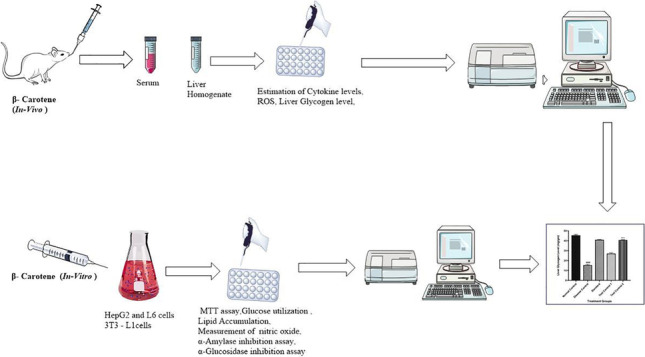
Graphical abstract
The present study was performed to investigate the therapeutic potential of β- Carotene against STZ induced diabetes by using in vivo and in vitro models. MTT assay was performed to check the cytotoxic effect of β- Carotene in HepG2 liver cells which were treated with β- Carotene (10, 20 μM). The anti-diabetic activity was examined by estimating different enzymes in cell lines. Further, we validated activity by using in vitro models. Male Albino Wistar rats were divided into five groups each group contain six animals (n = 6). The diabetes was induced via intraperitoneal injection of STZ and the β- Carotene was treated with daily doses of 10 and 20 mg/kg for 14 days. After the last dose of β- Carotene, rats were sacrificed and the biochemical parameters were estimated in liver homogenate. The disease control group showed an elevation in the level of cytokine as well as ROS and β- Carotene-treated animals showed a reduction in the level of cytokine and normal content of anti-oxidant enzyme in liver tissue homogenate. We found β- Carotene had no toxic effect on HepG2 liver cells. In the case of the glucose utilization assay, it was found that glucose uptake level was significantly increased with the increasing concentrations of β-Carotene. In conclusion β- Carotene improves glucose metabolism along with oxidative status in STZ-induced diabetic rats.
Keywords: β- Carotene, Oxidative stress, Cytokine, STZ, HepG2 liver cell lines
Introduction
Worldwide as due to lifestyle modifications, diabetes mellitus (DM) reaches a most common type of metabolic disorder affected with macro as well microvascular complications that leads to significant mortality. In diabetic patients insulin action and insulin secretion do not function properly, the action of insulin in insulin-sensitive tissues such as liver, muscle, and adipose tissue (insulin resistance in T2DM) and insulin secretion by pancreatic islet β-cells (β-cell dysfunction in T2DM) are affected, which results in abnormal blood levels of glucose [6]. In T2DM, insulin resistance contributes to increased glucose reduction in the liver and decreased glucose uptake in muscle and adipose tissue at a set insulin level. In addition, β-cell dysfunction results in reduced insulin release, which is insufficient for maintaining normal glucose levels [13]. Both insulin resistance and β-cell dysfunction occur early in the pathogenesis of T2DM, and their critical importance has been verified longitudinally in Pima Indian people progressing from normal glucose tolerance to impaired glucose tolerance to T2DM [21]. β-cell impairment in diabetic people mainly occurs due to low antioxidant capacity because β-cells are very sensitive to oxidative stress [4]. Numerous studies recommend that diabetes has a high prevalence and it is a common reason for mortality in the world [2]. The natural plant-derived small molecules have high demand in diabetic patients because of these remedies does not have any side effect when used with insulin as well hypoglycemic agents [10]. Several complications like changes in concentrations of cholesterol, plasma lipids and lipoprotein observed in Patients suffering from DM that lead to serious cardiac complications like ischemic heart disease. The high dietary content of fresh vegetables & fruits has been positively accompanying by a reduced incidence of ischemic heart diseases by several epidemiological studies [1]. Flavonoids are certainly a biomarker of the “health” and good diet plan (large contents of materials, greens, and simple sugars; low contents of lipids and Complex carbohydrate; low-calorie intake, etc); nevertheless, behind these “unspecific” impacts, current biochemical and clinical research has concentrated on some “specific” properties of flavonoids regarding a cardiac system that may clarify the lower CHD mortality in diabetic patients [8, 19]. In diabetes, mellites is especially known for insufficient secretion of insulin by the pancreas and efficient uptake of muscle, liver, and fat tissue counters the dangerous build-up of glucose within the blood and, finally leads to the onset of type-2 diabetes [14] which can be normalized using the antioxidant property of the chemical effects of Beta carotene. Beta-carotene (BC) is a member of naturally occurring ingredients known as carotenoids which are a small grouping of phytochemicals [14, 23]. This terpenoid acts as an herbal remedy to quench a single molecule of oxygen to inhibit peroxyl free-radical reactions. Beta-carotene is one of many kinds of carotene that is red-orange colored pigment & widely found in nature, rich in herbal mud fruit, particularly in orange berries. Indeed, in the insulin-producing islet of the Langerhans tissue of the pancreas, some proteins are fairly attached, which helps to transfer the zinc into those pancreatic tissues. Henceforth, it’s well established that, the chemical abilities of beta-carotene allow interesting topics in diabetic research. According to several studies reports that the antioxidant property of beta carotene, hence we hypothesized that the beta carotene will show the therapeutic potential against STZ induced diabetic rats. The present study aimed to examine the healing capabilities of β-Carotene by using in vitro & in Vivo assay through estimation of liver glycogen, anti-oxidant capacity, proinflammatory cytokines, and nitric oxide.
