Abstract
For such a thin tissue, the aortic valve possesses an exquisitely complex, multi-layered extracellular matrix (ECM), and disruptions to this structure constitute one of the earliest hallmarks of fibrocalcific aortic valve disease (CAVD). The native valve structure provides a challenging target for engineers to mimic, but the development of advanced, ECM-based scaffolds may enable mechanistic and therapeutic discoveries that are not feasible in other culture or in vivo platforms. This review first discusses the ECM changes that occur during heart valve development, normal aging, onset of early-stage disease, and progression to late-stage disease. We then provide an overview of the bottom-up tissue engineering strategies that have been used to mimic the valvular ECM, and opportunities for advancement in these areas.
Keywords: calcific aortic valve disease, tissue engineering, collagen, biomaterials
Introduction
The extracellular matrix (ECM) is a complex system of molecules that form the physical scaffolding to sustain tissues and organs. In addition to providing structural support, the ECM supplies mechanical and biochemical cues that direct cell function during tissue development, normal aging, and disease [1]. Depending upon the tissue, the ECM may be relatively simple and subjected to little stress, or it may possess highly complex and stratified structures that are subject to strong mechanical forces.
The aortic heart valve endures some of the most dynamic and rigorous conditions in the human body, opening and closing approximately 42 million times each year. The ECM of mature aortic valves possesses a highly organized trilaminar structure across its ~300 μm thickness; this arrangement provides the mechanical strength necessary to manage the hemodynamic stresses and pressure changes that occur as blood flows between the left ventricle and the aorta [2]. Disruptions to this structure are seen in calcific aortic valve disease (CAVD) [3], the most prevalent valvular disease in the developed world. CAVD progression is associated with extensive remodeling of the ECM, with unique patterns of ECM disorganization seen during each stage of disease. This review will provide an overview of the aortic valve ECM during development, aging, and disease, and highlight key engineering-based efforts to generate in vitro models of the aortic valve ECM.
ECM in Aortic Valve Development
During embryonic development, the ECM regulates cellular differentiation and the availability of soluble factors to create the aortic valve [4]. First, a cardiac jelly rich in glycosaminoglycans (GAGs) is deposited by embryonic progenitor cells in the space between the endocardium and myocardium (Figure 1) [5, 6]. The hydrophilic nature of GAGs causes swelling and protrusion of the interior lumen of the primitive heart tube, forming the endocardial cushions. These cushions are composed of endothelial cells that undergo differentiation to mesenchymal cells (EndMT), and these mesenchymal cells, in turn, are the precursors to the valvular interstitial cells (VICs) that comprise most of the mature aortic valve [7]. Hyaluronic acid (HA) is required for endocardial cushion formation; it has been shown that knockdown of hyaluronan synthase 2 (Has2) causes reduced EndMT and no formation of endocardial cushions in chicken embryos [5, 8]. As valvulogenesis continues, endocardial cushions remain rich in GAGs, HA, and versican, as well as basement membrane proteins, while mesenchymal cells remodel and alter their ECM by increasing production of multiple types of collagen (I, II, II, IV, VI, and IX), aggrecan, and periostin [6].
Figure 1: ECM changes during Aortic Valve Development.
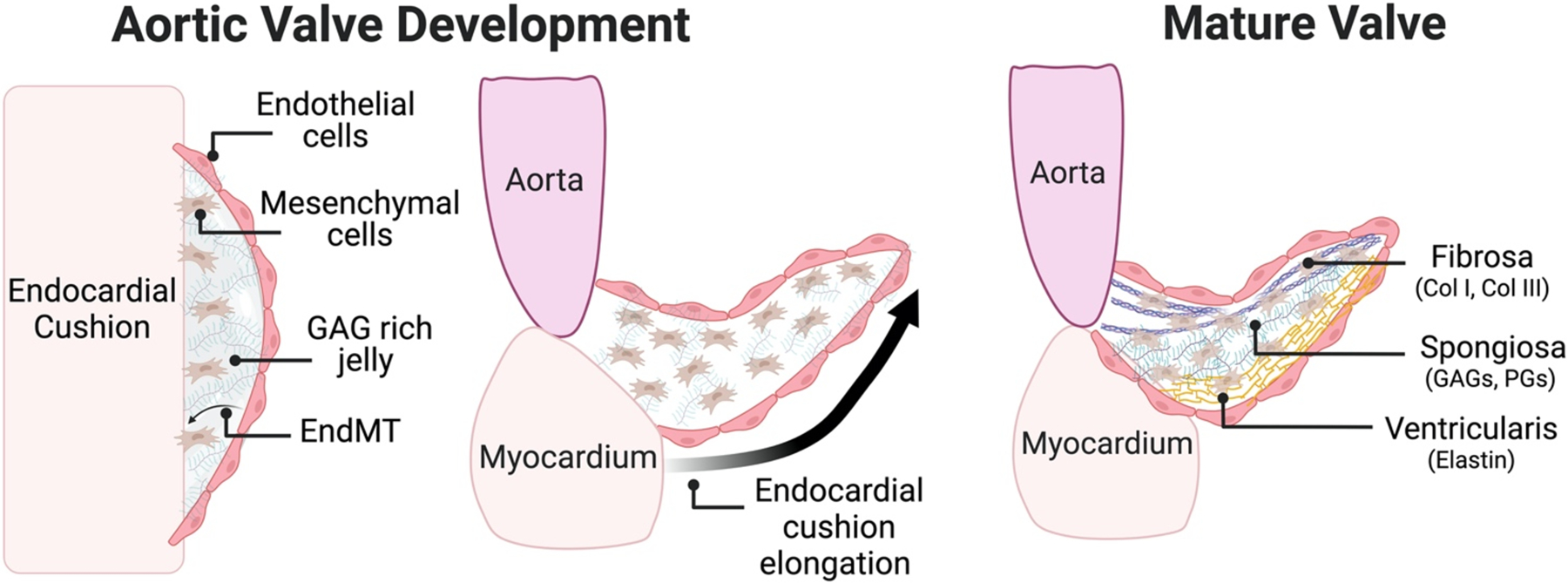
In early valve development endothelial cells undergo transition to mesenchymal cells in a GAG-rich jelly. As valvulogenesis continues, endocardial cushions begin to thin and elongate, remaining rich in GAGs. Lastly, the trilaminar structure of the valve begins to emerge in the postnatal period with enrichment of collagen I fibrils in the fibrosa, HA and chondroitin sulfate in the spongiosa, and elastin localized to the ventricularis.
Mesenchymal cells are highly proliferative at the beginning of aortic valve development and quickly begin to populate the endocardial cushions [5]. At the early stage of development, the ECM is loosely organized. As valvulogenesis continues, mesenchymal cells remain highly proliferative at the tip of the valve but exhibit attenuated proliferation everywhere else, leading to elongation of the developing valve [9]. Mesenchymal cells then gain expression of α-SMA and develop a phenotype more similar to aVICS [10]. These cells facilitate the remodeling of the loosely organized ECM as seen in endocardial cushions into a highly organized trilaminar structure, through the breakdown of GAG-rich endocardial cushions and synthesis of ECM proteins. The aortic-facing side of the valve becomes enriched in type I collagen fibrils, forming what will eventually become the fibrosa. As the aortic valve continues to develop, HA concentration decreases, and the remaining HA and chondroitin sulfate proteoglycans form the valve’s middle layer, the spongiosa. The small amount of elastin present in the fetal valve localizes to the ventricle-facing side, creating a layer which will become the ventricularis. Further production, remodeling, and stratification of the ECM continues in the post-natal valve, eventually forming the trilaminar structure of adult aortic valves (Figure 1) [2, 4, 5].
ECM in Healthy, Adult Aortic Valves
The aforementioned three layers of the adult aortic valve each possess a unique profile of ECM molecules, and this architecture is optimized to manage the mechanical stresses of pumping blood from the left ventricle to the aorta. The highest concentration of fibrillar collagen in the aortic valve is located in the fibrosa, which contains collagen types I and III. Collagen fiber bundles in the fibrosa provide the mechanical strength necessary to resist the stresses from mounting blood pressure within the left ventricle [11]. Collagen fibers are circumferentially arranged, which allows for complete closure of the aortic valve during diastole as fibers uncrimp. When the valve is closed during diastole, the taut collagen fibers transfer the stress load from the leaflets to the aortic wall [12]. Collagen also regulates the phenotype of VICs, and is thought to support a quiescent (qVIC) phenotype [13, 14].
