Abstract
Objective:
Ketamine is an anesthetic drug associated with dissociation. Decreased electroencephalogram alpha (8 to 13 Hz) and low-beta (13 to 20 Hz) oscillation power have been associated with ketamine-induced dissociation. We aimed to characterize surface electroencephalogram signatures that may serve as biomarkers for dissociation.
Methods:
We analyzed data from a single-site, open-label, high-density surface electroencephalogram study of ketamine anesthesia (2mg/kg, n = 15). We assessed dissociation longitudinally using the Clinician Administered Dissociation States Scale (CADSS) and administered midazolam to attenuate dissociation and enable causal inference. We analyzed spectral power and global coherence with multitaper spectral methods. Mixed effects models were used to assess whether electroencephalogram power and global coherence signatures of ketamine could be developed into dissociation-specific biomarkers.
Results:
Compared to baseline, ketamine unresponsiveness was associated with increased frontal power between 0.5 to 9.3 Hz, 12.2 to 16.6 Hz, and 24.4 to 50 Hz. As subjects transitioned into a responsive but dissociated state (mean CADSS ± SD, 22.1 ± 17), there was a decrease in power between 0.5 to 10.3 Hz and 11.7 to 50 Hz. Midazolam reduced dissociation scores (14.3 ± 11.6) and decreased power between 4.4 to 11.7 Hz and an increase in power between 14.2 to 50 Hz. Our mixed-effects model demonstrated a quadratic relationship between time and CADSS scores. When models (frontal power, occipital power, global coherence) were reanalyzed with midazolam and electroencephalogram features as covariates, only midazolam was retained.
Conclusions:
Ketamine is associated with structured electroencephalogram power and global coherence signatures that may enable principled anesthetic state but not dissociation monitoring.
Significance:
A neurophysiological biomarker for dissociation may lead to a better understanding of neuropsychiatric disorders.
Keywords: Ketamine, dissociation, EEG, spectral, CADSS
1. Introduction
Ketamine is associated with altered arousal and awareness of self, environment, and reality, termed dissociation (Domino, 2010, Li and Vlisides, 2016, Nowacka and Borczyk, 2019). Ketamine is also associated with acute analgesia, antihyperalgesia, and modulation of opioid-mediated analgesia (Heifets, 2020, Persson, 2013). In the United States, ketamine is typically administered as part of a balanced general anesthetic regimen to enhance post-operative analgesia. At high doses, ketamine is sometimes used to induce a state of general anesthesia (Akeju and Brown, 2017, Akeju et al., 2016b, Domino, 2010, Li and Vlisides, 2016). However, the dissociative properties of ketamine have largely limited its widespread use as an analgesic or general anesthetic medication (Gitlin et al., 2020).
Anesthetic drugs such as ketamine significantly modulate neural circuits to produce unconsciousness, amnesia, anti-nociception, and immobility (Akeju and Brown, 2017, Brown et al., 2011, Purdon et al., 2015). Drug modulation of anesthetic (Akeju and Brown, 2017, Akeju et al., 2016a, Akeju et al., 2016b, Akeju et al., 2014, Blain-Moraes et al., 2014, Chamadia et al., 2019, Duclos et al., 2021, Hayashi et al., 2007, Mashour et al., 2021, Pavone et al., 2017, Shortal et al., 2019), and potentially dissociation promoting neural circuits may be associated with unique electroencephalogram signatures. For example, the administration of ketamine as an anesthetic adjunct during steady-state general anesthesia maintained with a γ-aminobutyric acid modulating general anesthetic has been associated with decreased alpha and low-beta oscillation power (~8 to 20 Hz) and increased high-frequency oscillation power (Akeju et al., 2016a, Chamadia et al., 2019, Hayashi et al., 2007). Decreased alpha and low-beta oscillation power have also been associated with the dissociative state induced by subanesthetic ketamine (de la Salle et al., 2021, de la Salle et al., 2016, Vlisides et al., 2018). However, it is not clear whether these ketamine-induced surface electroencephalogram findings are specific to dissociation.
A neurophysiological biomarker for dissociation is expected to be of widespread clinical benefit. For example, it may be used to detect the onset of neuropsychiatric disorders like schizophrenia (O’Driscoll et al., 2014), panic disorders (Ball et al., 1997), and obsessive-compulsive disorder (Watson et al., 2004). Further, ketamine-induced dissociation was assessed using the Clinician Administered Dissociative States Scale (CADSS), and ketamine’s antidepressant response remains of interest (Ballard and Zarate, 2020). Therefore, we performed a single-site, open-label electroencephalogram study of ketamine anesthesia (n = 15). We assessed for dissociation longitudinally using the CADSS. The initial version of the CADSS consisted of 27 clinician-administered and observed items scored on a 0 to 4 Likert scale (Bremner et al., 1998, van Schalkwyk et al., 2018). Although objective cut points for dissociation were not defined per se, the CADSS enabled measurement of present-state dissociative symptoms, e.g., patients with post-traumatic distress syndrome had a mean CADSS of 21.9, which significantly increased to 35 after exposure to traumatic memories (Bremner et al., 1998). The version typically used for ketamine trials consists of 23 clinician-administered items also scored on a 0 to 4 Likert scale (van Schalkwyk et al., 2018). Because benzodiazepines are typically prescribed for attenuating ketamine-induced dissociation (Sener et al., 2011, Suzuki et al., 2000), we administered midazolam to enable causal inference. We hypothesized that surface electroencephalogram signatures of ketamine anesthesia might serve as biomarkers for dissociation.
2. Methods
2.1. Experimental protocol
The Partners Institutional Review Board approved this human research study (2018P000417, NCT03553758). All participants provided written informed consent. The electroencephalogram data analyzed in this manuscript have not previously been published.
Subject recruitment
As previously reported (Gitlin et al., 2020), this was a single-site, open-label study of ketamine anesthesia in 15 healthy subjects (8 males), age 24 ± 3.4 years, mean weight 70 ± 12 kg, and mean body mass index 23.5 ± 2.4 kg/m2. We conducted a complete medical history and a pre-anesthesia assessment in all subjects. The primary inclusion criterion for this study was meeting the American Society of Anesthesiology Physical Status I. Key exclusion criteria were pregnancy, personal or family history of anesthesia-related complications, suspected history of drug abuse, and neuropsychiatric diagnoses. We also performed a 12-lead electrocardiogram and the following screening laboratory tests: complete blood count, liver function, basic metabolic panel, urine toxicology, and urine pregnancy for females.
