Abstract
In Saccharomyces cerevisiae, POL3 encodes the catalytic subunit of DNA polymerase δ. While yeast POL3 mutant strains that lack the proofreading exonuclease activity of the polymerase have a strong mutator phenotype, little is known regarding the role of other Pol3p domains in mutation avoidance. We identified a number of pol3 mutations in regions outside of the exonuclease domain that have a mutator phenotype, substantially elevating the frequency of deletions. These deletions appear to reflect an increased frequency of DNA polymerase slippage. In addition, we demonstrate that reduction in the level of wild-type DNA polymerase results in a similar mutator phenotype. Lowered levels of DNA polymerase also result in increased sensitivity to the DNA-damaging agent methyl methane sulfonate. We conclude that both the quantity and the quality of DNA polymerase δ is important in ensuring genome stability.
The low mutation rate observed in wild-type cells reflects both the accuracy of DNA polymerases and the existence of DNA repair systems that remove misincorporated bases. Mutations affecting components of either of these systems can result in a mutator phenotype, a global elevation in mutation frequencies throughout the genome (27). In the yeast Saccharomyces cerevisiae, a mutator phenotype has been associated with certain mutations of POL3 and POL2, encoding the replicative DNA polymerases δ and ɛ, respectively (12, 22, 37, 55); these alleles can reside in either a DNA-proofreading exonuclease domain of POL3 (Exo I domain [Fig. 1]) or near domains required for nucleotide binding (domains II and VI). Certain mutant substitutions of POL30, encoding the DNA polymerase processivity factor PCNA, also have a strong mutator phenotype (8, 20, 23, 56). In addition, null mutations of RAD27 (encoding an Okazaki fragment-processing enzyme) or certain alleles of RPA1 (encoding the large subunit of a single-stranded DNA binding protein) substantially elevate mutation rates (7, 19, 22, 54). A number of mutants in Schizosaccharomyces pombe, including those affecting DNA polymerases α and δ and DNA ligase, also exhibit increased rates of mutation (33).
FIG. 1.
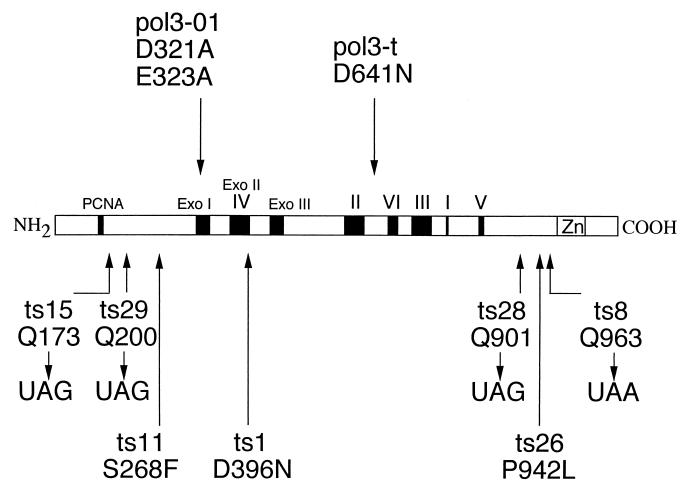
Arrangement of conserved domains in yeast Pol3p (16) and locations of amino acid alterations in POL3 mutants. POL3 encodes a polypeptide of 124 kDa (1,097 amino acids). The coding sequence includes three regions corresponding to the exonuclease proofreading active site (Exo I, II, and III; Exo II is contained within domain IV). Other conserved domains include a catalytic center (domain I), regions thought to be involved in nucleotide binding (domains II, III, and V), and a putative zinc finger DNA binding domain (Zn) (58). A putative PCNA-interacting domain (PCNA) is located in the N-terminal region of the protein (61). The locations of the mutator alleles pol3-01 (37) and pol3-t (12) are shown in the upper half of the diagram. The amino acid substitutions of the unique POL3 missense and nonsense alleles characterized in this paper are indicated in the lower half of the diagram.
In addition to mutations affecting DNA replication genes, mutations of the DNA mismatch repair genes have a mutator phenotype. Most mismatch repair in yeast involves two complexes, although other complexes have minor roles (24). Base-base mismatches are corrected by a heterotetramer involving Msh2p, Msh6p, Pms1p, and Mlh1p. Small DNA loops, resulting from DNA polymerase slippage events on simple repetitive DNA sequences (microsatellites) (Fig. 2A), are repaired by a complex that includes Msh2p, Msh3p, Pms1p, and Mlh1p. Failure to repair base-base mismatches results in an elevated frequency of single-base-pair substitutions, whereas failure to repair DNA loops results in an elevated frequency of deletions or insertions (47). Genetic and biochemical data indicate that the Msh2p-Msh3p Pms1p-Mlh1p complex can correct DNA loops up to 14 bases in size but is incapable of correcting loops that are 16 bases or greater (13, 35, 48, 49, 51, 52).
FIG. 2.
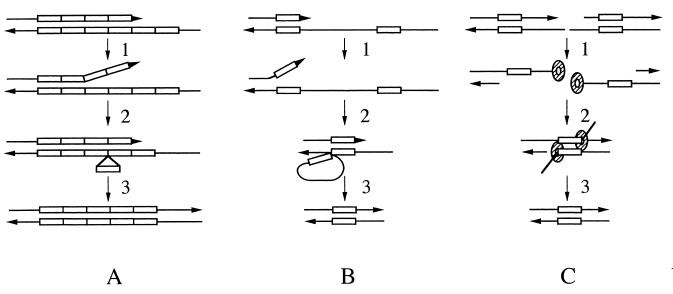
Deletions involving repetitive DNA elements. (A) Deletions generated within simple repetitive tracts by DNA polymerase slippage. The top strand of each pair represents the primer strand, and the bottom strand represents the complementary template. The horizontal arrows indicate the 3′ ends of the template strands, and the triangles show the 3′ ends of the primer strands. The rectangles illustrate the repeat units within the tracts. As replication proceeds through this repeat region, polymerase dissociation can allow strand separation (step 1) followed by misaligned reannealing (step 2), resulting in a loop of unpaired bases on the template strand consisting of an integral number of repeat units. Uncorrected loops would result in a deletion of a repeat unit(s) following the next round of replication (step 3). Insertions can also be generated if the unrepaired loop appears on the primer strand. (B) Deletions between nontandem direct repeats generated by DNA polymerase slippage. The rectangles represent two small repeats of identical sequence. Following replication of one of these units, polymerase dissociation and strand separation (step 1) can be followed by reannealing of the 3′ end of the primer to the complementary region of the downstream repeat unit. The resulting intermediate contains a loop on the template strand consisting of one of the repeat units and the unique sequence between the repeats (step 2). Failure to correct this loop results in a large deletion (step 3). (C) Deletions between nontandem direct repeats generated by SSA (40). The bottom and top strand of each structure represents the template strand and two adjacent Okazaki fragments, respectively, with DNA synthesis proceeding from left to right. The rectangles represent two small units of identical sequence. Prolonged exposure of single-stranded DNA on the template strand can result in cleavage within this region, forming a double-strand break. The 5′ ends present at the break can be resected by an exonuclease activity (step 1). Exposure of the complementary nontandem-repeat units allow these short tracts to anneal (step 2). This annealing may be promoted by Rad52p (shown as shaded circles). Processing of the 3′ noncomplementary tails (step 3) results in a deletion.
Although some mutators affect only DNA replication or DNA mismatch repair, other mutators are likely to affect both processes. For example, some pol30 alleles both reduce the efficiency of DNA mismatch repair and increase the rate of DNA polymerase slippage (8, 20, 56).
Mutators differ in two ways: the degree to which they elevate mutation rates and the types of mutations that are elevated. For purposes of this discussion, we will classify mutations as single-base-pair substitutions, frameshifts (additions or deletions of repeat units to a repetitive tract of DNA), or additions and deletions involving nonrepetitive DNA sequences. Mutant substitutions affecting the proofreading exonuclease domain of DNA polymerase (pol3-01 and pol2-4) greatly increase the frequency of base pair substitutions but have a modest effect on the stability of long microsatellites (37, 51). A different allele of POL3 (pol3-t) and several of the POL30 and RPA1 alleles greatly increase the rate of deletions involving nonrepetitive DNA sequences (7, 8, 12), whereas mutations in RAD27 increase the frequency of duplications (19, 22, 54). Mutations that affect DNA mismatch repair enzymes elevate both single-base-pair substitutions and frameshifts, but the elevation observed for frameshifts is much greater (47).
Since only two mutator alleles of POL3 have been described, we decided to do a genetic screen for new mutator alleles of this gene. Following random mutagenesis of the entire POL3 gene, we isolated and characterized three new missense mutants. We unexpectedly also isolated a number of other mutator alleles that were nonsense mutations within POL3. As discussed below, our analysis of these mutants and strains in which the level of DNA polymerase δ was reduced demonstrated a novel mechanism for producing a mutator phenotype.
MATERIALS AND METHODS
Media.
Standard enriched (YPD) or minimal (SD) growth medium for yeast was utilized (46). Media for the growth of strains containing POL3 under the control of the GAL1/10 promoter consisted of 1% Bacto Yeast Extract, 2% Bacto Peptone, 3% raffinose (YPR), and various concentrations of galactose as indicated.
Yeast strains and plasmids.
The yeast strains used in this study are listed in Table 1. All of the strains are isogenic with AMY125 (α ade5-1 leu2-3 trp1-289 ura3-52 his7-2 [51]) except for changes introduced by transformation. EAS63a (provided by E. Sia) was a derivative of MS71 in which the mating type was switched, the ade5-1 mutation was reverted, and a poly(GT) tract 29 bp in length was inserted into the ADE2 gene (ade2-100). RJK48 is a leu2 derivative of EAS63a constructed by targeted disruption of the LEU2 gene with BglII-treated pNKY85 (1).
TABLE 1.
