Abstract
Introduction
Inhaled corticosteroids (ICSs) play an important role in lowering the risk of acute exacerbation of chronic obstructive pulmonary disease (COPD). However, ICSs are known to increase the risk of pneumonia. Moreover, previous studies have shown that the incidence rate of pneumonia varies depending on the type of ICS. In this study, the risk of pneumonia according to the type of ICS was investigated in a population-based cohort.
Methods
A retrospective cohort study was conducted using claims data of the entire population from the Korean National Health Insurance Service. Patients who were newly diagnosed with COPD and prescribed fluticasone propionate or budesonide were enrolled as study subjects. Cumulative doses of ICSs were classified into categorical variables to analyze the risk of pneumonia within identical ICS doses.
Results
A total of 47,473 subjects were identified and allocated as 14,518 fluticasone propionate and 14,518 budesonide users through 1:1 propensity score matching. Fluticasone propionate users were more likely to develop pneumonia than budesonide users (14.22% vs 10.66%, p<0.0001). The incidence rate per 100,000 person-years was 2,914.77 for fluticasone propionate users and 2,102.90 for budesonide users. The hazard ratio (HR) of pneumonia in fluticasone propionate compared to budesonide was 1.34 (95% CI 1.26–1.43, p<0.0001). The risk of pneumonia for fluticasone propionate compared to budesonide increased with higher ICS cumulative doses: 1.06 (0.93–1.21), 1.41 (1.19–1.66), 1.41 (1.23–1.63), and 1.49 (1.33–1.66) from the lowest to highest quartiles, respectively.
Conclusion
ICS types and doses need to be carefully considered during treatment with ICSs in patients with COPD.
Keywords: inhaled corticosteroid, chronic obstructive pulmonary disease, pneumonia, fluticasone propionate, budesonide
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth largest cause of death worldwide, and is likely to be the third in the near future.1 Drugs such as long-acting muscarinic antagonist (LAMA), long-acting β-agonist (LABA), and inhaled corticosteroids (ICSs) have been developed and used to prevent COPD exacerbations, control symptoms, and improve survival rates.2 In the treatment of COPD, ICSs are used in combination with LABA and/or LAMA to improve quality of life by alleviating symptoms and reducing the frequency of acute exacerbation.3 In recent years, it has been found that triple therapy combining ICS/LABA/LAMA in a single inhaler reduced the frequency of exacerbation of COPD compared to LABA/LAMA dual therapy.4 Thus, it is expected to expand the prescription of ICSs for the management of COPD.5
Patients with COPD might experience various systemic side effects, such as hyperglycemia, adrenal suppression, glaucoma, cataract, and osteoporosis from the use of ICSs.6 Of these, pneumonia is a major trigger for acute exacerbation of COPD, although it has not been directly associated with a rise in the mortality of patients with COPD.7 In previous studies concerning the association between ICSs and the development of pneumonia, there were consistent results showing that the incidence of pneumonia increased in patients treated with ICSs compared to those treated without ICSs.8 However, there were conflicting results regarding whether the incidence of pneumonia varied depending on the type of ICS. In a meta-analysis, no significant difference was noted in the occurrence of pneumonia between fluticasone propionate and budesonide.9 On the other hand, in another study, fluticasone propionate was highly associated with the risk of pneumonia compared to budesonide through an indirect comparison.10
Considering the substantial discordance between the recommendations of guidelines and practice patterns in real-world clinics,11,12 robust evidence through direct comparison of ICS types in a real-world population is essential for the safe management of patients with COPD treated with ICSs. Therefore, this study aimed to examine whether the incidence of pneumonia differed depending on the type of ICS, fluticasone propionate and budesonide, in real-world clinical settings.
Methods
Data Source
The National Health Insurance Service (NHIS) is a single insurer that covers the 100% of the Korean population and is compulsory for all residents in Korea.13 Those insured under the NHIS need to pay monthly contributions according to their socioeconomic status. Healthcare providers offer healthcare services to the insured and submit healthcare claims to the NHIS for reimbursement. Diseases and healthcare services are electronically converted using a coding system to determine the claim costs. Diseases are classified into diagnostic codes according to the International Classification of Diseases, 10th revision (ICD-10). The claim data are reviewed to determine whether the healthcare services were appropriate, and the claimed costs are finally reimbursed to healthcare providers from the NHIS. The claim database contains general information, including patient demographics, diagnosis, diagnostic procedures, and status of outpatients or inpatients. In addition, it contains detailed information related to drugs, such as the names, dosages, prescription dates, durations, and routes of administration. NHIS data without individual identifiers were obtained. This study was approved by the Institutional Review Board of Wonju Severance Christian Hospital (CR318361). Since this was a retrospective study using anonymous claims data, the requirement for informed consent was waived.