GAGs are found throughout the leaflet, but are the predominant component of the spongiosa [2]. The most abundant GAGs in the healthy aortic valve are HA, chondroitin sulfate (CS), and dermatan sulfate (DS). With the exception of HA, all GAGs in the aortic valve are attached to core proteins to form proteoglycans. Versican, decorin, and biglycan are the most prevalent proteoglycans, with biglycan existing at the highest concentration. The most well-studied GAG in the aortic valve is HA. Prior work demonstrated that VIC culture on HA coatings supports a phenotype that is resistant to calcification [15] and culture on HA-containing hydrogels decreases VIC secretion of pro-inflammatory cytokines [16]. Moreover, enzymatic depletion of HA or inhibiting the ability of VICs to interact with HA (via blocking of the HA cell receptor, CD44) in 3-D ex vivo leaflet culture leads to cellular behaviors typically associated with valve pathology (e.g., VIC proliferation, apoptosis, alkaline phosphatase expression, and mineralization) [15]. Far fewer prospective studies have been performed to analyze the role of CS or DS in healthy valve function. However, data from disease-inspired ECM environments indicate that CS performs similar functions to HA with respect to maintaining a healthy VIC phenotype. Specifically, increasing levels of CS were associated with decreased production of multiple inflammatory cytokines by VICs [16]. Together, these findings suggest that the presence of GAGs is essential for supporting a healthy VIC phenotype.
Elastin is located mostly in the ventricularis [2]. Elastic fibers and collagen fibers in the ventricularis are oriented perpendicularly to the collagen fibers in the fibrosa. With this organization, collagen is able to provide mechanical support, and the elastic fibers facilitate leaflet recoil once the pressure from the ventricle is released [17, 18]. Most investigation of elastin has focused on its mechanical role in the valve, so relatively little is known about the biological functions of valvular elastin (e.g., how it interacts with VICs in the normal adult valve or influences their phenotype).
ECM in Aging Aortic Valves
Throughout the body, normal aging is accompanied by changes to the ECM, often resulting in alterations to tissue function. For many years, aortic valve calcification was mistakenly viewed as a degenerative process, where calcification was thought to be a consequence of normal aging [3]. Thus, many prior studies that examined “healthy” elderly valves actually included valves with calcification [19, 20], meaning that those data cannot be used to define the characteristics of a normal, aged valve. A renewed characterization of the aging aortic valve has started to emerge in recent years, although the majority of samples in these studies have come from individuals <60 years old, which is earlier than the age of typical CAVD onset.
Histological analysis of aged human aortic valves has revealed dysregulation of the extracellular matrix organization and loss of the characteristic trilaminar structure [21]. During aging, there is an increase in collagen crosslinking and fragmentation of elastin, concurrent with an increase in stiffness and decrease in extensibility [22–24]. These changes in ECM are due, in part, to changing hemodynamics, as an increase in diastolic blood pressure is seen in normal aging. Circumferential stress increases significantly with age, and it is possible that this increase in circumferential stretch drives the collagen expression and remodeling seen in aging human valves [22, 25]. Although the overall amount of sulfated GAGs does not significantly change between early and late adulthood, the expression levels of individual GAGs have been found to undergo changes with age. For example, enzymes that regulate HA synthesis and degradation are elevated in aged valves [26]. Immunohistochemical staining of 6-year-old pig valves revealed that decorin expression is increased in the spongiosa and iduronate expression also increases in aged valves [27]. In addition, Elastin becomes fragmented in the aging aortic valve [22], which is mediated by increased expression of MMPs and cathepsins [23].
As noted above, historical misconceptions about the origin of valvular disease mean that there has been relatively little characterization of normal, aged (>60 y.o.) human aortic valves. However, knowledge of other aged tissues can provide us with some insight into what other age-related changes might be occurring in the aortic valve ECM. A fairly consistent age-related feature is ECM stiffening due to non-enzymatic crosslinking and subsequent accumulation of advanced glycation end products (AGEs) [27–29]. Although AGEs have yet to be quantified in aged human aortic valves, their accumulation likely plays a role in the development of CAVD. In hypercholesterolemic rabbits, increased expression of AGEs and their receptors leads to an inflammatory response and promotion of an osteoblastic VIC phenotype [30].
ECM in CAVD
CAVD is the leading cause of valve replacement in the United States, affecting 2.5 million Americans [31]. It is a fibrocalcific disorder in which valve thickening and stiffening can ultimately lead to impairment of blood flow through the valve. The severity of CAVD can vary from mild, asymptomatic valvular sclerosis to severe flow obstruction in valvular stenosis, and prominent remodeling of the ECM is a critical hallmark across all of these stages of CAVD (Figure 2) [32]. Men are at greater risk of developing CAVD, and the disease exhibits sexual dimorphism in its pathology, wherein women develop more fibrosis and men have more prominent calcification [33, 34]; these differences provide further motivation for understanding ECM pathobiology in CAVD.
Figure 2: ECM changes in CAVD.
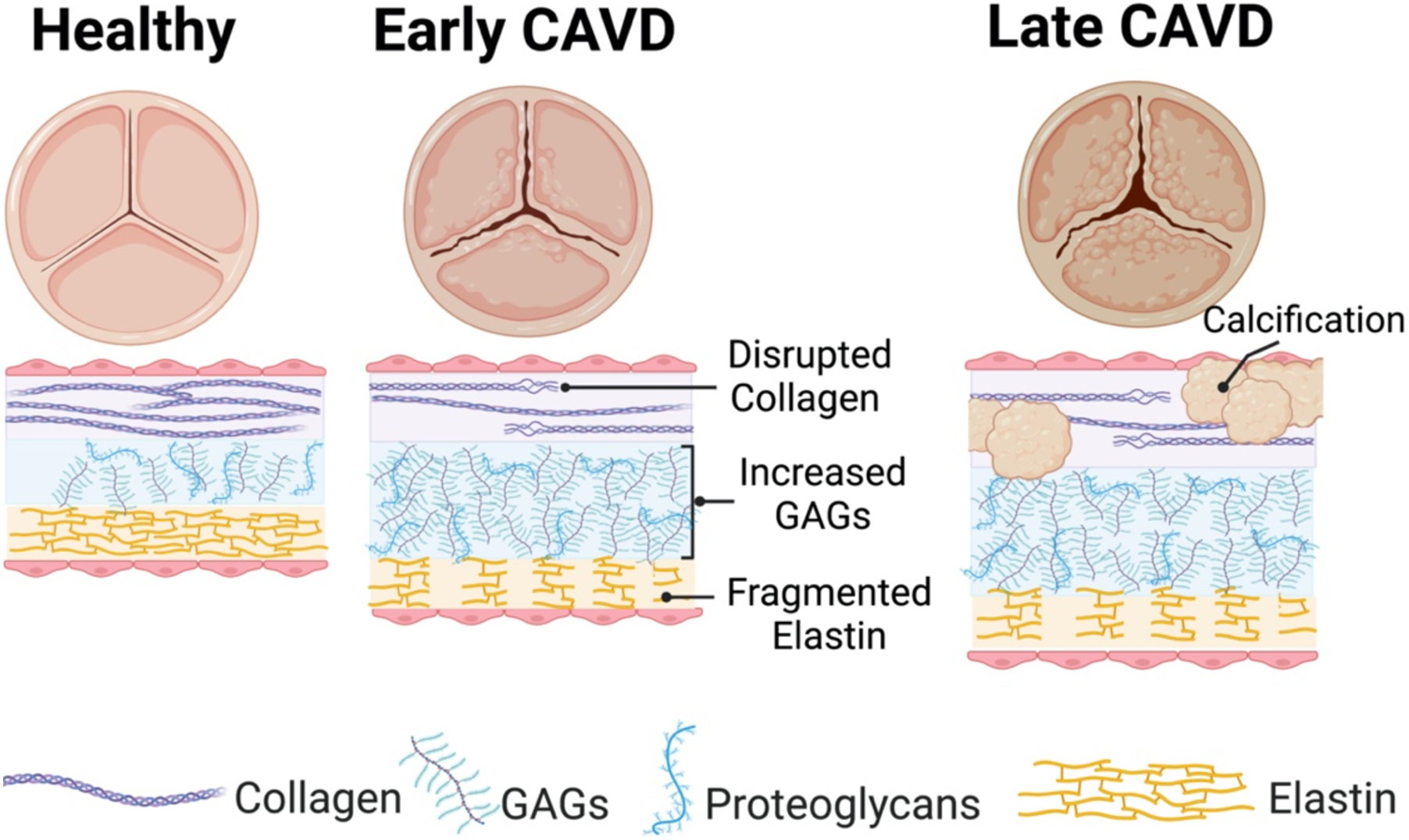
A healthy aortic valve has three layers: a collagen rich fibrosa, GAG rich spongiosa, and elastin filled ventricularis. Early in CAVD, collagen fibrils are disrupted, there is an increase in GAG concentration and elastin is fragmented. Late stage CAVD is associated with fibrosis throughout the valve and mineralization of the valve ECM, primarily in the fibrosa.