Yeast strains
| Strain | Relevant genotypea | Source or construction |
|---|---|---|
| MS71 | LEU2 | 52 |
| EAS63a | a ade2-100 ADE5 LEU2 | E. A. Sia |
| RJK48 | a ade2-100 ADE5 | Described in Materials and Methods |
| HM6 | LEU2 rad52Δ::hisG-URA3-hisG | H. Moore |
| GCY140 | LEU2 msh3Δ | 52 |
| RJK341 | LEU2 kanMX-GAL1-(3×HA)-POL3 | Described in Materials and Methods |
| RJK368-4 | LEU2 kanMX-GAL1-(3×HA)-POL3 pep4::URA3 | Described in Materials and Methods |
| MS71-pol3-t | LEU2 pol3-t | 22 |
| JED213-30 | LEU2 (3×HA)-POL3 | Described in Materials and Methods |
| RJK394 | LEU2 (3×HA)-POL3 pep4::URA3 | Described in Materials and Methods |
| RJK366-5 | LEU2 pep4::URA3 | Described in Materials and Methods |
| RJK370 | a rad52Δ::hisG-URA3-hisG | Spore from RJK364b |
| RJK396 | a LEU2 | Spore from RJK372b |
| RJK397 | rad52Δ::hisG-URA3-hisG | Spore from RJK372b |
| RJK398 | kanMX-GAL1-(3×HA)-POL3 | Spore from RJK372b |
| RJK399 | a LEU2 rad52Δ::hisG-URA3-hisG kanMX-GAL1-(3×HA)-POL3 | Spore from RJK372b |
| LS31 | rev3Δ::kanMX | J. Gerton |
| EAS102 | LEU2 rad1Δ | E. A. Sia |
| RJK67-5 | a ade2-100 ADE5 + pBL304[CEN4/URA3/POL3] | RJK48 transformed with pBL304 |
| RJK78-3 | a ade2-100 ADE5 + pBK100 [CEN4/LEU2/POL3] | Described in Materials and Methods |
| RJK122-3 | a ade2-100 ADE5 pol3::kanMX + pBL304 | Described in Materials and Methods |
| RJK160-1 | a ade2-100 ADE5 pol3::kanMX + pBK100 | Plasmid shuffle of RJK122-3c |
| RJK158-3 | a ade2-100 ADE5 pol3::kanMX + pBK101 [CEN4/LEU2/pol3-ts1] | Plasmid shuffle of RJK122-3c |
| RJK185-1 | a ade2-100 ADE5 pol3::kanMX + pBK102 [CEN4/LEU2/pol3-ts8] | Plasmid shuffle of RJK122-3c |
| RJK187-1 | a ade2-100 ADE5 pol3::kanMX + pBK103 [CEN4/LEU2/pol3-ts11] | Plasmid shuffle of RJK122-3c |
| RJK218-2 | a ade2-100 ADE5 pol3::kanMX + pBK104 [CEN4/LEU2/pol3-ts15] | Plasmid shuffle of RJK122-3c |
| RJK223-1 | a ade2-100 ADE5 pol3::kanMX + pBK105 [CEN4/LEU2/pol3-ts26] | Plasmid shuffle of RJK122-3c |
| RJK286-1 | a ade2-100 ADE5 pol3::kanMX + pBK106 [CEN4/LEU2/pol3-ts28] | Plasmid shuffle of RJK122-3c |
| RJK287-1 | a ade2-100 ADE5 pol3::kanMX + pBK107 [CEN4/LEU2/pol3-ts29] | Plasmid shuffle of RJK122-3c |
| RJK288 | a ade2-100 ADE5 pol3::kanMX rad52Δ::hisG-URA3-hisG + pBK101 | Spore from RJK272b |
| RJK334 | a ade2-100 ADE5 pol3::kanMX rad52Δ::hisG-URA3-hisG + pBK100 | Spore from RJK310b |
| RJK335 | a ade2-100 ADE5 pol3::kanMX rad52Δ::hisG-URA3-hisG + pBK100 | Spore from RJK310b |
| RJK357 | a ade2-100 ADE5 pol3::kanMX msh3Δ + pBK100 | Spore from RJK344b |
| RJK358 | a ade2-100 ADE5 pol3::kanMX msh3Δ + pBK101 | Spore from RJK345b |
| RJK363 | a ade2-100 ADE5 pol3::kanMX msh3Δ rad52Δ::hisG-URA3-hisG + pBK101 | Spore from RJK345b |
| RJK381 | a ade2-100 ADE5 pol3::kanMX msh3Δ rad52Δ::hisG-URA3-hisG + pBK101 | Spore from RJK373b |
| RJK379 | a ade2-100 ADE5 pol3::kanMX msh3Δ rad52Δ::hisG + pBK101 | 5-FOAr isolate of RJK363 |
| RJK242-1 | a ade2-100 ADE5 pol3::kanMX + pBK100 + pSH44 | RJK160-1 transformed with pSH44 |
| RJK166-4 | a ade2-100 ADE5 pol3::kanMX + pBK101 + pSH44 | RJK158-3 transformed with pSH44 |
| RJK202-3 | a ade2-100 ADE5 pol3::kanMX + pBK103 + pSH44 | RJK187-1 transformed with pSH44 |
| RJK232-5 | a ade2-100 ADE5 pol3::kanMX + pBK105 + pSH44 | RJK223-1 transformed with pSH44 |
| LS38-1 | a ade2-100 ADE5 pol3::kanMX msh3Δ + pBK100 + pSH44 | RJK357 transformed with pSH44 |
| LS40-1 | a ade2-100 ADE5 pol3::kanMX msh3Δ + pBK101 + pSH44 | RJK358 transformed with pSH44 |
| RJK380-1 | a ade2-100 ADE5 pol3::kanMX msh3Δ rad52Δ::hisG + pBK101 + pSH44 | RJK379 transformed with pSH44 |
All strains are isogenic with AMY125 (α trp1-289 his7-2 ura3-52 leu2-3 ade5-1 [51]) except for the changes introduced by transformation. Only alterations from the AMY125 genotype are shown.
Diploids referred to were constructed by the following crosses: RJK310 (RJK160-1 × HM6), RJK344 (RJK160-1 × GCY140), RJK345 (RJK288 × GCY140), RJK272 (RJK158-3 × HM6), RJK373 (RJK358 × HM6), RJK364 (LS31 × RJK288), and RJK372 (RJK341 × RJK370).
These strains were generated by transforming RJK122-3 with mutagenized pBK100, followed by screening for derivatives of the transformed strain that lost the pBL304 plasmid.
The plasmid pBL304 contains the wild-type POL3 gene cloned into YCp50. The plasmid pBK100, in which the URA3 of pBL304 was replaced with LEU2, was generated in vivo by transforming the strain RJK67-5 (RJK48 plus pBL304) with a PCR fragment containing the LEU2 gene flanked by 40 bp of URA3 homology; this fragment was generated by PCR using SalI/HindIII-digested pRS315 (LEU2) as a template and the primers 5′-AACCCTTGGCAGAACATATCCATCGCGTCCGCCATCTCCAtcaattgtcctgtacttcc and 5′-GTGATTCAT TCTGCTAACCAGTAAGGCAACCCCGCCAGCCtaaggccgtttctgacaga (the bases in lowercase indicate LEU2-specific sequences).
To delete the chromosomal copy of POL3, we transformed RJK67-5 with a PCR-generated fragment in which the kanMX gene is flanked by DNA sequences derived from the regions upstream and downstream of POL3. The oligonucleotides used for the amplification were 5′-TTGCTATTAAGCATTAATCTTTATACATATACGCACAGCAcgtacgctgcaggtcgac and (downstream) 5′-CCTTTCTTAATCCTAATATGATGTGCCACCCTATCGTTTTatcgatgaattcgagctcg (the lowercase letters correspond to kanMX sequences); the substrate for the amplification reaction was a plasmid containing Tn903 (57). The resulting strain was RJK122-3. Strains similar to RJK122-3 with plasmid-borne copies of POL3 or mutant pol3 genes were isolated by the plasmid shuffle technique described below. To create strains bearing rad52Δ and/or msh3Δ in combination with the plasmid-borne POL3 genes, we formed diploids by crossing appropriate haploid strains, sporulated the diploids, and identified spore colonies that contained the desired genotypes.
The strain RJK341 contains a version of POL3 in which an epitope (3 × HA) is inserted at the N terminus of POL3 and the promoter is replaced with the GAL1/10 promoter; a kanMX cassette is immediately upstream of the GAL1/10 promoter. This strain was constructed by transforming MS71 with a DNA fragment generated by PCR amplification of pFA6a-kanMX6-PGAL1-3HA as a template (34) using the primers 5′-TATTGAGCACTTGCTATTAAGCATTAATCTTTATACATATgaattcgagctcgtttaaac and 5′-TCTTCACATCAACCATGGGAAGGGATCTTTTTTCACTCATgcactgagcagcgtaatctg. The uppercase sequences correspond to nucleotides −49 to −10 and nucleotides +40 to +1 relative to the POL3-initiating AUG. The construction replaces the 9 nucleotides just upstream of the POL3-initiating AUG with the kanMX cassette, the GAL1/10 promoter, and the 3×HA tag. The epitope tag is present with its own initiating codon downstream of the GAL1/10 promoter and is in frame and directly upstream of the entire POL3 coding sequence.
The strain JED213-30 has a 3×HA epitope tag inserted into a wild-type POL3 gene. This strain was constructed by transforming the strain MS71-pol3-t (which has a temperature-sensitive pol3 allele) with a PCR fragment designed to simultaneously revert the pol3-t allele and insert the 3×HA epitope tag at the beginning of the POL3 coding sequence. This PCR fragment was generated using genomic DNA from strain RJK341 as a template with the following primers: upstream, 5′-AAATAGATATTGAGCACTTGCTATTAAGCATTAATCTTTATACATATACGCACAGCAatgtctttaattaacatcttt, and downstream, 5′-CAATAGAAACCAAGGAACAGGAATC. The sequence shown in uppercase on the first primer corresponds to nucleotides −57 to −1 relative to the POL3 start codon, and the lowercase letters correspond to the first 21 nucleotides of the 3×HA epitope tag coding sequence. The downstream primer consists of nucleotides 2545 to 2521 of POL3. Transformants were selected by growth at 37°C.
PEP4 was deleted by replacing it with URA3. The DNA fragment used for this replacement was generated by PCR using pRS306 (URA3) as the template and the following two primers (PEP4 sequences are capitalized): upstream, 5′-GTA TTTAATCCAAATAAAATTCAAACAAAAACCAAAACTAACcgcttttcaattca attc, and downstream, 5′-GCAGAAAAGGATAGGGCGGAGAAGTAAGAAAAGTTTAGCcagggtaataactgatataa.
To evaluate the effects of various mutations on dinucleotide repeat instability, we transformed appropriate strains with pSH44 [CEN TRP1] (15). This plasmid contains a 33-bp poly(GT) tract inserted in frame within the URA3 coding sequence.
POL3 mutagenesis and mutant isolation.
The POL3 gene was randomly mutagenized by treating pBK100 with hydroxylamine (50). Following mutagenesis of pBK100, POL3 mutants were isolated by the plasmid shuffle technique (50). Yeast strain RJK122-3 was transformed with 1 μg of the mutagenized pBK100, plated on solid minimal medium lacking leucine, and incubated at 22°C for 5 days. The Leu+ colonies were replica plated onto two plates containing minimal medium lacking leucine and containing 5-fluoroorotate (5-FOA). This selection (4) allows for growth of cells that have lost the pBL304 plasmid and retained the mutagenized plasmid. Isolates containing potential temperature-sensitive lethal POL3 mutants were distinguished by growth on 5-FOA medium at 22°C and loss of growth on this medium at 37°C.
Assays for cell viability and sensitivity to DNA damage.
For the experiments that tested for cell viability upon loss of [PSI+], strains were grown at 22°C on solid YPD medium to single colonies. The colonies were replica plated onto YPD medium with or without 5 mM guanidine hydrochloride (GuHCl) and grown overnight at 22°C. The replica plating was repeated twice more onto identical media. To test the damage sensitivity of the temperature-sensitive POL3 mutants, we pregrew yeast strains on solid YPD medium for 2 days at 22°C. For the similar experiments that utilized the strain bearing the GAL1-POL3 construct, strains were pregrown on solid YPR medium containing 0.05% galactose for 2 days at 30°C. Fivefold serial dilutions of each strain were spotted onto appropriate media. To test for UV sensitivity, we treated patches of cells of various serial dilutions with UV light from a germicidal lamp. MMS (methyl methane sulfonate) sensitivity was examined by spotting serial dilutions on appropriate solid medium containing 0.025% MMS. All plates were incubated for 3 to 4 days in the dark at 30°C.
Determination of spontaneous mutation rates and mutational spectra.
The forward mutation rate at the CAN1 1ocus was determined by standard methods (48), using at least 12 independent cultures for each rate estimate. Rates were calculated from the frequencies of canavanine-resistant mutants by using the method of the median (28). For strains bearing the GAL1/10 promoter upstream of POL3, the cells were pregrown in YPR medium containing appropriate concentrations of galactose.