Study Design and Subjects
Of the entire Korean population registered in the NHIS, patients who had a COPD diagnosis at least twice between January 2005 and December 2018 were initially selected. The diagnosis of COPD was based on the corresponding diagnostic codes J42–J44 (except for J430). Those who were diagnosed with lung cancer (C33–C39) and did not have a history of a lung function test 1 year before and after their COPD diagnosis were excluded. Finally, half of the participants were extracted and selected for this study. This 50% provision of the sampling data is a NHIS procedure that is applied to prevent healthcare databases from being used for commercial purposes. The other applied exclusion criteria were as follows: diagnostic history of COPD before 2005; patient age <40 years; no record of a prescription for the management of COPD; treatment with only oral medications without prescriptions of any inhalers; prescriptions of different types of ICSs simultaneously; death before prescription of ICSs; insufficient medication records; not treated with fluticasone propionate or budesonide; and diagnosis of pneumonia before the index date.
Fluticasone propionate was chosen as the component of fluticasone, while another component of fluticasone, fluticasone furoate, was not included in this study. Budesonide is another ICS component that act as a comparator of fluticasone propionate. Therefore, all study participants were treated with various ICS commercial products containing fluticasone propionate or budesonide for at least 1 month, regardless of the use of metered dose inhalers or dry powder inhalers. The index date was defined as the first date of fluticasone propionate or budesonide prescription in each group. The period from the COPD diagnosis to the index date was calculated. Data on the prescription of other inhaled medications treated together with ICSs such as fluticasone propionate and budesonide during 1 year after the index date were collected. These were classified as short-acting β-agonist (SABA), LABA, LAMA, and LABA/LAMA.
The diagnosis of comorbidities was based on the corresponding diagnostic codes for each disease. The comorbidities included in this study were diabetes (E10–E14), hypertension (I10), heart failure (I11, I50), stroke (I60–I69), chronic kidney disease (N17–N19), and chronic liver disease (K70–K76). In addition, Charlson comorbidity indices (CCIs) were calculated using diagnostic codes. Prescription records of oral corticosteroids (OCSs) were collected in terms of the proportion of subjects who had an OCS prescription and the mean duration of the OCS prescription during 1 year after the index date. These two factors were used to determine the baseline severity of COPD.
Outcome Variables
The objective of this study was to compare the incidence of pneumonia between participants treated with fluticasone propionate and those treated with budesonide. Patients admitted for the treatment of pneumonia were identified as having pneumonia in this study. Therefore, the date of first hospitalization with a diagnostic code of pneumonia (J10–J18) was defined as pneumonia.14 Cumulative doses of ICSs were calculated. Budesonide was converted to fluticasone propionate considering an ICS equivalent dose in which 50 μg fluticasone propionate was equivalent to 80 μg budesonide.15 The ICS cumulative dose was the sum of all prescribed ICSs for the entire study period and divided into four groups.
Statistical Analysis
To reduce selection bias, fluticasone propionate users and budesonide users were subjected to propensity score matching at a 1:1 ratio. The matching was conducted between the two groups by means of logistic regression of various variables such as age, sex, comorbidities, bronchodilator prescription types, OCS prescription rates and durations, and intervals from COPD diagnosis to the index dates. Clinical features of fluticasone propionate and budesonide users were compared using Student’s t-test for continuous variables and the chi-squared test for categorical variables. Survival analysis over time was analyzed through a Log rank test using Kaplan-Meier curves. Cox proportional hazards analyses were performed to analyze the hazard ratio (HR) and 95% confidence interval (CI) describing the relative risk of pneumonia between fluticasone propionate and budesonide users. Significant differences in baseline characteristics between the two groups after propensity score matching were included in the multivariate analysis. All statistical analyses were conducted using SAS 9.4 (version 9.4; SAS Institute Inc., Cary, NC, USA). Statistical significance was set at p < 0.05.
