Abstract
Background:
Succinic semialdehyde dehydrogenase deficiency (SSADHD) is a disorder of GABA degradation with use-dependent downregulation of postsynaptic GABAA/B receptors. We aim to measure the resulting cortical excitation:inhibition ratio using transcranial magnetic stimulation (TMS).
Methods:
In this single-center observational study, 18 subjects with SSADHD and 8 healthy controls underwent TMS. Resting motor threshold (rMT), cortical silent period (CSP), and long interval intracortical inhibition (LICI) were measured in both groups. rMT in focal epilepsy patients from an institutional TMS database were also included.
Results:
SSADHD subjects had higher rMT than healthy controls but lower relative to focal epilepsy patients. rMT decreased with age in all groups. CSP was longer in SSADHD subjects than in healthy controls. No difference was detected in LICI between the two groups.
Conclusion:
Findings suggest abnormal corticospinal tract (CST) physiology in SSADHD, but with preserved developmental trajectory for CST maturation. Defining features of these TMS metrics in SSADHD will be better elucidated through this on-going longitudinal study.
Background
Succinic semialdehyde dehydrogenase deficiency (SSADHD) is a rare metabolic disorder of the gamma aminobutyrate aminotransferase (GABA) degradation pathway characterized by a predominantly static encephalopathy, and core features of hypotonia, ataxia, and expressive language deficits. In rodent SSADHD models, the resulting accumulation of GABA and 4-hydroxybutyric acid (GHB) in the CNS has been shown to downregulate post-synaptic GABAA- and GABAB-receptor expression.[1–5] [11C]Flumazenil PET studies in human subjects with SSADHD showed reduced benzodiazepine receptor binding, further supporting the hypothesis of GABAA-receptor downregulation and/or dysfunction of GABAA receptors.[6] Approximately half of patients with SSADHD develop epilepsy.[7] These findings further supports the hypothesis that excess GABA in the CNS of patients with SSADHD results in downregulation of GABA-receptors and paradoxical net cortical hyperexcitability. The reduction in GABA-mediated cortical inhibition can be measured using transcranial magnetic stimulation (TMS).
TMS basics.
TMS is a noninvasive form of focal cortical stimulation in which an external magnet induces an intracranial electrical field over the stimulated region used to interrogate or modulate states of cortical excitation or inhibition. When delivered over the motor cortex, TMS elicits a motor evoked potential (MEP) that can be recorded by surface electromyogram (EMG) electrodes in the contralateral limb (Figure 1). Thus, TMS coupled with EMG (TMS-EMG) uniquely enables generation of “input-output” curves, in vivo and in humans, from which a range of cortical excitability and plasticity measures can be derived.
Figure 1. TMS-derived metrics.
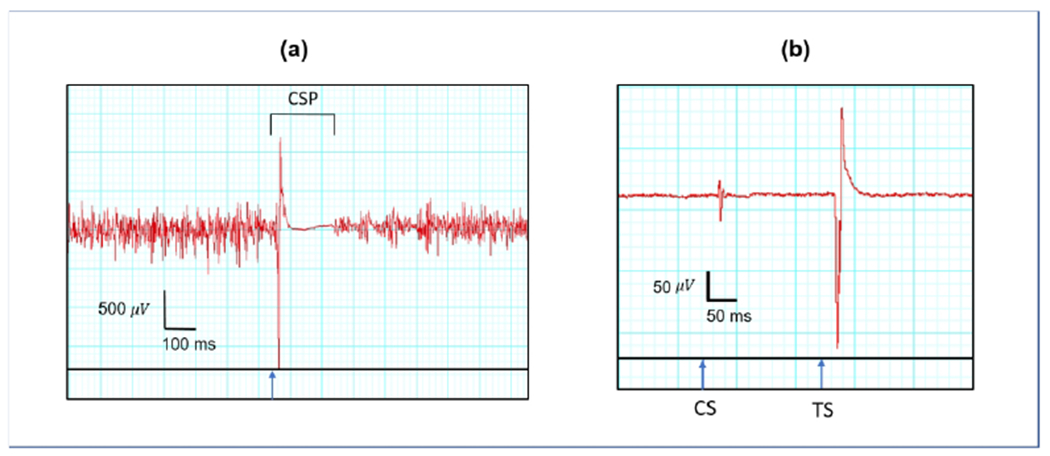
Tracings show APB activity over time recorded by a surface EMG electrode from a healthy control. Blue arrows indicated the time of stimulation. (a) CSP is the duration from time of stimulation to time of return of voluntary muscle contraction as depicted by surface EMG. The participant is asked to voluntarily activate the target muscle prior to stimulation. Shown in red is EMG activity followed by the MEP resultant from TMS pulse. (b) LICI is represented as a ratio of the MEP amplitude resultant from the test stimulus (TS) to the MEP amplitude resultant from the conditioning stimulus (CS) at suprathreshold intensity. The two MEPs resultant from the conditioning and test stimuli are depicted in red.
