Abstract
Background
Diabetes mellitus (DM) is a metabolic disorder characterized by chronic hyperglycemia resulting from insulin deficiency or dysfunction. The imbalance between free radicals and antioxidants known as oxidative stress has been implicated in the pathogenesis and complications associated with DM. Chrysophyllum albidum is a seasonal fruit found to be rich in natural antioxidants.
Methods
DM was induced by high-fat diet dietary supplementation for 14 days followed by intraperitoneal injection of streptozotocin (35 mg/kg). Thirty-five experimental rats were then divided into seven groups viz.: non-diabetic control; diabetic control; metformin; diabetic and non-diabetic fed with 5 and 10% C. albidum fruit pulp powder (CAFPP). Fasting blood glucose was done with an automatic auto-analyzer and weights were monitored at three-day intervals. The expressions of Nrf2, SOD, CAT, GST, TNF-α, DPP4, and insulin were investigated using RT-PCR. Schrödinger suites was used for docking of C. albidum phytocompounds with insulin.
Results
Diabetic rats fed with CAFPP for thirteen days have their blood glucose lowered significantly (p < 0.05) and gained weight compared to diabetic control. CAFPP significantly (p < 0.05) up-regulated Nrf2, CAT, GST, SOD, and insulin genes expression in the diabetic group relative to diabetic control with concomitant down-regulation of TNF-α and DPP4 genes expression. Molecular docking of compounds previously characterized from C. albidum revealed that they are potent ligands of insulin receptors.
Conclusion
The study revealed that CAFPP could be effective in the management of DM-related oxidative stress by up-regulating antioxidant and down-regulating pro-inflammatory genes expression. It also positively modulates genes associated with glucose metabolism.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-021-00921-0.
Keywords: Chrysophyllum albidum, Oxidative stress, Inflammation, Antioxidants, Gene expression
Introduction
Diabetes mellitus (DM) is a group of metabolic disorders whose main feature is chronic hyperglycemia (high blood glucose), either because insulin production is inadequate, or because the body’s cells do not respond properly to the insulin produced, or both [1]. DM is one of the five important causes of death worldwide, killing 1.6 million annually, and a major global health problem [2]. The global prevalence of diabetes among adults was reported to be 422 million in 2014, 463 million in 2019, and anticipated to reach 578 million by 2030, and 700 million by 2045. [3, 4]. This abnormality promotes auto-oxidation of glucose to form free radicals leading to oxidative modifications of cellular proteins. This eventually results in the development of insulin resistance, pancreatic β-cell dysfunction, and impaired glucose tolerance [5–7].
Oxidative stress and low levels of the antioxidant system play a pivotal role in the pathophysiology, development, and progression of diabetes mellitus [8, 9]. Hyperglycemia has been found to induce glucose auto-oxidation, this continues to recycle free radicals’ generation overwhelming the antioxidant system thus leading to oxidative stress [10]. When there is an imbalance between the radical-generating and radical-scavenging systems, such as increased free radical production or diminished antioxidant defense activity, or both, oxidative stress occurs [11]. Reports have shown the effects of free radicals and consequentially oxidative stress on β cells, β-cell destruction is a major cause of DM as evidenced by high blood sugar [12].
Drug and diet therapies are the most popular approaches applied in the management of diabetes mellitus (DM). However, synthetic drugs used in the treatment of DM are relatively expensive and associated with various side effects, they include; metformin, acarbose, sulfonylureas, thiazolidinedione, and meglitinides [13, 14]. Side effects of metformin include diarrhea, nausea, and abdominal pain. Acarbose, another conventional drug may cause flatulence and diarrhea. Treatment of DM with sulfonylureas has been associated with hypoglycemia, weight gain, skin reactions, acute porphyria, and hyponatremia has been seen in some cases. Meglitinides have been linked to weight gain and low blood sugar. Thiazolidinedione may also cause weight gain, liver disease, anemia, and swelling of legs or ankles during or after diabetic treatment [15]. Hence, the need to search for safe and effective antidiabetic agents from natural sources such as plants [16, 17]. Fruits, usually edible are from plants and found to be medicinal. They are found to be very rich in antioxidants [18].
Many ailments have been treated with edible plants, particularly fruits [19]. Antioxidant-rich meals can boost the body’s antioxidant systems, lowering the impacts of free radical production [11]. Fruits are a good source of minerals, dietary fiber, as well as antioxidant vitamins A, C, and E. Fruit and vegetable consumption has been linked to a lower incidence of diabetes, hypertension, coronary heart disease, cancer, and stroke [19, 20].
C. albidum (Africa Star Apple) fruit contains a very high content of ascorbic acid (an antioxidant) per 100 g of edible fruit, which is about 100 times that of oranges and 10 times of that of guava and cashew [18, 21]. Folklore and reports on different parts of C. albidum such as the leaves, seeds, and cotyledon indicated its antidiabetic potentials [22]. However, there is a paucity of information on the mechanisms involved.
Antioxidants are known to negate the effect of free radicals and prooxidants like reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive chlorine species [10]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is an antioxidant that is sensitive to oxidative imbalance. It can also, directly and indirectly, regulate other antioxidants [23]. Superoxide dismutase (SOD), Catalase (CAT), glutathione-S-transferase (GST), and Tumour necrosis factor (TNF-α) have been reported to be associated with body defense mechanisms in a diseased (pathological) and healthy (physiological) state [23]. The effects of CAFPP on the expression of genes associated with glucose metabolism like dipeptidyl peptidase-4 (DPP4) and insulin were also investigated in the pancreas of the experimental rats used in this study. Compounds previously characterized from C. albidum fruit pulp powder [19, 24, 25] were further docked into the active site of insulin using Schrödinger suites to investigate their binding modes and binding affinities.
Materials and methods
Apparatus/equipment used
Centrifuge (Model (D-37520 Osteorode), Kendro Laboratory products, Germany), UV absorbance spectrophotometer (Model AE-560-20, A & E LAB-UK), Microwave oven (model no: H2OMOWH, Hisense – China), Blue Box Transilluminator (Model {QP - 1700-01 Rev. 2}, USA), thermocycler/PCR Machine (EPPENDORF-AG 22331 Hamburg, Germany), Pipette (Labmate & Lichen- South Africa), Camera Phone (Infinix Smart) Glucometer machine, Gloves, Dissection set, Glucometer and strip, Weighing balance, insulin syringe, Cannula, Whatman filter paper, and Eppendorf tubes.
Chemical/reagents used
Streptozotocin (STZ) was procured from Santacruz’s, Ethanol, Citrate Buffer (0.1 M, pH 4.5), Aqueous solution, Primer sets (forward and reverse) procured from Inqaba biotech (South, Metformin, Formalin, distilled water, cotton wool, and methylated spirit.
Plant collection and preparation
Ripe C. albidum fruits were purchased in a local market in Ado-Ekiti, Ekiti State. They were identified and authenticated at the Department of Plant Science and Biotechnology, Ekiti State University, Ekiti State. The fruits were thoroughly washed, sorted out, and sliced. The seeds were extracted, the pulp scraped, freeze-dried, and milled (Figs. 1 and 2 in supplementary figures). The C. albidum fruit pulp powder was then sealed in a polythene bag and stored in a refrigerator at −10 °C before usage.
Feed preparation, animal grouping, and management
Thirty-five (35) Wistar rats weighing 185–210 g were purchased from the Department of Medical Biochemistry, College of Medicine, Ekiti State University, Ado-Ekiti, Nigeria. The animals were kept in well-ventilated cages. They were given access to water and food ad libitum and were maintained at 25 °C on a 12 h light/dark cycle. They were handled and used by the National Institute of Health (NIH) Guidelines for the Care and Use of Laboratory Animals.