Results
Baseline Characteristics of Study Subjects
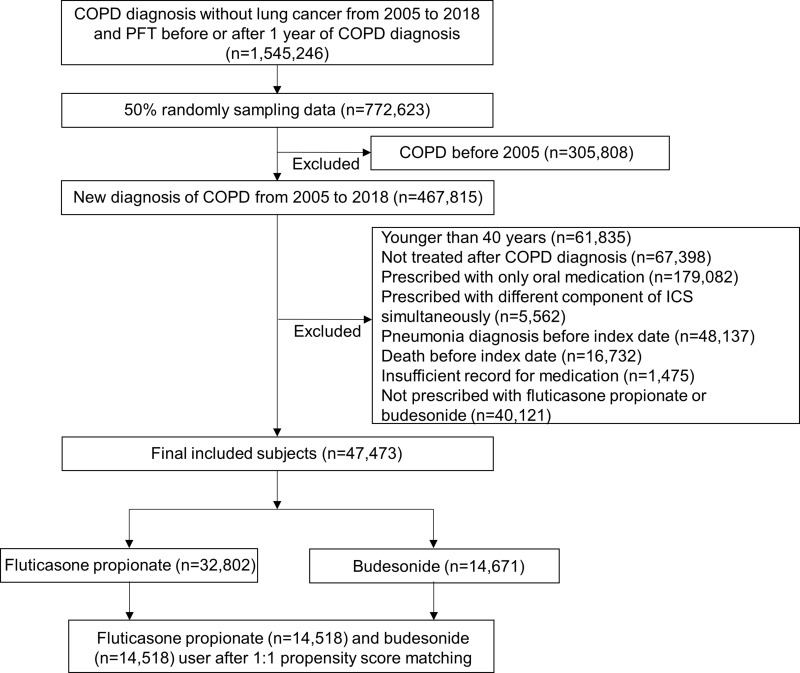
Among the entire Korean population, those who had a diagnosis of COPD without lung cancer between 2005 and 2018 and who had a pulmonary function test within 1 year before or after their COPD diagnosis were identified. A 50% random sampling of 772,623 patients was performed. Patients diagnosed with COPD before 2005 were excluded from the study. When the other exclusion criteria were applied, 47,473 participants were included in this study. Among them, 32,802 were treated with fluticasone propionate and 14,671 were treated with budesonide. After 1:1 propensity score matching, 14,518 fluticasone propionate and 14,518 budesonide users were selected as the target population for statistical analysis (Figure 1).
Figure 1.
Flowchart of patient selection.
The average age was 61.96 ± 11.53 years in fluticasone propionate users and 61.99 ± 11.35 years in budesonide users (p = 0.1341). Sex, comorbidities, and CCI did not differ between the two groups. SABA use was more frequent in budesonide users than in fluticasone propionate users (6.12% versus 2.12%, p < 0.0001). However, LABA use was higher in fluticasone propionate users than in budesonide users (78.27% versus 74.20%, p < 0.0001). A significant difference was not observed in terms of OCS prescription rates and durations of OCS prescriptions (Table 1).
Table 1.
Baseline Characteristics of Study Subjects
| Fluticasone Propionate (n=14,518) | Budesonide (n= 14,518) | p value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | |||||
| Mean (SD) | 61.96 (11.53) | 61.99 (11.35) | 0.1341 | ||
| 40–49 | 2491 | 17.16 | 2342 | 16.13 | 0.2124 |
| 50–59 | 3735 | 25.73 | 3793 | 26.13 | |
| 60–69 | 4286 | 29.52 | 4360 | 30.03 | |
| 70–79 | 3084 | 21.24 | 3110 | 21.42 | |
| ≥ 80 | 922 | 6.35 | 913 | 6.29 | |
| Sex | |||||
| Male | 8335 | 57.41 | 8294 | 57.13 | 0.6267 |
| Female | 6183 | 42.59 | 6224 | 42.87 | |
| Comorbidity | |||||
| Diabetes | 4414 | 30.40 | 4494 | 30.95 | 0.3087 |
| Hypertension | 4183 | 28.81 | 4200 | 28.93 | 0.8258 |
| Heart failure | 3660 | 25.21 | 3716 | 25.60 | 0.4503 |
| Stroke | 2955 | 20.35 | 3046 | 20.98 | 0.1872 |
| Chronic kidney disease | 715 | 4.92 | 720 | 4.96 | 0.8923 |
| Chronic liver disease | 5059 | 34.85 | 5057 | 34.83 | 0.6401 |
| CCI | |||||
| Mean (SD) | 3.01 (2.08) | 2.97 (2.06) | |||
| <2 | 4030 | 27.76 | 4112 | 28.32 | 0.2840 |
| ≥ 2 | 10,488 | 72.24 | 10,488 | 72.24 | |
| Bronchodilator | |||||
| SABA | 308 | 2.12 | 888 | 6.12 | <0.0001 |
| LAMA | 1876 | 12.92 | 1905 | 13.12 | 0.6131 |
| LABA | 11,363 | 78.27 | 10,772 | 74.20 | <0.0001 |
| LABA/LAMA | 971 | 6.69 | 953 | 6.56 | 0.6711 |
| OCS prescription | |||||
| Yes | 12,256 | 84.42 | 12,341 | 85.00 | 0.1657 |
| No | 2262 | 15.58 | 2177 | 15.00 | |
| OCS prescription day | |||||
| Mean (SD) | 11.08 (17.45) | 10.96 (17.57) | 0.5365 | ||
| Interval from COPD diagnosis to index date | |||||
| Mean (SD) | 441.4 (834.0) | 466.2 (911.5) | 0.0154 | ||
Abbreviations: SABA, short-acting β-agonist; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2 agonist; OCS, oral corticosteroid.