ECM in Early CAVD
Otto et al. first described the histopathological hallmarks of early calcific lesions in their 1994 study which identified the leaflet thickening, lipid accumulation, collagen fiber disarray, and calcific nodule formation in human valves that are characteristic of this disease [35]. However, thorough characterization of even earlier stages of CAVD (i.e., prior to any evidence of calcification) has been challenging due to the scarcity of human valve samples at this stage, as these patients do not meet criteria for valve replacement. As a result, much of what we know about early stages of CAVD has come from studying animal models. Work with swine models and limited studies with sclerotic human valve samples have revealed the presence of lipid accumulation, oxidative stress, inflammation, and significant ECM disorganization during early CAVD [36–39]. These ECM changes drive valve thickening and involve alterations in collagen organization, proteoglycan (PG) concentration, and elastin arrangement.
Both human histology and multiple different large animal models have shown that one of the earliest ECM alterations in CAVD is enrichment of GAGs and PGs [40, 41]. In atherosclerosis, these ECM molecules can bind to oxidized lipid species and promote their accumulation [42]; there is evidence that they are performing a similar role in CAVD [16, 35]. In addition, this increase in PGs and GAGs is associated with significant valvular thickening [35]. Additional ECM changes, such as disruption of collagen alignment and fragmentation of elastin, also start to emerge during early CAVD [43, 44].
ECM in Late Stage CAVD
Total valve replacement is commonly performed to treat CAVD, yielding valve samples that can be assessed to understand late-stage disease processes. In late-stage CAVD, the aforementioned early-stage alterations in ECM progress toward fibrosis and calcification, which ultimately lead to significant obstruction of aortic outflow [45]. The formation of calcific nodules tends to be localized to the fibrosa [46], while fibrosis occurs throughout the thickness of the valve.
Fibrillar collagen is the primary marker for fibrosis and undergoes significant changes in expression and crosslinking in end stage disease [44]. In the human valve fibrosa, collagen fibers decrease in length and there is an increase in lysyl oxidase expression, an enzyme that mediates collagen fiber crosslinking [47]. Collagen disruption is mediated largely by increased expression of MMPs. MMPs 1, 2, 3, and 9 are all associated with late-stage CAVD [48–50]. TIMPs 1 and 2 as well as cathepsins have also been associated with late stage CAVD in human valves and contribute to remodeling processes that ultimately cause collagen fiber disruption [49, 51]. Furthermore, the formation of calcific nodules in late-stage CAVD further disrupt collagen organization [49]. Alterations to collagen in CAVD are not isolated to the fibrosa. The spongiosa, which became highly enriched in GAGs during early CAVD, experiences a significant increase in fibrillar collagen as disease progresses [47].
As the valve becomes enriched in collagen during fibrosis, PGs and GAGs correspondingly make up a smaller proportion of the valve ECM. Late-stage CAVD is associated with increased heterogeneity of PG and GAG distribution and focal enrichment of these ECM components in the areas surrounding calcific nodules [26, 52]. Specifically, increased concentrations of decorin and biglycan around pre-nodules and increased expression of decorin, biglycan, versican, and hyaluronan around calcified nodules are seen in late-stage diseased human valves [53]. Molecules involved in HA homeostasis, such as HAS2 (which synthesizes HA), HYAL1 (which degrades HA), as well as HA-binding proteins and receptors, also become highly dysregulated as disease progresses [26].
Finally, the ventricularis ECM is also altered in late-stage CAVD. As CAVD progresses, elastic fibers become more fragmented and disorganized [44]. MMPs, including MMPs 2 and 9, modulate this fragmentation of elastin [48, 49]. Cathepsins S, K, V, and G are thought to further degrade elastin in the ventricularis [54, 55]. This loss of elastin leads to aortic valve cup distention, reduced extensibility and increased stiffness [56].
Engineering Valvular ECM
The dynamic and complex composition of native valve ECM makes it difficult to precisely pinpoint the contributions of each component and the sequence in which physiological and pathological events occur. Similarly, histological analyses of explanted human and animal specimens offer only snapshots that cannot fully capture the mechanisms involved in the many processes responsible for maintaining valve homeostasis. To better understand the role of the ECM in regulating leaflet function in health and disease, engineers and material scientists have endeavored to generate well-defined in vitro environments that mimic the native valve ECM. As described in the preceding sections, each stage of valve development and disease has unique ECM characteristics. A variety of engineering approaches are necessary to generate experimental platforms that accurately recreate the valve environment, and such platforms may serve as critical tools in building a detailed understanding of valvular (patho)biology. In the following sections, we focus our attention on bottom-up approaches that have been used to engineer ECM-mimetic environments in the context of the aortic valve (Figure 3).
Figure 3.
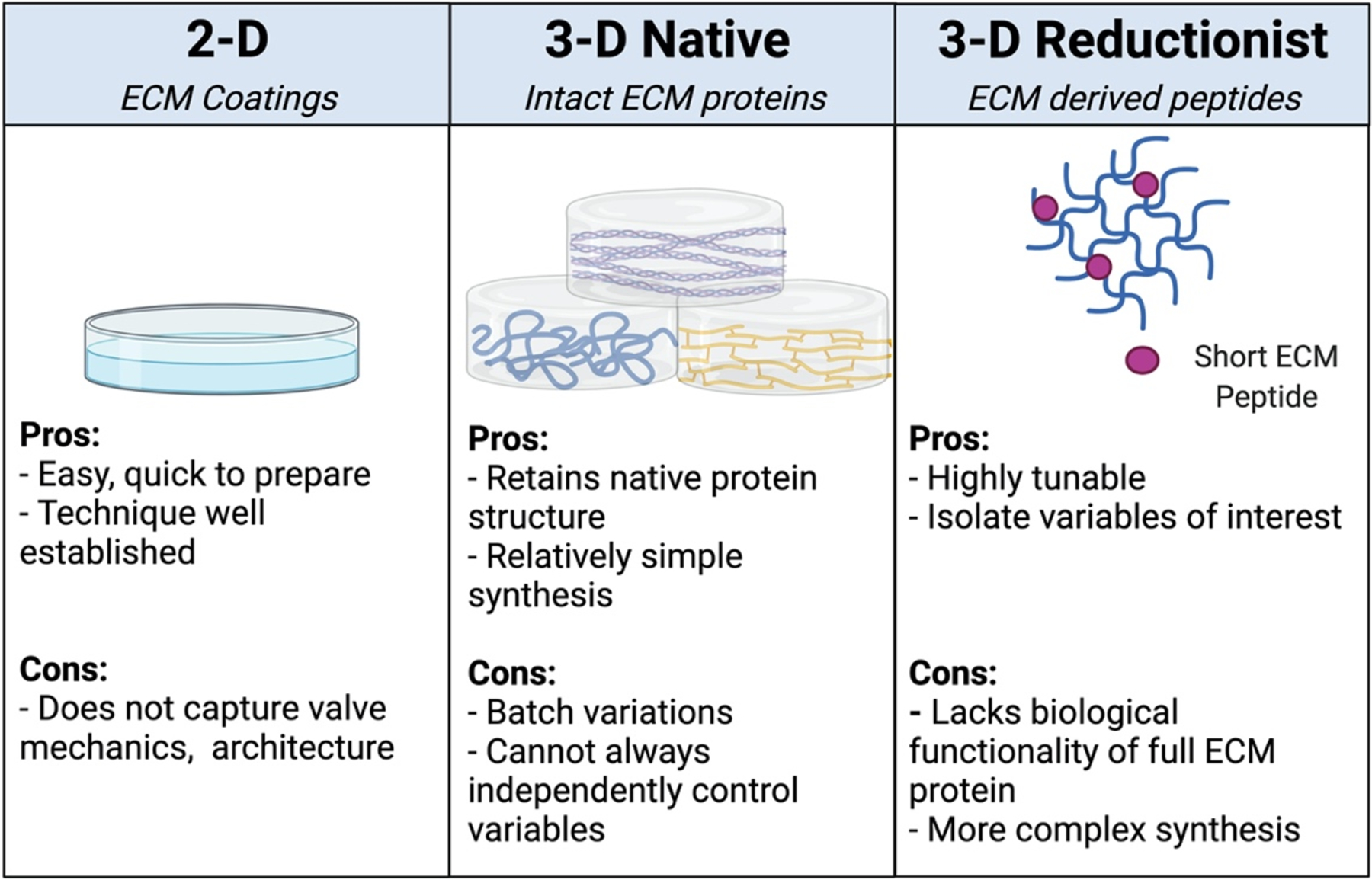
An overview of the general approaches used to create ECM-inspired culture environments to mimic the aortic valve.