A range of metrics that may provide both insight into the vulnerability for seizures, and also into mechanism of disease can be derived by TMS delivered to the motor cortex. Resting motor threshold (rMT) and cortical silent period (CSP) are derived using single-pulse TMS (spTMS). rMT is most often operationally defined as minimal stimulation intensity needed to produce an MEP > 50 μV from an intrinsic hand muscle in at least 50% of trials. It is measured as a percentage of total machine output (MO). The rMT reflects voltage-gated sodium channel-mediated cortical excitation, as it is increased following administration of voltage-gated sodium channel blockers.[8–14] CSP is a period of suppressed EMG activity until voluntary muscle activity returns following delivery of a suprathreshold stimulus that reflects GABAA- and GABAB-mediated inhibition.[15–19] A range of E:I ratio measures can also be obtained with paired-pulse (ppTMS) protocols where each test stimulus (TS) is preceded by a conditioning stimulus (CS) to the same motor cortex site. Long-interval intracortical inhibition (LICI), for example, reflects GABAB-mediated local inhibition and likely GABAA-mediated network inhibition[17, 18, 20, 21] To obtain LICI, CS and TS are delivered at an interstimulus interval (ISI) of 50-300 ms, with the degree of inhibition represented as a ratio of the second MEP amplitude (resulting from TS) to the first MEP amplitude (resulting from CS).
These metrics can be modulated by agents that alter GABAergic or glutamatergic tone, and can be measured to detect either abnormalities in disease or restoration by treatment.
Prior TMS studies in SSADHD:
The use of TMS paradigms to elucidate pathophysiology of SSADHD was first reported by Reis et al. in 2012.[22] In their study of 7 subjects with SSADHD, a figure-of-eight coil was placed over the left motor cortex “hot spot” for the first dorsal interosseous muscle for all TMS-EMG paradigms. CSP was calculated as an average over 20 trials following stimulation at 150% rMT during active muscle contraction. LICI was elicited using 150% rMT for both CS and TS at an ISI of 150 ms, and averaged over 15 trials randomly interspersed with control single test stimuli, delivered 4-8 seconds apart. rMT was higher in SSADHD subjects (age: 10-27 years) than in their parents or in adult controls, but not significantly different from age-matched “young” control subjects. CSP was shorter in SSADHD subjects compared to all other comparison groups. LICI was “virtually absent”, with 101.16 ± 8.7% of the test MEP in subjects with SSADHD, and thus significantly impaired relative to the comparison groups.
Despite differences in protocols (including coil type and placement and stimulation parameters), Schreiber et al. corroborated Reis et al.’s findings in a separate study in 2016 while studying the effects of taurine supplementation as a potential therapeutic in SSADHD.[23] Seven patients (age: 12-33 years) underwent TMS using a round coil placed over the motor “hot spot” for the FDI muscle prior to initiation of taurine. Ten trials each at a stimulation intensity of 110, 120, 130, and 140% rMT were used to elicit CSP. LICI protocol consisted of 120% rMT stimulus intensity for both stimuli, with an ISI of 100 ms, for a total of 10 trials. Although there was no control group, CSP was short (128 ms at 140% rMT) and LICI was minimal.
The above-mentioned studies support the feasibility of and potential role for TMS-derived biomarkers as metrics of disease severity and, perhaps, as measures of target engagement by therapeutics. Accordingly, we aim to quantify TMS measures of cortical excitatory and inhibitory tone in a larger population of patients with SSADHD, and compare them to age-matched healthy controls. This work is part of an observational natural history study in which recruitment is ongoing. Interim data analysis was presented at the SSADHD Virtual Conference in July 2020, and is summarized below.
