Abstract
Despite considerable effort, malaria remains a major public health burden. Malaria is caused by five Plasmodium species and is transmitted to humans via the female Anopheles mosquito. The development of malaria vaccines against the liver and blood stages has been challenging. Therefore, malaria elimination strategies advocate integrated measures, including transmission-blocking approaches. Designing an effective transmission-blocking strategy relies on a sophisticated understanding of the molecular mechanisms governing the interactions between the mosquito midgut molecules and the malaria parasite. Here we review recent advances in the biology of malaria transmission, focusing on molecular interactions between Plasmodium and Anopheles mosquito midgut proteins. We provide an overview of parasite and mosquito proteins that are either targets for drugs currently in clinical trials or candidates of promising transmission-blocking vaccines.
Subject terms: Protein vaccines, Parasitic infection
Introduction
According to the World Health Organization (WHO), there were approximately 229 million malaria cases worldwide in 2019, resulting in 409,000 deaths. Africa remains the heartland of malaria transmission, accounting for 94% of cases and deaths globally. Five Plasmodium species, P. falciparum, P. vivax, P. malaria, P. ovale, and P. knowlesi, cause malaria in humans, of which P. falciparum accounts for approximately 99.7% of malaria cases in Africa1–3. P. falciparum is also responsible for most malaria-related deaths, especially in children under five years old and in pregnant women2,4. Malaria is transmitted to humans by the bite of a female Anopheles mosquito. More than 30 species of Anopheles mosquitoes in various regions of the globe can transmit malaria. Anopheles gambiae is the major vector for the deadliest parasite, P. falciparum, in Africa5,6. Current malaria control practices rely heavily on clinical case management through a timely diagnosis and effective drug treatment in addition to vector-based measures such as insecticide-treated bed nets and indoor residual sprays of insecticides. However, the emergence of drug-resistant parasites and insecticide-resistant mosquitoes pose increasing challenges to malaria control and elimination7,8, demanding the development of innovative technologies.
Vaccine development against malaria has been on the research agenda for malaria control and elimination for decades. Malaria vaccines are generally divided into three categories according to the target stage of the parasite’s life cycle—pre-erythrocytic, blood-stage, and transmission-blocking vaccines (TBV). However, vaccine development against such a complicated eukaryotic parasite is challenging. Currently, the most advanced vaccine, RTS,S, which targets the P. falciparum circumsporozoite surface protein (PfCSP), showed <37% protection against P. falciparum malaria in phase 3 clinical trials9–11. Similarly, the first field trial of a Pfs25-based TBV in Malian adults revealed significant limitations12. Given such partial effectiveness of vaccines against single targets at individual stages, it is anticipated that a more efficacious approach would involve the combination of subunit vaccines targeting multiple antigens and multiple stages13. This principle is illustrated in a study showing that combining a partially effective pre-erythrocytic vaccine and TBV could synergistically reduce the prevalence of mosquito infections in laboratory mice14. With the growing recognition of effective transmission interruption as one key measure for malaria elimination3,15, TBVs have received increasing attention. Currently, there are only a few parasite sexual-stage antigens as the “priority” TBV candidates (Table 1), which emphasizes the need for strenuous efforts in antigen discovery to broaden the antigen repertoire16,17. These efforts will benefit from a better understanding of the malaria parasite–vector interactions during the midgut invasion process. Here we review recent advances in the identification of key parasite and mosquito molecules that play roles in midgut invasion, and we evaluate their potential for TBV development.
Table 1.
Malaria TBV candidates for sexual parasite proteins either under preclinical development or in clinical trials.
| Protein name | Expression location | Function | Current status | References |
|---|---|---|---|---|
| Pfs25 (PF3D7_1031000) | Extracellular gametocyte through ookinete | Ookinete survival in the mosquito midgut, penetration of the midgut epithelium, and transformation of ookinetes into oocysts | Phase I | 12,78,79,83,84,86–89,137 |
| Pfs28 (PF3D7_1014200) | Zygote and ookinete surface | Ookinete invasion in the mosquito midgut and protects ookinetes from midgut enzymes | Preclinical development | 90–92 |
| Pfs230 (PF3D7_0209000) | Gametocyte and gamete | Gamete–gamete interaction and male gamete fertility | Phase I, about to start phase II | 65,82,93,94,98 |
| Pfs48/45 (PF3D7_1346700) | Gametocyte and gamete | Essential for parasite fertilization | Preclinical development | 64,103,104,108 |
| Pfs47 (PF3D7_1346800) | Female gametocyte through ookinetes | Parasitic immune evasion | Preclinical development | 68,69,109–111,130,138 |
| HAP2 (PF3D7_1014200) | Male gametocytes and microgametes | Fusion and fertilization of gametes | Preclinical development | 71,82,113,115 |
Key: PF3D7 Plasmodium falciparum gene identifier, HAP2 HAPLESS2.
The malaria parasite development in the human host
The life cycle of Plasmodium parasites alternates between a female Anopheles mosquito and a vertebrate host, involving multiple unique developmental stages that target diverse host cell types18,19. In humans, the cycle begins after the injection of sporozoites into the bloodstream by an infected female Anopheles mosquito during its blood-feeding. Within 30 min, the sporozoites migrate from the bloodstream to the liver and invade hepatocytes. Inside the liver cells, the now intracellular parasite divides mitotically over several days and eventually develops into a schizont that contains thousands of merozoites1,20. Rupture of a schizont releases merozoites into the bloodstream to invade erythrocytes, which initiate the asexual erythrocytic stage of the replication cycle. Inside the red blood cells (RBCs), the parasites undergo schizogony, lasting over 48 h for P. falciparum, progressing through the ring, trophozoite, and schizont stages. The schizont subsequently ruptures, releasing 8–36 merozoites to invade other RBCs. This is the stage when the affected patient typically manifests clinical symptoms such as fever and chill in recurring episodes20–22.
During the asexual replication cycle within the RBCs, some parasites undergo sexual development and differentiate into male and female gametocytes, a process termed gametocytogenesis4,23,24. For P. falciparum, the gametocyte commitment generally starts before schizogony, wherein an individual schizont produces a progeny of merozoites that develops into either all asexual or all sexual forms25–27. Gametocytogenesis is influenced by a combination of host and environmental stress factors, including anemia28–31, drug treatment29–32, host immune response33,34, and high parasitemia26. In vitro, gametocytogenesis can be stimulated by manipulation of the parasite culture, for example by adding red blood cell lysate and lymphocytes together with serum. It was also shown that the nucleic acid synthesis inhibitor Berenil stimulates gametocytogenesis35–37.
Recent studies have provided new insights into the mechanisms of sexual commitment and differentiation. For example, sexual commitment in P. falciparum has been shown to be governed by the AP2-domain transcription factor PfAP2-G functioning as a master regulator38–40. PfAP2-G also regulates early gametocyte development, as it binds to the promoters of many early gametocyte genes such as Pfs16, etramp10.3, gexp05, Pfg14.744, and Pfg14.74838. The P. falciparum gametocyte development 1 (Pfgdv1) is an upstream activator of sexual commitment, antagonizing the heterochromatin protein 1-mediated gene silencing of Pfap2-g41–43. In addition, targeted gene disruption and complementation showed that P. falciparum gene implicated in gametocytogenesis (pfgig) also participates in gametocyte commitment44. Pfs16, a gametocyte-specific parasitophorous vacuole membrane (PVM) protein, is required for gametocyte development45,46. A comprehensive list of genes that are essential for gametocyte development has been reported24.
P. falciparum gametocytes take 7–10 days to traverse five morphologically distinct stages to reach maturity. Gametocytes in stages I–IV become sequestered in the extravascular spaces of the hematopoietic system such as bone marrow, spleen, brain, heart, and gut, whereas mature gametocytes are released back into the blood circulation4,47 where they can be taken up by an anopheline mosquito.
The Anopheles midgut as a trigger for parasite transformation
When a mosquito bites a human, the ingested blood meal regulates mosquito gene expression48. Specifically, when the human blood reaches the mosquito midgut, it stimulates the midgut epithelium to secrete factors including 3–13% chitin microfibrils and a variety of proteins49,50, which forms a peritrophic matrix (PM)51 that surrounds the ingested blood and separates the blood bolus from midgut epithelial cells. The newly formed PM provides another physical barrier that limits the infection of the mosquito by pathogens in the blood meal52.