After two weeks of acclimatization, the rats were allocated into two dietary regimens; normal control and high-fat diet (HFD). Feed preparation was carried out using the method of [26]. The dietary supplementation continued for 14 days after which the rats fed with HFD were injected intraperitoneally (i.p) with streptozotocin (STZ) freshly prepared in citrate buffer (0.1 M, pH 4.5) at a single dose of 35 mg/kg body weight for the induction of diabetes mellitus according to the method described previously [26]. The non-diabetic animals received 1 ml of 0.1 M citrate buffer intraperitoneally.
The diabetic state was checked 72 h after induction with STZ and blood samples taken by tail vein puncture, glucose level was monitored using automatic auto-analyzer (Fine test Auto-coding). Animals with blood glucose ≥200 mg/dl after 72 h were considered diabetic and used in the study. Rats were weighed with a weighing balance. C. albidum fruit pulp powder diet supplement started after confirmation of diabetes (3 days/72 h after induction) and continued for 13 days with fasting blood glucose and weight monitored at 3 days interval. The rats were divided into seven groups (n = 5) each as illustrated below:
Group 1: Normal control rats fed with basal diet (40% skimmed milk, 46% corn starch, 4% mineral and vitamin premix, and 10% groundnut oil).
Group 2: Untreated diabetic control rats placed on basal diet.
Group 3: Diabetic control rat placed on a basal diet with a standard drug is given (metformin) orally (25 mg/kg body weight).
Group 4: Diabetic control rats placed on a basal diet supplemented with 5% C. albidum fruit pulp powder.
Group 5: Diabetic control rats placed on a basal diet supplemented with 10% C. albidum fruit pulp powder.
Group 6: Non-diabetic rats placed on a basal diet supplemented with 5% C. albidum fruit pulp powder.
Group 7: Non-diabetic rats placed on a basal diet supplemented with 10% C. albidum fruit pulp powder.
C. albidum fruit pulp powder feed formulation and doses are based on a previous study as described previously [26].
Gene expression study
RNA isolation
The animals were sacrificed by cervical dislocation as described previously [27]. Each animal’s liver and pancreatic tissues were removed and homogenized in Eppendorf tubes containing Trizol reagent (Thermofisher Scientific). The homogenate was added to a gradient separation medium (chloroform) and centrifuged for 10 min at 10,000 rpm. The supernatant was mixed with 100 l of precipitating buffer and centrifuged for 30 min at 10,000 rpm. The RNA pellet was washed three times with 70% alcohol before being treated with DNase. The concentration and purity of the RNA were measured at 260 and 280 nm after it was reconstituted in nuclease-free water. RNA was converted to cDNA using the ProtoScript first-strand cDNA synthesis kit (NEB) according to the manufacturer’s instructions.
Polymerase chain reaction (PCR)
The cDNA was amplified using the OneTaq 2x Master Mix (NEB) and the gene-specific primer sets described below in a polymerase chain reaction (Table 1). Primer Express v2.0 was used to create the primers (Applied Biosystem, Foster City, Calif). - During the experiment, actin was utilized as a control.
Table 1.
Primer sequence
| Gene | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| Nrf2 | 5’-CACATCCAGACAGACACCAGT-3’ | 5’-CTACAAATGGGAATGTCTCTGC-3’ |
| SOD | 5’-AGGGCCTGTCCCATGATGTC-3’ | 5’-AGAAACCCGTTTGCCTCTACTGAA-3’ |
| CAT | 5’-GATGGTAACTGGGACCTTGTG-3’ | 5’-GTGGGTTTCTCTTCTGGCTATG-3’ |
| GST | 5’-CAGTTCCTCCAGGACAAAGAC-3’ | 5’-GATGCATCAGCTCCGTGATAG-3’ |
| TNF-α | 5’-ACCACGCTCTTCTGTCTACTG-3’ | 5’-CTTGGTGGTTTGCTACGAC-3’ |
| DPP4 | 5’-GCAAGACGTGGGTAATGATG-3’ | 5’-AGCCTGGTTGGGTTTGTATG-3’ |
| Insulin | 5’-GTCCTCTGGGAGCCAAG-3’ | 5’-ACAGAGCCTCCACCAGG-3’ |
| β-actin | 5’-CCTCTATGCCAACACAGTGC-3’ | 5’-CATCGTACTCCTGCTTGCTG-3’ |
The expressions of nuclear factor erythroid 2-related factor 2 (Nrf2), Superoxide dismutase (SOD), Catalase (CAT), Glutathione-S-transferase (GST), and Tumour necrosis factor (TNF-α) genes were investigated in the liver. Dipeptidyl peptidase-4 (DPP4) and insulin genes expression were investigated in the pancreas of the experimental rats.
Agarose gel electrophoresis
Polymerase chain reaction amplicons were subjected to 1% agarose gel electrophoresis. Snapshots revealing the relative density of DNA bands were taken with a camera under a blue box transilluminator.
Data analysis
The intensities of the bands were quantified densitometrically using Image J software (http://imagej.en.softonic.com/). Results were expressed as mean ± standard error of mean (SEM) [28]. Statistical analysis was carried out using PRISM 5.01 (Graph pad software, Inc.) followed by one-way analysis of variance (ANOVA) and post-hoc Dunnette’s multiple range test with p < 0.05 being considered as statistically significant.
Molecular docking
Ligand preparation
Compounds previously characterized from C. albidum were sketched in SDF format using Marvin Suite and prepared for docking using LigPrep [29] module in maestro, Schrödinger suites. Low-energy 3D structures with correct chirality were generated. The possible ionization states for each ligand structure were generated at a physiological pH of 7.2 ± 0.2 and minimized using the OPLS3 force field [30].
Molecular docking study
The receptor grid was created around the binding site of the insulin receptor (PDB ID: 3EKK) using the “receptor grid generation” option in the glide-v7.5 programme of Maestro-v11.5. Afterward, the prepared ligands were docked into the receptor grid using the extra precision (XP) [28, 31] workflow module of the Schrödinger suite with default parameters. The virtual Screening Workflow in Maestro was used to dock and score C. albidum derived compounds.
Results
Effect of CAFPP on the fasting blood glucose and weight patterns
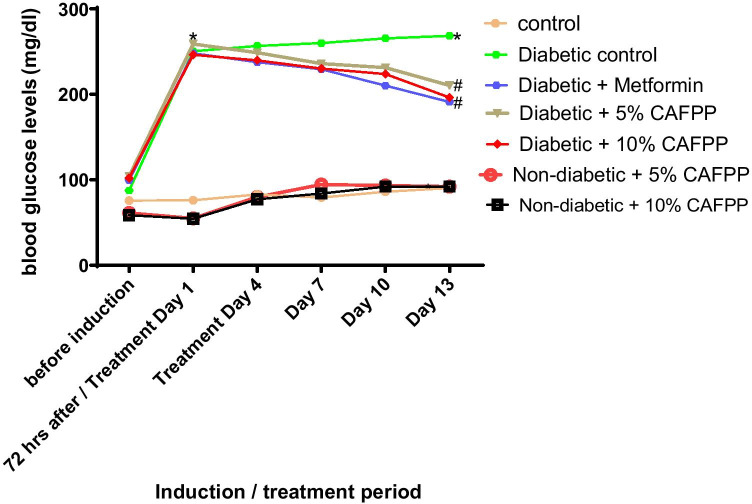
The result in Fig. 1 shows the effect of dietary C. albidum fruit pulp powder (CAFPP) on blood glucose levels in type-2 diabetic rats (mg/dl). Diabetes induction by streptozotocin significantly elevated blood in the induced group relative to non-diabetic control (Fig. 2, p < 0.05). By day 13, CAFPP significantly (p < 0.05) reduced blood glucose concentration in all treated diabetic groups when compared with the diabetic control group. There was weight loss seen in diabetic control relative to non-diabetic control after thirteen days (Table 2). The diabetic group fed with CAFPP (5 and 10%) fed rats gained weight compared to diabetic control at the same duration of treatment.
