Abstract
Background
People with opioid use disorder (OUD) are disproportionately burdened by HIV. The United States’ Centers for Disease Control and Prevention (CDC) has issued guidelines for pre-exposure prophylaxis (PrEP) indication. We know little about PrEP for people receiving medication for OUD. The objective of this study is to report PrEP indication, awareness, and uptake in patients engaged in outpatient OUD treatment with buprenorphine.
Methods
Adult patients (n=137) receiving buprenorphine for OUD at an outpatient substance use disorder treatment clinic completed a cross-sectional survey between July and September 2019. The study determined PrEP indication by 2017 CDC criteria. PrEP awareness and uptake were self-reported. The study assessed statistical differences in PrEP indicators by Pearson’s χ2 and Fisher’s exact.
Results
Nearly three-quarters (73.7%, n=101) of the study sample met CDC criteria for PrEP-indication based on past-year risk behaviors. Ninety-five percent of these participants reported inconsistent condom use, 21.0% engaged in commercial sex, 9.0% shared injection equipment, 8.9% reported a recent bacterial STI, and 4.0% had an HIV+ sexual partner. Of PrEP indicated participants (n=101), 19 had heard of PrEP prior to the survey, but only 1 participant reported past-year PrEP use.
Conclusions
Among a clinical population of people receiving buprenorphine for OUD, HIV risk behaviors were common, yet PrEP awareness and uptake were low. People engaged in treatment for OUD remain at high risk for HIV and are a priority population for PrEP. In light of the current opioid crisis, more research is needed to guide the integration of comprehensive HIV prevention into outpatient opioid treatment centers.
Keywords: Buprenorphine, Opioid use disorder, PrEP, HIV prevention, Substance use disorder treatment
1. Introduction
The United States is facing an opioid crisis; approximately 2 million people have an opioid use disorder (OUD) (Lipari, 2018), and opioid overdose deaths have continued to increase in recent years (CDC, 2019a; Scholl et al., 2018). While HIV incidence has decreased 31% over the past decade among people who inject drugs, due in part to harm-reduction efforts such as sterile syringe distribution (NCHHSTP AtlasPlus, 2019), people with OUD remain at high risk for HIV acquisition (Volkow and Montaner, 2011). As the prevalence of people with OUD increases in the United States, HIV incidence may again be on the rise.
People with OUD are more likely to have an increased number of sexual partners, less frequent condom use, and engage in high-risk sexual behaviors (e.g., exchanging sex for drugs, money, etc.) than people without OUD (Macafee et al., 2020; Strathdee and Sherman, 2003). OUD treatment utilizing medications such as methadone and buprenorphine decreases HIV risk behaviors (Gowing et al., 2013; Mitchell et al., 2012; Otiashvili et al., 2013; Pan et al., 2017; Woody et al., 2014). However, even after treatment engagement, patients continue to be at risk of HIV acquisition (Macafee et al., 2020; Mitchell et al., 2012; Terplan et al., 2016).
In 2012, the Food and Drug Administration (FDA) approved the use of combined tenofovir disoproxil fumarate and emtricitabine (Truvada) as pre-exposure prophylaxis (PrEP) for individuals at high risk of HIV transmission. Taken orally once daily, PrEP is highly effective in preventing HIV transmission (CDC, 2019). Daily adherence to PrEP can reduce HIV risk by 90% for sexual transmission and 70% for injection drug use (Campbell et al., 2013; Eisingerich et al., 2012; Page et al., 2015).
Since the FDA approved PrEP nearly a decade ago, the CDC has issued and revised guidelines for PrEP indication, recommending PrEP use for men who have sex with men (MSM), people who inject drugs, and others who are at “high-risk” for HIV (Table 1). The CDC aims to provide PrEP to at least half of PrEP-indicated people (about 600,000) by 2030, but as of 2018, only 18% (219,700) of PrEP-indicated people were prescribed PrEP (CDC, 2019b). Efforts to scale-up PrEP use have seen some success, largely within MSM subpopulations (Finlayson, 2019; Mayer et al., 2018). Unfortunately, PrEP has not reached women and men with OUD on such a scale. With the expansion of treatment for OUD in response to the growing opioid crisis, SAMHSA’s recent Treatment Improvement Protocol 63: Medications for Opioid Use Disorder recommends evaluating HIV negative patients in OUD treatment for pre-exposure prophylaxis (Treatment Improvement Protocol (TIP) 63, n.d.). However, only 9.5% of opioid treatment programs in the United States provide PrEP (Jones et al., 2019).
