solution and 200 μl of the overnight cultured bacteria were added to TSA top agar and poured onto the plates. After 24 h, the number of plaques in each plate was counted and the bacteriophage titers were calculated using the following formula:
Where C was the bacteriophage titer, n was the plaque count, d was the dilution factor and v was the volume of dilution added.
Bacteriophage genome extraction.
Briefly, 10 μl of the bacteriophage solution were filtered and mixed with DNase 1 and RNase A. This was incubated at 37°C for 30 min and then mixed with 4 μl of 20% PEG 6000. The mixture was incubated on ice for 1 h and centrifuged at 20,000g for 30 min. The supernatant was discarded and the precipitate was dissolved in 600 μl of SM buffer. Then, 25 μl of phenol, 24 μl of chloroform, and 1 μl of isoamyl alcohol were added to the solution. The solution was centrifuged at 10,000 g for 20 min and then mixed with 600 μl of chloroform and re-centrifuged at 10,000 g for 20 min. Then,600 μl of isopropyl alcohol was added to the mixture and incubated at −80°C for 12 min. After 20 min of centrifugation of the solution and discarding of the supernatant, 700 μl of ethyl alcohol were added to the precipitate. After 20 min of centrifugation of the solution and discarding of the supernatant, the precipitate was dried under the hood for 10 min and then dissolved in 50 μl of TE buffer.
Bacteriophage genome typing.
First, the purity of the extracted bacteriophage genome was measured at 260/280 nm and 260/230 nm. Then, 1 U of each DNase 1, RNase A, and endonuclease S1 enzymes was added to 1 μg of the extracted genomes and incubated at 37°C for 2–3 h. In the next step, 10 μl of each reaction was electrophoresed on 1% agarose gels and the resulting bands were analyzed under UV light.
Host specificity of bacteriophages.
To investigate host specificity of the bacteriophages, the isolated bacteriophages were also examined on Escherichia coli (ATCC 35218), Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 29213), Salmonella enterica (ATCC 13076), Salmonella Typhimurium (ATCC 14028), and Streptococcus dysgalactiae (ATCC 27957) standard strains. These strains were kindly provided by the Faculty of Veterinary Medicine, University of Tehran, previously characterized and used in other studies.
Transmission electron microscopy.
To prepare bacteriophage samples for electron microscopy, bacteriophage plaques were diluted to 10−8 in sterile microtubes using SM buffer. Microtubes were centrifuged at 4,480 g for 10 min and the supernatants were transferred to fresh sterile microtubes. Then, 200 μl of each sample was used for bacteriophage staining. For the bacteriophage staining, 2% uranyl acetate dye and carbon-coated grids were used. After staining, samples were studied using transmission electron microscopy (TEM) (Philips EM208S, The Netherlands) at 100 kV(Partow Rayan Rastak, Iran).
RESULTS
In general, 25 Enterococcus strains were isolated from the clinical samples. These enterococcal species, including 14 E. faecium, 10 E. faecalis, and one E. gallinarum, were isolated from blood samples. In this study, most of the isolated strains were resistant to vancomycin, erythromycin, and clindamycin. Enterococcus faecium EntfacYE was sensitive to linezolid and resistant to vancomycin, erythromycin, clindamycin, ceftriaxone, and cefoxitin. The tuf gene partial sequencing results verified the primary characterization of the isolated bacteria. Overall, three bacteriophages were isolated on one of the enterococcal isolates using two various wastewater sources of public ponds in Tehran (Table 1). Two bacteriophages included isometric shapes, one with a long flexible tail (Siphoviridae) (Entfac.YE1) and another one with a non-flexible tail (Myoviridae) (Entfac.YE2) (Figs. 1 and 2). The third bacteriophage was filamentous (Inoviridae) (Entfac.YE3) (Fig. 3). These isolated bacteriophages belonged to the Caudovirales order. The bacteriophages genomes were cut using the enzymatic restriction method and their genome types were investigated. The two tailed bacteriophages contained double-strand DNA (dsDNA) and the filamentous bacteriophage single-strand DNA (ssDNA) genomes. In the host specificity assay, all the three bacteriophages were able to lyse the isolated enterococci as well as Streptococcus dysgalactiae ATCC 27957; however, the bacteriophages were not able to lysis the other five standard bacteria of this study.
Table 1.
Characteristics of the isolated enterococcal bacteriophages
| Bacteriophage | Nomenclature | Head length (nm) | Head width (nm) | Tail length (nm) | Tail width (nm) | Genome |
|---|---|---|---|---|---|---|
| Entfac.YE1 | Siphoviridae | 96 | 90 | 192 | 13 | dsDNA |
| Entfac.YE2 | Myoviridae | 81 | 81 | 121 | 13 | dsDNA |
| Entfac.YE3 | Inoviridae | N/A | N/A | 800 | 15 | ssDNA |
N/A, not applicable; dsDNA, double-stranded DNA; ssDNA, single-stranded DNA
Fig. 1.