Materials and methods
Animals
Thirty-six male Albino Wistar rats weighing 180–220 g were used for this experimental examination. Animals were kept in nicely-ventilated polypropylene cages with a controlled temperature of 25 ± 2 °C, 12-h light/ dark cycle inside the animal house. Animals provided free access to pelletized feed (Amrut rodent Feed, Pune) and drinking water ad libitum.
Chemicals and drugs
The chemicals and drugs were used are Streptozotocin (Sigma Aldrich, USA), β-carotene, carboxymethylcellulose, metformin (Sigma Aldrich, USA). creatinine kit, Glucose Kit, Albumin Kit, Urea/BUN Kit, and Total Protein Kit were purchased from ERBA (Mumbai, India). TNF-α (Tumor necrosis factor-alpha) and IL-6 (interleukin-6) were purchased from Loba Chemicals Pvt. Ltd. (Mumbai, India). All other needed chemicals and reagents for the study were of analytical grade.
Induction of diabetes
All animals except the control group were treated with Streptozotocin (STZ) at a dose of 55 mg/kg via an intraperitoneal route of administration leads to the initiation of diabetes. In the dark conditions’ cold solution of phosphate buffer (pH -7.4) was applicable for the formulation of STZ solution. Induction of diabetic was confirmed by fasting blood glucose level was estimated in animals after 72 h and animals with fasting blood glucose level above 200 mg/dL was used in the study [10].
Experimental design
All the animals were divided randomly into 5 groups (n = 6).
Group I: Control Group - received 0.5% Carboxymethyl cellulose
Group II: Disease Control Group– STZ (i.p. 55 mg/kg)
Group III: Standard Group– STZ (i.p. 55 mg/kg) & metformin (70 mg/kg)
Group IV: Test Control Group- STZ (i.p. 55 mg/kg) and 10 mg/kg of β- Carotene
Group V: Test Control Group- STZ (i.p. 55 mg/kg) and 20 mg/kg of β- Carotene
In the current study, Metformin and β- Carotene administered orally for 14 days of the study after induction of diabetes. After the 14th day of study, to estimate the biochemical parameters the animals were euthanized using a CO2 chamber, then their livers were isolated. Then, ice-cold phosphate buffer saline solution (pH 7.4) was used for the preparation of the liver tissue homogenate (10%), furthermore, the supernatant was prepared after centrifugation of the homogenate at 10000 rpm for 10 min and 4 °C. Then the supernatant was bifurcated for and used for further assessment of anti-oxidant enzymes as well as cytokines [5].
Physiological parameters
Weekly the bodyweight of each animal was recorded by using an electronic weighing balance.
Liver glycogen
For the assessment of Liver glycogen content on the last day of study, the animals were euthanized and sacrificed. Then, the liver of the rat was isolated and washed using a cold saline solution. In 80% ethanol, the hepatic cells were minced and then homogenized at a tissue concentration of 100 mg/mL then centrifuged at 10000 rpm for 15 min. Then there were added water mL and perchloric acid 52%. From this solution, the precipitate was collected, dried, and extracted. For the regaining of supernatant, the collected extract was centrifuged at 9500 rpm for 15 min. 0.2 mL of supernatant +1 mL water (distilled water) and reagent (anthrone reagent) was added, and then heated and finally cooled at room temperature & at 630 nm of intensity, a solution was examined and recorded. With the help of glucose solution standard curved was prepared and lastly, the glycogen content was determined [1].
Biochemical estimations of antioxidant parameters in liver homogenates
Assessment of antioxidant biomarkers like Glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA) was done according to kits that are commercially available in the market & guidelines given the manufacturers, while Catalase was assessed with the help of UV spectroscopic methods. Into the hydroperoxide solution (H2O2), the liver homogenate supernatant (10 μL) was added. Using a UV spectrophotometer (Mithras LB 940, Berthold, Germany) at 240 nm the reduced optical density was measure in this mixture of supernatant and hydrogen peroxide. The reduced optical density in 3 min after the addition of liver homogenate was considered as an indicator of catalase activity that exists in the homogenate [9, 17].
Estimation of Proinflammatory cytokine parameters
Assessment of Proinflammatory cytokines like Tumor Necrosis Factor -α Interleukin-6 was assessed into liver homogenate supernatant by ELISA sandwich kits as per the guidelines given by the manufacturer and final concentration was estimated with the help of a standard curve [18].
Cell culture and reagent
HepG2 liver cells were cultured in RPMI-1640 supplemented with 10% FBS, 1.5 mM L-glutamine, and 1% antibiotics (100 U/ml of penicillin, 10 mg/ml of streptomycin).L6 myoblasts cells had been cultured within an antibiotic-free growth that is composed of RPMI 1640 supplemented with 10% FBS. 3 T3-L1 cells were cultured in DMEM with 10% fetal bovine serum. RAW 264.7 macrophages tissue happened to be cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing L-glutamine formulated with 10% FBS and 1% antibiotics. All cellular lines were cultured until 90% confluency is attained within a soaked incubator in 5% CO2 at 37 °C and then they were trypsinized and utilized relating to their unique requirements [11]. All the cellular reagents like media, antibiotics, L-glutamine, trypsins were purchased from Gibco. Toxins like MTT [3-(4, 5-dimethylthiazol-2yl-)-2, 5-diphenyl tetrazoliumbromide] and β-Carotene are procured from Sigma.
