Abstract
CFTR function is required for normal mucociliary clearance (MCC) and cough-assisted clearance (CC). Lumacaftor-ivacaftor is approved for use in people with cystic fibrosis (CF) carrying two copies of F508del-CFTR. In this observational study performed at four study sites, we characterized the effect of lumacaftor-ivacaftor on mucociliary and cough clearance and related this to other clinical and research endpoints after one month of treatment. Twenty-five adolescents and adults were enrolled. No effect on whole lung MCC was observed, but CC was significantly increased. Sweat chloride improved by 18 mEq/L in this group, indicating a modest restoration of CFTR activity, but no demonstrable change in FEV1 or lung clearance index was observed. We speculate that the modest effect of lumacaftor-ivacaftor on CFTR function was insufficient to yield an improvement in MCC.
The cystic fibrosis transmembrane conductance regulator (CFTR) is a critical anion transporter that regulates the properties of airway secretions. Mucus clearance relies upon this function and when absent in cystic fibrosis (CF) leads to airway mucus obstruction and chronic infection. Small molecule CFTR modulators are now changing how CF lung disease is treated and improving outcomes. Phase three clinical studies of lumacaftor-ivacaftor for F508del homozygotes demonstrated modest improvement in FEV1 and reduced pulmonary exacerbation rates(1, 2). When compared to earlier studies of the highly effective CFTR modulator ivacaftor in patients with responsive CFTR gating mutations(3, 4), the effect of lumacaftor-ivacaftor on CFTR activity and clinical outcomes were modest.
The previously reported multicenter prospective longitudinal study of CFTR-dependent disease profiling (PROSPECT; http://clinicaltrials.gov/ct2/show/NCT02477319) investigated the real-world clinical effects of lumacaftor-ivacaftor(5). PROSPECT included multiple sub-studies, including one that demonstrated an improvement in lung clearance index (LCI), a measure of ventilation inhomogeneity stemming from small airway obstruction, despite the absence of a significant FEV1 improvement in that population (6). Here, we report on an additional sub-study that characterized the effect of lumacaftor-ivacaftor on mucociliary clearance (MCC). Relating MCC to clinical endpoints and CFTR activity biomarkers is critically important to our understanding of the relationship between the level of CFTR functional restoration and the clinical results that follow.
The PROSPECT MCC sub-study was performed at 4 study sites. Study endpoints were measured at screening and approximately one month after beginning clinically prescribed lumacaftor-ivacaftor. Clinical endpoints included spirometry, sweat chloride and anthropometrics. Both LCI, by multiple breath washout (MBW) of nitrogen(7), and the fraction of exhaled nitric oxide (FeNO)(8) were measured to allow correlations between airway obstruction, an indirect index of ciliary activity and MCC. Study participants performing MCC were a subset of those enrolled in the previously reported core clinical study(5) and the PROSPECT-MBW sub-study(6). Enrollment criteria required that participants be homozygous for F508del-CFTR, ≥12 years of age, and clinically stable within 2 weeks of the baseline visit. They must also have had an FEV1 that was consistently ≥30% predicted during the 6 months prior to enrollment and be willing to discontinue use of hypertonic saline and dornase alfa for at least 12 hours prior to each MCC scan. MCC was quantitated using gamma scintigraphy as previously described based on a standardized protocol(9) and centralized image analysis. The change in the average rate of radiotracer clearance through 60 minutes in the whole right lung compartment (WL-AveClr60) was the pre-specified primary outcome. Cough-assisted clearance between 60-90 minutes (CC60-90) was normalized to the particle retention value at t=60 min and expressed as % clearance/min. Statistical analyses were performed with JMP Pro 15 (SAS, Cary NC). Endpoints are described by the mean and standard deviation (SD). Comparison of mean values were made with paired Student t-tests. Reported p values from multiple comparisons are provided to describe the data and were not corrected for multiple comparisons. Correlations between variables were assessed by calculating Pearson correlation coefficients. WL-AveClr60 and CC60-90 were also analyzed with repeated measures models, with visit (i.e., treatment status), a deposition descriptor (C/P or skew) and the interaction term between visit and the selected deposition indicator as fixed effects. The study was funded by the CF Foundation.