Methods
Participants with SSADHD were enrolled as part of the Natural History Study of Patients with SSADHD (ClinicalTrials.gov Identifier: NCT03758521). Diagnostic confirmation of disease by genetic (biallelic pathogenic variants in ALDH5A1) and metabolic (elevated urine GHB levels) evaluation was performed by study physicians. Participants less than 2 years old or with any contraindication to TMS (e.g. metal hardware not known to be TMS-compatible) were excluded from the study. Healthy controls with neurotypical development and no history of neurological disorder or brain injury who were within 2 years of the age of one of our participants were also recruited. Unfortunately, age-matched controls for the 3 participants 3-4 years of age were unable to be recruited during this timeframe. Specifically for comparisons of the rMT, data obtained as part of routine clinical care (as part of a presurgical evaluation) from age-matched patients with focal epilepsy, also within 2 years of the age of one of our participants with SSADHD, from the Boston Children’s Hospital TMS laboratory database were also included as an additional comparison group. No patient with focal epilepsy existed in our database to age-match the 27 and 39-year old participants. Measuring CSP and LICI is not part of the standard clinical TMS evaluation completed for these patients.
TMS
Neuronavigated TMS requires use of an anatomical MRI scan to be used in conjunction with TMS. Co-registration was performed against each participant’s T1-weighted MRI sequence that was converted to a 3D head surface and brain reconstruction using Nexstim 4.3.1 software (Nexstim, Finland). For healthy controls, if an MRI was not obtained, an anonymized brain MRI with comparable head circumference available in the TMS lab repository was used for co-registration. Surface electromyography (EMG) electrodes were placed on 6 bilateral and symmetric locations of each participant’s body: (i) thumb (over the abductor pollicis brevis [APB]), (ii) shoulder (over the deltoid), (iii) leg (over the tibialis anterior). An additional grounding electrode was placed on the underside of the right forearm. spTMS and ppTMS protocols were performed using a figure-of-eight cooled magnetic coil positioned over the APB hotspot in either hemisphere identified for each participant.
spTMS
The motor hotspot was identified for abductor pollicis brevis (APB) and deltoid muscles in each hemisphere. rMT was obtained by determining the minimum machine output required to elicit an MEP ≥ 50 μV from the target muscle at rest in >50% of trials of stimulation over the designated hotspot. If rMT was greater than maximum machine output (100% MO), rMT was recorded as 100%. To measure CSP a suprathreshold stimulus at 120% rMT up to a maximum of 100%MO was delivered over the motor hotspot of the target muscle, with the target muscle in a controlled pre-activated state. CSP was calculated as the duration from the time of stimulation to return of spontaneous muscle activity as depicted by EMG (Figure 1). To control for variability in degree of pre-stimulus APB activation, during post-hoc analysis of CSP data, root mean square of voltages in the 100 milliseconds prior to the resultant MEP was calculated. Maintaining a consistent level of APB muscle contraction for CSP measurements is challenging in the pediatric population, and use of a hand-held dynamometer was not feasible or practical in this study. Therefore, CSP duration from trials with RMS between 50-150 in the 100 ms prior to the MEP were included.
ppTMS
To elicit LICI each CS was followed 200 milliseconds later by the TS, both at 150% rMT up to a maximum of 100% MO, over the site corresponding to peak MEP activation for the target muscle (Figure 1). LICI is expressed as a log transformation of the ratio of the peak-to-peak MEP amplitude resultant from each TS divided by the peak-to-peak MEP amplitude resultant from the preceding CS, averaged per muscle group.
Statistical analysis:
Data was analyzed by SPSS version 26. Frequency tables were generated for rMT, CSP, and LICI per cohort, as well as per subgroup of participants with SSADHD with and without epilepsy. Paired Student t-test was used to compare right and left APB rMT within each cohort. One-way ANOVA with Bonferroni correction was performed to compare APB rMT in subjects with SSADHD, age-matched healthy controls, and in the affected hemisphere of age-matched patients with focal epilepsy from the single-center database. Independent samples t-test was performed to evaluate for differences between APB CSP and LICI in SSADHD participants and healthy controls. All analyses were performed with significance thresholded at 5% and p-value < 0.05 deemed statically significant.
