Abstract
Purpose
The antidiabetic activities of Ocimum gratissimum (OG) leaf extract are well documented in experimental diabetes induced by beta cell destruction resulting in hypoinsulinemia. There is however paucity of data on its effect in conditions characterized by hyperinsulinemia. This study therefore investigated the effect of OG on insulin resistance induced by dexamethasone in male Wistar rats.
Method
Twenty male Wistar rats grouped as control, normal + OG, Dex and Dex + OG were used. Control and normal + OG received normal saline while Dex and Dex + OG received dexamethasone (1 mg/kg, i.p) followed by distilled water or OG (400 mg/kg) for 10 days. Levels of fasting blood glucose (FBG), insulin, HOMA-IR, liver and muscle glycogen, hexokinase activities, hepatic HMG CoA reductase activity were obtained. Histopathology of pancreas and liver tissues was carried out using standard procedures.
Results
Body weight reduced significantly in the Dex and Dex + OG groups compared with the control. FBG (147.8 ± 9.93 mg/dL), insulin (2.98 ± 0.49 µIU/ml) and HOMA-IR (1.11 ± 0.22) of Dex animals were higher than the control (FBG = 89.22 ± 6.53 mg/dL; insulin = 1.70 ± 0.49 µIU/ml; HOMA-IR = 0.37 ± 0.04). These were significantly reduced in the Dex + OG (FBG = 115.31 ± 5.93 mg/dL; insulin = 1.85 ± 0.11µIU/ml; HOMA-IR = 0.53 ± 0.08) compared with Dex. Glycogen content and hexokinase activities were increased in the Dex + OG. Increased pancreatic islet size, hepatic steatosis and HMG Co A reductase activity were observed in the Dex but reduced in Dex + OG.
Conclusion
OG promotes cellular glucose utilization and reduces hepatic fat accumulation in Wistar rats with insulin resistance induced by dexamethasone. Further study to identify the involved signal transduction will throw more light on the observed effects.
Keywords: Insulin Resistance, HMG coA reductase, Hexokinase, Glycogen, Ocimum gratissimum
Introduction
Ocimum gratissimum (OG) Linn. leaf extract has been documented by several researchers to be a potent antidiabetic agent [1–7]. We have also shown that OG inhibits hepatic glycogen phosphorylase activity in rats with streptozotocin-induced diabetes [8], reverses diabetes-induced intestinal hyperplasia [9] and lowers fructosamine level as an indicator of good glycemic control in alloxan-induced diabetic rats [10]. In a recent study using human intestinal epithelial cell model (Caco-2 cells), it was shown that OG inhibited sodium-dependent glucose transporter (SGLT) 1-mediated glucose uptake [11] which may contribute to its hypoglycemic activities. Whereas, earlier studies on OG and diabetes used animal models characterized by pancreatic beta cell destructions to create a hypoinsulinemic condition, to the best of our knowledge there is no report on the effect of OG on conditions characterized by hyperglycemia and hyperinsulinemia.
Insulin resistance is the hallmark of metabolic syndrome with a global incidence that parallels the incidence of obesity and type 2 diabetes mellitus [12]. It occurs when the metabolic response of insulin to stimulate glucose uptake is defective and/or inability of insulin to suppress hepatic gluconeogenesis and glucose released into circulation [13]. Insulin resistance is pathway-selective in that when it fails to suppress hepatic glucose production, it promotes hepatic lipogenesis and fat accumulation thus, hypertriglyceridemia and hepatic steatosis are associated with insulin resistance [14–16]. Functionally, insulin promotes glucose utilization in the liver, skeletal muscle and adipose tissue. Although insulin is required for glucose uptake in the skeletal muscle and adipose tissue, it is not required for glucose uptake in the liver. Insulin action on hepatic glucose utilization is by stimulating glucokinase gene expression through the inhibition of the suppressive effects of Forkhead transcription factor (Foxo1) on gene expression [17]. Unabatedly, Foxo1 protein interacts with the DNA binding sites in the promoter region of genes, coding several enzymes involved in gluconeogenesis [18, 19] to favour hepatic glucose production over utilization. Hence, disruption of its activity by insulin favours glucose utilization [20, 21]. Defective insulin action on Foxo1 protein will not only reduce hepatic glucose utilization but also favours assembly of very low-density lipoprotein (VLDL) and reduces peripheral triglyceride catabolism (by the induction of microsomal triglyceride transfer protein and apolipoprotein CIII, respectively) [22]. Thus, through Foxo1 protein, there is a link between insulin resistance, VLDL overproduction and hypertriglyceridemia. Owing to the fact that glycogenesis is also hampered during insulin resistance, therefore restoration of insulin sensitivity will include promotion of glucose utilization, enhancement of glycogenesis and elimination of excess lipid production.
Glucocorticoid induction of metabolic syndrome is associated with accumulation of excess lipids within the hepatocytes [23] through the inhibition of Activating Transcription Factor 3 [24] thereby contributing to the dyslipidemia associated with metabolic syndrome. Ocimum gratissimum leaf extract has been documented to reverse diabetes-induced dyslipidemia in Wistar rats [5, 25], reduce plasma lipid level in high cholesterol fed hamsters [26] and mitigate estrogen deficiency-induced adiposity in female Sprague dawley rats [27]. Oxidative stress plays pivotal role in the development of insulin resistance and the progression of diabetes complications [28–30] thus several antioxidant rich plants have been documented to mitigate insulin resistance [31, 32]. Phytochemical screening of OG showed that it is rich in antioxidants such as flavonoids and polyphenols [33] which had been found beneficial in several conditions [6, 34–38]. Earlier on, we evaluated the total antioxidant capacity of OG [9] and found that its ability to ameliorate diabetes-induced intestinal hyperplasia may be hinged on its high antioxidant capacity. We therefore hypothesized that Ocimum gratissimum leaf extract’s hypoglycemic, hypolipidemic and antioxidant properties could ameliorate insulin resistance induced by glucocorticoid. Preliminary data from the current study indicated that OG caused significant decrease in the blood glucose level of the glucocorticoid-induced insulin resistant rats [39].
Materials and methods
Twenty male Wistar rats (130 ± 20 g) were used for the study. The animals were kept in well aerated plastic cages covered with wire mesh in the animal house of the Department of Physiology, College of Medicine, University of Ibadan, Ibadan, Nigeria. They were kept under ambient temperature of 25 ± 2 0 C, humidity of 46% and light/dark cycle of 12/12 h. They were given standard pelletized rats feed (Ladokun feeds®, Composition: 26.5% protein, 40% carbohydrate, 29% Fat and 4.5% crude fibre) and water ad libitum. Acclimatization was done for 2 weeks prior to the commencement of the study. All experimental protocols and handling were in compliance with the University of Ibadan Ethical code on animal use and handling for experiment which were in accordance with the NIH publication No. 85–23 guidelines.