A total of 25 subjects were enrolled. Females accounted for 56% of the group, and median age was 18.7 (range 12-56) years. Baseline FEV1 % predicted was 77.2 (24.9). Hypertonic saline and dornase alfa were used by most participants (80% and 84%, respectively). Study endpoints are shown in Table 1. As observed in the core PROSPECT study cohort, a significant reduction in sweat chloride without a change in FEV1 % predicted was observed in this sub-study cohort. No change in LCI was observed, in contrast to the improvement in LCI reported by Shaw and colleagues in the larger PROSPECT MBW cohort (N=49). However, that group of subjects was substantially younger and more mildly affected(6). Fractional exhaled NO also was not meaningfully changed.
Table 1:
Summary of key study endpoints. N = number of paired data points. WL = whole lung; CC60-90 = cough assisted clearance between 60-90 min; C/P = central/peripheral; CL = central lung; PL = peripheral lung; FeNO = fractional exhaled NO. Listed p values are not corrected for multiple comparisons.
| Variable | N | Baseline | 1-month | Paired t-test P-values |
|---|---|---|---|---|
| Sweat Chloride - mEq/L | 24 | 99.4 ± 9.0 | 81.6 ± 12.0 | 1.8E-07 |
| FEV1 - L | 25 | 2.53 ± 0.92 | 2.55 ± 0.96 | 0.70 |
| FVC - L | 25 | 3.34 ± 1.06 | 3.39 ± 1.06 | 0.42 |
| FEV1 - % pred | 25 | 77.2 ± 24.4 | 77.1 ± 23.9 | 0.98 |
| FVC - % pred | 24 | 86.9 ± 18.4 | 87.3 ± 15.8 | 0.75 |
| FeNO | 21 | 11.2 ± 6.5 | 12.3 ± 8.8 | 0.40 |
| LCI2.5% | 21 | 12.1 ± 5.3 | 11.8 ± 4.7 | 0.56 |
| WL_Ave60 % | 25 | 11.9 ± 10.4 | 10.6 ± 8.1 | 0.45 |
| WL_Ave90 % | 25 | 13.9 ± 11.4 | 13.2 ± 8.1 | 0.70 |
| WL_24hr % | 25 | 28.7 ± 18.2 | 30.2 ± 16.3 | 0.76 |
| CC60-90 %/min | 25 | 0.07 ± 0.24 | 0.21 ± 0.31 | 0.02 |
| Deposition C/P ratio | 25 | 2.37 ± 0.91 | 2.19 ± 0.65 | 0.26 |
| Deposition skew | 25 | 2.08 ± 1.65 | 1.76 ± 1.14 | 0.11 |
| CL_Ave60 % | 25 | 16.4 ± 14.7 | 16.5 ± 11.0 | 0.98 |
| CL_Ave90 % | 25 | 19.6 ± 15.6 | 20.5 ± 11.0 | 0.70 |
| PL_Ave60 % | 25 | 7.1 ± 5.9 | 5.7 ± 7.3 | 0.35 |
| PL_Ave90 % | 25 | 8.1 ± 6.4 | 7.3 ± 7.3 | 0.58 |
With regard to MCC, no statistically significant change in clearance was observed in WL-AveClr60, 24-hr clearance, or clearance from any other region of interest (central or peripheral) through 60 or 90 minutes. However, cough-assisted clearance (CC60-90) was low at baseline and improved after lumacaftor-ivacaftor (Figure 1). Because aerosol deposition patterns impact MCC and CC rates (i.e., more central and heterogenous aerosol deposition patterns are associated with faster MCC and CC rates), we also used repeated measure models to determine whether subtle changes in aerosol deposition might have influenced MCC results. Each of these models confirmed a possible effect of lumacaftor-ivacaftor on CC60-90 (p < 0.005) and the absence of an effect on WL-AveClr60. The correlation between changes in MCC/CC and other endpoints (FEV1% predicted, LCI, FeNO, sweat chloride) also were investigated. Not surprisingly, given the overall lack of change in clinical endpoints other than sweat chloride, none were observed.