This study was approved by the Boston Children’s Hospital Institutional Review Board (IRB-P00029917).
Results
Participants:
Of the 19 subjects with SSADHD enrolled in the study who underwent TMS, 18 were included in the analysis. One participant was excluded due to technical challenges eliciting the TMS metrics described above. Median age of subjects with SSADHD was 9.1 years (range: 3.6-27.1 years, mean 11.0 years). Five (28%) of the 18 participants with SSADHD had epilepsy, 4 of whom were on antiseizure medications. Eight age-matched healthy controls were recruited, but the healthy control age-matched to the excluded SSADHD patient was not included in data analysis. Median age of healthy controls was 16.8 years (range: 6.8-25.1 years). The median age of the forty-four age-matched patients with focal epilepsy was 9.5 years (range: 3.2-22.3 years), all of whom were on at least 1 antiseizure medication.
rMT:
rMT was measured in all participants. There were no left/right differences in APB rMT within the SSADHD cohort or healthy controls so right APB rMT measurements were used for comparison between groups. In all 3 groups, rMT declined with age (Figure 2). Average APB rMT in subjects with SSADHD was higher than that of healthy controls (67 ± 23 vs 43 ± 15% MO, p=0.02). When constrained to the subgroup without epilepsy, average rMT in patients with SSADHD was still higher (73 ± 19%) than in age-matched controls (p = 0.002). However, patients with focal epilepsy had a higher mean rMT in the affected hemisphere (81 ± 19%) than subjects with SSADHD (p = 0.02) (Figure 3). Even when comparing the rMT only in those subjects with SSADHD and epilepsy to those age-matched with focal epilepsy, rMT was greater in the focal epilepsy cohort (81 ± 19 vs. 52 ± 29 %, p = 0.04). A comparison of rMT in SSADHD participants with and without epilepsy was underpowered to identify a significant difference between groups (52 ± 29 % vs. 73 ± 19%, p = 0.08).
Figure 2.
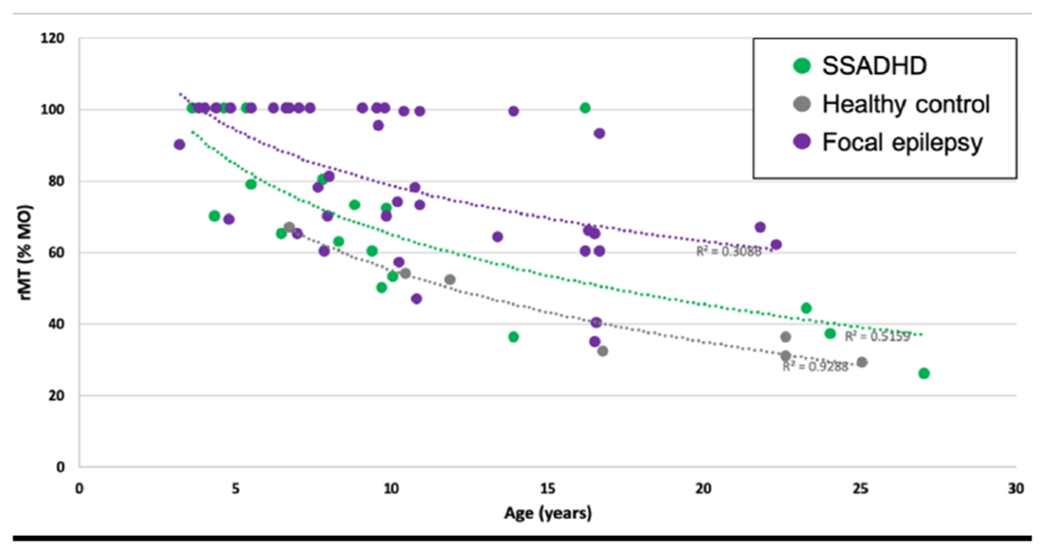
Average rMT for APB as a function of age per group. The dotted lines represent the logarithmic trendlines per group.
Figure 3.