Preparation of Ocimum gratissimum aqueous extract
Fresh leaves of Ocimum gratissimum (OG) were gathered around Ibadan metropolis. Identified and authenticated at the Forest Research Institute of Nigeria by Mr Shasanya O.S and Mr Ekundayo A.A. The identified plant was issued herbarium number FHI 110,026. The leaves were washed and air-dried for 3 weeks, then pulverized into powdery form. The powder was soaked for 24 h in distilled water, filtered and the filtrate was concentrated using a rotary evaporator to yield 9.2% (9.2 g/100 g) aqueous extract. The dose of 400 mg/kg body weight of Ocimum gratissimum administered was based on our previous studies [6, 8, 9] and the LD50 of 1250 mg/kg reported by Omotosho et al. [34].
Induction of insulin resistance and experimental design
Insulin resistance was induced according to the method described by Martínez et al. [40]. The Wistar rats were randomly divided in to 4 groups of 5 rats each as the control, OG, Dex only and Dex + OG. Animals in the Dex and Dex + OG groups were given 1 mg/kg body weight of dexamethasone (Dexamethasone Sodium Phosphate, Zanelb®) (i.p), 30 min before oral administration of distilled water or 400 mg/kg body weight of the extract of Ocimum gratissimum for 10 consecutive days. The control and OG animals were given normal saline (i.p) 30 min prior to the administration of distilled water or OG for the same period of 10 days.
Sampling
Body weights before and after treatments were obtained. After the 10 days of treatments, fasting blood glucose (FBG) and insulin levels were determined from blood obtained through the retroorbital sinus using One Touch Ultra glucometer® and commercially available insulin kit (Calbiotech®), respectively. Under anaesthesia by sodium thiopental (50 mg/kg, i.p.), liver, pancreas and soleus muscles were harvested. Glycogen content and hexokinase activity were determined in liver and muscle samples; HMG co A reductase activity was determined in the liver sample while the remaining liver sample and pancreas were fixed for histopathology. Homeostatic Index of Insulin Resistance (HOMA-IR) was calculated from the FBG and insulin concentration as (serum insulin X FBG) / 22.5 according to Antunes et al. [41]. Samples for hexokinase and HMG CoA reductase activities were homogenized in Phosphate Buffered Saline (pH 7.4). Protein content of the homogenate was determined using the biuret method.
Assays
Determination of glycogen content
The glycogen content in the liver and muscle samples were determined as previously reported [8]. Briefly, the liver or muscle weight was noted and 1 g was obtained for digestion in an Erlenmeyer flask containing 10 ml of 30% KOH over boiling water. The tissue digest was cooled and 4 ml aliquot was washed by adding 5 ml of 95% ethanol in a test-tube. After shaking the mixture thoroughly, it was centrifuged for 10 min. The supernatant was decanted and the precipitate was reconstituted with 0.5 ml distilled water and rewashed. The final precipitate was reconstituted with 2 ml of distilled water and used for the glycogen content determination. Into a test-tube, 0.5 ml of the reconstituted glycogen sample was added followed by stepwise addition of 0.5 ml each of concentrated hydrochloric acid, 88% formic acid and Anthrone reagent dissolved in 80% Sulphuric acid. After shaking the mixture thoroughly, it was incubated in boiling water for 10 min to obtain a blue-coloured solution. A serially diluted glycogen standard and distilled water (blank) were treated similarly. Absorbance of the sample or standard was obtained at 630 nm against the blank using a UV/Visible spectrophotometer (721D Searchtech Instrument®). Glycogen concentration of each sample was obtained from a glycogen standard curve plotted from the serially diluted standards.
Determination of hexokinase activity
Hexokinase activities of the liver and muscle samples were determined as described earlier [42]
Briefly, 2 ml of Glucose buffer [0.0025 M glucose, 0.0025 M MgCl2, 0.01 M K2HP04, 0.077 M KCl, and 0.03 M Trizma base, pH 8] was pippetted into a test tube followed by 0.1 ml of 0.18 M ATP solution and 0.9 ml of distilled water. The mixture was preheated in water for 5 min at 38 0 C, 1 ml of liver homogenate was added and 100 µl of the homogenate-buffer substrate mixture was taken immediately for initial glucose analysis. The mixture was incubated at 380C for 30 min and another 100 µl was taken for final glucose analysis. The difference in the level of glucose was calculated and hexokinase activity was expressed as glucose metabolized /mg. pr/30 min. All assays were carried out in duplicates. In this assay, glucose was assayed using a commercially available Glucose GOD-PAP kit (Fortress Diagnostic®, United Kingdom).
Determination of HMG Co A reductase activity
HMG Co A reductase (the rate limiting enzyme in cholesterol synthesis) activity was determined by the indirect method reported by Erejuwa et al. [43] using the ratio of HMG CoA to Mevalonate as an index of its activities. Briefly, equal volumes of liver tissue homogenate and diluted perchloric acid were mixed and allowed to stand for 5 min prior to centrifugation at 2000 rev/min for 10 min to obtain the filtrate. To determine HMG Co A, 1.0 ml of the filtrate was taken into a test tube and 0.5 ml of freshly prepared acidic hydroxylamine reagent (pH 2.1) was added, mixed and allowed to stand for 5 min followed by addition of 1.5 ml of ferric chloride reagent. After shaking the mixture thoroughly, it was allowed to stand for 10 min and the absorbance at 540 nm was obtained against a similarly treated saline arsenate blank. Similar procedure was carried to obtain Mevalonate except that alkaline hydroxylamine reagent (pH 5.5) was used in place of the acidic hydroxylamine reagent. The ratio of the absorbances of HMG CoA to Mevalonate was obtained as index of HMG CoA reductase activity. An inverse relationship exists between the HMG CoA: Mevalonate ratio and HMG CoA reductase activity thus, increased HMG CoA: Mevalonate ratio indicates decreased HMG CoA reductase activity which by extension indicates decreased cholesterol synthesis and vice versa [43].
Histology
Pancreas and liver samples were immediately placed in 10% formal saline and sent to Histopathology Department, University College Hospital, Ibadan. Each tissue was dehydrated in ascending grade of absolute ethanol and then placed in xylene to clear. It was embedded in paraffin from which a 5µ section was made using a microtome. The section was floated on 20% ethanol at 50C, picked with grease free microscope slide and allowed to drain. It was stained with Haematoxylin and Eosin (H & E) and mounted on the slide. Photomicrographs of the sections were made to observe morphological changes across the groups using a microscope at × 400.
