Abstract
LIM domain-containing proteins play critical roles in vertebrate development and cellular differentiation. Recently, four members of the four and one-half LIM protein (FHL) family have been identified and cloned. One of these, FHL2, is expressed in a restricted manner in the cardiovascular system throughout development into adulthood, suggesting that FHL2 may play an important role in cardiovascular development and function. Here we describe the generation and analysis of mice carrying a null mutation of the FHL2 gene. FHL2-deficient mice are viable and maintain normal cardiac function both before and after acute mechanical stress induced by aortic constriction. These data suggest that FHL2 is not essential for cardiac development and function.
The LIM domain has been found in variety of proteins (8, 9, 13, 22) and mediates protein-protein interaction (1). Some LIM proteins are transcription factors involved in cell lineage determination and pattern formation. Others are associated with the cytoskeleton and play a role in adhesion plaque and actin microfilament organization (4, 8, 16, 23).
Functional roles for LIM domain proteins have been demonstrated by genetic studies. Disruption of several homeodomain-containing LIM genes has demonstrated a critical role in development of neuronal lineages (16). Mice homozygous for deficiency of the LIM-only protein LMO2 die at embryonic day 10.5 due to lack of erythropoiesis (21). Muscle LIM protein, which is expressed abundantly in heart and skeletal muscle, consists of two LIM domains only (2). Recently it has been shown that mice lacking muscle LIM protein develop dilated cardiomyopathy with hypertrophy and heart failure after birth (3).
We hypothesized that other LIM domain-containing proteins may also play important roles in cardiac function. Characterization of these proteins will improve our understanding of the function of LIM domains and may identify candidate genes for cardiomyopathy. A combined GenBank and expressed sequence tag database search revealed a newly identified group of LIM-only proteins with four and one-half LIM domains (the FHL family) (5, 14), which are enriched in striated muscle. This group consists of four family members. Recently, we reported the expression patterns of murine FHL family members, FHL1, -2, and -3, which suggest important functions of this family in skeletal muscle and the cardiovascular system (7).
To address the in vivo functions of FHL2, we generated an FHL2-deficient mouse through homologous recombination in embryonic stem (ES) cells. In this study, we demonstrate that FHL2-deficient mice exhibit no obvious phenotype before or at 15 months of age compared to their wild-type littermates. The hearts and blood vessels of homozygous null mice appear normal by histological analysis; hearts of homozygous null mice exhibit normal function on echocardiographic and electrocardiographic analyses, with normal heart weight/body weight ratios, compared to hearts from wild-type littermates. Moreover, homozygous null mice respond to acute pressure overload induced by transverse aortic constriction (TAC) in the same manner as wild-type littermate controls. Taken together, these data suggest that FHL2 is dispensable for normal cardiovascular system development and function.
MATERIALS AND METHODS
Generation of FHL2 null mice.
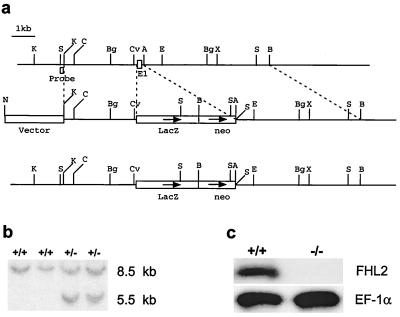
A genomic DNA clone was isolated from a mouse 129-SV/J genomic DNA library (Stratagene, La Jolla, Calif.), using a 410-bp probe from the 5′ cDNA sequence of FHL2 (7). PCR-based mutagenesis was used to convert the approximately 300-bp sequence from two base pairs 5′ of the translation start codon ATG to the ApoI site in intron 1 to an XhoI site. A cDNA encoding LacZ and containing a pGKneo cassette was then inserted into this XhoI site. Orientation of this cassette was confirmed by restriction enzyme digestion and DNA sequencing (Fig. 1a). In this manner, the lacZ cDNA would be brought under the control of the endogenous FHL2 promoter while also ablating the endogenous FHL2 gene (Fig. 1a).
FIG. 1.
