Abstract
Background
There is controversial evidence for the beneficial effects of antioxidative vitamins (vits) on dyslipidemia. In this regard, we aimed to systematically review all meta-analyses of trials on this topic.
Methods
We comprehensively searched PubMed, Web of Science, Scopus, and Cochrane Library databases until January 2021 to explore the published English meta-analyses of trials conducted to assess the effects of single or combined vits C, D and E consumption on lipid profile. The meta-analyses of observational, in vivo/in vitro, or case-report studies were excluded. Search results were reported based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flowchart.
Results
Overall, 25 meta-analyses including 32,177 individuals with different underlying disorders met our inclusion criteria. Numerous studies had assessed supplementation with Vit-D or its combination with other agents on lipid profile. Consumption of 400 IU/day (d) to 50,000 IU/week (w) Vit-D for at least eight weeks improved the levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG) in type 2 diabetes mellitus or polycystic ovary syndrome (PCOS) patients. This treatment reduced the levels of TC and TG in patients with chronic kidney disease. A significant increase in high-density lipoprotein cholesterol (HDL-C) levels was only observed in coronary artery disease patients. Sole intake of 500–2000 mg/d Vit-C for at least 3 weeks improved LDL-C and TG values in hypercholesterolemic patients. Nevertheless, sole intake of Vit-E had controversial effects on lipid profile. The combination of 400–1800 IU/d omega-3 free fatty acid (FFA) and 400 IU/d Vit-E significantly reduced the levels of LDL-C and TG in overweight individuals, without any significant effect on other components. A significant improvement of TG values was observed after consumption of 1000–2000 mg/d omega-3 FFA plus 400 IU/d Vit-E along with 50,000 IU/each 2w Vit-D for at least 6 weeks in diabetic patients.
Conclusion
The beneficial effects of antioxidative vitamins (C, D, E) or their combination with other agents on lipid profile varied based on their dosage, intake duration, and the health status of the individuals.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-021-00919-8.
Keywords: Cardiovascular diseases, Dyslipidemia, Antioxidative vitamin, Systematic review
Background
The increasing prevalence of cardiovascular diseases (CVDs), as one of the most common non-communicable diseases (NCDs), is one of the main current health problems. Several modifiable metabolic risk factors are known for CVDs. One of these risk factors is dyslipidemia, which presents as high serum levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), and low serum levels of high-density lipoprotein cholesterol (HDL-C) [1]. Therefore, focusing on the reduction of the mentioned risk factor is the suggested way to prevent and control CVDs.
The initial event in atherosclerosis is the accumulation of the lipid cells in the arterial walls. Atherosclerosis studies have indicated that the development of the disease is linked with free radical reactions, producing lipid peroxides and other substances secondary to dietary lipids [2]. Furthermore, augmented intracellular production of reactive oxygen species (ROS) plays a crucial role in chronic inflammatory reactions secondary to atherosclerosis [3].
ROS can also interact with biomolecules such as carbohydrates, proteins, and lipids; thus, it disrupts the normal function of the cells. Oxidative stress is an imbalance between antioxidants and ROS. It has been acknowledged as a part of the mechanisms inducing cellular and molecular tissue damage, happening in a wide range of diseases such as diabetes, CVDs, and other NCDs [4, 5]. Studies have reported that individuals suffering from hyperlipidemia have higher serum levels of certain oxygenated compounds like malondialdehyde (MDA); the consumption of dietary supplementations with antioxidants, however, could reduce its serum values [6, 7].
Antioxidants protect cells against oxidant-related damage, and are classified into enzymatic and non-enzymatic types [8]. Superoxide dismutase (SOD) and catalase (CAT) are enzymatic antioxidants; though, vitamins, minerals, and polyphenols count as non-enzymatic ones [9, 10]. It is suggested that dietary antioxidants prevent diseases caused by oxidative stress. Until now, several studies [11] have investigated the beneficial properties of supplementary antioxidants including vitamins E, C, and D on lipid profile. Some have reported the beneficial effects of the antioxidants on lipid modulation [11, 12], while others have stated a weak or even no such effect [12]. Similarly, several systematic review/meta-analysis studies conducted on this topic, have reported controversial results. In a recent meta-analysis, Jin and his colleagues [13] compared the effects of vitamin D (Vit-D) supplementation with placebo in female adults with polycystic ovary syndrome (PCOS). They stated the beneficial effects of Vit-D on TG, TC, and LDL-C levels, but not on HDL-C levels. Another meta-analysis of the clinical trials, on the other hand, revealed no significant changes in this regard when evaluating the effects of Vit-D plus calcium on lipid profile [14].
The current systematic review aimed to critically assess all the previous meta-analysis studies evaluating the effects of antioxidative vitamins on dyslipidemia, considering the discrepancies found in their results.
Methods
Search strategy
A comprehensive search of published English literature was conducted through Web of Science, PubMed, Scopus, and Cochrane Library Databases to find relevant studies published before 1 January 2021. The search terms were “lipid", “cardiovascular”, and the name of each "antioxidative vitamin", and their equivalents. The search strategy is presented as supplementary, Table S1. To report the search results, we used the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram [15] (Supplementary 1). All retrieved papers were imported into the Endnote library version X8. In case of not having access to the full texts, the corresponding authors were contacted through e-mail. All PRISMA steps as well as the reference list of the included papers were independently checked by two researchers. Any disagreements were discussed and resolved by consensus or consult with the expert.
Eligibility criteria
We included all meta-analyses of clinical trials that had assessed the effects of antioxidative vitamins (C, D, E) alone or in combination with the minerals or other agents on lipid profile of healthy or non-healthy individuals. The meta-analyses conducted on observational studies, in vivo/ in vitro studies, or case reports were excluded.