Insights from 2D ECM Coatings
Some progress towards understanding the role of the ECM within the context of the aortic valve has been achieved via the use of ECM coatings on traditional tissue culture polystyrene (TCPS). Although TCPS does not capture many of the biophysical characteristics of the valvular ECM (e.g., mechanics, microarchitecture), this approach has yielded insight into the ability of the ECM to regulate VIC function. For example, ECM coatings alone have the ability to drive or inhibit the formation of calcific nodules by VICs. When VICs were seeded on TCPS coated with fibrin, laminin, or heparin, VIC aggregation into nodule-like structures was promoted in a concentration-dependent manner [57, 58]. Meanwhile, culture of porcine VICs on type I collagen, HA, or plasma fibronectin resulted in decreased nodule formation; for HA, this effect was dependent upon HA molecular weight [15]. As a coating on TCPS, collagen I in particular appears to provide a protective environment for porcine VICs, as its presence assists in maintenance of a quiescent porcine VIC phenotype in vitro [14], and VICs cultured on type I collagen coatings demonstrate reduced sensitivity to pro-calcific stimuli [57]. 2D studies have revealed that the response of porcine VICs to various exogenous factors can vary with the ECM environment. Specifically, ECM coatings affected VIC behavior in response to the pro-fibrotic stimulus transforming growth factor beta-1 (TGF-β1) [58] and the cholesterol-lowering drug simvastatin [59]. However, while 2D studies employing ECM coatings have provided a foundation from which to build an understanding of the aortic valve microenvironment, more mechanically and physiologically relevant environments are necessary to develop adequate models of tissue function.
3D ECM-based scaffolds
Engineered models of developing and healthy valves provide a platform to investigate the molecular mechanisms that maintain valve homeostasis as well as those that may regulate transitions to diseased states. While many of these approaches first arose with the end goal of engineering an organ for valve replacement, they provide an important foundation upon which more complex or disease-inspired structures can be built. ECM-based systems may be preferred over purely synthetic systems because they more closely mimic native valve architecture, allow for the sequestering of growth factors and cellular signals, and inherently promote cell adhesion.
Type I collagen was one of the first scaffold materials used in a tissue engineering context [60]. Its historical usage in tissue engineering applications, combined with it being the most abundant ECM molecule in the mature, healthy valve, have yielded a wide range of efforts to apply collagen-based scaffolds to valvular tissue engineering. Collagen-based hydrogels can be formed via several methods [61]. One common approach is to adjust the temperature and pH of an acidified solution of native collagen to reach physiological conditions, and VICs can be readily encapsulated during this gelation process. The resulting scaffold provides not only the most prevalent ECM protein in the native valve but also fibrillar topography, which is important for directing cell function. However, the elastic modulus of collagen-only gels tends to be low (<6 kPa). The dominant usage of collagen-only gels in the context of aortic valve investigations is to provide a platform for assessing VIC contractility in response to stimuli. Although collagen type I coatings can promote the maintenance of a quiescent VIC phenotype, this does not hold true for 3D collagen gels, where encapsulated porcine VICs readily transition to a myofibroblast phenotype and contract the gels [62–65]. Collagen gels have also been used to probe stiffness-dependent VIC behaviors; in the presence of calcifying media, VICs on compliant (2 kPa) type I collagen gels tended to express markers related to osteogenesis, while the stiffer (6 kPa) collagen gels supported transition to a myofibroblastic phenotype and greater formation of calcified aggregates [66]. Collagen gels may also be subjected to mechanical loading to investigate VIC response to applied strain [67]; this approach has been used more often with porcine mitral valve cells [68, 69].
Despite the advantages of collagen with respect to its biological and architectural properties, the robust contraction of these gels by VICs, combined with challenges in tuning collagen scaffold properties, can limit their applicability to mimic valvular conditions. Thus, researchers have used modified gelatin as an alternative to collagen [70–72], as gelatin is inexpensive and more amenable to synthetic modification [61]. Scaffolds made from methacrylated gelatin (GelMA) possess cellular adhesion sequences, are easily modified, and have tunable stiffness, which can be further enhanced through copolymerization or reinforcement with other materials [61, 73]. GelMA scaffolds support porcine VIC viability and spreading [70], although, similar to collagen gels, VICs in 5.7 kPa GelMA scaffolds spontaneously differentiate into myofibroblasts [71]. Scaffolds made from GelMA have been used to support VIC-VEC co-culture in an organ-on-a-chip system [74], and to examine inflammatory and remodeling outcomes in response to chronic hyperglycemia [75].
To design culture environments that mimic multiple components of the native valve ECM, it is becoming increasingly common to combine GelMA with modified HA. On its own, HA has been attractive as a valve-mimicking biomaterial due to its critical role in valvulogenesis and abundance in the spongiosa of healthy adult valves. With no high order structure to maintain, HA can be easily modified to introduce crosslinking capabilities [76], and the resulting scaffolds modified with adhesion peptides to better support cell spreading [77]. Scaffolds fabricated from methacrylated HA (HAMA) support porcine VIC viability and ECM biosynthesis [78, 79]; they also support maintenance of a less activated VIC phenotype, as well as secretion of elastin, which can be difficult to achieve in vitro [77]. Hybrid scaffolds of GelMA and HAMA offer many opportunities as a platform to further explore valvular pathobiology. Porcine VICs encapsulated in GelMA-HAMA scaffolds (ranging from 2–100 kPa) retain a quiescent phenotype and require exogenous stimulation to a myofibroblast phenotype, rendering this environment highly useful to investigate causes of VIC activation and progression of early CAVD [71, 79, 80]. Bioprinting of GelMA and HAMA has been used to create a multi-layered leaflet model that recapitulated layer-specific mechanics [81]. These scaffolds supported the development of microcalcifications in response to treatment with an osteogenic stimulus. GelMA can also be combined with other GAGs to mimic the valvular environment. For example, chondroitin sulfate (CS) can be modified in a manner similar to HA to enable methacrylate-based crosslinking. Using this approach, scaffolds were constructed to mimic the GAG-enriched early disease state of CAVD [16], and it was found that enrichment in CS did not directly induce pathological behaviors in porcine VICs, but rather trapped oxidized lipids which, in turn, promoted inflammatory outcomes. Meanwhile, enrichment in HA was not associated with significant lipid entrapment, but did stimulate production of pro-angiogenic factors by VICs [16]. In another study, a composite system of 20 kPa GelMA-HAMA was used to examine the transition between porcine qVICs and osteoblastic VICs. Within these scaffolds, treatment with osteogenic media and TNFɑ resulted in the formation of calcified nodules and increased aSMA expression [80].