Statistical analysis
The data were expressed as Mean ± SEM. Difference in the mean values were compared by One-way ANOVA and Turkey’s post hoc test using GraphPad prism® Version 7. P value less than 0.05 was considered significant.
Results
Body weight, FBG, insulin and HOMA-IR
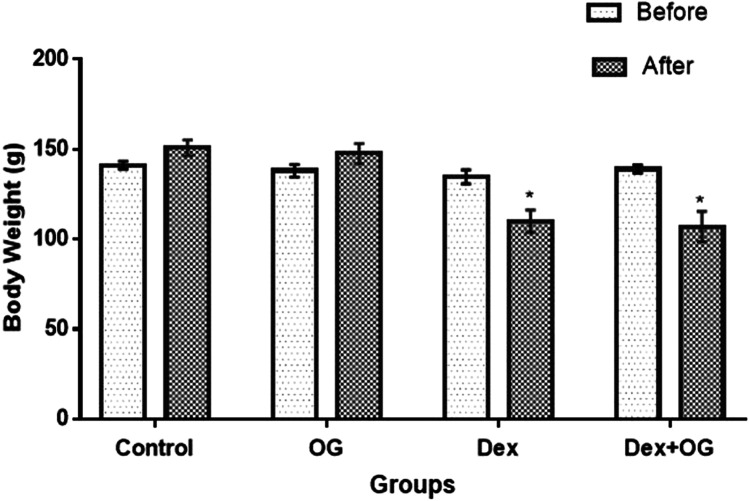
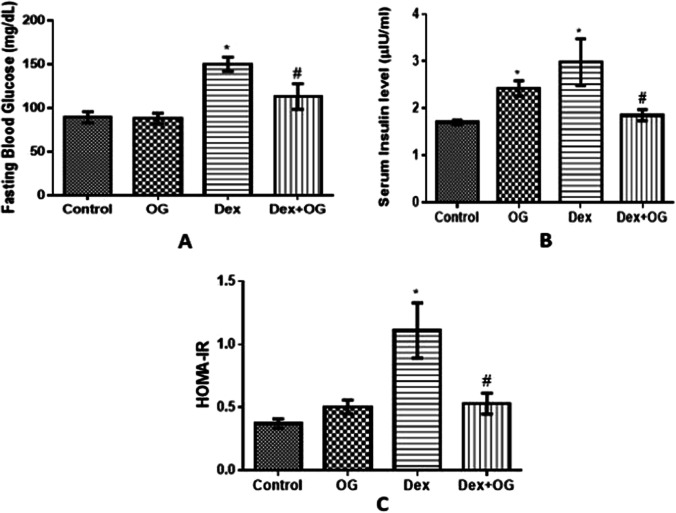
Body weight was significantly reduced in the Dex and Dex + OG group compared with the control (Fig. 1). As shown in Fig. 2, FBG (147.8 ± 9.93 mg/dl), insulin (2.98 ± 0.49 µIU/ml) and HOMA-IR (1.11 ± 0.22) of Dex animals were significantly higher than the control (FBG = 89.22 ± 6.53 mg/dl; insulin = 1.70 ± 0.49 µIU/ml; HOMA-IR = 0.37 ± 0.04); these were however reduced after OG treatment in the Dex + OG (FBG = 115.31 ± 5.93 mg/dl; insulin = 1.85 ± 0.11µIU/ml; HOMA-IR = 0.53 ± 0.08) compared with Dex.
Fig. 1.
Effect of Ocimum gratissimum on body weight of normal and dexamethasone-induced insulin resistant rats. n = 5, *P < 0.05 Before Vs After
Fig. 2.
Effect of Ocimum gratissimum on fasting blood glucose (A), serum insulin (B) and HOMA-IR (C) of normal and dexamethasone-induced insulin resistant rats. n = 5 *P < 0.05 Control Vs Dex; #P < 0.05 Dex Vs Dex + OG
Glycogen content
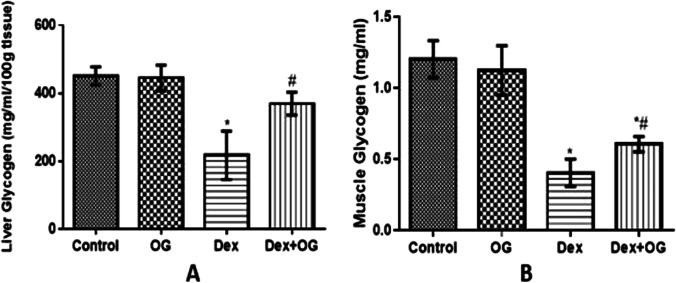
Glycogen contents in the liver and soleus muscle of animals in the Dex group were significantly depleted when compared with the control. The depleted liver glycogen content was however reversed in the Dex + OG group when compared with Dex only group while the increased muscle glycogen observed in Dex + OG group was not significantly different from the Dex only group (Fig. 3).
Fig. 3.
Effect of Ocimum gratissimum on liver (A) and muscle (B) glycogen content of normal and dexamethasone-induced insulin resistant rats. n = 5 *P < 0.05 Control Vs Dex; #P < 0.05 Dex Vs Dex + OG
Hexokinase activity
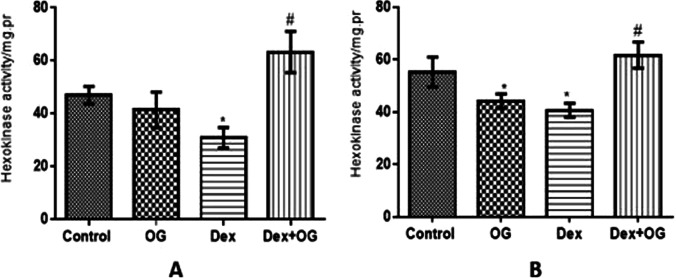
As shown in Fig. 4, hexokinase activities were significantly decreased in the liver and muscles of the Dex animals compared with the control. These were however increased by treatment with OG in the Dex + OG animals compared with the Dex only.
Fig. 4.
Effect of Ocimum gratissimum on hexokinase activity in liver (A) and muscle (B) of normal and dexamethasone-induced insulin resistant rats. n = 5 *P < 0.05 Control Vs Dex + OG, #P < 0.05 Vs Dex
Hepatic HMG Co A reductase activity
As shown Fig. 5, there was a significant decrease in the level of HMG Co A:Mevalonate ratio in the Dex only group when compared with control group (by interpretation, HMG Co A reductase activity was significantly increased in the Dex group when compared with the control). The decreased HMG Co A:Mevalonate ratio observed in the dexamethasone only group was increased by treatment with Ocimum gratissimum in the Dex + OG group.