Fig. 1.
Effect of dietary C. albidum Fruit Pulp Powder (CAFPP) on blood glucose level (mg/dl) in diabetic rats. *indicates statistical difference (p < 0.05) to non-diabetic control while # indicates statistical difference (p < 0.05) to diabetic control
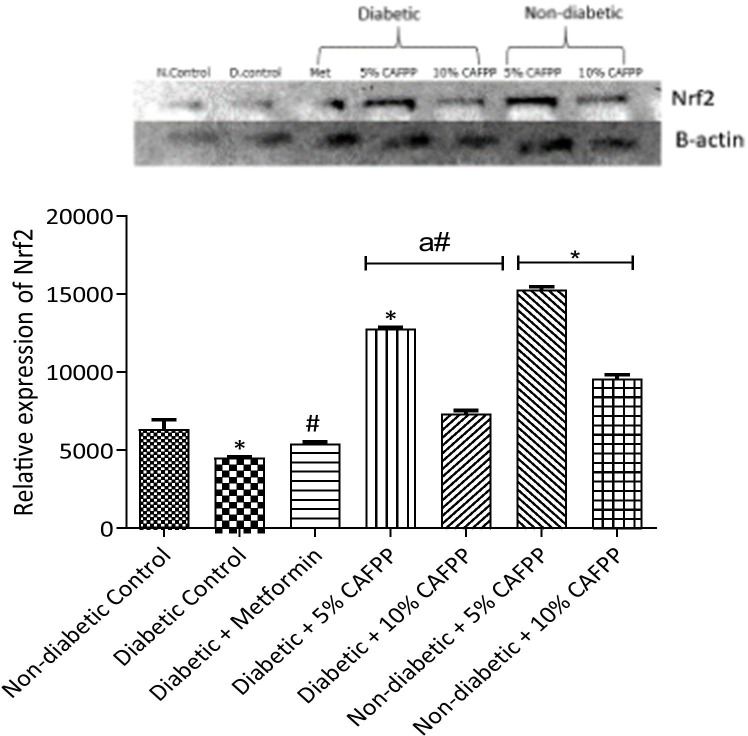
Fig. 2.
Qualitative-PCR analysis of Nuclear factor erythroid 2-related factor 2 (Nrf2) expression in the Liver of rats from different groups. Snapshot representation of RT-PCR agarose gel electrophoresis for Nrf2 gene followed by densitometric analysis. *indicates statistical difference (p < 0.05) to non-diabetic control, # indicates statistical difference (p < 0.05) to diabetic control and ‘a’ indicates statistical difference (p < 0.05) to diabetic + metformin
Table 2.
Average weight (g) of Type 2 Diabetic Rats Fed Chrysophyllum albidum Fruits Pulp Powder Diets
| Groups | Initial Weight (g) |
Final Weight (g) |
Weight Gain/loss (g) |
|---|---|---|---|
| Non-diabetic Control | 219.17 ± 5.31 | 225.48 ± 6.86 | 6.31 |
| Diabetic Control | 228.53 ± 5.57 | 224.87 ± 5.25 | −3.66 |
| Diabetic + Metformin | 217.68 ± 7.23 | 222.07 ± 5.49 | 4.39 |
| Diabetic +5% CAFPP | 227.18 ± 8.90 | 230.72 ± 8.29 | 3.54 |
| Diabetic +10% CAFPP | 219.65 ± 5.07 | 223.75 ± 5.71 | 4.1 |
| Non-diabetic +5% CAFPP | 215.02 ± 6.75 | 221.01 ± 5.02 | 5.99 |
| Non-diabetic +10% CAFPP | 219.17 ± 5.31 | 225.48 ± 6.86 | 6.31 |
Keys: STZ streptozotocin, CAFPP C. albidum fruits pulp powder
Effect of CAFPP on Nrf2 signaling and other endogenous antioxidant enzymes in the liver
This study revealed a significant down-regulation of nuclear factor erythroid-derived factor 2-related factor 2 (Nrf2) antioxidant gene in the liver of diabetic control relative to normal control rats (Fig. 2, p < 0.05). This points to the dysregulation of the Nrf2 signaling pathway in diabetic rats. C. albidum fruit pulp powder diet inclusion was able to significantly up-regulate the expression of Nrf2 in diabetic rats compared with diabetic control (Fig. 2, p < 0.05). The inclusion of C. albidum fruit pulp powder in the diets of the rats at varying percentages significantly increased the expression of catalase, GST, and SOD genes in the non-diabetic and diabetic state when compared to both normal control and diabetic control groups respectively (Figs. 3, 4 and 5, p < 0.05). The expression of GST in diabetic control was significantly repressed relative to the normal control (Fig. 4, p < 0.05). This could be a pointer of oxidative stress in the diabetic state.
Fig. 3.
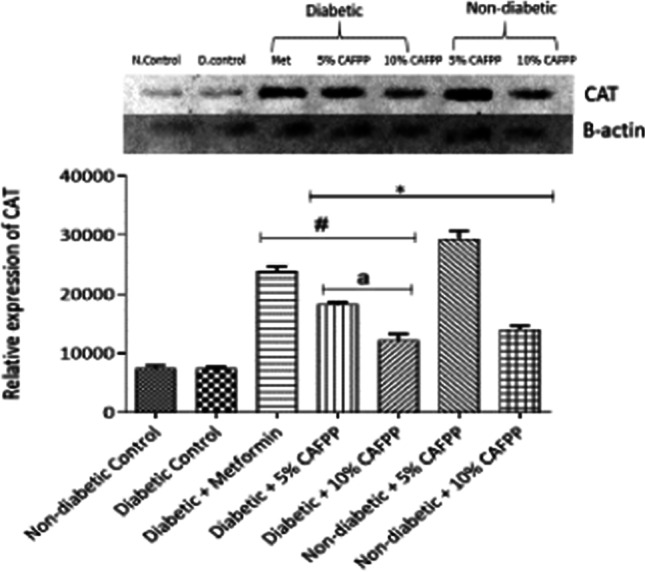
Qualitative-PCR analysis of catalase (CAT) gene expression in the Liver of rats from different groups. Snapshot representation of RT-PCR agarose gel electrophoresis for CAT gene followed by densitometric analysis. *indicates statistical difference (p < 0.05) to non-diabetic control, # indicates statistical difference (p < 0.05) to diabetic control and ‘a’ indicates statistical difference (p < 0.05) to diabetic + metformin
Fig. 4.
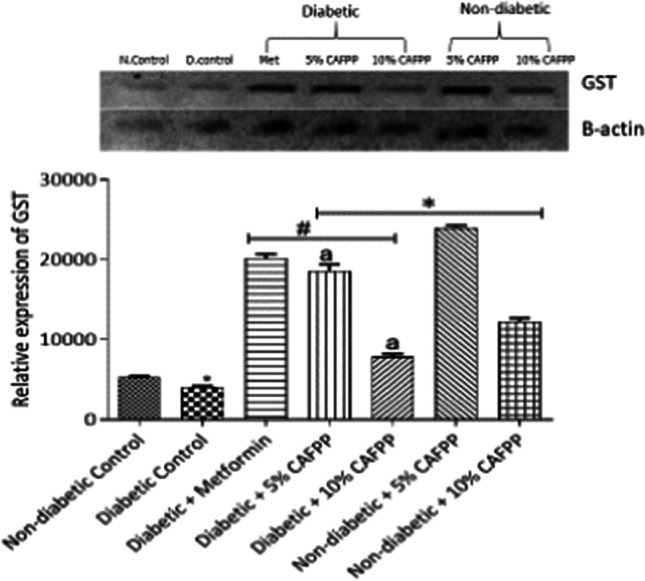
Qualitative-PCR analysis of glutathione-S-transferase (GST) gene expression in the Liver of rats from different groups. Snapshot representation of RT-PCR agarose gel electrophoresis for GST gene followed by densitometric analysis. *indicates statistical difference (p < 0.05) to non-diabetic control, # indicates statistical difference (p < 0.05) to diabetic control and ‘a’ indicates statistical difference (p < 0.05) to diabetic + metformin
Fig. 5.