The average cumulative ICS dose was 185,065 ± 524,205 μg for fluticasone propionate users and 141,148 ± 318,030 μg for budesonide users. The cumulative ICS doses were divided into quartiles: 0–15,000 μg (Q1), 15,001–36,000 μg (Q2), 36,001–135,000 μg (Q3), and >135,000 μg (Q4) (Table 2).
Table 2.
Proportion of Subjects According to the Cumulative Doses of ICS
| Fluticasone Propionate (n=14,518) | Budesonide (n= 14,518) | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| ICS cumulative dose (μg) | |||||
| Mean (SD) | 185,065 (524,205) | 141,148 (318,030) | |||
| 0–15,000 | 4801 | 33.07 | 4944 | 34.05 | |
| 15,001–36,000 | 2354 | 16.21 | 2935 | 20.22 | |
| 36,001–135,000 | 3625 | 24.97 | 3160 | 21.77 | |
| >135,000 | 3738 | 25.75 | 3479 | 23.96 | |
Incidence and Risk of Pneumonia
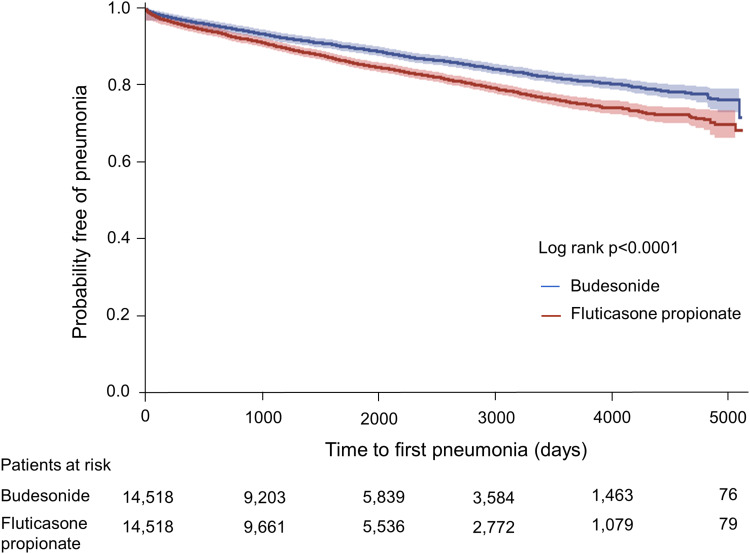
Pneumonia was significantly more common in those treated with fluticasone propionate than in those treated with budesonide for an observational period of approximately 13 years (p < 0.0001) (Figure 2). For fluticasone propionate users, the total number of pneumonia cases was 2,064 and the incidence rate was 2,914.77 per 100,000 person-years. For budesonide users, there were 1548 pneumonia cases and the incidence rate was 2,102.90 per 100,000 person-years. The incidence of pneumonia increased with higher cumulative ICS doses in both groups. The incidence rates of pneumonia were 2,138.25 (Q1), 2,737.23 (Q2), 3,139.52 (Q3), and 3,625.99 (Q4) for fluticasone propionate users, while those of budesonide users were 1,957.91 (Q1), 1,917.65 (Q2), 2,021.29 (Q3), and 2,434.71 (Q4) (Table 3).
Figure 2.
The probability free of pneumonia over time in patients treated with different types of ICSs.
Table 3.