Targeting the FHL2 gene. (a) Restriction maps of the FHL2 genomic region of interest (top), the targeting construct (center), and the mutated locus after homologous recombination (bottom). A, ApoI; B, BamHI; Bg, BglII; C, ClaI; Cv, CvnI; E, EcoRI; K, KpnI; N, NotI; S, SstI; X, XbaI. (b) Detection of the heterozygous recombinant FHL2 allele by Southern blot analysis. DNAs from neo-positive ES cells were digested with SstI and analyzed by Southern blotting with the probe shown in the top line of panel a. (c) Detection of FHL2 mRNA by Northern blot analysis. Aliquots of 10 μg of total RNA isolated from adult ventricles of wild-type (+/+) and FHL2-deficient (−/−) mice were analyzed by using a cDNA probe spanning the entire FHL2 coding region. EF-1α cDNA was used as a control probe to normalize for loading.
The linearized targeting construct was electroporated into 129-SV/J-derived ES cells. The ES cells were maintained on feeder layers under the selective pressure of G418 and ganciclovir as described previously (6). G418-resistant ES clones were screened for homologous recombination by Southern blotting with a probe as shown in Fig. 1b. Cells from two independent targeted clones were microinjected into C57BL/6J blastocysts and transferred into pseudopregnant recipients. Chimeric animals resulting from the microinjections were bred to Black Swiss mice, and agouti pups were screened for germ line transmission of the mutant allele. The genotypes from these and all subsequent matings were determined by PCR on DNA from tail biopsy specimens (data not shown). For PCR analysis, oligonucleotides for neo cDNA (neo1, 5′-GGATCGGCCATTGAACAAGATG-3′; neo2, 5′-GAGCAAGGTGAGATGACAGGAG-3′) and the FHL2 gene (FHL2-P2, 5′-AGCATGACTGAACGCTTTGACT-3′; FHL2-P3, 5′-GGTAACCAGAACAGGGAGAGTG-3′) were used. PCR conditions were as follows: denaturation at 94°C for 4 min, followed by 31 cycles of 30 s at 95°C, 30 s at 60°C, and 1 min at 72°C. We obtained identical results from both targeted lines. Analysis thus far has been carried out on the hybrid Black Swiss–129-SV/J background. All animals were housed in the animal care unit of the University of California, San Diego, Department of Medicine, according to animal care guidelines.
RNA isolation and Northern blot analysis.
Total RNA was isolated from the left ventricles of mice, using a polytron with RNAzol B (Tel-Test) as reported previously (7). Ten micrograms of total RNA was electrophoresed on 1% agarose gels and transferred to a nylon membrane, which was subsequently hybridized with [α-32P]dATP-labeled FHL1, -2, and -3 probes in QuickHyb solution (Stratagene) as described by the company.
Histological analysis.
Cardiac, skeletal, and smooth muscle from the aorta and intestine was dissected from wild-type, heterozygous, and FHL2-deficient mice and rapidly frozen in liquid nitrogen-cooled isopentane. Ten-micrometer cryosections were analyzed by staining with hematoxylin and eosin.
Microsurgical techniques to introduce pressure overload cardiac hypertrophy.
Pressure overload was produced in mice at 8 weeks of age by performing TAC as described previously (18). At 7 days following surgery, the gradient of the arterial blood pressure between the constriction was measured. Six homozygous FHL2 mutants and nine wild-type mice showing an adequate pressure gradient (>40 mm of Hg) were subjected to further studies.
ECG analysis of FHL2-deficient mice.
Each mouse was anesthetized intraperitoneally with a mixture of pentobarbital and ketamine (0.015 and 0.033 mg/g of body weight, respectively), and a six-lead electrocardiogram (ECG) was obtained by placing 27-gauge needle electrodes subcutaneously in each limb.
Echocardiographic analysis.
Mice were anesthetized with 2.5% Avertin (1 ml/g of body weight given intraperitoneally), and transthoracic echocardiography was performed before and 7 days after TAC as described in detail elsewhere (19).
Statistical analysis.
Data are indicated as average ± standard deviation. Statistical comparisons between the wild-type mice and FHL2 mutants were done by unpaired Student t test. A P value of <0.05 was considered significant.
RESULTS
Generation of FHL2-deficient mice.