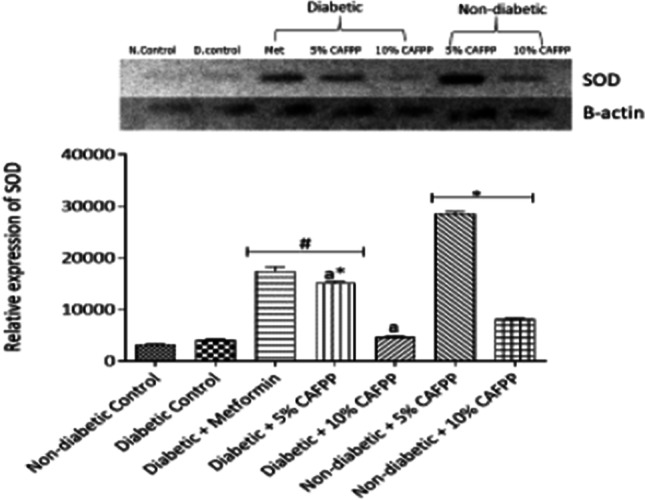
Qualitative-PCR analysis of superoxide dismutase (SOD) gene expression in the Liver of rats from different groups. Snapshot representation of RT-PCR agarose gel electrophoresis for SOD gene followed by densitometric analysis. *indicates statistical difference (p < 0.05) to non-diabetic control, # indicates statistical difference (p < 0.05) to diabetic control and ‘a’ indicates statistical difference (p < 0.05) to diabetic + metformin
Effect of CAFPP on the expression of tumour necrosis factor (TNF-α) in the liver
The expression of TNF-α in the liver was significantly up-regulated in the diabetic control relative to control (Fig. 6, p < 0.05). CAFPP (10%) inclusion in diet significantly down-regulated TNF-α expression in diabetic and non-diabetic rats when compared with diabetic control (Fig. 6, p < 0.05). This revealed the possible potential of CAFPP in combating diabetes-induced inflammation.
Fig. 6.
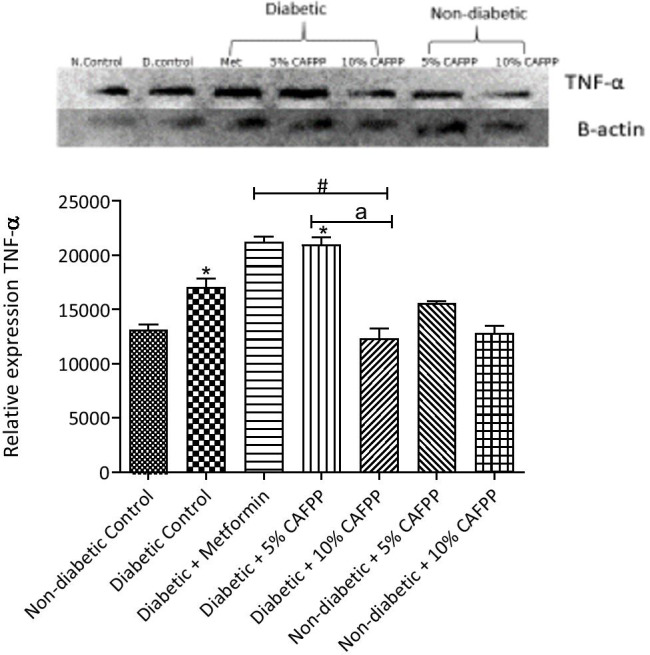
Qualitative-PCR analysis of tumour necrosis factor-alpha (TNF-α) gene expression in the Liver of rats from different groups. Snapshot representation of RT-PCR agarose gel electrophoresis for TNF-α gene followed by densitometric analysis. *indicates statistical difference (p < 0.05) to non-diabetic control, # indicates statistical difference (p < 0.05) to diabetic control and ‘a’ indicates statistical difference (p < 0.05) to diabetic + metformin
Effect of CAFPP on the expression of genes associated with glucose metabolism in the pancreas
Insulin expression was significantly up-regulated by dietary inclusion of C. albidum fruit pulp powder (CAFPP) in the pancreas of diabetic (10%) and non-diabetic rats (5%) relative to the diabetic control (Fig. 7, p < 0.05). This means CAFPP could enhance insulin secretion in both diabetic and non-diabetic states.
Fig. 7.
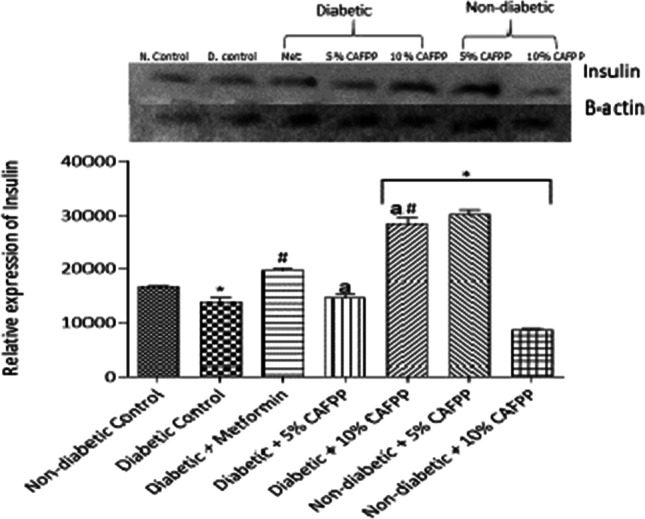
Qualitative-PCR analysis of insulin gene expression in the pancreas of rats from different groups. Snapshot representation of RT-PCR agarose gel electrophoresis for insulin gene followed by densitometric analysis. *indicates statistical difference (p < 0.05) to non-diabetic control, # indicates statistical difference (p < 0.05) to diabetic control and ‘a’ indicates statistical difference (p < 0.05) to diabetic + metformin
Dipeptidyl peptidase-4 (DPP-4) expression was significantly up-regulated in the diabetic control and metformin-treated groups in comparison to the non-diabetic control (Fig. 8, p < 0.05) which is an indication of disturbance in glucose metabolism. This abnormality was however significantly managed by 5% CAFPP inclusion in diabetic rats’ diet as evidenced by the down-regulation of DPP-4 expression relative to diabetic control (Fig. 8, p < 0.05).
Fig. 8.