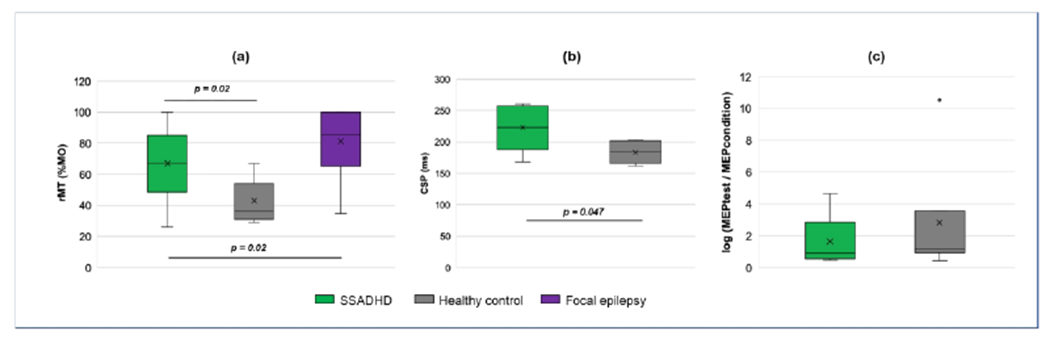
Boxplots illustrating TMS metrics in subjects with SSADHD, healthy controls, and focal epilepsy. “X” represents the mean value, with height of each box representing the 25th and 75th quartiles. (a) Significant differences in rMT were found between subjects with SSADHD and healthy controls, and independently with patients with focal epilepsy. (b) CSP was also significantly longer in subjects with SSADHD than in healthy controls. (c) No difference in LICI was detected between the two groups, however. Log ratios >1 = facilitation; <1 = inhibition.
CSP:
APB CSP was elicited in 5 SSADHD subjects and 6 healthy controls. The mean CSP duration in SSADHD subjects was longer compared to that in healthy controls (222.8 ± 37.4 vs. 183.7 ± 17.5 ms, p = 0.047) as shown in Figure 3.
LICI:
APB LICI was elicited in 6 SSADHD subjects and all 7 healthy controls. No significant difference was present between LICI of the two groups (p= 0.46; Figure 3). In both groups, a mean paradoxical faciliatory response was elicited. However, at the individual level, 3 of 6 subjects with SSADHD demonstrated an inhibitory response (ratio < 1), while only 1 of 7 healthy controls had a net inhibitory response (Figure 4).
Figure 4. LICI scatter plot.
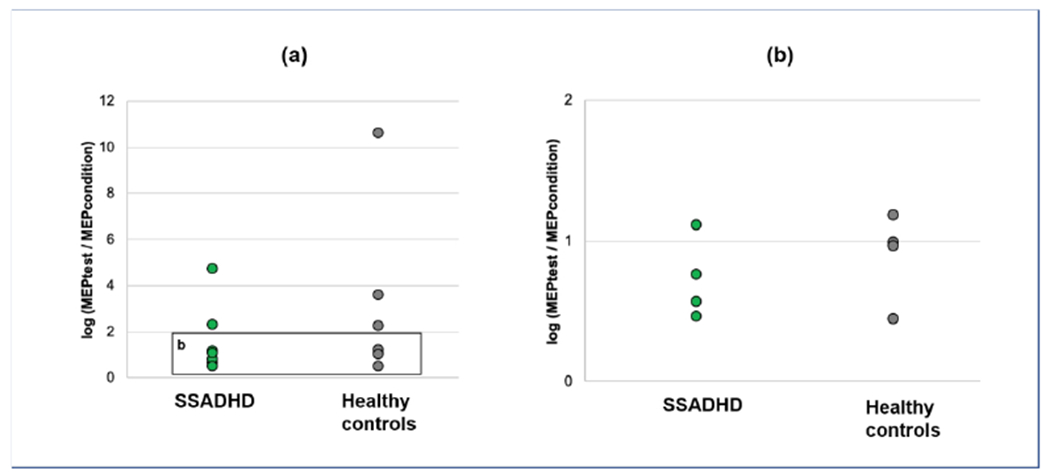
Log ratios >1 indicate cortical facilitation; ratios <1 indicate cortical inhibition. (a) Average LICI per participant varied from net inhibition to net facilitation in both SSADHD and health controls. (b) Magnified view of a subset of LICI ratios show 3 of 6 subjects with SSADHD and 1 of 7 healthy controls demonstrated net inhibition.