Table 1.
2017 CDC summary of guidance for PrEP use.
| Men Who Have Sex with Men (MSM) | Heterosexual Women and Men | Persons Who Inject Drugs | |
|---|---|---|---|
| Detecting substantial risk of acquiring HIV infection† | HIV-positive sexual partner Recent bacterial STI* High number of sex partners History of inconsistent or no condom use Commercial sex work |
HIV-positive sexual partner Recent bacterial STI** High number of sex partners History of inconsistent or no condom use Commercial sex work In high HIV prevalence area or network |
HIV-positive injecting partner Sharing injection equipment |
Patients are said to meet CDC criteria for PrEP indication if they both 1) belong to one or more of the 3 listed subpopulations and 2) meet one or more of the risk behaviors listed for that subpopulation
Gonorrhea, chlamydia, syphilis for MSM
Gonorrhea, syphilis for heterosexual women and men
Promising work aimed at closing this gap has focused on individuals receiving methadone, highlighting patients’ willingness to take PrEP and barriers and facilitators to PrEP adherence. To our knowledge, no study has described PrEP indication among people receiving buprenorphine, another medication for opioid use disorder (MOUD) that practitioners are increasingly using (Alderks, 2017). Given differences between buprenorphine and methadone-based treatment settings (e.g., office-based prescription vs. daily clinic dosing) and typical patient populations (e.g., higher socioeconomic status vs. lower socioeconomic status; Rhee, 2019), contemporary PrEP data from a buprenorphine patient population is needed to guide comprehensive HIV prevention efforts in the opioid crisis. The objective of this study is to assess the indication, awareness, and uptake of PrEP in people engaged in outpatient OUD treatment with buprenorphine.
2. Methods
2.1. Participants
The study recruited adult patients at a comprehensive, outpatient substance use treatment clinic in Richmond, Virginia, for a one-time cross-sectional survey between July and September 2019 (N=162; 97% response rate). The current analysis included all participants (n=137) who met the following criteria: a) HIV-negative; b) prescribed buprenorphine for OUD; and c) answered questions on HIV risk behaviors and PrEP.
2.2. Study design
The study recruited participants through fliers posted in the clinic, information at the study table, and referrals by clinic staff. Participants completed the survey in the clinic or a private room according to preference, and those who requested assistance had the survey administered to them by a research assistant. Participants received $20 cash compensation. The Virginia Commonwealth University Institutional Review Board approved this study, and all participants provided verbal consent.
2.3. Measures
The study collected survey data (demographics, self-reported risk behaviors, and PrEP/HTV variables) in REDCap (Peterson et al., 2019; Shrestha et al., 2017; Stein et al., 2014) via electronic tablets. Self-reported sex was dichotomous as male or female. Participants self-reported race/ethnicity, employment, income, education, and marital status. We took questions on HIV risk and PrEP from the National HIV Behavioral Surveillance System. The 2017 CDC criteria outlined the HIV risk factors, and the study defined PrEP indication as having any risk factor (according to participant membership to one or more of the listed high-risk subpopulations) in the past 12 months. Study staff obtained buprenorphine prescription data and insurance status by electronic medical record chart abstraction; we defined length of treatment as the number of days between the date of the buprenorphine prescription or first clinic visit and the survey date.
2.4. Analysis
Statistical differences in categorical variables between PrEP-indicated and non–indicated groups were assessed by Pearson’s χ2 or Fisher’s exact where appropriate by cell size. Research staff assessed group differences for continuous variables by T-tests or Mann Whitney U per normality of distributions. We set the significance level at 5%. The study summarized data using SAS 9.4 (SAS Institute Inc., Cary, NC).
