Abstract
Purpose
Physical exercise is necessary in the handling DM, but it is not distinct which kind of physical exercise can improve cardiac autonomic modulation in T2DM. The purpose of this study was to compare the effects of three different modalities of exercise (aerobic, resistant, and combined) on cardiac autonomic modulation as measured by HRV in patients with type 2 diabetic neuropathy.
Methods
The participants were 45 men (age: 55.24 ± 8.11 years, weight: 89.5 ± 13.4 kg, height: 171.85 ± 6.98 cm, duration of diabetes: 12.51 ± 6.46 years) with type 2 diabetic neuropathy who were clinically free from signs and symptoms of cardiovascular disease. Participants were randomly assigned to one of four groups: aerobic-training (n = 11), dynamic resistant-training (n = 11), Combined-training (n = 11), or a non-exercise control group (n = 12). The exercise groups performed aerobic and resistant exercise for 25–45 min per day, 3–5 times per week for 12 weeks. Anthropometrics, biochemical markers (FBS, HbA1c, Lipid Profile, and Insulin), and heart rate variability in the exercise laboratory and under ambulatory conditions by 3-channel ECG digital Holter recorder were examined.
Results
All time and frequency-domain HRV parameters (except LF power) were significantly improved in the exercise groups, compared with the control group (p < 0.05). SDNN, rMSSD, and HF power were boosted, LF power was not different, and the LF/HF ratio decreased after versus before exercise training, independent of exercise modality. HbA1c in aerobic and resistant-training groups, and insulin and LDL in the resistant-training group were decreased after exercise training (p < 0.05).
Conclusions
Exercise training, independent of modality, in patients with type 2 diabetic neuropathy who were clinically free of the cardiovascular disease cause to significant progress in cardiovascular autonomic function assessed by HRV via enhancing cardio-vagal and reducing cardio-sympathetic tone.
Keywords: Aerobic Training, Combined-training, Diabetes Mellitus, Heart Rate Variability, Resistant Training
Introduction
Type 2 diabetes mellitus (T2DM) has become one of the leading public health problems in modern society, partly due to low levels of physical activity [1]. It has been predicted that, all over, it will be the seventh, dominant cause of fatality by 2030 [2]. The feasibility of cardiovascular disease in subjects with diabetes is 2 to 4 times more than those without diabetes [3]. One of the most frequent complications and outcomes of chronic hyperglycemia is peripheral neuropathy [4], a serious cause of morbidity and mortality [5].
Screening for cardiac autonomic neuropathy (CAN) has been prescribed in cases with T2DM [6]. Early diagnosing malfunctions in the autonomic nervous system is critical to attenuate the progression of the CAN process [7]. Although standard cardiovascular reflex tests still being the golden norm for the valuation of cardiovascular autonomic neuropathy [8], one of the most comfortable and reliable methods to determine cardiac autonomic neuropathy is the estimation of the heart rate variability (HRV). HRV is the variation between two successive beats: the higher the variety, the greater the parasympathetic activity. A high HRV reveals that an individual can regularly accommodate micro-environmental changes [9]. Thus, low HRV is an indicator of cardiovascular danger [10]. Patients with T2DM exhibit a significant decrease in HRV due to reduced sympathetic and parasympathetic activity compared to non-T2DM patients [6], reflecting impairment in cardiovascular autonomic function [11].
Physical exercise is necessary in the handling of DM [12]; it promotes physical fitness, and functional capacity [13, 14], quality of life and well-being [15], increases metabolic regulation and insulin sensitivity [16], reduces inflammatory markers [17] and neuropathy symptoms [18], and increases the regenerative capacity of cutaneous axons, slowing or inhibiting neuropathy advancement [19]. Moreover, exercise can promote HRV in different populations, including healthy individuals and a variety of clinical populations, including patients with T2DM [20–23], but it is not distinct which kind of physical exercise can improve HRV in T2DM [24, 25]. Earlier trials have assessed the effect of aerobic-training [26–28] and of resistant-training [29, 30] on HRV among patients with T2DM. Only a few studies have investigated resistant-training combined with aerobic-training termed combined exercise training. Thus, the purpose of this study was to compare the effects of three different modalities of exercise (aerobic, resistant, and combined) on cardiac autonomic modulation as measured by HRV in patients with type 2 diabetic neuropathy.
Methods
Setting
The exercise interventions took place at three community-based exercise facilities in the University of Tabriz, Iran. The exercise was supervised by three exercise specialist trainers.
