Abstract
Purpose
The present study aimed to verify the clustering of cardiometabolic risk factors for cardiovascular diseases (CVD) and its relationship with the continuous cardiometabolic risk score (cMetS).
Methods
Cross-sectional study with 631 children aged 6 to 9 years. Cardiorespiratory fitness, glucose, systolic blood pressure, total cholesterol/high-density lipoprotein cholesterol ratio, triglycerides, and waist circumference were assessed. The number of children in whom the risk factors were not independently distributed was analyzed. Z-scores were computed for each risk factor to calculate the cMetS.
Results
There was a high proportion of children with clustering of risk factors for CVD. The clustering of risk factors was apparent in 11.3% of the children for four or more risk factors, and 21.9% had three or more risk factors. The cMetS showed a linear relationship with the increase in the number of risk factors. A cMetS value higher than 0.91 indicated clustering of cardiometabolic risk factors amongst children.
Conclusion
The use of clustering of cardiometabolic risk factors identified a high proportion of children with the presence of relevant cardiometabolic alterations. A cMetS value higher than 0.91 (relative to an international standard) indicated higher clustering of cardiometabolic risk factors amongst children.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-021-00845-9.
Keywords: Cardiovascular diseases, Child, Metabolic syndrome, Pediatric
Introduction
Cardiometabolic disorders have mainly been a focus in adults, but there is also evidence of their existence in childhood [1]. Metabolic syndrome (MS) is now recognized as a problem during childhood [2]. Intensive efforts have been directed, in recent years, to identify the presence of cardiometabolic risk factors in childhood and adolescence [1, 3–5]. For children younger than 10 years of age, the diagnosis of MS is characterized using family history data of MS, type II diabetes, dyslipidemia, cardiovascular diseases (CVD), hypertension, and obesity, without considering the child's laboratory information [6]. It is also noteworthy that risk factor levels are already high in some children and youth, especially in relation to obesity, blood pressure and lipid indicators [7, 8]. However, there is no consensus concerning the MS definition in children, and the present criteria used to diagnose MS in childhood and youth populations have a low agreement [7].
These cardiometabolic alterations appear slowly and progressively in the infant population, and early screening is important, regardless of family history, especially in overweight children [9]. Most people do not show CVD outcomes in early childhood and adolescence, but they are already in the early stages of risk factors development for CVD [3], largely due to behavioral habits [10, 11]. Clustering means that elevated levels of multiple cardiometabolic risk factors for CVD can be found simultaneously in the same children [1], and it can be viewed as a sign of poor cardiometabolic health [3]. Furthermore, an unhealthy cardiometabolic profile during this stage of life can reduce life expectancy and increase the odds of Type II Diabetes and CVD development during adult life [12].
There are several ways to define children and adolescents ‘at risk’, and there is no consensus for a definition. In one approach, Andersen et al. [1] calculated whether there were more children with multiple CVD risk factors than would be expected given a random distribution. Another approach to define cardiometabolic health has been highly used in international studies. The continuous cardiometabolic risk score (cMetS) sums standardized values of the traditional cardiometabolic risk factors to provide a better measure of the true risk for CVD [2] and MS amongst children and adolescents [3]. Thus, the present study aims to verify the clustering of cardiometabolic risk factors for CVD and its relationship with cMetS amongst children from Southern Brazil.