MTT assay
MTT assay was performed. Briefly, cells were dislodged by short exposure to 0.25% Trypsin in phosphate-buffered saline, counted, suspended in a new growth medium, and then approximately, 8000 to 10,000 HepG2 and L6 cells were seeded in 96-well plates in triplicate and grown to 60–70% confluency. Then, cells were treated with the increasing concentrations of β-Carotene for 48 h. After that, media was removed, 20 μL MTT reagent (5 mg/mL) was added to each well and incubated at 37 °C for 2 h for the formation of purple formazan crystals [16]. After removing 15the MTT solution, purple formazan crystals were dissolved in isopropanol. The color intensity was measured by spectrophotometer at 570 nm using a microplate reader (Mithras LB 940, Berthold, Germany).
The % viability 1was calculated as mentioned below:
Glucose utilization experimental procedure
The glucose utilization both in HepG2 and L6 cells were assessed to ascertain the In vitro anti-diabetic property of β-Carotene. In brief, HepG2 and L6 cells comprise seeded in 96 wells then given treated 5, 10, 15 μg/mL concentrations of β-carotene. Two rows that was cell-free and included to serve as blanks [15]. After 48 h incubation, the spent culture medium was got rid of and replaced with a 25 μl incubation buffer (RPMI medium diluted with PBS, 0.1% BSA, and 8 mM glucose) and further incubated for the additional 3 h at 37 °C. Metformin (0.1 μg/mL) and insulin (6 μg/mL) were used as a control that was dispositive Hep G2 and L-6 cells, respectively to compare the effectiveness of β-carotene. After incubation, 10 μl on the incubation medium had been removed from each well and transmitted into the newest plate that is a 96-well plate which 200 μL of glucose oxidase reagent was included to assess the amount of glucose into the medium. After 15 min of incubation at 37 °C, the absorbance got measured at 492 nm. The amount of glucose used was determined while the difference in the cell-free and cell-containing wells. The portion of glucose utilization got computed concerning the untreated controls.
Lipid accumulation in 3 T3-L1 Preadipocytes
Nearly, 8000 3 T3 - L1cells seeded per well into a 24 - well culture plate and permitted to grow until 60–70% confluence got reached. Subsequently, the preadipocytes were treated with the above-mentioned concentrations of β-Carotene along with positive controls (Rosiglitazone; 0.4 μg/ml) for 48 h. The cells were then cultured for the extra period of ten days in a culture medium (DMEM with 10% FBS) as well as the medium got changed every 2 to 3 days. The spent culture medium was removed and gently washed with PBS after ten days. The cells comprise subsequently permitted to fix at room temperature for nearly 1 h by adding 250 μl per well of 10% formaldehyde in PBS. The fix solution was aspirated and later stained by adding 200 μl of pre-warmed oil red working solution [6 ml of stock solution (0.5 g oil red dye in 100 ml isopropanol) in 4 ml of distilled water] for 15 min at 37 °C. After 15 min of incubation, the extra colorant ended up being thoroughly washed with water & plate dried in an oven at 37 °C. The dye was further extracted with the addition of isopropanol (150 μl a perfectly) followed by 100 μl ended up being utilized in a 96-well dish and the absorbance was calculated at 520 nm [7].
Measurement of nitric oxide (NO)
Nitric oxide has been reported to contribute to the pathogenesis of diabetes and hence inhibition of NO is important to monitor anti-diabetes efficacy. RAW cells (100,000 cells/well) were cultured in 6 well plates and incubated with LPS to induce NO production. After the LPS supplementation, cells were treated with the treatment concentrations of β-Carotene for 48 h. After that cells were lysed with RIPA lysis buffer and protein concentrations were measured. 50 μg of proteins from different treatment groups were taken in 96 well plates on which 50 μL of Griess reagent I and Griess reagent II were added and incubated for 15 min in dark for the pink color formation. After that, absorbance was taken spectrophotometrically at 560 nm and represented graphically.
α-Amylase inhibition assay
The α-amylase assay was performed to check if our drug is affecting the term of inhibiting α-amylase. Briefly, 15 μl of the β-Carotene at different concentrations (5, 10 and 15 μg/mL) was added to 5 μl of enzyme porcine pancreatic solution into 96-well plate.
After 10 min of incubation at 37 °C, the reaction was initiated by adding 20 μl of starch solution and further incubated for 30 min at 37 °C (Wickramaratne, Punchihewa, and Wickramaratne 2016). The reaction was then stopped by adding 10 μl 1 M of HCl to each well followed by 75 μl of iodine reagent. A blank containing phosphate buffer (pH 6.9) instead of the extract and positive control (acarbose, 64 μg/ml) was prepared. No enzyme control and no starch control were included for each test sample. The absorbance was measured at 580 nm and the percentage inhibitory activity was calculated by using the following equation:
α-Glucosidase inhibition assay
For the assessment of β-Carotene mediated α- glucosidase inhibition, this assay is very useful. In brief, 5 μl of the β-Carotene of the above-mentioned treatment concentrations were added to 20 μl of 50 μg/ml alpha-glucosidase solution into a well of a 96- well plate. After that, 60 μl of 67 mM potassium phosphate buffer solution (pH 6.8) was then added. After 5 min of incubation, 10 μl of 10 mM p-nitrophenyl-α-D-glucoside solution (PNPGLUC) was then added and additionally incubated for 20 min at 37 °C. 25 μl of 100 mM Na2CO3 was added and the absorbance was measured at 405 nm [20] After incubation. A blank and sample blank was also prepared by adding 5 μl of deionized water instead of plant extract and 20 μl of deionized water instead of an enzyme, respectively. Epigallocatechin gallate (10 μg/ml) was used as a positive control. The percentage inhibition was calculated using the following equation:
Statistical analysis
Statistical analysis was performed using Graph Pad Prism 5.0 software, USA. Results represented the mean ± SEM of TWO separate experiments. Data were analyzed using one-way, Two-way ANOVA, and repeated measures ANOVA followed by Bonferroni’s multiple comparison test. Statistical significance of difference in the central tendencies compared to control groups was designated as‘*’(p < 0.05),‘**’(p < 0.001) and‘***’(p < 0.0001).