The mutant substitutions in CAN1 were analyzed by PCR amplification of the 1.8-kb CAN1 gene. Primers located at three positions within the gene were used in the sequence analysis. For some experiments, the 1.8-kb CAN1 gene was treated with SspI and the resulting fragments (with sizes of 515, 652, and 718 bp) were examined by electrophoresis using 2% Metaphor (FMC Corp.) agarose gels; this method can detect deletions as small as 8 bp. The extents of deletions that removed one or both ends of CAN1 were determined using 14 primer pairs to amplify sequences centromere-distal to CAN1 (7). The sequence of deletions followed by telomeric additions was determined by a PCR procedure utilizing a series of degenerate primers (7).
Analysis of plasmid-borne simple-repeat instability.
Yeast strains were transformed with pSH44 to determine the rate of instability within a 33-bp poly(GT) tract by methods described previously (15). Alterations within the repetitive tract were determined by PCR amplification of the repetitive region and analysis by gel electrophoresis as described previously (48).
Western blot analysis.
Liquid cultures for RJK368-4 were grown in YPR medium containing various concentrations of galactose and those for RJK366-5 and RJK394 were grown in YPD medium to mid-log phase. Protein extracts from spheroplasts of these strains were prepared as described previously (2). Proteins were fractionated on a sodium dodecyl sulfate–7.5% polyacrylamide gel, transferred to nitrocellulose, and blocked by standard procedures (43). Membranes were probed with mouse antihemagglutinin (anti-HA) monoclonal antibody (clone 12CA5) (Boehringer Mannheim). Detection of antibody binding was performed using horseradish peroxidase-linked anti-mouse immunoglobulin G (Amersham). Quantitation of protein bands was performed on a scanning densitometer (Molecular Dynamics). Quantitation was based on the intensity of a band specific to Pol3p normalized by the intensity of a band representing a protein that is nonspecifically recognized by the HA antibody.
Statistical methods.
Calculation of 95% confidence intervals was done as described previously (22). Fisher exact tests were performed using the InStat version 1.12 program.
RESULTS
Isolation and phenotypes of yeast POL3 missense mutants.
In order to identify new mutator alleles of DNA polymerase δ, we isolated mutants of the yeast POL3 gene by the plasmid shuffle technique (50). The plasmid pBK100 (YCp POL3 LEU2) was randomly mutagenized by treating the plasmid DNA with hydroxylamine. This mutagenized plasmid was transformed into a yeast strain containing pBL304 (YCp POL3 URA3) and a deletion of the POL3 chromosomal locus. Cells derived from each transformant that lost pBL304 were detected on medium containing 5-FOA, which selects against Ura+ cells (4). In order to increase the probability of identifying strains with a mutagenized POL3 gene, we screened the transformants containing mutagenized pBK100 (7,500 strains examined) for temperature sensitivity (loss of growth at 37°C) on minimal growth medium containing 5-FOA; POL3 is an essential gene in yeast, and temperature-sensitive alleles of POL3 were previously isolated (14). Ten independent strains with a temperature-sensitive pol3 mutation were identified. DNA sequence analysis of these mutant alleles showed that four (pol3-ts1, pol3-ts11, pol3-ts18, and pol3-ts26), representing three different alterations (pol3-ts1 is equivalent to pol3-ts18), contained single missense mutations within the POL3 gene. The positions and amino acid alterations of these mutations are shown in Fig. 1.
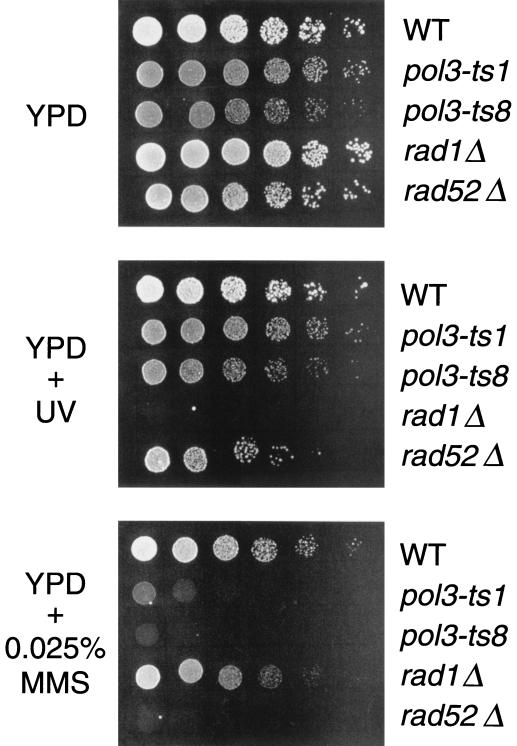
These POL3 missense mutants were examined for abnormal cell growth at the semipermissive temperature (30°C) and were screened for sensitivity to DNA damage. Many of the mutant cells had buds approximately the same size as the mother cell. The percentages of cells with this morphology at 30°C for the wild type, pol3-ts1, pol3-ts11, and pol3-ts26 were 7, 39, 18, and 63%, respectively (500 cells were counted). After the incubation of cells at 37°C for 20 h, the percentages of cells with this morphology for the wild-type, pol3-ts1, pol3-ts11, and pol3-ts26 were 6, 98, 95, and 95%, respectively (500 cells were counted). The accumulation of large-budded cells at 37°C suggests that the growth defect associated with these mutant strains is due to a defect in DNA replication that does not allow for efficient completion of S phase, since a similar position of cell cycle arrest is observed for other types of mutations affecting DNA synthesis (29). Each POL3 mutant also exhibited moderate to high sensitivity to MMS but no sensitivity to UV radiation, suggesting that the mutant polymerases confer specific defects in the repair of damaged DNA. An example of these phenotypes for the pol3-ts1 mutants is shown in Fig. 3. Strains with rad1Δ (conferring sensitivity to UV) or rad52Δ (conferring sensitivity to MMS) mutations are shown as controls.
FIG. 3.
Sensitivity of pol3 mutant strains to DNA damage. Fivefold serial dilutions of each strain were spotted onto YPD plates in the presence of MMS or subjected to UV irradiation. The strains examined were RJK160-1 (wild-type [WT]; top rows), RJK158-3 (missense mutant pol3-ts1; second rows), RJK185-1 (nonsense mutant pol3-ts8, third rows), EAS102 (rad1Δ; fourth rows), and HM6 (rad52Δ, bottom rows). The results were similar for all POL3 missense and nonsense mutants identified in this study. The rad1Δ strain was used as a control for UV sensitivity, and the rad52Δ strain was the control for MMS sensitivity.
POL3 missense mutants exhibit mutator phenotypes and dissimilarities in mutational spectra.
To determine if the strains bearing the POL3 missense mutations conferred a mutator phenotype, we assayed for the forward mutation rate at the CAN1 locus in vivo. Wild-type cells are sensitive to the arginine analog canavanine, and any mutation that inactivates the arginine permease encoded by CAN1 results in canavanine resistance. We found that strains bearing any of the three POL3 alleles exhibit four- to sixfold-elevated rates of mutation at CAN1 (Table 2).
TABLE 2.
Forward mutation rates at CAN1 in strains with POL3 missense mutations and/or mutations affecting DNA repair or recombination
| Strain genotypea | Rate of canavanine resistance (10−7 per cell division)b | 95% Confidence interval (10−7 per cell division) |
|---|---|---|
| WT | 2.4 (1) | 2.3–2.9 |
| pol3-ts1 | 14 (6) | 10–18 |
| pol3-ts11 | 10 (4) | 9.4–13 |
| pol3-ts26 | 9.7 (4) | 8.5–11 |
| msh3 | 2.5 (1) | 2.1–3.2 |
| rad52 isolate 1 | 27 (11) | 24–33 |
| rad52 isolate 2 | 29 (12) | 25–40 |
| pol3-ts1 msh3 | 47 (20) | 42–62 |
| pol3-ts1 rad52 | 63 (26) | 48–77 |
| pol3-ts1 msh3 rad52 isolate 1 | 50 (21) | 45–75 |
| pol3-ts1 msh3 rad52 isolate 2 | 42 (18) | 22–48 |
Strain names are as follows: RJK160-1 (wild type [WT]), RJK158-3 (pol3-ts1), RJK187-1 (pol3-ts11), RJK223-1 (pol3-ts26), RJK357 (msh3), RJK334 (rad52 isolate 1), RJK335 (rad52 isolate 2), RJK358 (pol3-ts1 msh3), RJK288 (pol3-ts1 rad52), RJK363 (pol3-ts1 msh3 rad52 isolate 1), and RJK381 (pol3-ts1 msh3 rad52 isolate 2).
Numbers given in parentheses are fold increase over wild type.
Although all of these strains are mutators, they do not display the same spectrum of mutations. The mutational spectrum for each strain was determined by sequencing the CAN1 gene from approximately 20 independent canavanine-resistant (Canr) isolates. As shown in Table 3, over one-half of the mutations generated in the pol3-ts1 and -ts11 strains were deletions larger than a few base pairs. The deletions within CAN1 in the pol3-ts1 strain ranged from 16 to 336 bp in length, while the pol3-ts11 mutation resulted in deletions of 16 to 864 bp. One distinguishing characteristic of each of the sequenced CAN1 deletions is the presence of an imperfect direct repeat flanking each deletion. These repeat motifs range from 5 to 11 bp (Table 4); with one exception, each pair of flanking repeats has a perfect match of at least 75% of the bases. These types of deletions have been previously observed in strains bearing the rfa1-t29 allele of S. cerevisiae (7) and several pol3 alleles of S. pombe (33). In addition, one canavanine-resistant isolate in the pol3-ts11 strain exhibited a complete deletion of approximately 35,000 bp of one end of chromosome V (including the entire CAN1 gene) and the addition of a telomeric sequence (repeats of TG1–3); the breakpoint of this deletion mutation occurred in a GT-rich region of the chromosome (Table 3). Telomeric additions have previously been observed in other strains with mutations affecting DNA repair or replication (6, 7) or associated with repair of an HO-induced DNA break (25). Only 20% of the mutations generated within these pol3 strains are base substitutions. Since 55% of the sequenced mutations in an isogenic wild-type strain are substitutions, it appears that pol3-ts1 and -ts11 have a small (or no) effect on the accumulation of this class of mutation.
TABLE 3.