3. Results
Table 1 summarizes participant demographics (n=137) by sex and PrEP indication as determined by CDC risk criteria. Participants were 54.0% female (n=74) and 46% male (n=63). The majority of the sample was Black (70.8%), married (68.6%), unemployed or on disability (72.2%), with a high school diploma (51.1%), and reported an annual household income of less than $5,000 (46.7%). The average length of participants’ current buprenorphine treatment episode was 404.3±250.7 days.
Almost three-quarters of our sample (n=101; 73.7%) met CDC criteria for PrEP indication (Table 1). Notably, OUD treatment duration was not different between PrEP-indicated and non–indicated participants (p=0.152). Younger females were more likely to be PrEP-indicated than older females (38.1±11.6 vs. 47.4±9.4, p=0.004); PrEP indication in males did not vary by age. Males receiving Medicaid were more likely to be PrEP-indicated than males with other types of insurance coverage (p=0.014); this difference was not significant in females.
Of participants meeting PrEP indication, 95.0% reported inconsistent condom use, 21.0% engaged in transactional sex, 9.0% shared injection equipment, 8.9% reported a recent bacterial sexually transmitted infection, and 4.0% had an HIV+ sexual partner (Table 2). Further, of the 101 PrEP-indicated participants, 19 had heard of PrEP prior to the survey, and only one participant reported past-year PrEP use (Figure 1). Two other participants reported past-year PrEP use but did not meet CDC criteria. PrEP indication, knowledge, uptake, and inaccessibility did not vary significantly by sex. Two participants reported an unsuccessful attempt to obtain PrEP or postexposure prophylaxis in the past year (data not shown).
Table 2.
Study population demographics by PrEP indication and sex.
| Total n (%) n=137 | Females n (%) n=74 | Males n (%) n=63 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Met CDC criteria for PrEP indication n=101 |
Did not meet CDC criteria for PrEP indication n=36 |
P value1,2 | Met CDC criteria for PrEP indication n=57 |
Did not meet CDC criteria for PrEP indication n=17 |
P value1,2 | Met CDC criteria for PrEP indication n=44 |
Did not meet CDC criteria for PrEP indication n=19 |
P value1,2 | ||
| Age (years) | mean±SD | 41.8±12.2 | 47.6±11.9 | 0.014* | 38.1±11.6 | 47.4±9.4 | 0.004* | 46.4±11.4 | 47.8±14.0 | 0.688 |
| Race | White | 23 (23.2) | 7 (19.4) | 0.968 | 17 (30.4) | 4 (23.5) | 0.920 | 6 (14.0) | 3 (15.8) | 1.00 |