Result
Effect of β- Carotene on body weight in STZ-induced diabetic rats
In the Present Investigation, we have determined the effect of STZ and β- Carotene on change in body weight. The bodyweight of the animals significantly decreased in the disease control group as compared to the normal group. Treatment with β- Carotene increased the body weight as compared to the disease control (P < 0.001) shown in Fig. 1, Whereas the metformin-treated group showed an increase in the body weight as compared to the disease control group (P < 0.001).
Fig. 1.
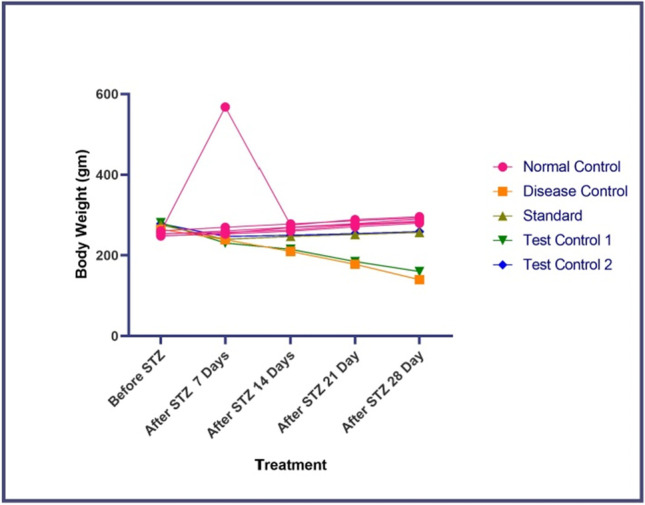
Effect of β-carotene on change in body weight in STZ-induced diabetic rats. Disease control- STZ treated, Standard- Metformin treated, Test Control 10- mg/kg of β- Carotene treated, Test Control 2–20 mg/kg of β- Carotene treated. Each point represents mean ± S.E.M. (N-6). The result values were statistically analyzed by using repeated-measures ANOVA considering time as a repeated factor followed by Bonferroni’s test for multiple comparisons
Effect of β- Carotene on liver glycogen level in STZ-induced diabetic rats
In the Present Investigation, we have determined the effect of STZ and β- Carotene on Liver Glycogen levels significantly decreased in the disease control group as compared to the normal group. Treatment with β- Carotene increased the Liver Glycogen Levels compared to the disease control group (P < 0.001) shown in Fig. 2, Whereas the metformin-treated group showed an increase in the Liver Glycogen Level as compared to the disease control group (P < 0.001).
Fig. 2.
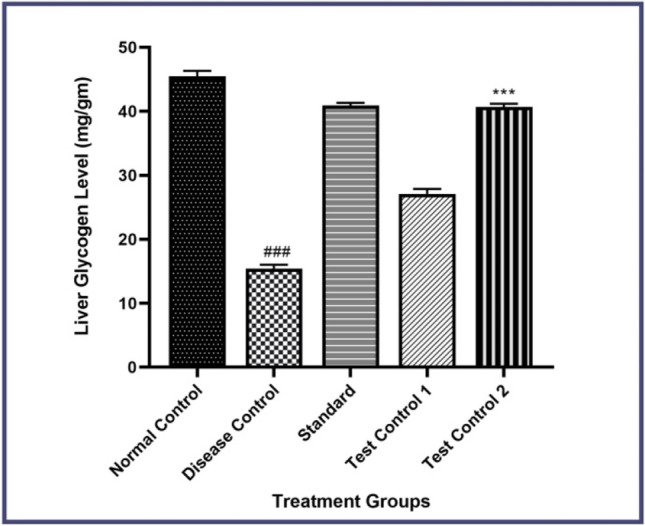
Effect of β-carotene on liver glycogen level in STZ-induced diabetic rats. Disease control- STZ treated, Standard- Metformin treated, Test Control 10- mg/kg of β- Carotene treated, Test Control 2–20 mg/kg of β- Carotene treated. Data were presented as mean ± S.E.M. (n-6). The result values were statistically analyzed by using a one-way ANOVA drug as an independent factor, followed by Bonferroni’s test for multiple comparisons (P < 0.001)
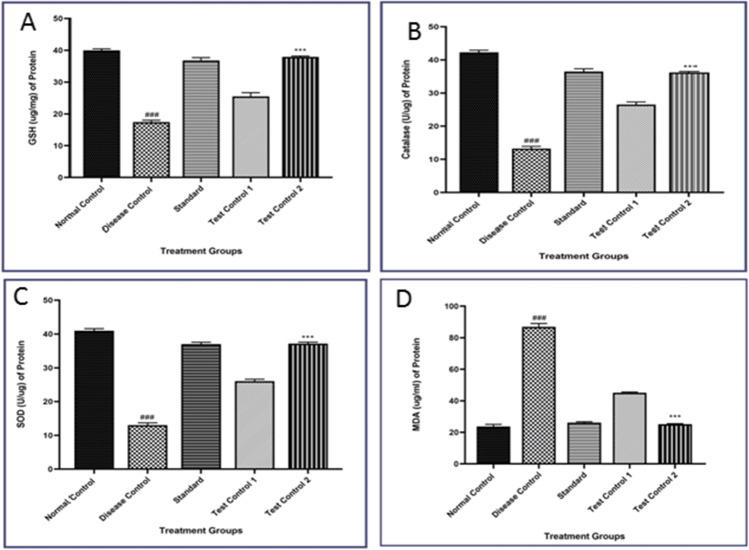
Effect of β- Carotene on oxidative stress in STZ-induced diabetic rats
As shown in Fig. 3, lipid peroxidation was significantly high in the disease control group as suggested by a marked increase in Liver MDA levels as compared to the normal control group. (P < 0.001). Administration of β- Carotene to the disease control group significantly restored the increased lipid peroxidation levels as compared to the disease control group (P < 0.001). The disease control group showed a significant decrease in SOD activities and reduced GSH level and catalase activities (P < 0.001) in the Liver tissues. Metformin-treated rats show a similar effect to normal rats. Administration of β- Carotene into disease control significantly increases the activities of SOD, reduced GSH, and Liver catalase activities as compared with the disease control group (P < 0.001).