Crude Incidence Rate of Pneumonia According to the ICS Type and Dose
| Variables | Fluticasone Propionate | Budesonide | ||||
|---|---|---|---|---|---|---|
| Person-Year | Pneumonia Cases | Incidence Rate (per 100,000) | Person-Year | Pneumonia Cases | Incidence Rate (per 100,000) | |
| Total | 70,811.79 | 2064 | 2,914.77 | 73,612.70 | 1548 | 2,102.90 |
| ICS cumulative dose (μg) | ||||||
| 0–15,000 | 21,606.49 | 462 | 2,138.25 | 22,421.82 | 439 | 1,957.91 |
| 15,001–36,000 | 10,996.52 | 301 | 2,737.23 | 14,340.45 | 275 | 1,917.65 |
| 36,001–135,000 | 17,359.32 | 545 | 3,139.52 | 15,287.24 | 309 | 2,021.29 |
| >135,000 | 20,849.45 | 756 | 3,625.99 | 21,563.18 | 525 | 2,434.71 |
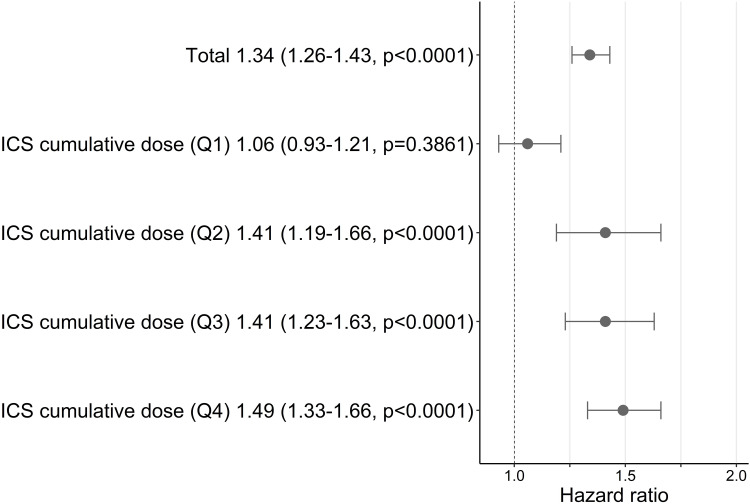
Fluticasone propionate was associated with increased risk of pneumonia compared to budesonide (HR 1.34, 95% CI 1.26–1.43, p < 0.0001) (Supplementary Table S1). Additional analyses were conducted to compare the risk of pneumonia with identical ICS doses (Supplementary Table S2). The risk of pneumonia for fluticasone propionate increased with higher ICS cumulative doses compared to budesonide: the HRs were 1.06 (0.93–1.21) (Q1), 1.41 (1.19–1.66) (Q2), 1.41 (1.23–1.63) (Q3), and 1.49 (1.33–1.66) (Q4) (Figure 3).
Figure 3.
Risk of pneumonia in fluticasone propionate users compared with budesonide users. Hazard ratios were calculated as the ratio of the risk of pneumonia in fluticasone propionate compared to budesonide (reference) within identical doses and presented with 95% CIs and p value.
Discussion
In the comparison of the risk of pneumonia, fluticasone propionate was significantly more associated with pneumonia than budesonide in patients with COPD. The development of pneumonia increased with higher cumulative ICS doses in both fluticasone propionate and budesonide users. In the comparison of identical ICS doses, the risk of pneumonia increased with higher ICS cumulative doses in those treated with fluticasone propionate compared to those treated with budesonide.
ICSs have been found to cause systemic side effects and pulmonary complications. Among them, pneumonia is a well-known pulmonary complication that has been demonstrated in all study designs, including randomized controlled-trials (RCTs), population-based case-control studies, cohort-studies, and meta-analyses.16 In recent two meta-analyses, ICSs significantly increased the risk of pneumonia with the relative risk of 1.43 (1.34–1.53) and 1.59 (1.33–1.90).17,18 In observational studies, the relative risk of pneumonia from ICS use ranged from 1.11 (1.05–1.18) to 3.26 (1.07–9.98).8 On the other hand, the differences in the risk of pneumonia among the different types of ICSs were controversial. In meta-analyses, fluticasone propionate was highly associated with pneumonia, with a relative risk of pneumonia ranging from 1.43 (1.13–1.81) to 1.84 (1.47–2.30); in contrast, budesonide was not found to be associated with pneumonia.10,17–22 However, these studies consisted of RCTs with indirect comparison between ICSs and LABA or placebo. In addition, the other meta-analysis with indirect comparisons did not find a difference in the risk of pneumonia between fluticasone propionate and budesonide.9 In a recent meta-analysis including one RCT (fluticasone furoate) and five observational cohort studies (fluticasone propionate) with direct comparisons between fluticasone and budesonide, an increased risk of pneumonia was associated with fluticasone, with a relative risk of 1.14 (1.09–1.20) for serious pneumonia.23 Thus, the observed increased risk of pneumonia with fluticasone propionate administration compared to budesonide that was found in our study is consistent with the results of previous observational studies. Although RCTs demonstrating different risks of pneumonia through head-to-head comparisons are still sparse, our observational study with a large number of subjects and a long-term observational period consolidates the evidence for the risk of pneumonia between fluticasone propionate and budesonide. Moreover, in a prospective population-based cohort study, the annual incidence of hospitalized patients with community-acquired pneumonia was approximately 10-fold higher in patients with COPD than in the general population: 634 per 100,000 adults in the general population and 5832 per 100,000 in patients with COPD.24 Considering the higher risk of pneumonia in COPD and overall study results regarding ICSs, physicians need to pay close attention to ICSs themselves as well as the types of ICSs at the start of ICS treatment.
