Abstract
Purpose:
We examined the association of Stroke Belt birth state with late-life cognition in The Study of Healthy Aging in African Americans (STAR).
Methods:
STAR enrolled 764 Black Americans ages 50+ who were long-term Kaiser Permanente Northern California members. Participants completed Multiphasic Health Check-ups (MHC;1964–1985) where early-life overweight/obesity, hypertension, diabetes, and hyperlipidemia were measured. At STAR (2018), birth state, self-reported early-life socioeconomic status (SES), and executive function, verbal episodic memory, and semantic memory scores were collected. We used linear regression to examine the association between Stroke Belt birth and late-life cognition adjusting for birth year, gender, and parental education. We evaluated early-life SES and cardiovascular risk factors (CVRF) as potential mechanisms.
Results:
Twenty-seven percent of participants were born in the Stroke Belt with a mean age of 69(SD=9) at STAR. Stroke Belt birth was associated with worse late-life executive function (β(95% CI):−0.18(−0.33,−0.02)) and semantic memory (−0.37(−0.53, −0.21)), but not verbal episodic memory (−0.04(−0.20, 0.12)). Adjustment for SES and CVRF attenuated associations of Stroke Belt birth with cognition (executive function (−0.05(−0.25, 0.14)); semantic memory (−0.28(−0.49, −0.07))).
Conclusion:
Black Americans born in the Stroke Belt had worse late-life cognition than those born elsewhere, underscoring the importance of early-life exposures on brain health.
Keywords: Dementia, Minority Health, Health Status Disparities, Cardiovascular Diseases, Heart Disease Risk Factors, Socioeconomic Status, Cognitive Dysfunction, Alzheimer Disease, Cerebrovascular Disease, Social Determinants of Health
Introduction:
Immense geographic disparities in health outcomes across the United States have persisted for decades.1,2 The Southeastern U.S. is a region colloquially known as the “Stroke Belt” for its disproportionately high rates of stroke and cardiovascular disease morbidity and mortality.2–4 This geographic health disparity is particularly relevant for Black Americans who represent 26% of the Stroke Belt population and 10% of the population of the rest of the U.S.2 Studies suggest that the poor health outcomes in this region are driven, in part, by socioeconomic status (SES) and cardiovascular risk factors (CVRF).5,6
Due to historical and on-going de jure and de facto racism, Black Americans are more likely to experience lower income, poorer education quality, lower educational attainment, and higher levels of unemployment compared to White Americans, and these inequalities are amplified in the Stroke Belt.7,8 Low SES is associated with a number of poor health outcomes including elevated risk of cognitive impairment and dementia.9,10 In addition, Black Americans have higher prevalence of CVRF compared to other racial/ethnic groups; almost half of Black adults ages 20 and older are obese, 42% are hypertensive, and 20% have diabetes.11–16 These common CVRF, typically developed in midlife, are associated with elevated risk of late-life cognitive impairment and dementia.17–20 The higher prevalence of low SES and CVRF among Black Americans may contribute to the disproportionate burden of dementia in this population, particularly among those living in the Stroke Belt.4,17–19,21
Stroke Belt birth is associated with increased risk of dementia and dementia-related mortality in Black Americans, even among individuals who were born in the region but have since moved elsewhere.4,22 However, the extent to which Stroke Belt birth is associated with late-life cognition after adjustment for early-life SES and CVRF is less clear. Using data from the Study of Healthy Aging in African Americans (STAR) we aimed to characterize the association between Stroke Belt birth with late-life cognition before and after accounting for early-life SES and CVRF potentially on the causal pathway (Figure 1). We hypothesized that Black Americans born in the Stroke Belt would have lower late-life cognitive function compared to Black Americans born elsewhere. We hypothesized that those born in the Stroke Belt would have a higher prevalence of low SES and CVRF, and that adjusting for these factors would attenuate the associations between birth region and cognition, suggesting mediation.
Figure 1.
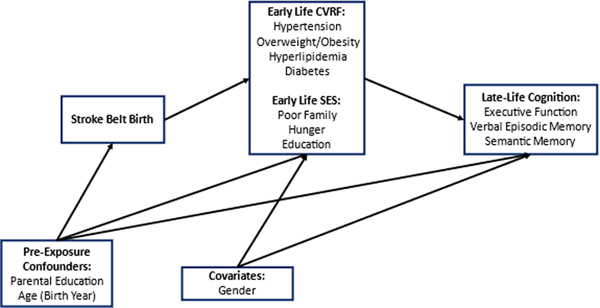
Directed acyclic graph showing the relationship between birth in the Stroke Belt with late-life cognition
Methods:
The STAR cohort includes community-dwelling older Black adults residing in the San Francisco Bay Area of California, primarily the cities of Oakland and Richmond. Individuals eligible for STAR were long-term members of Kaiser Permanente Northern California, an integrated healthcare delivery system, who identified as Black American, were age 50 years or older on January 1, 2018, and had previously participated in Kaiser Permanente Multiphasic Health Checkup (MHC) exams between 1964–1985. STAR wave 1 was conducted from 2018–2019. Stratified random sampling by age and education was used with the goal of recruiting approximately equal proportions of participants ages 50–64 and 65 and older (range 53–95 years). Exclusion criteria included electronic medical record diagnosis of dementia or other neurodegenerative diseases (frontotemporal dementia, Lewy body disease, Pick’s disease, Parkinson’s disease with dementia, Huntington’s disease) and presence of health conditions that would impede participation in study interviews (defined by hospice activity in the past 12 months, history of severe chronic obstructive pulmonary disease in the past 6 months, congestive heart failure hospitalizations in the past 6 months, and history of end stage renal disease or dialysis in the past 12 months). This study was conducted after IRB approval, and all participants provided informed consent.