Inside the mosquito midgut, the co-ingested mature gametocytes undergo gametogenesis, gamete fertilization, and ookinete development before penetrating the midgut epithelium and landing at the destination between the midgut endothelium and basal lamina53,54. Several mechanistic details of this sequence of events are now understood. Thus, immediately after ingestion, the gametocytes are activated collectively by environmental signals such as a sharp drop in temperature of approximately 5 °C, a rise in pH from 7.4 to 8.0, and a mosquito-derived molecule xanthurenic acid (XA)55. Each female extracellular gametocyte forms a single immotile macrogamete, while a male extracellular gametocyte generates up to eight flagella-like microgametes in a process called “exflagellation”. Gametocyte egress from the RBC occurs via an inside‐out fashion with the rupture of the PVM first and the erythrocyte membrane later56. Recent studies revealed some of the molecular details of the gametogenesis process. XA activates membrane guanyl cyclases in gametocytes to synthesize cyclic guanosine monophosphate (cGMP), consecutively stimulating the cGMP-dependent protein kinase G (PKG) pathway and significantly increasing the intracellular calcium57. A recent CRISPR/Cas9-based functional study demonstrated that an intracellular membrane protein, the gametogenesis essential protein 1 (GEP1), is essential for XA-stimulated gametogenesis regulation in P. yoelii58. Other signaling molecules identified to be essential for gametogenesis include the male gametocyte-specific kinase PfMAP-259. Cytosolic Ca2+ triggers the release of a perforin-like protein PfPLP2, which in turn permeabilizes the erythrocyte membrane, leading to P. falciparum gametocyte egress from erythrocytes60. P. falciparum patatin-like phospholipase 1 (PfPATPL1) with phospholipase A2 (PLA2) activity was found to play a crucial role in gametogenesis by mediating PfPLP2 secretion, gametocyte rounding up, and male gamete exflagellation61. Pfs16 was described as an important gene for in vitro male gametocyte exflagellation46. In a more recent study, antibodies to Pfs16 resulted in a significant reduction in the number of oocysts. In the same study, five additional parasite proteins including PF3D7_0303900, PF3D7_1204400 (Pfs37), PF3D7_1214800, PF3D7_1239400, and PF3D7_1472800 were found to interact with the mosquito midgut62. It has been shown that knocking out Pbg37 (PBANKA_060330) in P. berghei, the ortholog of Pfs37, led to a significant reduction in the formation of oocysts63. These proteins, along with the previously discussed Plasmodium proteins, may serve as novel targets for blocking malaria transmission.
Several Plasmodium proteins involved in fertilization and zygote development have been reported. Pfs48/45 is expressed in both male and female gametes, and a gene disruption study of Pfs48/45 revealed that only the male gamete fertility was altered and failed to fertilize the macrogamete64. Pfs230 is another critical protein for male fertility and exflagellation65,66. A male-specific protein, a disulfide isomerase (PDI-Trans/PBANKA_0820300), is reportedly crucial for fertilization67. The female-specific protein P47 is required for the fertility of the macrogametes in P. berghei66, but not in P. falciparum68. However, Pfs47 in P. falciparum does play a fundamental role in parasitic survival by mediating the evasion of the immune system of the vector69. Similarly, the P. berghei P47 is also important during ookinete-to-oocyst transition by protecting ookinetes from the complement-like response of mosquitoes70. Fertilization starts when a microgamete attaches to a macrogamete and undergoes membrane fusion. HAP2, a conserved protein of protozoan parasites, plants, and algae, has been reported to be essential for P. berghei gamete fusion71.
Following fertilization, the zygotes undergo meiosis and transform into motile and invasive ookinetes. Expression of over 500 genes in ookinetes is regulated by the AP2 family Plasmodium transcription factor AP2-O72, which affects ookinete development, motility, midgut penetration, mosquito immunity evasion, and oocyst development initiation73. Further studies demonstrated the function of these genes (Table 2), including ookinete surface‐associated proteins like P25 and P28; secretory proteins like chitinase, perforins, PPLP3‐5, and PSOPs74; adhesive proteins like the secreted ookinete adhesive protein (SOAP)75, the von Willebrand factor A domain‐related protein (WARP)76, circumsporozoite- and TRAP-related protein (CTRP)77; mobile proteins like glideosome-associated proteins (GAP) and cell traversal protein for ookinetes and sporozoites (CelTOS); and heat shock protein (HSP) 20, 40, 70, and 9073. P25 and P28 share multiple functions and are crucial for ookinete development, midgut traversal, and ookinete to oocyst transformation. P25 and P28 are among the top candidates for TBV development78,79, a topic that is discussed in greater detail later in this review. The secreted enzymes like chitinase digest the physical barrier of the PM, assisting the ookinete to invade the mosquito midgut80. In terms of mobility, P. yoelii encoded guanylate cyclase β (GCβ) is expressed on the ookinetes and localized polarly at the ookinete extrados site (OES) and found to be essential for ookinete gliding. GCβ contains the N-terminal P4-ATPase-like domain (ALD) and the C-terminal guanylate cyclase domain (GCD), which are required for its polymerization and subsequent ookinete gliding. During ookinete development, CDC50A, as a co-factor of P4-ATPase, stabilizes GCβ. IMC sub-compartment protein 1 (ISP1) was found to be a crucial molecule for anchoring the GCβ/CDC50A complex at the OES of mature ookinetes81.
Table 2.
Summary of parasite proteins discovered to be involved in Anopheles midgut survival and dissemination.
| Protein name | Expression stage | Function | References |
|---|---|---|---|
| Pfgdv1 (PF3D7_0935400) | Gametocytes | Plays a role in transcriptional activation of PfAP2-G | 41 |
| PfAP2-G (PF3D7_1222600) | Gametocytes | Regulation of early gametocyte genes and gametocytogenesis | 38,40 |
| Pfgig (PF3D7_0935600) | Gametocytes | Regulation of gametocyte commitment | 44 |
| Pfs16 (PF3D7_0406200) | Gametocytes and gametes | Gametocyte development and male gametocyte exflagellation | 46,62 |
| PfPLP2 (PF3D7_1216700) | Gametocytes | Key to gametocytes egress from erythrocytes | 60 |
| PfPATPL1 (PF3D7_0209100) | Gametocytes | Function in gametogenesis by mediating PfPLP2 secretion, gametocyte rounding up, and male gamete exflagellation | 61 |
| PfMAP-2 (PF3D7_1113900) | Male gametocytes | Gametogenesis and exflagellation | 59 |
| GEP1 (PF3D7_0515500) | Gametocytes | XA-stimulated gametogenesis regulation | 58 |
| PDI-Trans/PBANKA_0820300 | Male gametocytes | Fertilization and malarial transmission to the mosquito | 67 |
| Pfs37 (PF3D7_1204400) | Sexual stage-specific protein | May play role in gametogenesis and exflagellation | 62,63 |
| PF3D7_0303900 | ?? | Phosphatidylethanolamine-binding protein | 62 |
| PF3D7_1214800 | ?? | unknown | 62 |
| PF3D7_1239400 | ?? | unknown | 62 |
| PF3D7_1472800 | ?? | HSP20-like chaperone | 62 |
| PfCHT1 (PF3D7_1252200) | Ookinetes | Midgut PM invasion | 139,140 |
| PfCelTOS (PF3D7_1216600) | Ookinetes and sporozoites | Plays a crucial role in establishing malaria infections in both mosquito and vertebrate hosts | 141–143 |
| SOAP (PF3D7_1404300) | Ookinetes and young oocysts | Interacts with midgut basal laminin and plays a role in oocyst development | 75 |
| WARP (PF3D7_0801300) | Ookinetes | Midgut invasion | 144 |
Key: Pfgdv1 P. falciparum gametocyte development 1 gene, PfAP2-G P. falciparum AP2 transcription factor, PfMAP-2 P. falciparum mitogen-activated protein kinase-2, GEP1 gametogenesis essential protein 1, Pfgig P. falciparum gene implicated in gametocytogenesis, PfPLP2 P. falciparum perforin-like protein 2, PfPATPL1 P. falciparum patatin-like phospholipase, CHT1 chitinase, PfCelTOS cell traversal protein for ookinetes and sporozoites, SOAP secreted ookinete adhesive protein, WARP von Willebrand factor A domain-related protein. Note: Italics format represents gene name.