Figure 1:
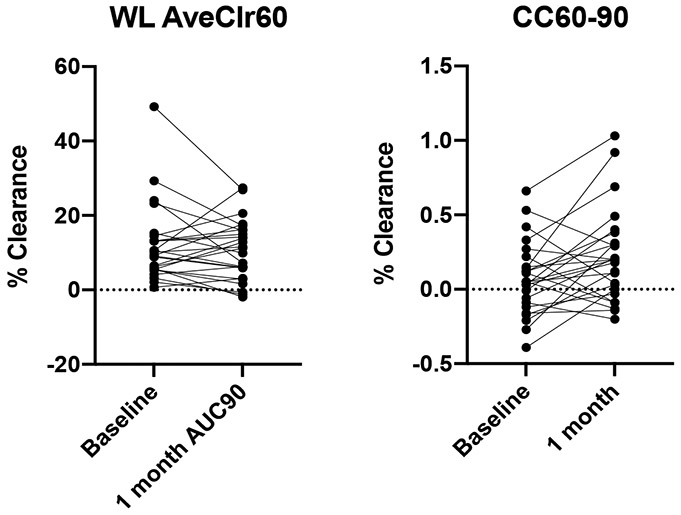
Scatter plot of whole lung clearance (WL AveClr60) and cough-assisted clearance (CC60-90)
Importantly, these data should be considered in the context of results obtained with ivacaftor in G551D-CFTR patients where more robust restoration of CFTR function is achieved. With ivacaftor, sweat chloride values typically drop from ~100 to ~50 mEq/L, and lung function improvements are robust(10). WL MCC more than doubled, while an independent effect on CC was not detected(10, 11). In vitro studies of human bronchial epithelial (HBE) cells demonstrated that ~50% of wild type CFTR function is restored, ciliary beating is increased, and mucus transport is accelerated (12, 13). Intestinal current measurements (ICM) also suggest that ~50% of wild type (WT) CFTR function is restored in GI epithelia(14). In human airways, in vivo nasal potential difference (NPD) measurements with the clinically approved dose of ivacaftor revealed a −4mV CFTR response, reflecting ~20% of WT CFTR function (15). In contrast, lumacaftor-ivacaftor in people homozygous for F508del, reduced sweat chloride by ~18 mEq/L (5, 16), and ~15% of WT CFTR function is restored according to in vitro HBE and ICM studies (16, 17). Lumacaftor-ivacaftor induced a small but significant increase in chloride secretion measured in vivo with NPD (−1.3 mV)(16) that would be estimated to be <10% of WT. The absence of an effect on MCC without cough may, therefore, reflect the modest effect of lumacaftor-ivacaftor on CFTR function in F508del homozygotes. Although we observed an improvement in CC in this study, a similar effect with ivacaftor in G511D-CFTR patients was not observed in prior work (11), raising the possibility that the observed CC effect with lumacaftor-ivacaftor occurred by chance, as this was one of multiple MCC endpoints that were compared.
Others have shown that lumacaftor-ivacaftor clearly protects against pulmonary exacerbations(1, 18, 19), independent of FEV1 changes(20). It may be that the MCC assay in its current form is insufficiently sensitive to detect local/regional effects on mucus clearance that are associated with protection against disease exacerbations. Alternatively, improvement in other host defenses may also play a role in the drug’s protective effects.
Future use of MCC/CC measurements may play a particularly important role in the assessment of inhaled agents that target CFTR function (e.g., CFTR mRNA, CFTR gene editing), as sweat chloride measures will not be useful in this context. While a change in MCC/CC is predicated on restoration of sufficient CFTR activity, it is likely that a similar degree of restoration will be needed to improve clinical outcomes (e.g., FEV1). Combining MCC/CC with other sensitive endpoints (e.g. LCI or emerging functional imaging modalities) will likely be needed to identify novel therapies with the greatest promise in small study populations limited by restricted genotype eligibility or CFTR modulator treatment status.
Highlights.
The effect of lumacaftor-ivacaftor (L-I) on mucociliary clearance and clinical endpoints was tested
L-I did not improve whole lung mucociliary clearance in F508del homozygotes
Only cough-assisted clearance improved without apparent effects on other MCC outcomes
Sweat chloride, but no other clinical endpoints (e.g. FEV1, LCI), improved.
Lack of MCC improvement after L-I differs from that measured after highly effective modulators
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
All authors were supported by grants from the Cystic Fibrosis Foundation for the submitted work.
Dr. Donaldson reports grants from Vertex Pharmaceuticals, grants from Astra Zeneca, personal fees from Chiesi, personal fees from Boehringer Ingleheim, grants and personal fees from Calithera.