Stroke Belt Birth:
State of birth was ascertained at STAR Wave 1 when participants were asked to self-report their place of birth. Individuals who reported being born in North Carolina, South Carolina, Georgia, Tennessee, Alabama, Mississippi, Arkansas, or Louisiana were classified as being born in the Stroke Belt. These eight states had an age-adjusted stroke mortality 10% above the national average from 1999 through 2016 (with the exception of Louisiana for one year)2,23 and have been used in prior literature to define the Stroke Belt, including in the Reasons for Geographic and Racial Differences in Stroke Study.24 Participants who reported being born in states outside of the eight listed above were identified as being born elsewhere (i.e., outside of the Stroke Belt) and foreign-born participants were excluded.
Cognitive Assessment:
Cognitive function was assessed using the Spanish and English Neuropsychological Assessment Scales (SENAS), a battery of cognitive tests that has undergone extensive development using item response theory methodology for valid comparisons of cognition and cognitive change across racial/ethnic and linguistically diverse groups.25–27 Three cognitive domains (executive function, verbal episodic memory, and semantic memory) were derived from the SENAS, and each domain was z-standardized using the mean and standard deviation from the full baseline sample. Executive function is a measure of reasoning and problem solving, verbal episodic memory measures recollection of events and personal experiences, and semantic memory measures memory of general facts, concepts, and ideas.28 Administration procedures, development, and psychometric characteristics have been described in detail elsewhere.25,26
Covariates:
MHC exams were conducted as part of routine care at KPNC and administered in five waves from 1964–1973, 1973–1977, 1977–1985, 1985–1992, and 1992–1996. Only data from each participant’s first MHC were available for this analysis. All first visits fell during the first three MHC waves (1964–1985). Data collection included blood and urine samples, physical measurements (such as weight and height), and questionnaires about lifestyle, health behaviors, and health history. These data were used to ascertain young adulthood (mean age = 27 (SD = 7)) CVRF. Hypertension (defined as a systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, self-report of high blood pressure diagnosis, or self-report of antihypertensive medication use), overweight/obesity (defined as a body mass index of ≥ 25 (kg/m2)), hyperlipidemia (defined as a fasting serum total cholesterol ≥ 200 mg/dL), and diabetes (defined as a fasting serum glucose ≥ 126 mg/dL or a non-fasting serum glucose of ≥ 200 mg/dL, self-report of a diabetes, or self-report of insulin use) were measured by clinical staff using standard methods and dichotomized (yes/no).A categorical CVRF variable based on the number of risk factors each participant had at their first MHC was created with categories of 0 risk factors, 1 risk factor, or 2+ risk factors.
At STAR wave 1, participants were asked about conditions in their childhood that were used to ascertain early-life SES. Participants were asked whether from birth to age 16 they would say their family was financially well off, average, or poor. Responses were dichotomized as poor childhood vs. average/well-off childhood. Participants were asked how often they had to skip a meal or go hungry while growing up and responses were dichotomized as never (no childhood hunger) vs. any other response (rarely, sometimes, often, or very often). Participant educational attainment was reported as the last or highest level of school completed for credit and dichotomized as some college or less vs. college graduate or more. A categorical SES variable was created using the number of SES risk factors each participant reported. These risk factors included a financially poor childhood, experience of childhood hunger, and participant educational attainment and a composite score of 0, 1, or 2+ risk factors was determined.
We considered additional covariates as potential confounders including birth year (ascertained from medical records), gender (male or female based on self-report or medical records), parental education, and age at MHC. Parental education was defined as the highest level of education attained by each participant’s mother (or woman that raised them) or father (or man that raised them), whichever was higher or non-missing. A dichotomous variable was created to indicate parental education of high school or less versus more than high school.