Mutational spectra at CAN1 in strains with POL3 missense mutations and/or mutations affecting DNA repair or recombination
| Strain genotypea | Type and frequency of mutation
|
|||
|---|---|---|---|---|
| Base substitutions | Alterations within simple repeats | Deletions (bp) flanked by direct repeats | Other | |
| WT | ||||
| Total | 55% (11/20) | 15% (3/20) | 5% (1/20) | 25% (5/20) |
| C to T (3/20) | G3 to G4 (1/20) | 18 (1/20) | 1-bp deletion (1/20) | |
| T to A (2/20) | A6 to A5 (1/20) | 5-bp insertion (1/20) | ||
| G to T (2/20) | A5 to A4 (1/20) | 5-bp duplication (1/20)b | ||
| G to A (2/20) | 23-bp duplication (1/20) | |||
| T to C (1/20) | 32-bp duplication (1/20) | |||
| C to G (1/20) | ||||
| pol3-ts1 | ||||
| Total | 20% (4/20) | 5% (1/20) | 65% (13/20) | 10% (2/20) |
| G to C (1/20) | A4 to A3 (1/20) | 16 (1/20) | CCAGGT to TTCTCCA (1/20) | |
| C to G (1/20) | 18 (3/20) | GGT to TGG (1/20) | ||
| C to A (1/20) | 38 (2/20) | |||
| C to T (1/20) | 42 (1/20) | |||
| 49 (1/20) | ||||
| 51 (1/20) | ||||
| 59 (1/20) | ||||
| 99 (1/20) | ||||
| 108 (1/20) | ||||
| 336 (1/20) | ||||
| pol3-ts11 | ||||
| Total | 21% (4/19) | 5% (1/19) | 58% (11/19) | 16% (3/19) |
| G to C (1/19) | T6 to T5 (1/19) | 16 (1/19) | 1-bp deletion (1/19) | |
| C to T (1/19) | 18 (1/19) | 40-bp deletion (1/19) | ||
| C to G (1/19) | 27 (1/19) | Telomere addition (1/19)c | ||
| G to T (1/19) | 34 (1/19) | |||
| 38 (1/19) | ||||
| 49 (1/19) | ||||
| 142 (1/19) | ||||
| 239 (1/19) | ||||
| 662 (1/19) | ||||
| 804 (1/19) | ||||
| 864 (1/19) | ||||
| pol3-ts26 | ||||
| Total | 70% (14/20) | 15% (3/20) | 15% (3/20) | 0% (0/20) |
| C to G (3/20) | T6 to T7 (1/20) | 8 (1/20) | ||
| G to C (3/20) | A4 to A3 (1/20) | 16 (1/20) | ||
| G to A (3/20) | (GTT)2 to (GTT)1 (1/20) | 27 (1/20) | ||
| T to A (2/20) | ||||
| A to T (2/20) | ||||
| C to T (1/20) | ||||
| msh3Δ | ||||
| Total | 32% (6/19) | 25% (5/19) | 11% (2/19) | 32% (6/19) |
| C to T (2/19) | A2 to A1 (1/19) | 8 (1/19) | TTCC to CCC (1/19) | |
| G to T (2/19) | C3 to C2 (1/19) | 10 (1/19) | TAT to CAG (1/19) | |
| G to A (1/19) | T4 to T3 (1/19) | 1-bp deletion (1/19) | ||
| C to G (1/19) | (AT)2 to (AT)1 (1/19) | 6-bp duplication (1/19) | ||
| (TTTA)2 to (TTTA)1 (1/19) | 11-bp duplication (1/19) | |||
| 49-bp duplication (1/19) | ||||
| rad52Δ | ||||
| Total | 80% (16/20) | 5% (1/20) | 0% (0/20) | 15% (3/20) |
| G to C (4/20) | (AT)2 to (AT)1 (1/20) | TC to AA (1/20) | ||
| C to A (3/20) | TTATT to ATATTT (1/20) | |||
| G to A (3/20) | Two base substitutions: G to A and T to A (1/20) | |||
| A to T (2/20) | ||||
| A to G (1/20) | ||||
| C to G (1/20) | ||||
| A to C (1/20) | ||||
| C to T (1/20) | ||||
| pol3-ts1 msh3Δ | ||||
| Total | 13% (5/39) | 49% (19/39) | 36% (14/39) | 2% (1/39) |
| C to A (1/39) | C2 to C1 (1/19) | 8 (5/39) | Two base substitutions: T to G and C to T (1/39) | |
| C to T (1/39) | C3 to C2 (2/39) | 12 (3/39) | ||
| T to A (1/39) | C4 to C3 (1/39) | 13 (1/39) | ||
| G to T (1/39) | (AG)4 to (AG)3 (10/39) | 16 (1/39) | ||
| C to G (1/39) | (TTTA)2 to (TTTA)1 (2/39) | 26 (1/39) | ||
| (GGCTT)2 to (GGCTT)1 (1/39) | 29 (1/39) | |||
| (CATTAT)2 to (CATTAT)1 (2/39) | 38 (2/39) | |||
| pol3-ts1 rad52Δ | ||||
| Total | 85% (17/20) | 5% (1/20) | 0% (0/20) | 10% (2/20) |
| C to G (6/20) | T6 to T5 (1/20) | GT5GTAT to AT6TTAT (1/20) | ||
| T to A (3/20) | 1-bp deletion (1/20) | |||
| G to A (2/20) | ||||
| C to A (2/20) | ||||
| G to C (2/20) | ||||
| A to T (1/20) | ||||
| T to C (1/20) | ||||
| pol3-ts1 msh3Δ rad52Δ (isolate 1) | ||||
| Total | 61% (11/18) | 17% (3/18) | 11% (2/18) | 11% (2/18) |
| C to A (4/18) | G2 to G1 (1/18) | 12 (1/18) | 1-bp deletion (2/18) | |
| C to T (2/18) | T4 to T5 (1/18) | 16 (1/18) | ||
| C to G (2/18) | (AG)4 to (AG)3 (1/18) | |||
| A to C (1/18) | ||||
| G to C (1/18) | ||||
| T to G (1/18) | ||||
| pol3-ts1 msh3Δ rad52Δ (isolate 2) | ||||
| Total | 37% (7/19) | 32% (6/19) | 11% (2/19) | 21% (4/19) |
| C to T (2/19) | A3 to A4 (1/19) | 8 (1/19) | TTTAAA to TTTA (1/19) | |
| T to G (2/19) | G3 to G2 (1/19) | 18 (1/19) | C1 to C0 (1/19) | |
| G to T (2/19) | T6 to T5 (1/19) | T1 to T0 (1/19) | ||
| C to G (1/19) | T4 to T6 (1/19) | A5C to A6G (1/19) | ||
| T3 to T2 (1/19) | ||||
| (TTTA)2 to (TTTA)1 (1/19) | ||||
Strain names are as follows: RJK160-1 (wild type [WT]), RJK158-3 (pol3-ts1), RJK187-1 (pol3-ts11), RJK223-1 (pol3-ts26), RJK357 (msh3), RJK334 (rad52), RJK358 (pol3-ts1 msh3), RJK288 (pol3-ts1 rad52), RJK363 (pol3-ts1 msh3 rad52 isolate 1), and RJK381 (pol3-ts1 msh3 rad52 isolate 2).
All duplications had short direct repeats flanking the duplicated segment, similar to those observed in rad27 strains (54).
Telomere addition. This isolate contains a loss of chromosome V DNA up to nucleotide 34842 (as defined by Stanford Saccharomyces Genome Database) and the addition of a telomere of indeterminate length. The DNA sequence at this breakpoint is as follows (capital letters represent chromosome V DNA, and lowercase letters represent the new telomeric sequence): ACCTGCAGTGGAGGGTGTggtgtggtgtgtg.
TABLE 4.
Sequences flanking large deletions observed in pol3-ts1 strain
| Deletion size (bp) | Sequencea | Extent of homology between direct repeats (bases) |
|---|---|---|
| 16 | CATGGAGACAtctactggtggtgacaAAGTTTTCGA | 7 of 8 |
| 18 | ATTTACGTTGGTtcccgtattttatttggtCTATCAAAG | 8 of 11 |
| 18 | CTTCTACATTGGctctctattattcattggACTTTTAGT | 9 of 11 |
| 18 | ATATCTCTGTTTtcctgttcttagctgtttGGATCTTAT | 6 of 6 |
| 38 | TATTTACGTTGGttcc…gaacaagttggCTCCTAAA | 6 of 7 |
| 38 | CGTTGGTTCCCgtat…caagttggctcctAAATTCCT | 8 of 10 |
| 42 | GTTCCATACATtgca…tttggcttacatGGAGACATC | 6 of 7 |
| 49 | GGTGCTGGGGTtacc…gtgcctggggtCCAGGTAT | 7 of 7 |
| 51 | GATTAACGCTGCcttc…tggtgaagctgcAAACCCCA | 7 of 9 |
| 59 | AATGCCCGGCTTggct…gtttcacggcttTTGCACCA | 8 of 10 |
| 99 | CCATACATTGCAGtttt…ctggtgttgcagGCTTTTTT | 6 of 6 |
| 108 | AAAGGTGGTGTTccat…tatcactggtgttGCAGGCTT | 7 of 7 |
| 336 | TGTATTGGTTTTCttgg…taggttgggtttcCTCTTTGAT | 8 of 9 |
Deleted sequence is shown in lowercase. Imperfect direct repeats are underlined. Bases that differ within each repeat pair are shown in italics.
In contrast to the results obtained with pol3-ts1 and -ts11, 70% of all Canr isolates derived from the pol3-ts26 allele are single-base substitutions. Fifteen percent of the CAN1 mutations in this strain background were deletions flanked by imperfect direct repeats; however, these deletions are much smaller (8 to 27 bp) than those observed in pol3-ts1 and -ts11. Thus, it appears that this mutation near the C terminus of the protein primarily increases the rate of base substitutions.
We also examined the effects of these polymerase mutations on the instability of a 33-bp poly(GT) tract. This instability is measured by a frameshift assay (15) utilizing a plasmid (pSH44) containing a repetitive GT sequence fused upstream and in frame to URA3. In yeast strains transformed with this plasmid, additions or deletions of repeat units within the poly(GT) tract that result in an out-of-frame URA3 sequence can be detected by growth in medium containing 5-FOA. This assay has previously been used to demonstrate that mutations in genes required for DNA mismatch repair (48, 51, 52) and DNA replication (RAD27 and POL30) (19, 20, 22, 23, 56) significantly enhance the rate of microsatellite instability. We found that pol3-ts1 elevates the frequency of tract instability about twofold and that neither pol3-ts11 nor pol3-ts26 had any significant effect (Table 5).
TABLE 5.
Effect of pol3 missense mutations on 33-bp dinucleotide repeat instability
| Strain genotypea | Rate of 5-FOAr (10−6 per cell division)b | 95% Confidence interval (10−6 per cell division) |
|---|---|---|
| WT | 4.3 (1) | 1.6–7.3 |
| pol3-ts1 | 10 (2.3) | 6.3–13 |
| pol3-ts11 | 5.0 (1.2) | 3.3–6.2 |
| pol3-ts26 | 5.6 (1.3) | 2.3–10 |
| msh3 | 200 (47) | 160–310 |
| pol3-ts1 msh3 | 660 (150) | 530–990 |
| pol3-ts1 msh3 rad52 | 240 (55) | 170–350 |
Strains containing transformed pSH44 are as follows RJK242-1 (wild type [WT]), RJK166-4 (pol3-ts1), RJK202-3 (pol3-ts11), RJK232-5 (pol3-ts26), LS38-1 (msh3), LS40-1 (pol3-ts1 msh3), and RJK380-1 (pol3-ts1 msh3 rad52).
Numbers given in parentheses are fold increase over wild type.
Effect of msh3 mutation on accumulation of genomic deletions in pol3-ts1 and -ts11 strains.
A possible source of the deletions observed in the strains bearing pol3-ts1 and -ts11 is DNA polymerase slippage. This mechanism has commonly been invoked to explain the instability of simple repetitive DNA (Fig. 2A) (53). A deletion flanked by imperfect repeats may also occur by this mechanism (Fig. 2B). Once replication proceeds through one homologous region, reannealing of separated strands may occur between the newly synthesized primer strand and a homologous region further downstream on the template, resulting in formation of a DNA loop. Failure to repair this loop would ultimately result in a deletion of the type that is prevalent in pol3-ts1 and -ts11 strains.
It has previously been shown that the DNA mismatch repair system efficiently corrects loops ranging from 1 to 14 nucleotides in length (49, 60). All of the deletions in the “deletions flanked by direct repeats” class in the pol3-ts1 and -ts11 strains were 16 bp or greater (Table 3). One explanation of this observation is that slippage events resulting in DNA loops of less than 16 bp are not observed because such loops are efficiently repaired by the DNA mismatch repair system. This interpretation predicts that deletions of less than 16 bp would be observed in the polymerase mutant strains in the absence of DNA mismatch repair. To test this hypothesis, we combined pol3-ts1 with a null mutation of MSH3, a MutS homolog that specifically functions in the correction of DNA loops up to 14 nucleotides in length (48, 49).