The different risk of pneumonia between fluticasone propionate and budesonide may be explained by the pharmacokinetic and pharmacodynamic properties of each ICS component, influencing local immunity against bacterial infection. First, ICS particles are dissolved in the mucosal lining fluid and then absorbed into the airway tissue.25 Dissolution is determined by its aqueous solubility or lipophilicity. Fluticasone propionate is highly lipophilic, whereas budesonide is less lipophilic. In an in vitro study examining the dissolution rate of ICSs, budesonide dissolved quickly in human bronchial fluid (6 min), while fluticasone propionate dissolved very slowly (>8 h).26 In a human study, patients with severe COPD received treatment with budesonide/formoterol or fluticasone propionate/salmeterol, and ICS concentration was measured in spontaneously expectorated sputum.27 Most of the budesonide was recovered within the first 2 h, whereas the fluticasone propionate in the expectorated sputum continuously increased over 6 h. Although budesonide has less lipophilic and rapid absorption properties, it also acts for a long duration, similar to highly lipophilic fluticasone propionate. Because budesonide conjugates with the fatty acids in the airway tissues, it becomes highly lipophilic.28,29 Activated human bronchial epithelial cells and alveolar macrophages in response to swine dust or lipopolysaccharides release pro-inflammatory cytokines such as interleukin-6 (IL-6), IL-8, and tumor necrosis factor-α. ICSs were found to inhibit the release of pro-inflammatory cytokines from bronchial epithelial cells and alveolar macrophages. In this regard, fluticasone propionate had an approximately 10 times potent immunosuppressive effect than budesonide.30 In addition, fluticasone propionate had a 10–80 times greater inhibitory effect than budesonide on the expression of cell surface adhesion molecules such as E-selectin and vascular cell adhesion molecule-1, which are related to the recruitment of leukocytes.31,32 Collectively, budesonide’s rapid absorption to airway tissues, conjugation with fatty acids, and less potent immunosuppressive effects may contribute to its associated decreased risk of pneumonia in COPD.
Our study had several strengths. First, this is a population-based cohort study with a large number of study subjects and a long-term follow-up duration of approximately 13 years. To our knowledge, our study results are based on a large number of populations, compared to previous observational studies describing the risk of pneumonia with direct comparisons between fluticasone propionate and budesonide.23 Second, a dose-response risk of pneumonia with ICSs was demonstrated. Studies on different risks according to cumulative ICS doses are sparse. Previous observational studies did not describe the different risk of pneumonia according to ICS doses.23 On the contrary, we found that the risk of pneumonia increased more with higher cumulative doses of fluticasone propionate compared to budesonide through analyses within identical ICS cumulative doses.
This study has several limitations. First, pneumonia was defined as the first hospitalization with a diagnosis of pneumonia and diagnosis of pneumonia in outpatient settings was not included as a pneumonia case. As radiologically proven pneumonia was not included as a pneumonia case recruitment, the incidence of pneumonia in this study might not reflect the exact statistics of pneumonia. However, in the validation study of claims-based pneumonia diagnosis, the diagnostic accuracy estimated as positive predictive value was overall 80% and greater in inpatient hospitalization compared to outpatient visit (87.6% versus 73.4%).33 Second, there might be confounding factors that interfered with the comparison between fluticasone propionate and budesonide. Lung function and COPD exacerbation which could both increase the risk of pneumonia were not included in the baseline characteristics of study subjects. In addition, actual intake of the ICS which could be a possible source of difference in the risk of pneumonia were unknown. Instead, we collected data on OCS prescriptions as a surrogate of COPD exacerbation to adjust the severity of COPD at baseline. Moreover, comorbidities and bronchodilator usage were also adjusted through propensity score matching and multivariate analysis for a more exact comparison of the risk of pneumonia between the two groups. Third, potential influence of eosinophils on the risk of pneumonia was not reported. After treatment with fluticasone propionate for 1 year in patients with stable COPD, sputum bacterial load increased only in patients with lower baseline sputum or blood eosinophils (<2%).34 In the observational cohort study, blood eosinophils less than 100/μL was found to be an independent factor associated with the risk of pneumonia.35 Interaction between eosinophils and risk of pneumonia should be thoroughly investigated in large number of patients with COPD stratified by blood or sputum eosinophil count.