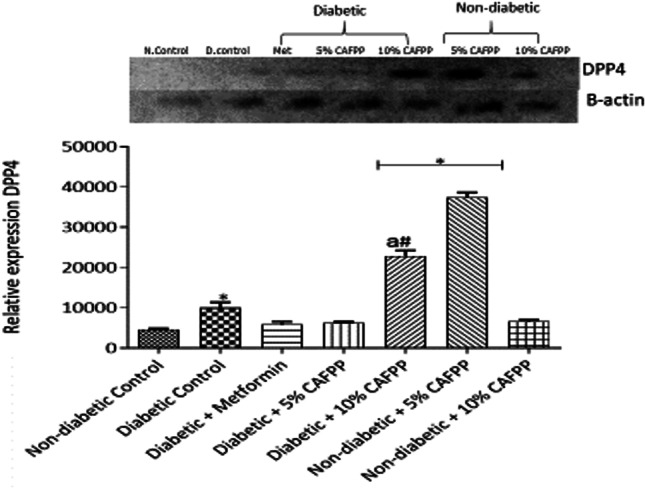
Qualitative-PCR analysis of dipeptidyl peptidase-4 (DPP4) gene expression in the pancreas of rats from different groups. Snapshot representation of RT-PCR agarose gel electrophoresis for DPP4 gene followed by densitometric analysis. *indicates statistical difference (p < 0.05) to non-diabetic control, # indicates statistical difference (p < 0.05) to diabetic control and ‘a’ indicates statistical difference (p < 0.05) to diabetic + metformin
Effect of CAFPP on glucose level and weight of STZ-induced diabetic and non-diabetic rats
Effect of dietary administration of C. albidum fruit pulp powder (CAFPP) on the expression of antioxidant genes in the liver of STZ-induced diabetic and non-diabetic rats
Effect of dietary administration of C. albidum fruit pulp powder (CAFPP) on the expression of tumour necrosis factor (TNF-α) in the liver of STZ-induced diabetic and non-diabetic rats
Effect of dietary administration of C. albidum fruit pulp powder (CAFPP) on the expression of genes associated with glucose metabolism in the pancreas of STZ-induced diabetic and non-diabetic rats
Docking results of Chrysophyllum albidum fruit pulp powder (CAFPP) - derived compounds as potential antidiabetics
Molecular docking simulation of compounds previously characterized from C. albidum fruit pulp powder (CAFPP) (Fig. 3 of supplementary material) with anti-diabetic targets insulin revealed that they are potent ligands of this target (Table 3) [19, 24, 25]. Epigallocatechin and Epicatechin ranked highest with binding affinities of −8.519 Kcal/mol and − 7.774 Kcal/mol respectively when docked with the 3D structure of insulin. Panel A of Figs. 4, 5, 6, 7, 8, 9 and 10 in the supplementary material showed the binding pose and binding site of the phytochemicals with insulin receptor while Panel B showed the molecular interaction of the phytochemicals with amino acid residues within the binding pocket of the protein structure.
Table 3.
Binding affinity, H- bonding, and hydrophobic interaction of C. albidum fruit-derived compounds with insulin
| No | Phytochemical | Hydrogen bonding | Binding energy (KCAL/MOL) | Hydrophobic interaction |
|---|---|---|---|---|
| 1 | Epigallocatechin | 2 (LEU 1002, ASP 1083) | −8.519 | 7 (MET 1079, LEU 1078, MET 1076, ALA 1028, MET 1139, VAL 1060, VAL 1010) |
| 2 | Epicatechin | 2(ASP 1083, ASP 1050) | −7.774 | 6 (LEU 1078, MET 1079, ALA 1080, LEU 1002, VAL 1010, MET 1139) |
| 3 | 1,1-Octadecanoic acid methyl ester | −6.427 | 9 (ALA 1080, MET 1079, LEU 1078, MET 1076, ALA 1028, LEU 1002, VAL 1010, VAL 1060, MET 1139) | |
| 4 | Octadecanoic acid,methylester | 2(MET 1079, GLU 1077) | −6.136 | 8 (LEU 1002, MET 1079, LEU 1078, MET 1076, MET 1139, ALA 1028, VAL 1060, VAL 1010) |
| 5 | Sulfurous acid Octadecyl-2-propyl ester | −6.036 | 7 (ALA 1028, VAL 1010, ALA 1080, MET 1079, LEU 1002, TYR 1087, MET 1139) | |
| 6 | 9,12-Octadecadienoic acid methylester | 1 (SER 1086) | −5.918 | 7 (VAL 1010, ALA 1028, LEU 1082, ALA 1080, MET 1079, LEU 1078, MET 1139) |
| 7 | Stigmasterol | −5.377 | 8 (MET 1079, LEU 1078, MET 1076, LEU 1002, ALA 1028, MET 1139, VAL 1060, VAL 1010) |
The table above shows the binding affinities of the listed previously characterized CAFPP phytochemicals with Insulin. It also shows the amino acids involved in hydrogen bonding and hydrophobic interaction of these phytochemicals with insulin
Discussion
Dietary modification is and could be an important factor in the prevention and management of chronic metabolic diseases such as diabetes. Regular consumption of diets rich in fruits and vegetables has been linked to a lowered risk of diabetes and other metabolic or lifestyle diseases due to the presence of different bioactive compounds [32]. C. albidum fruit parts have been reported in several studies and folklore to possess anti-oxidative and anti-hyperglycemic properties but there is a dearth of information on the mechanisms involved [24, 26, 33]. Hyperglycaemia is a major hallmark of diabetes mellitus (DM), thus many antidiabetic drug kinds of research and diet therapies are targeted at glycaemic control and oxidative stress reduction as these decrease the risk and complications associated with DM [34, 35].
In this study, type 2 diabetes mellitus was induced by a combination of a high-fat diet (HFD) and a low dose of streptozotocin (STZ) (35 mg/kg) after which the animals were subjected to different treatment regimens including CAFPP-supplemented diets (5 and 10%). The blood glucose of rats administered HFD and STZ was significantly increased compared to the non-diabetic control (Fig. 1). The high blood glucose could be a result of the decrease in the pancreas beta-cells mass caused by STZ. This mechanism of beta-cell destruction could be by a continuous cycle of oxidative stress caused by STZ. This is supported by the work of [28] who reported the cytotoxic effect of STZ on beta-cells. Metformin and CAFPP-diet (5 and 10%) significantly reduced the fasting blood glucose of STZ-induced diabetic rats when compared with the untreated diabetic group after 13 days of treatment (Fig. 1). This suggests that CAFPP possesses anti-hyperglycemic properties which can be attributed to the presence of polyphenols and support what was previously reported to be present in the pulp by [19]. Other parts of C. albidum such as the seed and leaf extracts have also been reported to lower blood glucose levels in diabetic rats [22]. This study is further in line with reports of fruit-rich diets suggested playing a defensive role in glycemic control in diabetes [14, 35, 36]. There was no significant difference between the blood glucose levels of non-diabetic control and non-diabetic rats fed with a 5% and 10% CAFPP-supplemented diet. This suggests that CAFPP is capable of maintaining euglycemia in non-diabetic or normal physiology states.
Untreated diabetic rats experienced weight loss compared to the non-diabetic control while there was no major difference in the final and initial weight measurements of the other groups treated with metformin and CAFPP –diets compared to the non-diabetic control group. Significant weight loss in rats fed with a high-fat diet before induction of type 2 diabetes with STZ has been reported by [37]. The weight loss in untreated diabetic rats (Group 2) indicates a disruption in the glucose control mechanism. Diabetes-induced Oxidative stress generates free radicals like reactive oxygen species which react with unsaturated fatty acids in the cell membrane compromising the membrane integrity. This could lead to the death of cells and apoptosis of body cells could progress to weight loss. This is supported by the work of [10].