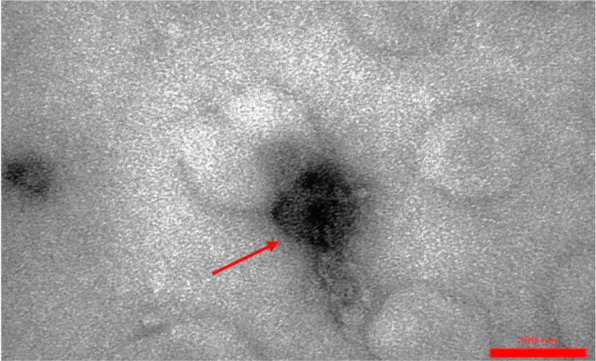
Entfac.YE1 enterococcal bacteriophage. An isometric head with a flexible tail
Fig. 2.
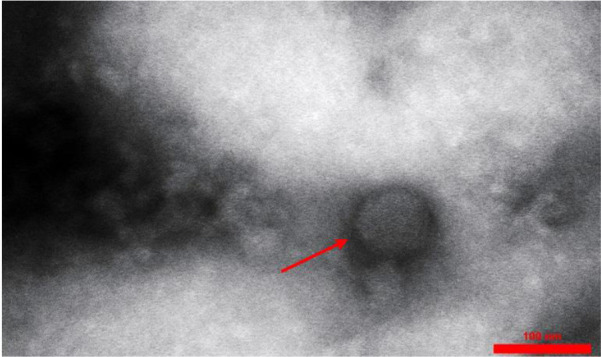
Entfac.YE2 enterococcal bacteriophage. An isometric head with a non-flexible tail
Fig. 3.
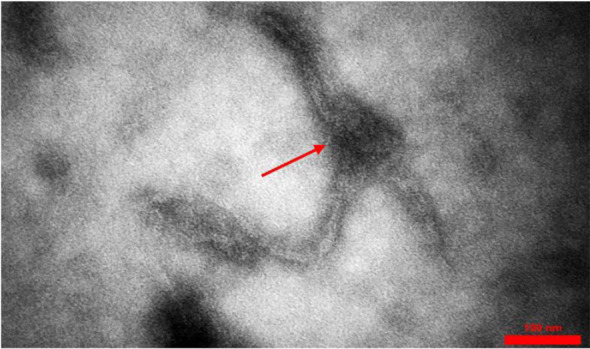
Entfac.YE3 enterococcal bacteriophage. A long filamentous bacteriophage
DISCUSSION
In this study, we have isolated and identified 14 E. faecium, 10 E. faecalis, and one E. gallinarum while, in 2005, Mohanty et al. identified 24 E. faecium, 7 E. feacalis, and one E. gallinarum from 38 enterococci strains (18). In 2019, Karna et al. characterized four E. faecium and one E. faecalis in five enterococci isolates (19). They identified 74 E. faecalis and 46 E. faecium from a total number of 120 enterococci isolates. According to these studies, it can be concluded that the prevalence of E. faecium and E. faecalis in enterococcal infections are higher than other species. Since enterococcal infections are common in patients, further studies on bacteriophages as alternatives to antibiotics can be beneficial. In the current study, genetic identification of the enterococcal isolates was carried out by using a pair of specific primers for the enterococcal tuf gene and Sanger partial sequencing method. Previously, Li et al. (2012) used the tuf gene as an appropriate target for the identification of Gram-positive cocci (20). Therefore, the study of specific genes in the bacterial genome using specific primers can verify the genus and species of the pathogen. Enterococcus faecium is a gut commensal of humans and animals but is also enlisted in the global priority list of multidrug-resistant pathogens by the World Health Organization (WHO) (21). In the current study, isolated E. faecium strains were resistant to vancomycin, erythromycin, clindamycin, ceftriaxone, and cefoxitin. Based on the published studies, the isolated E. faecium mean resistance to vancomycin and erythromycin from 2001 to 2016 was 47% and 76.3%, respectively in Iran (22). Based on the previous studies, the increased resistance profile of enterococci to common antibiotics can cause serious problems for the treatment of infections; therefore, the use of novel therapies such as bacteriophages can be a good solution.