Study selection and data extraction
Following data were extracted into predefined forms: first author, year of publication, number of trials in each meta-analysis, participants’ characteristics (sample size, age, sex), type of intervention and control groups, dosage, duration of the intervention, reported effective dosage, quality score, and main outcome.
Critical appraisal of included studies
The risk of bias was evaluated by using the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) tool for all included meta-analyses [16], Table S2. The AMSTAR checklist consisted of 11- validated questions. The articles were considered to be of “good quality” when scored 8–11; medium quality with a score of 4–7, and poor quality in case of scores below 3 [16].
The risk of bias assessment was also performed independently by two researchers.
Results
Overall, 2749 relevant publications were recognized through a comprehensive electronic search. After excluding duplicated publications, 2050 articles were screened by title and abstract, among which 53 studies met the eligibility criteria and were considered for full-text assessment. Finally, 25 meta-analyses were included [13, 14, 17–39]. The PRISMA flow diagram for the literature search is presented in Fig. 1.
Fig. 1.
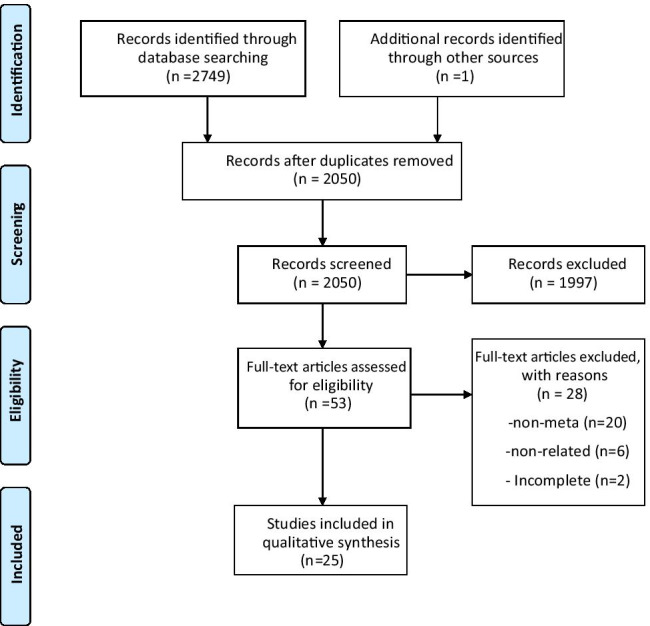
Flow diagram of the study selection process
According to the included meta-analysis studies, 32,177 individuals participated from two weeks to five years in trials assessing the effects of vitamins C, E, D with/without omega-3 free fatty acid (FFA), calcium, or magnesium on lipid profile. Most of the participants were adults (≥ 18 years old) of both genders, with health status varying from being healthy to suffering from disorders such as type 2 diabetes mellitus (T2DM), obesity, hypertension, chronic kidney disease (CKD), polycystic ovary syndrome (PCOS), or non-alcoholic fatty liver disease (NAFLD). The majority of the meta-analyses had good quality (AMSTAR score ≥ 8). The characteristics of the included meta-analyses are accessible in Table 1.
Table 1.
Characteristics of anti-oxidative vitamins’ effects on lipid profiles in meta-analysis of clinical trials
| Study | anti-oxidative vitamin/Control | meta-analyzed studies (n)/ disorders | Participants | Intervention | Main outcome | Effective dose | Quality assessment | AMSTAR score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size (n) | Age (yr) | Sex | Dose/Frequency | Duration (w) | |||||||
| Vitamin D | |||||||||||
| Wang et al. 2012 [19] | Vit. D / placebo | 12/ Healthy, obese, T2DM overweight | 1346 | 18–80 | both | 300–3332 IU /d | 6–48 | ↑sig. TC (3.23, 95%CI: 0.55, 5.9) non-sig effect on others | NR | Yes | 10 |
| Manousopoulou et al. 2015 [20] | Vit. D/ placebo | 8/ obesity | 4827 | 26–67 | both | 1000—7000 IU/d; 20,000—50,000 IU/ w; 120,000 IU fortnightly | 6 –192 |
↑sig. LDL-C (0.77, 95% CI: 2.71, 23.59) non-sig effect on others |
NR | Yes | 11 |
| Jafari et al. 2016 [22] | Vit. D/ placebo | 17/T2DM | 1365 | UN | both | 1000—7000 IU/d | 8 – 192 |
↓ sig. TG (-3.74, 95% CI: -7.13, -0.34), ↓ sig. LDL-C (-2.55, 95% CI: -4.83, -0.26) non-sig effect on others |
≤ 2000 IU/d | Yes | 9 |
| Tabrizi et al. 2017 [23] | Vit. D/ placebo | 7/ NAFLD | 452 | 18–75 | both | 1000 IU/d-50000 IU/w | 10–12 | non-sig change | NR | Yes | 10 |
| Xu et al. 2017 [24] | Vit. D/ placebo | 6/ PCOS | 156 | 18–30 | F | 400 IU/d-50000 IU/w | 3–24 |
↓ sig. TG (-38.09, 95% CI: -17.72, -57.57) non-sig effect on LDL-C |
< 50000 IU once weekly | Yes | 10 |
| Mirhosseiniet al. 2018 [39] | Vit D3/placebo | 38/ Healthy, T2DM, overweight, obese, PCOS, hypercholesteremia | 4734 | 22–72 | both | 400–12,000 IU/d | 12–240 |
↓ sig. TC (-5.8, 95% CI: -9.667, -1.546) ↓ sig. LDL-C (-3.867, 95% CI:-7.734, -0.116) ↓ sig. TG (-4.64, 95% CI: -8.89, -0.12) ↑ sig. HDL-C (3.48, 95% CI: 0.00, 6.57) |
< 4000 IU/d for TC, and HDL-C | Yes | 9 |
| Swart et al. 2018 [25] | Vit. D/ placebo | 12/obese, prediabetes, osteoporotic | 2994 | 20- > 70 | both | 200 IU/d-40000 IU/w | 16–48 | ↓ sig. LDL-C by different serum level of Vit D:(-3.87, 95% CI: − 7.73, − 0.00) by Vit D < 75 nmol/l | NR | Yes | 9 |