We found that while the mutation rate at the CAN1 locus was unaffected by msh3Δ in a wild-type strain, there was a synergistic effect between pol3-ts1 and msh3Δ. While the rate of mutation for pol3-ts1 was 6-fold greater than that of the wild type, a 20-fold effect was observed for pol3-ts1 msh3Δ (Table 2). Sequencing of 39 Canr isolates from the double-mutant strain reveals two classes of mutations rarely seen in the pol3-ts1 single mutant (Table 3). One class consists of deletions in microsatellite sequences within CAN1 in which the size of the repeat unit ranges from 1 to 6 bp. Approximately one-half (19 of 39) of the Canr isolates in the pol3-ts1 msh3 strain are of this class, which is significantly different from the frequency seen in the pol3-ts1 single mutant (P = 0.001; Fisher exact test). One common deletion that appeared in 25% of the independent isolates is an (AG)4 to (AG)3 deletion at positions 254 to 261 of the CAN1 open reading frame. We also transformed the pol3-ts1 msh3 strain with pSH44 in order to assay the effects of these two mutations on the instability of a 33-bp poly(GT) tract (Table 5). We found that the effects of these two mutations are multiplicative. This strain exhibited a 150-fold enhancement in instability over the wild type for this dinucleotide repeat. Sequencing of the repeat tract from 10 independent 5-FOAr isolates showed that 70% of the mutations were frameshifts resulting from 2-bp deletions within the repeat tract. These results are similar to the effects seen for these two mutations on the rate of deletion formation within CAN1 microsatellites.
The second class of mutations consists of deletions flanked by imperfect direct repeats in which the size of the deletion is less than 16 bp. These deletions were observed in approximately one-quarter (9 of 39) of the mutations in the pol3-ts1 msh3 strain. This distribution is significantly different from that seen in the pol3-ts1 strain, in which this deletion class is entirely absent (0 of 20) (P = 0.02). In addition to these two unique classes of mutations, larger deletions flanked by direct repeats persist in the pol3-ts1 msh3 strain, but they are less frequent than in the pol3-ts1 strain (5 of 39). In addition, the sizes of these deletions in the double-mutant strain appear to be generally smaller (16 to 38 bp) than the deletions in the pol3-ts1 strain. We did a more limited analysis of an additional 60 independent Canr isolates in the pol3-ts1 msh3 strain. Rather than sequencing all of these isolates, only those that exhibited apparent deletions by a PCR-restriction digest screen were chosen for sequencing. This screening allowed for detection of deletions as small as 8 bp by gel electrophoresis. Of these 60 isolates, 11 were deletions ranging from 8 to 14 bp and 9 were larger deletions (18 to 135 bp). The frequencies at which these classes of deletions were present were similar to those that were observed in the original 39 Canr isolates.
High rate of deletion formation in the pol3-ts1 strain depends on Rad52p.
To investigate the role of Rad52p in the generation of deletions in the pol3-ts1 strain, we combined rad52Δ with the polymerase mutation. This double-mutant strain grew much more slowly than either of the two single-mutant strains. We also found that the effect of these two mutations on the overall mutation rate at CAN1 was additive (Table 2). In addition, loss of Rad52p activity eliminated all of the deletions between direct repeats that are generated in the pol3-ts1 strain (Table 3). A similar effect was previously observed when the rfal-29 allele was combined with rad52Δ (7). These data indicate that Rad52p is required for the formation of deletions in the polymerase mutant strain.
When rad52Δ was combined with pol3-ts1 msh3, we found that for two independently derived isolates, the overall rate of mutation was identical to that of pol3-ts1 msh3 (Table 2). In the triple-mutant strain, however, there is a significant reduction in the frequency at which frameshifts within microsatellites and deletions flanked by direct repeats are generated relative to a pol3-ts1 msh3 strain (P < 0.0001 for comparison to RJK363 and P = 0.0017 for comparison to RJK381) (Table 3). In addition, the high rate of poly(GT) instability seen in the pol3-ts1 msh3 strain decreased by a factor of 3 in the absence of Rad52p (Table 5).
Although rad52Δ in combination with pol3-ts1 has its most striking effect on the rate of deletions, loss of Rad52p also substantially increased the rate at which base substitutions are generated in pol3-ts1. Most (85%) of the mutations in pol3-ts1 rad52 were base substitutions, while only 20% of the mutations in pol3-ts1 were of this class. Based on the overall rates of mutation and the fraction of mutations that are base substitutions for each strain, we found that the rates at which base substitutions are generated are 1.3 × 10−7 (wild type; relative rate of 1×), 2.8 × 10−7 (pol3-ts1; 2×), 2.2 × 10−6 (rad52; 17×), and 5.4 × 10−6 (pol3-ts1 rad52; 42×) per cell division. This result suggests that the effects of the mutant polymerase and the loss of Rad52p activity operate synergistically to generate a high rate of point mutations.
POL3 nonsense mutations exhibit a mutator phenotype and are viable as a consequence of PSI-mediated nonsense suppression.
In addition to the missense mutants described above, we also identified five mutants (pol3-ts8, -tsl5, -ts27, -ts28, and -ts29) representing four unique nonsense mutants of POL3 (pol3-ts27 is equivalent to pol3-ts29). The positions of these mutations are shown in Fig. 1. One mutant (pol3-17), which was not investigated further, had three mutant substitutions (silent mutation at Y336, L781F, and Q963UAA). All four nonsense mutants examined were temperature sensitive and MMS sensitive (Fig. 3). In addition, a large fraction of large budded cells were observed at both 30 and 37°C. The percentages of cells with this morphology at 30°C for the wild type and pol3-ts8, -ts15, -ts28, and -ts29 were 7, 37, 35, 42, and 39%, respectively (500 cells were counted). After incubation of the cells at 37°C for 20 h, the percentages of cells with this morphology for the wild type and pol3-ts8, -ts15, -ts28, and -ts29 were 6, 83, 42, 83, and 65%, respectively (500 cells were counted). The pol3-ts15 mutant had a smaller fraction of large-budded cells than the other mutants at 37°C and also displayed a less severe growth defect at this temperature than the other mutants.
Each of these nonsense mutants elevated the rate of mutation at the CAN1 locus between 5- and 14-fold. The mutation rates (all 10−7/cell division; 95% confidence limits are shown in parentheses) for the wild type and pol3-ts8, -ts15, -ts28, and -ts29 were 2.4 (2.3 to 2.9), 20 (17 to 22), 12 (8 to 14), 20 (16 to 26), and 33 (30 to 55). We examined the mutational spectra at CAN1 for the nonsense mutants pol3-ts8 and pol3-ts15. As was observed for pol3-ts1 and pol3-ts11, over one-half of the mutations within CAN1 generated in strains bearing either of these two nonsense mutations were deletions of 16 bp or greater (Table 6) flanked by imperfect direct repeats (data not shown). One mutation identified in the pol3-ts8 strain had a deletion followed by a telomere addition that was similar to one of the observed mutations in the pol3-ts11 strain.
TABLE 6.
Mutational spectra at CAN1 in strains with POL3 nonsense mutations
| Strain genotypea | Type and frequency of mutation
|
|||
|---|---|---|---|---|
| Base substitutions | Alterations within simple repeats | Deletions (bp) flanked by direct repeats | Other | |
| pol3-ts8 | ||||
| Total | 22% (2/9) | 11% (1/9) | 56% (5/9) | 11% (1/9) |
| C to A (1/9) | (AT)2 to (AT)1 (1/9) | 18 (1/9) | Telomere addition (1/9)b | |
| A to T (1/9) | 27 (1/9) | |||
| 30 (1/9) | ||||
| 38 (1/9) | ||||
| 662 (1/9) | ||||
| pol3-ts15 | ||||
| Total | 33% (3/9) | 0% (0/9) | 56% (5/9) | 11% (1/9) |
| C to T (1/9) | 16 (2/9) | 17-bp duplication (1/9)c | ||
| G to A (1/9) | 18 (1/9) | |||
| C to G (1/9) | 268 (1/9) | |||
| 426 (1/9) | ||||
Strain names are as follows: RJK185-1 (pol3-ts8) and RJK218-2 (pol3-ts15).
Telomere addition. This isolate contains a loss of chromosome V DNA up to nucleotide 34831 (as defined by Stanford Saccharomyces Genome Database) and the addition of a telomere of indeterminate length. The DNA sequence at this breakpoint is as follows (capital letters represent chromosome V DNA, and lowercase letters represent the new telomeric sequence): GTGGAGGGTGTTGTTGTGGAGTgggtgtggtgtgtg.
All duplications had short direct repeats flanking the duplicated segment, similar to those observed in rad27 strains (54).
Since POL3 is an essential gene and several of the mutations were located upstream of the catalytic domain of Pol3p, our recovery of viable cells with these mutations was very surprising. One plausible explanation for viability, the existence of a nonsense-suppressing tRNA gene in the genetic background, was unlikely, since such suppressors in yeasts are codon specific (17) and we had identified both amber (UAG) and ochre (UAA) pol3 mutants.
A different type of suppressor, which can elevate the readthrough of all nonsense codons, is the prion-like [PSI+] factor (31, 32, 59). The [PSI+] factor, encoded by the SUP35 gene, is a misfolded form of the translational termination factor eRF-3. [PSI+] can be cured from a yeast strain by plating cells on enriched medium containing low levels of GuHCl (59). To test for the presence of a [PSI+]-like element in our host strain, we examined the viability of the strains containing the POL3 nonsense mutations on medium containing GuHCl. We found that all of the temperature-sensitive POL3 nonsense mutants failed to grow on enriched medium containing GuHCl while an isogenic POL3+ strain and all of the POL3 missense mutants grew normally (data not shown). Since loss of viability under growth conditions that cure yeast of [PSI+] was specific to the presence of POL3 nonsense mutations, it appears that [PSI+]-mediated nonsense suppression is responsible for the viability of strains containing these POL3 mutations.
Reduced expression of POL3 elevates mutation rates.
There are two explanations of the mutator phenotype exhibited by the pol3 nonsense mutations. Since [PSI+]-mediated suppression involves the insertion of an amino acid in place of the termination codon, this substitution may yield a mutator DNA polymerase. Alternatively, since the efficiency of [PSI+]-mediated suppression in yeast is reported to be very low (∼1%) (30), the mutator phenotype may reflect a very low level of fully functional DNA polymerase. To distinguish between these alternatives, we replaced the native promoter of POL3 with the galactose-inducible GAL1/10 promoter; in addition, we inserted an epitope tag (HA) at the beginning of the gene in order to monitor the level of the protein. From previous studies, it has been shown that the level of gene expression from the GAL1/10 promoter can be regulated by altering the concentration of galactose (3). In most of our experiments, cells were grown in a constant level of the noninducing carbon source raffinose (3%) and the level of galactose was varied from 0.5 to 0%. In a few experiments, we grew cells in 2% glucose. In glucose-containing medium, the level of expression from the GAL1/10 promoter is even lower than that from cells grown in 3% raffinose in the absence of glucose because glucose-grown cells repress expression of the GAL1/10 promoter (45).