In the present study, three various types of bacteriophages were isolated from a clinical strain of E. faecium using two various wastewater sources. In previous studies, bacteriophages have been isolated from various wastewater sources such as raw, hospital, and municipal wastewaters (23–25). This means that various sources of wastewaters are rich in bacteriophages. Overall, three various bacteriophages were isolated on E. faecium, namely Entfac.YE1–3. These bacteriophages respectively belonged to the Siphoviridae, Myoviridae, and Inoviridae families of the Caudovirales order. Based on the literature, these bacteriophages are mostly isolated on E. faecalis and E. faecium (26). In a study in 2020, a Siphoviridae bacteriophage was isolated on E. faecalis, which was previously identified in 2012 (27, 28). In 2019 Wandro et al. carried out a study on the coevolution of E. faecium isolates from healthy human stools in the absence and presence of Myoviridae bacteriophages (29). In the current study, specificity assessment of the isolated bacteriophages demonstrated that the bacteriophages could lyse Streptococcus spp. as well as the Enterococcus spp. This broader host-specify of the isolated bacteriophages could be important due to the horizontal transfer of antimicrobial resistance genes and other virulence genes between various bacterial genera (15). Moreover, this may be significant regarding the treatment of a wider spectrum of bacterial pathogens using a single bacteriophage, instead of a bacteriophage cocktail. Further characterization of the bacterial responses to bacteriophage infections is important for understanding how bacteriophages modulate bacterial physiology, which opens new horizons toward effective phage therapies against multiple-drug-resistant bacteria (30). Phage therapy, could be an alternative to antimicrobial treatment of bacterial diseases and it is becoming further popular, especially due to the rapid spread of antimicrobial resistance in bacteria and recent restrictions on antimicrobial uses (31).
CONCLUSION
Bacteriophages are spread in the environment, especially in wastewaters. Nowadays, clinically isolated bacteria show multiple resistance to available antibiotics. Several infectious diseases are caused by Enterococcus species, especially by two common species of E. faecalis and E. faecium. Resistance of these bacteria to common antibiotics, especially vancomycin, has significantly increased in recent decades. This has become a serious problem for medical specialists and patients. As shown in this study, bacteriophages from wastewater sources are capable of lysing multiple-resistant enterococci from clinical samples as well as destroying Streptococcus spp. Most of the isolated bacteriophages on enterococcal isolates belong to head-tailed bacteriophages, as two head-tailed bacteriophages were identified in this study. Since effects of bacteriophages on eukaryotic cells have not been reported and due to the extensive resistance of most enterococcal isolates to common antimicrobials, bacteriophages are suggested as appropriate alternatives to routine antimicrobials for the treatment of enterococcal infections. In conclusion, further studies with various pathogens and phage sources can help more effectively treat a wide spectrum of common bacterial infections.
ACKNOWLEDGEMENTS
The authors thank staff within the Microbiology Laboratory for their kind helps. The current study was financially supported by a grant from the Deputy Dean for Research, Tehran University of Medical Sciences (grant no. 97-02-27-39571).
REFERENCES
- 1.Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci: from commensals to leading causes of drug-resistant infection [Internet]. Boston: Massachusetts Eye and Ear Infirmary; 2014. [PubMed] [Google Scholar]
- 2.Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012;10:266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013;34:1–4. [DOI] [PubMed] [Google Scholar]
- 4.Harwood VJ, Delahoya NC, Ulrich RM, Kramer MF, Whitlock JE, Garey JR, et al. Molecular confirmation of Enterococcus faecalis and E. faecium from clinical, faecal and environmental sources. Lett Appl Microbiol 2004;38:476–482. [DOI] [PubMed] [Google Scholar]
- 5.Domig KJ, Mayer HK, Kneifel W. Methods used for the isolation, enumeration, characterization and identification of Enterococcus spp.: 2. pheno and genotypic criteria. Int J Food Microbiol 2003;88:165–188. [DOI] [PubMed] [Google Scholar]
- 6.Bortolaia V, Guardabassi L. (2015). Zoonotic transmission of antimicrobial resistant enterococci: a threat to public health or an overemphasisedrisk? In: Zoonoses-infections affecting humans and animals. Ed, Sing A. Springer, 1st ed. Dordrecht, Netherlands, pp. 407–431. [Google Scholar]
- 7.Kataoka Y, Umino Y, Ochi H, Harada K, Sawada T. Antimicrobial susceptibility of enterococcal species isolated from antibiotic-treated dogs and cats. J Vet Med Sci 2014;76:1399–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh A, Dowd SE, Zurek L. Dogs leaving the ICU carry a very large multi-drug resistant enterococcal population with capacity for biofilm formation and horizontal gene transfer. PLoS One 2011;6(7):e22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilmore MS, Lebreton F, van Schaik W. Genomic transition of enterococci from gut commensals to leading causes of multidrug-resistant hospital infection in the antibiotic era. Curr Opin Microbiol 2013;16:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med 2003;348:1342–1347. [DOI] [PubMed] [Google Scholar]