P. falciparum TBV candidate proteins
Pfs25 and Pfs28
The expression of Pfs25 and Pfs28 begins in the extracellular gametocytes within the mosquito vector82. Pfs25 is a 25 kDa sexual stage protein mostly expressed on the surface of macrogametes, zygotes, and ookinetes of P. falciparum inside the mosquito midgut83. Pfs25 was the first protein to progress to a clinical trial; however, the phase 1 trial of this protein, formulated in the adjuvant Montanide ISA51, was found to be reactogenic84. Ensuing research focused on combining Pfs25 with other proteins. Fusion of Pfs25 antigen to IMX313, a protein multimerization technology, formulated a nanoparticle with enhanced immunogenicity85. A similar study demonstrated stimulation of functional antibody response against malaria infection and transmission in mice by combining Pfs25-IMX313 with RTS, S/AS0186. Several studies involving other recombinant products have moved into phase 1 clinical trial, including one involving the conjugation product of Pfs25 linked to a detoxified form of Pseudomonas aeruginosa exoprotein A (EPA)87, Pfs25-EPA conjugates formulated with Alhydrogel® 88, and a chimeric virus-like particle (VLP) containing Pfs25 fused to the alfalfa mosaic virus coat protein89. However, the antibody titers in these trials and the effectiveness of transmission-blocking (TB) potential were insufficient. Hence there is a need for an alternative adjuvant.
Similarly, Pfs28 is a 28 kDa protein mainly expressed on the surface of zygotes and ookinetes of P. falciparum90. Chemical conjugation of Pfs28 to a mutant EPA significantly enhanced immunogenicity in mice immunized with conjugated Pfs2891. A 39-kDa chimeric recombinant protein produced by the fusion of Pfs25 and Pfs28 proteins secreted by Saccharomyces cerevisiae was found to be more potent than the two candidate proteins alone92.
Pfs230 and Pfs48/45
The expression of the gametocyte antigens Pfs230 and Pfs48/45 starts intracellularly within the human host82. Pfs230 and Pfs48/45 are 6-cys family proteins expressed on the surface of gametocytes and gametes of P. falciparum93,94. Pfs48/45 is expressed by both male and female gametes; however, it is required only for male fertility64. Despite their important role in malaria transmission, the development of these TBV candidate antigens has been hampered due to a lack of properly folded recombinant proteins95. Although the expression of a full-length recombinant Pfs230 has been hindered by its large size and a large number of disulfide bonds96,97, different domains at the N-terminal region of Pfs230 showed TB activities98. The Pfs230 N-terminal prodomain, Pfs230C (amino acids 443 to 1132), synthesized using a cell-free system, showed a sufficient complement-dependent malaria TB activity98. Antibodies generated against Pfs230C1, another region of the Pfs230 N-terminal (amino acids 443 to 731), significantly reduced the number of oocysts99. Pfs230D1+ , a modified form of Pfs230C1 constructed by eliminating the glycosylation property, was found to be homogeneous and more stable than Pfs230C1. Pfs230D1+ also demonstrated higher expression yield and transmission-reducing activity similar to Pfs230C1100. Another construct of Pfs230 domain C, 230CMB, produced using a plant-based expression system, showed strong TB activity101. In another study, conjugation of Pfs230 with an outer membrane protein complex of Neisseria meningitidis was found to have enhanced immunogenicity and functional activity of the Pfs230 protein102.
Similar to Pfs230, expression of a full-length recombinant protein Pfs48/45 has been difficult due to its size and complexity, which forced researchers to focus on protein domains. A C-terminal Pfs48/45 expressed in baculovirus demonstrated significant transmission-reducing activity and was found to be homogeneous with respect to its size, conformation, glycosylation, and folding103. Several studies showed that chimeras of the 6 C subunit of Pfs48/45 produced in Lactococcus lactis are a strong candidate for TBV104–106. In another study, monoclonal antibody 85RF45.1 against Pfs48/45 demonstrated a strong reduction of parasite transmission107. A recent study reported that a chimeric protein construct of Pfs230 and Pfs48/45, formed by the fusion of the Prodomain of Pfs230 and 6 C fragment of Pfs48/45, showed a higher TB potency than the single proteins alone108. This finding bolsters the previous findings with the Pfs25 and Pfs28 fusion protein. All together we can assume that protein chimeras of two or more different proteins can be used to generate antibodies with higher TB activity.
Pfs47 and HAP2
Pfs47 and HAP2 are other recently identified TBV target proteins. Pfs47 is a member of the 6-cys family of proteins expressed only in macrogametocytes, macrogametes, and ookinetes94. Although Pfs47 was found to be dispensable for female gamete fertility, it mediates parasite evasion of the mosquito immune system by suppressing the effect of midgut nitration, a crucial reaction to activate the complement-like system68,69,109. While monoclonal antibodies against the full-length Pfs47 protein failed to show TB activity68, antibodies targeting a specific region of Pfs47 revealed a significant TB activity110. More importantly, conjugation of Pfs47 with AP205 VLP enhanced its immunogenicity and TB activity111. Likewise, an in vivo study identified a specific region of P. berghei P47 (Pbs47) that offers protection against mosquito immunity. This particular domain has similar immunogenicity to that of Pfs47. Conjugation of the protective antigenic region to a bacteriophage AP205-VLP enhanced immunogenicity and TB activity112. A conjugated form of different vaccine candidates has shown higher immunogenicity and better TB activity. Therefore, a conjugated vaccine form of candidate antigens may help resolve the issue of poor immunogenicity during the development of an effective malaria TBV.
On the other hand, PfHAP2, a family member of HAP2 family protein, is expressed only in male gametocytes and activated male gametes113. The PfHAP2 recombinant protein-induced IgG antibodies have a significant TB activity114. In P. berghei, PbHAP2 induced antibodies also showed potent in vitro and in vivo TB activity113. A recent study also showed that the anti-Plasmodium vivax HAP2 (PvHAP2) antisera significantly reduced the oocyst numbers in mosquito feeding assays using clinical P. vivax isolates115. Collectively, these findings emphasize that HAP2 is a promising TBV candidate that warrants further investigation and trials.
Midgut protein interactions affecting Plasmodium transmission
Much of TBV research effort has focused on discovering parasite proteins involved in mosquito invasion. However, recently a concerted effort has been made to elucidate mosquito midgut proteins involved in the parasite invasion process. Essential molecular interactions between the mosquito and the parasite have been discovered, revealing prime TBV candidates such as the AnAPN1, FREP1, and AgPfs47Rec (Fig. 1) as promising TBV targets. Besides these major players, in this section, we will also describe newly discovered mosquito midgut proteins that play a role in the P. falciparum transmission process.
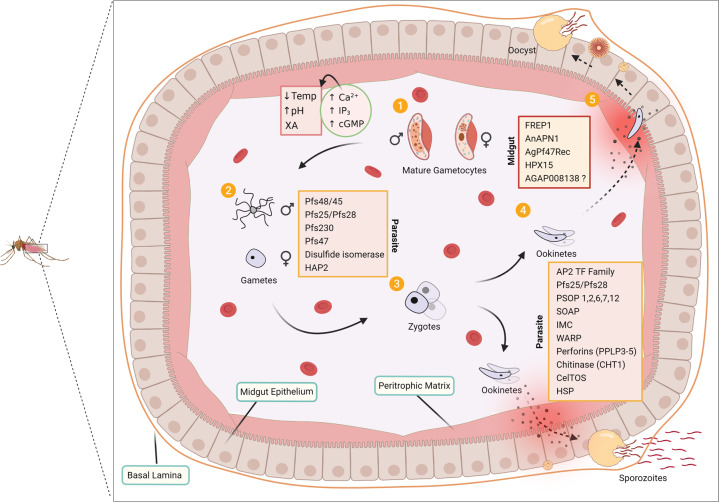
Fig. 1. Key proteins responsible for the development of P. falciparum parasites inside the Anopheles mosquito midgut.
1 The male and female gametocytes ingested during a blood meal egress from the RBCs and become extracellular gametocytes which then develop into male and female gametes via numerous factors such as an increase in pH, a decrease in temperature, and the presence of XA in the midgut environment. This leads to a rise in intracellular Ca2+, IP3, and cGMP signaling and to the development of gametes. 2, 3 The microgametes (male) and the macrogametes (female) increase expression of certain key proteins such as Pfs48/45 and Pfs47 and generate a complete zygote after fertilization. 4 Zygotes develop into motile ookinetes that can penetrate the PM by breaking its chitin structure through chitinases (CHT1), perforins (PPLP3-5), and many other enzymes. 5 Several midgut proteins such as AnAPN1 and FREP1 also help parasites penetrate the peritrophic membrane and midgut epithelium to develop into oocysts at the basal lamina side.