| African American or Black | 70 (70.7) | 27 (75.0) | 36 (64.3) | 12 (70.6) | 34 (79.1) | 15 (78.9) | ||||
| Other | 6 (6.1) | 2 (5.6) | 3 (5.4) | 1 (5.9) | 3 (7.0) | 1 (5.3) | ||||
| Employment | Employed | 30 (29.7) | 8 (22.2) | 0.227 | 13 (22.8) | 1 (5.9) | 0.063 | 17 (38.6) | 7 (36.8) | 1.00 |
| Unemployed | 54 (53.5) | 17 (47.2) | 33 (57.9) | 8 (47.1) | 21 (47.7) | 9 (47.4) | ||||
| On Disability | 17 (16.8) | 11 (30.6) | 11 (19.3) | 8 (47.1) | 6 (13.6) | 3 (15.8) | ||||
| Annual Income ($) | <5,000 | 50 (50.5) | 14 (41.2) | 0.277 | 30 (53.6) | 6 (37.5) | 0.143 | 20 (46.5) | 8 (44.4) | 0.967 |
| 5,001-10,000 | 15 (15.2) | 9 (26.5) | 8 (14.3) | 6 (37.5) | 7 (16.3) | 3 (16.7) | ||||
| >10,000 | 34 (34.3) | 11 (32.4) | 18 (32.1) | 4 (25.0) | 16 (37.2) | 7 (38.9) | ||||
| Education Level | Less than High School | 23 (22.8) | 5 (13.9) | 0.124 | 12 (21.1) | 2 (11.8) | 0.214 | 11 (25.0) | 3 (15.8) | 0.518 |
| HS Diploma/GED equivalent | 54 (53.5) | 16 (44.4) | 31 (54.4) | 7 (41.2) | 23 (52.3) | 9 (47.4) | ||||
| Greater than HS | 24 (23.8) | 15 (41.7) | 14 (24.6) | 8 (47.1) | 10 (22.7) | 7 (36.8) | ||||
| Diploma | ||||||||||
| Insurance Status | Medicaid | 55 (54.5) | 13 (36.1) | 0.048* | 28 (49.1) | 9 (52.9) | 0.122 | 27 (61.4) | 4 (21.1) | 0.014* |
| Medicare | 5 (5.0) | 7 (19.4) | 3 (5.3) | 4 (23.5) | 2 (4.6) | 3 (15.8) | ||||
| Private | 12 (11.9) | 12 (11.9) | 9 (15.8) | 1 (5.9) | 3 (6.8) | 3 (15.8) | ||||
| None | 29 (28.7) | 12 (33.3) | 17 (29.8) | 3 (17.7) | 12 (27.3) | 9 (47.4) | ||||
| Married | Yes | 71 (70.3) | 23 (63.9) | 0.532 | 41 (71.9) | 13 (76.5) | 1.00 | 30 (68.2) | 10 (52.6) | 0.267 |
| Past yr. same-sex contact | Yes | 34 (33.7) | 13 (36.1) | 0.839 | 27 (47.4) | 11 (64.7) | 0.273 | 7 (15.9) | 2 (10.5) | 0.711 |
| Days in OUD Treatment | Current buprenorphine treatment episode mean±SD | 395.3±263.1 | 428.5±215.4 | 0.467 | 433.4±269.3 | 448.8±171.8 | 0.786 | 344.0±248.6 | 411.4±249.8 | 0.339 |
P-values for categorical variables are calculated by chi square or Fisher’s exact testing based cell size
P-values for numeric variables (days in buprenorphine treatment, age) assessed by Mann Whitney U or T-test based on distribution
significant at α=0.05
Figure 1. PrEP “Cascade” among study participants in outpatient OUD treatment receiving buprenorphine.
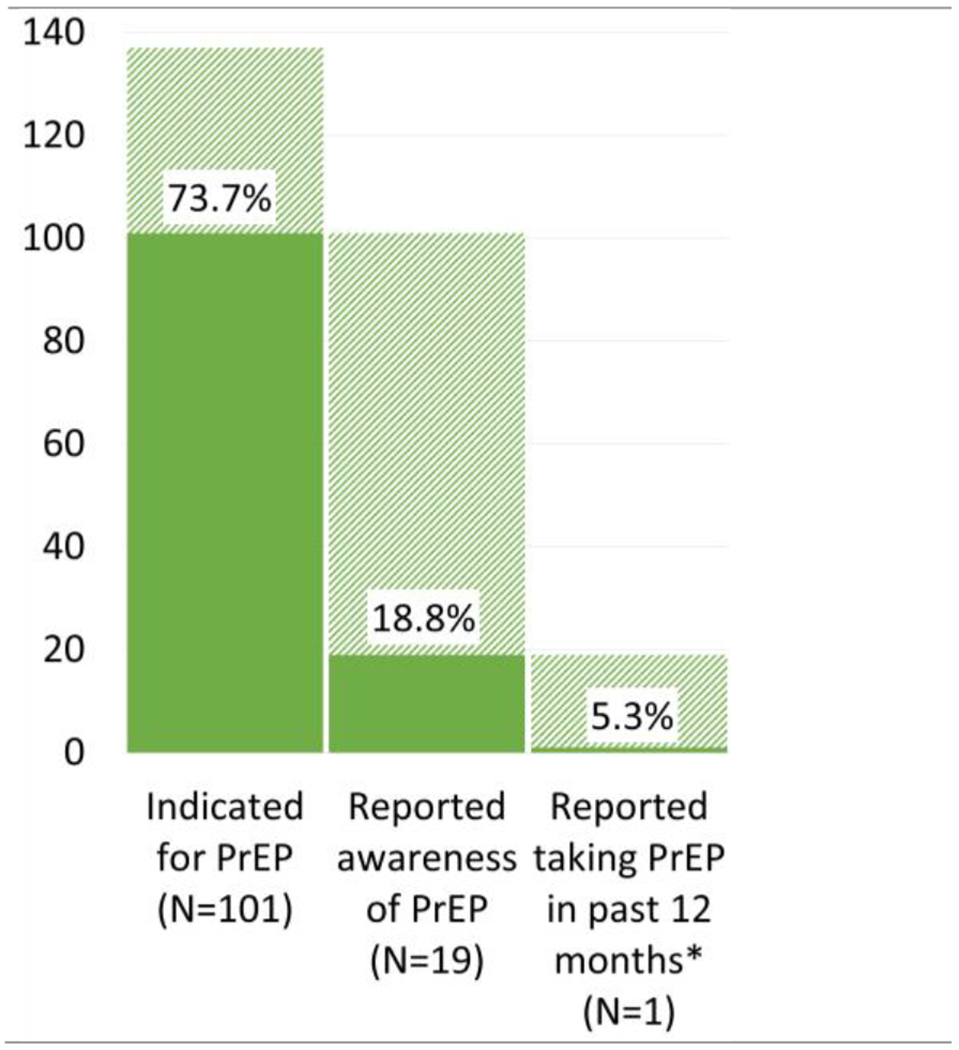
*1 subject met inclusion criteria, PrEP indication, and reported awareness and use (2 others met study criteria, reported PrEP awareness and use, but did not meet CDC criteria)
4. Discussion
4.1. Findings