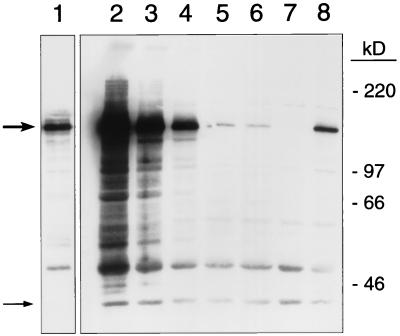
To monitor the level of DNA polymerase δ, we performed Western blot analysis (using antibodies directed against the HA epitope) on protein extracts of cells grown in various types of media. As expected, the level of DNA polymerase δ was related to the concentration of galactose in the medium (Fig. 4). As a control, we monitored the level of DNA polymerase δ in a strain in which the same HA tag was inserted into a POL3 gene with its native promoter (Fig. 4, lane 8). Giving the level of DNA polymerase in this control strain a value/1, the approximate levels of DNA polymerase in cells grown in 3% raffinose plus 0.5% galactose (lanes 1 and 2), 3% raffinose plus 0.05% galactose (lane 3), 3% raffinose plus 0.1% galactose (lane 4), 3% raffinose plus 0.005% galactose (lane 5), and 3% raffinose without galactose (lane 6) were 27, 8, 3, 0.07, and 0.04, respectively.
FIG. 4.
Effect of galactose concentration on Pol3p levels in GAL1-POL3 strain. Cell lysates were prepared and probed for 3×HA-Pol3p by Western blot analysis as described in Materials and Methods. The location of 3×HA-Pol3p is indicated with a thick arrow, and the position of a protein nonspecifically recognized by the HA antibody (used as a loading control) is indicated with a thin arrow. The positions of the molecular mass standards are shown on the right. Lanes 1 to 6 contain lysates of RJK368-4 (GAL1-3×HA-POL3 pep4) grown in enriched medium containing 3% raffinose and the following galactose concentrations: lanes 1 and 2, 0.5%; lane 3, 0.05%; lane 4, 0.01%; lane 5, 0.005%; lane 6, no galactose. Lane 7 contains a lysate of RJK366-5 (POL3 pep4) grown in 2% glucose; lane 8 contains a lysate of RJK394 (3×HA-POL3 pep4) grown in 2% glucose. The lysates in lanes 7 and 8 are from strains in which POL3 transcription is driven from the native POL3 promoter. The amount of lysate loaded was approximately 30 μg in lanes 2 to 8 and 6 μg in lane 1. The band pattern shown in lane 1 was generated from a much shorter film exposure than those shown in lanes 2 to 8. The intensities of the Pol3p bands in lanes 2 to 6 relative to the Pol3p band intensity in lane 8 are 32, 11, 2.8, 0.084, and 0.060, respectively. Similar relative intensities derived from a second independent set of lysates were 6.1, 3.0, 3.0, 0.052, and 0.022. From a third set of lysates, the relative intensities of the Pol3p band for the samples in lanes 2 and 3 were 44 and 11, respectively. Averages among all the data sets for each growth condition are presented in Results.
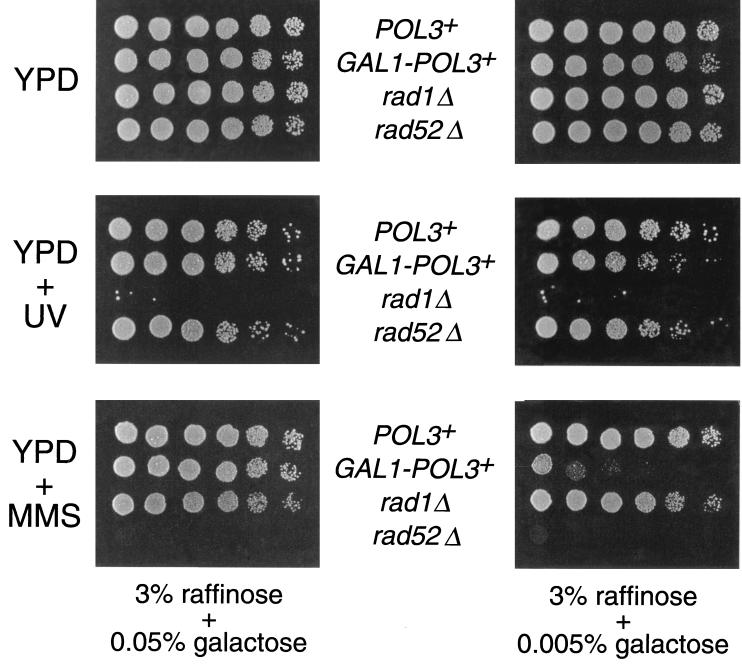
The effects of reducing POL3 expression on cell growth and sensitivity to DNA damage were evaluated. When it was grown with reduced levels of galactose (0.005%), we found the yeast strain containing this GAL1-POL3 construct exhibited a fairly high level of sensitivity to MMS but no apparent UV sensitivity (Fig. 5). In addition, cell viability was sensitive to the level of DNA polymerase δ in the absence of Rad52p. As shown in Fig. 6, lowering the galactose concentration from 0.05 to 0.005% noticeably lowered the ability of a GAL1-POL3 rad52Δ strain to sustain growth. One interpretation of this result is that a reduction in the level of DNA polymerase δ leads to an increase in the level of DNA double-strand breaks.
FIG. 5.
Effects of a reduction in the level of DNA polymerase δ on DNA repair processes. Fivefold serial dilutions of strains MS71 (POL3; top row of each panel), RJK341 (GAL1-POL3; second row), EAS102 (rad1Δ; third row), and HM6 (rad52Δ; bottom row) were spotted onto YPR plates containing the indicated concentrations of galactose in the presence of MMS or subjected to UV irradiation.
FIG. 6.
A reduction in the level of Pol3p reduces cell viability in the absence of Rad52p. Fivefold serial dilutions of strains RJK396 (POL3 RAD52; top row of each panel), RJK397 (POL3 rad52Δ; second row), RJK398 (GAL1-POL3 RAD52; third row), and RJK399 (GAL1-POL3 rad52Δ; bottom row) were spotted onto plates containing the indicated medium. All four strains were derived from the same tetrad following sporulation and dissection of diploid strain RJK372. The growth pattern shown here is identical to that from two additional tetrads of the same genotypes.
Surprisingly, we observed no apparent loss in viability or temperature sensitivity when the growth medium contained low levels of galactose in a RAD52 strain with the GAL1-POL3 construct (RJK398) (Fig. 6 and data not shown). There was, however, a larger percentage of large-budded cells (40%) present during growth with 0.005% galactose compared to that seen during growth with 0.05% galactose (10% large-budded cells). In the absence of galactose (3% raffinose), a condition in which there is a very low level of DNA polymerase δ, the growth rate is substantially reduced at both 30 and 37°C, although there was still no apparent temperature sensitivity under these growth conditions. In addition, a much higher percentage of cells grown in the absence of galactose are large budded (65%). Furthermore, most of the unbudded cells are enlarged and irregular in shape. The above-mentioned growth defects in the absence of galactose are further exacerbated when this strain is grown in enriched medium containing only glucose (YPD). Cells incubated in YPD medium grew very slowly and were not further evaluated.
When the strain bearing the GAL1-POL3 construct was examined for the CAN1 mutation rate, we observed a gradual increase in the mutator phenotype as the level of Pol3p was reduced. The rates of can1 mutations in the strain with the GAL1/10-POL3 gene (all 10−7/cell division; 95% confidence limits are in parentheses) when grown in 3% raffinose plus 0.5% galactose, 3% raffinose plus 0.05% galactose, 3% raffinose plus 0.01% galactose, 3% raffinose plus 0.005% galactose, and 3% raffinose without galactose were 1.5 (1.3 to 1.6), 1.7 (1.6 to 2.1), 5.6 (4.9 to 7.4), 15 (11 to 18), and 56 (43 to 95), respectively. When the strain with the HA-tagged POL3 gene and the native promoter was grown in 3% raffinose plus 0.05% galactose, 3% raffinose without galactose, and YPD medium, the mutation rates (all 10−7/cell division; 95% confidence limits are in parentheses) were 2.6 (2.2 to 3.6), 2.6 (2.1 to 3.6), and 4.4 (3.4 to 5.7), respectively. Finally, when a strain with an untagged version of the POL3 gene with its native promoter was grown in medium containing 3% raffinose plus 0.05% galactose, we observed a rate of mutation of 1.4 (1.1 to 1.9) × 10−7/cell division. We conclude from these studies that reducing the level of fully functional DNA polymerase δ can substantially elevate global mutation rates, whereas overexpression of DNA polymerase δ does not result in a mutator phenotype.
We also examined the spectra of mutations generated in cells with low levels of DNA polymerase (Table 7). Although cells grown in medium with 3% raffinose plus 0.05% galactose (no mutator effect) had a spectrum of alterations similar to that observed in the wild-type strain, the same strain grown in medium with 3% raffinose and 0.005% galactose or no galactose had a significantly elevated frequency of deletions between direct repeats. Thus, the mutator phenotype in strains with reduced levels of Pol3p resembles that observed for some of the POL3 missense mutations and the POL3 nonsense mutations.
TABLE 7.
Effect of POL3 expression level on mutational spectra at CAN1 in yeast strain RJK341
| Growth media | Type and frequency of mutation
|
|||
|---|---|---|---|---|
| Base substitutions | Alterations within simple repeats | Deletions (bp) flanked by direct repeats | Other | |
| YPR + 0.05% galactose | ||||
| Total | 84% (16/19) | 0% (0/19) | 5% (1/19) | 11% (2/19) |
| G to T (4/19) | 206 (1/19) | ATA to TT (1/19) | ||
| G to A (3/19) | TT to ATA (1/19) | |||
| C to T (3/19) | ||||
| C to A (2/19) | ||||
| A to T (1/19) | ||||
| T to C (1/19) | ||||
| T to G (1/19) | ||||
| T to A (1/19) | ||||
| YPR + 0.005% galactose | ||||
| Total | 32% (6/19) | 5% (1/19) | 58% (11/19) | 5% (1/19) |
| G to C (2/19) | T5 to T4 (1/19) | 15 (1/19) | 1-bp deletion (1/19) | |
| T to A (2/19) | 16 (2/19) | |||
| C to G (1/19) | 18 (2/19) | |||
| C to T (1/19) | 24 (1/19) | |||
| 39 (2/19) | ||||
| 49 (1/19) | ||||
| 53 (2/19) | ||||
| YPR | ||||
| Total | 40% (8/20) | 5% (1/20) | 45% (9/20) | 10% (2/20) |
| G to C (3/20) | A5 to A6 (1/20) | 8 (2/20) | GTA to TTT (1/20) | |
| T to A (2/20) | 16 (2/20) | Two base substitutions: A to C and G to C (1/20) | ||
| C to G (1/20) | 17 (1/20) | |||
| A to T (1/20) | 29 (1/20) | |||
| C to A (1/20) | 59 (1/20) | |||
| 81 (1/20) | ||||
| 1,077 (1/20) | ||||
DISCUSSION
The low level of mutations observed in wild-type cells is a consequence of the serial operation of multiple levels of control of the accuracy of DNA replication: base selectivity by DNA polymerase, exonucleolytic proofreading, and postreplicative DNA mismatch repair (44). If frameshifts within simple repetitive DNA sequences and deletions between direct repeats reflect DNA polymerase slippage events, the low level of such alterations in wild-type cells is likely to reflect the processivity of replicative DNA polymerases and the efficiency of postreplicative DNA mismatch repair. One might expect, therefore, mutator alleles of DNA polymerase that reduce base selectivity, the efficiency of proofreading, or the processivity of replication. In addition, a DNA polymerase mutation could indirectly result in a mutator phenotype. For example, if the level of an accurate replicative DNA polymerase was lowered, its role could be partially taken over by an error-prone DNA polymerase.