Statistical Analysis:
After exclusions for missing cognitive test scores (n=5) and missing birth state (including those born outside of the United States; n=31), 728 STAR participants were in the analytic sample. Descriptive statistics were presented and stratified by birth region (Stroke Belt birth vs. born elsewhere). Using linear regression, we estimated the mean difference in cognitive test z-score between those born in the Stroke Belt compared to those born elsewhere. Z-scores were used so that test scores were standardized and comparable across the three cognitive domains. Cognitive domains were tested separately, and all models were adjusted for birth year, gender, and parental education. In recognition that SES factors and CVRF may be on the causal pathway between Stroke Belt birth and cognition, we first adjusted models for demographics (birth year, gender, parental education), then added adjustments for SES and CVRF composite scores (using 0 risk factors as the reference). We also tested individual SES factors and CVRF, though results were similar to models using composite measures and are presented as supplemental tables. Effect estimates and 95% confidence intervals were generated using bootstrapping with 10,000 replications due to SES factors and CVRF being potential mediators. Interactions between birth region and SES factors, CVRF, and composite SES and CVRF variables were tested; there were no statistically significant interactions (p > 0.05), so interaction terms were dropped from final models (results not shown). All models with CVRF variables were additionally adjusted for age at MHC (i.e., age when CVRF were assessed). Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results:
Of 728 participants, 27% (n=197) were born in a Stroke Belt State. Mean age at cognitive assessment was 69(SD=9) years with those born in the Stroke Belt being older (75 years (SD=8) versus 66 (SD=8)) (Table 1). Thirty-one percent of participants were male, which was similar across birth region groups. Fifty-one percent of those born in the Stroke Belt had parents with a high school education or less compared to 37% of participants born elsewhere. Resource poor childhood, childhood hunger, and having less than a college degree were more common among participants born in the Stroke Belt than those born elsewhere. Fifty-six percent of those born in the Stroke Belt had two or more SES risk factors compared to 40% of those born outside the Stroke Belt. Overweight/obesity, hypertension, hyperlipidemia, and diabetes were all more prevalent among those born in the Stroke Belt. Thirty-eight percent of participants born in the Stroke Belt had two or more CVRF compared to 21% of those born elsewhere. Stroke Belt born participants were more likely to have 2+ CVRF across levels of SES compared to those born elsewhere (Figure 2).
Table 1.
Participant baseline characteristics stratified by birth region
| Variables | Overall | Born in Stroke Belt | Born Elsewhere |
|---|---|---|---|
| n=728 | n = 197 | n = 531 | |
| Age at MHC * , years ± SD | 26.7 ± 7.3 | 32.0 ± 7.2 | 24.7 ± 6.3 |
| Age at STAR, years ± SD | 68.8 ± 8.8 | 75.3 ± 8.1 | 66.3 ± 7.7 |
| Male, % (n) | 30.5 (222) | 31.0 (61) | 30.3 (161) |
| Parental Education ≤ H.S., % (n) | 40.4 (294) | 50.8 (100) | 36.5 (194) |
| Socioeconomic Factors | |||
| Resource Poor Childhood, % (n) | 33.3 (242) | 43.7 (86) | 29.4 (156) |
| Childhood Hunger, % (n) | 8.8 (64) | 11.2 (22) | 7.9 (42) |
| College Graduate or more, % (n) | 35.2 (256) | 31.0 (61) | 36.7 (195) |
| Socioeconomic Composite | |||
| 0 Risk Factors, % (n) | 15.7 (111) | 9.0 (17) | 18.1 (94) |
| 1 Risk Factor, % (n) | 39.8 (282) | 34.9 (66) | 41.5 (216) |
| 2+ Risk Factors, % (n) | 44.6 (316) | 56.1 (106) | 40.4 (210) |
| Cardiovascular Risk Factors | |||
| Overweight/Obesity † , % (n) | 33.4 (243) | 41.1 (81) | 30.5 (162) |
| Hypertension ‡ , % (n) | 21.0 (152) | 25. 4 (50) | 19.3 (102) |
| Hypercholesteremia § , % (n) | 36.3 (258) | 47.6 (89) | 32.3 (169) |
| Diabetes ∥ , % (n) | 2.3 (17) | 5.1 (10) | 1.3 (7) |
| Cardiovascular Composite | |||
| 0 Risk Factors, % (n) | 38.1 (270) | 30.0 (56) | 41.1 (214) |
| 1 Risk Factor, % (n) | 36.4 (258) | 32.1 (60) | 38.0 (198) |
| 2+ Risk Factors, % (n) | 25.4 (180) | 38.0 (71) | 20.9 (109) |
Multiphasic Health Check-ups (MHC) conducted as part of routine care between 1964–1985
Overweight/Obesity defined as BMI of ≥ 25 (kg/m2)
Hypertension defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, self-report of high blood pressure diagnosis, or self-report of antihypertensive medication use
Hyperlipidemia defined as serum total cholesterol ≥ 200 mg/dL
Diabetes defined as defined as a fasting serum glucose ≥ 126 mg/dL or a non-fasting serum glucose of ≥ 200 mg/dL, self-report of diabetes diagnosis, or self-report of insulin medication use
Figure 2.