The linearized targeting construct (Fig. 1a) was electroporated into R1 ES cells, and two independent clones which were positive for homologous recombination were used to generate chimeric founder mice. Heterozygous mice from the F1 generation were identified by PCR analysis and were crossed to obtain FHL2-deficient mice. The frequency of homozygous FHL2-deficient mice born was consistent with a predicted Mendelian distribution.
Northern blot analysis of RNA from ventricular muscle of wild-type and homozygous FHL2-deficient mice revealed that the FHL2 transcript was absent in homozygous null mice (Fig. 1c).
mRNA levels of FHL1 and FHL3 do not increase in hearts of FHL2 homozygous null mice.
To determine whether or not two highly related FHL genes, FHL1 and FHL2 (7), are up-regulated to compensate for the absence of FHL2 in the ventricle, Northern blot analysis was performed with probes for FHL1 and FHL3 mRNAs. No significant differences were observed between the homozygous null mice and the wild-type controls (data not shown).
FHL2-deficient mice have no detectable cardiac phenotype and maintain a normal cardiac hypertrophic response to acute pressure overload.
Cryosections (10 μm) of heart and aorta from homozygous null mice and wild-type littermates (12 mice per group; ranging in age from 7 to 11 months old) were stained with hematoxylin and eosin. All specimens appeared normal, exhibiting no signs of hypertrophy, infarction, necrosis, fibrosis, calcification, or fat infiltration (data not shown). There were no differences in the heart weight-to-body weight ratio in the wild-type (0.39 ± 0.18), heterozygous (0.37 ± 0.13), and homozygous null (0.39 ± 0.08) groups of mice. Thus far, we have analyzed FHL2-deficient mice up to 15 months of age and have consistently found that homozygous null mice are indistinguishable from wild-type or heterozygous mice.
To analyze the stress response of FHL2-deficient mice, we performed acute pressure overload induced by TAC. Upon echocardiographic analyses, no statistically significant differences were observed between wild-type and homozygous mice, either in the basal state or following TAC (Table 1).
TABLE 1.
Analysis of in vivo cardiac size and function by echocardiography in FHL2-deficient mice at basal level and 7 days following TAC
| Mouse group | Avg ± SDa
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body wt (mg) | Heart rate (beats/min) | IVS (mm) | PW (mm) | LVEDD (mm) | LVESD (mm) | FS% | Vcf (circumferences/s) | PG (mm of Hg) | Heart wt/ body wt | |
| Wild type | ||||||||||
| Basal (n = 9) | 36.5 ± 5.4 | 488 ± 40 | 0.70 ± 0.08 | 0.73 ± 0.08 | 3.63 ± 0.53 | 2.12 ± 0.56 | 42.4 ± 8.9 | 7.69 ± 1.83 | ||
| After TAC (n = 9) | 34.5 ± 4.32 | 425 ± 38 | 0.94 ± 0.11b | 1.00 ± 0.15b | 3.10 ± 0.32c | 1.51 ± 0.31c | 51.5 ± 8.2c | 9.18 ± 1.25 | 67 ± 28 | 0.41 ± 0.07 |
| Homozygous | ||||||||||
| Basal (n = 6) | 34.5 ± 6.5 | 414 ± 63 | 0.71 ± 0.04 | 0.70 ± 0.11 | 3.91 ± 0.45 | 2.45 ± 0.46 | 37.6 ± 6.2 | 7.16 ± 1.01 | ||
| After TAC (n = 6) | 34.2 ± 7.08 | 427 ± 46 | 0.90 ± 0.24d | 0.88 ± 0.25d | 3.04 ± 0.43d | 1.66 ± 0.5d | 46.0 ± 12.2d | 8.05 ± 2.74 | 85 ± 47 | 0.38 ± 0.06 |
IVS, interventricular wall thickness; PW, left ventricular posterior wall thickness; LVEDD, end-diastolic left ventricular dimension; LVESD, end-systolic left ventricular dimension; FS%, left ventricular percent fractional shortening; Vcf, velocity of circumferential fiber shortening; PG, pressure gradient across the aortic constriction.
P < 0.001, wild type after TAC versus basal.
P < 0.05, wild type after TAC versus basal.
P < 0.05, homozygous after TAC versus basal.
FHL2-deficient mice maintain a normal cardiac conduction system.