One of the POL3 missense mutants, pol3-ts26, elevated the rate of base substitution mutations with only a minor effect on the rate of deletions. This mutant polymerase could have a partial defect in base selectivity or proofreading. Strains with the other two missense mutations (pol3-ts1 and pol3-ts11), the nonsense pol3 mutations, or reduced levels of DNA polymerase δ had elevated levels of deletions, as well as base pair substitutions; these deletions, like those observed in rfa1 strains (7), involved interactions between short dispersed repeats.
Mechanism of deletion formation.
Deletions could result from DNA polymerase slippage (Fig. 2B) or single-strand annealing (SSA) (Fig. 2C). Our conclusion that many of the deletions result from DNA polymerase slippage is based primarily on a comparison of the sizes of the deletions generated in pol3 mutant strains and in strains with both pol3 and msh3 mutations. In single-mutant pol3-ts1 and pol3-ts11 strains, all deletion mutations involving the CAN1 locus were single-base-pair deletions in homopolymeric regions or deletions of at least 16 bp. In contrast, in a strain with both the pol3-ts1 and msh3 mutations, over 70% of the deletions were more than 1 bp but less than 16 bp (Table 3). Msh3p is involved in the repair of DNA loops ranging in size from 1 to 14 bp (48, 60). We suggest, therefore, that the pol3-ts1 and pol3-ts11 mutations result in increased levels of DNA polymerase slippage and increased rates of formation of DNA loops. Since DNA loops smaller than 16 bp are efficiently corrected in single-mutant pol3 strains, we observe an increase in mutation frequency only in deletions of 16 bp or larger. In the double-mutant pol3 msh3 strains, deletions of both classes are observed, as expected by the DNA polymerase slippage model. This model also predicts the synergistic effect of pol3-ts1 and msh3 on mutation rates at the CAN1 locus and the synergistic destabilization of the poly(GT) tract.
An alternative model for the generation of deletions is by SSA (Fig. 2C). Although SSA requires MSH3 (as well as MSH2, RAD1, and RAD10), if the distance between interacting repeats is greater than 60 bp (39), deletions smaller than 60 bp could be generated by this mechanism. However, since this mechanism does not explain the lack of deletions of less than 16 bp in the pol3-ts1 strain and the prevalence of these deletions in the pol3-ts1 msh3 strain, we prefer the DNA polymerase slippage model. Several additional points concerning this conclusion should be mentioned. First, our conclusion is rigorous only for deletions of 16 bp or less, although it seems reasonable that somewhat larger deletions are also generated by DNA polymerase slippage. Second, it is likely that very large deletions (greater than 1 kb) represent SSA events. Chen et al. (7) found that a mutant allele of RFA1 elevated frequencies of large deletions (mostly larger than 6 kb) in the CAN1 locus; they reported that deletion formation was dependent on Rad10p, as expected for SSA events. It should be pointed out that, although Rad1p and Rad10p have roles in the meiotic repair of large DNA loops (21), rad1 strains do not appear to be deficient in the mitotic repair of large DNA loops (E. Sia and T. Petes, unpublished data).
Rad52p is required for most types of homologous recombination in yeast, including SSA (40). In strains with mutations in POL3 and RAD52, very few deletions are observed. This observation can be interpreted in two ways. One possibility is that deletions are generated by SSA; as described above, this possibility is unlikely for deletions of less than 16 bp. An alternative possibility is that Rad52p promotes DNA polymerase slippage between short dispersed repeats. Following DNA strand dissociation, Rad52p could help the nascent strand seek out regions of homology in single-stranded regions of the replication fork. Using a different assay for mutations, Tran et al. (55) observed Rad52p dependence of deletion formation and suggested a nonrecombinational role for Rad52p in this process.
We observed a substantial increase in the rate of base substitutions in a rad52Δ strain and a synergistic effect between pol3-ts1 and rad52. It has previously been shown that loss of Rad52p function also increases the rate of base substitutions at the SUP4 locus and that this increase is dependent on the presence of the error-prone DNA polymerase ζ (42). Since DNA polymerase ζ normally functions in translesion DNA synthesis (11), the elevated mutation rates observed in the rad52Δ strain may be a consequence of error-prone repair synthesis at regions of spontaneous DNA damage. The synergistic effect of pol3-ts1 and rad52Δ on the rate of base substitutions may reflect an increase in DNA damage associated with the pol3-ts1 mutation.
One interpretation of the phenotypes associated with the pol3-ts1 and -ts11 mutations is that the mutant polymerases are less processive. Reduced processivity associated with phage T7 DNA polymerase lacking the processivity factor thioredoxin and a mutant of the Klenow fragment DNA polymerase has been correlated with increased microsatellite instability (26, 36). For the yeast DNA polymerase mutants, reductions in processivity may be a consequence of defective interactions with the processivity factor PCNA. Regions within the N-terminal region of Pol3p appear to interact with PCNA, and the processivity of DNA polymerase δ depends on this interaction (5, 61). Alternatively, increases in frameshift mutagenesis could reflect defective interactions within the polymerase active site, as observed for a mutant human DNA polymerase β (38). A final alternative is that the pol3-ts1 and -ts11 mutations result in lower levels of DNA polymerase, producing a mutator phenotype by the mechanisms described below for strains with low levels of DNA polymerase δ.
Nonsense mutations of POL3.
We recovered viable POL3 nonsense mutations because of [PSI]-mediated readthrough of the nonsense codons. Since this suppression is inefficient (30), the mutator phenotype of the POL3 nonsense mutations (increased frequencies of deletions with a smaller effect on base pair substitutions) presumably reflects the same mechanisms (described below) observed in strains with low levels of DNA polymerase δ. The temperature-sensitive phenotype of the nonsense mutations is likely to be a consequence of the temperature-sensitive efficiency of [PSI]-dependent nonsense suppression (18). One interesting consequence of this mechanism is the fact that, in strains with the nonsense POL3 mutations, the [PSI] factor (usually dispensable) is essential.
Mutator phenotype generated by depleted levels of DNA polymerase δ.
When the level of DNA polymerase δ was reduced, we observed elevated rates of deletions (presumably reflecting elevated rates of DNA polymerase slippage) and base pair substitutions. Since slippage could occur during the transition between discontinuous synthesis (associated with dissociation of DNA polymerase α from the template) and continuous synthesis (associated with attachment of DNA polymerase δ to the template), a reduction in the level of wild-type DNA polymerase molecules could result in increased slippage. Similarly, delayed DNA synthesis on the lagging strand could lead to increased single-stranded regions in the replication fork and the opportunity for the occurrence of slippage events that involve dispersed small repeats, resulting in deletions.
Although there is a strong inverse correlation between polymerase levels and mutation rates in the GAL1-POL3 strain, there is one apparent discrepancy. Growth of this strain in YPR medium containing 0.01% galactose results in a threefold increase in the level of DNA polymerase δ over that observed in a strain in which POL3 is normally expressed under the control of its native promoter. However, the mutation rate of the GAL1-POL3 strain under these growth conditions is twice that of the normal observed rate. One possible explanation is that GAL1-POL3 expression is constitutive while POL3 expression under its native promoter occurs primarily during S phase.
Although the most obvious mutator phenotype of reducing the level of DNA polymerase δ was an effect on the rate of deletion formation, base pair substitutions were also elevated. If reducing the level of DNA polymerase δ increases the size of single-stranded regions at the replication fork, there may be more spontaneous DNA damage. Such damage could have two consequences: (i) reduction in viability in strains with a rad52 mutation (as observed) and (ii) increased levels of mutations resulting from repair of damaged DNA by an error-prone DNA polymerase, such as DNA polymerase ζ. It is somewhat surprising that gross overproduction of DNA polymerase δ had no mutator phenotype, since dominant-negative effects caused by overproducing one subunit of a multienzyme complex are quite common in yeast (41).
Our observations may be relevant to understanding certain human diseases associated with genomic instability. One type of genomic instability (microsatellite instability) in patients with hereditary nonpolyposis colorectal cancer is associated with mutations in genes involved in DNA mismatch repair (9). We suggest that mutator variants of DNA polymerase δ or epigenetic reduction in the level of DNA polymerase δ could be another source of genome destabilization. Mutations of the human DNA polymerase δ gene have been detected in cancer cell lines, although the functional significance of these mutations has not yet been established (10). In order for mutations in DNA polymerase genes to promote tumor formation, they would need to have a strong mutator phenotype without a strong growth defect, possibly a difficult balance to achieve.
ACKNOWLEDGMENTS
We thank the members of the Petes lab for helpful discussions and suggestions throughout the course of this project, A. McKenzie III for advice on the construction of the GAL1-POL3 strain, P. Burgers for supplying pBL304, and R. Rybczynski for advice on the use of the scanning densitometer. We also thank Y. Chernoff for helpful discussions regarding [PSI].
This work was supported by NIH grants GM52319 (T.D.P.) and GM17879 (R.J.K.).