Finally, scaffolds may also be made to include elastin, the third major ECM category of the aortic valve. Collagen and solubilized elastin have been combined to form scaffolds via spontaneous assembly at physiological conditions, and these materials supported culture of both VICs and VECs [82]. However, unlike other ECM molecules, the intact elastin molecule presents significant obstacles to its use in vitro, such as a high level of hydrophobicity and a tendency to induce calcification [83, 84], which has limited its implementation in tissue-engineered valvular materials.
Reductionist Approaches to Mimic the Valve ECM
Natural polymers may be ideal in mimicking the biochemical nature of the valve, but they can be large, unwieldy, and difficult to precisely control. Although fully synthetic polymer systems have shown promise in the development of an engineered valve [85, 86], a system that combines natural and synthetic elements may better capture the biophysical and biochemical properties of the aortic valve microenvironment. A reductionist approach, which involves the incorporation of ECM-derived peptides, rather than whole ECM molecules, may be employed to simplify scaffold fabrication while maintaining biofunctionality. Use of a reductionist approach also simplifies the isolation of variables to gain mechanistic insight. The synthetic polymer polyethylene glycol (PEG) is frequently used as the base material for reductionist approaches; bioactive peptides that regulate VIC adhesion, proliferation, and differentiation can be appended to PEG-based matrices.
PEG-based scaffolds can be modified to include both adhesive sequences and degradation targets that are reminiscent of the native ECM. PEG scaffolds containing adhesive peptides and MMP-degradable peptide sequences support porcine VIC attachment, spreading, and migration [87], and VIC behavior can be modulated via changing the peptide density or identity [88, 89]. Meanwhile, tailoring the degradation of PEG-based scaffolds can influence both the VIC ECM production profile and the distribution of ECM in the construct [90]. PEG-based systems have also been used to demonstrate that VIC responsiveness to adhesive peptides varies with platform dimensionality, and that soft (<5 kPa) 3D PEG systems can promote a more quiescent porcine VIC phenotype than their 2D counterpart [91]. A microarray-based gene expression analysis revealed further differences between VICs on 2D vs. within 3D PEG-peptide scaffolds [92]. The response of VICs in PEG-peptide scaffolds to exogenous soluble factors is consistent with physiological expectations, where TGF-β1 increases myofibroblastic differentiation, while fibroblast growth factor-2 (FGF-2) supports maintenance of a more quiescent phenotype [93]. Moreover, these scaffolds can be combined with native ECM components (e.g., fibrillar collagen) to yield more complex biomimetic environments that can serve as platforms to assess not only mineralization, but also the ability of drugs to stop this process [94]. Finally, PEG-peptide gels present a useful platform for analyzing the effects of altered stiffness on porcine VIC function. Consistent with findings on collagen gels, stiffer (10 kPa) PEG hydrogels supported more myofibroblastic differentiation of VICs [95].
PEG-based systems are also amenable to spatial patterning and temporal regulation of scaffold properties. For example, patterning of scaffold mechanical properties can be used to mimic the anisotropic environment of the native valve [88] and has revealed that spatially disorganized elasticity increased porcine VIC proliferation [95]. Meanwhile, adhesive ligand patterning has been used to spatially pattern VICs and VECs in order to better mimic native aortic valve architecture [96]. PEG scaffolds can also be dynamically tuned while in culture, offering researchers the ability to further probe the impact of ECM microenvironment changes on VIC function. For example, dynamic stiffening may be employed to mimic the progression of fibrosis and can be achieved by diffusing in additional PEG polymer and initiating crosslinking [97]. Dynamic softening of PEG matrices can also be used to modulate porcine VIC phenotype between myofibroblastic and more quiescent states [98]. A summary of the different materials discussed herein is presented in Table 1.
Table 1.
Common Biomaterials Used to Mimic Aortic Valve Architecture
| Type | Roles | Pros | Cons | |
|---|---|---|---|---|
| Coll I | Native |
|
|
|
| HA and HAMA | Native and Native - Modified |
|
|
|
| Elastin | Native |
|
|
|
| GelMA | Native - Modified |
|
|
|
| PEG | Synthetic |
|
|
|
Future Directions
ECM-mimicking 3D environments are becoming increasingly common as platforms to examine valvular cell function and pathology. As discussed in the previous section, these scaffolds can be constructed to mimic various compositional and architectural features of native healthy and diseased valves. However, there remain many opportunities to expand these engineering efforts to advance our understanding – and potential future treatment – of valvular dysfunction.
Engineering Scaffolds to Mimic the Aged ECM
As with many diseases, advanced age is the primary risk factor for CAVD. Also, as with many diseases, our in vitro culture approaches typically fail to account for this aged environment. Because of the complicated history of valvular characterization, where normal aging was thought to include calcification, a complete picture of the healthy aged valve ECM is still emerging. But, our knowledge of aged valve ECM thus far suggests that we can mimic these changes via a combination of tuning existing valvular scaffold platforms and borrowing tools from other fields. Existing chemistries may be tailored to mimic major ECM changes associated with aging, such as increased collagen and HA concentrations and valve stiffening/thickening. In tumor engineering, interpenetrating networks of fibrillar collagen I with other materials have been formed to modulate fiber density independently of other scaffold features [99]; a similar approach was recently applied to valves [94]. With respect to other aging features, it has yet to be determined how much AGEs and ECM crosslinking enzymes are involved in forming the normal aged valve environment, and these classes of molecules have not yet been combined with valvular tissue engineering. However, work on other diseases and tissues has included strategies to form AGE-modified ECM scaffolds [100] and lysyl oxidase-treated materials [101], which may be translated to inform valvular approaches. Overall, the needs and targets for engineering the aged valve ECM will evolve as we further quantify these features in native valves.
Moving Toward a Trilaminar Engineered ECM
The trilayered architecture of the native valve has been mimicked in valve scaffolds constructed from synthetic polymers [102, 103]. However, current ECM-based valve scaffold approaches rarely incorporate defined layers. The trilaminar valve structure helps to dictate the mechanical behavior of the valve; moreover, valve pathophysiology occurs in a layer-specific manner, so the presence of a layered structure may be important for recapitulating this element of CAVD. It may be possible to modify existing approaches to create a multi-layered valve scaffold, for example by photocrosslinking a GelMA-rich fibrosa layer atop a spongiosa-mimicking HAMA scaffold. However, as with all efforts that increase culture platform complexity, one should also be mindful of overengineering the system, weighing whether the increased complexity is necessary for the biological question at hand [104].
Another element of the native valve that is infrequently represented in existing scaffolds is the elastin-rich ventricularis. Engineering the ventricularis may receive less attention in part because elastin-based scaffolds are less common in general, but also because this layer is not the site for many pathological events in CAVD. Nevertheless, it is possible that the ventricularis makes meaningful biochemical and biophysical contributions to CAVD pathogenesis, which could be examined in an in vitro system. Self-assembling elastin-like polypeptides (ELPs) have been used to mimic various features of elastin in other engineered tissues [105], although it does not appear that ELPs have been used in valvular scaffolds. It is also possible that the mechanical aspects of elastic fibers could be incorporated into valve scaffolds via introduction of entirely synthetic fibrillar materials [106].
Engineering the Spectrum of Disease
Finally, it is expected that the current engineering approaches to mimic the valvular ECM will evolve to recapitulate a continuum of disease states. As noted in a previous section, the development of dynamic scaffold platforms enables one to simulate biochemical and physical changes seen in disease progression. An important consideration as this research moves forward is how to introduce fibrosis-mimicking architecture in these scaffolds. Many of the scaffolds currently in use for VIC culture do not incorporate a fibrillar element; or, if they do, the topography and arrangement of these fibers are not spatially controlled. Methods such as bioprinting [107] or other fiber patterning techniques [108] may offer opportunities to better mimic the fibrotic element of CAVD. More precise control over collagen structure can also open the door to mimicking other valvular diseases, such as congenital aortic valve stenosis [109].