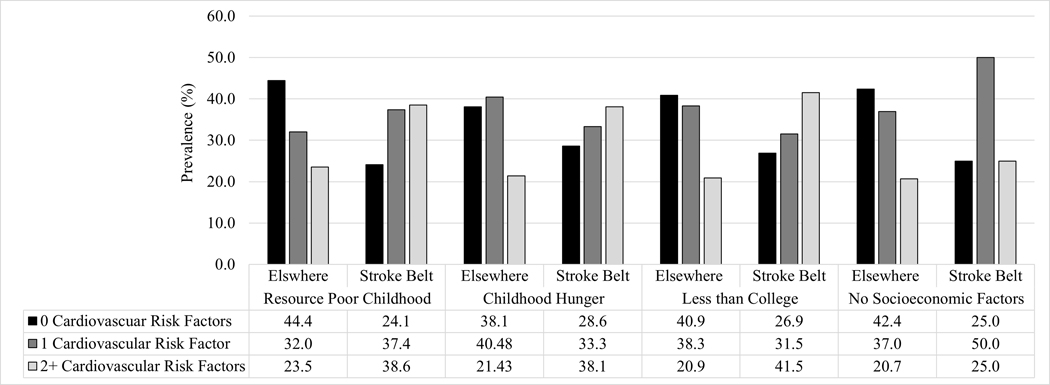
Prevalence of cardiovascular risk factors stratified by socioeconomic factors and birth region
Executive Function:
Stroke Belt birth was associated with a 0.18 lower (95% CI: −0.33, −0.02) executive function z-score compared to being born elsewhere after adjusting for birth year, gender, and parental education (Figure 3). Birth region explained 8% of the variance in executive function whereas potential confounders (birthyear, gender, and parental education) explained 26%, CVRF explained 7%, and childhood SES explained 12%. Compared to having 0 SES risk factors, having 1 SES factor (β (95% CI): −0.37 (−0.60, −0.14)) or 2+ SES factors (β (95% CI): −0.66 (−0.91, −0.41)) was associated with significantly worse late-life executive function (Table 2). Adjustment for SES attenuated the association between birth region and executive function slightly (β (95% CI): −0.16 (−0.35, 0.04)) such that the beta was no longer statistically significant. Compared to having 0 CVRF, late-life executive function did not significantly differ from those with 1 CVRF (β (95% CI): −0.04 (−0.22, 0.14)), but was associated with significantly worse executive function among those with 2+ CVRFs (β (95% CI): −0.27 (−0.47, −0.06)). Adjustment for number of CVRF attenuated the association between Stroke Belt birth and executive function (β (95% CI): −0.08 (−0.28, 0.12)) (Figure 3). The association between Stroke Belt birth and late-life executive function ((95% CI): −0.05 (−0.25, 0.14)) remained non-significant when adjusting for composite SES and CVRF concurrently. Similar attenuation was seen in the association of Stroke Belt birth and cognition when adjusting for individual SES and CVRF (Supplemental Table 1).
Figure 3.
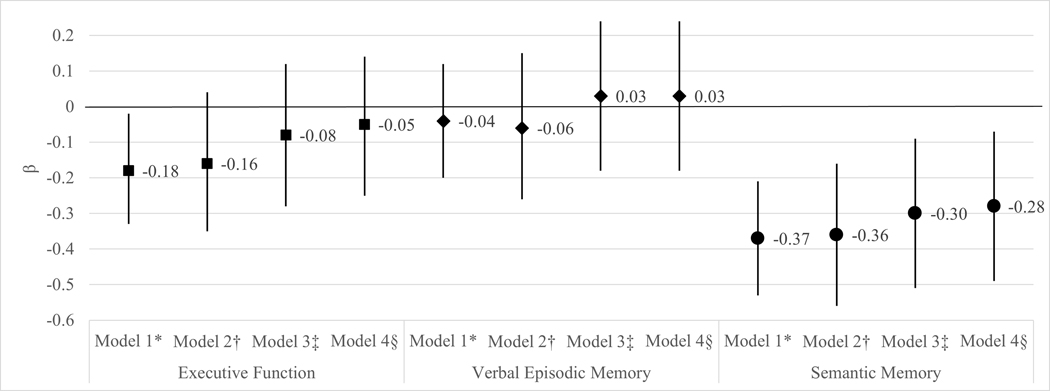
Linear regression of the mean difference (β (95% CI)) in late-life cognitive test z-score between those born in the Stroke Belt compared to those born elsewhere (reference)
*Model 1 adjusted for birth year, gender, and parental education
†Model 2 adjusted for Model 1 and SES composite
‡Model 3 adjusted for Model 1 and CVRF composite
§Model 4 adjusted for Model 1, SES composite, and CVRF composite
Table 2.