Conclusion
Fluticasone propionate is associated with an increased risk of pneumonia compared to budesonide in patients with COPD. In addition, the risk of pneumonia increased at higher ICS doses. Therefore, the types of ICSs and ICS doses need to be carefully considered in the treatment of COPD with ICS.
Funding Statement
There was no funding for this research.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Kim C, Kim Y, Yang DW, et al. Direct and indirect costs of chronic obstructive pulmonary disease in Korea. Tuberc Respir Dis. 2019;82(1):27–34. doi: 10.4046/trd.2018.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matera MG, Page CP, Calzetta L, et al. Pharmacology and therapeutics of bronchodilators revisited. Pharmacol Rev. 2020;72(1):218–252. doi: 10.1124/pr.119.018150 [DOI] [PubMed] [Google Scholar]
- 3.Singh D. Blood eosinophil counts in chronic obstructive pulmonary disease: a biomarker of inhaled corticosteroid effects. Tuberc Respir Dis. 2020;83(3):185–194. doi: 10.4046/trd.2020.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 5.Vanfleteren L, Fabbri LM, Papi A, et al. Triple therapy (ICS/LABA/LAMA) in COPD: time for a reappraisal. Int J Chron Obstruct Pulmon Dis. 2018;13:3971–3981. doi: 10.2147/COPD.S185975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matera MG, Cardaci V, Cazzola M, et al. Safety of inhaled corticosteroids for treating chronic obstructive pulmonary disease. Expert Opin Drug Saf. 2015;14(4):533–541. doi: 10.1517/14740338.2015.1001363 [DOI] [PubMed] [Google Scholar]
- 7.Malo de Molina R, Mortensen EM, Restrepo MI, et al. Inhaled corticosteroid use is associated with lower mortality for subjects with COPD and hospitalised with pneumonia. Eur Respir J. 2010;36(4):751–757. doi: 10.1183/09031936.00077509 [DOI] [PubMed] [Google Scholar]
- 8.Finney L, Berry M, Singanayagam A, et al. Inhaled corticosteroids and pneumonia in chronic obstructive pulmonary disease. Lancet Respir Med. 2014;2(11):919–932. doi: 10.1016/S2213-2600(14)70169-9 [DOI] [PubMed] [Google Scholar]
- 9.Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(3):Cd010115. doi: 10.1002/14651858.CD010115.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halpin DM, Gray J, Edwards SJ, et al. Budesonide/formoterol vs. salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomised controlled trials. Int J Clin Pract. 2011;65(7):764–774. doi: 10.1111/j.1742-1241.2011.02685.x [DOI] [PubMed] [Google Scholar]
- 11.Sehl J, O’Doherty J, O’Connor R, et al. Adherence to COPD management guidelines in general practice? A review of the literature. Ir J Med Sci. 2018;187(2):403–407. doi: 10.1007/s11845-017-1651-7 [DOI] [PubMed] [Google Scholar]
- 12.Palmiotti GA, Lacedonia D, Liotino V, et al. Adherence to GOLD guidelines in real-life COPD management in the Puglia region of Italy. Int J Chron Obstruct Pulmon Dis. 2018;13:2455–2462. doi: 10.2147/COPD.S157779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song SO, Jung CH, Song YD, et al. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab. 2014;38(5):395–403. doi: 10.4093/dmj.2014.38.5.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suissa S, Patenaude V, Lapi F, et al. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029–1036. doi: 10.1136/thoraxjnl-2012-202872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CH, Kim K, Hyun MK, et al. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax. 2013;68(12):1105–1113. doi: 10.1136/thoraxjnl-2012-203175 [DOI] [PubMed] [Google Scholar]