Discussion
This cohort is the largest one to date of subjects with SSADHD in whom TMS has been performed. In contrast to prior studies, the age range of subjects in this study is broader which allows for evaluation of age-dependent findings in cortical excitability and inhibition. A decline in rMT, evidence of a maturational trajectory, is well-established in typically developing individuals, in the unaffected hemisphere of patients with focal epilepsy, and in the less affected hemisphere of patients with hemiplegic cerebral palsy.[24–27] Based on this cross-sectional analysis, subjects with SSADHD still also retain this maturational trajectory, which we interpret as an encouraging sign that at least this aspect of cortical excitability remains under normal developmental control. However, to assess whether the rate of maturation or the time to full maturation is similar to that seen in healthy controls rMT in a larger sample size over a broader age range is needed. Maturational trajectory will also be evaluated prospectively with repeated rMT measurements per subject over the course of Natural History Study of Patients with SSADHD. Per subject rMT trajectory in conjunction with clinical history may also shed light on the clinical significance of the maturational trajectory in people with SSADHD.
The overall higher average rMT in the SSADHD group, when compared to healthy controls reflects a decrease in cortical excitability that, by pharmacologic studies, is contingent in large part on voltage-gated sodium channel tone. Given the propensity of epilepsy in SSADHD, the high rMT may be a compensatory response akin to the high rMT seen in the hemisphere that is ipsilateral to the seizure focus in patients with focal epilepsy.[28] The elevated rMT was not specific to SSADHD subjects with epilepsy, but could support the known increased risk of seizures in this patient population.
While rMT findings were similar to what was reported in prior TMS studies in SSADHD patients, notable differences were present in our CSP and LICI data compared to published data. The prolonged CSP in SSADHD subjects in our cohort suggests enhanced GABA-mediated inhibition at either GABAA or GABAB or both receptor subtypes. In contrast, Reis et al. and Schreiber et al. both found shortened CSP in this population, which was concordant with rodent models and flumazenil-PET studies suggesting down-regulation of post-synaptic GABAA and GABAB-receptors.[22, 23] Absence of difference in LICI response between SSADHD subjects and healthy controls also differs from prior reports.
Such discrepancies may be due to differences in TMS protocols. A longer ISI duration could result in less of an inhibitory effect of the CS on the second MEP.[29] The suprathreshold stimulation intensity of 150% rMT may have resulted in stimulation of a greater density of neurons more reliably also resulting in a decreased inhibitory response.[30] The combination of these two parameters in our LICI protocol may have contributed to the trend towards facilitation in both subjects with SSADHD and healthy controls. The variance was smaller in the SSADHD population, with 50% demonstrating net inhibitory LICI. A dedicated study to determine optimal LICI protocol parameters to reliably obtain expected inhibitory response in healthy control subjects is needed to further refine stimulation parameters for LICI in this population.
However, the discrepancy between our CSP findings and those previously reported are difficult to reconcile. Based on these findings we also cannot distinguish between the role that GABAA and GABAB-mediated inhibition may play in SSADHD. Further studies using murine SSADHD models to correlate TMS data with immunohistochemical and electrophysiologic data will be useful to better elucidate this inconsistency.[5, 31, 32]
Limitations of this study include the small, heterogeneous sample size of subjects with SSADHD in whom CSP and LICI measurements were able to be obtained. However, this cohort of SSADHD participants is the largest one in whom rMT has been measured. Recruitment of additional subjects is ongoing. In addition, stimulation intensity to obtain LICI and CSP in SSADHD subjects with high rMT was less than 150% rMT when that value exceeded 100% MO. Increasing the sample size and future methods that utilize a low suprathreshold intensity for LICI and CSP to provide uniformity across subjects will be considered. Unique brain MRIs were not available for each participant (though anonymized brain MRI of a subject with similar head circumference stored in the TMS lab repository was used as needed).
Conclusion
Threshold for cortical excitability is higher in participants with SSADHD than in age-matched healthy controls but lower than in the epileptic (and non-epileptic) hemisphere of patients with focal epilepsy, the significance of which remains uncertain. However, preserved corticospinal tract maturational trajectory suggests that aspects of neurodevelopment are maintained. The discordant CSP measurements recorded thus far in SSADHD participants compared to prior published results may be due to differences in stimulation parameters or differences in the age of participants. The longer CSP is, however, consistent with a hyper-GABAergic state that could result from the excess CNS GABA. Animal models that can correlate CSP measurements with in-vivo alterations in GABAA/B expression or function may be helpful. The absence of differences in LICI between SSADHD participants and healthy controls thus far remains preliminary and may be due to suboptimal stimulation parameters and small sample size.