Linear regression of the mean difference (β (95% CI)) in late-life cognitive test z-score by composite socioeconomic and cardiovascular risk factors
| Executive Function | Verbal Episodic Memory | Semantic Memory | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Models* | Model 1† | Model 2‡ | Model 3§ | Model 1† | Model 2‡ | Model 3§ | Model 1† | Model 2‡ | Model 3§ |
| Born in Stroke Belt (ref: Born Elsewhere) | −0.16 (−0.35, 0.04) | −0.08 (−0.28, 0.12) | −0.05 (−0.25, 0.14) | −0.06 (−0.26, 0.15) | 0.03 (−0.18, 0.24) | 0.03 (−0.18, 0.24) | −0.36 (−0.56, −0.16) | −0.30 (−0.51, −0.09) | −0.28 (−0.49, −0.07) |
|
| |||||||||
| Socioeconomic Composite (ref: 0 Risk Factors) | |||||||||
|
| |||||||||
| 1 Risk Factor | −0.37 (−0.60, −0.14) | −0.45 (−0.68, −0.22) | −0.33 (−0.57, −0.09) | −0.37 (−0.62, −0.13) | −0.48(−0.72, −0.24) | −0.49 (−0.74, −0.24) | |||
|
|
|||||||||
| 2+ Risk Factors | −0.66 (−0.91, −0.41) | −0.72 (−0.97, −0.48) | −0.36 (−0.62, −0.09) | −0.38 (−0.65, −0.11) | −0.63 (−0.89, −0.37) | −0.61 (−0.87, −0.34) | |||
|
| |||||||||
| Cardiovascular Composite (ref: 0 Risk Factors) | |||||||||
|
| |||||||||
| 1 Risk Factor | −0.04 (−0.22, 0.14) | −0.04 (−0.22, 0.14) | 0.03 (−0.16, 0.21) | 0.00 (−0.19, 0.19) | −0.02 (−0.21, 0.17) | −0.02 (−0.21, 0.17) | |||
|
|
|||||||||
| 2+ Risk Factors | −0.27 (−0.47, −0.06) | −0.26 (−0.46, −0.05) | −0.26 (−0.48, −0.04) | −0.25 (−0.47, −0.03) | −0.26 (−0.48, −0.04) | −0.24 (−0.45, −0.02) | |||
All models adjusted for birth year, gender, and parental education
Model 1 adjusted for SES composite
Model 2 adjusted for CVRF composite
Model 3 adjusted for SES composite and CVRF composite
Verbal Episodic Memory:
Stroke Belt birth was not statistically significantly associated with verbal episodic memory (β (95% CI): −0.04 (−0.20, 0.12)) (Figure 3; Supplemental Table 2).
Semantic Memory:
Participants born in the Stroke Belt had a 0.37 lower (95% CI: −0.53, −0.21) semantic memory z-score compared to individuals born elsewhere after adjusting for covariates (Figure 3). Birth region explained 9% of the variance in semantic memory whereas potential confounders explained 16%, CVRF explained 3%, and childhood SES explained 7%. Compared to 0 SES risk factors, having 1 risk factor (β (95% CI): −0.48 (−0.72, −0.24)) or 2+ risk factors (β (95% CI): −0.63 (−0.89, −0.37)) was associated with significantly worse late-life semantic memory (Table 2). Compared to having no CVRF, semantic memory did not significantly differ from those with 1 CVRF (β (95% CI): −0.02 (−0.21, 0.17)) and was significantly lower in those with 2+ CVRF (β (95% CI): −0.26 (−0.48, −0.04)). The association of Stroke Belt birth with semantic memory was attenuated after adjustment for SES and CVRF (β (95% CI): −0.28 (−0.49, −0.07)) (Figure 3). Similar attenuation was seen in the association of Stroke Belt birth and cognition when adjusting for individual SES factors and CVRF (Supplemental Table 3).