Fig. 5.
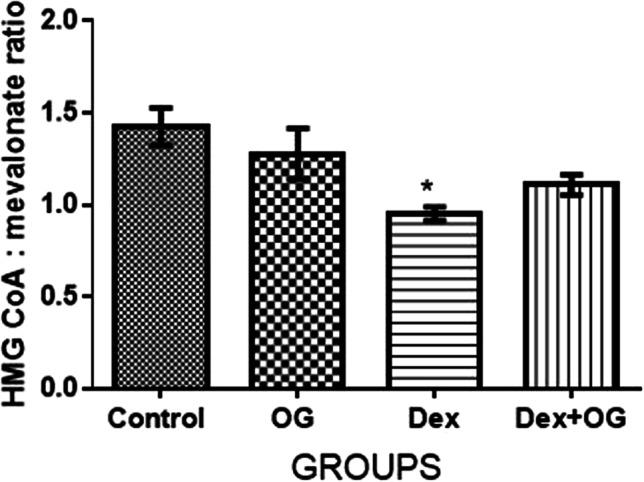
Effect of Ocimum gratissimum on hepatic HMG CoA: Mevalonate ratio of normal and dexamethasone-induced insulin resistant rats. n = 5 *P < 0.05 Control Vs Dex
Histopathology of the pancreas and liver
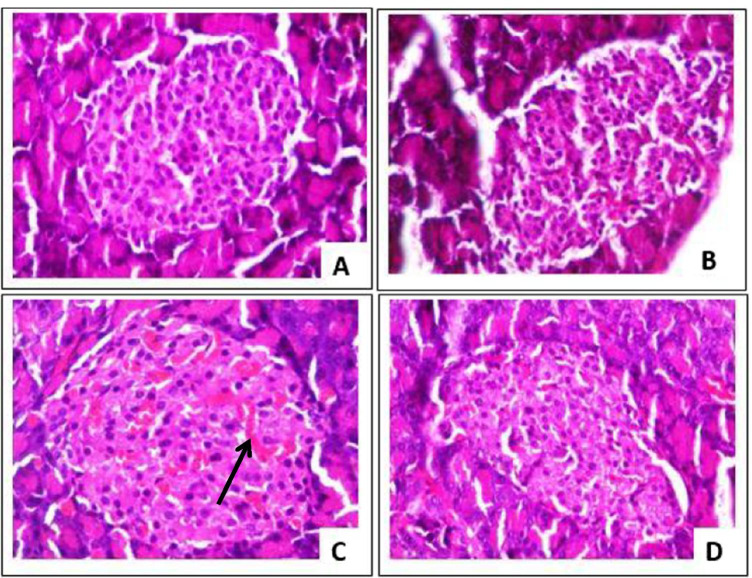
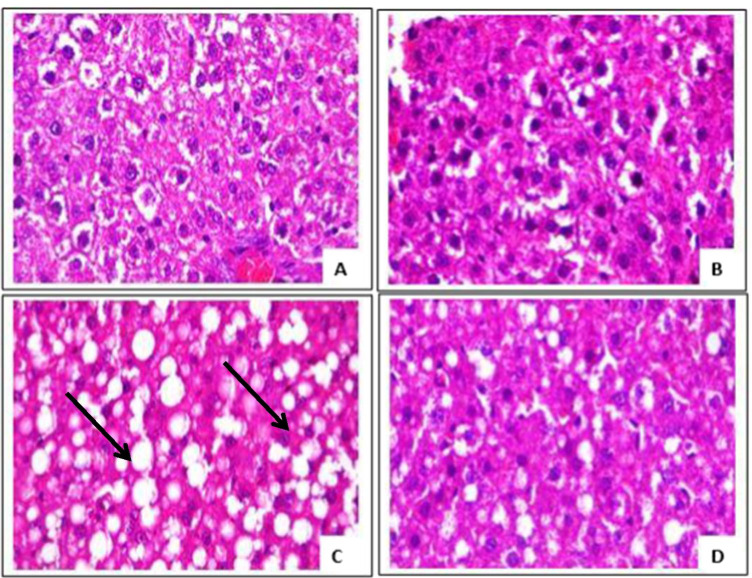
Histopathological analysis of the H & E-stained pancreas showed increased size of the islet, islet congestion and mild vascular congestion in the Dex group compared with the control. Distortion in the pancreatic cytoarchitecture was absent in the Dex + OG group (Fig. 6). Widespread hepatic steatosis was visible in the liver of Dex group and this was dissipated in the Dex + OG group (Fig. 7).
Fig. 6.
Photomicrographs of the pancreas from (A) control, (B) OG, (C) Dex and (D) Dex + OG showing enlarged islet and congestions (black arrow) in the Dex only group. H & E, × 400
Fig. 7.
Photomicrographs of the liver from (A) control, (B) OG, (C) Dex and (D) Dex + OG. Hepatic steatosis (black arrow) is evident in the Dex group (C) and this was markedly reduced in the Dex + OG group (D). H & E, × 400
Discussion
Dexamethasone and other glucocorticoids have differential effects on body weight in human and animals. It causes weight gain in human [44] while its net effect is weight loss in animals [45] despite increased percentage body fat composition. Accordingly, significant reduction in body weight was observed in all the dexamethasone treated rats in this study. Reduction in appetite, food intake and energy as well as inhibition of protein synthesis are probable contributors to the observed decreased body weight following dexamethasone administration [46–48]. Amino acids involved in the inhibition of protein synthesis and protein degradation were increased in the metabolomic profile of dexamethasone treated rats and this translated to increased muscular atrophy that was obvious in vivo [49]. The Malkawi et al. [49] and our findings corroborated the earlier report of Wu et al. [50] that dexamethasone treatment in rats causes severe reduction in age-related development. Ocimum gratissimum however did not have any effect on the observed reduction in body weight following dexamethasone treatment.