Fig. 3.
Effect of β- Carotene on A) GSH level, B) Catalase activity, C) SOD activity, and D) MDA level in the liver of STZ-induced diabetic rats. Disease control- STZ treated, Standard- Metformin treated, Test Control 10- mg/kg of β- Carotene treated, Test Control 2–20 mg/kg of β- Carotene treated. Data were presented as mean ± S.E.M. (n-6). The result values were statistically analyzed by using one-way ANOVA drug as an independent factor, followed by Bonferroni’s test for multiple comparisons
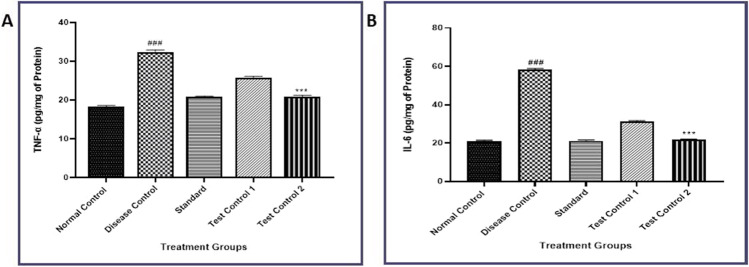
Effect of β- Carotene on TNF-α and IL-6 proinflammatory cytokines levels in STZ-induced diabetic rats
The level of Pro-inflammatory cytokine was measured by ELISA-Sandwich assay and showed a significant rise in TNF-α and IL-6 in STZ-treated rats. This was indicated by a marked increase in Liver cytokine levels as compared to the disease control group (P < 0.001). β- Carotene treatment at different doses in the disease control group significantly restored the increased cytokine levels as compared to the disease control group (P < 0.001) (Fig. 4).
Fig. 4.
Effect of β-carotene on A) TNF-α, B) IL-6 levels in STZ-induced diabetic rats. Disease control- STZ treated, Standard- Metformin treated, Test Control 10- mg/kg of β- Carotene treated, Test Control 2–20 mg/kg of β- Carotene treated. Data were presented as mean ± S.E.M. (n-6). The result values were statistically analyzed by using one-way ANOVA drug as an independent factor, followed by Bonferroni’s test for multiple comparisons
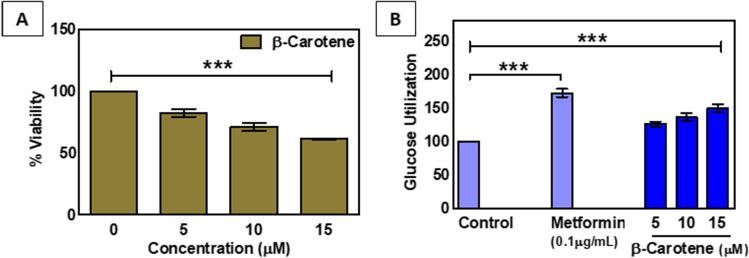
Β-Carotene enhances the glucose utilization in HepG2 liver cell line
Research suggests that a potent anti-diabetic substance enhances glucose utilization in the liver to decrease the high blood glucose level. In our current study when we have exposed β-carotene in the HepG2 cell line, the IC50 value was not reached up to the treatment concentration of 15 μM (Fig. 5A). The cytotoxicity data suggested that β- Carotene displayed a low level of cytotoxicity in HepG2 cells.
Fig. 5.
A. MTT cell viability after the exposure of different concentrations of β-carotene in HepG2 liver cells. B. Effect of β-carotene on glucose utilization in HepG2 hepatocytes. Cells were treated for 48 h in the presence or absence of varying concentrations of β-carotene. Metformin serves as a positive control. Data expressed as mean ± S.E.M. ((n = 3). ‘***’ indicates statistical significance (p < 0.0001)
Further, when we performed the glucose utilization experiment, it was found that glucose uptake level was significantly increased with the increasing concentrations of β-Carotene. Approximately 1.49 fold enhanced glucose utilization was found upon the treatment of 15 μM β-Carotene (Fig. 5B). To compare the efficacy of our drug, we have used Metformin as a positive control. 0.1 μg/ml Metformin exposure caused 1.72 fold elevated level of glucose uptake in our treatment procedure and concerning this, our drug of interest showed comparably lower elevation of glucose utilization (Fig. 5B).