| Dibaba et al. 2019 [27] | Vit. D/ placebo | 41/ healthy, obese or overweight | 3434 | ≥ 18 | both | 20–8570 IU/d | 8–144 |
↓ sig. TC (-6.57, 95% CI: –10.83, –2.32) ↓ sig. LDL-C (-4.64, 95% CI: -8.89, –0.39) ↓ sig. TG (-13.29, 95% CI: –21.26, –5.31) non-sig effect on HDL-C |
NR | Yes | 9 |
| Gasparri et al. 2019 [28] | Vit. D-fortified yogurt /non-fortified yogurt | 5/ T2DM, prediabetes, MetS | 469 | ≥ 18 | both | 400 -2000 IU/d | 8 -16 |
↓ sig. TC (-13.38, 95% CI: − 20.19, − 6.56) ↓ sig. LDL-C (-7.86, 95% CI: − 15.35, − 0.37) ↓ sig. TG (-30.12, 95% CI: − 43.22, − 17.02) |
NR | Yes | 10 |
| Ostadmohammadi et al. 2019 [29] | Vit. D/Placebo | 8/CAD | 630 | 46–78 | both | 50000 IU/ 2w | 8–36 | ↑sig. HDL-C: (3.08; 95% CI: 1.42, 4.73) non-sig effect on others | NR | Yes | 9 |
| Milajerdi et al. 2019 [30] | Vit D3/placebo | 17/ CKD | 1781 | 18–78 | both | 0.03–0.5 mcg/d calcitriol, 40,000–300,000 IU/w in different times | 3–52 |
↓sig. TG: (− 32.52; 95% CI: − 57.57, − 7.47), ↓sig. TC: (− 7.93; 95% CI: − 13.03, − 2.83), non-sig effect on others |
NR | Yes | 11 |
| AlAnouti et al. 2020 [32] | Vit. D/ placebo | 4/ MetS | 232 | > 18 | both | 2000 IU/d-50000 IU/w | 8—48 |
↑sig. TG (30.67, 95% CI: 4.89, 56.45) non-sig effect on others |
NR | Yes | 11 |
| Guo et al. 2020 [34] | Vit. D/ placebo | 10/ NAFLD | 544 | 25–65 | both | 1000 IU/d-50000 IU/w | 10–48 | non-sig change | NR | Yes | 10 |
| Hauger et al. 2020 [35] | Vit. D/ placebo | 14/obese, overweight, normal weight children, adolescents | 1088 | 4–19 | both | 10–125 µg/d, 1250–7500 µg/w | 4–26 |
↑sig. LDL-C (4.25, 95% CI: 0.77, 7.73) |
NR | Yes | 11 |
| Jin et al. 2020 [13] | Vit. D/ placebo | 8/PCOS | 467 | UN | F | 2500–12,000 IU/d | 8–24 |
↓ sig. TG (-11.88, 95% CI: - 17.03, -6.73) ↓ sig. TC (-9.09, 95% CI: - 14.90, -3.29) ↓ sig. LDL-C (-5.22, 95% CI:- 10.32, -0.13) ↓ sig. VLDL-C (-2.43, 95% CI:- 3.69, -1.17) non-sig effect on HDL-C |
< 4000 IU/d for TC, LDL-C, VLDL-C | Yes | 11 |
| Miao et al. 2020 [37] | Vit. D/ placebo | 11/PCOS | 285 | 18–40 | F | 400 IU/d- 50,000 IU/w | 8–24 |
↓ sig. TC (-11.90, 95% CI: -15.67 to -8.13) ↓ sig. LDL-C (-4.54, 95% CI:-7.29 to -1.80) non-sig effect on others |
NR | Yes | 10 |
| Vitamin C | |||||||||||
| McRae et al. 2008 [18] | Vit. C/ placebo | 13/ hypercholesterolemia | 549 | 48–82 | both | 500–2000 mg/d | 3–24 |
↓ sig. LDL-C (-7.9, 95% CI: -12.3, -3.5) ↓ sig. TG (-20.1, 95% CI: -33.3, -6.8) non-sig effect on HDL-C |
NR | Yes | 7 |
| Ashor et al. 2016 [21] | Vit. C/ placebo | 40/ healthy, T2DM, CKD, HTN, CAD, hyperlipidemia | 1981 | 20–81 | both | 125–4500 mg/d | 2–240 |
No significant change in lipid profile ↓sig. TC/↓sig. TG in subgroup (TC serum > 205 mg/dl: (-11.6, 95%CI: -19.72, -3.48), TG (-15.06, 95%CI: -26.57, -4.43) |
NR | Yes | 11 |
| Vitamin E | |||||||||||
| Huang et al. 2015 [17] | Vit E-coated dialyzer/ non-Vit. E coated | 3/ hemodialysis patients | 111 | 39–75 | both | NR | 6–10 | non-sig change | NR | Yes | 11 |
| Zuo et al. 2020 [38] | Tocotrienols (Vit. E family) / placebo | 15/healthy, DM, fatty liver, MetS, hyperlipidemia, other disorders | 931 | > 18 | both | 50—600 mg/d | 4–72 | ↑sig. HDL-C: (5.65, 95% CI: 2.55, 8.7), non-sig. effect on others | ↓sig. TG and ↑sig. HDL-C in doses ≥ 200 mg/d | Yes | 9 |
| Combination | |||||||||||
| Chen et al. 2017 [14] | Vit D + calcium/placebo | 9/ Healthy, overweight, T2DM | 2336 | 20–73 | both | Dietary calcium + 125 IU/d-50000 IU/w Vit D | 2–240 | non-sig change | NR | Yes | 10 |
| Asbaghi et al. 2019 [26] | Omega-3 FAs + Vit. E/ placebo | 5/ metabolic disorders, overweight | 254 | 24–65 | both | Vit. E 400 IU/d, Omega-3 400–1800 mg/d | 8–12 |
↓ sig. LDL-C (-8.07, 95% CI:-15.10, -1.05) ↓ sig. TG (-28.34, 95% CI: -37.44, -19.22) non-sig effect on others |
NR | Yes | 10 |
| Sepidarkish et al. 2019 [31] | Omega-3 FAs + Vit. E/ placebo | 9/ healthy, GDM, T2DM, PCOS, hemodialysis patients, CVDs | 640 | 19–76 | both | 1000–4000 mg FAS + 400 IU Vit. E/d | 6–13 | ↓sig. VLDL-C: (-2.96, 95%CI: -5.84, -0.09), non-sig. effect on others | NR | Yes | 9 |
| Dehbalaei et al. 2020 [33] | Mg + Vit. E/ placebo | 4/ DF, PCOS, GDM | 237 | 25–58 | both | Mg 250 mg/d, Vit. E 400 IU/d | 6 -12 |