These interim TMS results from the ongoing observational study in participants with SSADHD presented at the Virtual SSADHD Conference in July 2020 and at the Virtual Joint International Child Neurology Conference and Child Neurology Society Meeting in October 2020. Continued recruitment and repeat TMS testing at different time points will provide an opportunity to better define the maturational trajectory in SSADHD and confirm intrasubject test-retest reliability, in addition to correlating TMS metrics with metabolite levels being collected in the biorepository of the natural history study. Ultimately, we may be able to identify TMS-derived metrics of disease severity that may also serve as biomarkers of therapeutic target engagement.
Acknowledgements:
A.R.’s work is also supported by a research grant from the SSADH Association.
Funding:
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number: R01HD091142. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of conflicting interests:
The Authors declare that there is no conflict of interest.
References
- 1.Buzzi A, Wu Y, Frantseva MV, et al. , Succinic semialdehyde dehydrogenase deficiency: GABAB receptor-mediated function. Brain Res, 2006. 1090(1): p. 15–22. [DOI] [PubMed] [Google Scholar]
- 2.Pearl PL, Gibson KM, Cortez MA, et al. , Succinic semialdehyde dehydrogenase deficiency: lessons from mice and men. J Inherit Metab Dis, 2009. 32(3): p. 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, Buzzi A, Frantseva M, et al. , Status epilepticus in mice deficient for succinate semialdehyde dehydrogenase: GABAA receptor-mediated mechanisms. Ann Neurol, 2006. 59(1): p. 42–52. [DOI] [PubMed] [Google Scholar]
- 4.Vardya I, Drasbek KR, Gibson KM, and Jensen K, Plasticity of postsynaptic, but not presynaptic, GABAB receptors in SSADH deficient mice. Exp Neurol, 2010. 225(1): p. 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel KR, Ainslie GR, Walters DC, et al. , Succinic semialdehyde dehydrogenase deficiency, a disorder of GABA metabolism: an update on pharmacological and enzyme-replacement therapeutic strategies. J Inherit Metab Dis, 2018. 41(4): p. 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearl PL, Gibson KM, Quezado Z, et al. , Decreased GABA-A binding on FMZ-PET in succinic semialdehyde dehydrogenase deficiency. Neurology, 2009. 73(6): p. 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lapalme-Remis S, Lewis EC, De Meulemeester C, et al. , Natural history of succinic semialdehyde dehydrogenase deficiency through adulthood. Neurology, 2015. 85(10): p. 861–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R, Samii A, Canos M, Wassermann EM, and Hallett M, Effects of phenytoin on cortical excitability in humans. Neurology, 1997. 49(3): p. 881–883. [DOI] [PubMed] [Google Scholar]
- 9.Manganotti P, Bongiovanni LG, Zanette G, Turazzini M, and Fiaschi A, Cortical excitability in patients after loading doses of lamotrigine: a study with magnetic brain stimulation. Epilepsia, 1999. 40(3): p. 316–321. [DOI] [PubMed] [Google Scholar]
- 10.Kazis DA, Kimiskidis VK, Papagiannopoulos S, et al. , The effect of valproate on silent period and corticomotor excitability. Epileptic Disord, 2006. 8(2): p. 136–142. [PubMed] [Google Scholar]
- 11.Lee HW, Seo HJ, Cohen LG, Bagic A, and Theodore WH, Cortical excitability during prolonged antiepileptic drug treatment and drug withdrawal. Clin Neurophysiol, 2005. 116(5): p. 1105–1112. [DOI] [PubMed] [Google Scholar]
- 12.Lang N, Rothkegel H, Peckolt H, and Deuschl G, Effects of lacosamide and carbamazepine on human motor cortex excitability: a double-blind, placebo-controlled transcranial magnetic stimulation study. Seizure, 2013. 22(9): p. 726–730. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Ricci R, Large CH, Anderson B, Nahas Z, and George MS, Lamotrigine and valproic acid have different effects on motorcortical neuronal excitability. J Neural Transm (Vienna), 2009. 116(4): p. 423–429. [DOI] [PubMed] [Google Scholar]