The significant increase in blood glucose level observed in the dexamethasone treated rats is consistent with the hyperglycemic effect of dexamethasone [51]. Similarly, reversal of the dexamethasone-induced hyperglycemia by administration of OG is consistent with its well documented hypoglycemic properties [39]. As it was also observed in this study, increased insulin level is associated with dexamethasone treatment in vivo [52, 53] and in vitro [47]. The increased insulin level could be an adaptive response of the pancreatic islet to hyperglycemia. Augmented glucose-stimulated insulin secretion in rats given dexamethasone [54] has been reported to involve an increased expression of gap junction protein, connexin Cx36 while glucose transporter (Glut 2) remained unaltered [55]. Increased pancreatic β- cell proliferation and islet size were also shown to contribute to the increased insulin production [56]. Interestingly, histological findings from the current study also showed marked enlargement of the islet in the dexamethasone treated rats. A dose-dependent increase in pancreatic islet size of dexamethasone treated rats has been established and at dose of 1 mg/kg (similar to the dose used in the current study), the increase could be up to 2.7 folds [56]. The reduction in the serum insulin level of OG-treated dexamethasone-exposed rats in this study marches the reduction in the hyperglycemia and the relatively unaltered pancreatic islet size. It is worthy of note from this study that serum insulin level of normal rats administered OG was also increased despite being normoglycemic and having intact pancreatic islet, this observation might not be unrelated to the insulin stimulatory effect of OG earlier posited by Casanova et al. [57].
The validity of HOMA-IR in the prediction of insulin resistance in rodents has been established by several workers [41, 58]. The relationship between HOMA-IR and insulin resistance is linear, thus increased HOMA-IR indicates insulin resistance while its decrease (relative to the control) indicates insulin sensitivity. These decision rules were depicted in the Dex group and the Dex + OG group of this study, respectively. In other words, OG treatment following dexamethasone administration reversed insulin resistance observed in the rats given dexamethasone only. The effect of OG on HOMA-IR observed in the current study following OG treatment is similar to the decreased HOMA-IR observed following OG treatment of diabetes (induced by streptozotocin and high-fat feeding) [7] as well as obesity (induced by monosodium glutamate) in rats [59].
The reversal of insulin resistance in the Dex + OG was further corroborated by our present findings on glycogen content and hexokinase activity. Hepatic glycogen depletion observed in the Dex only rats is consistent with earlier findings [52, 60]. Although, some studies [47, 61] reported increased hepatic glycogen as a consequence of dexamethasone treatment, Zheng et al. [62] showed that hepatic glycogen metabolism in response to dexamethasone is biphasic depending on dose and duration. At low dose, its effect is glycogenesis while at higher dose, it is glycogenolysis. For instance, at a dose of 0.1 mg/kg of dexamethasone [47], hepatic glycogen was increased while at 1 mg/kg used in this study, hepatic glycogen was decreased despite the two studies having similar duration of 10 days. The hepatic glycogenolysis may probably be due to activation of glycogen phosphorylase A activity. In cultured hepatocytes, dexamethasone has been documented to cause a sustained activation of glycogen phosphorylase A activity [63]. The increase in the hepatic glycogen content of Dex + OG rats may therefore not surprising since we had earlier documented the inhibitory effect of OG on glycogen phosphorylase activity in streptozotocin-induced diabetic rats [8].
Hexokinase catalyzes the formation of glucose-6-phosphate which can be channeled into 3 major glucose disposal pathways: glycolysis, glycogenesis and pentose phosphate pathway [64] therefore, its activity will have bearing on the 3 pathways. Different isomers are found in the liver and muscles, however, the kinetic method used in assaying its activities in the current study measured metabolized glucose within a specific time to eliminate bias of tissue specificity. In this study, hepatic hexokinase activity was significantly reduced in the Dex only animals in tandem with the documented reports that glucocorticoids inhibit hexokinase activity [65], decrease glucose oxidation [66, 67] and switch cellular glucose oxidation to gluconeogenesis [68]. Interestingly, hexokinase activity was increased in both the liver and muscle of Dex + OG. As far as we know, this is the first study to establish that OG may promote glucose utilization by increasing activity of a glycolytic enzyme. Such increase glucose utilization following OG treatment was postulated by Egesie et al. [2]. A recent study in obese rats showed that OG treatment increased GLUT-4 gene expression [59] which may probably increase cellular glucose uptake. A well-known phenomenon is that until an enzyme is saturated, the rate of its activity is directly proportional to substrate availability [69]. Therefore, it may be possible that increased glucose uptake occurred in the OG treated rats to stimulate the observed increased hexokinase activity.
Hepatic steatosis features prominently in dexamethasone treatments and contributes largely to induction of metabolic syndrome and its associated dyslipidemia [23, 24, 70]. Widespread hepatic steatosis was evident in the Dex rats of this study. Using HMG co A: Mevalonate ratio as an indirect method of assessing cholesterol synthesis, Dex rats had significantly increased cholesterol synthesis to march the steatosis observed in the histology of their liver. HMG co A: Mevalonate ratio is an indicator of the activities of HMG co A reductase which is the rate limiting enzyme in cholesterol synthesis. Inhibition of its activity has been found effective in lowering cholesterol in human and is a major target for therapeutic treatment of high cholesterol [71, 72]. The dissipation of hepatic steatosis observed histologically in the Dex + OG also marched the decreased cholesterol synthesis extrapolated from the HMG coA: Mevalonate ratio. Previous studies have documented similar anticholesterolemic activities of OG [5, 7, 25–27]. The observed decreased hepatic steatosis in the OG treated rats may be associated with its widely reported antioxidant activities [6, 34–38] given that several studies have reported that antioxidants of plant/diet origin are potential therapeutic candidates for the treatment of hepatic steatosis [73].
With the reduction in hepatic cholesterol synthesis coupled with increased insulin sensitivity showcased by reduced HOMA-IR, increased glycogen content and increased hexokinase activity observed in the Dex + OG animals, we therefore conclude that Ocimum gratissimum leaf extract may promote cellular glucose metabolism in Wistar rats with insulin resistance induced by dexamethasone. Studies to identify the involved signaling pathway/s will shed more light on the observed role of Ocimum gratissimum. It is also imperative to identify the metabolite (s) of Ocimum gratissimum that may be involved.
Data availability
Data for the study is available with STS.