Study Design
Briefly and as previously reported (Gitlin et al., 2020), we performed urine toxicology and pregnancy testing immediately before administering ketamine. All subjects were required to avoid food and water intake for at least 8 hours before ketamine administration. We monitored blood pressure using a standard non-invasive pneumatic cuff, oxygen saturation using pulse oximetry, ventilation using capnography, and cardiac rhythm using a 5-lead electrocardiogram. We administered a bolus dose of intravenous ketamine (2mg/kg) to induce unresponsiveness and longitudinally acquired electroencephalogram and CADSS data. The airway was not instrumented, and all subjects breathed spontaneously throughout the study protocol without assistance. Supplemental oxygen was provided using a face mask. A board-certified anesthesiologist was present during all study procedures. Figure 1a illustrates the schematic of our study design.
Figure 1.
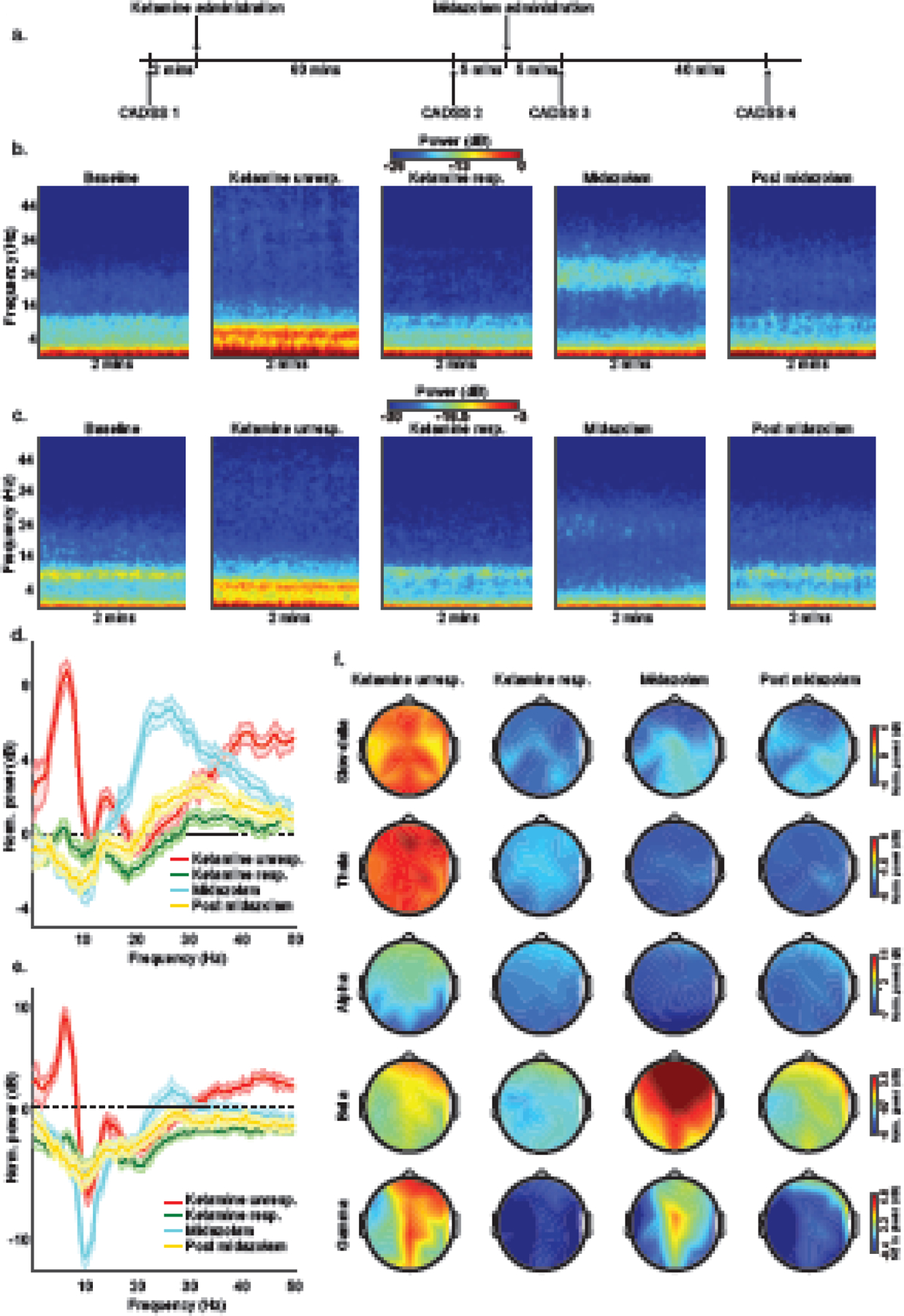
Schematic of the study protocol, spectrograms, spectral analyses, and spatial plots. (a) We acquired data using an open-label and single-site study design in (n = 15) healthy subjects. Subjects were induced to lose responsiveness with 2mg/kg of intravenous ketamine. We also administered 2mg of intravenous midazolam to attenuate ketamine-induced dissociation. We assessed dissociative symptoms using the Clinician Administered Dissociative States Scale (CADSS). Visual representation of group-level (b) frontal and (c) occipital spectrogram computed from 2 minutes EEG segments illustrates that ketamine induces structured electroencephalogram oscillations that may be used to monitor the anesthetic state. Baseline-normalized group-level spectral comparison of (d) frontal and (e) occipital channel with shaded regions representing 99% confidence interval bounds. (f) Baseline-normalized power spectral-spatial plots. dB = decibel; Hz = Hertz; CADSS = Clinician Administered Dissociative States Scale; unresp. = unresponsiveness; resp. = responsiveness; mins = minutes; Norm. = normalized.