Materials and methods
Participants
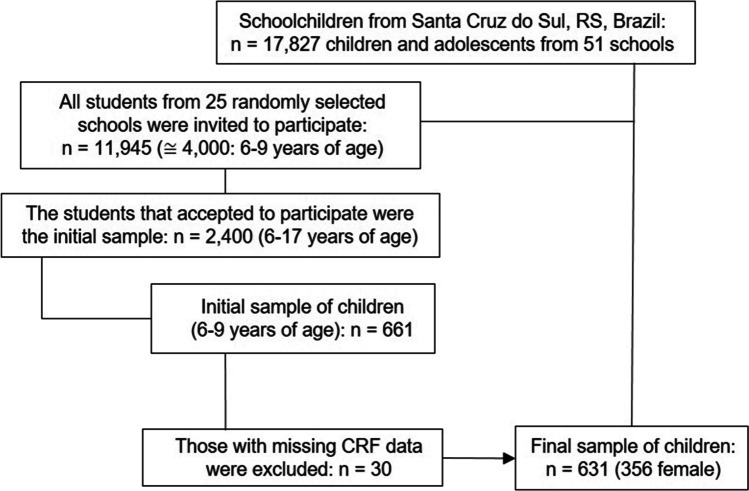
In this cross-sectional study, data from 631 children (336 female) were used. The sample consists of schoolchildren aged six to nine years, from public and private schools in the city of Santa Cruz do Sul, Rio Grande do Sul State in the southern region of Brazil. The sample was randomly selected, stratified by conglomerates (center, north, south, east and west regions of the city) and urban and rural areas. All children from 25 randomly selected schools were invited to participate. A sample of 661 children was evaluated. Those who did not perform the fitness test were excluded (n = 30). Children with complete data and whose parents or guardians signed the consent form were enrolled in the study (Fig. 1).
Fig. 1.
Flow chart showing the population and sampling design
Measurements
Body mass index (BMI) was calculated by the ratio between body mass in kilograms and the height squared in meters (weight/[height]2). Waist circumference (WC) was assessed for obesity evaluation. A non-elastic tape with resolution of 1 mm was used to measure the circumference of the narrowest part of the trunk between the ribs and hips [13]. Systolic blood pressure (SBP) was evaluated at rest with a sphygmomanometer and stethoscope on the right arm and a cuff suitable for the children according to the VI Brazilian Guidelines of Hypertension [14]. Glucose and blood lipoprotein-lipids (high-density lipoprotein cholesterol [HDL-C], total cholesterol [TC], and triglycerides) were determined through blood collection. The six-minute run-walk test assessed cardiorespiratory fitness level (CRF), as recommended by Projeto Esporte Brasil [15], with subsequent calculation of the peak oxygen uptake (VO2peak) in mL/kg/min using the following formula [16]:
where Test is the distance performed by the student in meters, and the values of 1 and 0 are used for males and females, respectively.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, v. 23.0, IBM, Armonk, NY, USA). First, children were recoded into two age groups: 6–7 years and 8–9 years old. However, as no significant differences were found, we analyzed all children together. The continuous variables were described as mean and standard deviation (SD) or mean and 95% confidence interval (CI). Mann–Whitney U test (for continuous variables) or chi-square test (for categorical variables) were used to compare the sample characteristics by sex. The p-values of p < 0.05 were considered significant in all analyses.
WC, TC/HDL-C ratio, and triglycerides (skewed variables) were logarithmically transformed by the natural logarithm. Z-scores were computed for all risk factors (glucose, SBP, TC/HDL-C ratio, triglycerides, WC, and CRF) according to sex- and age-specific international reference values to calculate the standardized level of each child using the following formula:
A cMetS was calculated by summing the glucose, SBP, TC/HDL-C ratio, triglycerides, WC, and CRF (VO2peak inversed) scores and divided by six [17].
For the clustering analysis, we analyzed the number of children where the risk factors were not independently distributed. This part was done by defining the upper quartile of glucose, SBP, TC/HDL-C, triglycerides, and WC and the lower quartile of CRF to be at risk. From this, we calculated how many risk factors each child had and then compared the number we observed with a specific number of risk factors we would expect if they were independent of each other. The latter was calculated from a binomial distribution. The proportion of expected children with 0 to 6 risk factors was calculated using the binomial formula [18]:
used by Andersen et al. [1], where n was the possible number of risk factors (6 risk factors), p was the probability of having a quartile at risk (¼ = 0.25), and r was the number of the risk factors which the probability was calculated for (0 to 6 risk factors). The expected proportions from a binomial distribution were: 0.178, 0.356, 0.297, 0.132, 0.033, 0.004, and 0.0002 for 0 to 6 risk factors, respectively. Additionally, we merged 3 or more risk factors, 4 or more risk factors, and 5 or more risk factors; the expected proportions from a binomial distribution for those were: 0.169, 0.038, and 0.005, respectively. The observed number of children in each group (0 to 6 risk factors) was compared to the expected proportion from a binomial distribution by calculating the ratio between observed and expected with 95% confidence intervals for each group. After we found out how many children had clustering (4 or more cardiometabolic risk factors) defined as non-independent distribution of risk factors, we made a cMetS cutpoint which selected the exact value of children with clustering. Finally, we compared children between the groups (lower cardiometabolic risk < cMetS cutpoint; and higher cardiometabolic risk > cMetS cutpoint) using the the Independent t-test or the Mann–Whitney U test considering significant differences for p < 0.05.