As expression of FHL2 in the heart is highest in cardiac septa and in the region adjacent to the atrioventricular bundle, we postulated that FHL2 might play a critical role in conduction system function (7). To assess whether FHL2-deficient mice have any cardiac conduction system defects, we performed ECG studies on homozygous null FHL2 mice and wild-type littermates. We were unable to detect any significant changes in FHL2 mutant mice in heart rate, P-R interval, QRS complex, or Q-T interval (data not shown).
DISCUSSION
An accumulating body of evidence indicates that FHL proteins may play important roles in both cardiac and striated muscle. Recent studies indicate that a splice variant of FHL1/KyoT, termed KyoT2, can repress transcriptional activation by the Notch signaling pathway (20). The Notch pathway has been shown to be important for both striated muscle development and differentiation (11, 15). FHL1 mRNA levels are up-regulated dramatically in both hypertrophic and failing hearts of human patients (12). FHL2, also named DRAL (down-regulated in rhabdomyosarcoma LIM protein), is downregulated in rhabdomyosarcomas and is primarily expressed in striated muscles (10). It has also been shown recently that FHL2 is a novel tissue-specific coactivator of the androgen receptor (17).
Despite an intensive analysis of FHL2 null mice, we have not been able to detect any abnormality. Partially overlapping expression patterns of FHL1, -2, and -3 genes could explain the lack of the phenotype of FHL2 null mutants (7), although FHL1 and -3 are not up-regulated in the FHL2 null mutant heart at the mRNA level. We have generated FHL1 knockout mice and are in the process of making FHL3 gene-targeted mice. It will be interesting to examine the phenotypes of mice with double and triple deficiencies of FHL1, -2, and -3.
ACKNOWLEDGMENTS
We thank Sylvia Evans for critical reading of the manuscript. We thank Nancy Dalton for echocardiographic studies and Judith Canicio for looking after the transgenic mice.
J. Chen is a recipient of a grant-in-aid from the American Heart Association. P.-H. Chu was supported by a grant from the Chung Gang Memorial Hospital at Taiwan.
REFERENCES
- 1.Arber S, Caroni P. Specificity of single LIM motifs in targeting and LIM/LIM interactions in situ. Genes Dev. 1996;10:289–300. doi: 10.1101/gad.10.3.289. [DOI] [PubMed] [Google Scholar]
- 2.Arber S, Halder G, Caroni P. Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell. 1994;79:221–231. doi: 10.1016/0092-8674(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 3.Arber S, Hunter J J, Ross J, Jr, Hongo M, Sansig G, Borg J, Perriard J C, Chien K R, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. doi: 10.1016/s0092-8674(00)81878-4. [DOI] [PubMed] [Google Scholar]
- 4.Beckerle M C. Zyxin: zinc fingers at sites of cell adhesion. Bioessays. 1997;19:949–957. doi: 10.1002/bies.950191104. [DOI] [PubMed] [Google Scholar]
- 5.Chan K K, Tsui S K, Lee S M, Luk S C, Liew C C, Fung K P, Waye M M, Lee C Y. Molecular cloning and characterization of FHL2, a novel LIM domain protein preferentially expressed in human heart. Gene. 1998;210:345–350. doi: 10.1016/s0378-1119(97)00644-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Kubalak S W, Chien K R. Ventricular muscle-restricted targeting of the RXRalpha gene reveals a non-cell-autonomous requirement in cardiac chamber morphogenesis. Development. 1998;125:1943–1949. doi: 10.1242/dev.125.10.1943. [DOI] [PubMed] [Google Scholar]
- 7.Chu P-H, Ruiz-Lozano P, Zhou Q, Cai C, Chen J. Expression patterns of FHL/SLIM family members suggest important roles in skeletal muscle and cardiovascular system. Mech Dev. 2000;95:259–265. doi: 10.1016/s0925-4773(00)00341-5. [DOI] [PubMed] [Google Scholar]
- 8.Dawid I B, Breen J J, Toyama R. LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet. 1998;14:156–162. doi: 10.1016/s0168-9525(98)01424-3. [DOI] [PubMed] [Google Scholar]