- 16.Avdeev S, Aisanov Z, Arkhipov V, et al. Withdrawal of inhaled corticosteroids in COPD patients: rationale and algorithms. Int J Chron Obstruct Pulmon Dis. 2019;14:1267–1280. doi: 10.2147/COPD.S207775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang M, Du Y, Chen H, et al. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Int Immunopharmacol. 2019;77:105950. doi: 10.1016/j.intimp.2019.105950 [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Sun J, Huang Q, et al. Inhaled corticosteroids and the pneumonia risk in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Front Pharmacol. 2021;12:691621. doi: 10.3389/fphar.2021.691621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S, Loke YK. Risk of pneumonia associated with long-term use of inhaled corticosteroids in chronic obstructive pulmonary disease: a critical review and update. Curr Opin Pulm Med. 2010;16(2):118–122. doi: 10.1097/MCP.0b013e328334c085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spencer S, Karner C, Cates CJ, et al. Inhaled corticosteroids versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;2011(12):Cd007033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012(9):Cd006829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nannini LJ, Poole P, Milan SJ, et al. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;2013(11):Cd003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodise TP, Li J, Gandhi HN, et al. Intraclass difference in pneumonia risk with fluticasone and budesonide in COPD: a systematic review of evidence from direct-comparison studies. Int J Chron Obstruct Pulmon Dis. 2020;15:2889–2900. doi: 10.2147/COPD.S269637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806–1812. doi: 10.1093/cid/cix647 [DOI] [PubMed] [Google Scholar]
- 25.Janson C, Stratelis G, Miller-Larsson A, et al. Scientific rationale for the possible inhaled corticosteroid intraclass difference in the risk of pneumonia in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3055–3064. doi: 10.2147/COPD.S143656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson M. Pharmacodynamics and pharmacokinetics of inhaled glucocorticoids. J Allergy Clin Immunol. 1996;97(1 Pt 2):169–176. doi: 10.1016/S0091-6749(96)80217-X [DOI] [PubMed] [Google Scholar]
- 27.Dalby C, Polanowski T, Larsson T, et al. The bioavailability and airway clearance of the steroid component of budesonide/formoterol and salmeterol/fluticasone after inhaled administration in patients with COPD and healthy subjects: a randomized controlled trial. Respir Res. 2009;10(1):104. doi: 10.1186/1465-9921-10-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brattsand R, Miller-Larsson A. The role of intracellular esterification in budesonide once-daily dosing and airway selectivity. Clin Ther. 2003;25(SupplC):C28–41. doi: 10.1016/S0149-2918(03)80304-1 [DOI] [PubMed] [Google Scholar]
- 29.van den Brink KI, Boorsma M, Staal-van den Brekel AJ, et al. Evidence of the in vivo esterification of budesonide in human airways. Br J Clin Pharmacol. 2008;66(1):27–35. doi: 10.1111/j.1365-2125.2008.03164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ek A, Larsson K, Siljerud S, et al. Fluticasone and budesonide inhibit cytokine release in human lung epithelial cells and alveolar macrophages. Allergy. 1999;54(7):691–699. doi: 10.1034/j.1398-9995.1999.00087.x [DOI] [PubMed] [Google Scholar]
- 31.Ray KP, Farrow S, Daly M, et al. Induction of the E-selectin promoter by interleukin 1 and tumour necrosis factor alpha, and inhibition by glucocorticoids. Biochem J. 1997;328(Pt 2):707–715. doi: 10.1042/bj3280707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atsuta J, Plitt J, Bochner BS, et al. Inhibition of VCAM-1 expression in human bronchial epithelial cells by glucocorticoids. Am J Respir Cell Mol Biol. 1999;20(4):643–650. doi: 10.1165/ajrcmb.20.4.3265 [DOI] [PubMed] [Google Scholar]
- 33.Kern DM, Davis J, Williams SA, et al. Validation of an administrative claims-based diagnostic code for pneumonia in a US-based commercially insured COPD population. Int J Chron Obstruct Pulmon Dis. 2015;10:1417–1425. doi: 10.2147/COPD.S83135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Contoli M, Pauletti A, Rossi MR, et al. Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD. Eur Respir J. 2017;50(4):1700451. doi: 10.1183/13993003.00451-2017 [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Garcia MA, Faner R, Oscullo G, et al. Inhaled steroids, circulating eosinophils, chronic airway infection, and pneumonia risk in chronic obstructive pulmonary disease. a network analysis. Am J Respir Crit Care Med. 2020;201(9):1078–1085. doi: 10.1164/rccm.201908-1550OC [DOI] [PubMed] [Google Scholar]