2.2. Dissociation Assessments
We assessed dissociation using the CADSS, which was developed to measure perceptual, behavioral, and attentional alterations during dissociative experiences (Bremner et al., 1998). The CADSS questionnaire was administered by a trained clinical research coordinator (JG). As previously reported (Gitlin et al., 2020) the first CADSS assessment (baseline) occurred before the administration of ketamine. The second assessment occurred approximately 60min after the administration of ketamine. After the second assessment, we administered 2mg of midazolam to attenuate ketamine-associated dissociation. The third assessment occurred approximately 75min after the administration of ketamine (approximately 5min post midazolam). The fourth assessment occurred approximately 120min after the administration of ketamine. One subject did not perform assessments after recovery of responsiveness.
2.3. EEG acquisition and processing
We recorded eyes-closed high-density EEG signals using the Waveguard system with a standard EEG cap (64 channels, ANT Neuro, Netherlands) and electrode impedances of <5 kΩ. We acquired the EEG data at 512 Hz, down-sampled to 250 Hz for analysis, and remontaged using the nearest neighbor Laplacian referencing scheme. We averaged data from three channels to approximate frontal (Fz) and occipital (Pz) channel locations. Anesthetic drugs are associated with a transition from occipitally dominant awake-alpha oscillations to frontally dominant alpha and beta oscillations termed anteriorization. Therefore, we selected frontal and occipital channel locations to place our findings in context with anteriorization and previous neurophysiological studies. Five 2-min periods of interest were extracted: baseline (2 minutes before ketamine administration), ketamine unresponsiveness (4 minutes post ketamine), ketamine responsiveness (2 minutes before midazolam administration), midazolam (3 minutes post midazolam administration), and post midazolam (45 minutes post midazolam administration). We instructed all subjects to close their eyes throughout the data acquisition period to reduce blink and muscle artifacts.
We used the Chronux toolbox in MATLAB (MathWorks) to compute the multitaper spectral estimates. The spectral parameters were: TW or time-bandwidth product = 3, K or number of tapers = 5, and T or window size = 2 s with no overlapping windows for statistical inference. For visualization, spectrograms with overlapping windows were computed. We obtained a frequency vector for each subject by computing the median baseline spectral estimates for each frequency bin across time. We divided the spectral estimates obtained from the ketamine and midazolam states by this frequency vector to obtain our baseline normalized estimates. We computed the median of the spectral estimate across all subjects for group-level visualization. We summed the spectral estimates across frequency and weighted them by the frequency difference to obtain point estimates of spectral power in frequency bands of interest. We next obtained the median estimates across time. The topoplot function in EEGLab was used to generate spatial head plots. Global coherence was estimated using methods previously described (Chamadia et al., 2019). Briefly, we computed the cross-spectral matrix for every subject over every non-overlapping moving window using multitaper methods. Next, we computed the median of the imaginary and real components of the cross-spectral matrix. Finally, we computed the singular value decomposition of the cross-spectral matrix for each frequency and time window. We normalized the dominant singular value by the trace of the singular value matrix to obtain global coherence for a given frequency and time window. We obtained the median global coherence estimate across frequency and then time to obtain point estimates of global coherence in frequency bands of interest. Data from one subject during the ketamine unresponsiveness state and three subjects during the post midazolam state were excluded due to artifacts.
2.4. Statistical Methods
We employed an empirical bootstrap approach to enable statistical inferences for spectral power and global coherence. First, we bootstrapped the estimates of each non-overlapping window. Next, we computed a median of the bootstrapped estimates at the subject level and then computed the group median. We computed the median difference between groups and then iterated the above procedure 5,000 times to obtain a distribution of the median difference between groups. We computed the 99% Confidence interval of this distribution.
Associations between spectral power, global coherence, and CADSS were computed using Spearman’s correlation. We also ran a limited backward elimination mixed random and fixed effects longitudinal analysis (Locascio and Atri, 2011). The fixed covariate of interest was the quadratic effect of time (the lower order effect of time was also included). Random terms were subject intercepts and the interaction of subjects with time, which were allowed to be correlated. Separate models with the time-varying fixed binary covariate of pre-midazolam versus post-midazolam states were run for frontal power, occipital power, and global coherence. Our frequency bands of interest were: slow-delta [0.5 to 4 Hz], theta [4 to 8 Hz], alpha [8 to 13 Hz], low beta [13 to 20 Hz], high beta [12 to 30 Hz], and gamma [30 to 50 Hz]). An initial model including all the above terms was run, followed by a limited backward elimination model, excluding the least significant term sequentially until only significant terms remained or nonsignificant terms subsumed within significant higher-order terms. Residuals from all fit models were examined for conformance to assumptions of normality. JMP Pro 14 (Cary, NC, US) was the statistical software employed for all analyses. All p-values were computed based on the two-sided tests. P-values were not corrected for the number of models that were assessed. The significance level was set at 0.05.
3. Results
No serious adverse events were reported during this study.
Spectral analysis
Group-average frontal spectrograms, occipital spectrograms, baseline-normalized frontal spectra, baseline-normalized occipital spectra, and baseline-normalized spatial head plots are presented in Figure 1.
Frontal
We first directly compared ketamine responsiveness (dissociated) and midazolam (dissociation treated) states to baseline. Ketamine responsiveness was associated with decreased power between 13.7 to 23 Hz. Midazolam was associated with decreased power between 3.4 to 12.2 Hz and increased power between 17.6 to 50 Hz. These data are summarized in Supplementary Figure 1a.
Next, to enable inferences on the temporal evolution of electroencephalogram signatures, we compared electroencephalogram spectra across consecutive behavioral states: baseline versus ketamine unresponsiveness, ketamine unresponsiveness versus ketamine responsiveness, ketamine responsiveness versus midazolam, and midazolam versus post midazolam. Compared to baseline, ketamine unresponsiveness was associated with increased power between 0.5 to 9.3 Hz, 12.2 to 16.6 Hz, and 24.4 to 50 Hz. As subjects transitioned into being responsive, there was a decrease in power between 0.5 to 10.3 Hz and 11.7 to 50 Hz. Midazolam resulted in a further decrease in power between 4.4 to 11.7 Hz and an increase in power between 14.2 to 50 Hz. Approximately 45 minutes later, the effects of midazolam had begun to dissipate such that there was an increase in power between 7.8 to 11.7 Hz and a decrease in power between 15.6 to 45.4 Hz. These data are summarized in figure 2 a–d.