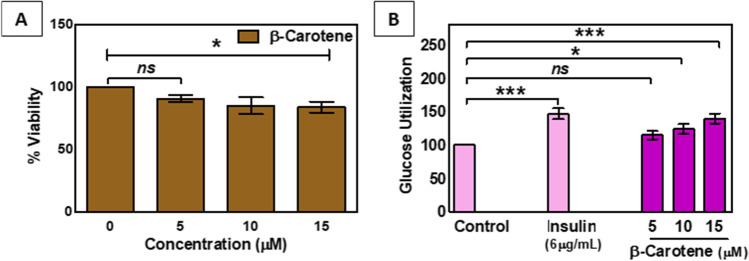
Β-Carotene potentiates the glucose utilization in L6 myoblast cell line
Many potent anti-diabetic agents lower the blood glucose level by enhancing the glucose uptake in muscle cells. In our experiment, to evaluate the anti-diabetic efficacy, we have checked the glucose utilization in the L6 myoblast cell line. Figure 6A shows the β-Carotene mediated decrease in cell viability of myoblast cells. Data suggested an approximately 1.18 fold decrease in cell viability with the highest treatment concentration of β-Carotene (15 μM), which suggests the low level of cell toxicity in muscle cells.
Fig. 6.
A. MTT cell viability after the exposure of different concentrations of β-carotene in L6 myoblast cells. B. Effect of β-carotene on glucose utilization in L6 myoblast cells. Cells were treated for 48 h in the presence or absence of varying concentrations of β- carotene. Insulin serves as a positive control. Data expressed as mean ± S.E.M.(n = 3).‘ns’,‘*’, ‘***’ indicates non-significance (p > 0.05) and statistical significance (p < 0.05 and p < 0.0001,respectively)
In addition, it was also found that with the increasing concentrations of β-Carotene, glucose utilization was also increased in muscle cells. Upon 15 μM treatment concentration of β-Carotene, 1.4 fold elevated level of glucose uptake was found in myoblast cells (Fig. 6B). Insulin (6 μg/ml) was used as a positive control, which enhanced the glucose utilization 1.47 fold in with compare to this our drug also showed a good potential effect (Fig. 6B).
Β-Carotene increases lipid accumulation in 3 T3-L1 preadipocytes
The above observation supported the fact that β-Carotene shows the minimum level of cytotoxicity in normal cells. Further to support the anti-diabetic potentiality of β-Carotene, we have checked the triglyceride accumulation in 3 T3-L1 per adipocytes. Figure 7 displayed an increase in lipid accumulation with the increasing treatment concentrations of β-Carotene. At a 15 μM treatment concentration of β- Carotene,1.46 fold enhanced lipid accumulation was found which was slightly less than Rosiglitazone (1.56 fold, approximately).
Fig. 7.
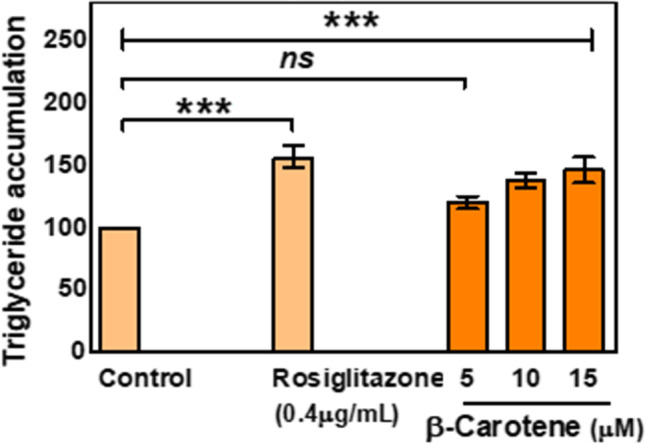
Effect of β-carotene on triglyceride accumulation in 3 T3-L1 preadipocytes. Cells were treated for 48 h in the presence or absence of varying concentrations of β-carotene. Data were expressed as mean ± S.E.M. (n = 3). The result values were statistically analyzed by using one-way ANOVA drug as independent factor ‘ns’, ‘***’ indicates statistical non-significance (p > 0.05) and statistical significance (p < 0.0001). Rosiglitazone serves as a positive control
β-Carotene inhibits nitric oxide (NO) production in RAW 264.7 cell line
Inhibition of NO was found to be one of the central mechanisms in many anti-diabetic substances. In our current study, when we wanted to check the effect of β-carotene in a macrophage cell line, it was found that nitric oxide production was significantly affected with the increasing treatment concentrations of β-carotene. 15 μM β-carotene caused an approximately 2.15 fold reduction in NO production in the RAW cell lines (Fig. 8). To compare the effectiveness of β-carotene in terms of reducing NO production, a positive control, Aminoguanidine was used.4 μg/ml Amino guanidine, caused an approximately3.4 fold decrease in NO production, which suggests that our drug of interest is having a great impact on the reduction of NO production (Fig. 8).
Fig. 8.