- 14.Ziemann U, Reis J, Schwenkreis P, et al. , TMS and drugs revisited 2014. Clin Neurophysiol, 2015. 126(10): p. 1847–1868. [DOI] [PubMed] [Google Scholar]
- 15.Ziemann U, Lonnecker S, Steinhoff BJ, and Paulus W, The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res, 1996. 109(1): p. 127–135. [DOI] [PubMed] [Google Scholar]
- 16.Siebner HR, Dressnandt J, Auer C, and Conrad B, Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve, 1998. 21(9): p. 1209–1212. [DOI] [PubMed] [Google Scholar]
- 17.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, and Classen J, Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol, 1999. 517 ( Pt 2): p. 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierantozzi M, Marciani MG, Palmieri MG, et al. , Effect of Vigabatrin on motor responses to transcranial magnetic stimulation: an effective tool to investigate in vivo GABAergic cortical inhibition in humans. Brain Res, 2004. 1028(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 19.Kimiskidis VK, Papagiannopoulos S, Kazis DA, et al. , Lorazepam-induced effects on silent period and corticomotor excitability. Exp Brain Res, 2006. 173(4): p. 603–611. [DOI] [PubMed] [Google Scholar]
- 20.Mohammadi B, Krampfl K, Petri S, et al. , Selective and nonselective benzodiazepine agonists have different effects on motor cortex excitability. Muscle Nerve, 2006. 33(6): p. 778–784. [DOI] [PubMed] [Google Scholar]
- 21.McDonnell MN, Orekhov Y, and Ziemann U, The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res, 2006. 173(1): p. 86–93. [DOI] [PubMed] [Google Scholar]
- 22.Reis J, Cohen LG, Pearl PL, et al. , GABAB-ergic motor cortex dysfunction in SSADH deficiency. Neurology, 2012. 79(1): p. 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreiber JM, Pearl PL, Dustin I, et al. , Biomarkers in a Taurine Trial for Succinic Semialdehyde Dehydrogenase Deficiency. JIMD Rep, 2016. 30: p. 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garvey MA and Mall V, Transcranial magnetic stimulation in children. Clin Neurophysiol, 2008. 119(5): p. 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hameed MQ, Dhamne SC, Gersner R, et al. , Transcranial Magnetic and Direct Current Stimulation in Children. Curr Neurol Neurosci Rep, 2017. 17(2): p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadelis C, Kaye H, Shore B, Snyder B, Grant PE, and Rotenberg A, Maturation of Corticospinal Tracts in Children With Hemiplegic Cerebral Palsy Assessed by Diffusion Tensor Imaging and Transcranial Magnetic Stimulation. Front Hum Neurosci, 2019. 13: p. 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saisanen L, Maatta S, Julkunen P, et al. , Functional and structural asymmetry in primary motor cortex in Asperger syndrome: a navigated TMS and imaging study. Brain Topogr, 2019. 32(3): p. 504–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badawy RA, Jackson GD, Berkovic SF, and Macdonell RA, Cortical excitability and refractory epilepsy: a three-year longitudinal transcranial magnetic stimulation study. Int J Neural Syst, 2013. 23(1): p. 1250030. [DOI] [PubMed] [Google Scholar]
- 29.de Goede AA and van Putten M, Repeatability of long intracortical inhibition in healthy subjects. Clin Neurophysiol Pract, 2017. 2: p. 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeil CJ, Martin PG, Gandevia SC, and Taylor JL, Long-interval intracortical inhibition in a human hand muscle. Exp Brain Res, 2011. 209(2): p. 287–297. [DOI] [PubMed] [Google Scholar]
- 31.Lee HHC, Pearl PL, and Rotenberg A, Novel genetic tools to model functional enzyme restoration in succinic semialdehyde dehydrogenase deficiency (SSADHD). bioRxiv, 2020. [Google Scholar]
- 32.Vogel KR, Ainslie GR, McConnell A, Roullet JB, and Gibson KM, Toxicologic/transport properties of NCS-382, a gamma-hydroxybutyrate (GHB) receptor ligand, in neuronal and epithelial cells: Therapeutic implications for SSADH deficiency, a GABA metabolic disorder. Toxicol In Vitro, 2018. 46: p. 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]