Discussion:
In this all-Black cohort of older adults living in Northern California, we found that Stroke Belt birth was associated with significantly worse late-life executive function and semantic memory. Participants from the Stroke Belt were older, had lower educational attainment, and had lower parental educational attainment compared to those born elsewhere. Participants born in the Stroke Belt were also more likely to report having early-life low SES and CVRF. Adjustment for SES and CVRF attenuated the associations of Stroke Belt birth with executive function by 5.8% and semantic memory by 3.5%. CVRF appeared to have a stronger attenuating effect on birth region than SES. Early-life CVRF may be a stronger predictor of late-life brain health than early-life SES. However, these results should be interpreted with caution given the possibility of uncontrolled confounding and measurement error, particularly for SES. For executive function, adjustment for risk factors caused the association between birth region and cognition to become non-significant; the association with semantic memory, while attenuated, remained statistically significant. These findings suggest that while measured SES and CVRF may be on the causal pathway, they do not fully explain the association between Stroke Belt birth and cognition. Our results are consistent with previous work showing an association between birth or residence in a Stroke Belt state with late-life cognitive impairment, dementia, and dementia mortality even after adjustment for covariates including SES and CVRF.4,22,29–31
We found associations of Stroke Belt birth with cognitive domains of executive function and semantic memory, but not verbal episodic memory. While deficits in verbal episodic memory are often considered a hallmark of Alzheimer’s disease (AD), studies of individuals with preclinical AD have consistently shown subtle deficits in executive function and semantic memory.32 Further, semantic memory represents “world knowledge” and is developed through abstraction of experiences and encounters.33,34 This domain is heavily influenced by childhood experiences and environment which could explain why the relationship between Stroke Belt birth and late-life semantic memory was only slightly attenuated by adjustments for SES and CVRF.34 Understanding the health contributions of birth in the Stroke Belt on cognition is particularly important for older Black Americans who are now reaching late-life and at elevated risk of Alzheimer’s disease and related dementias (ADRD).35 Many Black Americans who were born in the Stroke Belt over the course of the 20th century have since moved elsewhere as part of the Great Migration (1910–1970).36 Understanding how residential history influences cognitive aging may help in identifying higher risk populations as well as geographic differences in risk factors that contribute to racial/ethnic health disparities.37 The Stroke Belt region has had the greatest proportion of residents living in poverty throughout U.S. history with 17.5% living in poverty in 1971 compared to 9.5%, 10.3%, and 11.4% in the Northeast, Midwest, and West, respectively.38 While this trend is narrowing, higher rates of poverty in the South have persisted over the last 50 years.38 Poverty in early life is associated with low birthweight39, poor nutrition40, poor education quality and lower educational attainment41, environmental toxicants such as lead42, as well as chronic stress,43 all of which are associated with poor cognitive development and function.44 In addition to higher rates of poverty in the Stroke Belt, the region has elevated prevalence of cardiovascular risk factors and disease including overweight/obesity, hypertension, diabetes, and coronary heart disease, which disproportionately affect Black residents.2,6,11 The association between midlife CVRF and cognitive impairment and dementia is well documented.17,19,21 Our analysis suggests that even when developed in childhood and early adulthood, CVRF have a negative impact on late-life cognitive function.37
STAR participants lived the majority of their lives in Northern California with most moving to the region between 1960–1980. This suggests there are likely additional factors influencing the relationship between birth region and cognition that need to be identified. Education is strongly linked to cognitive outcomes and higher levels of educational attainment are believed to enhance cognitive reserve and resilience.45 Several studies have shown that mandatory schooling46, school desegregation31,47, and school quality41 are associated with better late-life cognitive function. The impact of these policies is heavily influenced by secular and geographic trends, and they should be investigated as potential mediators of the relationship between Stroke Belt birth with cognitive aging. Additionally, geographic differences in the relationship between lifestyle and environmental factors with cognitive function are understudied and may influence the relationship between birth region and cognition.2,48,49 Studies have shown associations of dietary patterns50 and air pollution51 with cognitive impairment and dementia, but further research with emphasis on geographic differences is needed.
This work has several strengths including measures of early-life risk factors, a cohort whose participants had equal access to healthcare, and a comprehensive and sensitive measure of cognition using the SENAS. Limitations include an inability to ascertain when participants moved from the Stroke Belt to Northern California (where all participants resided by the 1980s). We are unable to assess whether time spent living in the Stroke Belt had a cumulative effect on cognition. Effect estimates of the association between birth region and cognition may be susceptible to selection bias due to differences between Black participants born in the Stroke Belt who were able to leave (eventually residing in Northern California) and those without an opportunity or desire to move elsewhere. This may cause underestimation of the effect of Stroke Belt birth on late-life cognition if individuals who moved away from the region were healthier, had higher SES, or other advantages associated with better late-life cognitive function.52 In addition, there is limited generalizability to Black populations that moved from the Stroke Belt to other parts of the United States. We were limited to assessment of cognition at one time point. Consequently, we measured current cognition and did not directly measure cognitive impairment, which entails a decline from a previous level of cognitive function. Thus, we cannot determine whether Stroke Belt birth is associated with cognitive decline or dementia, though previous studies suggest that may be the case.4,22 Finally, our assessment of early life SES was based on self-report in late-life and limited to a small number of factors. We cannot account for potential information and measurement bias in measures of early life SES and CVRF, nor the possibility of residual confounding.