Code availability
NA
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aguiyi JC, Obi CI, Gyang SS, Igweh AC. Hypoglycaemic activity of Ocimum gratissimum in rats. Fitoterapia. 2000;71:444–446. doi: 10.1016/s0367-326x(00)00143-x. [DOI] [PubMed] [Google Scholar]
- 2.Egesie UG, Adelaiye AB, Ibu JO, Egesie OJ. Safety and hypoglycaemic properties of aqueous leaf extract of Ocimum gratissimum in streptozotocin induced diabetic rats. Niger J Physiol Sci. 2006;21:31–35. doi: 10.4314/njps.v21i1-2.53971. [DOI] [PubMed] [Google Scholar]
- 3.Owoyele BV, Funsho MA, Soladoye AO. Effect of aqueous leaves extract of Ocimum gratissimum (sweet basil) on alloxan induced diabetic rats. Phcog Mag. 2005;1:62–63. [Google Scholar]
- 4.Mohammed A, Tanko Y, Okasha MA, Magaji RA, Yaro AH. Effects of aqueous leaves extract of Ocimum gratissimum on blood glucose levels of streptozotocin-induced diabetic Wistar rats. Afr J Biotechnol. 2007;6:2087–2090. [Google Scholar]
- 5.Ayinla MT, Dada SO, Shittu ST, Olayaki LA, Akiode AO, Ojulari SL. Anti-hyperlipidemic effect of aqueous leaf extract of ocimum gratissimum in alloxan induced diabetic rats. Int J Med Med Sci. 2011;3(12):360–363. [Google Scholar]
- 6.Shittu ST, Oyeyemi WA, Lasisi TJ, Shittu SA, Lawal TT, Olujobi ST. Aqueous leaf extract of Ocimum gratissimum improves hematological parameters in alloxan-induced diabetic rats via its antioxidant properties. Int J App Basic Med Res. 2016;6:96–100. doi: 10.4103/2229-516X.179016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okoduwa SIR, Umar IA, James DB, Inuwa HM. Anti-Diabetic Potential of Ocimum gratissimum Leaf Fractions in Fortified Diet-Fed Streptozotocin Treated Rat Model of Type-2 Diabetes. Medicines (Basel) 2017;4(4):73. doi: 10.3390/medicines4040073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shittu ST, Oyeyemi WA, Shittu SA, Lasisi TJ. Ocimum gratissimum inhibits glycogen phosphorylase activity without changes in hepatic nuclear factor kappa B (NF-kB) and inducible nitric oxide synthase (iNOS) in streptozotocin-induced diabetic rats. Nig Med Pract. 2018;73:10–17. [Google Scholar]
- 9.Shittu ST, Lasisi TJ, Shittu SA, Ogundiran AA. Reversal of diabetes-induced intestinal hyperplasia in male rats treated with Ocimum gratissimum leaf extract. Nig Med Pract. 2019;75(1–3):10–19. [Google Scholar]
- 10.Shittu ST, Shittu SA, Olatunji AA, Oyeyemi WA. Ocimum gratissimum leaf extract may precipitate infertility in male diabetic Wistar rats. JBRA Assist Repro. 2019;23(1):37–44. doi: 10.5935/1518-0557.20180072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimada H, Kuma C, Iseri T, Matsumura S, Kawase A, Matsuura M, Iwaki M. Inhibitory Effect of Ocimum gratissimum Leaf Extract on Postprandial Increase of Blood Glucose. Nat Pro Commun. 2019;14(10):1–5. [Google Scholar]
- 12.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20(2):12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol. 2008;159(Suppl 1):S67–S74. doi: 10.1530/EJE-08-0245. [DOI] [PubMed] [Google Scholar]
- 14.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Otero YF, Stafford JM, Owen P, McGuinness OP. Pathway-selective insulin resistance and metabolic disease: the importance of nutrient flux. J Bio Chem. 2014;289:20462–20469. doi: 10.1074/jbc.R114.576355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 18.Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG. Regulation of glucose6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J Biol Chem. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- 19.Hall RK, Yamasaki T, Kucera T, Waltner-Law M, O'Brien R, Granner DK. Regulation of phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein-1 gene expression by insulin. The role of winged helix/forkhead proteins. J Biol Chem 2000; 275(39):30169–75 [DOI] [PubMed]
- 20.Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8(1):65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, Ahima RS, Ueki K, Kahn CR, Birnbaum MJ. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18(3):388–395. doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sparks JD, Dong HH. FoxO1 and hepatic lipid metabolism. Curr Opin Lipidol. 2009;20(3):217–226. doi: 10.1097/MOL.0b013e32832b3f4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woods CP, Hazlehurst JM, Tomlinson JW. Glucocorticoids and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol. 2015;154:94–103. doi: 10.1016/j.jsbmb.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Wei J, Shi M, Jiang H, Zhou J. Glucocorticoid induces hepatic steatosis by inhibiting activating transcription factor 3 (ATF3)/S100A9 protein signaling in granulocytic myeloid-derived suppressor cells. J Biol Chem. 2016;291(41):21771–21785. doi: 10.1074/jbc.M116.726364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nwogor UA. Effects of Prolonged administration of aqueous extract of Ocimum gratissimum (scent leaf) on blood glucose and lipid profile in alloxan induced diabetic albino rats. Am J Biomed Life Sci. 2016;2016(4):30–34. [Google Scholar]
- 26.Chao PY, Lin JA, Ting WJ, Lee HH, Hsieh K, Chiu YW, Lai TJ, Hwang JM, Liu JR, Huang CY. Ocimum gratissmum aqueous extract reduces plasma lipid in hypercholesterol-fed hamsters. Int J Med Sci. 2016;13(11):819–824. doi: 10.7150/ijms.16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao PY, Chiang TI, Chang IC, Tsai FL, Lee HH, Hsieh K, Chiu YW, Lai TJ, Liu JY, Hsu LS, Shih YC. Amelioration of estrogen-deficiency-induced obesity by Ocimum gratissimum. Int J Med Sci. 2017;14(9):896–901. doi: 10.7150/ijms.19933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 29.Fridlyand LE, Philipson LH. Reactive species and early manifestation of insulin resistance in type 2 diabetes. Diabetes Obes Metab. 2006;8(2):136–145. doi: 10.1111/j.1463-1326.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- 30.Mahjoub S, Masrour-Roudsari J. Role of oxidative stress in pathogenesis of metabolic syndrome. Caspian J Intern Med. 2012;3(1):386–396. [PMC free article] [PubMed] [Google Scholar]