AnAPN1
Anopheles alanyl aminopeptidase N (AnAPN1) is considered an important and promising mosquito-based TBV candidate. It is found in the midgut of A. gambiae and acts as a ligand for P. falciparum and P. berghei ookinete invasion116,117. The midgut microvilli glycol-conjugates interact with the ookinete and enhances infection of the mosquito117. Mosquito midgut glycoproteins are extensively glycosylated with a high proportion of N-linked N-acetylglucosamine (GlcNAc) and N-acetylgalactosamine (GalNAc) terminal oligosaccharides. Glycans, such as GalNAc, and lectins like jacalin, reduce parasite invasion inside the mosquito midgut118,119. AnAPN1 at the A. gambiae midgut luminal surface is the principal jacalin target and plays an important role in ookinete invasion. The conserved role of AnAPN1 in ookinete invasion of the mosquito midgut explained by the ability of anti-AnAPN1 IgG to strongly inhibit both P. berghei and P. falciparum development in various mosquito species117. Identifying the specific domain of the ligand that interacts with the parasite is important for designing an appropriate TBV.
The AnAPN1 monoclonal antibody, 4H5B7 mAb, showed a potent TB activity at low concentrations116. The recombinant AnAPN1 demonstrated acceptable vaccine potency and immunogenicity without immunization-related histopathologies in mice. The AnAPN1 protein has four domains (I to IV), and antibodies against the N-terminal domain-I were found to interfere with the sexual cycle of P. falciparum and P. berghei117,120. Although more research is still required to identify the critical interactions, the resolution of the structure of AnAPN1 is a major advance, which will guide structure-based vaccine design. One such attempt involved using only the relevant domain I of AnAPN1 as the immunogen to reduce unspecific immune stimulation. The domain I construct elicited adequate immune responses in mice, and the IgG antibodies showed potent TB activity121.
FREP1
FREP1 presents another leading candidate for a promising mosquito-based TBV. FREP1 belongs to the fibrinogen-related protein (FREP/FBN) family. In mammals, FREPs play a fundamental role in blood coagulation, while in mosquitoes, they generally act as pattern recognition receptors122. Several FREPs activate the mosquito immune system by attaching to external pathogens such as parasites, bacteria, and fungi to activate downstream defense signaling pathways123,124. FBN9 and FBN30 were found to inhibit Plasmodium infection in midgut epithelial cells. Their functions were illustrated by the increased Plasmodium infection of the midgut when FBN9 or FBN30 was knocked down125,126. FBN30 is a secreted octameric protein and specifically interacts with the P. berghei blood stages and ookinetes, but not sporozoites127. Intriguingly, FBN30 was found to interact with the clinically circulating P. falciparum, but no interaction occurred with the NF54 P. falciparum laboratory strain127. Understanding the underlying mechanism for this difference may be helpful for TBV development.
Anopheles FREP1 was discovered through an association study using clinically circulating P. falciparum and wild-derived A. gambiae126. Unlike FBN9 and FBN30, which hinder Plasmodium infection, FREP1 serves as a molecular anchor in the PM, assisting P. falciparum ookinete invasion of the mosquito midgut128. The Plasmodium α-tubulin-1 protein was found to strongly interact with FREP1 during in vitro studies, and antibodies against α-tubulin-1 blocked malaria transmission inside the mosquito129. An increased understanding of the molecular interaction of FREP1 with the α-tubulin-1 may offer another promising TBV candidate. Lastly, it was shown that FREP1 CRISPR-Cas9 knockout mosquitoes exhibited a profound suppression of parasite infection at both the oocyst and sporozoite stages. Yet, inactivation of the FREP1 gene inflicted substantial fitness costs on blood-feeding propensity, longevity following a blood meal, fecundity, as well as the rate of egg hatching53. A study identified direct interactions of the fibrinogen-like (FBG) domain of FREP1 with P. falciparum gametocytes and ookinetes. FBG is highly conserved in different species of Anopheles mosquitoes and presents as an effective TBV target against infection from a variety of Plasmodium species124. The FBG region between amino acids 463 and 677 of A. gambiae FREP1 shows more than 90% identity in protein sequence with the FREP1 FBG in 13 anopheline species, suggesting that anti-FBG IgG may potentially block malaria transmission in these 13 mosquito species5. Notably, anti-FBG serum showed greater than 81% TB efficiency against P. falciparum in A. gambiae and ~67% TB efficiency against P. vivax in A. dirus23. This finding further supports that FREP1, specifically the FBG domain, is an ideal TBV target.
AgPfs47Rec (AGAP006398)
A recent study revealed that Pfs47 mediates immune evasion in mosquitoes through interaction with the Anopheles midgut receptor protein AgPf47Rec (AGAP006398)130. The interaction allows the parasite to evade the mosquito immune system by disrupting the c-Jun-N-terminal kinase (JNK) signaling pathway and eventually suppressing the effect of midgut nitration, a crucial reaction to activate the mosquito complement-like system68,69,109,131,132. Further studies are needed to determine if antibodies against AgPfs47Rec possess TB activity. In addition, the identification of mosquito species-specific AgPfs47Rec and its interaction with various Pfs47 haplotypes provide increasing evidence for a highly specific lock and key model between the ligand and receptor130, demonstrating a natural selection of Pfs47 haplotypes in the P. falciparum adaptation to different mosquito vectors.
Other midgut proteins
An increasing number of midgut proteins interacting with the parasite and mediating parasite transmission to mosquitoes have been discovered (Table 3). In a recent study, the Anopheles midgut protein AGAP008138 was found to interact with sexual-stage Plasmodium parasites and, upon knockdown, increased mosquito susceptibility to P. falciparum133. Likewise, the evolutionarily conserved heme peroxidase gene HPX15134 was found to interact with the P. falciparum sexual stages133, and knocking down its expression in the midgut after a bloodmeal decreased the oocyst load in A. gambiae due to the reduced integrity of the mucin barrier and subsequent activation of midgut immunity pathways135. In addition, some immunoglobulin-like secreted proteins such as AGAP002848 and AGAP002851 are pattern-recognition molecules and possibly potent inhibitors of parasite infection133. Some structure-related proteins such as AGAP006972 and AGAP006268 are needed to maintain the integrity of the midgut PM and suppress parasitic invasion133,136. These newly identified midgut proteins are all worthwhile of further investigation to determine their TB potential and suitability.
Table 3.
Recognized Anopheles midgut proteins involved in malaria transmission.
| Protein name | Function | Current status | References |
|---|---|---|---|
| AnAPN1(AGAP004809) | Ligand for ookinete invasion into the midgut | Preclinical development | 116,117 |
| FREP1(AGAP007031) | Enabling ookinete invasion into the midgut | Preclinical development | 5,128 |
| HPX15(AGAP013327) | Supports parasite development inside the midgut lumen | Novel candidate | 133–135 |
| AgP47Rec (AGAP006398) | Plays a critical role in parasite survival by interacting with Pfs47 | Novel candidate | 130 |
| AGAP008138 | Solely ookinete invasion facilitating | Novel candidate | 133 |
| AGAP002848 | Protective Immune Niemann Pick Type C2 | Novel candidate | 133 |
| AGAP002851 | Protective Immune Niemann Pick Type C2 | Novel candidate | 133 |
| AGAP006972 | Unknown | Novel candidate | 133 |
| AGAP006268 | Involved in peritrophic matrix formation | Novel candidate | 133 |
Key: AGAP Anopheles gambiae gene identifier, AgP47Rec Pfs47 receptor, AnAPN1 Anopheles alanyl aminopeptidase N 1, FREP1 fibrinogen-related protein 1, HPX15 Heme peroxidase 15.
Conclusion
Here we reviewed recent advances in the study of malaria transmission, focusing specifically on molecular mechanisms of interactions between P. falciparum and A. gambiae mosquito midgut proteins. Overall, TBVs can halt the transmission cycle of the malaria parasite between the mosquito and its vertebrate host. Although TBV development has met many challenges, recent studies have identified novel protein candidates from both the parasite and vector sides. Further investigation of the molecular mechanisms of interaction between the mosquito midgut proteins and the malaria parasite will provide fundamental insights into malaria transmission and accelerate TBV development, ultimately bringing us one step closer to eradicating this devastating disease from this planet.