Structural racism is a key determinant of health.53 Black Americans have higher rates of cognitive impairment and dementia and are more likely to experience low SES and CVRF.2,7,11,35 Geographic differences in these exposures and outcomes further contribute to racial/ethnic health disparities, but geographic contributions are not fully understood and often overlooked. Public health initiatives to improve cognitive outcomes in Black Americans must consider the role of geography on risk and risk factors. Future research is needed to continue to characterize the influence of geography on life course risk factors for dementia, particularly among older Black Americans many of whom were part of the Great Migration, one of the largest migrations of people in history.36 Epidemiologic studies of cognitive function and dementia have underscored the importance of assessing lifecourse risk factors, and our work further supports that conditions from birth through late-life contribute to late-life brain health.
Supplementary Material
Acknowledgements and Funding:
Dr. George was supported by the National Institutes of Health (NIH)/National Institute on Aging (NIA) Neuroscience of Cognitive Aging Training Grant (T32AG050061). This work was funded by the NIH/NIA under grant numbers RF1AG05078202 (PI: Whitmer) and R00AG053410 (PI: Mayeda).
Abbreviations:
- SES
Socioeconomic status
- CVRF
Cardiovascular risk factors
- STAR
Study of Health Aging in African Americans
- MHC
Multiphasic Health Check-ups
- SENAS
Spanish and English Neuropsychological Assessment Scales
References:
- 1.Murray CJ, Kulkarni SC, Michaud C, et al. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med. 2006;3(9):e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard G, Howard VJ. Twenty Years of Progress Toward Understanding the Stroke Belt. Stroke. 2020;51(3):742–750. [DOI] [PubMed] [Google Scholar]
- 3.Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, et al. Trends and Patterns of Geographic Variation in Cardiovascular Mortality Among US Counties, 1980–2014. JAMA. 2017;317(19):1976–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glymour MM, Kosheleva A, Wadley VG, Weiss C, Manly JJ. Geographic distribution of dementia mortality: elevated mortality rates for black and white Americans by place of birth. Alzheimer Dis Assoc Disord. 2011;25(3):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard G. Why do we have a stroke belt in the southeastern United States? A review of unlikely and uninvestigated potential causes. Am J Med Sci. 1999;317(3):160–167. [DOI] [PubMed] [Google Scholar]
- 6.Liao Y, Greenlund KJ, Croft JB, Keenan NL, Giles WH. Factors explaining excess stroke prevalence in the US Stroke Belt. Stroke. 2009;40(10):3336–3341. [DOI] [PubMed] [Google Scholar]
- 7.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol. 2016;35(4):407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard VJ, McClure LA, Kleindorfer DO, et al. Neighborhood socioeconomic index and stroke incidence in a national cohort of blacks and whites. Neurology. 2016;87(22):2340–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marden JR, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM. Contribution of Socioeconomic Status at 3 Life-Course Periods to Late-Life Memory Function and Decline: Early and Late Predictors of Dementia Risk. Am J Epidemiol. 2017;186(7):805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George KM, Lutsey PL, Kucharska-Newton A, et al. Life-Course Individual and Neighborhood Socioeconomic Status and Risk of Dementia in the Atherosclerosis Risk in Communities Neurocognitive Study. Am J Epidemiol. 2020;189(10):1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prevention CfDCa. Health, United States Spotlight: Racial and Ethnic Disparities in Heart Disease Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/hus/spotlight/HeartDiseaseSpotlight_2019_0404.pdf. Published 2019. Accessed.
- 12.Carnethon MR, Pu J, Howard G, et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation. 2017;136(21):e393–e423. [DOI] [PubMed] [Google Scholar]
- 13.Effoe VS, Carnethon MR, Echouffo-Tcheugui JB, et al. The American Heart Association Ideal Cardiovascular Health and Incident Type 2 Diabetes Mellitus Among Blacks: The Jackson Heart Study. J Am Heart Assoc. 2017;6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ommerborn MJ, Blackshear CT, Hickson DA, et al. Ideal Cardiovascular Health and Incident Cardiovascular Events: The Jackson Heart Study. Am J Prev Med. 2016;51(4):502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong ND, Zhao Y, Patel R, et al. Cardiovascular Risk Factor Targets and Cardiovascular Disease Event Risk in Diabetes: A Pooling Project of the Atherosclerosis Risk in Communities Study, Multi-Ethnic Study of Atherosclerosis, and Jackson Heart Study. Diabetes Care. 2016;39(5):668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xanthakis V, Sung JH, Samdarshi TE, et al. Relations between subclinical disease markers and type 2 diabetes, metabolic syndrome, and incident cardiovascular disease: the Jackson Heart Study. Diabetes Care. 2015;38(6):1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. [DOI] [PubMed] [Google Scholar]
- 18.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42–48. [DOI] [PubMed] [Google Scholar]