Conclusions
Recent years have brought numerous advancements in engineering valvular ECM structures, and continued characterization of both healthy and diseased valves enable further biomimicry and refinement of these approaches. Opportunities exist to expand these efforts to recapitulate the full continuum of valvular disease and decipher CAVD pathogenesis. The treatment of CAVD is still limited to surgical valve replacement, so the further development of engineered, disease-mimicking scaffolds could be transformative in the pursuit of novel drug discovery and testing for this disease.
Acknowledgements
This work was supported by the National Institutes of Health (R01 HL141181 to K.S.M. and TL1TR002375 to A.J.S.) and the American Heart Association (15PRE 22170006 to A.M.P.). All figures were created using Biorender.
Cited References
- 1.Alberts B, Johnson A, Lewis J. The Extracellular Matrix of Animals. 2002. In: Molecular Biology of the Cell [Internet]. New York: Garland Science. 4th edition. [https://www.ncbi.nlm.nih.gov/books/NBK26810/]. [Google Scholar]
- 2.Kodigepalli KM, Thatcher K, West T, Howsmon DP, Schoen FJ, Sacks MS, et al. Biology and Biomechanics of the Heart Valve Extracellular Matrix. J Cardiovasc Dev Dis. 2020;7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124(16):1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valves: coordination by extracellular matrix and growth factor signaling. J Mol Med (Berl). 2003;81(7):392–403. [DOI] [PubMed] [Google Scholar]
- 6.Camenisch TD, Biesterfeldt J, Brehm-Gibson T, Bradley J, McDonald JA. Regulation of cardiac cushion development by hyaluronan. Exp Clin Cardiol. 2001;6(1):4–10. [PMC free article] [PubMed] [Google Scholar]
- 7.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105(5):408–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A Jr., et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. The Journal of clinical investigation. 2000;106(3):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butcher JT, McQuinn TC, Sedmera D, Turner D, Markwald RR. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ Res. 2007;100(10):1503–11. [DOI] [PubMed] [Google Scholar]
- 10.Horne TE, VandeKopple M, Sauls K, Koenig SN, Anstine LJ, Garg V, et al. Dynamic Heterogeneity of the Heart Valve Interstitial Cell Population in Mitral Valve Health and Disease. J Cardiovasc Dev Dis. 2015;2(3):214–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balguid A, Rubbens MP, Mol A, Bank RA, Bogers AJ, van Kats JP, et al. The role of collagen cross-links in biomechanical behavior of human aortic heart valve leaflets--relevance for tissue engineering. Tissue Eng. 2007;13(7):1501–11. [DOI] [PubMed] [Google Scholar]
- 12.Sacks MS, David Merryman W, Schmidt DE. On the biomechanics of heart valve function. J Biomech. 2009;42(12):1804–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez KJ, Piechura LM, Porras AM, Masters KS. Manipulation of valve composition to elucidate the role of collagen in aortic valve calcification. BMC Cardiovasc Disord. 2014;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porras AM, van Engeland NC, Marchbanks E, McCormack A, Bouten CV, Yacoub MH, et al. Robust Generation of Quiescent Porcine Valvular Interstitial Cell Cultures. J Am Heart Assoc. 2017;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez KJ, Piechura LM, Masters KS. Regulation of valvular interstitial cell phenotype and function by hyaluronic acid in 2-D and 3-D culture environments. Matrix Biol. 2011;30(1):70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porras AM, Westlund JA, Evans AD, Masters KS. Creation of disease-inspired biomaterial environments to mimic pathological events in early calcific aortic valve disease. Proc Natl Acad Sci U S A. 2018;115(3):E363–E71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stella JA, Sacks MS. On the biaxial mechanical properties of the layers of the aortic valve leaflet. J Biomech Eng. 2007;129(5):757–66. [DOI] [PubMed] [Google Scholar]
- 18.Sacks MS, Smith DB, Hiester ED. The aortic valve microstructure: effects of transvalvular pressure. J Biomed Mater Res. 1998;41(1):131–41. [DOI] [PubMed] [Google Scholar]
- 19.Sell S, Scully RE. Aging Changes in the Aortic and Mitral Valves. Histologic and Histochemical Studies, with Observations on the Pathogenesis of Calcific Aortic Stenosis and Calcification of the Mitral Annulus. Am J Pathol. 1965;46:345–65. [PMC free article] [PubMed] [Google Scholar]
- 20.Waller BF. The old-age heart: normal aging changes which can produce or mimic cardiac disease. Clin Cardiol. 1988;11(8):513–7. [DOI] [PubMed] [Google Scholar]
- 21.Balaoing LR, Post AD, Liu H, Minn KT, Grande-Allen KJ. Age-related changes in aortic valve hemostatic protein regulation. Arterioscler Thromb Vasc Biol. 2014;34(1):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Geemen D, Soares AL, Oomen PJ, Driessen-Mol A, Janssen-van den Broek MW, van den Bogaerdt AJ, et al. Age-Dependent Changes in Geometry, Tissue Composition and Mechanical Properties of Fetal to Adult Cryopreserved Human Heart Valves. PLoS One. 2016;11(2):e0149020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spadaccio C, Mozetic P, Nappi F, Nenna A, Sutherland F, Trombetta M, et al. Cells and extracellular matrix interplay in cardiac valve disease: because age matters. Basic Res Cardiol. 2016;111(2):16. [DOI] [PubMed] [Google Scholar]
- 24.Spadaccio C, Rainer A, Mozetic P, Trombetta M, Dion RA, Barbato R, et al. The role of extracellular matrix in age-related conduction disorders: a forgotten player? J Geriatr Cardiol. 2015;12(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oomen PJ, Loerakker S, van Geemen D, Neggers J, Goumans MJ, van den Bogaerdt AJ, et al. Age-dependent changes of stress and strain in the human heart valve and their relation with collagen remodeling. Acta Biomater. 2016;29:161–9. [DOI] [PubMed] [Google Scholar]
- 26.Krishnamurthy VK, Stout AJ, Sapp MC, Matuska B, Lauer ME, Grande-Allen KJ. Dysregulation of hyaluronan homeostasis during aortic valve disease. Matrix Biol. 2017;62:40–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens EH, Grande-Allen KJ. Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis. 2007;16(6):672–82. [PubMed] [Google Scholar]
- 28.Stephens EH, Chu CK, Grande-Allen KJ. Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: Relevance to an age-specific tissue-engineered heart valve. Acta Biomater. 2008;4(5):1148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275(50):39027–31. [DOI] [PubMed] [Google Scholar]
- 30.Li F, Cai Z, Chen F, Shi X, Zhang Q, Chen S, et al. Pioglitazone attenuates progression of aortic valve calcification via down-regulating receptor for advanced glycation end products. Basic Res Cardiol. 2012;107(6):306. [DOI] [PubMed] [Google Scholar]
- 31.Coffey S, Cox B, Williams MJ. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol. 2014;63(25 Pt A):2852–61. [DOI] [PubMed] [Google Scholar]
- 32.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111(24):3316–26. [DOI] [PubMed] [Google Scholar]
- 33.Voisine M, Hervault M, Shen M, Boilard AJ, Filion B, Rosa M, et al. Age, Sex, and Valve Phenotype Differences in Fibro-Calcific Remodeling of Calcified Aortic Valve. J Am Heart Assoc. 2020;9(10):e015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simard L, Cote N, Dagenais F, Mathieu P, Couture C, Trahan S, et al. Sex-Related Discordance Between Aortic Valve Calcification and Hemodynamic Severity of Aortic Stenosis: Is Valvular Fibrosis the Explanation? Circ Res. 2017;120(4):681–91. [DOI] [PubMed] [Google Scholar]
- 35.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90(2):844–53. [DOI] [PubMed] [Google Scholar]
- 36.Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, et al. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho KI, Sakuma I, Sohn IS, Jo SH, Koh KK. Inflammatory and metabolic mechanisms underlying the calcific aortic valve disease. Atherosclerosis. 2018;277:60–5. [DOI] [PubMed] [Google Scholar]