- 31.Lin D, Xiao M, Zhao J, et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules. 2016;21(10):1374. doi: 10.3390/molecules21101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aryaeian N, Sedehi SK, Arablou T. Polyphenols and their effects on diabetes management: a review. Med J Islam Repub Iran. 2017;31:134. doi: 10.14196/mjiri.31.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alabi QK, Akomolafe RO, Omole JG, Adefisayo MA, Ogundipe OL, Aturamu A, Sanya JO. Polyphenol-rich extract of Ocimum gratissimum leaves ameliorates colitis via attenuating colonic mucosa injury and regulating pro-inflammatory cytokines production and oxidative stress. Biomed Pharmacother. 2018;103:812–822. doi: 10.1016/j.biopha.2018.04.071. [DOI] [PubMed] [Google Scholar]
- 34.Omotosho IO, Henshaw AN, Adeagbo G. Effect of natural antioxidant – Ocimum gratissimum in modulating neurodegenerative changes in rats fed with high concentration of lead acetate. J Med Plants Res. 2011;5(13):2743–2747. [Google Scholar]
- 35.Chen YH, Chiu YW, Shyu JC, Tsai CC, Lee HH, Hung CC, Hwang JM, Liu JY, Wang WH. Protective effects of Ocimum gratissimum polyphenol extract on carbon tetrachloride-induced liver fibrosis in rats. Chin J Physiol. 2015;58(1):55–63. doi: 10.4077/CJP.2015.BAD285. [DOI] [PubMed] [Google Scholar]
- 36.Oyem JC, Chris-Ozoko LE, Enaohwo MT, Otabor FO, Okudayo VA, Udi OA. Antioxidative properties of Ocimum gratissimum alters Lead acetate induced oxidative damage in lymphoid tissues and hematological parameters of adult Wistar rats. Toxicol Rep. 2021;2021(8):215–222. doi: 10.1016/j.toxrep.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okechukwu GN, Ezor E, Finbarrs-Bello E, Ebube LN, Uzomba GC, Ibegbu AO. Effects of aqueous extract of Ocimum gratissimum leaves and vitamin C on lead acetate-induced changes in the thymus of adult Wistar rats. Int J Biochem Res Rev. 2019;26(1):1–9. doi: 10.9734/ijbcrr/2019/v26i130087. [DOI] [Google Scholar]
- 38.Igbinosa EO, Uzunuigbe EO, Igbinosa IH, Odjadjare EE, Igiehon NO, Emuedo OA. In vitro assessment of antioxidant, phytochemical and nutritional properties of extracts from the leaves of Ocimum gratissimum (Linn) Afr J Tradit Complement Altern Med. 2013;10(5):292–298. [PMC free article] [PubMed] [Google Scholar]
- 39.Shittu ST, Lasisi TJ, Shittu SA, Adeyemi A, Alada ARA. Insulin sensitivity was enhanced by Ocimum gratissimum leaf extract in Wistar rats with dexamethasone-induced metabolic syndrome. Proc Physiol Soc. 2019;43:PC155. [Google Scholar]
- 40.Martínez BB, Pereira AC, Muzetti JH, Telles P, Mundim FG, Teixeira MA. Experimental model of glucocorticoid-induced insulin resistance. Acta Cir Bras. 2016;31(10):645–649. doi: 10.1590/S0102-865020160100000001. [DOI] [PubMed] [Google Scholar]
- 41.Antunes LC, Elkfury JL, Jornada MN, Foletto KC, Bertoluci MC. Validation of HOMA-IR in a model of insulin-resistance induced by a high-fat diet in Wistar rats. Arch Endocrinol Metab. 2016;60(2):138–142. doi: 10.1590/2359-3997000000169. [DOI] [PubMed] [Google Scholar]
- 42.Shittu ST, Alada ARA, Oyebola DDO. Metabolic fate of glucose taken up by the intestine during induced hyperglycaemia in dogs. Niger J Physiol Sci. 2018;33(1):37–49. [PubMed] [Google Scholar]
- 43.Erejuwa OO, Akpan JL, Uwaezuoke NJI, Nwobodo NN, Ezeokpo BC, Erhiano E, Araromi EJ, Ude UN, AbdulWahab M, Sulaiman SA. Effects of honey on postprandial hyperlipidemia and oxidative stress in Wistar rats: role of HMG-CoA reductase inhibition and antioxidant effect. Niger J Physiol Sci. 2018;33(2):129–138. [PubMed] [Google Scholar]
- 44.Chong PK, Jung RT, Scrimgeour CM, Rennie MJ. The effect of pharmacological dosages of glucocorticoids on free living total energy expenditure in man. Clin Endocrinol. 1994;40:577–581. doi: 10.1111/j.1365-2265.1994.tb03007.x. [DOI] [PubMed] [Google Scholar]
- 45.Dimitriadis G, Leighton B, Parry-Billings M, Sasson S, Young M, Krause U, Bevan S, Piva T, Wegener G, Newsholme EA. Effects of glucocorticoid excess on the sensitivity of glucose transport and metabolism to insulin in rat skeletal muscle. Biochem J. 1997;321:707–712. doi: 10.1042/bj3210707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poggioli R, Ueta CB, Drigo RA, Castillo M, Fonseca TL, Bianco AC. Dexamethasone Reduces Energy Expenditure And Increases Susceptibility To Diet-Induced Obesity in Mice. Obesity (Silver Spring) 2013;21(9):E415–E420. doi: 10.1002/oby.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bighetti BB, d Assis GF, Vieira DC, Violato NM, Cestari TM, Taga R, Bosqueiro JR, Rafacho A. Long-term dexamethasone treatment alters the histomorphology of acinar cells in rat parotid and submandibular glands. Int J Exp Pathol. 2014;95(5):351–363. doi: 10.1111/iep.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morimoto Y, Kondo Y, Kataoka H, Honda Y, Kozu R, Sakamoto J, et al. Heat treatment inhibits skeletal muscle atrophy of glucocorticoid-induced myopathy in rats. Physiol Res. 2015;2015(64):897–905. doi: 10.33549/physiolres.932942. [DOI] [PubMed] [Google Scholar]
- 49.Malkawi AK, Alzoubi KH, Jacob M, Matic G, Ali A, Al Faraj A, Almuhanna F, Dasouki M, Abdel Rahman AM. Metabolomics based profiling of dexamethasone side effects in rats. Front Pharmacol. 2018;9:46. doi: 10.3389/fphar.2018.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu T, Yang L, Jiang J, Ni Y, Zhu J, Zheng X, et al. Chronic glucocorticoid treatment induced circadian clock disorder leads to lipid metabolism and gut microbiota alterations in rats. Life Sci. 2017;192:173–182. doi: 10.1016/j.lfs.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 51.Tamez-Pérez HE, Quintanilla-Flores DL, Rodríguez-Gutiérrez R, González-González JG, Tamez-Peña AL. Steroid hyperglycemia: Prevalence, early detection and therapeutic recommendations: a narrative review. World J Diabetes. 2015;6(8):1073–1081. doi: 10.4239/wjd.v6.i8.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbera M, Fierabracci V, Novelli M, Bombara M, Masiello P, Bergamini E, De Tata V. Dexamethasone-induced insulin resistance and pancreatic adaptive response in aging rats are not modified by oral vanadyl sulfate treatment. Eur J Endocrinol. 2001;145:799–806. doi: 10.1530/eje.0.1450799. [DOI] [PubMed] [Google Scholar]