Figure 2.
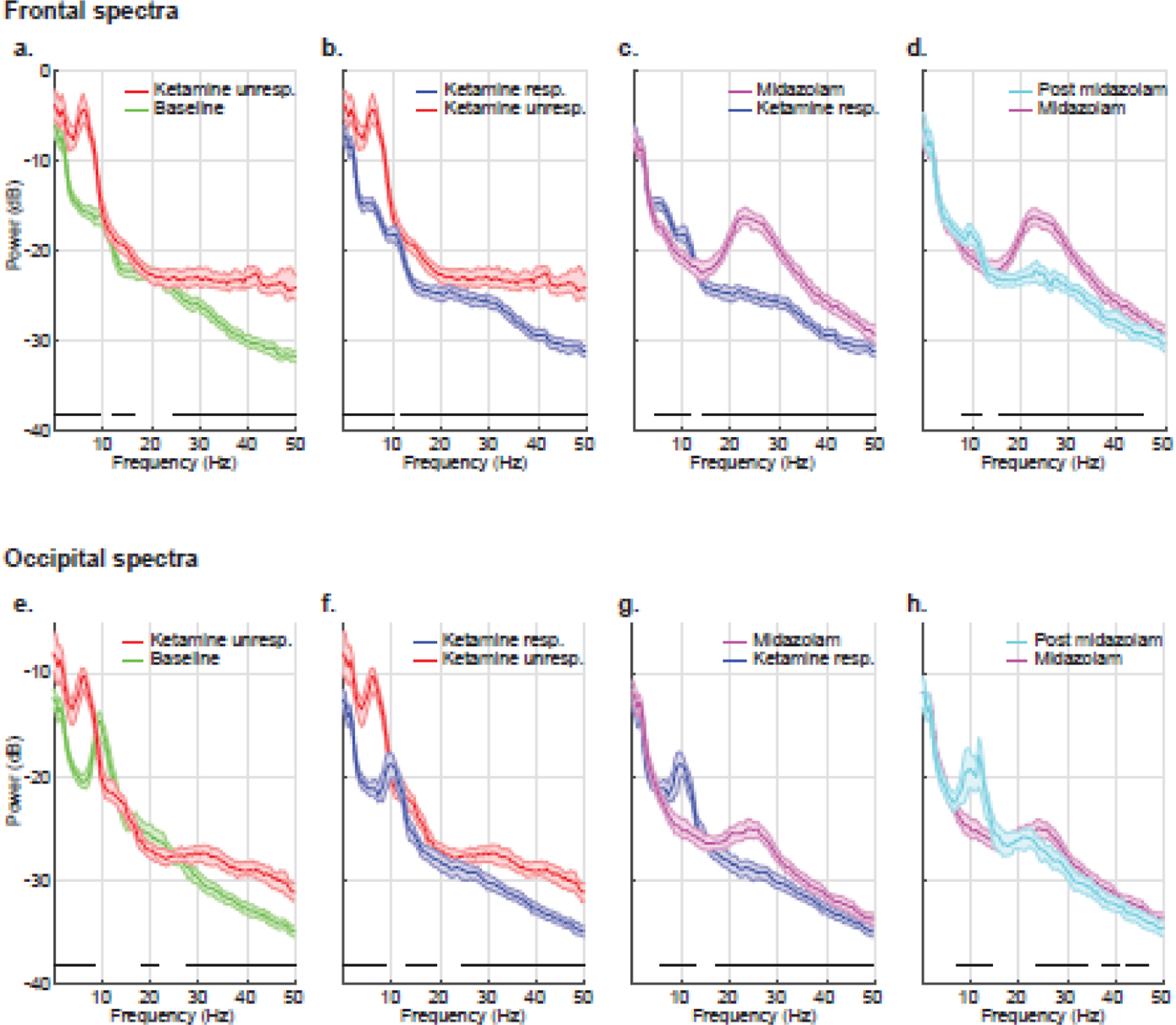
Group-level spectral comparison of consecutive behavioral states. (a) baseline (green) versus ketamine unresponsiveness (red), (b) ketamine unresponsiveness (red) versus ketamine responsiveness (blue), (c) ketamine responsiveness (blue) versus midazolam (magenta), and (d) midazolam (magenta) versus post midazolam (cyan). The bottom panel (e-h) illustrates the corresponding group-level occipital channel spectral comparison. The shaded regions represent the 99% confidence interval for the uncertainty around each median spectrum. Horizontal solid black lines represent frequency ranges at which significant differences existed. dB = decibel; Hz = hertz; unresp. = unresponsiveness; resp. = responsiveness.
Occipital
Compared to baseline, ketamine responsiveness was associated with decreased power between 2.4 to 5.4 Hz and 7.3 to 26.4 Hz. Midazolam decreased power between 4.9 to 18.6 Hz and increased power between 23.4 to 50 Hz. These data are summarized in Supplementary Figure 1b.
Analysis of consecutive behavioral states showed that compared to baseline, ketamine unresponsiveness was associated with increased power between 0.5 to 8.3 Hz and 27.3 to 50 Hz and decreased power between 18.6 to 21.5 Hz. As subjects transitioned into being responsive, there was a decrease in power between 0.5 to 8.9 Hz, 13.2 to 19 Hz, and 24.4 to 50 Hz. Midazolam resulted in a further decrease in power between 5.9 to 13.2 Hz and an increase in power between 17.1 to 50 Hz. Approximately 45 minutes later, the effects of midazolam had begun to dissipate such that there was an increase in power between 7.3 to 14.2 Hz and a decrease in power between 23.4 to 34.2 Hz, 37.1 to 40 Hz, and 42.5 to 46.4 Hz. These data are summarized in figure 2 e–h.
Global Coherence Analysis
Group-average global coherograms, coherence spectra, and spatial head plots are presented in Figure 3.
Figure 3.