↓ sig. TC (-15.89, 95% CI: -24.39, -7.39) ↓ sig. LDL-C (-11.37, 95% CI:-19.32,-3.41) ↓ sig. TG (-26.97, 95% CI: -46.03,-7.90) non-sig effect on HDL-C |
Mg 250 mg/d, Vit. E 400 IU/d | Yes | 9 |
| Jiang et al. 2020 [36] | Omega-3 FAs + Vit.(D, E)/ placebo | 5/GDM, prediabetes | 334 | 25–55 | F | 1000–2000 mg omega-3/ 400 IU vit. E/ 50,000 IU vit. D every 2w | 6–8 |
↓ sig. TG (-28.29, 95% CI: -38.94, -17.64) non-sig effect on others |
NR | Yes | 10 |
Legend: Vit. vitamin, TC total cholesterol, TG triglyceride, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, NAFLD Non-alcoholic fatty liver disease, PCOS polycystic ovary syndrome, GDM gestational diabetes mellitus, T2DM Type 2 diabetes mellitus, MetS Metabolic syndrome, DF diabetic foot, Fas Fatty acids, CKD Chronic kidney disease, CVDs Cardiovascular diseases, CAD Coronary artery disease, Mg Magnesium, NR Not reported, NA Not access, F Female, d Day, w week
Effects of vitamin D on lipid profile
The effects of Vit-D were assessed in 16 meta-analyses [13, 19, 20, 22–25, 27–30, 32, 34, 35, 37, 39], including healthy, obese individuals, or patients with T2DM, CKD, PCOS, or NAFLD. In 7 meta-analyses [13, 22, 25, 27, 28, 37, 39], the additional effects of Vit-D compared to placebo helped reduce LDL-C levels with a standardized mean difference (SMD) of -2.55 to -7.86 mg/dl in patients with different underlying disorders. The SMD of LDL-C varied from -2.55 to -7.86 mg/dl in type 2 diabetics [22, 28] and from -4.54 to -5.22 mg/dl in patients suffering from PCOS [13, 37]. Another two studies [20, 35], on the other hand, reported a significant increase in LDL-C levels (0.77 and 4.25 mg/dl) in overweight/obese individuals. Seven meta-analyses [13, 22, 24, 27, 28, 30, 39] detected a significant reduction in serum TG levels following Vit-D consumption, compared with placebo (SMD -3.74, -38.09 mg/dl) in a heterogeneous population. The SMD of TG level ranged from -3.74 to -30.12 mg/dl in patients with diabetes type 2 [22, 28], from -11.88 to -38.09 mg/dl in PCOS patients [13, 24], but -32.52 in CKD patients [30]. A significant increase in TG levels (SMD 30.67 and CI95%: 4.89, 56.45), though, was reported in a single meta-analysis containing metabolic syndrome (MetS) participants [32]. Six meta-analyses [13, 27, 28, 30, 37, 39] revealed a statistically significant decrease in TC levels of the participants receiving Vit-D regardless of their health status (SMD: from -5.8 to -13.38 mg/dl). The SMD of TC ranged from -9.09 to -11.90 mg/dl in PCOS patients [13, 37], -13.38 mg/dl in patients with diabetes type 2 [28], and.
-7.93 in those suffering from CKD [30]. In two meta-analyses on participants with coronary artery disease [29] or other health statuses [39], a statistically significant increase in HDL-C levels was noted in the group receiving Vit-D (SMD from 3.08 to 3.48). In two other meta-analyses, no meaningful were found effects for Vit-D intake on lipid profile of NAFLD patients [23, 34]. The effective dose for improving lipid profile was reported to be ≤ 4000 IU/d for at least 12 weeks in T2DM patients, and ≤ 4000 IU/d or < 50,000 IU/once weekly for at least three weeks in PCOS individuals [13, 24, 39].
Effects of vitamin C on lipid profile
Two meta-analyses evaluated the effects of supplementation with Vit-C on lipid profile in hypercholesterolemic [18] or patients with various disorders [21]. One meta-analysis reported data synthesis from 13 trials consisting of 549 hypercholesterolemic individuals aged between 48–82 years. It was revealed that supplementation with at least 500 mg/d of Vit-C for 3–24 weeks can result in a significant reduction in serum levels of LDL–C and TG with SMD (-7.9), and (-20.1) mg/dl, respectively. However, nonsignificant elevation was detected in HDL-C values [18]. Ashor et al. [21] pooled data from 40 trials conducted on 1981 individuals. The authors reported no significant effect for consuming from 2 to 240 weeks of Vit-C on the serum lipid profile. Nevertheless, in meta-regression, a significant reduction in TC and TG levels was observed in hypercholesterolemic subjects with TC concentrations higher than 205 mg/dl at baseline; SMD (-11.6) and (-15.06) mg/dl, respectively. The effective dose was not stated in the included meta-analyses.