REFERENCES
- 1.Alani E A, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alarcon C M, Heitman J, Cardenas M E. Protein kinase activity and identification of a toxic effector domain of the target of rapamycin TOR proteins in yeast. Mol Biol Cell. 1999;10:2531–2546. doi: 10.1091/mbc.10.8.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aparicio O M, Gottschling D E. Overcoming telomeric silencing: a trans-activator competes to establish gene expression in a cell cycle-dependent way. Genes Dev. 1994;8:1133–1146. doi: 10.1101/gad.8.10.1133. [DOI] [PubMed] [Google Scholar]
- 4.Boeke J D, Lacroute F, Fink G R. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoroorotic acid resistance. Mol Gen Genet. 1984;167:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 5.Brown W C, Campbell J L. Interaction of proliferating cell nuclear antigen with yeast DNA polymerase δ. J Biol Chem. 1993;268:21706–21710. [PubMed] [Google Scholar]
- 6.Chen C, Kolodner R D. Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat Genet. 1999;23:81–85. doi: 10.1038/12687. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Umezu K, Kolodner R D. Chromosomal rearrangements occur in S. cerevisiae rfa1 mutator mutants due to mutagenic lesions processed by double-strand-break repair. Mol Cell. 1998;2:9–22. doi: 10.1016/s1097-2765(00)80109-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Merrill B J, Lau P L, Holm C, Kolodner R D. Saccharomyces cerevisiae pol30 (proliferating cell nuclear antigen) mutations impair replication fidelity and mismatch repair. Mol Cell Biol. 1999;19:7801–7815. doi: 10.1128/mcb.19.11.7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eshleman J R, Markowitz S D. Microsatellite instability in inherited and sporadic neoplasms. Curr Opin Oncol. 1995;7:83–89. [PubMed] [Google Scholar]
- 10.Flohr T, Dai J C, Buttner J, Popanda O, Hagmuller E, Thielmann H W. Detection of mutations in the DNA polymerase δ gene of human sporadic colorectal cancers and colon cancer cell lines. Int J Cancer. 1999;80:919–929. doi: 10.1002/(sici)1097-0215(19990315)80:6<919::aid-ijc19>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Friedberg E C, Gerlach V L. Novel DNA polymerases offer clues to the molecular basis of mutagenesis. Cell. 1999;98:413–416. doi: 10.1016/s0092-8674(00)81970-4. [DOI] [PubMed] [Google Scholar]
- 12.Gordenin D A, Malkova A L, Peterzen A, Kulikov V N, Pavlov Y I, Perkins E, Resnick M A. Transposon Tn5 excision in yeast: influence of DNA polymerases α, δ, and ɛ repair genes. Proc Natl Acad Sci USA. 1992;89:3785–3789. doi: 10.1073/pnas.89.9.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habraken Y, Sung P, Prakash L, Prakash S. Binding of insertion/deletion DNA mismatches by the heterodimer of yeast mismatch repair proteins MSH2 and MSH3. Curr Biol. 1996;6:1185–1187. doi: 10.1016/s0960-9822(02)70686-6. [DOI] [PubMed] [Google Scholar]
- 14.Hartwell L H. Sequential function of gene products relative to DNA synthesis in the yeast cell cycle. J Mol Biol. 1976;104:803–817. doi: 10.1016/0022-2836(76)90183-2. [DOI] [PubMed] [Google Scholar]
- 15.Henderson S, Petes T D. Instability of simple sequence DNA in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:2749–2757. doi: 10.1128/mcb.12.6.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hindges R, Hubscher U. DNA polymerase δ, an essential enzyme for DNA transactions. Biol Chem. 1997;378:345–362. doi: 10.1515/bchm.1997.378.5.345. [DOI] [PubMed] [Google Scholar]
- 17.Hinnebusch A G, Liebman S W. Protein synthesis and translational control in Saccharomyces cerevisiae. In: Broach J R, Pringle J R, Jones E W, editors. The molecular and cellular biology of the yeast Saccharomyces: genome dynamics, protein synthesis, and energetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1991. pp. 627–735. [Google Scholar]
- 18.Inge-Vechtomov S G, Mironova L N, Ter-Avanesian M D. Ambiguity of translation: an eukaryotic version? Russian J Genet. 1994;30:890–902. [PubMed] [Google Scholar]
- 19.Johnson R E, Kovvali G K, Prakash L, Prakash S. Requirement of the yeast RTH1 5′ to 3′ exonuclease for the stability of simple repetitive DNA. Science. 1995;269:238–240. doi: 10.1126/science.7618086. [DOI] [PubMed] [Google Scholar]
- 20.Johnson R E, Kovvali G K, Guzder S N, Amin N S, Holm C, Habraken Y, Sung P, Prakash L, Prakash S. Evidence for involvement of yeast proliferating cell nuclear antigen in DNA mismatch repair. J Biol Chem. 1996;271:27987–27990. doi: 10.1074/jbc.271.45.27987. [DOI] [PubMed] [Google Scholar]
- 21.Kirkpatrick D, Petes T D. Repair of DNA loops involves DNA mismatch and nucleotide excision repair proteins. Nature. 1997;387:929–931. doi: 10.1038/43225. [DOI] [PubMed] [Google Scholar]
- 22.Kokoska R J, Stefanovic L, Tran H T, Resnick M A, Gordenin D A, Petes T D. Destabilization of yeast micro- and minisatellite DNA sequences by mutations affecting a nuclease involved in Okazaki fragment processing (rad27) and DNA polymerase δ (pol3-t) Mol Cell Biol. 1998;18:2779–2788. doi: 10.1128/mcb.18.5.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokoska R J, Stefanovic L, Buermeyer A B, Liskay R M, Petes T D. A mutation of the yeast gene encoding PCNA destabilizes both microsatellite and minisatellite DNA sequences. Genetics. 1999;151:511–519. doi: 10.1093/genetics/151.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolodner R. Biochemistry and genetics of eukaryotic mismatch repair. Genes Dev. 1996;10:1433–1442. doi: 10.1101/gad.10.12.1433. [DOI] [PubMed] [Google Scholar]
- 25.Kramer K M, Haber J E. New telomeres in yeast are initiated with a highly selected subset of TG1–3. Genes Dev. 1993;7:2345–2356. doi: 10.1101/gad.7.12a.2345. [DOI] [PubMed] [Google Scholar]
- 26.Kunkel T A, Patel S S, Johnson K A. Error-prone replication of repeated DNA sequences by T7 DNA polymerase in the absence of its processivity unit. Proc Natl Acad Sci USA. 1994;91:6830–6834. doi: 10.1073/pnas.91.15.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunz B A, Ramachandran K, Vonarx E J. DNA sequence analysis of spontaneous mutagenesis in Saccharomyces cerevisiae. Genetics. 1998;148:1491–1505. doi: 10.1093/genetics/148.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lea D E, Coulson C A. The distribution of the number of mutants in bacterial populations. J Genet. 1949;49:264–285. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- 29.Lew D J, Weinert T, Pringle J R. Cell cycle control in Saccharomyces cerevisiae. In: Pringle J R, Broach J R, Jones E W, editors. The molecular and cellular biology of the yeast Saccharomyces: cell cycle and cell biology. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1997. pp. 607–695. [Google Scholar]
- 30.Liebman S W, Sherman F. Extrachromosomal PSI+ determinant suppresses nonsense mutations in yeast. J Bacteriol. 1979;139:1068–1071. doi: 10.1128/jb.139.3.1068-1071.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindquist S. Mad cows meet psi-chotic yeast: the expansion of the prion hypothesis. Cell. 1997;89:495–498. doi: 10.1016/s0092-8674(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 32.Lindquist S, Patino M M, Chernoff Y O, Kowal A S, Singer M A, Liebman S W, Lee K H, Blake T. The role of Hsp104 in stress tolerance and [PSI+] propagation in Saccharomyces cerevisiae. Cold Spring Harbor Symp Quant Biol. 1995;60:451–460. doi: 10.1101/sqb.1995.060.01.050. [DOI] [PubMed] [Google Scholar]
- 33.Liu V F, Bhaumik D, Wang T S-F. Mutator phenotype induced by aberrant replication. Mol Cell Biol. 1999;19:1126–1135. doi: 10.1128/mcb.19.2.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longtine M S, McKenzie III A, Demarini D J, Shah N G, Wach A, Brachat A, Philippsen P, Pringle J R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 35.Marsischky G T, Filosi N, Kane M F, Kolodner R D. Redundancy of Saccharomyces cerevisiae MSH3 and MSH6 in MSH2-dependent mismatch repair. Genes Dev. 1996;10:407–420. doi: 10.1101/gad.10.4.407. [DOI] [PubMed] [Google Scholar]
- 36.Minnick D T, Astatke M, Joyce C M, Kunkel T A. A thumb subdomain mutant of the large fragment of Escherichia coli DNA polymerase I with reduced DNA binding affinity, processivity, and frameshift fidelity. J Biol Chem. 1996;271:24954–24961. doi: 10.1074/jbc.271.40.24954. [DOI] [PubMed] [Google Scholar]
- 37.Morrison A, Sugino A. The 3′ to 5′ exonucleases of both DNA polymerases δ and ɛ participate in correcting errors in DNA replication in Saccharomyces cerevisiae. Mol Gen Genet. 1994;242:289–296. doi: 10.1007/BF00280418. [DOI] [PubMed] [Google Scholar]
- 38.Osheroff, W. P., W. A. Beard, S. Yin, S. H. Wilson, and T. A. Kunkel. Minor groove interactions at the DNA polymerase β active site modulate single-base deletion error rates. J. Biol. Chem., in press. [DOI] [PubMed]
- 39.Paques F, Haber J E. Two pathways for removal of nonhomologous DNA ends during double-strand break repair in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:6765–6771. doi: 10.1128/mcb.17.11.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paques F, Haber J E. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rine J. Gene overexpression in studies of Saccharomyces cerevisiae. In: Guthrie C, Fink G R, editors. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press; 1991. pp. 239–251. [Google Scholar]
- 42.Roche H, Gietz R D, Kunz B A. Specificities of the Saccharomyces cerevisiae rad6, rad18, and rad52 mutators exhibit different degrees of dependence on the REV3 gene product, a putative nonessential DNA polymerase. Genetics. 1995;140:443–456. doi: 10.1093/genetics/140.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 44.Schaaper R M. Base selection, proofreading, and mismatch repair during DNA replication in Escherichia coli. J Biol Chem. 1993;268:23762–23765. [PubMed] [Google Scholar]
- 45.Schneider J C, Guarente L. Vectors for expression of cloned genes in yeast: regulation, overproduction, and underproduction. In: Guthrie C, Fink G R, editors. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press; 1991. pp. 373–388. [DOI] [PubMed] [Google Scholar]
- 46.Sherman F. Getting started with yeast. In: Guthrie C, Fink G R, editors. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press; 1991. pp. 3–21. [Google Scholar]
- 47.Sia E A, Jinks-Robertson S, Petes T D. Genetic control of microsatellite stability. Mutat Res. 1997;383:61–70. doi: 10.1016/s0921-8777(96)00046-8. [DOI] [PubMed] [Google Scholar]
- 48.Sia E A, Kokoska R J, Dominska M, Greenwell P, Petes T D. Microsatellite instability in yeast: dependence on repeat unit size and DNA mismatch repair genes. Mol Cell Biol. 1997;17:2851–2858. doi: 10.1128/mcb.17.5.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sia E A, Kokoska R J, Dominska M, Greenwell P, Petes T D. Genetic control of microsatellite instability in Saccharomyces cerevisiae. In: Sarma R H, Sarma M H, editors. Structure, motion, interaction and expression of biological macromolecules. Albany, N.Y: Adenine Press; 1998. pp. 209–213. [Google Scholar]
- 50.Sikorski R S, Boeke J D. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. In: Guthrie C, Fink G R, editors. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press; 1991. pp. 302–318. [DOI] [PubMed] [Google Scholar]
- 51.Strand M, Prolla T A, Liskay R M, Petes T D. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature. 1993;365:274–276. doi: 10.1038/365274a0. [DOI] [PubMed] [Google Scholar]
- 52.Strand M, Earley M C, Crouse G F, Petes T D. Mutations in the MSH3 gene preferentially lead to deletions within tracts of simple repetitive DNA in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1995;92:10418–10421. doi: 10.1073/pnas.92.22.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Streisinger G, Okada Y, Emrich J, Newton J, Tsugita A, Terzaghi E, Inouye M. Frameshift mutations and the genetic code. Cold Spring Harbor Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Tishkoff D X, Filosi N, Gaida G M, Kolodner R D. A novel mutational avoidance mechanism dependent on S. cerevisiae RAD27 is distinct from mismatch repair. Cell. 1997;88:253–263. doi: 10.1016/s0092-8674(00)81846-2. [DOI] [PubMed] [Google Scholar]
- 55.Tran H T, Degtyareva N P, Koloteva N N, Sugino A, Masumoto H, Gordenin D A, Resnick M A. Replication slippage between distant short repeats in Saccharomyces cerevisiae depends on the direction of replication and the RAD50 and RAD52 genes. Mol Cell Biol. 1995;15:5607–5617. doi: 10.1128/mcb.15.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Umar A, Buermeyer A B, Simon J A, Thomas D C, Clark A B, Liskay R M, Kunkel T A. Requirement for PCNA in DNA mismatch repair at a step preceding DNA synthesis. Cell. 1996;87:65–73. doi: 10.1016/s0092-8674(00)81323-9. [DOI] [PubMed] [Google Scholar]
- 57.Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 58.Wang T S-F. Cellular DNA polymerases. In: DePamphilis M L, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 461–493. [Google Scholar]
- 59.Wickner R B. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 60.Wierdl M, Dominska M, Petes T D. Microsatellite instability in yeast: dependence on the length of the microsatellite. Genetics. 1997;146:769–779. doi: 10.1093/genetics/146.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang P, Mo J-Y, Perez A, Leon A, Liu L, Mazloum N, Xu H, Lee M Y W T. Direct interaction of proliferating cell nuclear antigen with p124 catalytic subunit of mammalian DNA polymerase δ. J Biol Chem. 1999;274:26647–26653. doi: 10.1074/jbc.274.38.26647. [DOI] [PubMed] [Google Scholar]