Results
Table 1 shows the sample characteristics, stratified by sex. Girls presented less favorable cardiorespiratory fitness (p < 0.001), TC/HDL-C ratio (p < 0.001), and triglycerides (p = 0.008) than boys, whereas boys exhibited less favorable glucose (p = 0.004) than girls.
Table 1.
Sample characteristics
| Male | Female | p | Total | |
|---|---|---|---|---|
| n = 295 | n = 336 | n = 631 | ||
| n (%) | n (%) | n (%) | ||
| Living area | ||||
| Urban | 242 (82.0) | 280 (83.3) | 0.667 | 522 (82.7) |
| Rural | 53 (18.0) | 56 (16.7) | 109 (17.3) | |
| Socioeconomic level | ||||
| High | 136 (46.1) | 148 (44.0) | 0.781 | 284 (45.0) |
| Middle | 150 (50.8) | 175 (52.1) | 325 (51.5) | |
| Low | 9 (3.1) | 13 (3.9) | 22 (3.5) | |
| Skin color | ||||
| White | 231 (78.3) | 266 (79.2) | 0.482 | 497 (78.8) |
| Black | 23 (7.8) | 31 (9.2) | 54 (8.6) | |
| Brown/mulatto | 37 (12.5) | 32 (9.5) | 69 (10.9) | |
| Yellow | 4 (1.4) | 5 (1.5) | 9 (1.4) | |
| Indigenous | - | 2 (0.6) | 2 (0.3) | |
| Mean (SD) | Mean (SD) | p | Mean (SD) | |
| Cardiorespiratory fitness (mL/kg/min) | 47.14 (5.11) | 42.87 (4.90) | < 0.001 | 44.86 (5.43) |
| Glucose (mmol/L) | 4.91 (0.54) | 4.83 (0.59) | 0.004 | 4.86 (0.57) |
| Systolic Blood Pressure (mmHg) | 100.20 (12.27) | 98.60 (12.63) | 0.126 | 99.35 (12.47) |
| TC/HDL-C (mmol/L) | 2.54 (0.52) | 2.79 (0.65) | < 0.001 | 2.67 (0.60) |
| Triglycerides (mmol/L) | 0.70 (0.35) | 0.75 (0.33) | 0.008 | 0.73 (0.34) |
| Waist Circumference (cm) | 61.28 (8.55) | 60.54 (8.81) | 0.122 | 60.89 (8.69) |
| Continuous cardiometabolic risk score | 0.03 (0.60) | 0.07 (0.67) | - | 0.05 (0.64) |
Chi-square test for categorical variables; Mann–Whitney U test for continuous variables; significant differences for p < 0.05. SD: standard deviation; mL/kg/min: Milliliters per kilogram per minute; mmol/L: Milimol per liter; mmHg: Millimeters of mercury; TC/HDL-C: Total cholesterol/High-density lipoprotein cholesterol ratio; cm: centimeters
The presence of clustered risk factors in children was 11.3% (4 or more risk factors). The degree of clustering risk factors was assessed between the ratio of observed/expected risk factors (Table 2). It shows 1.97 (95% CI: 1.44 to 2.71), 9.74 (95% CI: 6.62 to 14.32), and 19.47 (95% CI: 6.26 to 60.54) times as many as expected of children having clustering of 4, 5, and 6 risk factors, respectively.
Table 2.