Uncontrolled hyperglycemia promotes auto-oxidation of glucose to form free radicals which ultimately leads to oxidative stress when the generation of free radicals exceeds the scavenging abilities of endogenous antioxidant defenses [38]. Oxidative stress contributes to the oxidative modification of cellular proteins which consequentially leads to pancreatic β-cell dysfunction and insulin resistance [6]. Nuclear factor erythroid-derived factor 2-related factor 2 (Nrf2) serves as a master regulator in inducing the expression of multiple antioxidant enzymes in response to oxidative imbalance [39, 40]. The expression of nuclear factor erythroid-derived factor 2-related factor 2 (Nrf2) gene in the liver of diabetic rats was significantly down-regulated relative to non-diabetic control in the study (Fig. 2). The repression of this antioxidant signaling gene could result in oxidative stress or damage resulting in some disorders and complications seen in diabetic cases; this is supported by the work of [7]. Endogenous antioxidants have also been reported to be significantly reduced in diabetes, thus exacerbating complications associated with diabetes mellitus [40]. Nrf2 expression was significantly up-regulated in diabetic and non-diabetic groups fed with 5% and 10% CAFPP-supplemented diets relative to diabetic control and non-diabetic control groups respectively. This suggests that CAFPP boosts antioxidant production, enhance body defense mechanisms in both diabetic and healthy state. Activation of Nrf2 in maintaining oxidative balance has also been reported [28, 41]. This showed that the fruit may exhibit the potentials to neutralize and prevent oxidative stress.
Catalase (CAT), superoxide dismutase (SOD), and glutathione S-transferase (GST) are endogenous antioxidant enzymes capable of mopping up free radicals. They all can also be activated by Nrf2 signaling directly or indirectly. There was significant repression in GST in diabetic control relative to non-diabetic control. This suppressed expression and activity of this enzyme could be caused by increased free radical production and oxidative stress caused by glucose auto-oxidation in hyperglycemic conditions [5]. Reduced antioxidant enzymes activities in diabetic states have also been reported [11]. In this study, all the three enzymes investigated were significantly up-regulated in the CAFPP-fed diabetic group relative to diabetic control (Figs. 3, 4 and 5). This shows that CAFPP could hence be capable of resuscitating and enhancing the body’s defense or immune system against oxidative stress by promoting antioxidants function. CAFPP (5 and 10%) also significantly up-regulated the expression of the three enzymes in the non-diabetic group relative to the non-diabetic control group (Figs. 3, 4 and 5). This further suggests that this fruit is capable of boosting the antioxidants system in normal physiological conditions. This supports the work of scientists who have reported the antioxidant potentials of C. albidum [18, 35].
Tumour necrosis factor-alpha (TNF-α), a pro-inflammatory cytokine gene was significantly up-regulated in diabetic control rats when compared with non-diabetic control rats (Fig. 6). Chronic hyperglycemia induces inflammation, which increases the production of cytokines, eventually leading to pancreatic dysfunction (destruction of beta-cells). Upregulation of pro-inflammatory cytokines in pathological states has also been reported [42]. CAFPP (10%) significantly down-regulated TNF-α gene expression in the diabetic state relative to diabetic control and the diabetic group treated with metformin. This suggests that CAFPP is capable of reducing hyperglycemia-induced inflammation and is a better anti-inflammatory agent compared to metformin (a standard drug) in diabetic conditions.
Insulin is a peptide hormone made by the pancreas’ beta-cells. It helps in glucose metabolism by allowing the entry of blood glucose into cells for fuel or storage [43]. The expression of insulin in the pancreas as shown in Fig. 7 decreased significantly in diabetic control rats compared to non-diabetic control. The reduction in insulin expression in diabetic control rats could be due to pancreatic β-cell dysfunction associated with diabetic mellitus. Insulin secretion is down-regulated in diabetic states could also be due to a variety of metabolic complications. Several studies have also shown that type 2 diabetes mellitus is associated with a reduction in insulin secretion among other factors [34, 44]. In this study, a significant up-regulation in expression of insulin gene was observed on treatment with metformin and inclusion of 10% of CAFFP in the diet of diabetic animals compared to diabetic control. Metformin which is a standard antidiabetic drug significantly mitigated this dysfunction as evidenced by the up-regulation of insulin secretion in the group treated with metformin. Metformin has been revealed to up-regulate insulin gene expression [45]. CAFPP (10%) in the diet of diabetic rats significantly up-regulated insulin secretion compared with standard drug-metformin suggesting CAFPP is a better antidiabetic agent at that dose. Dietary supplementation with 5% CAFPP significantly up-regulated insulin gene expression relative to non-diabetic control while 10% CAFPP did not. These findings suggest that moderate intake of the fruit in a healthy state maintains and ensure normoglycemia while a higher dose is needed to enhance insulin function in the diabetic state.
Dipeptidyl peptidase-4 (DPP-4) exists as a cell surface membrane-bound peptidase which is ubiquitously expressed in many tissues of the body including the pancreas. It inhibits insulin secretion [46]. In this study (Fig. 8), DPP-4 expression in diabetic control rats was significantly up-regulated in comparison with non-diabetic control rats thus suggesting reduced insulin sensitivity and secretion leading to elevated blood glucose as observed in this study. A similar result has been reported [47]. The expression of the DPP-4 gene was significantly down-regulated in CAFPP (5%) fed rats relative to diabetic control. This suggested that CAFPP potentially enhances insulin secretion. This is corroborated by other studies [48, 49].
Numerous sphytocompounds have been characterized from C. albidum fruit pulp (Fig. 3 of supplementary material) [9, 24, 25]. To identify the possible bioactive compound(s) responsible for the anti-oxidative and antidiabetic effect of C. albidum, the binding modes of C. albidum derived compounds to the three-dimensional structure of insulin receptor were investigated by molecular docking experiment. The docking results (Table 3) showed that epigallocatechin (EGC) and epicatechin (EC) ranked highest with binding affinities of −8.519 Kcal/mol and − 7.774 Kcal/mol respectively when docked with the insulin receptor. The results of this in silico study suggest that epigallocatechin and epicatechin may be the major anti-diabetic compounds in C. albidum (Figs. 4 and 5; supplementary material). During the docking simulation of epigallocatechin with the receptor, 2 residues (LEU 1002, ASP 1083) within the active site were responsible for H-bond formulation while 7 residues (MET 1079, LEU 1078, MET 1076, ALA 1028, MET 1139, VAL 1060, VAL 1010) contributed to the hydrophobic interactions of epigallocatechin with insulin (Fig. 4 of supplementary material). The hydrogen bonding interaction of epicatechin with insulin was enhanced by 2 residues (ASP 1083, ASP 1050) at the active site. Six residues (LEU 1078, MET 1079, ALA 1080, LEU 1002, VAL 1010, MET 1139) were responsible for its hydrophobic interactions with insulin (Table 3).
Epicatechin (EC) and epigallocatechin (EGC) are flavonoids that are polyphenolic phytochemical compounds [50]. Their structures contain double bonds and hydroxyl groups which help in scavenging free radicals which have been implicated in the pathogenesis of type 2 diabetes mellitus. The ability of the hydroxyl groups in the structure of EC and EGC to mop up free radicals reduces oxidative stress, inhibits pancreatic beta-cell death, and improves insulin production. This is supported by the work of [51] who reported that EC boosts the activity of antioxidant enzymes. EGC has also been reported to alleviate oxidative stress by increasing the level of CAT and SOD [52, 53]. This could be the reason for CAFPP’s potential to exhibit anti-oxidative, anti-inflammatory, and anti-diabetic actions as shown in this study. Hence, C. albidum fruit pulp-derived compounds improve insulin status and function, as evidenced by the significant up-regulation of its gene expression upon CAFPP inclusion in both diabetic (10%) and non-diabetic (5%) groups compared to diabetic control and non-diabetic control rats respectively. The molecular interactions of other phytocompounds from C. albidum with insulin are presented in Figs. 4, 5, 6, 7, 8, 9 and 10 in the supplementary material.