Effects of vitamin E on lipid profile
Two meta-analyses [17, 38] reported the pooled effects of Vit-E intake alone on lipid profile. Meta-analyses of 15 trials including 931 individuals with varying health status by Zuo, et al. [38] demonstrated a significant change in HDL-C concentrations (5.65 mg/dl) after tocotrienol consumption. The authors detected a greater improvement in serum levels of HDL-C and TG following the intake of ≥ 200 mg/day of the Vit-E family. In another meta-analysis [17], no significant effect for Vit-E on lipid profile was reported in hemodialysis patients. The effective dose of Vit-E (≥ 200 mg/d for at least 4 weeks) was reported in a single meta-analysis conducted on healthy individuals as well as patients with hyperlipidemia, or diabetes [38].
Effects of combination of vitamins with other agents on lipid profile
Five meta-analyses [14, 26, 31, 33, 36] assessed the effects of supplementation with the combination of vitamins and other agents on individuals with different health statuses. Vit-D plus calcium did not result in any significant change in lipid profile of healthy individuals, overweight or diabetic patients [14]. The combination of omega-3 FFA and Vit-E compounds significantly reduced TG (-28.34), LDL-C (-8.07), and very-low-density lipoprotein cholesterol (VLDL-C) (-2.96) mg/dl [26, 31] levels, without any significant effect on other components of lipid profile regardless of underlying health status. The combination of 1,000–2,000 mg/d omega-3 FFA and 400 IU/d Vit-E compounds plus 50,000 IU Vit-D for every two weeks significantly reduced TG levels (-28.29) mg/dl [36], without any considerable effect on other components of lipid profile in pre-/gestational diabetic patients. A significant improvement in TC, LDL-C, and TG values was observed after the consumption of the combination of 250 mg/d magnesium (Mg) and 400 IU/d Vit-E for at least 1.5 months in a single meta-analysis [33] on patients with PCOS or gestational diabetes.
Discussion
The current systematic review summarized the evidence of meta-analyses assessing the effects of antioxidative vitamins C, D, and E supplementations on lipid profile. Our findings, on one side, illustrated the significant beneficial effects of Vit-D alone or in combination with Vit-E, omega-3 FFA, or Mg on lipid profile and, on the other hand, the controversial effects of sole intake of Vit-E.
Overall, 10 out of 17 meta-analyses supported the beneficial effects of Vit-D supplementation on lipid profile. Conversely, seven studies reported a significant increase in the values of TC, LDL-C, TG, without any significant changes in other lipid components. It should also be mentioned that baseline serum levels of Vit-D might have affected the conclusion. Moreover, the demonstration of beneficial effects of such supplementations in populations with sufficient Vit-D levels is difficult. Several additional reasons for such different observations could be the variations noted in the length of intervention, dosage, participants’ characteristics, and even geographical latitudes of the study sites [40]. In Manousopoulou et al. study [20], the cardioprotective effects of Vit-D was observed at doses of 1,000 IU/day to 120,000 IU biweekly after eight weeks up to one year of consumption. This is while several studies have failed to report any effect for approximately similar dose, frequency (1100 IU / day to 50 000 IU /week) and duration (8 weeks to 4 years) of Vit-D consumption [20]. Four meta-analyses calculated the effective dosage of Vit-D, suggesting the beneficial effects only with low doses [13, 22, 24, 39]. Moreover, the impact of Vit-D on the transcriptional activity of insulin-induced gene-2 (Insig-2) and Vit-D receptors is supposed to be the mechanism behind this vitamin’s effect on the circulating cholesterol levels. Insig-2 reduces the sterol regulatory element activation of protein 2 binding (SREBP-2) and inhibits the expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR). This results in reduced cholesterol synthesis by affecting HMGR, a vital enzyme for this process [41]. Moreover, several genetic variations including DHCR7 and CYP2R1 have an essential effect on the response to the Vit-D treatment among individuals with various health statues [42, 43]. However, none of the included meta-analysis studies had assessed the influence of the genetic variants on the responses to vitamins supplementations.
The additional effects of Vit-D compared to placebo or its active control on reducing LDL-C were reported in 7 meta-analyses. Two meta-analyses showed no statistically significant increase in HDL-C levels in the group receiving Vit-D [13, 22, 25, 27–29, 37, 39].
The evidence of the influence of Vit-C supplementation on improving lipid profile is limited. As a water-soluble antioxidant, ascorbic acid’s (Vit-C) pro- or antioxidative effects could vary based on the dosage [44]. The highest reduction in the TC, LDL-C, and TG levels was found following the consumption of a maximum dose of Vit-C; 2000 mg/d for six months, according to two meta-analyses [18, 21]. In sub-group analyses, in conditions such as low concentrations of Vit-C or high serum levels of TC/ TG at baseline and subjects suffering from T2DM, the beneficial effects of Vit-C supplementation on lipid profile were noted [18, 21]. According to Ashor et al. [21], participants older than 52 years old were less likely to benefit from Vit-C supplementations due to the lower absorption rate of Vit-C in older individuals [45]. Hence, more Vit-C intake is required to counteract the higher oxidative stress due to the age-related mitochondrial dysfunction [46]. Nevertheless, there is only weak evidence regarding the impact of Vit-C supplementation on risk factors of cardiovascular disease such as dyslipidemia. These results highlighted the potential importance of a personalized approach to Vit-C in the management of dyslipidemia. A mendelian randomization study was conducted on 97,203 white individuals. It was observed that higher consumption of vegetables and fruits is associated with a lower risk of cardiovascular diseases, despite the same effect size for genetically high content of Vit-C in serum [47].