Acknowledgements
Our Fig. 1 was created with Biorender.com and applicable license. J.L. was supported by grant R01AI125657 from NIAID and the NSF Career Award 1742644. L.C. was supported by a grant U19AI089672 from NIAID, NIH. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Y.K. and J.R. are both considered co-first authors. Conceptualization: J.L.; Writing: Y.K., J.R., and J.L.; Visualization: J.R.; Editing: L.C. and J.L. All authors read and approved the final manuscript.
Data availability
All data are provided in the main text, tables and the figure.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cowman AF, Tonkin CJ, Tham WH, Duraisingh MT. The molecular basis of erythrocyte invasion by malaria parasites. Cell Host Microbe. 2017;22:232–245. doi: 10.1016/j.chom.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 2.WHO. World Malaria Report 2019. (2019).
- 3.Alonso, P. L. et al. A research agenda to underpin malaria eradication. PLoS Med.10.1371/journal.pmed.1000406 (2011). [DOI] [PMC free article] [PubMed]
- 4.Gardiner DL, Trenholme KR. Plasmodium falciparum gametocytes: playing hide and seek. Ann. Transl. Med. 2015;3:8–10. doi: 10.3978/j.issn.2305-5839.2015.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niu G, et al. The fibrinogen-like domain of FREP1 protein is a broad-spectrum malaria transmission-blocking vaccine antigen. J. Biol. Chem. 2017;292:11960–11969. doi: 10.1074/jbc.M116.773564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The malERA, C. G. o. V. A research agenda for malaria eradication: Vaccines. PLoS Med.10.1371/journal.pmed.1000398 (2011). [DOI] [PMC free article] [PubMed]
- 7.Conrad MD, Rosenthal PJ. Antimalarial drug resistance in Africa: the calm before the storm? Lancet Infect. Dis. 2019;19:e338–e351. doi: 10.1016/S1473-3099(19)30261-0. [DOI] [PubMed] [Google Scholar]
- 8.Alout, H., Roche, B., Dabiré, R. K. & Cohuet, A. Consequences of insecticide resistance on malaria transmission. PloS Pathog.10.1371/journal.ppat.1006499 (2017). [DOI] [PMC free article] [PubMed]
- 9.Adepoju P. RTS,S malaria vaccine pilots in three African countries. Lancet. 2019;393:1685–1685. doi: 10.1016/S0140-6736(19)30937-7. [DOI] [PubMed] [Google Scholar]
- 10.Chedraui PA, et al. Final results from a phase 3, individually randomised, controlled trial of the RTS,S/AS01 malaria vaccine in African infants and children, including an evaluation of the efficacy of a booster dose. UpToDate. 2016;374:2519–2529. [Google Scholar]
- 11.Vandoolaeghe P, Schuerman L. [The RTS,S/AS01 malaria vaccine in children aged 5-17 months at first vaccination] PanAfr. Med. J. 2018;30:142. doi: 10.11604/pamj.2018.30.142.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagara I, et al. Safety and immunogenicity of Pfs25H-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum: a randomised, double-blind, comparator-controlled, dose-escalation study in healthy Malian adults. Lancet Infect. Dis. 2018;18:969–982. doi: 10.1016/S1473-3099(18)30344-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osier FH, et al. New antigens for a multicomponent blood-stage malaria vaccine. Sci. Transl. Med. 2014;6:247ra102. doi: 10.1126/scitranslmed.3008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherrard-Smith E, et al. Synergy in anti-malarial pre-erythrocytic and transmission-blocking antibodies is achieved by reducing parasite density. Elife. 2018;7:e35213. doi: 10.7554/eLife.35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabinovich RN, et al. malERA: An updated research agenda for malaria elimination and eradication. PLoS Med. 2017;14:e1002456. doi: 10.1371/journal.pmed.1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draper SJ, et al. Malaria vaccines: recent advances and new horizons. Cell Host Microbe. 2018;24:43–56. doi: 10.1016/j.chom.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delves MJ, Angrisano F, Blagborough AM. Antimalarial transmission-blocking interventions: Past, present, and future. Trends Parasitol. 2018;34:735–746. doi: 10.1016/j.pt.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Alaganan AÃ, Singh PÃ, Chitnis CE. Molecular mechanisms that mediate invasion and egress of malaria parasites from red blood cells. Curr. Opin. Hematol. 2017;24:208–214. doi: 10.1097/MOH.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 19.Belachew, E. B. Immune Response and Evasion Mechanisms of Plasmodium falciparum Parasites. J. Immunol. Res.10.1155/2018/6529681 (2018). [DOI] [PMC free article] [PubMed]
- 20.Crutcher, J. M. & Hoffman, S. L. Medical Microbiology for Malaria (University of Texas Medical Branch at Galveston, 1996). [PubMed]
- 21.Soulard, V. et al. Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice. Nat. Commun.10.1038/ncomms8690 (2015). [DOI] [PMC free article] [PubMed]
- 22.Klein EY. Antimalarial drug resistance: a review of the biology and strategies to delay emergence and spread. Int. J. Antimicrob. Agents. 2013;41:311–317. doi: 10.1016/j.ijantimicag.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Natalie, J. & Nokoff, N. P. S. D. E. S. Gametocytogenesis in malaria parasites: commitment, development and regulation. Futur. Microbiol.6, 1351–1369 (2011). [DOI] [PMC free article] [PubMed]
- 24.Josling GA, Williamson KC, Llinas M. Regulation of sexual commitment and gametocytogenesis in malaria parasites. Annu Rev. Microbiol. 2018;72:501–519. doi: 10.1146/annurev-micro-090817-062712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brancucci, N. M. B. et al. Probing Plasmodium falciparum sexual commitment at the single-cell level [version 4; referees: 2 approved]. Wellcome Open Res.10.12688/wellcomeopenres.14645.4 (2018). [DOI] [PMC free article] [PubMed]
- 26.Bruce MC, Alano P, Duthie S, Carter R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990;100:191–200. doi: 10.1017/s0031182000061199. [DOI] [PubMed] [Google Scholar]
- 27.Ngwa, C. J., Rosa, T. F. d. A. & Pradel, G. in Current Topics in Malaria (ed. Alfonso J. Rodriguez-Morales) (Autonomous Univ. Foundation of the Americas, 2016).