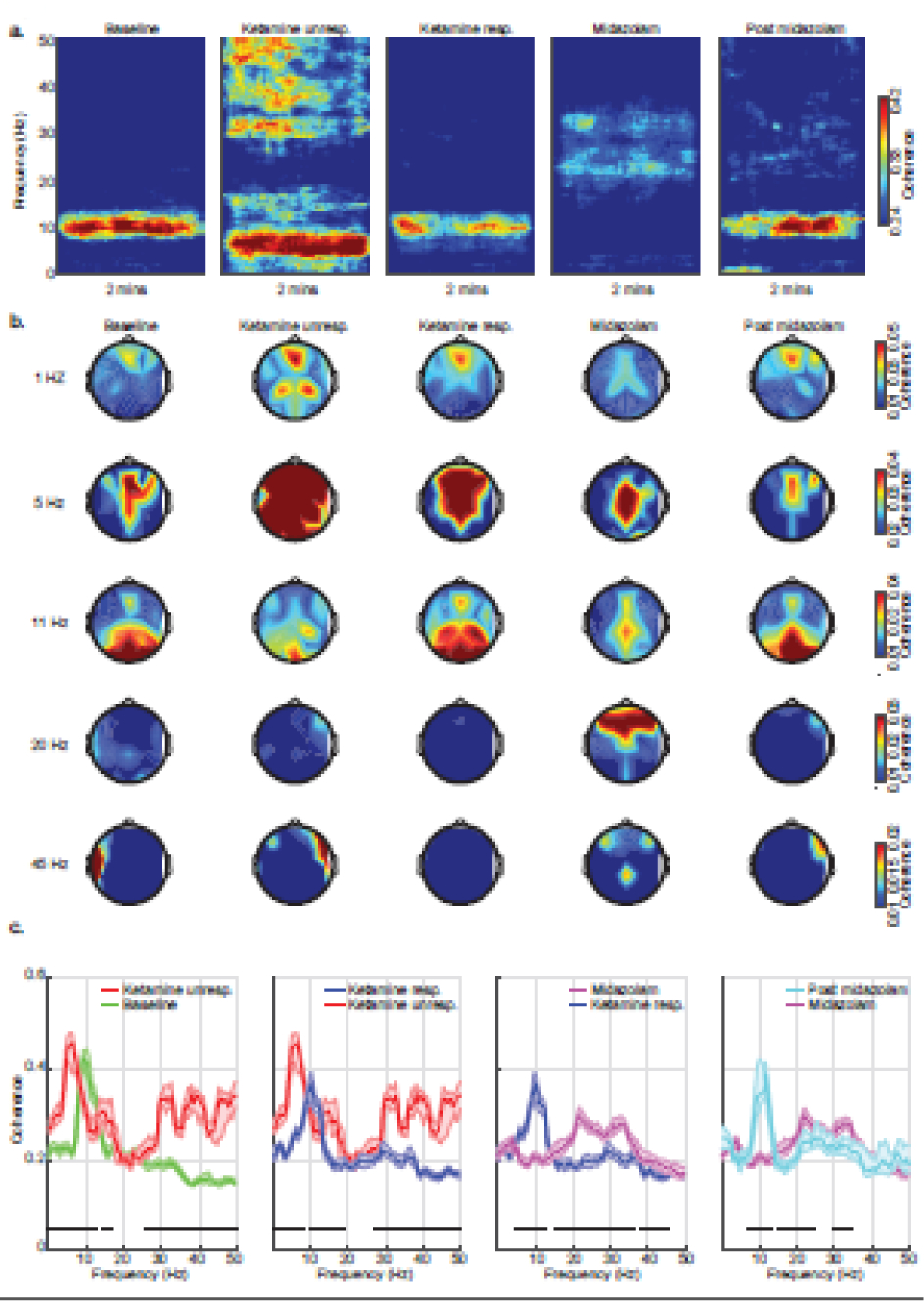
Group-level global coherence analyses. (a) Group level median coherograms, (b) global coherence-spatial plots, and (c) Global coherence spectral comparison across various behavioral states computed from 2 minutes EEG segments. Occipital alpha coherence (eye closed EEG signature) was evident during baseline, transitioning to globally coherent theta oscillations upon ketamine administration. Midazolam-induced beta oscillations were globally coherent with frontal dominance. The shaded regions in the bottom panel (c) represent the 99% confidence interval for the uncertainty around each median coherence. Horizontal solid black lines represent frequency ranges at which significant differences existed. dB = decibel; Hz = hertz; unresp. = unresponsiveness; resp. = responsiveness.
Similar to the spectral analysis, we first directly compared ketamine responsiveness and midazolam states to baseline. Ketamine responsiveness was associated with decreased coherence between 13.2 to 23 Hz and increased coherence between 27.3 to 31.7 Hz, 34.7 to 41 Hz, and 44.4 to 50 Hz. Midazolam was associated with decreased coherence between 6 to 14.2 Hz (occipital alpha) and increased coherence between 18.1 to 50 Hz (frontal). These data are summarized in Supplementary Figure 2.
Finally, to assess the temporal evolution of coherence, we compared electroencephalogram coherence across consecutive behavioral states. Compared to baseline, ketamine unresponsiveness was associated with decreased coherence between 8.8 to 12.7 Hz (occipital alpha) and increased coherence between 0.5 to 7.8 Hz, 14.2 to 17.1 Hz, and 23.4 to 50 Hz. As subjects became responsive to verbal cues, there was decreased coherence between 0.5 to 8.3 Hz, 13.2 to 19 Hz, and 25.4 to 50 Hz, and increased coherence between 9.8 to 12.7 Hz (occipital alpha). Midazolam decreased coherence between 4.9 to 13.2 Hz (including loss of occipital alpha) and increased coherence between 15.1 to 36.1 Hz and 38.1 to 45.4 Hz. The effect of dissipating midazolam was evident by increased coherence between 6.8 to 13.7 Hz (occipital alpha) with a decreased coherence between 15.1 to 24.4 Hz and 29.8 to 34.7 Hz. These data are summarized in figure 3.