Huang et al. [17] did not observe any significant improvement in lipid profile of hemodialysis patients, although several meta-analyses had supported the beneficial effects of Vit-E on certain lipid components. Vit-E is known as the strongest antioxidant in reducing atherosclerotic lesions. It stimulates the peroxisome proliferator-activated receptor-gamma signaling pathway, suppressing oxidized LDL-C production, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, and cholesterol synthesis as well as its absorption in the intestines [48, 49]. Similar to Vit-C, the effects of Vit-E on lipid components are dose-dependent. One meta-analysis indicated that supplementation with 400 IU/day of Vit-E for 6–12 weeks improved TC, LDL-C, and TG serum levels without any beneficial effects on HDL-C levels [33]. Conversely, Zou et al. [38] observed that consumption of more than 300 IU/day Vit-E for less than eight weeks can significantly increase HDL-C levels while reducing TG serum levels.
Vitamin co-supplementation has shown positive effects on certain disorders; for instance, the combination of vitamin C and E is accompanied by an increase in the glycemic status of T2DM patients [10]. In the current systematic review, the beneficial effects of several co-supplementations on several lipid parameters were established. Additionally, the cardioprotective effects of omega-3 free fatty acids (FFA) and Vit-E co-supplementation were noted in several meta-analyses [26, 31, 36]. Such outcome may be related to the synergistic protective effects of these compounds against CVDs. In other words, the cardioprotective effects of Vit-E consist of its anti-hypertensive and anti-inflammatory properties along with improved endothelial function by inhibiting the oxidation of omega-3 FFA [26]. However, due to inadequate evidence, such interpretations should be made with caution.
Our systematic review had several limitations. The main limitation was the heterogeneity of the meta-analyses in terms of studied populations along with the prescribed dosages and intervention durations. The absence of the effective dosage of antioxidants data on lipid profile in most of the included meta-analyses was another limitation. Our study does not have a PROSPERO registration code since it is an overview of the published meta-analyses without re-calculating the data. This is while nearly all the included meta-analyses had a high quality and provided reliable results.
Conclusions
In conclusion, this systematic review highlights that the consumption of antioxidative Vit-D alone or in combination with other agents might improve lipid profile. However, it should be borne in mind that the beneficial effects of antioxidative vitamins can vary based on their dosage, intake duration, and even the health status of the studied individuals. The effective dose for improving lipid profile was reported to be ≤ 4,000 IU/d for at least 12 weeks in T2DM patients, and ≤ 4000 IU/d or < 50,000 IU/weekly for at least three weeks in PCOS individuals.
As for Vit-C, it was revealed that 3–24 weeks supplementation with at least 500 mg/d of Vit-C can result in a significant reduction in serum levels of LDL– C and TG, respectively. The effective dose of Vit-E (≥ 200 mg/d for at least four weeks) was reported in a single meta-analysis conducted on individuals with various health conditions [38]. Significant improvements in TC, LDL-C, and TG values were observed after the usage of the combination of 250 mg/d Mg and 400 IU/d Vit-E for at least 1.5 months [33] in patients with PCOS or gestational diabetes.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary 1 PRISMA 2020 checklist (DOCX 36 KB)
Table S1 Search strategy in PubMed database (DOCX 13 KB)
Abbreviations
- CVDs
Cardiovascular diseases
- ROS
Reactive oxygen species
- Vits
Vitamins
- CTs
Clinical trials
- CKD
Chronic kidney diseases
- NCDs
Non-communicable diseases
- TC
Total cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- TG
Triglycerides
- HDL-C
Low serum levels of high-density lipoprotein cholesterol
- VLDL-C
Very-low-density lipoprotein cholesterol
- MDA
Malondialdehyde
- SOD
Superoxide dismutase
- CAT
Catalase
- MeSH
Medical Subject Headings
- T2DM
Type 2 diabetes mellitus
- PCOS
Polycystic ovary syndrome
- NAFLD
Non-alcoholic fatty liver disease
- AMSTAR
Assessing the Methodological Quality of Systematic Reviews
- HMG-CoA
Hydroxy-3-methylglutaryl coenzyme A
- Insig-2
Insulin induced gene-2
- SREBP-2
Sterol regulatory element activation protein 2 binding
- HMGR
3-Hydroxy-3-methylglutaryl coenzyme A reductase
- FFA
Free Fatty Acid
Authors’ contributions
OTM conceived and coordinated the study. OTM and BL participated in design of the study. SHM, OTM, and ZHSH extracted data from the published articles and drafted the manuscript. ZHSH, PK, MRMT and BL helped to edit of the manuscript draft. SHM, OTM, PK and BL critically reviewed the manuscript and helped in quality assessment. All authors read and approved the final manuscript.
Funding
This study is in-home study without any financial support.
Data availability
Not applicable. All data analyzed in the current systematic reviews are extracted from published articles in PubMed, Web of Science, Scopus, and Cochrane Library web databases.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
All authors declare that there are no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller M. Niacin as a component of combination therapy for dyslipidemia. Mayo Clin Proc. 2003;78(6):735–742. doi: 10.4065/78.6.735. [DOI] [PubMed] [Google Scholar]
- 2.Lönn ME, Dennis JM, Stocker R. Actions of “antioxidants” in the protection against atherosclerosis. Free Radic Biol Med. 2012;53(4):863–884. doi: 10.1016/j.freeradbiomed.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Rad Biol Med. 2000;28(12):1815–1826. doi: 10.1016/S0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 4.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Saeidnia S, Abdollahi M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol Appl Pharmacol. 2013;273:442–455. doi: 10.1016/j.taap.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Yang R, Le G, Li A, Zheng J, Shi Y. Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition. 2006;22(11–12):1185–1191. doi: 10.1016/j.nut.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Yang RL, Shi YH, Hao G, li W, Le GW. Increasing Oxidative Stress with Progressive Hyperlipidemia in Human: Relation between Malondialdehyde and Atherogenic Index. J Clin Biochem Nutr. 2008; 43(3): 154–8. 10.3164/jcbn.2008044. [DOI] [PMC free article] [PubMed]
- 8.Halliwell B, Gutteridge JM. Free radicals in biology and medicine: Oxford University Press, USA; 2015.