This study reports indication, awareness, and uptake of PrEP among a clinical population of people in outpatient OUD treatment receiving buprenorphine. Although relatively few participants reported sharing injection equipment in the past year (n=9), many participants reported high-risk sexual behaviors, and nearly three-quarters of the sample (n=101) had an indication for PrEP. However, fewer than 1 in 5 participants were aware of PrEP prior to the study (n=19), and only 1 participant in this study sample had taken PrEP, a significantly lower uptake than the 18% in the U.S. population of PrEP-indicated people and the CDC’s goal of 50% (CDC, 2019b).
Consistent with prior work among people receiving methadone for OUD (Shrestha and Copenhaver, 2018), the majority of our sample of people receiving buprenorphine for OUD met CDC criteria for PrEP indication, due in large part to heterosexual risk behaviors. Further, we found that length of OUD treatment was not associated with PrEP indication, highlighting how HIV risk persists for people with OUD even after treatment engagement. Our study adds to the existing, growing literature regarding HIV risk and PrEP among people receiving methadone for OUD to also include the increasing number of people receiving buprenorphine from an outpatient provider in the current opioid crisis. Namely, we highlight how people across different OUD treatment settings, not only methadone opioid treatment programs, remain a vulnerable population for HIV and are a high priority for PrEP coverage.
Our low prevalence of PrEP awareness is comparable to prior studies for people in treatment for OUD, in which awareness ranges from 7% to 23% (Peterson et al., 2019; Shrestha et al., 2017; Stein et al., 2014). A similar study of 400 patients receiving methadone treatment in a Connecticut outpatient facility found that only 18% of patients had heard of PrEP (Shrestha and Copenhaver, 2018). As past and current PrEP campaigns primarily target MSM in serodiscordant partnerships (Shrestha et al., 2017), they may be less likely to reach an OUD clinic sample like ours, where MSM are a minority. Low PrEP awareness among patients engaged in buprenorphine or methadone-based OUD treatment programs represents a missed opportunity for patient education, as these individuals are regularly interfacing with the health care system. Therefore, within the opioid crisis, new, innovative PrEP awareness efforts are needed to reach this unique and growing at-risk population (Barnes et al., 2020).