Ketamine-induced electroencephalogram signatures were not dissociation-specific
CADSS scores are presented in figure 4. During the ketamine responsive (dissociated state), we did not find any significant correlations between CADSS and frontal alpha power (Spearman rho = 0.30, p = 0.294), frontal low-beta (Spearman rho = −0.14, p = 0.63) occipital alpha (Spearman rho = 0.33, p = 0.25), or occipital low beta (Spearman rho = 0.01, p = 0.958). Also, we did not find any significant correlations between CADSS and alpha global coherence (Spearman rho = 0.1, p = 0.730) or low beta global coherence (Spearman rho = 0.25, p = 0.399). The final backward elimination models for frontal power, occipital power, and global coherence all converged to a previously reported model that demonstrated a quadratic relationship between time and dissociation affected by midazolam. Specifically, and as previously reported (Gitlin et al., 2020), midazolam resulted in a 10.3 ([95%CI: 3.4 to 17.1], p = 0.005) point reduction in CADSS adjusted means. Residuals from this model did not differ markedly from normality.
Figure 4.
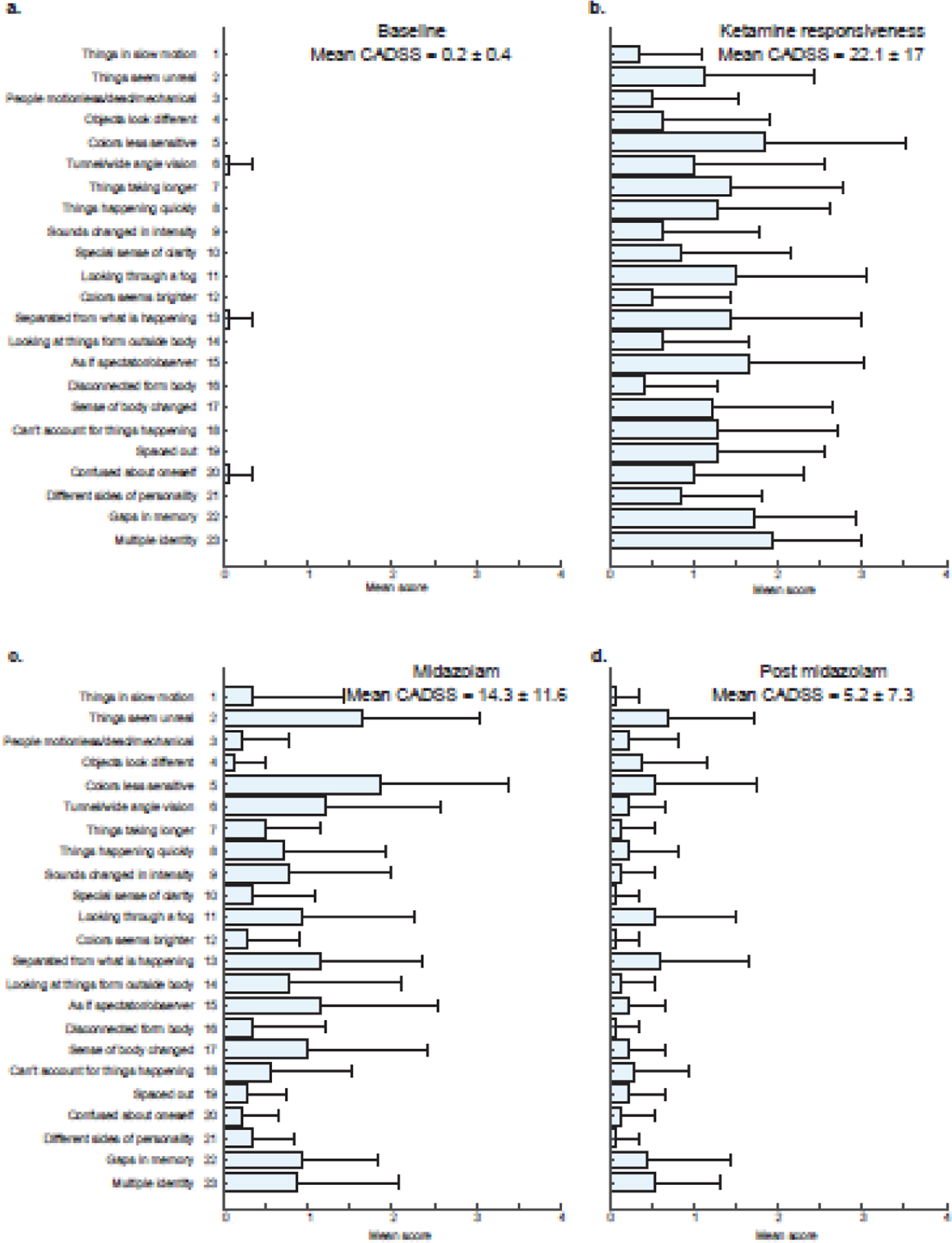
Mean CADSS scores. (a) baseline, (b) ketamine responsiveness, (c) midazolam, and (d) post midazolam behavioral states. Horizontal error bars represent standard deviation in CADSS subscale across subjects. 0 = not at all, 2 = moderately, 3 = considerably, and 4 = extremely. CADSS = Clinician Administered Dissociative States Scale.
4. Discussion
In this investigation, we studied the spectral and global coherence electroencephalogram signatures of ketamine anesthesia. Similar to previous studies and compared to baseline, we found that ketamine unresponsiveness was associated with loss of occipital alpha and increased power in slow-delta, theta, beta, and gamma frequency bands (Akeju et al., 2016a, Blain-Moraes et al., 2014). Theta oscillations were coherent and widespread across the scalp. The ketamine responsive yet dissociated state was associated with decreased alpha and low-beta power. Treating dissociation with midazolam resulted in frontally dominant coherent beta oscillations. However, our primary finding was that CADSS scores had no strong correlation with ketamine-induced power or global coherence signature.
Decreased alpha and low-beta oscillation power have previously been associated with the ketamine-induced dissociative state (de la Salle et al., 2021, de la Salle et al., 2016, Vlisides et al., 2018). However, it has been unclear whether this finding is a biomarker of dissociation as measured by CADSS. Consistent with our findings, Vesuna et al. recently showed that a 1–3 Hz rhythm in layer five neurons of the retrosplenial cortex underlies dissociation-like behavior in mice (Solt and Akeju, 2020, Vesuna et al., 2020). The authors further confirmed that this deep brain rhythm was associated with dissociation in a patient with epilepsy by eliciting this rhythm from electrodes located in deep, but not superficial, posteromedial cortex locations (Solt and Akeju, 2020, Vesuna et al., 2020). Thus, deep brain recordings may be necessary to elicit putative neurophysiological biomarkers that directly underly dissociation.