- 28.Nacher M, et al. Decreased hemoglobin concentrations, hyperparasitemia, and severe malaria are associated with increased Plasmodium falciparum gametocyte carriage. J. Parasitol. 2002;88:97–101. doi: 10.1645/0022-3395(2002)088[0097:DHCHAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Price R, et al. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 1999;60:1019–1023. doi: 10.4269/ajtmh.1999.60.1019. [DOI] [PubMed] [Google Scholar]
- 30.Stepniewska K, et al. Plasmodium falciparum gametocyte dynamics in areas of different malaria endemicity. Malar. J. 2008;7:249–249. doi: 10.1186/1475-2875-7-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Von Seidlein L, Drakeley C, Greenwood B, Walraven G, Targett G. Risk factors for gametocyte carriage in Gambian children. Am. J. Trop. Med Hyg. 2001;65:523–527. doi: 10.4269/ajtmh.2001.65.523. [DOI] [PubMed] [Google Scholar]
- 32.Buckling A, Ranford-Cartwright LC, Miles A, Read AF. Chloroquine increases Plasmodium falciparum gametocytogenesis in vitro. Parasitology. 1999;118:339–346. doi: 10.1017/s0031182099003960. [DOI] [PubMed] [Google Scholar]
- 33.Bousema J, Drakeley C, Sauerwein R. Sexual-stage antibody responses to P. falciparum in endemic populations. Curr. Mol. Med. 2006;6:223–229. doi: 10.2174/156652406776055140. [DOI] [PubMed] [Google Scholar]
- 34.Meibalan, E. & Marti, M. Biology of malaria transmission. Cold Spring Harb. Perspect. Med.10.1101/cshperspect.a025452 (2017). [DOI] [PMC free article] [PubMed]
- 35.Carter R, Miller LH. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull. World Health Organ. 1979;57:37–52. [PMC free article] [PubMed] [Google Scholar]
- 36.Ono T, Ohnishi Y, Nagamune K, Kano M. Gametocytogenesis induction by Berenil in cultured Plasmodium falciparum. Exp. Parasitol. 1993;77:74–78. doi: 10.1006/expr.1993.1062. [DOI] [PubMed] [Google Scholar]
- 37.Smalley ME, Brown J. Plasmodium falciparum gametocytogenesis stimulated by lymphocytes and serum from infected Gambian children. Trans. R. Soc. Trop. Med. Hyg. 1981;75:316–317. doi: 10.1016/0035-9203(81)90348-5. [DOI] [PubMed] [Google Scholar]
- 38.Josling GA, et al. Dissecting the role of PfAP2-G in malaria gametocytogenesis. Nat. Commun. 2020;11:1–13. doi: 10.1038/s41467-020-15026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kafsack BFC, et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507:248–252. doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Llorà-Batlle O, et al. Conditional expression of PfAP2-G for controlled massive sexual conversion in Plasmodium falciparum. Sci. Adv. 2020;6:eaaz5057–eaaz5057. doi: 10.1126/sciadv.aaz5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eksi, S. et al. Plasmodium falciparum Gametocyte Development 1 (Pfgdv1) and gametocytogenesis early gene identification and commitment to sexual development. PLoS Pathog.10.1371/journal.ppat.1002964 (2012). [DOI] [PMC free article] [PubMed]
- 42.Filarsky M, et al. GDV1 induces sexual commitment of malaria parasites by antagonizing HP1-dependent gene silencing. Science. 2018;359:1259–1263. doi: 10.1126/science.aan6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Usui M, et al. Plasmodium falciparum sexual differentiation in malaria patients is associated with host factors and GDV1-dependent genes. Nat. Commun. 2019;10:2140. doi: 10.1038/s41467-019-10172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardiner, D. L. et al. Implication of a Plasmodium falciparum gene in the switch between asexual reproduction and gametocytogenesis. Mol. Biochem. Parasitol.10.1016/j.molbiopara.2004.12.010 (2005). [DOI] [PubMed]
- 45.Dechering KJ, Thompson J, Dodemont HJ, Eling W, Konings RNH. Developmentally regulated expression of pfs16, a marker for sexual differentiation of the human malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 1997;89:235–244. doi: 10.1016/s0166-6851(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 46.Kongkasuriyachai D, Fujioka H, Kumar N. Functional analysis of Plasmodium falciparum parasitophorous vacuole membrane protein (Pfs16) during gametocytogenesis and gametogenesis by targeted gene disruption. Mol. Biochem. Parasitol. 2004;133:275–285. doi: 10.1016/j.molbiopara.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Joice, R. et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med.10.1126/scitranslmed.3008882 (2014). [DOI] [PMC free article] [PubMed]
- 48.Christophides GK, Vlachou D, Kafatos FC. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol. Rev. 2004;198:127–148. doi: 10.1111/j.0105-2896.2004.0127.x. [DOI] [PubMed] [Google Scholar]
- 49.Toprak U, Baldwin D, Erlandson M, Gillott C, Hegedus DD. Insect intestinal mucins and serine proteases associated with the peritrophic matrix from feeding, starved and moulting Mamestra configurata larvae. Insect Mol. Biol. 2010;19:163–175. doi: 10.1111/j.1365-2583.2009.00966.x. [DOI] [PubMed] [Google Scholar]
- 50.Dinglasan RR, et al. The Anopheles gambiae adult midgut peritrophic matrix proteome. Insect Biochem. Mol. Biol. 2009;39:125–134. doi: 10.1016/j.ibmb.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Billingsley PF. Blood digestion in the mosquito, Anopheles stephensi Liston (Diptera: Culicidae): partial characterization and post-feeding activity of midgut aminopeptidases. Arch. Insect Biochem. Physiol. 1990;15:149–163. doi: 10.1002/arch.940150304. [DOI] [PubMed] [Google Scholar]
- 52.Shao L, Devenport M, Jacobs-Lorena M. The peritrophic matrix of hematophagous insects. Arch. Insect Biochem. Physiol. 2001;47:119–125. doi: 10.1002/arch.1042. [DOI] [PubMed] [Google Scholar]
- 53.Dong Y, Simoes ML, Marois E, Dimopoulos G. CRISPR/Cas9-mediated gene knockout of Anopheles gambiae FREP1 suppresses malaria parasite infection. PLoS Pathog. 2018;14:e1006898–e1006898. doi: 10.1371/journal.ppat.1006898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghosh A, Edwards MJ, Jacobs-Lorena M. The journey of the malaria parasite in the mosquito: hopes for the new century. Parasitol. Today. 2000;16:196–201. doi: 10.1016/s0169-4758(99)01626-9. [DOI] [PubMed] [Google Scholar]
- 55.Billker O, et al. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- 56.Sologub L, et al. Malaria proteases mediate inside-out egress of gametocytes from red blood cells following parasite transmission to the mosquito. Cell. Microbiol. 2011;13:897–912. doi: 10.1111/j.1462-5822.2011.01588.x. [DOI] [PubMed] [Google Scholar]
- 57.McRobert L, et al. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol. 2008;6:e139. doi: 10.1371/journal.pbio.0060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang Y, et al. An intracellular membrane protein GEP1 regulates xanthurenic acid induced gametogenesis of malaria parasites. Nat. Commun. 2020;11:1–15. doi: 10.1038/s41467-020-15479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hitz E, Balestra AC, Brochet M, Voss TS. PfMAP-2 is essential for male gametogenesis in the malaria parasite Plasmodium falciparum. Sci. Rep. 2020;10:1–16. doi: 10.1038/s41598-020-68717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wirth CC, et al. Perforin-like protein PPLP2 permeabilizes the red blood cell membrane during egress of Plasmodium falciparum gametocytes. Cell. Microbiol. 2014;16:709–733. doi: 10.1111/cmi.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh P, et al. Role of a patatin-like phospholipase in Plasmodium falciparum gametogenesis and malaria transmission. Proc. Natl Acad. Sci. USA. 2019;116:17498–17508. doi: 10.1073/pnas.1900266116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niu, G., Cui, Y., Wang, X., Keleta, Y. & Li, J. Studies of the parasite-midgut interaction reveal plasmodium proteins important for malaria transmission to mosquitoes. Front. Cell. Infect. Microbiol.10.3389/fcimb.2021.654216 (2021). [DOI] [PMC free article] [PubMed]
- 63.Liu F, et al. Characterization of Plasmodium berghei Pbg37 as both a pre- and postfertilization antigen with transmission-blocking potential. Infect. Immun. 2018;86:IAI.00785–00717. doi: 10.1128/IAI.00785-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Dijk MR, et al. A central role for P48/45 in malaria parasite male gamete fertility. Cell. 2001;104:153–164. doi: 10.1016/s0092-8674(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 65.Eksi S, et al. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol. Microbiol. 2006;61:991–998. doi: 10.1111/j.1365-2958.2006.05284.x. [DOI] [PubMed] [Google Scholar]