- 19.Knopman DS, Gottesman RF, Sharrett AR, et al. Midlife vascular risk factors and midlife cognitive status in relation to prevalence of mild cognitive impairment and dementia in later life: The Atherosclerosis Risk in Communities Study. Alzheimers Dement. 2018;14(11):1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66(10):1210–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 2014;10(5):562–570. [DOI] [PubMed] [Google Scholar]
- 22.Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Association Between Birth in a High Stroke Mortality State, Race, and Risk of Dementia. JAMA Neurol. 2017;74(9):1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karp DN, Wolff CS, Wiebe DJ, Branas CC, Carr BG, Mullen MT. Reassessing the Stroke Belt: Using Small Area Spatial Statistics to Identify Clusters of High Stroke Mortality in the United States. Stroke. 2016;47(7):1939–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. [DOI] [PubMed] [Google Scholar]
- 25.Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16(4):347–359. [DOI] [PubMed] [Google Scholar]
- 26.Mungas D, Reed BR, Haan MN, Gonzalez H. Spanish and English neuropsychological assessment scales: relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005;19(4):466–475. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez Saucedo H, Whitmer RA, Glymour M, et al. Measuring cognitive health in ethnically diverse older adults. J Gerontol B Psychol Sci Soc Sci. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey PD. Domains of cognition and their assessment Dialogues Clin Neurosci. 2019;21(3):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadley VG, Unverzagt FW, McGuire LC, et al. Incident cognitive impairment is elevated in the stroke belt: the REGARDS study. Ann Neurol. 2011;70(2):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thacker EL, Gillett SR, Wadley VG, et al. The American Heart Association Life’s Simple 7 and incident cognitive impairment: The REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2014;3(3):e000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamar M, Lerner AJ, James BD, et al. Relationship of Early-Life Residence and Educational Experience to Level and Change in Cognitive Functioning: Results of the Minority Aging Research Study. J Gerontol B Psychol Sci Soc Sci. 2020;75(7):e81–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer’s disease. J Intern Med. 2004;256(3):195–204. [DOI] [PubMed] [Google Scholar]
- 33.Kumar AA. Semantic memory: A review of methods, models, and current challenges. Psychon Bull Rev. 2021;28(1):40–80. [DOI] [PubMed] [Google Scholar]
- 34.Unger L, Vales C, Fisher AV. The Role of Co-Occurrence Statistics in Developing Semantic Knowledge. Cogn Sci. 2020;44(9):e12894. [DOI] [PubMed] [Google Scholar]
- 35.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leibbrand C, Massey C, Alexander JT, Genadek KR, Tolnay S. The Great Migration and Residential Segregation in American Cities during the Twentieth Century. Soc Sci Hist. 2020;44(1):19–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18(3):223–254. [DOI] [PubMed] [Google Scholar]
- 38.Chaudry A, Wimer C, Macartney S, et al. Poverty in the United States: 50-Year Trends and Safety Net Impacts. In: Services DoHaH, ed. March 2016 ed: Office or Human Services Policy; 2016:1–47. [Google Scholar]
- 39.Barker DJ. The fetal origins of coronary heart disease. Acta Paediatr Suppl. 1997;422:78–82. [DOI] [PubMed] [Google Scholar]
- 40.Olson CM. Nutrition and health outcomes associated with food insecurity and hunger. J Nutr. 1999;129(2S Suppl):521S–524S. [DOI] [PubMed] [Google Scholar]
- 41.Liu SY, Glymour MM, Zahodne LB, Weiss C, Manly JJ. Role of Place in Explaining Racial Heterogeneity in Cognitive Outcomes among Older Adults. J Int Neuropsychol Soc. 2015;21(9):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cecil KM, Brubaker CJ, Adler CM, et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5(5):e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proc Natl Acad Sci U S A. 2009;106(16):6545–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13(2):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glymour MM, Kawachi I, Jencks CS, Berkman LF. Does childhood schooling affect old age memory or mental status? Using state schooling laws as natural experiments. J Epidemiol Community Health. 2008;62(6):532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson RL, George KM, Barnes LL, et al. Timing of School Desegregation Experience and Late-Life Cognition in the Study of Healthy Aging in African Americans (STAR) Cohort. Alzheimer’s & Dementia. 2020;16(S10):e045354. [Google Scholar]
- 48.Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology. 2005;25(1):8–14. [DOI] [PubMed] [Google Scholar]
- 49.Wilson RS, Scherr PA, Bienias JL, et al. Socioeconomic characteristics of the community in childhood and cognition in old age. Exp Aging Res. 2005;31(4):393–407. [DOI] [PubMed] [Google Scholar]
- 50.Gustaw-Rothenberg K. Dietary patterns associated with Alzheimer’s disease: population based study. Int J Environ Res Public Health. 2009;6(4):1335–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. Air Pollution and Dementia: A Systematic Review. J Alzheimers Dis. 2019;70(s1):S145–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Black DA, Sanders SG, Taylor EJ, Taylor LJ. The Impact of the Great Migration on Mortality of African Americans: Evidence from the Deep South. Am Econ Rev. 2015;105(2):477–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