A transient gamma-burst electroencephalogram pattern was previously associated with a bolus administration of ketamine (Akeju et al., 2016a). However, a theta predominant oscillatory pattern was associated with the state we defined as ketamine unresponsive. Because this oscillatory dynamic was not spatially restricted, we speculate that ketamine-induced theta oscillations may underly the large-scale network disruption necessary to maintain the ketamine unresponsive state. We note that inhaled anesthetic vapors in common clinical use (desflurane, isoflurane, sevoflurane) are also associated with theta oscillations at high doses (Akeju et al., 2016a, Akeju et al., 2014, Chamadia et al., 2019, Shortal et al., 2019). However, anesthetic vapor theta oscillations are spatially restricted to frontal electrode locations, which suggests that the neural circuit mechanisms underlying ketamine theta are different from anesthetic vapor theta.
The 23-item CADSS has been used to assess dissociation in numerous clinical trials that have studied ketamine as a treatment for depression (Ballard and Zarate, 2020, Fava et al., 2020, van Schalkwyk et al., 2018). In these studies, sub-anesthetic ketamine has been typically dosed at 0.5mg/kg infused intravenously over approximately 40 minutes (Ballard and Zarate, 2020). In a recent study, the mean CADSS score measured at the 40-minute infusion point of ketamine was 13.6 (van Schalkwyk et al., 2018). Using the same dosing paradigm, Fava et al (Fava et al., 2020) demonstrated a dose-response curve with respect to the dissociative symptoms elicited by ketamine at the 40-minute infusion point: 0.1mg/kg (mean CADSS ~3), 0.2mg/kg (mean CADSS ~4), 0.5mg/kg (mean CADSS ~14), and 1mg/kg (mean CADSS ~24). These findings are consistent with the mean CADSS score of 22 we reported approximately 60 minutes after administering an anesthetic dose of ketamine. Taken together, these dose-dependent findings suggest that the CADSS measures ketamine-induced dissociation. However, it is unclear if all aspects of ketamine-induced dissociation are captured by the items on the CADSS (Singh et al., 2016, van Schalkwyk et al., 2018).
A major strength of our study is that we longitudinally conducted structured dissociation assessments. However, our study has several important limitations. First, although CADSS is a widely used instrument to measure ketamine-induced dissociation, it was initially introduced into clinical practice to discriminate patients with dissociative disorders from patients with other psychiatric disorders and healthy patients. Second, midazolam may reduce dissociation through neural circuits distinct from those necessary for inducing or maintaining dissociation. However, electroencephalogram features extracted during the dissociated state did not correlate with CADSS. Third, to obtain point estimates of power and global coherence, studies such as ours reduce the complexity of the electroencephalogram. Thus, future studies in various patient populations that couple machine learning techniques to an expansive set of electroencephalogram features may be necessary to predict the presence and severity of dissociation.
We conclude that ketamine is associated with structured electroencephalogram power and global coherence signatures that may enable principled anesthetic state but not dissociation monitoring.
Supplementary Material
Supplementary Figure 1. Group-level spectral comparison of behavioral states compared to baseline. (a) baseline (green) versus ketamine unresponsiveness (red), (b) baseline (green) versus ketamine responsiveness (blue), (c) baseline (green) versus midazolam (magenta), and (d) baseline (green) versus post midazolam (cyan). The bottom panel (e-h) illustrates the corresponding group-level occipital spectral comparison. The shaded regions represent the 99% confidence interval for the uncertainty around each median spectrum. Horizontal solid black lines represent frequency ranges at which significant differences existed. dB = decibel; Hz = hertz; unresp. = unresponsiveness; resp. = responsiveness.
Supplementary Figure 2. Group-level global coherence comparison of behavioral states compared to baseline. (a) baseline (green) versus ketamine unresponsiveness (red), (b) baseline (green) versus ketamine responsiveness (blue), (c) baseline (green) versus midazolam (magenta), and (d) baseline (green) versus post midazolam (cyan). The shaded regions represent the 99% confidence interval for the uncertainty around each median coherence. Horizontal solid black lines represent frequency ranges at which significant differences existed. dB = decibel; Hz = hertz; unresp. = unresponsiveness; resp. = responsiveness.
Highlights.
Ketamine is an anesthetic drug with dissociative properties.
Ketamine induces highly structured oscillations that may be used to monitor drug-induced altered arousal.
Surface electroencephalogram oscillations are unlikely to underly ketamine-induced dissociation.
Acknowledgments
NIH NIA RO1AG053582 to OA; and, Innovation funds from the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital to OA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
OA has received speaker’s honoraria from Masimo Corporation and is an inventor on EEG monitoring patents assigned to Massachusetts General Hospital. All other authors declare that no competing interests exist.