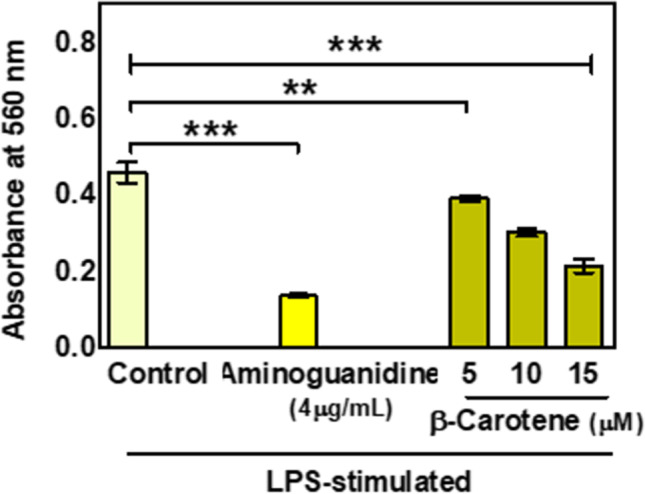
The effect of β-carotene on NO production by LPS-stimulated RAW macrophage cells. Data are expressed as mean ± S.E.M. (n = 3). The result values were statistically analyzed by using a one-way ANOVA drug as independent factor ‘**’,’***’ indicates statistical significance (p < 0.001 and p < 0.0001, respectively). Aminoguanidine serves as a positive control
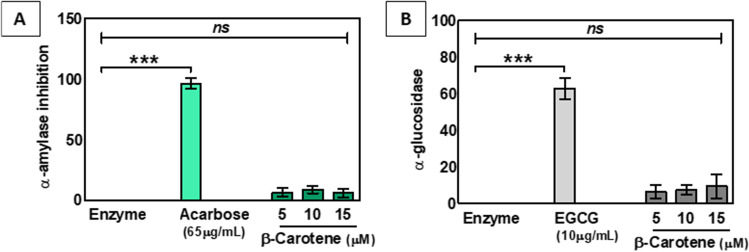
Effect of β-Carotene in alpha-amylase and alpha-glucosidase
Research suggests that some of the anti-diabetic drugs lower the blood glucose level by down regulating the expression of two enzymes, α-amylase, and α-glucosidase. In this study, when we wanted to illustrate the molecular mechanism behind the way of action of β-carotene by measuring the level of α-amylase and α-glucosidase, it was found that β-carotene had no significant effect to alter the expression of the set coenzymes (Fig. 9A, B).
Fig. 9.
The effect of β-carotene on α-amylase (A) and α-glucosidase (B) activity. Data expressed as mean ± S.E.M. (n = 3). The result values were statistically analyzed by using one-way ANOVA drug as independent factor ‘ns’,’***’ indicates statistical non-significance (p > 0.05) and statistical significance (p < 0.0001). Acarbose and Epigallocatechin gallate (EGCG) act as positive controls, respectively
Discussion
In this study, we have systematically studied the effect of β-carotene to establish it as a potent anti-diabetic substance. Β-carotene is a natural anti-oxidant having many commercial values. Being a carotenoid and an antioxidant, it was thought to be non-toxic or less toxic [14]. STZ induced diabetes is well known preclinical model for screening of anti-diabetic potential of the drug. Diabetes was induced at the dose of 55 mg/kg body weight of rats. It was found an elevated level of glucose was in rats [10]. Meanwhile, rats treated with β-carotene showed a normal level of glucose in the blood. Also, the rats treated with STZ showed an increased level of cytokine and β-carotene treated rats showed a decrease in the cytokine level as compared to the control group.
In this present study, when we exposed β-carotene to liver cells and muscle cells, very little β-carotene mediated cytotoxicity was noted. Moreover, up to the treatment concentration of 15 μM, the IC50value of β-carotene was not found. Hence, the relatively low level of toxicity exhibited by β-carotene raises prospects that β-carotene could be potentially safe for the users. Further, various biochemical assays were used to evaluate the probable antidiabetic action of β-carotene [1]. Glucose utilization experiments suggested that β-carotene enhances the glucose uptake in liver and muscle cells may be through activating glucokinase-2 in liver or muscle cells, which promotes glucose uptake and utilization in these cells mainly via glycogen synthesis and maintaining a gradient for glucose transport into the cells, however it needs further investigation. Metformin and Insulin were used to compare the effectiveness of β-carotene to enhance the glucose uptake in liver and muscle cells, respectively. Although β-carotene was not found to be that much effective as Metformin and Insulin, it showed a significant impact in enhancing glucose uptake. Data obtained from lipid accumulation, further showed that β-carotene has some role in enhancing triglyceride accumulation this may be due to inhibiting lipolysis of adipose tissue. The potentiality of adipose tissue to lodge excess lipid can be exceeded in obese patients, which results in the abnormal accumulation of lipid in muscle, liver, and pancreatic islet leading to lipotoxicity. Higher levels of lipid accumulation in 3 T3-L1 adipocytes suggesting that β-carotene might be a good therapeutic agent to lower the lipid profiles [7]. The above observations suggested the anti-diabetic potentiality of β-carotene. However, to evaluate the molecular mechanism of β-carotene mediated anti-diabetic action, some experiments were carried out. Nitric oxide plays an important role in the onset of diabetes. So, we wanted to check if β-carotene has some effect in lowering the NO production in RAW cells or not. Our study suggested that β-carotene has a significant role to reduce the NO level; it may be due to its antioxidant activity [3]. Which could be the potential way of action of β- carotene mediated anti-diabetic activity. At present, several anti-diabetic drugs that are used to manage diabetes exhibit the inhibition of alpha-amylase and/or alpha-glucosidase. Malathi proved that metformin has potent alpha-amylase and alpha-glucosidase inhibitory activity [12]. However, our data demonstrated that β-carotene has no significant inhibition on alpha-amylase and alpha-glucosidase [20, 22].
Conclusion
In summary, our findings suggest that β-carotene exerts its hypoglycemic activity by enhancing glucose utilization in liver and muscle cells and enhancing lipid accumulation. Although β-carotene does not affect the level of alpha-amylase and alpha-glucosidase, it has a great role in reducing nitric oxide production. This reduction of nitric oxide production could be probably through inhibition of iNOS but further research needs to be done to confirm that.
Authors’ contributions
Not applicable.
Data availability
All data and material are available upon request.
Declarations
All authors read and approved the final manuscript.
Ethics approval and consent to participate
The experimental protocol was approved by the institutional animal ethical committee of Dr. VVPF’s College of Pharmacy, Ahmednagar, Maharashtra, India with protocol no. Coph/IAEC/2020/01.