Presence of clustered risk in children and odds ratio for observed/expected risk factors
| Probability expected of children having 0 to 6 risk factors |
Observed risk factors clustering n (%) |
OR (95% CI) | |
|---|---|---|---|
| 0 risk factors | 0.178 | 192 (30.4) | 1.71 (1.44 to 2.03) |
| 1 risk factors | 0.356 | 193 (30.6) | 0.86 (0.73 to 1.02) |
| 2 risk factors | 0.297 | 108 (17.1) | 0.58 (0.47 to 0.71) |
| 3 risk factors | 0.132 | 67 (10.6) | 0.81 (0.63 to 1.04) |
| 4 risk factors | 0.033 | 41 (6.5) | 1.97 (1.44 to 2.71) |
| 5 risk factors | 0.004 | 27 (4.3) | 9.74 (6.62 to 14.32) |
| 6 risk factors | 0.0002 | 3 (0.5) | 19.47 (6.26 to 60.54) |
| 3 or more risk factors | 0.169 | 138 (21.9) | 1.29 (1.07 to 1.56) |
| 4 or more risk factors | 0.038 | 71 (11.3) | 2.99 (2.34 to 3.83) |
| 5 or more risk factors | 0.005 | 30 (4.8) | 10.25 (7.10 to 14.79) |
OR: Odds ratio of observed/expected; 95% CI: 95% confidence interval
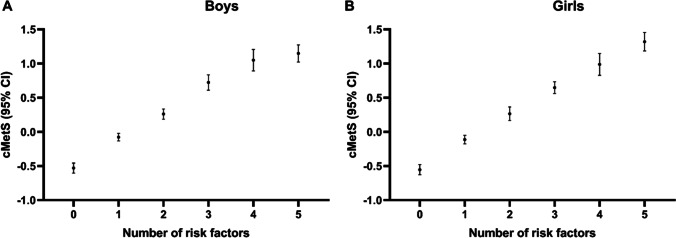
The comparison of cMetS with number of risk factors clustered shows a linear relationship for boys (Fig. 2A) and girls (Fig. 2B). For higher number of risk factors observed, less favorable means of cMetS were observed as well. Children without any risk factors presented negative cMetS means (boys: -0.53 [95% CI: -0.60 to -0.46]; girls: -0.55 [95% CI: -0.63 to -0.48]. For five risk factors, the means of cMetS were 1.15 for boys (95% CI: 1.02 to 1.27) and 1.32 for girls (95% CI: 1.18 to 1.45). The figure does not present cMetS means and confidence intervals for 6 risk factors because we only observed three boys with clustering of 6 risk factors.
Fig. 2.
Comparison of cMetS with number of risk factors clustered for boys and girls
The cMetS cutpoint (0.91) selected exactly 11.3% of the sample. Overall, 560 children (265 boys) had scores lower than the established cutpoint, and 71 (30 boys) had score above the cutpoint. Children classified as lower risk had more favorable levels for all cardiometabolic risk factors (Table 3).
Table 3.