References
- Akeju O, Brown EN. Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr Opin Neurobiol 2017;44:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeju O, Hamilos AE, Song AH, Pavone KJ, Purdon PL, Brown EN. GABAA circuit mechanisms are associated with ether anesthesia-induced unconsciousness. Clin Neurophysiol 2016a;127(6):2472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeju O, Song AH, Hamilos AE, Pavone KJ, Flores FJ, Brown EN, et al. Electroencephalogram signatures of ketamine anesthesia-induced unconsciousness. Clin Neurophysiol 2016b;127(6):2414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeju O, Westover MB, Pavone KJ, Sampson AL, Hartnack KE, Brown EN, et al. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology 2014;121(5):990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball S, Robinson A, Shekhar A, Walsh K. Dissociative symptoms in panic disorder. J Nerv Ment Dis 1997;185(12):755–60. [DOI] [PubMed] [Google Scholar]
- Ballard ED, Zarate CA Jr. The role of dissociation in ketamine’s antidepressant effects. Nat Commun 2020;11(1):6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain-Moraes S, Lee U, Ku S, Noh G, Mashour GA. Electroencephalographic effects of ketamine on power, cross-frequency coupling, and connectivity in the alpha bandwidth. Front Syst Neurosci 2014;8:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 1998;11(1):125–36. [DOI] [PubMed] [Google Scholar]
- Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci 2011;34:601–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamadia S, Pedemonte JC, Hahm EY, Mekonnen J, Ibala R, Gitlin J, et al. Delta oscillations phase limit neural activity during sevoflurane anesthesia. Commun Biol 2019;2:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Salle S, Choueiry J, Shah D, Bowers H, McIntosh J, Ilivitsky V, et al. Resting-state functional EEG connectivity in salience and default mode networks and their relationship to dissociative symptoms during NMDA receptor antagonism. Pharmacol Biochem Behav 2021;201:173092. [DOI] [PubMed] [Google Scholar]
- de la Salle S, Choueiry J, Shah D, Bowers H, McIntosh J, Ilivitsky V, et al. Effects of Ketamine on Resting-State EEG Activity and Their Relationship to Perceptual/Dissociative Symptoms in Healthy Humans. Front Pharmacol 2016;7:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF. Taming the ketamine tiger. 1965. Anesthesiology 2010;113(3):678–84. [DOI] [PubMed] [Google Scholar]
- Duclos C, Maschke C, Mahdid Y, Berkun K, Castanheira JDS, Tarnal V, et al. Differential classification of states of consciousness using envelope- and phase-based functional connectivity. Neuroimage 2021:118171. [DOI] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry 2020;25(7):1592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin J, Chamadia S, Locascio JJ, Ethridge BR, Pedemonte JC, Hahm EY, et al. Dissociative and Analgesic Properties of Ketamine Are Independent. Anesthesiology 2020;133(5):1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Tsuda N, Sawa T, Hagihira S. Ketamine increases the frequency of electroencephalographic bicoherence peak on the alpha spindle area induced with propofol. Br J Anaesth 2007;99(3):389–95. [DOI] [PubMed] [Google Scholar]
- Heifets BD. Piercing the Ketamine Cloud. Anesthesiology 2020;133(5):970–972. [DOI] [PubMed] [Google Scholar]
- Li L, Vlisides PE. Ketamine: 50 Years of Modulating the Mind. Front Hum Neurosci 2016;10:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio JJ, Atri A. An overview of longitudinal data analysis methods for neurological research. Dement Geriatr Cogn Dis Extra 2011;1(1):330–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour GA, Palanca BJ, Basner M, Li D, Wang W, Blain-Moraes S, et al. Recovery of consciousness and cognition after general anesthesia in humans. Elife 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacka A, Borczyk M. Ketamine applications beyond anesthesia - A literature review. Eur J Pharmacol 2019;860:172547. [DOI] [PubMed] [Google Scholar]
- O’Driscoll C, Laing J, Mason O. Cognitive emotion regulation strategies, alexithymia and dissociation in schizophrenia, a review and meta-analysis. Clin Psychol Rev 2014;34(6):482–95. [DOI] [PubMed] [Google Scholar]
- Pavone KJ, Su L, Gao L, Eromo E, Vazquez R, Rhee J, et al. Lack of Responsiveness during the Onset and Offset of Sevoflurane Anesthesia Is Associated with Decreased Awake-Alpha Oscillation Power. Front Syst Neurosci 2017;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J Ketamine in pain management. CNS Neurosci Ther 2013;19(6):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical Electroencephalography for Anesthesiologists: Part I: Background and Basic Signatures. Anesthesiology 2015;123(4):937–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener S, Eken C, Schultz CH, Serinken M, Ozsarac M. Ketamine with and without midazolam for emergency department sedation in adults: a randomized controlled trial. Ann Emerg Med 2011;57(2):109–14 e2. [DOI] [PubMed] [Google Scholar]
- Shortal BP, Hickman LB, Mak-McCully RA, Wang W, Brennan C, Ung H, et al. Duration of EEG suppression does not predict recovery time or degree of cognitive impairment after general anaesthesia in human volunteers. Br J Anaesth 2019;123(2):206–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, et al. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am J Psychiatry 2016;173(8):816–26. [DOI] [PubMed] [Google Scholar]
- Solt K, Akeju O. The brain rhythms that detach us from reality. Nature 2020;586(7827):31–2. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Tsueda K, Lansing PS, Tolan MM, Fuhrman TM, Sheppard RA, et al. Midazolam attenuates ketamine-induced abnormal perception and thought process but not mood changes. Can J Anaesth 2000;47(9):866–74. [DOI] [PubMed] [Google Scholar]
- van Schalkwyk GI, Wilkinson ST, Davidson L, Silverman WK, Sanacora G. Acute psychoactive effects of intravenous ketamine during treatment of mood disorders: Analysis of the Clinician Administered Dissociative State Scale. J Affect Disord 2018;227:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesuna S, Kauvar IV, Richman E, Gore F, Oskotsky T, Sava-Segal C, et al. Deep posteromedial cortical rhythm in dissociation. Nature 2020;586(7827):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlisides PE, Bel-Bahar T, Nelson A, Chilton K, Smith E, Janke E, et al. Subanaesthetic ketamine and altered states of consciousness in humans. Br J Anaesth 2018;121(1):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Wu KD, Cutshall C. Symptom subtypes of obsessive-compulsive disorder and their relation to dissociation. J Anxiety Disord 2004;18(4):435–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Group-level spectral comparison of behavioral states compared to baseline. (a) baseline (green) versus ketamine unresponsiveness (red), (b) baseline (green) versus ketamine responsiveness (blue), (c) baseline (green) versus midazolam (magenta), and (d) baseline (green) versus post midazolam (cyan). The bottom panel (e-h) illustrates the corresponding group-level occipital spectral comparison. The shaded regions represent the 99% confidence interval for the uncertainty around each median spectrum. Horizontal solid black lines represent frequency ranges at which significant differences existed. dB = decibel; Hz = hertz; unresp. = unresponsiveness; resp. = responsiveness.
Supplementary Figure 2. Group-level global coherence comparison of behavioral states compared to baseline. (a) baseline (green) versus ketamine unresponsiveness (red), (b) baseline (green) versus ketamine responsiveness (blue), (c) baseline (green) versus midazolam (magenta), and (d) baseline (green) versus post midazolam (cyan). The shaded regions represent the 99% confidence interval for the uncertainty around each median coherence. Horizontal solid black lines represent frequency ranges at which significant differences existed. dB = decibel; Hz = hertz; unresp. = unresponsiveness; resp. = responsiveness.