Dr. Rowe reports grants and personal fees from Novartis, grants and personal fees from Bayer, grants from Translate Bio, non-financial support from Proteostasis, grants, personal fees and non-financial support from Galapagos/Abbvie, grants, personal fees and other from Synedgen/Synspira, grants from Eloxx, grants and personal fees from Celtaxsys, grants, personal fees, non-financial support and other from Vertex Pharmaceuticals Inc, personal fees from Renovion, grants and personal fees from Arrowhead, grants and other from Ionis, grants from Astra Zeneca, personal fees from Cystetic Medicines, personal fees from Arcturus.
Dr. Mogayzel reports grants from Vertex Pharmaceutics and Eloxx Pharmaceuticals.
REFERENCES
- 1.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N Engl J Med. 2015;373(3):220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratjen F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6-11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. The Lancet Respiratory medicine. 2017;5(7):557–67. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363(21):1991–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sagel SD, Khan U, Heltshe SL, Clancy JP, Borowitz D, Gelfond D, et al. Clinical Effectiveness of Lumacaftor/Ivacaftor in Patients with Cystic Fibrosis Homozygous for F508del-CFTR. A Clinical Trial. Ann Am Thorac Soc. 2021;18(1):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw M, Khan U, Clancy JP, Donaldson SH, Sagel SD, Rowe SM, et al. Changes in LCI in F508del/F508del patients treated with lumacaftor/ivacaftor: Results from the prospect study. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2020;19(6):931–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson PD, Latzin P, Verbanck S, Hall GL, Horsley A, Gappa M, et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur Respir J. 2013;41(3):507–22. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic S, European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–30. [DOI] [PubMed] [Google Scholar]
- 9.Bennett WD, Laube BL, Corcoran T, Zeman K, Sharpless G, Thomas K, et al. Multisite comparison of mucociliary and cough clearance measures using standardized methods. Journal of aerosol medicine and pulmonary drug delivery. 2013;26(3):157–64. [DOI] [PubMed] [Google Scholar]
- 10.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190(2):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donaldson SH, Laube BL, Corcoran TE, Bhambhvani P, Zeman K, Ceppe A, et al. Effect of ivacaftor on mucociliary clearance and clinical outcomes in cystic fibrosis patients with G551D-CFTR. JCI Insight. 2018;3(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106(44):18825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birket SE, Chu KK, Houser GH, Liu L, Fernandez CM, Solomon GM, et al. Combination therapy with cystic fibrosis transmembrane conductance regulator modulators augment the airway functional microanatomy. American journal of physiology Lung cellular and molecular physiology. 2016;310(10):L928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graeber SY, Hug MJ, Sommerburg O, Hirtz S, Hentschel J, Heinzmann A, et al. Intestinal Current Measurements Detect Activation of Mutant CFTR in Patients with Cystic Fibrosis with the G551D Mutation Treated with Ivacaftor. Am J Respir Crit Care Med. 2015;192(10):1252–5. [DOI] [PubMed] [Google Scholar]
- 15.Rowe SM, Liu B, Hill A, Hathorne H, Cohen M, Beamer JR, et al. Optimizing nasal potential difference analysis for CFTR modulator development: assessment of ivacaftor in CF subjects with the G551D-CFTR mutation. PloS one. 2013;8(7):e66955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graeber SY, Dopfer C, Naehrlich L, Gyulumyan L, Scheuermann H, Hirtz S, et al. Effects of Lumacaftor-Ivacaftor Therapy on Cystic Fibrosis Transmembrane Conductance Regulator Function in Phe508del Homozygous Patients with Cystic Fibrosis. Am J Respir Crit Care Med. 2018;197(11):1433–42. [DOI] [PubMed] [Google Scholar]
- 17.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A. 2011;108(46):18843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgel PR, Durieu I, Chiron R, Mely L, Prevotat A, Murris-Espin M, et al. Clinical response to lumacaftor-ivacaftor in patients with cystic fibrosis according to baseline lung function. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2020. [DOI] [PubMed] [Google Scholar]
- 19.Tong K, Barker D, France M, Burr L, Greville H, Visser S, et al. Lumacaftor/ivacaftor reduces exacerbations in adults homozygous for Phe508del mutation with severe lung disease. J Cyst Fibros. 2020;19(3):415–20. [DOI] [PubMed] [Google Scholar]
- 20.McColley SA, Konstan MW, Ramsey BW, Stuart Elborn J, Boyle MP, Wainwright CE, et al. Lumacaftor/Ivacaftor reduces pulmonary exacerbations in patients irrespective of initial changes in FEV1. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2019;18(1):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]