Conclusion
This study suggests that C. albidum fruit pulp powder (CAFPP) regulates oxidative stress-induced diabetic state through the activation and increased expression of Nrf2, leading to enhanced antioxidant enzymes production to combat the cytotoxic effects of free radicals induced by hyperglycemia. The work also suggests that CAFPP is effective in the management of diabetes-induced oxidative stress. C. albidum fruit pulp shows promising potentials in reducing hepatic inflammation caused by DM by down-regulating the expression of TNF-α thereby protecting the liver from oxidative damage. This study further revealed that CAFPP phytocompounds effectively bind to insulin receptors and thus act as insulin receptor agonists resulting in up-regulation of insulin expression. This confirms C. albidum use in folklore as an antidiabetic agent. Further studies are required to investigate the most effective CAFPP dosage in animals and human subjects.
Recommendation
The oral consumption of foods and fruits rich in antioxidants such as C. albidum (African Star Apple) is highly encouraged and recommended as a means of diabetes management (pathological state) and to improve antioxidants status in physiological state.
Supplementary Information
(DOCX 11969 kb)
Abbreviations
- CAFPP
C. albidum fruit pulp powder
- STZ
Streptozotocin
- DM
Diabetes mellitus
- Nrf2
Nuclear factor erythroid 2-related factor 2
- SOD
Superoxide dismutase
- CAT
Catalase
- GST
Glutathione-S-transferase
- TNF-α
Tumour necrosis factor-alpha
- DPP4
Dipeptidyl peptidase-4
- RT-PCR
Reverse transcriptase-polymerase chain reaction
Authors’ contributions
This work was carried out in collaboration between all authors. Authors FLO and SFA designed the study and supervised the work. Authors IAO, FOJ, and OOE did the laboratories work. OOE provided laboratory facilities. Authors MOA and FOJ drafted the manuscript and performed the statistical analysis. Authors OOE and FLO edited the manuscript.
Funding
Not applicable. No funding was received for this work.
Data availability
The datasets generated and/or analyzed in this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
The experimental protocol was approved by the Ethical Committee of Ekiti State University, Ado-Ekiti.
Consent for publication
Not applicable.
Competing interests
The authors hereby declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elekofehinti OO, Ariyo EO, Akinjiyan MO, Olayeriju OS, Lawal AO, Adanlowo IG, Rocha JBT. Potential use of bitter melon (Momordica charantia) derived compounds as antidiabetics: in silico and in vivo studies. Pathophysiology. 2018;945:1–7. doi: 10.1016/j.pathophys.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Talib WH, Mahmod AI, Abuarab SF, Hasen E, Munaim AA, Haif SK, Kury LTA. Diabetes and Cancer: metabolic association, therapeutic challenges, and the role of natural products. Molecules. 2021;26(8):2179. doi: 10.3390/molecules26082179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.Alamri A Sr, Alharbi K, Hassan K, Alhakami S, Alosaimi M, Rofidi K, Ahmed I. Frequency of neuropathic sensory symptoms among patients with uncontrolled diabetes mellitus in security forces hospital, Riyadh, Saudi Arabia. Cureus. 2021;13(8):e17528. [DOI] [PMC free article] [PubMed]
- 5.Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerf MEBC. Dysfunction and insulin resistance. Front Endocrinol. 2013;4:37. doi: 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia, and type 2 diabetes mellitus. World J Diabetes. 2015;6:456–480. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarangarajan R, Meera S, Rukkumani R, Sankar P, Anuradha G. Antioxidants: friend or foe? Asian Pac J Trop Med. 2017;10(12):1111–1116. doi: 10.1016/j.apjtm.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Cai Z, Yuan S, Zhong Y, Deng L, Li J, Tan X, Feng J. Amelioration of endothelial dysfunction in diabetes: role of Takeda G protein-coupled receptor 5. Front Pharmacol. 2021;12:637051. doi: 10.3389/fphar.2021.637051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-a concise review. Saudi Pharm J. 2016;24(5):547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh YS, Jun H-S. Effects of glucagon-like Peptide-1 on oxidative stress and Nrf2 signaling. Int J Mol Sci. 2018;19(1):26. doi: 10.3390/ijms19010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nahdi AT, Arwa MT, John A, Raza H. Elucidation of molecular mechanisms of Streptozotocin-induced oxidative stress, apoptosis, and mitochondrial dysfunction in Rin-5F pancreatic β-cells. Oxidative Med Cell Longevity. 2017;20:15. doi: 10.1155/2017/7054272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolman KG, Fonseca V, Dalpiaz A, Tan MH. The spectrum of liver disease in type 2 diabetes and Management of Patients with diabetes and liver disease. Diabetes Care. 2007;30(3):734–743. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 14.Tiwari N, Thakur AK, Kumar V, Dey A, Kumar V. Therapeutic targets for diabetes mellitus: an update. Clin Pharmacol Biopharm. 2014;3:117. [Google Scholar]
- 15.Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: a review on recent drug-based therapeutics. Biomed Pharmacother. 2020;131:110708. doi: 10.1016/j.biopha.2020.110708. [DOI] [PubMed] [Google Scholar]
- 16.Ahn K. The worldwide trend of using botanical drugs and strategies for developing global drugs. BMB Rep. 2017;50(3):111–116. doi: 10.5483/BMBRep.2017.50.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee N, Yoo H, Yang H. Cluster analysis of medicinal plants and targets based on multipartite network. Biomolecules. 2021;11(4):546. doi: 10.3390/biom11040546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyetayo FL, Akomolafe SF, Balogun GB. Effects of Chrysophyllum albidum fruit pulp on hemodynamic parameters, pro-inflammatory markers, antioxidant parameters, and critical biomolecules associated with hypertension-in vivo. Inflammopharmacology. 2021;29(3):825–839. doi: 10.1007/s10787-021-00830-x. [DOI] [PubMed] [Google Scholar]
- 19.Ajayi OB, Oyetayo FL, Akomolafe SF. Starch composition, glycemic indices, antioxidant properties, and carbohydrate hydrolyzing enzymes activities of African star apple fruit parts. BMC Complement Med Ther. 2020;20:260. doi: 10.1186/s12906-020-03053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Southon S. Increased fruit and vegetable consumption within the EU: potential health benefits. Food Res Int. 2000;33:211–217. [Google Scholar]
- 21.Emudainohwo JO, Erhirhie EO, Moke EG, Edje KE. A comprehensive review on ethno-medicine, Phytochemistry and Ethnopharmacology of Chrysophyllum albidum. JAMPS. 2015;3(4):147–154. [Google Scholar]
- 22.Engwa AG, Marcellus U, Aniakor UE, Osuji AG, Agbafor NK, Olayinka AA, Okoh A. Antioxidant and antidiabetic activities of the seed and leaf extracts of Chrysophyllum albidum. Asian Pacific J Trop Dis. 2016;6(8):642–649. [Google Scholar]
- 23.Staurengo-Ferrari L, Badaro-Garcia S, Hohmann MSN, Manchope MF, Zaninelli TH, Casagrande R, Verri WAJ. Contribution of Nrf2 modulation to the mechanism of action of analgesic and anti-inflammatory drugs in pre-clinical and clinical stages. Front Pharmacol. 2019;9:1536. doi: 10.3389/fphar.2018.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Idowu TO, Ogundaini AO, Adesanya SA, Onawunmi GO, Osungunna MO, Obuotor EM, Abegaz BM. Isolation and characterization of chemical constituents from Chrysophyllum albidum G. Don-Holl. Stem-bark extracts and their antioxidant andantibacterial properties. Afr J Tradit Complement Altern Med. 2016;13(5):182–189. doi: 10.21010/ajtcam.v13i5.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harley BK, Dickson RA, Amponsah IK, Ngala RA, Berkoh D, Fleischer TC. Antidiabetic effect of Chrysophyllum albidum is mediated by enzyme inhibition and enhancement of glucose uptake via 3T3-L1 adipocytes and C2C12 myotubes. Asian Pac J Trop Biomed. 2020;10:387–396. [Google Scholar]