Comparison of cardiometabolic risk factors between lower and higher risk groups
| cMetS cutpoint | p | ||
|---|---|---|---|
| Lower risk (< 0.91) |
Higher risk (> 0.91) |
||
| Mean (SD) | Mean (SD) | ||
| All participants | n = 560 | n = 71 | |
| Cardiorespiratory fitness (mL/kg/min) | 45.81 (4.77) | 37.42 (4.45) | < 0.001* |
| Glucose (mmol/L) | 4.81 (0.51) | 5.30 (0.77) | < 0.001 |
| Systolic Blood Pressure (mmHg) | 97.64 (11.53) | 112.82 (11.52) | < 0.001 |
| TC/HDL-C (mmol/L) | 2.60 (0.54) | 3.25 (0.76) | < 0.001 |
| Triglycerides (mmol/L) | 0.68 (0.30) | 1.11 (0.40) | < 0.001 |
| Waist Circumference (cm) | 59.04 (6.68) | 75.44 (9.02) | < 0.001 |
| Boys | n = 265 | n = 30 | |
| Cardiorespiratory fitness (mL/kg/min) | 48.06 (4.33) | 38.96 (4.15) | < 0.001 |
| Glucose (mmol/L) | 4.86 (0.54) | 5.28 (0.38) | < 0.001 |
| Systolic Blood Pressure (mmHg) | 98.44 (10.87) | 115.73 (13.09) | < 0.001 |
| TC/HDL-C (mmol/L) | 2.50 (0.49) | 2.92 (0.58) | < 0.001 |
| Triglycerides (mmol/L) | 0.66 (0.32) | 1.05 (0.38) | < 0.001 |
| Waist Circumference (cm) | 59.54 (6.59) | 76.64 (8.60) | < 0.001 |
| Girls | n = 295 | n = 41 | |
| Cardiorespiratory fitness (mL/kg/min) | 43.78 (4.23) | 36.30 (4.37) | < 0.001 |
| Glucose (mmol/L) | 4.76 (0.48) | 5.31 (0.96) | < 0.001 |
| Systolic Blood Pressure (mmHg) | 96.93 (12.05) | 110.68 (9.84) | < 0.001 |
| TC/HDL-C (mmol/L) | 2.69 (0.56) | 3.50 (0.79) | < 0.001 |
| Triglycerides (mmol/L) | 0.69 (0.28) | 1.16 (0.41) | < 0.001 |
| Waist Circumference (cm) | 58.60 (6.74) | 74.56 (9.32) | < 0.001 |
Mann–Whitney U test or *Independent t-test; significant differences for p < 0.05. SD: standard deviation; mL/kg/min: Milliliters per kilogram per minute; mmol/L: Milimol per liter; mmHg: Millimeters of mercury; TC/HDL-C: Total cholesterol/High-density lipoprotein cholesterol ratio; cm: centimeters
Discussion
The present study demonstrated that the clustering of cardiometabolic risk factors was evident in 11.3% of the children evaluated (presence of 4 or more risk factors). Similarly, Andersen et al. [19] (using the following risk factors: SBP, triglycerides, TC/HDL-C ratio, insulin resistance, sum of four skinfolds, and CRF [VO2peak measured by a cycle test]) found that 10.6% of students in Denmark, Estonia and Portugal had at least four risk factors. The use of this approach can detect a higher proportion of children with cardiometabolic risk and provide a better view on cardiometabolic health, since the presence of MS, according to the current diagnostic criteria, presents a low prevalence (0.9%) in the juvenile population [3]. However, the International Diabetes Federation (IDF) based their definition on expert opinion [6] while ours is based on a biological rationale of clustering.
Analyzing the ratio between observed and expected values for risk factors, we observed 19.47 times more than expected for the presence of six risk factors, and 9.74 and 1.97 times for five and four risk factors, respectively. In Danish children and adolescents, Andersen et al. [1] also found higher values than expected, for five (around 8–9 times more than expected) and four (around 3 times more than expected) risk factors. Our study included CRF levels in the aggregation of risk factors, because it represents an important factor for the cardiometabolic health of children [19] and is inversely associated with the cMetS itself [20–23]. Andersen et al. [3] stated that CRF plays an important role in the MS diagnosis, and it is strongly related to other cardiometabolic risk factors as much as adiposity. Other authors indicate that CRF may pose as a greater risk to overall health than excess adiposity [24]. For example, Lerum et al. [25] also suggest that CRF should be part of the cardiometabolic risk assessment, since this component is a strong predictor of CVD, improving the early identification of children who may be at risk. In this sense, the inclusion of CRF in the evaluation of cardiometabolic risk improves MS diagnosis [3].
A positive and direct relationship between the mean value of cMetS and the number of MS components was observed both in boys and girls. The cMetS has been widely used in European countries [3, 5, 17, 19, 26] and in the United States [27–29] for the evaluation of cardiometabolic risk in schoolchildren. In Brazil, a study validated the cMetS for children (n = 348), using the following components: WC, HOMA, HDL-C, triglycerides, and the mean of arterial pressure. The authors indicated a cutoff point of 1.86 (sensitivity: 96.7%, specificity: 82.7%, AUC: 0.96) for their continuous score [30]. Also, Reuter et al. [4], using the same risk factors from the present study, showed the cutoff points for cMetS presented high sensitivity (100.0%) and specificity (92.5%) to predict MS (defined by Cook et al. [31] criteria) in adolescents.