Last, of the 101 participants with a PrEP indication, only one reported receiving PrEP in the last 12 months, similar to prior work among patients receiving methadone where only 2% reported ever taking PrEP (Shrestha and Copenhaver, 2018). This extremely low uptake likely reflects a cumulation of deficiencies along the PrEP cascade, including a lack of assessments for PrEP indication by treatment providers, PrEP patient education, and provider training in PrEP provision. Most opioid treatment programs in the United States provide HIV testing and risk reduction counseling, but fewer than 1 in 10 provide PrEP (Jones et al., 2019). Prior studies have shown that people in treatment for OUD tend to be receptive to PrEP uptake, at rates ranging from 31–60% (Peterson et al., 2019; Shrestha et al., 2017). Thus, public health measures focused on closing systematic and provider-level gaps along the PrEP cascade have potential to ultimately improve PrEP uptake among people engaged in OUD treatment.
4.2. Limitations
This study has several limitations. First, participants completed this survey at a single outpatient substance use disorder treatment clinic, limiting the generalizability of study results. Second, the low prevalence of PrEP awareness and uptake limit statistical modeling options to explore associated factors. Third, we based risk behaviors used to identify individuals with PrEP indication on self-report of past year behaviors and subject to recall bias. Fourth, the cross-sectional nature of the survey prevents our understanding of chronology of risk behaviors and outcomes. Finally, although the survey was confidential and de-identified, risk behaviors may have been underreported due to stigmatization and unwillingness of participants to disclose sensitive information.
5. Conclusion
Among a clinical population of people receiving buprenorphine treatment for OUD, many participants met CDC criteria for PrEP indication largely due to HIV sexual risk behaviors. However, PrEP awareness and uptake were very low. PrEP outreach needs to extend to people with OUD. Targeting PrEP awareness and provision efforts at outpatient-based opioid treatment programs represents an ideal opportunity to take significant steps toward achieving the CDC PrEP goal, given the high frequency of health care interactions by people receiving MOUD. More research on patient, provider, and system-level facilitators and challenges to PrEP provision for people receiving buprenorphine for OUD should help to guide evidence-based efforts to integrate OUD treatment and HIV prevention.
Table 3.
Past 12-month HIV risk behaviors among study participants meeting CDC criteria for PrEP indication.
| Total n (%) n=101 | Females n (%) n=57 | Males n (%) n=44 | P value1 | |
|---|---|---|---|---|
| Engaged in transactional sex | 21 (21.0) | 14 (25.0) | 7 (15.9) | 0.327 |
| Diagnosed with bacterial STI | 9 (8.9) | 5 (8.8) | 4 (9.1) | 1.00 |
| Injected drugs with needle that had been used by someone who may be HIV+ | 9 (9.0) | 6 (10.7) | 3 (6.8) | 0.727 |
| Inconsistent condom use | 95 (95.0) | 54 (94.7) | 41 (95.4) | 1.00 |
| Had condomless sex with someone who was known to be HIV+ | 4 (4.0) | 3 (5.3) | 1 (2.3) | 0.630 |
| Had condomless sex with someone with unknown HIV status | 13 (13.0) | 8 (14.0) | 5 (11.6) | 0.773 |
P-values are calculated by chi square or Fisher’s exact testing based on cell size
Women who have sex with women are not indicated for PrEP per CDC guidelines
significant at α=0.05
CDC: United States Centers for Disease Control and Prevention
PrEP: Pre-exposure prophylaxis
STI: Sexually transmitted infection
HIV: Human immunodeficiency virus
Column percent totals do not add to 100% due to participants’ endorsing more than one risk behavior
Highlights.
After engaging in OUD treatment, a high risk of HIV transmission persists.
People receiving buprenorphine for OUD demonstrate very low awareness of PrEP.
Many people with OUD meet CDC PrEP indication criteria but do not receive PrEP.
More research is needed to guide comprehensive HIV prevention within OUD treatment.