- 9.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabatabaei-Malazy O, Nikfar S, Larijani B, Abdollahi M. Influence of ascorbic acid supplementation on type 2 diabetes mellitus in observational and randomized controlled trials; a systematic review with meta-analysis. J Pharm Pharm Sci. 2014;17(4):554–582. doi: 10.18433/J3ZG6R. [DOI] [PubMed] [Google Scholar]
- 11.Asemi Z, Soleimani A, Bahmani F, Shakeri H, Mazroii N, Abedi F, et al. Effect of the omega-3 fatty acid plus vitamin E supplementation on subjective global assessment score, glucose metabolism, and lipid concentrations in chronic hemodialysis patients. Mol Nutr Food Res. 2016;60(2):390–398. doi: 10.1002/mnfr.201500584. [DOI] [PubMed] [Google Scholar]
- 12.Tamadon MR, soleimani A, keneshlou F, Mojarrad MZ, Bahmani F, Naseri A, et al. Clinical Trial on the Effects of Vitamin D Supplementation on Metabolic Profiles in Diabetic Hemodialysis. Horm Metab Res. 2018;50(1):50–5. [DOI] [PubMed]
- 13.Jin B, Qian L, Fu X, Zhu J, Shu J. Influence of vitamin D supplementation on lipid levels in polycystic ovary syndrome patients: a meta-analysis of randomized controlled trials. J Int Med Res. 2020;48(8):300060520935313. doi: 10.1177/0300060520935313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CL, Ge S, Li S, Wu L, Liu t, Li C. The Effects of Dietary Calcium Supplements Alone or With Vitamin D on Cholesterol Metabolism A Meta-Analysis of Randomized Controlled Trials. J Cardiovasc Nurs. 2017;32(5): 496–506. [DOI] [PubMed]
- 15.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7(1):10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Yi B, Li AM, Zhang H. Effects of vitamin E-coated dialysis membranes on anemia, nutrition and dyslipidemia status in hemodialysis patients: a meta-analysis. Ren Fail. 2015;37(3):398–407. doi: 10.3109/0886022X.2014.1001281. [DOI] [PubMed] [Google Scholar]
- 18.McRae MP. Vitamin C supplementation lowers serum low-density lipoprotein cholesterol and triglycerides: a meta-analysis of 13 randomized controlled trials. J Chiropr Med. 2008;7(2):48–58. doi: 10.1016/j.jcme.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Xia N, Yang Y, Peng DQ. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids Health Dis. 2012;11:42. doi: 10.1186/1476-511X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manousopoulou A, Al-Daghri NM, Garbis SD, Chrousos GP. Vitamin D and cardiovascular risk among adults with obesity: a systematic review and meta-analysis. Eur J Clin Invest. 2015;45(10):1113–1126. doi: 10.1111/eci.12510. [DOI] [PubMed] [Google Scholar]
- 21.Ashor AW, Siervo M, van der Velde F, Willis ND, Mathers JC. Systematic review and meta-analysis of randomised controlled trials testing the effects of vitamin C supplementation on blood lipids. Clin Nutr. 2016;35(3):626–637. doi: 10.1016/j.clnu.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Jafari T, Fallah AA, Barani A. Effects of vitamin D on serum lipid profile in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Clin Nutr. 2016;35(6):1259–1268. doi: 10.1016/j.clnu.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Tabrizi R, Moosazadeh M, Lankarani KB, Akbari M, Heydari ST, Kolahdooz F, et al. The effects of vitamin D supplementation on metabolic profiles and liver function in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. 2017;11(Suppl 2):S975–s982. doi: 10.1016/j.dsx.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Xue Y, Xu P, Xue K, Duan X, Cao J, Luan T, et al. Effect of vitamin D on biochemical parameters in polycystic ovary syndrome women: a meta-analysis. Arch Gynecol Obstet. 2017;295(2):487–496. doi: 10.1007/s00404-016-4247-y. [DOI] [PubMed] [Google Scholar]
- 25.Swart KMA, Lips P, Brouwer IA, Jorde R, Heymans MW, Grimnes G, et al. Effects of vitamin D supplementation on markers for cardiovascular disease and type 2 diabetes: an individual participant data meta-analysis of randomized controlled trials. Am J Clin Nutr. 2018;107(6):1043–1053. doi: 10.1093/ajcn/nqy078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asbaghi O, Choghakhori R, Abbasnezhad A. Effect of Omega-3 and vitamin E co-supplementation on serum lipids concentrations in overweight patients with metabolic disorders: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr Clin Res Rev. 2019;13(4):2525–2531. doi: 10.1016/j.dsx.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Dibaba DT. Effect of vitamin D supplementation on serum lipid profiles: a systematic review and meta-analysis. Nutr Rev. 2019;77(12):890–902. doi: 10.1093/nutrit/nuz037. [DOI] [PubMed] [Google Scholar]
- 28.Gasparri C, Perna S, Spadaccini D, Alalwan T, Girometta C, Infantino V, et al. Is vitamin D-fortified yogurt a value-added strategy for improving human health? A systematic review and meta-analysis of randomized trials. J dairy Sci. 2019;102(10):8587–603. [DOI] [PubMed]
- 29.Ostadmohammadi V, Milajerdi A, Ghayour-Mobarhan M, Ferns G, Taghizadeh M, Badehnoosh B, et al. The Effects of Vitamin D Supplementation on Glycemic Control, Lipid Profiles and C-Reactive Protein Among Patients with Cardiovascular Disease: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr Pharm Des. 2019;25(2):201–210. doi: 10.2174/1381612825666190308152943. [DOI] [PubMed] [Google Scholar]