Unique to our study was the fact that we used an international sex- and age-standardized method to define cardiometabolic health amongst children [17] instead of traditional criteria to diagnose MS. We decided to make a cMetS cutpoint based on how many children had clustering, defined as risk factors that were not independently distributed. The approach presented in the present study, therefore, is necessary, since the current cutoff points for the diagnosis of MS are not concordant [7], and there is no consensus on the best criteria to be used [32]. In addition, the diagnosis of MS in children younger than 10 years is currently by IDF made only by family history of MS, type II diabetes, dyslipidemia, CVD, arterial hypertension and/or obesity, without considering any laboratory data of the child [6]. However, many children have a risk profile in which many risk factors are elevated in the same child compared to the level of their peers, and risk factors show a pattern of clustering even in children below 10 years of age. Also, we emphasize the importance of using the continuous variable, which is statistically more sensitive and less prone to errors, compared to the categorized form [33, 34].
The study presents strengths, such as the presentation of a randomly selected sample of children from a municipality in southern Brazil. To our knowledge, this is the first study to evaluate the aggregation among risk factors for CVD in a sample of Brazilian children. Lastly, our study used age- and sex-specific international reference values [17] to standardize Z-scores for each risk factors for CVD instead of using a sample-specific approach. On the other hand, we also highlight some limitations. We used glucose as a marker of glycemic metabolism. It is known that changes in glucose profile are still subtle in childhood [31], because even children with severe insulin resistance are able to produce a sufficient amount of insulin to regulate glucose. These children have normal fasting glucose but very high fasting insulin. The cross-sectional design also makes it impossible to state which parameter is most important for the development of cardiometabolic risk factors [10].
Conclusion
In conclusion, the use of clustering of cardiometabolic risk factors identified a high proportion of children with the presence of relevant cardiometabolic alterations. Also, the cMetS assessment showed a linear relationship with number of risk factors for CVD both in boys and girls. Finally, a cMetS value higher than 0.91 (relative to an international standard) indicated higher clustering of cardiometabolic risk factors amongst children.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
The present study did not receive funding resources.
Availability of data and material
Data are available under reasonable request with the corresponding author.
Declarations
Ethics approval
The study was approved by the Committee of Ethics in Research with Human Subjects of the University of Santa Cruz do Sul (UNISC), under number 37338314.6.0000.5343.
Consent to participate and for publication
All parents or guardians were informed about the study objectives and signed informed consent.
Conflicts of interest
The authors state no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andersen LB, Wedderkopp N, Hansen HS, Cooper AR, Froberg K. Biological cardiovascular risk factors cluster in danish children and adolescents: The European youth heart study. Prev Med (Baltim) 2003;37:363–367. doi: 10.1016/s0091-7435(03)00145-2. [DOI] [PubMed] [Google Scholar]
- 2.Vanlancker T, Schaubroeck E, Vyncke K, et al. Comparison of definitions for the metabolic syndrome in adolescents. The HELENA study. Eur J Pediatr. 2017;176:241–252. doi: 10.1007/s00431-016-2831-6. [DOI] [PubMed] [Google Scholar]