Funding
This study was supported by the Jeanann Dunlap Foundation and by CTSA award No. UL1TR002649 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. Anna Beth Parlier-Ahmad reports receiving NIDA T32DA007027 award (PI: Dr. William Dewey). Dr. Martin is supported by CTSA award No. KL2TR002648 from the National Center for Advancing Translational Sciences. Funders were not directly involved in the preparation of this research manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Alderks CE, 2017. Trends in the Use of Methadone, Buprenorphine, and Extended-release Naltrexone at Substance Abuse Treatment Facilities: 2003-2015 (Update). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [PubMed] [Google Scholar]
- Barnes AJ, Cunningham PJ, Saxe-Walker L, Britton E, Sheng Y, Boynton M, Harper K, Harrell A, Bachireddy C, Montz E, Neuhausen K, 2020. Hospital Use Declines After Implementation Of Virginia Medicaid’s Addiction And Recovery Treatment Services. Health Aff Proj. Hope 39, 238–246. 10.1377/hlthaff.2019.00525 [DOI] [PubMed] [Google Scholar]
- Campbell JD, Herbst JH, Koppenhaver RT, Smith DK, 2013. Antiretroviral Prophylaxis for Sexual and Injection Drug Use Acquisition of HIV. Am. J. Prev. Med. 44, S63–S69. 10.1016/j.amepre.2012.09.045 [DOI] [PubMed] [Google Scholar]
- CDC, 2019a. NSFG [WWW Document], URL https://www.cdc.gov/nchs/nsfg/nsfg_2013_2015_questionnaires.htm (accessed 4.7.20).
- CDC, 2019b. Test, Treat, Prevent to End HIV [WWW Document], Cent. Dis. Control Prev. URL https://www.cdc.gov/vitalsigns/test-treat-prevent/index.html (accessed 4.7.20).
- Eisingerich AB, Wheelock A, Gomez GB, Garnett GP, Dybul M, Piot PK, 2012. Attitudes and Acceptance of Oral and Parenteral HIV Preexposure Prophylaxis among Potential User Groups: A Multinational Study. Plos One 7. 10.1371/joumal.pone.0028238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson T, 2019. Changes in HIV Preexposure Prophylaxis Awareness and Use Among Men Who Have Sex with Men — 20 Urban Areas, 2014 and 2017. MMWR Morb. Mortal. Wkly. Rep 68. 10.15585/mmwr.mm6827a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing LR, Hickman M, Degenhardt L, 2013. Mitigating the risk of HIV infection with opioid substitution treatment. World Health Organ. Bull. World Health Organ. Geneva 91, 148–9. 10.2471/BLT.12.109553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Byrd DJ, Clarke TJ, Campbell TB, Ohuoha C, Mccance-Katz EF, 2019. Characteristics and current clinical practices of opioid treatment programs in the United States. Drug Alcohol Depend. 205, 107616. 10.1016/j.drugalcdep.2019.107616 [DOI] [PubMed] [Google Scholar]
- Lipari RN, 2018. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health 82. [Google Scholar]
- Macafee K, Harfmann F, Cannon M, Kolenic K, Kusunoki K, Terplan K, Dalton K, 2020. Sexual and Reproductive Health Characteristics of Women in Substance Use Treatment in Michigan. Obstet. Gynecol. 135, 361–369. 10.1097/AOG.0000000000003666 [DOI] [PubMed] [Google Scholar]
- Mayer H, Chan A, Patel R, Flash A, Krakower S, S., 2018. Evolving Models and Ongoing Challenges for HIV Preexposure Prophylaxis Implementation in the United States. JAIDS J. Acquir. Immune Defic. Syndr. 77, 119–127. 10.1097/QAI.0000000000001579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SG, Kelly SM, Brown BS, O’Grady KE, Schwartz RP, 2012. HIV Sex-Risk Behaviors among In- versus Out-of-Treatment Heroin-Addicted Adults. Am. J. Drug Alcohol Abuse 38, 328–333. 10.3109/00952990.2011.643993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCHHSTP AtlasPlus. US Centers for Disease Control and Prevention [WWW Document], 2019. URL https://www.cdc.gov/nchhstp/atlas/index.htm (accessed 10.17.20).