- 38.Parisi V, Leosco D, Ferro G, Bevilacqua A, Pagano G, de Lucia C, et al. The lipid theory in the pathogenesis of calcific aortic stenosis. Nutr Metab Cardiovasc Dis. 2015;25(6):519–25. [DOI] [PubMed] [Google Scholar]
- 39.Small A, Kiss D, Giri J, Anwaruddin S, Siddiqi H, Guerraty M, et al. Biomarkers of Calcific Aortic Valve Disease. Arterioscler Thromb Vasc Biol. 2017;37(4):623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porras AM, Shanmuganayagam D, Meudt JJ, Krueger CG, Hacker TA, Rahko PS, et al. Development of Aortic Valve Disease in Familial Hypercholesterolemic Swine: Implications for Elucidating Disease Etiology. J Am Heart Assoc. 2015;4(10):e002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sider KL, Zhu C, Kwong AV, Mirzaei Z, de Lange CF, Simmons CA. Evaluation of a porcine model of early aortic valve sclerosis. Cardiovasc Pathol. 2014;23(5):289–97. [DOI] [PubMed] [Google Scholar]
- 42.Scuruchi M, Poti F, Rodriguez-Carrio J, Campo GM, Mandraffino G. Biglycan and atherosclerosis: Lessons from high cardiovascular risk conditions. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865(2):158545. [DOI] [PubMed] [Google Scholar]
- 43.Anselmo W, Branchetti E, Grau JB, Li G, Ayoub S, Lai EK, et al. Porphyrin-Based SOD Mimic MnTnBu OE −2-PyP(5+) Inhibits Mechanisms of Aortic Valve Remodeling in Human and Murine Models of Aortic Valve Sclerosis. J Am Heart Assoc. 2018;7(20):e007861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Vito A, Donato A, Presta I, Mancuso T, Brunetti FS, Mastroroberto P, et al. Extracellular Matrix in Calcific Aortic Valve Disease: Architecture, Dynamic and Perspectives. Int J Mol Sci. 2021;22(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Latif N, Sarathchandra P, Taylor PM, Antoniw J, Yacoub MH. Localization and pattern of expression of extracellular matrix components in human heart valves. J Heart Valve Dis. 2005;14(2):218–27. [PubMed] [Google Scholar]
- 46.Bertazzo S, Gentleman E, Cloyd KL, Chester AH, Yacoub MH, Stevens MM. Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nat Mater. 2013;12(6):576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutson HN, Marohl T, Anderson M, Eliceiri K, Campagnola P, Masters KS. Calcific Aortic Valve Disease Is Associated with Layer-Specific Alterations in Collagen Architecture. PLoS One. 2016;11(9):e0163858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edep ME, Shirani J, Wolf P, Brown DL. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc Pathol. 2000;9(5):281–6. [DOI] [PubMed] [Google Scholar]
- 49.Fondard O, Detaint D, Iung B, Choqueux C, Adle-Biassette H, Jarraya M, et al. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J. 2005;26(13):1333–41. [DOI] [PubMed] [Google Scholar]
- 50.Kaden JJ, Vocke DC, Fischer CS, Grobholz R, Brueckmann M, Vahl CF, et al. Expression and activity of matrix metalloproteinase-2 in calcific aortic stenosis. Z Kardiol. 2004;93(2):124–30. [DOI] [PubMed] [Google Scholar]
- 51.Kaden JJ, Dempfle CE, Grobholz R, Fischer CS, Vocke DC, Kilic R, et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol. 2005;14(2):80–7. [DOI] [PubMed] [Google Scholar]
- 52.Hinton RB Jr., Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98(11):1431–8. [DOI] [PubMed] [Google Scholar]
- 53.Stephens EH, Saltarrelli JG, Baggett LS, Nandi I, Kuo JJ, Davis AR, et al. Differential proteoglycan and hyaluronan distribution in calcified aortic valves. Cardiovasc Pathol. 2011;20(6):334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helske S, Syvaranta S, Kupari M, Lappalainen J, Laine M, Lommi J, et al. Possible role for mast cell-derived cathepsin G in the adverse remodelling of stenotic aortic valves. Eur Heart J. 2006;27(12):1495–504. [DOI] [PubMed] [Google Scholar]
- 55.Helske S, Syvaranta S, Lindstedt KA, Lappalainen J, Oorni K, Mayranpaa MI, et al. Increased expression of elastolytic cathepsins S, K, and V and their inhibitor cystatin C in stenotic aortic valves. Arterioscler Thromb Vasc Biol. 2006;26(8):1791–8. [DOI] [PubMed] [Google Scholar]
- 56.Lee TC, Midura RJ, Hascall VC, Vesely I. The effect of elastin damage on the mechanics of the aortic valve. J Biomech. 2001;34(2):203–10. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez KJ, Masters KS. Regulation of valvular interstitial cell calcification by components of the extracellular matrix. J Biomed Mater Res A. 2009;90(4):1043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benton JA, Kern HB, Anseth KS. Substrate properties influence calcification in valvular interstitial cell culture. J Heart Valve Dis. 2008;17(6):689–99. [PMC free article] [PubMed] [Google Scholar]
- 59.Monzack EL, Gu X, Masters KS. Efficacy of simvastatin treatment of valvular interstitial cells varies with the extracellular environment. Arterioscler Thromb Vasc Biol. 2009;29(2):246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231(4736):397–400. [DOI] [PubMed] [Google Scholar]
- 61.Patil VA, Masters KS. Engineered Collagen Matrices. Bioengineering (Basel). 2020;7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butcher JT, Nerem RM. Porcine aortic valve interstitial cells in three-dimensional culture: comparison of phenotype with aortic smooth muscle cells. J Heart Valve Dis. 2004;13(3):478–85; discussion 85–6. [PubMed] [Google Scholar]
- 63.Kamel PI, Qu X, Geiszler AM, Nagrath D, Harmancey R, Taegtmeyer H, et al. Metabolic regulation of collagen gel contraction by porcine aortic valvular interstitial cells. J R Soc Interface. 2014;11(101):20140852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim J, Ehsanipour A, Hsu JJ, Lu J, Pedego T, Wu A, et al. Inflammation Drives Retraction, Stiffening, and Nodule Formation via Cytoskeletal Machinery in a Three-Dimensional Culture Model of Aortic Stenosis. Am J Pathol. 2016;186(9):2378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res. 2004;95(3):253–60. [DOI] [PubMed] [Google Scholar]
- 66.Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29(6):936–42. [DOI] [PubMed] [Google Scholar]
- 67.Gould RA, Chin K, Santisakultarm TP, Dropkin A, Richards JM, Schaffer CB, et al. Cyclic strain anisotropy regulates valvular interstitial cell phenotype and tissue remodeling in three-dimensional culture. Acta Biomater. 2012;8(5):1710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gupta V, Werdenberg JA, Blevins TL, Grande-Allen KJ. Synthesis of glycosaminoglycans in differently loaded regions of collagen gels seeded with valvular interstitial cells. Tissue Eng. 2007;13(1):41–9. [DOI] [PubMed] [Google Scholar]
- 69.Gupta V, Werdenberg JA, Mendez JS, Jane Grande-Allen K. Influence of strain on proteoglycan synthesis by valvular interstitial cells in three-dimensional culture. Acta Biomater. 2008;4(1):88–96. [DOI] [PubMed] [Google Scholar]
- 70.Benton JA, DeForest CA, Vivekanandan V, Anseth KS. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng Part A. 2009;15(11):3221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hjortnaes J, Camci-Unal G, Hutcheson JD, Jung SM, Schoen FJ, Kluin J, et al. Directing valvular interstitial cell myofibroblast-like differentiation in a hybrid hydrogel platform. Adv Healthc Mater. 2015;4(1):121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loessner D, Meinert C, Kaemmerer E, Martine LC, Yue K, Levett PA, et al. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat Protoc. 2016;11(4):727–46. [DOI] [PubMed] [Google Scholar]
- 73.Eslami M, Vrana NE, Zorlutuna P, Sant S, Jung S, Masoumi N, et al. Fiber-reinforced hydrogel scaffolds for heart valve tissue engineering. J Biomater Appl. 2014;29(3):399–410. [DOI] [PubMed] [Google Scholar]
- 74.Chen MB, Srigunapalan S, Wheeler AR, Simmons CA. A 3D microfluidic platform incorporating methacrylated gelatin hydrogels to study physiological cardiovascular cell-cell interactions. Lab Chip. 2013;13(13):2591–8. [DOI] [PubMed] [Google Scholar]