- 26.Oyetayo FL, Akomolafe SF, Odeniyi IA. Effects of dietary supplementation of Chrysophyllum albidum fruit pulp powder on some biochemical parameters in a type 2 diabetes rat model. Vegetos. 2019;32:190–199. [Google Scholar]
- 27.Elekofehinti OO, Lawal AO, Ejelonu OC, Molehin OR, Famusiwa CD. Involvement of fat mass and obesity gene (FTO) in the anti-obesity action of Annona muricata Annonaceae: in silico and in vivo studies. J Diabetes Metabol Disord. 2020;19:197–204. doi: 10.1007/s40200-020-00491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elekofehinti OO, Onunkun AT, Olaleye MT. Cymbopogon citratus (DC) Stapf mitigates ER-stress induced by streptozotocin in rats via down-regulation of GRP78 and up-regulation of NRF2 signaling. J Ethnopharmacol. 2020;262:113130. doi: 10.1016/j.jep.2020.113130. [DOI] [PubMed] [Google Scholar]
- 29.Lam PC, Abagyan R, Totrov M. Ligand-biased ensemble receptor docking (LigBEnD): a hybrid ligand/receptor structure-based approach. J Comput Aided Mol Des. 2018;32(1):187–198. doi: 10.1007/s10822-017-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwaloye O, Elekofehinti OO, Kikiowo B, Fadipe TM, Akinjiyan MO, Ariyo EO, Aiyeku OO, Adewumi NA. Discovery of traditional Chinese medicine derived compounds as wild type and mutant plasmodium falciparum Dihydrofolate reductase inhibitors: induced fit docking and ADME studies. Curr Drug Discov Technol. 2021;18(4):554–569. doi: 10.2174/1570163817999200729122753. [DOI] [PubMed] [Google Scholar]
- 31.Arowosegbe MA, Amusan OT, Adeola SA, Adu OB, Akinola IA, Ogungbe BF, Omotuyi OI, Saibu GM, Ogunleye AJ, Kanmodi RI, Lugbe NE, Ogunmola OJ, Ajayi DC, Ogun SO, Oyende FO, Bello AO, Ishola PG, Obasieke PE. Kaempferol as a potential PAK4 inhibitor in triple-negative breast Cancer: extra precision glide docking and free energy calculation. Curr Drug Discov Technol. 2020;17(5):682–695. doi: 10.2174/1570163816666190823135948. [DOI] [PubMed] [Google Scholar]
- 32.Ibrahim HO, Osilesi O, Adebawo OO, Onajobi FD, Karigidi KO, Muhammad LB. Nutrients compositions and phytochemical contents of edible parts of Chrysophyllum albidum fruit. J Nutr Food Sci. 2017;7:1–9. [Google Scholar]
- 33.Oboh G, Adebayo AA, Ejakpovi II, Ogunsuyi OB, Boligon AA. Phenolic profiling and in vitro antioxidant, anticholinesterase, and antimonoamine oxidase properties of aqueous extract of African star apple (Chrysophyllum albidum) fruit parts. J Food Biochem. 2018;42(4):e12568. [Google Scholar]
- 34.Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66:241–255. doi: 10.2337/db16-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ajayi AM, Adedapo ADA, Badaki VB, Oyagbemi AA, Adedapo AA. Chrysophyllum albidum fruit ethanol extract ameliorates hyperglycaemia and elevated blood pressure in streptozotocin-induced diabetic rats through modulation of oxidative stress, NF-κB and PPAR-γ. Biomed Pharmacother. 2021;141:111879. doi: 10.1016/j.biopha.2021.111879. [DOI] [PubMed] [Google Scholar]
- 36.Akomolafe SF, Odeniyi IA, Oyetayo FL, Ajayi OB. African star apple fruit pulp-supplemented diet modulates fertility-related biomolecules in the testis and epididymis of high-fat diet/streptozotocin-induced diabetic rats. J Food Biochem. 2019;43(9):e12969. doi: 10.1111/jfbc.12969. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramaro P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type-2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Ruhe RC, McDonald RB. Use of antioxidant nutrients in the prevention and treatment of type 2 diabetes. J Am Coll Nutr. 2001;20:363S–3639. doi: 10.1080/07315724.2001.10719169. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzym Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Fu J, Hou Y, Xue P, Wang H, Xu Y, Qu W, Zhang Q, Pi J. Nrf2 in type 2 diabetes and diabetic complications: yin and Yang. Curr Opinion Toxicol. 2016;1:9–19. [Google Scholar]
- 41.Maher J, Yamamoto M. The rise of antioxidant signaling–the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2011;244(1):4–15. doi: 10.1016/j.taap.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Ahrens B. Antibodies in metabolic diseases. New Biotechnol. 2011;28(5):530–537. doi: 10.1016/j.nbt.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Elekofehinti OO, Akinjiyan MO. Effects of Momordica charantia Silver nanoparticles on genes associated with lipid metabolism and nephrotoxicity in Streptozotocin-induced diabetic rats. Nig J Biotech. 2020;37(2):126–133. [Google Scholar]
- 44.Al-Zubairi AS, Eid MEE. Molecular targets in the development of antidiabetic drugs. Int J Pharmacol. 2010;6(6):784–795. [Google Scholar]
- 45.Hashemitabar M, Bahramzadeh S, Saremy S, Nejaddehbashi F. Glucose plus metformin compared with glucose alone on beta-cell function in mouse pancreatic islets. Biomed Rep. 2015;3:721–725. doi: 10.3892/br.2015.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omar BA, Liehua L, Yamada Y, Seino Y, Marchetti P, Ahren B. Dipeptidyl peptidase-4 (DPP-4) is expressed in mouse and human islets and its activity is decreased in human islets from individuals with type 2 diabetes. Diabetologia. 2014;57(9):1876–1883. doi: 10.1007/s00125-014-3299-4. [DOI] [PubMed] [Google Scholar]
- 47.Deacon CF. Physiology and pharmacology of DPP-4 in glucose homeostasis and the treatment of type 2 diabetes. Front Endocrinol. 2019;10:80. doi: 10.3389/fendo.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koliaki C, Doupis J. Incretin-based therapy: a powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther Res Treat Educ Diabetes Relat Disord. 2011;2:101–121. doi: 10.1007/s13300-011-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duez H, Cariou B, Staels B. DPP-4 inhibitors in the treatment of type 2 diabetes. Biochem Pharmacol, Elsevier. 2012;83(7):823–832. doi: 10.1016/j.bcp.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 50.Yang K, Chan CB. Epicatechin potentiation of glucose-stimulated insulin secretion in INS-1 cells is not dependent on its antioxidant activity. Acta Pharmacol Sin. 2018;39(5):893–902. doi: 10.1038/aps.2017.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martín MÁ, Fernández-Millán E, Ramos S, Bravo L, Goya L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol Nutr Food Res. 2014;58:447–456. doi: 10.1002/mnfr.201300291. [DOI] [PubMed] [Google Scholar]
- 52.Wang S, Su R, Nie S, Sun M, Zhang J, Wu D, Moustaid-Moussa N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem. 2014;25(4):363–376. doi: 10.1016/j.jnutbio.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Othman AI, El-Sawi MR, El-Missiry MA, Abukhalil MH. Epigallocatechin-3-gallate protects against diabetic cardiomyopathy through modulating the cardiometabolic risk factors, oxidative stress, inflammation, cell death and fibrosis in streptozotocin-nicotinamide-induced diabetic rats. Biomed Pharmacother. 2017;94:362–373. doi: 10.1016/j.biopha.2017.07.129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 11969 kb)
Data Availability Statement
The datasets generated and/or analyzed in this study are available from the corresponding author on reasonable request.