- 9.Freyd G, Kim S K, Horvitz H R. Novel cysteine-rich motif and homeodomain in the product of the Caenorhabditis elegans cell lineage gene lin-11. Nature. 1990;344:876–879. doi: 10.1038/344876a0. [DOI] [PubMed] [Google Scholar]
- 10.Genini M, Schwalbe P, Scholl F A, Remppis A, Mattei M G, Schäfer B W. Subtractive cloning and characterization of DRAL, a novel LIM-domain protein down-regulated in rhabdomyosarcoma. DNA Cell Biol. 1997;16:433–442. doi: 10.1089/dna.1997.16.433. [DOI] [PubMed] [Google Scholar]
- 11.Hartenstein A Y, Rugendorff A, Tepass U, Hartenstein V. The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development. 1992;116:1203–1220. doi: 10.1242/dev.116.4.1203. [DOI] [PubMed] [Google Scholar]
- 12.Hwang D M, Dempsey A A, Wang R X, Rezvani M, Barrans J D, Dai K S, Wang H Y, Ma H, Cukerman E, Liu Y Q, Gu J R, Zhang J H, Tsui S K, Waye M M, Fung K P, Lee C Y, Liew C C. A genome-based resource for molecular cardiovascular medicine: toward a compendium of cardiovascular genes. Circulation. 1997;96:4146–4203. doi: 10.1161/01.cir.96.12.4146. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson O, Thor S, Norberg T, Ohlsson H, Edlund T. Insulin gene enhancer binding protein Isl-1 is a member of a novel class of proteins containing both a homeo- and a Cys-His domain. Nature. 1990;344:879–882. doi: 10.1038/344879a0. [DOI] [PubMed] [Google Scholar]
- 14.Lee S M, Tsui S K, Chan K K, Garcia-Barcelo M, Waye M M, Fung K P, Liew C C, Lee C Y. Chromosomal mapping, tissue distribution and cDNA sequence of four-and-a-half LIM domain protein 1 (FHL1) Gene. 1998;216:163–170. doi: 10.1016/s0378-1119(98)00302-3. [DOI] [PubMed] [Google Scholar]
- 15.Lindsell C E, Shawber C J, Boulter J, Weinmaster G. Jagged: a mammalian ligand that activates Notch1. Cell. 1995;80:909–917. doi: 10.1016/0092-8674(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 16.Lumsden A. Neural development. A ‘LIM code’ for motor neurons? Curr Biol. 1995;5:491–495. doi: 10.1016/s0960-9822(95)00100-x. [DOI] [PubMed] [Google Scholar]
- 17.Muller J M, Isele U, Metzger E, Rempel A, Moser M, Pscherer A, Breyer T, Holubarsch C, Buettner R, Schule R. FHL2, a novel tissue-specific coactivator of the androgen receptor. EMBO J. 2000;19:359–369. doi: 10.1093/emboj/19.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockman H A, Ono S, Ross R S, Jones L R, Karimi M, Bhargava V, Ross J, Jr, Chien K R. Molecular and physiological alterations in murine ventricular dysfunction. Proc Natl Acad Sci USA. 1994;91:2694–2698. doi: 10.1073/pnas.91.7.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka N, Dalton N, Mao L, Rockman H A, Peterson K L, Gottshall K R, Hunter J J, Chien K R, Ross J., Jr Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation. 1996;94:1109–1117. doi: 10.1161/01.cir.94.5.1109. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi Y, Furukawa T, Tun T, Han H, Honjo T. LIM protein KyoT2 negatively regulates transcription by association with the RBP-J DNA-binding protein. Mol Cell Biol. 1998;18:644–654. doi: 10.1128/mcb.18.1.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren A J, Colledge W H, Carlton M B, Evans M J, Smith A J, Rabbitts T H. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell. 1994;78:45–57. doi: 10.1016/0092-8674(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 22.Way J C, Chalfie M. mec-3, a homeobox-containing gene that specifies differentiation of the touch receptor neurons in C. elegans. Cell. 1988;54:5–16. doi: 10.1016/0092-8674(88)90174-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Q, Ruiz-Lozano P, Martone M E, Chen J. Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to alpha-actinin-2 and protein kinase C. J Biol Chem. 1999;274:19807–19813. doi: 10.1074/jbc.274.28.19807. [DOI] [PubMed] [Google Scholar]