- 3.Andersen LB, Lauersen JB, Brønd JC, et al (2015) A new approach to define and diagnose cardiometabolic disorder in children. J Diabetes Res 1–10. 10.1155/2015/539835 [DOI] [PMC free article] [PubMed]
- 4.Reuter CP, Andersen LB, de Moura Valim AR, Reuter ÉM, Borfe L, Renner JDP, de Mello ED (2019) Cutoff points for continuous metabolic risk score in adolescents from southern Brazil. Am J Hum Biol. 10.1002/ajhb.23211 [DOI] [PubMed]
- 5.Stavnsbo M, Skrede T, Aadland E, Aadland KN, Chinapaw M, Anderssen SA, Andersen LB, Resaland GK (2019) Cardiometabolic risk factor levels in Norwegian children compared to international reference values: The ASK study. PLoS One. 10.1371/journal.pone.0220239 [DOI] [PMC free article] [PubMed]
- 6.IDF (2007) The IDF consensus definition of the Metabolic Syndrome in children and adolescents. https://www.idf.org/e-library/consensus-statements/61-idf-consensus-definition-of-metabolic-syndrome-in-children-and-adolescents.html. Accessed 12 Feb 2021
- 7.Reuter CP, Burgos MS, Barbian CD, Renner JDP, Franke SIR, de Mello ED. Comparison between different criteria for metabolic syndrome in schoolchildren from Southern Brazil. Eur J Pediatr. 2018;177:1471–1477. doi: 10.1007/s00431-018-3202-2. [DOI] [PubMed] [Google Scholar]
- 8.Welser L, Lima RA, Silveira JF de C, Andersen LB, Pfeiffer KA, Renner JDP, Reuter CP (2021) Cardiometabolic risk factors in children and adolescents from southern Brazil: comparison to international reference values. J Pediatr Endocrinol Metab. Ahead of Print [DOI] [PubMed]
- 9.Damiani D, Kuba VM, Cominato L, Damiani D, Dichtchekenian V, de Menezes Filho HC. Síndrome metabólica em crianças e adolescentes: Dúvidas na terminologia, mas não nos riscos cardiometabólicos. Arq Bras Endocrinol Metabol. 2011;55:576–582. doi: 10.1590/S0004-27302011000800011. [DOI] [PubMed] [Google Scholar]
- 10.Andersen LB, Sardinha L, Froberg K, Riddoch CJ, Page AS, Anderssen SA. Fitness, fatness and clustering of cardiovascular risk factors in children from Denmark, Estonia and Portugal: The European Youth Heart Study. Int J Pediatr Obes. 2008;3:58–66. doi: 10.1080/17477160801896366. [DOI] [PubMed] [Google Scholar]
- 11.Stabelini Neto A, de Campos W, dos Santos GC, Mazzardo Junior O. Metabolic syndrome risk score and time expended in moderate to vigorous physical activity in adolescents. BMC Pediatr. 2014;14:42. doi: 10.1186/1471-2431-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen LB, Bugge A, Dencker M, Eiberg S, El-Naaman B. The association between physical activity, physical fitness and development of metabolic disorders. Int J Pediatr Obes. 2011;6:29–34. doi: 10.3109/17477166.2011.606816. [DOI] [PubMed] [Google Scholar]
- 13.Taylor RW, Jones IE, Williams SM, Goulding A. Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3–19 y. Am J Clin Nutr. 2000;72:490–495. doi: 10.1093/ajcn/72.2.490. [DOI] [PubMed] [Google Scholar]
- 14.Sociedade Brasileira de Cardiologia, Sociedade Brasileira de Hipertensão, Sociedade Brasileira de Nefrologia VI Diretrizes Brasileiras de Hipertensão. Arq Bras Cardiol. 2010;1:1–51. [PubMed] [Google Scholar]
- 15.PROESP-BR. Projeto Esporte Brasil (2015) Manual de testes e avaliação - Versão 2015. https://www.ufrgs.br/proesp/. Accessed 16 Jan 2016
- 16.Bergmann G, Bergmann M, De Castro A, Lorenzi T, Pinheiro E, Moreira R, Marques A, Gaya A (2014) Use of the 6-minute walk/run test to predict peak oxygen uptake in adolescents. Rev Bras Atividade Física Saúde. 10.12820/rbafs.v.19n1p64
- 17.Stavnsbo M, Resaland GK, Anderssen SA, et al. Reference values for cardiometabolic risk scores in children and adolescents: Suggesting a common standard. Atherosclerosis. 2018;278:299–306. doi: 10.1016/j.atherosclerosis.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Altman DG (1991) Theoretical distributions. In: Pract Stat Med Res. London: Chapman & Hall. pp 68–70