- 75.Ciortan L, Macarie RD, Cecoltan S, Vadana M, Tucureanu MM, Mihaila AC, et al. Chronic High Glucose Concentration Induces Inflammatory and Remodeling Changes in Valvular Endothelial Cells and Valvular Interstitial Cells in a Gelatin Methacrylate 3D Model of the Human Aortic Valve. Polymers (Basel). 2020;12(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trombino S, Servidio C, Curcio F, Cassano R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics. 2019;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masters KS, Shah DN, Leinwand LA, Anseth KS. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials. 2005;26(15):2517–25. [DOI] [PubMed] [Google Scholar]
- 78.Puperi DS, O’Connell RW, Punske ZE, Wu Y, West JL, Grande-Allen KJ. Hyaluronan Hydrogels for a Biomimetic Spongiosa Layer of Tissue Engineered Heart Valve Scaffolds. Biomacromolecules. 2016;17(5):1766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duan B, Hockaday LA, Kapetanovic E, Kang KH, Butcher JT. Stiffness and adhesivity control aortic valve interstitial cell behavior within hyaluronic acid based hydrogels. Acta Biomater. 2013;9(8):7640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hjortnaes J, Goettsch C, Hutcheson JD, Camci-Unal G, Lax L, Scherer K, et al. Simulation of early calcific aortic valve disease in a 3D platform: A role for myofibroblast differentiation. J Mol Cell Cardiol. 2016;94:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Valk DC, van der Ven CFT, Blaser MC, Grolman JM, Wu PJ, Fenton OS, et al. Engineering a 3D-Bioprinted Model of Human Heart Valve Disease Using Nanoindentation-Based Biomechanics. Nanomaterials (Basel). 2018;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X, Ali MS, Lacerda CMR. A Three-Dimensional Collagen-Elastin Scaffold for Heart Valve Tissue Engineering. Bioengineering (Basel). 2018;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koutsopoulos S, Paschalakis PC, Dalas E. The Calcification of Elastin in vitro. Langmuir. 1994;10(7):2423–8. [Google Scholar]
- 84.Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation. 2004;110(22):3480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ksiazek AA, Mitchell KJ, Cesarovic N, Schwarzwald CC, Hoerstrup SP, Weber B. PGA (polyglycolic acid)-P4HB (poly-4-hydroxybutyrate)-Based Bioengineered Valves in the Rat Aortic Circulation. J Heart Valve Dis. 2016;25(3):380–8. [PubMed] [Google Scholar]
- 86.Oveissi F, Naficy S, Lee A, Winlaw DS, Dehghani F. Materials and manufacturing perspectives in engineering heart valves: a review. Mater Today Bio. 2020;5:100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Benton JA, Fairbanks BD, Anseth KS. Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials. 2009;30(34):6593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang X, Xu B, Puperi DS, Yonezawa AL, Wu Y, Tseng H, et al. Integrating valve-inspired design features into poly(ethylene glycol) hydrogel scaffolds for heart valve tissue engineering. Acta Biomater. 2015;14:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gould ST, Anseth KS. Role of cell-matrix interactions on VIC phenotype and tissue deposition in 3D PEG hydrogels. J Tissue Eng Regen Med. 2016;10(10):E443–E53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah DN, Recktenwall-Work SM, Anseth KS. The effect of bioactive hydrogels on the secretion of extracellular matrix molecules by valvular interstitial cells. Biomaterials. 2008;29(13):2060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu Y, Grande-Allen KJ, West JL. Adhesive Peptide Sequences Regulate Valve Interstitial Cell Adhesion, Phenotype and Extracellular Matrix Deposition. Cell Mol Bioeng. 2016;9(4):479–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mabry KM, Payne SZ, Anseth KS. Microarray analyses to quantify advantages of 2D and 3D hydrogel culture systems in maintaining the native valvular interstitial cell phenotype. Biomaterials. 2016;74:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonzalez Rodriguez A, Schroeder ME, Walker CJ, Anseth KS. FGF-2 inhibits contractile properties of valvular interstitial cell myofibroblasts encapsulated in 3D MMP-degradable hydrogels. APL Bioeng. 2018;2(4):046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schroeder ME, Gonzalez Rodriguez A, Speckl KF, Walker CJ, Midekssa FS, Grim JC, et al. Collagen networks within 3D PEG hydrogels support valvular interstitial cell matrix mineralization. Acta Biomater. 2021;119:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma H, Killaars AR, DelRio FW, Yang C, Anseth KS. Myofibroblastic activation of valvular interstitial cells is modulated by spatial variations in matrix elasticity and its organization. Biomaterials. 2017;131:131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Puperi DS, Balaoing LR, O’Connell RW, West JL, Grande-Allen KJ. 3-Dimensional spatially organized PEG-based hydrogels for an aortic valve co-culture model. Biomaterials. 2015;67:354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mabry KM, Lawrence RL, Anseth KS. Dynamic stiffening of poly(ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment. Biomaterials. 2015;49:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kirschner CM, Alge DL, Gould ST, Anseth KS. Clickable, photodegradable hydrogels to dynamically modulate valvular interstitial cell phenotype. Adv Healthc Mater. 2014;3(5):649–57. [DOI] [PubMed] [Google Scholar]
- 99.Berger AJ, Linsmeier KM, Kreeger PK, Masters KS. Decoupling the effects of stiffness and fiber density on cellular behaviors via an interpenetrating network of gelatin-methacrylate and collagen. Biomaterials. 2017;141:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strieder-Barboza C, Baker NA, Flesher CG, Karmakar M, Neeley CK, Polsinelli D, et al. Advanced glycation end-products regulate extracellular matrix-adipocyte metabolic crosstalk in diabetes. Sci Rep. 2019;9(1):19748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mitra D, Yasui OW, Harvestine JN, Link JM, Hu JC, Athanasiou KA, et al. Exogenous Lysyl Oxidase-Like 2 and Perfusion Culture Induce Collagen Crosslink Formation in Osteogenic Grafts. Biotechnol J. 2019;14(3):e1700763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jana S, Lerman A. Behavior of valvular interstitial cells on trilayered nanofibrous substrate mimicking morphologies of heart valve leaflet. Acta Biomater. 2019;85:142–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Masoumi N, Annabi N, Assmann A, Larson BL, Hjortnaes J, Alemdar N, et al. Tri-layered elastomeric scaffolds for engineering heart valve leaflets. Biomaterials. 2014;35(27):7774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chester AH, Grande-Allen KJ. Which Biological Properties of Heart Valves Are Relevant to Tissue Engineering? Front Cardiovasc Med. 2020;7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varanko AK, Su JC, Chilkoti A. Elastin-Like Polypeptides for Biomedical Applications. Annu Rev Biomed Eng. 2020;22:343–69. [DOI] [PubMed] [Google Scholar]
- 106.Masoumi N, Larson BL, Annabi N, Kharaziha M, Zamanian B, Shapero KS, et al. Electrospun PGS:PCL microfibers align human valvular interstitial cells and provide tunable scaffold anisotropy. Adv Healthc Mater. 2014;3(6):929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rider P, Kacarevic ZP, Alkildani S, Retnasingh S, Barbeck M. Bioprinting of tissue engineering scaffolds. J Tissue Eng. 2018;9:2041731418802090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kristen M, Ainsworth MJ, Chirico N, van der Ven CFT, Doevendans PA, Sluijter JPG, et al. Fiber Scaffold Patterning for Mending Hearts: 3D Organization Bringing the Next Step. Adv Healthc Mater. 2020;9(1):e1900775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clift CL, Su YR, Bichell D, Jensen Smith HC, Bethard JR, Norris-Caneda K, et al. Collagen fiber regulation in human pediatric aortic valve development and disease. Sci Rep. 2021;11(1):9751. [DOI] [PMC free article] [PubMed] [Google Scholar]