- 66.van Dijk MR, et al. Three members of the 6-cys protein family of plasmodium play a role in gamete fertility. PLoS Pathog. 2010;6:1–13. doi: 10.1371/journal.ppat.1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Angrisano F, Sala KA, Tapanelli S, Christophides GK, Blagborough AM. Male-specific protein disulphide isomerase function is essential for plasmodium transmission and a vulnerable target for intervention. Sci. Rep. 2019;9:1–14. doi: 10.1038/s41598-019-54613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Schaijk BCL, et al. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 2006;149:216–222. doi: 10.1016/j.molbiopara.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 69.Molina-Cruz A, et al. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science. 2013;340:984–987. doi: 10.1126/science.1235264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ukegbu CV, et al. Plasmodium berghei P47 is essential for ookinete protection from the Anopheles gambiae complement-like response. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-05917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 2008;22:1051–1068. doi: 10.1101/gad.1656508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuda M, et al. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Mol. Microbiol. 2009;71:1402–1414. doi: 10.1111/j.1365-2958.2009.06609.x. [DOI] [PubMed] [Google Scholar]
- 73.Kaneko I, Iwanaga S, Kato T, Kobayashi I, Yuda M. Genome-wide identification of the target genes of AP2-O, a plasmodium AP2-family transcription factor. PLoS Pathog. 2015;11:1004905–1004905. doi: 10.1371/journal.ppat.1004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bennink S, Kiesow MJ, Pradel G. The development of malaria parasites in the mosquito midgut. Cell Microbiol. 2016;18:905–918. doi: 10.1111/cmi.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dessens JT, et al. Soap, a novel malaria ookinete protein involved in mosquito midgut invasion and oocyst development. Mol. Microbiol. 2003;49:319–329. doi: 10.1046/j.1365-2958.2003.03566.x. [DOI] [PubMed] [Google Scholar]
- 76.Yuda M, Yano K, Tsuboi T, Torii M, Chinzei Y. von Willebrand Factor A domain-related protein, a novel microneme protein of the malaria ookinete highly conserved throughout Plasmodium parasites. Mol. Biochem. Parasitol. 2001;116:65–72. doi: 10.1016/s0166-6851(01)00304-8. [DOI] [PubMed] [Google Scholar]
- 77.Dessens JT, et al. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 1999;18:6221–6227. doi: 10.1093/emboj/18.22.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tomas AM, et al. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J. 2001;20:3975–3983. doi: 10.1093/emboj/20.15.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saxena AK, Wu Y, Garboczi DN. Plasmodium P25 and P28 surface proteins: potential transmission-blocking vaccines. Eukaryot. Cell. 2007;6:1260–1265. doi: 10.1128/EC.00060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Langer RC, Vinetz JM. Plasmodium ookinete-secreted chitinase and parasite penetration of the mosquito peritrophic matrix. Trends Parasitol. 2001;17:269–272. doi: 10.1016/s1471-4922(01)01918-3. [DOI] [PubMed] [Google Scholar]
- 81.Gao, H. et al. ISP1-anchored polarization of GCb/CDC50A complex initiates malaria ookinete gliding motility article ISP1-anchored polarization of GCb/CDC50A complex initiates malaria ookinete gliding motility. Current Biol.10.1016/j.cub.2018.06.069 (2018). [DOI] [PubMed]
- 82.Acquah, F. K., Adjah, J., Williamson, K. C. & Amoah, L. E. Transmission-blocking vaccines: old friends and new prospects. Infect Immun.10.1128/IAI.00775-18 (2019). [DOI] [PMC free article] [PubMed]
- 83.Kaslow DC, et al. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 84.Wu, Y. et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs 25 formulated with montanide ISA 51. PLoS ONE10.1371/journal.pone.0002636 (2008). [DOI] [PMC free article] [PubMed]
- 85.Li, F., Bounkeua, V., Pettersen, K. & Vinetz, J. M. Plasmodium falciparum ookinete expression of plasmepsin VII and plasmepsin X. Malar. J.10.1186/s12936-016-1161-5 (2016). [DOI] [PMC free article] [PubMed]
- 86.Brod F, et al. Combination of RTS,S and Pfs25-IMX313 induces a functional antibody response against malaria infection and transmission in mice. Front. Immunol. 2018;9:2780–2780. doi: 10.3389/fimmu.2018.02780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shimp RL, et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine. 2013;31:2954–2962. doi: 10.1016/j.vaccine.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Talaat, K. R. et al. Safety and immunogenicityof Pfs25-EPA/ alhydrogel1, a transmission blocking vaccine against Plasmodium falciparum: an open label study in malaria naïve adults. PLoS ONE10.1371/journal.pone.0163144 (2016). [DOI] [PMC free article] [PubMed]
- 89.Chichester JA, et al. Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: a phase 1 dose-escalation study in healthy adults. Vaccine. 2018;36:5865–5871. doi: 10.1016/j.vaccine.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duffy PE, Kaslow DC. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect. Immun. 1997;65:1109–1113. doi: 10.1128/iai.65.3.1109-1113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qian F, et al. Enhanced antibody responses to Plasmodium falciparum Pfs28 induced in mice by conjugation to ExoProtein A of Pseudomonas aeruginosa with an improved procedure. Microbes Infect. 2009;11:408–412. doi: 10.1016/j.micinf.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gozar MM, Price VL, Kaslow DC. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infect. Immun. 1998;66:59–64. doi: 10.1128/iai.66.1.59-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaslow DC. Transmission-blocking vaccines: uses and current status of development. Int. J. Parasitol. 1997;27:183–189. doi: 10.1016/s0020-7519(96)00148-8. [DOI] [PubMed] [Google Scholar]
- 94.Templeton TJ, Kaslow DC. Identification of additional members define a Plasmodium falciparum gene superfamily which includes Pfs48/45 and Pfs230. Mol. Biochem. Parasitol. 1999;101:223–227. doi: 10.1016/s0166-6851(99)00066-3. [DOI] [PubMed] [Google Scholar]
- 95.Acquah, F. K. et al. Antibody responses to two new Lactococcus lactis-produced recombinant Pfs48/45 and Pfs230 proteins increase with age in malaria patients living in the Central Region of Ghana. Malar. J.10.1186/s12936-017-1955-0 (2017). [DOI] [PMC free article] [PubMed]
- 96.Carter R, Coulson A, Bhatti S, Taylor BJ, Elliott JF. Predicted disulfide-bonded structures for three uniquely related proteins of Plasmodium falciparum, Pfs230, Pfs4845 and Pf12. Mol. Biochem. Parasitol. 1995;71:203–210. doi: 10.1016/0166-6851(94)00054-q. [DOI] [PubMed] [Google Scholar]
- 97.Gerloff DL, Creasey A, Maslau S, Carter R. Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. Proc. Natl Acad. Sci. USA. 2005;102:13598–13603. doi: 10.1073/pnas.0502378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tachibana M, et al. N-terminal prodomain of Pfs230 synthesized using a cell-free system is sufficient to induce complement-dependent malaria transmission-blocking activity. Clin. Vaccin. Immunol. 2011;18:1343–1350. doi: 10.1128/CVI.05104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee, S. M. et al. N-terminal Pfs230 domain produced in baculovirus as a biological active transmission-blocking vaccine candidate. Clin. Vaccine Immunol. 10.1128/CVI.00140-17 (2017). [DOI] [PMC free article] [PubMed]
- 100.Lee, S.-M. et al. The Pfs230 N-terminal fragment, Pfs230D1+: expression and characterization of a potential malaria transmission-blocking vaccine candidate. Malar. J.10.1186/s12936-019-2989-2 (2019). [DOI] [PMC free article] [PubMed]
- 101.Farrance CE, et al. A plant-produced Pfs230 vaccine candidate blocks transmission of Plasmodium falciparum. Clin. Vaccine Immunol. 2011;18:1351–1357. doi: 10.1128/CVI.05105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scaria PV, et al. Outer membrane protein complex as a carrier for malaria transmission blocking antigen Pfs230. npj Vaccines. 2019;4:24–24. doi: 10.1038/s41541-019-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee, S.-M. et al. A C-terminal Pfs48/45 malaria transmission-blocking vaccine candidate produced in the baculovirus expression system. Sci. Rep.10.1038/s41598-019-57384-w (2020). [DOI] [PMC free article] [PubMed]
- 104.Singh SK, et al. A Plasmodium falciparum 48/45 single epitope R0.6C subunit protein elicits high levels of transmission blocking antibodies. Vaccine. 2015;33:1981–1986. doi: 10.1016/j.vaccine.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 105.Singh, S. K. et al. Construct design, production, and characterization of Plasmodium falciparum 48/45 R0.6C subunit protein produced in Lactococcus lactis as candidate vaccine. Microb. Cell Factories10.1186/s12934-017-0710-0 (2017). [DOI] [PMC free article] [PubMed]
- 106.Theisen M, et al. A multi-stage malaria vaccine candidate targeting both transmission and asexual parasite life-cycle stages. Vaccine. 2014;32:2623–2630. doi: 10.1016/j.vaccine.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 107.Kundu, P. et al. Structural delineation of potent transmission-blocking epitope I on malaria antigen Pfs48/45. Nat. Commun.10.1038/s41467-018-06742-9 (2018). [DOI] [PMC free article] [PubMed]