- 11.Clokie MR, Millard AD, Letarov AV, Heaphy S. Phages in nature. Bacteriophage 2011;1:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viertel TM, Ritter K, Horz HP. Viruses versus bacteria—novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J Antimicrob Chemother 2014;69:2326–2336. [DOI] [PubMed] [Google Scholar]
- 13.Rose T, Verbeken G, Vos DD, Merabishvili M, Vaneechoutte M, Lavigne R, et al. Experimental phage therapy of burn wound infection: difficult first steps. Int J Burns Trauma 2014;4:66–73. [PMC free article] [PubMed] [Google Scholar]
- 14.Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage 2011;1:111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazaheri Nezhad Fard R, Barton MD, Heuzenroeder MW. Novel bacteriophages in Enterococcus spp. Curr Microbiol 2010;60:400–406. [DOI] [PubMed] [Google Scholar]
- 16.Larsson MC, Karlsson E, Woksepp H, Frölander K, Mårtensson A, Rashed F, et al. Rapid identification of pneumococci, enterococci, beta-haemolytic streptococci and S. aureus from positive blood cultures enabling early reports. BMC Infect Dis 2014;14:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adeniji OO, Sibanda T, Okoh AI. Recreational water quality status of the Kidd’s beach as determined by its physicochemical and bacteriological quality parameters. Heliyon 2019;5(6):e01893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohanty S, Jose S, Singhal R, Sood S, Dhawan B, Das BK, et al. Species prevalence and antimicrobial susceptibility of enterococci isolated in a tertiary care hospital of north India. Southeast Asian J Trop Med Public Health 2005;36:962–965. [PubMed] [Google Scholar]
- 19.Karna A, Baral R, Khanal B. Characterization of clinical isolates of enterococci with special reference to glycopeptide susceptibility at a tertiary care center of eastern Nepal. Int J Microbiol 2019;2019:7936156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Xing J, Li B, Wang P, Liu J. Use of tuf as a target for sequence-based identification of gram-positive cocci of the genus enterococcus, streptococcus, coagulase-negative staphylococcus, and lactococcus. Ann Clin Microbiol Antimicrob 2012;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatterjee A, Willett JLE, Nguyen UT, Monogue B, Palmer KL, Dunny GM, et al. Parallel genomics uncover novel enterococcal-bacteriophage interactions. mBio 2020;11(2):e03120–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asadollahi P, Razavi S, Asadollahi K, Pourshafie MR, Talebi M. Rise of antibiotic resistance in clinical enterococcal isolates during 2001–2016 in Iran: a review. New Microbes New Infect 2018;26:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aghaee BL, Mirzaei MK, Alikhani MY, Mojtahedi A. Sewage and sewage-contaminated environments are the most prominent sources to isolate phages against Pseudomonas aeruginosa. BMC Microbiol 2021;21:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maal KB, Delfan AS, Salmanizadeh S. Isolation and identification of two novel Escherichia coli bacteriophages and their application in wastewater treatment and coliform’s phage therapy. Jundishapur J Microbiol 2015;8(3):e14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon ND, Kumar MS, Satheesh Babu TG, Bose S, Vijayakumar G, Baswe M, et al. A novel N4-Like bacteriophage isolated from a wastewater source in South India with activity against several multidrug-resistant clinical Pseudomonas aeruginosaisolates. mSphere 2021;6(1):e01215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arredondo-Alonso S, Top J, McNally A, Puranen S, Pesonen M, Pensar J, et al. Plasmids shaped the recent emergence of the major nosocomial pathogen Enterococcus faecium. mBio 2020;11(1):e03284–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhardwaj SB, Mehta M, Sood S, Sharma J. Isolation of a novel phage and targeting biofilms of drug-resistant oral enterococci. J Glob Infect Dis 2020;12:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horiuchi T, Sakka M, Hayashi A, Shimada T, Kimura T, Sakka K. Complete genome sequence of bacteriophage BC-611 specifically infecting Enterococcus faecalis strain NP-10011. J Virol 2012;86:9538–9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wandro S, Oliver A, Gallagher T, Weihe C, England W, Martiny JBH, et al. Predictable molecular adaptation of coevolving Enterococcus faecium and lytic phage EfV12-phi1. Front Microbiol 2019;9:3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kowalska JD, Kazimierczak J, Sowinska PM, Wojcik EA, Siwicki AK, Dastych J. Growing trend of fighting infections in aquaculture environment—opportunities and challenges of phage therapy. Antibiotics (Basel) 2020;9:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero-Calle D, GuimarãesBenevides R, Góes-Neto A, Billington C. Bacteriophages as alternatives to antibiotics in clinical care. Antibiotics (Basel) 2019;8:138. [DOI] [PMC free article] [PubMed] [Google Scholar]