- 30.Milajerdi A, ostadmohammadi V, Amirjani S, Kolahdooz F, Asemi Z. The effects of vitamin D treatment on glycemic control, serum lipid profiles, and C-reactive protein in patients with chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Int Urol Nephrol. 2019; 51(9): 1567–80. [DOI] [PubMed]
- 31.Sepidarkish M, Morvaridzadeh M, Akbari-Fakhrabadi M, Almasi-Hashiani A, Rezaeinejad M, Heshmati J. Effect of omega-3 fatty acid plus vitamin E Co-Supplementation on lipid profile: A systematic review and meta-analysis. Diabetes Metab Syndr. 2019;13(2):1649–1656. doi: 10.1016/j.dsx.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 32.AlAnouti F, Abboud M, Papandreou D, Mahboub N, Haidar S, Rizk R. Effects of vitamin D supplementation on lipid profile in adults with the metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2020;12(11):3352. doi: 10.3390/nu12113352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dehbalaei MG, Ashtary-Larky D, Amarpoor Mesrkanlou H, Talebi S, Asbaghi O. The effects of magnesium and vitamin E co-supplementation on some cardiovascular risk factors: A meta-analysis. Clin Nutr ESPEN. 2021;41:110–117. doi: 10.1016/j.clnesp.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 34.Guo XF, Wang C, Yang T, Li S, Li KL, Li D. Vitamin D and non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Food Funct. 2020;11(9):p.7389–99. [DOI] [PubMed]
- 35.Hauger H, Laursen RP, Ritz C, Mølgaard C, Lind MV, Damsgaard CT. Effects of vitamin D supplementation on cardiometabolic outcomes in children and adolescents: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr. 2020;59(3):873–884. doi: 10.1007/s00394-019-02150-x. [DOI] [PubMed] [Google Scholar]
- 36.Jiang L, Gao C, Yan P, Chen P, Jiang C, Xu Y, et al. Omega-3 fatty acids plus vitamin for women with gestational diabetes or prediabetes: a meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2020;1–8. [DOI] [PubMed]
- 37.Miao CY, Fang XJ, Chen Y, Zhang Q. Effect of vitamin D supplementation on polycystic ovary syndrome: A meta-analysis. Exp Ther Med. 2020;19(4):2641–2649. doi: 10.3892/etm.2020.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuo SP, Wang G, Han Q, Xiao H, O Santos H, Avelar Rodriguez D, et al. The effects of tocotrienol supplementation on lipid profile: A meta-analysis of randomized controlled trials. Complement Ther Med. 2020; 52:102450. [DOI] [PubMed]
- 39.Mirhosseini N, rainsbury J, Kimball SM. Vitamin D supplementation, serum 25(OH)D concentrations and cardiovascular disease risk factors: a systematic review and meta-analysis. Front Cardiovasc Med. 2018;5:87. [DOI] [PMC free article] [PubMed]
- 40.Tabrizi R, Moosazadeh M, Akbari M, Dabbaghmanesh MH, Mohamadkhani M, Asemi Z, Heydari ST, Akbari M, Lankarani KB. High Prevalence of Vitamin D Deficiency among Iranian Population: A Systematic Review and Meta-Analysis. Iran J Med Sci. 2018;43(2):125–139. [PMC free article] [PubMed] [Google Scholar]
- 41.Li S, He Y, Lin S, Hao L, Ye Y, Lv L, et al. Increase of circulating cholesterol in vitamin D deficiency is linked to reduced vitamin D receptor activity via the Insig-2/SREBP-2 pathway. Mol Nutr Food Res. 2016;60(4):798–809. doi: 10.1002/mnfr.201500425. [DOI] [PubMed] [Google Scholar]
- 42.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet (London, England) 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wimalawansa SJ. Vitamin D deficiency: effects on oxidative stress, epigenetics, gene regulation, and aging. Biology (Basel) 2019;8(2):30. doi: 10.3390/biology8020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khodaeian M, Tabatabaei-Malazy O, Qorbani M, Farzadfar F, Amini P, Larijani B. Effect of vitamins C and E on insulin resistanc in diabetes: a meta-analysis study. Eur J Clin Invest. 2015;45(11):1161–1174. doi: 10.1111/eci.12534. [DOI] [PubMed] [Google Scholar]
- 45.Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr. 2005;25:105–125. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- 46.Puca AA, Carrizzo A, Villa F, Ferrario A, Casaburo M, Maciąg A, et al. Vascular ageing: the role of oxidative stress. Int J Biochem Cell Biol. 2013;45(3):556–559. doi: 10.1016/j.biocel.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 47.Kobylecki CJ, Afzal S, Davey Smith G, Nordestgaard BG. Genetically high plasma vitamin C, intake of fruit and vegetables, and risk of ischemic heart disease and all-cause mortality: a Mendelian randomization study. Am J Clin Nutr. 2015;101(6):1135–1143. doi: 10.3945/ajcn.114.104497. [DOI] [PubMed] [Google Scholar]
- 48.Pang KL, Chin KY. The role of tocotrienol in protecting against metabolic diseases. Molecules. 2019;24(5):293. doi: 10.3390/molecules24050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bozaykut P, Karademir B, Yazgan B, Sozen E, Siow RC, Mann GE, et al. Effects of vitamin E on peroxisome proliferator-activated receptor γ and nuclear factor-erythroid 2-related factor 2 in hypercholesterolemia-induced atherosclerosis. Free Radic Biol Med. 2014;70:174–181. doi: 10.1016/j.freeradbiomed.2014.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary 1 PRISMA 2020 checklist (DOCX 36 KB)
Table S1 Search strategy in PubMed database (DOCX 13 KB)
Data Availability Statement
Not applicable. All data analyzed in the current systematic reviews are extracted from published articles in PubMed, Web of Science, Scopus, and Cochrane Library web databases.