- 108.Singh SK, et al. Pfs230 and Pfs48/45 fusion proteins elicit strong transmission-blocking antibody responses against Plasmodium falciparum. Front. Immunol. 2019;10:1256–1256. doi: 10.3389/fimmu.2019.01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Molina-Cruz A, Canepa GE, Barillas-Mury C. Plasmodium P47: a key gene for malaria transmission by mosquito vectors. Curr. Opin. Microbiol. 2017;40:168–174. doi: 10.1016/j.mib.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Canepa, G. E. et al. Antibody targeting of a specific region of Pfs47 blocks Plasmodium falciparum malaria transmission. npj Vaccines10.1038/s41541-018-0065-5 (2018). [DOI] [PMC free article] [PubMed]
- 111.Yenkoidiok-Douti, L., Williams, A. E., Canepa, G. E., Molina-Cruz, A. & Barillas-Mury, C. Engineering a virus-like particle as an antigenic platform for a Pfs47-targeted malaria transmission-blocking vaccine. Sci. Rep.10.1038/s41598-019-53208-z (2019). [DOI] [PMC free article] [PubMed]
- 112.Yenkoidiok-Douti, L., Canepa, G. E., Barletta, A. B. F. & Barillas-Mury, C. In vivo characterization of Plasmodium berghei P47 (Pbs47) as a malaria transmission-blocking vaccine target. Front. Microbiol.10.3389/fmicb.2020.01496 (2020). [DOI] [PMC free article] [PubMed]
- 113.Blagborough AM, Sinden RE. Plasmodium berghei HAP2 induces strong malaria transmission-blocking immunity in vivo and in vitro. Vaccine. 2009;27:5187–5194. doi: 10.1016/j.vaccine.2009.06.069. [DOI] [PubMed] [Google Scholar]
- 114.Miura K, et al. Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect. Immun. 2013;81:4377–4382. doi: 10.1128/IAI.01056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qiu Y, et al. Evaluation of Plasmodium vivax HAP2 as a transmission-blocking vaccine candidate. Vaccine. 2020;38:2841–2848. doi: 10.1016/j.vaccine.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Atkinson SC, et al. The Anopheles-midgut APN1 structure reveals a new malaria transmission-blocking vaccine epitope. Nat. Struct. Mol. Biol. 2015;22:532–539. doi: 10.1038/nsmb.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dinglasan RR, et al. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. PNAS. 2007;104:13461–13466. doi: 10.1073/pnas.0702239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wilkins S, Billingsley PF. Partial characterization of oligosaccharides expressed on midgut microvillar glycoproteins of the mosquito, Anopheles stephensi Liston. Insect Biochem. Mol. Biol. 2001;31:937–948. doi: 10.1016/s0965-1748(01)00040-6. [DOI] [PubMed] [Google Scholar]
- 119.Wilkins, S. & Billingsley, P. F. Mosquito cell line glycoproteins: an unsuitable model system for the Plasmodium ookinete-mosquito midgut interaction? Parasites Vectors.10.1186/1756-3305-3-22 (2010). [DOI] [PMC free article] [PubMed]
- 120.Mathias DK, et al. Expression, immunogenicity, histopathology, and potency of a mosquito-based malaria transmission-blocking recombinant vaccine. Infect. Immun. 2012;80:1606–1614. doi: 10.1128/IAI.06212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bender, N. G. et al. Immunofocusing humoral immunity potentiates the functional efficacy of the AnAPN1 malaria transmission-blocking vaccine antigen. npj Vaccines10.1038/s41541-021-00309-4 (2021). [DOI] [PMC free article] [PubMed]
- 122.Hanington PC, Zhang S-M. The primary role of fibrinogen-related proteins in invertebrates is defense, not coagulation. J. Innate Immun. 2011;3:17–27. doi: 10.1159/000321882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Doolittle RF, McNamara K, Lin K. Correlating structure and function during the evolution of fibrinogen-related domains. Protein Sci. 2012;21:1808–1823. doi: 10.1002/pro.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang, X., Zhao, Q. & Christensen, B. M. Identification and characterization of the fibronogen-like domain of fibronogen-related proteins in the mosquito, Anopheles gambiae, and the fruitfly, Drosophila melanogaster, genomes. BMC Genom.10.1186/1471-2164-6-114 (2005). [DOI] [PMC free article] [PubMed]
- 125.Dong Y, Dimopoulos G. Anopheles fibrinogen-related proteins provide expanded pattern recognition capacity against bacteria and malaria parasites. J. Biol. Chem. 2009;284:9835–9844. doi: 10.1074/jbc.M807084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li J, et al. Genome-block expression-assisted association studies discover malaria resistance genes in Anopheles gambiae. Proc. Natl Acad. Sci. USA. 2013;110:20675–20680. doi: 10.1073/pnas.1321024110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Niu, G. et al. FBN30 in wild Anopheles gambiae functions as a pathogen recognition molecule against clinically circulating Plasmodium falciparum in malaria endemic areas in Kenya. Sci. Rep.10.1038/s41598-017-09017-3 (2017). [DOI] [PMC free article] [PubMed]
- 128.Zhang G, et al. Anopheles midgut FREP1 mediates Plasmodium invasion. J. Biol. Chem. 2015;290:16490–16501. doi: 10.1074/jbc.M114.623165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang, G., Niu, G., Perez, L., Wang, X. & Li, J. Malaria transmission assisted by interaction between Plasmodium α-tubulin-1 and Anopheles FREP1 protein. Preprint at bioRxivhttps://www.biorxiv.org. (2019).
- 130.Molina-Cruz A, et al. Plasmodium falciparum evades immunity of anopheline mosquitoes by interacting with a Pfs47 midgut receptor. Proc. Natl Acad. Sci. 2020;117:2597–2605. doi: 10.1073/pnas.1917042117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith RC, Jacobs-Lorena M. Malaria parasite Pfs47 disrupts JNK signaling to escape mosquito immunity. Proc. Natl Acad. Sci. USA. 2015;112:1250–1251. doi: 10.1073/pnas.1424227112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramphul UN, Garver LS, Molina-Cruz A, Canepa GE, Barillas-Mury C. Plasmodium falciparum evades mosquito immunity by disrupting JNK-mediated apoptosis of invaded midgut cells. Proc. Natl Acad. Sci. USA. 2015;112:1273–1280. doi: 10.1073/pnas.1423586112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cui Y, Niu G, Li VL, Wang X, Li J. Analysis of blood-induced Anopheles gambiae midgut proteins and sexual stage Plasmodium falciparum interaction reveals mosquito genes important for malaria transmission. Sci. Rep. 2020;10:14316–14316. doi: 10.1038/s41598-020-71186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kajla, M. M., Gupta, K. & Kakani, P. Identification of an anopheles lineage-specific unique heme peroxidase HPX15: a plausible candidate for arresting malaria parasite development. J. Phylogenetics Evol. Biol.10.4172/2329-9002.1000160 (2015).
- 135.Kajla, M. et al. Anopheles stephensi heme peroxidase HPX15 suppresses midgut immunity to support plasmodium development. Front. Immunol.10.3389/fimmu.2017.00249 (2017). [DOI] [PMC free article] [PubMed]
- 136.Tellam RL, Wijffels G, Willadsen P. Peritrophic matrix proteins. Insect Biochem. Mol. Biol. 1999;29:87–101. doi: 10.1016/s0965-1748(98)00123-4. [DOI] [PubMed] [Google Scholar]
- 137.Siden-Kiamos I, Louis C. Interactions between malaria parasites and their mosquito hosts in the midgut. Insect Biochem. Mol. Biol. 2004;34:679–685. doi: 10.1016/j.ibmb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 138.Molina-Cruz A, et al. Plasmodium evasion of mosquito immunity and global malaria transmission: the lock-and-key theory. Proc. Natl Acad. Sci. 2015;112:15178–15183. doi: 10.1073/pnas.1520426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Langer RC, Li F, Popov V, Kurosky A, Vinetz JM. Monoclonal antibody against the Plasmodium falciparum chitinase, PfCHT1, recognizes a malaria transmission-blocking epitope in Plasmodium gallinaceum ookinetes unrelated to the chitinase PgCHT1. Infect. Immun. 2002;70:1581–1590. doi: 10.1128/IAI.70.3.1581-1590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tsai Y-L, Hayward RE, Langer RC, Fidock DA, Vinetz JM. Disruption of Plasmodium falciparum chitinase markedly impairs parasite invasion of mosquito midgut. Infect. Immun. 2001;69:4048–4054. doi: 10.1128/IAI.69.6.4048-4054.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Espinosa, D. A. et al. The Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites as a candidate for preerythrocytic and transmission-blocking vaccines. Infect. Immun.10.1128/IAI.00498-16 (2017). [DOI] [PMC free article] [PubMed]
- 142.Kariu T, Ishino T, Yano K, Chinzei Y, Yuda M. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol. Microbiol. 2006;59:1369–1379. doi: 10.1111/j.1365-2958.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- 143.Pirahmadi S, et al. Cell-traversal protein for ookinetes and sporozoites (CelTOS) formulated with potent TLR adjuvants induces high-affinity antibodies that inhibit Plasmodium falciparum infection in Anopheles stephensi. Malar. J. 2019;18:146–146. doi: 10.1186/s12936-019-2773-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li F, et al. Plasmodium ookinete-secreted proteins secreted through a common micronemal pathway are targets of blocking malaria transmission. J. Biol. Chem. 2004;279:26635–26644. doi: 10.1074/jbc.M401385200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are provided in the main text, tables and the figure.