- 53.Severino C, Brizzi P, Solinas A, Secchi G, Maioli M, Tonolo G. Low-dose dexamethasone in the rat: a model to study insulin resistance. Am J Physiol Endocrinol Metab. 2002;283:E367–E373. doi: 10.1152/ajpendo.00185.2001. [DOI] [PubMed] [Google Scholar]
- 54.Holness MJ, Smith ND, Greenwood GK, Sugden MC. Interactive influences of peroxisome proliferator-activated receptor alpha activation and glucocorticoids on pancreatic beta cell compensation in insulin resistance induced by dietary saturated fat in the rat. Diabetologia. 2005;48:2062–2068. doi: 10.1007/s00125-005-1894-0. [DOI] [PubMed] [Google Scholar]
- 55.Rafacho A, Roma LP, Taboga SR, Boschero AC, Bosqueiro JR. Dexamethasone-induced insulin resistance is associated with increased connexin 36 mRNA and protein expression in pancreatic rat islets. Can J Physiol Pharmacol. 2007;2007(85):536–545. doi: 10.1139/y07-037. [DOI] [PubMed] [Google Scholar]
- 56.Rafacho A, Cestari TM, Taboga SR, Boschero AC, Bosqueiro JR. High doses of dexamethasone induce increased beta-cell proliferation in pancreatic rat islets. Am J Physiol Endocrinol Metab. 2009;296(4):E681–E689. doi: 10.1152/ajpendo.90931.2008. [DOI] [PubMed] [Google Scholar]
- 57.Casanova LM, Gu W, Costa SS, Jeppesen PB. Phenolic substances from Ocimum species enhance glucose-stimulated insulin secretion and modulate the expression of key insulin regulatory genes in mice pancreatic islets. J Nat Prod. 2017;80(12):3267–3275. doi: 10.1021/acs.jnatprod.7b00699. [DOI] [PubMed] [Google Scholar]
- 58.Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2008;295:E1269–E1276. doi: 10.1152/ajpendo.90207.2008. [DOI] [PubMed] [Google Scholar]
- 59.Bako HY , Farouk HU, Rufa’i R, Shehu S, Balarabe AH, Mohammed JS.The Effect of methanolic crude extract of Ocimum gratisimum leaves on insulin resistance and GLUT-4 gene expression in monosodium glutamate induced obese rats. SWJ 2020; 15(No 4): 48–53
- 60.Hammon HM, Philipona C, Zbinden Y, Blum JW, Donkin SS. Effects of dexamethasone and growth hormone treatment on hepatic Gluconeogenic enzymes in Calves. JDS. 2005;88(6):2107–2116. doi: 10.3168/jds.S0022-0302(05)72887-3. [DOI] [PubMed] [Google Scholar]
- 61.Niu L, Chen Q, Hua C, Geng Y, Cai L, Tao S, Ni Y, Zhao R. Effect of chronic dexamethasone administration on hyperglycemia and insulin release in goats. J Anim Sci Biotechnol. 2018;9:26. doi: 10.1186/s40104-018-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng XF, Liu L, Zhou J, Zhu D, Xia ZF, Jiang CL. Biphasic effects of dexamethasone on glycogen metabolism in primary cultured rat hepatocytes. J Endocrinol Invest. 2009;32(9):756–758. doi: 10.1007/BF03346532. [DOI] [PubMed] [Google Scholar]
- 63.Baqué S, Roca A, Guinovart JJ, Gomez-Foix AM. Direct activating effects of dexamethasone on glycogen metabolizing enzymes in primary cultured rat hepatocytes. Eur J Biochem. 1996;236:772–777. doi: 10.1111/j.1432-1033.1996.t01-1-00772.x. [DOI] [PubMed] [Google Scholar]
- 64.Berg JM, Tymoczko JL, Stryer L. Section 16.2, The glycolytic pathway is tightly controlled. Biochemistry. 5th edition. New York: W H Freeman. 2002. Available from: https://www.ncbi.nlm.nih.gov/books/NBK22395/. Accessed 20 Sept 2020
- 65.Ilyin VS. Hormonal regulation of liver hexokinase activity. Adv Enzym Regul. 1964;2:151–175. doi: 10.1016/s0065-2571(64)80011-x. [DOI] [PubMed] [Google Scholar]
- 66.Tappy L, Randin D, Vollenweider P, Vollenweider L, Paquot N, Scherrer U, Schneiter P, Nicod P, Jéquier E. Mechanisms of dexamethasone-induced insulin resistance in healthy humans. J Clin Endocrinol Metab. 1994;79(4):1063–1069. doi: 10.1210/jcem.79.4.7962275. [DOI] [PubMed] [Google Scholar]
- 67.Kuo T, McQueen A, Chen TC, Wang JC. Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med Biol. 2015;2015(872):99–126. doi: 10.1007/978-1-4939-2895-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma R, Zhang W, Tang K, Zhang H, Zhang Y, Li D, Li Y, Xu P, Luo S, Cai W, Ji T, Katirai F, Ye D, Huang B. Switch of glycolysis to gluconeogenesis by dexamethasone for treatment of hepatocarcinoma. Nat Commun. 2013;4:2508. doi: 10.1038/ncomms3508. [DOI] [PubMed] [Google Scholar]
- 69.Robinson PK. Enzymes: principles and biotechnological applications [published correction appears in Essays Biochem. 2015;59:75] Essays Biochem. 2015;59:1–41. doi: 10.1042/bse0590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marino JS, Stechschulte LA, Stec DE, Nestor-Kalinoski A, Coleman S, Hinds TD., Jr Glucocorticoid receptor β induces hepatic steatosis by augmenting inflammation and inhibition of the Peroxisome Proliferator-activated Receptor (PPAR) α. J Bio Chem. 2016;291:25776–25788. doi: 10.1074/jbc.M116.752311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Debose-Boyd RA. Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res. 2008;18(6):609–621. doi: 10.1038/cr.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jo Y, Debose-Boyd RA. Control of cholesterol synthesis through regulated ER-associated degradation of HMG CoA reductase. Crit Rev Biochem Mol Biol. 2010;45(3):185–198. doi: 10.3109/10409238.2010.485605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferramosca A, Di Giacomo M, Zara V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J Gastroenterol. 2017;23(23):4146–4157. doi: 10.3748/wjg.v23.i23.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for the study is available with STS.
NA