- 19.Andersen LB, Harro M, Sardinha LB, Froberg K, Ekelund U, Brage S, Anderssen SA. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study) Lancet. 2006;368:299–304. doi: 10.1016/S0140-6736(06)69075-2. [DOI] [PubMed] [Google Scholar]
- 20.Machado-Rodrigues AM, Leite N, Coelho-E-Silva MJ, Martins RA, Valente-Dos-Santos J, Mascarenhas LPG, Boguszewski MCS, Padez C, Malina RM. Independent association of clustered metabolic risk factors with cardiorespiratory fitness in youth aged 11–17 years. Ann Hum Biol. 2014;41:271–276. doi: 10.3109/03014460.2013.856471. [DOI] [PubMed] [Google Scholar]
- 21.Marques KC, de C Silveira JF, Schneiders LDB, Souza S, de Mello ED, Reuter CP. Escore contínuo de risco metabólico em escolares com diferentes níveis de aptidão cardiorrespiratória. Rev Andaluza Med del Deport. 2019;12:354–357. [Google Scholar]
- 22.Saldanha Filho N, Reuter CP, Renner JDP, Barbian CD, de C Silveira JF, de BorbaSchneiders L, Pohl HH. Low levels of cardiorespiratory fitness and abdominal resistance are associated with metabolic risk in schoolchildren. J Pediatr Endocrinol Metab. 2019;32:455–460. doi: 10.1515/jpem-2018-0236. [DOI] [PubMed] [Google Scholar]
- 23.Silveira JF de C, Reuter CP, Welser L, Pfeiffer KA, Andersen LB, Pohl HH, Lima RA (2021) Tracking of cardiometabolic risk in a Brazilian schoolchildren cohort: a 3-year longitudinal study. J Sports Med Phys Fitness. 10.23736/S0022-4707.20.11479-8 [DOI] [PubMed]
- 24.Kennedy AB, Lavie CJ, Blair SN. Fitness or fatness which is more important? JAMA - J Am Med Assoc. 2018;319:231–232. doi: 10.1001/jama.2017.21649. [DOI] [PubMed] [Google Scholar]
- 25.Lerum Ø, Aadland E, Andersen LB, Anderssen SA, Resaland GK. Validity of noninvasive composite scores to assess cardiovascular risk in 10-year-old children. Scand J Med Sci Sport. 2017;27:865–872. doi: 10.1111/sms.12826. [DOI] [PubMed] [Google Scholar]
- 26.Viitasalo A, Lakka TA, Laaksonen DE, et al. Validation of metabolic syndrome score by confirmatory factor analysis in children and adults and prediction of cardiometabolic outcomes in adults. Diabetologia. 2014;57:940–949. doi: 10.1007/s00125-014-3172-5. [DOI] [PubMed] [Google Scholar]
- 27.Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol. 2008;7:17. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenmann JC, Laurson KR, Dubose KD, Smith BK, Donnelly JE. Construct validity of a continuous metabolic syndrome score in children. Diabetol Metab Syndr. 2010;2:8. doi: 10.1186/1758-5996-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okosun IS, Lyn R, Davis-Smith M, Eriksen M, Seale P. Validity of a continuous metabolic risk score as an index for modeling metabolic syndrome in adolescents. Ann Epidemiol. 2010;20:843–851. doi: 10.1016/j.annepidem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Villa JKD, Silva ARE, Santos TSS, Ribeiro AQ, Sant’Ana LFDR. Risco de síndrome metabólica em crianças: uso de um escore único. Rev Paul Pediatr. 2015;33:187–193. doi: 10.1016/j.rpped.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third national health and nutrition examination survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 32.Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it? Ann N Y Acad Sci. 2013;1281:123–140. doi: 10.1111/nyas.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragland DR. Dichotomizing continuous outcome variables: Dependence of the magnitude of association and statistical power on the cutpoint. Epidemiology. 1992;3:434–440. doi: 10.1097/00001648-199209000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Bakhshi E, McArdle B, Mohammad K, Seifi B, Biglarian A (2012) Let continuous outcome variables remain continuous. Comput Math Methods Med 1–13. 10.1155/2012/639124 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available under reasonable request with the corresponding author.