Consent for publication
Not applicable.
Competing interests
Authors does not have a conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmad U, Rabia SA. Anti-diabetic property of aqueous extract of Stevia rebaudiana Bertoni leaves in Streptozotocin-induced diabetes in albino rats. BMC Complement Aalter Med. 2018;18:1–11. doi: 10.1186/s12906-017-2057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aruljothi B, Samipillai SS. Antidiabetic activity of Catharanthus roseus in alloxan-induced diabetic rats. Int J Modn Res Revs. 2016;4:1121–1124. [Google Scholar]
- 3.Bast A, Van der Plas RM, Van Den Berg H, Haenen GR. Beta-carotene as antioxidant. Euro J Clin Nutri. 1996;50:S54–S56. [PubMed] [Google Scholar]
- 4.Drews MG, Krippeit-Drews P, Dufer M. Oxidative stress and beta-cell dysfunction. Pflug Arch Eur J Phy. 2010;460(4):703–718. doi: 10.1007/s00424-010-0862-9. [DOI] [PubMed] [Google Scholar]
- 5.Golder FJ, Robertson SA, Valverde A, Bolser DC. Urethane anesthesia in adult female rats: preliminary observations. Veter Anaesthe Analg. 2003;30:115–115. doi: 10.1046/j.1467-2995.2003.13335.x. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein BJ, Müller-Wieland D. Type 2 diabetes: principles and practice. 2. CRC Press; 2016. [Google Scholar]
- 7.Gustafson B, Ulf S. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J Biol Chem. 2006;281:9507–9516. doi: 10.1074/jbc.M512077200. [DOI] [PubMed] [Google Scholar]
- 8.Hertog MGL, Feskens EJM, Kromhout D, PCH H, Katan MB. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen elderly study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- 9.Jain PG, Mahajan UB, Shinde SD, Surana SJ. Cardioprotective role of FA against isoproterenol induced cardiac toxicity. Mole Biol Rep. 2018;45(5):1357–1365. doi: 10.1007/s11033-018-4297-2. [DOI] [PubMed] [Google Scholar]
- 10.Jain PG, Nayse PG, Patil DJ, Shinde SD, Surana SJ. The possible antioxidant capabilities of formononetin in guarding against streptozotocin-induced diabetic nephropathy in rats. Fut J Pharmaceut Sci. 2020;6:1–9. doi: 10.1186/s43094-019-0015-8. [DOI] [Google Scholar]
- 11.Luna-Vital DA, de Mejia EG. Anthocyanins from purple corn activate free fatty acid-receptor 1 and glucokinase enhancing in vitro insulin secretion and hepatic glucose uptake. PLoS One. 2018;13:e0200449. doi: 10.1371/journal.pone.0200449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malathi V, Devi SS, Revathi K. Anti diabetic activity by the in vitro alpha amylase and alpha-glucosidase inhibitory activity of catharanthus roseus. Bioscan. 2010;5(4):655–659. [Google Scholar]
- 13.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 14.Sayahi M, Saeed S. The antidiabetic and antioxidant effects of carotenoids: a review. Asian J Pharm Res Health Care. 2017;9:186–191. doi: 10.18311/ajprhc/2017/7689. [DOI] [Google Scholar]
- 15.Semaan DG, Igoli JO, Young L, Gray AI, Rowan EG, Marrero E. In vitro anti-diabetic effect of flavonoids and pheophytins from Allophylus cominia Sw. On the glucose uptake assays by HepG2, L6, 3T3-L1 and fat accumulation in 3T3-L1 adipocytes. J Ethnopharmacol. 2018;216:8–17. doi: 10.1016/j.jep.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Sharath Babu GR, Tamatam A, Ilaiyaraja N, Farhath K, Gopalan N. Pelargonidin modulates Keap1/Nrf2 pathway gene expression and ameliorates citrinin-induced oxidative stress in HepG2 cells. Fron Pharmacol. 2017;8:868. doi: 10.3389/fphar.2017.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinde SD, Cheke RS, Tathe PR, Jain PG, Narkhede RR. The Berberis aristata ameliorates oxazolone induced contact dermatitis: in-vivo and in silico evidences. Adv Trad Med. 2020:1–8.
- 18.Shinde SD, Jain PG, Cheke RS, Surana SJ, Gunjegaonkar SM. Abrogation of cisplatin-induced nephrotoxicity in rats and HEK-293 cell lines by formononetin: in vivo and in vitro study. Compar Clin Pathol. 2021:1–9.
- 19.Suen J, Thomas J, Kranz A, Vun S, Miller M. Effect of flavonoids on oxidative stress and inflammation in adults at risk of cardiovascular disease: a systematic review. In Healthcare. 2016;69 [DOI] [PMC free article] [PubMed]
- 20.Vongsak B, Kongkiatpaiboon S, Jaisamut S, Machana S, Pattarapanich C. In vitro alpha glucosidase inhibition and free-radical scavenging activity of propolis from Thai stingless bees in mangosteen orchard. Rev bras Farmacogn. 2015;25:445–450. doi: 10.1016/j.bjp.2015.07.004. [DOI] [Google Scholar]
- 21.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickramaratne M, Nirmali JC, Punchihewa WDBM. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement Altern Med. 2016;16:1–5. doi: 10.1186/s12906-016-1452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L, Ouyang L, Lin S, Chen S, Liu YJ, Zhou W, Wang X. Protective role of β-carotene against oxidative stress and neuroinflammation in a rat model of spinal cord injury. Int Immunopharmacol. 2018;61:92–99. doi: 10.1016/j.intimp.2018.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material are available upon request.