- Otiashvili D, Piralishvili G, Sikharulidze Z, Kamkamidze G, Poole S, Woody GE, 2013. Methadone and buprenorphine-naloxone are effective in reducing illicit buprenorphine and other opioid use, and reducing HIV risk behavior—Outcomes of a randomized trial. Drug Alcohol Depend. 133, 376–382. 10.1016/j.drugalcdep.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page A, Tsui A, Maher A, Choopanya A, Vanichseni A, Mock A, Celum A, Martin A, 2015. Biomedical HIV Prevention Including Pre-exposure Prophylaxis and Opiate Agonist Therapy for Women Who Inject Drugs: State of Research and Future Directions. JAIDS J. Acquir. Immune Defic. Syndr. 69, S169–S175. 10.1097/QAI.0000000000000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Metsch L, Wang W, Wang K-S, Duan R, Kyle T, Gooden L, Feaster D, 2017. Gender Differences in HIV Sexual Risk Behaviors Among Clients of Substance Use Disorder Treatment Programs in the U.S. Arch. Sex. Behav. 46, 1151–1158. 10.1007/s10508-015-0686-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M, Macmadu A, Truong AQ, Rich J, Pognon K, Lurie M, Clarke JG, Brinkley-Rubinstein L, 2019. Pre-exposure prophylaxis awareness and interest among participants in a medications for addiction treatment program in a unified jail and prison setting in Rhode Island. J. Subst. Abuse Treat. 106, 73–78. 10.1016/j.jsat.2019.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee TG, Rosenheck RA, 2019. Buprenorphine prescribing for opioid use disorder in medical practices: can office-based out-patient care address the opiate crisis in the United States? Addiction, 114(11). 10.1111/add.14733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G, 2018. Drug and Opioid-Involved Overdose Deaths — United States, 2013–2017. Morb. Mortal. Wkly. Rep. 67, 1419–1427. 10.15585/mmwr.mm675152el [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha R, Copenhaver M, 2018. Exploring the Use of Pre-exposure Prophylaxis (PrEP) for HIV Prevention Among High-Risk People Who Use Drugs in Treatment. Front. Public Health 6, 195. 10.3389/fpubh.2018.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha R, Karki P, Altice FL, Huedo-Medina TB, Meyer JP, Madden L, Copenhaver M, 2017. Correlates of willingness to initiate pre-exposure prophylaxis and anticipation of practicing safer drug- and sex-related behaviors among high-risk drug users on methadone treatment. Drug Alcohol Depend. 173, 107–116. 10.1016/j.drugalcdep.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Thurmond P, Bailey G, 2014. Willingness to Use HIV Pre-Exposure Prophylaxis Among Opiate Users. AIDS Behav. 18, 1694–1700. 10.1007/s10461-014-0778-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Sherman SG, 2003. The role of sexual transmission of HIV infection among injection and non-injection drug users. J. Urban Health Bull. N. Y. Acad. Med 80, iii7–iii14. 10.1093/jurban/jtg078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terplan B, Lawental E, Connah E, Martin E, 2016. Reproductive Health Needs Among Substance Else Disorder Treatment Clients. J. Addict. Med. 10, 20–25. 10.1097/ADM.0000000000000175 [DOI] [PubMed] [Google Scholar]
- Treatment Improvement Protocol (TIP) 63: Medications for Opioid Use Disorder, 1st ed, n.d. . USDeptof Health and Human Services. [Google Scholar]
- Volkow ND, Montaner J, 2011. The Urgency of Providing Comprehensive And Integrated Treatment For Substance Abusers With HIV. Health Aff (Millwood) 30, 1411–1419. 10.1377/hlthaff.2011.0663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody G, Bruce D, Korthuis P, Chhatre S, Hillhouse M, Jacobs P, Sorenson J, Saxon A, Poole S, Metzger D, Ling W, 2014. HIV Risk Reduction With Buprenorphine-Naloxone or Methadone: Findings from A Randomized Trial. Neuropsychopharmacology 39, S337–S338. [DOI] [PMC free article] [PubMed] [Google Scholar]