Participants
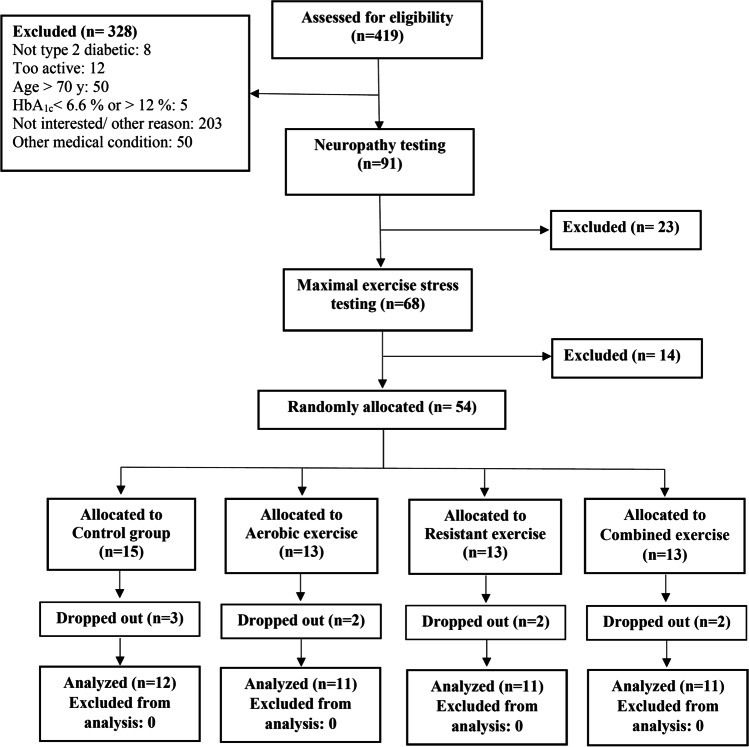
Previously physically inactive patients with T2DM aged 34 to 67 years recruited through advertising, physicians, and word of mouth. Inclusion criteria included having T2DM (as defined by the American Diabetes Association [31]) for more than 3 years, a baseline hemoglobin A1c (HbA1c) value of 6.6% to 12% (normal range, 4.0% to 6.0%), and the presence of symptoms of peripheral neuropathy. Exclusion criteria were participation in exercise two or more times weekly for 20 min or longer per session or in any resistant-training during the previous six months; resting blood pressure greater than 160/95 mm Hg; restrictions in physical activity because of disease; or presence of other medical conditions that makes participation inadvisable. After initial screening by telephone, requests for HbA1c and neuropathy testing were mailed to potentially eligible individuals. Those with screening HbA1c values of 6.6% to 12% and peripheral neuropathy were invited for in-person assessment where informed consent was obtained, followed by medical history and physical examination. Participants returned on a separate day for a maximal graded exercise stress test with electrocardiographic monitoring on a treadmill using a standard test (Bruce protocol). Persons who were clinically free of any cardiovascular disease on this test were allowed to proceed in the trial only if cleared by a cardiologist. Finally, 52 male participants (age: 55.24 ± 8.17 years, weight: 89.54 ± 13.41 kg, height: 171.85 ± 6.98 cm, duration of diseases: 12.51 ± 6.46 years) randomly allocated to the groups (Fig. 1).
Fig. 1.
Study flow diagram
Run-in phase
Before randomization, all participants entered a 2-week run-in phase to assess adherence. Participants performed 15 to 20 min of aerobic training, 1 or 2 sets of 9 resistant-training exercises and combined aerobic and resistant-training at moderate intensity with supervision. Only persons who attended 4 or more of the scheduled six run-in sessions were eligible for randomization.
Randomization
Participants were stratified based on HbA1c value, then randomly from each stratify; participants were allocated to equal numbers to the aerobic training, resistant training, combined-training, and control groups.
Intervention
Individual exercise supervision was provided weekly for the first four weeks after randomization and biweekly thereafter. See Table 1 for the details of the exercise interventions. The aerobic-training group exercised three times weekly. Training progressed gradually in duration and intensity. Participants progressed from 25 to 45 min per session at 70% of the maximum heart rate (MHR) to 40 min per session at 75% of the MHR, as determined from the maximal treadmill exercise test. Heart-rate monitors (Polar Electro Oy, Kempele, Finland) were used to adjust the workload to achieve the target heart rate. The resistant-training group exercised 3 times weekly, and training progressed gradually in duration, and intensity performed 9 different exercises on weight machines each session, progressing to 2 to 3 sets of each exercise at the maximum weight that could be lifted 8 to 12 times (2 sets of 4 upper-body exercises (bench press, seated row, shoulder press, and pull down), 3 sets of 3 leg exercises (leg press, extension, and flexion) and 2 sets each of abdominal crunches and back extensions).
Table 1.
Exercise program during the run-in and intervention phases
| Week | Aerobic Training | Resistant Training | Combined-training | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration, min/d | Intensity % of maximum heart rate* | Frequency, d/wk | Sets, n | Repetitions, n | Weight, maximum repetitions† | Frequency, session/wk | Aerobic Training | Resistant Training | |||||||||
| Duration, min/d | Intensity, % of maximum heart rate* | Frequency, d/wk | Sets, n | Repetitions, n | Weight, maximum repetitions† | Frequency, session/wk | |||||||||||
| Run-in phase | |||||||||||||||||
| 1–2 | 15–20 | 60 | 3 | 1–2 | 15 | 15 | 3 | 15–20 | 60 | 3 | 1–2 | 15 | 15 | 2 | |||
| Intervention phase | |||||||||||||||||
| 3–4 | 25 | 70 | 3 | 2–3 | 12 | 12 | 3 | 25 | 70 | 3 | 1 | 12 | 12 | 2 | |||
| 5–6 | 30 | 70 | 3 | 2–3 | 12 | 12 | 3 | 30 | 70 | 3 | 1 | 12 | 12 | 2 | |||
| 7–8 | 35 | 70 | 3 | 2–3 | 12 | 12 | 3 | 35 | 70 | 3 | 1 | 12 | 12 | 2 | |||
| 9–10 | 40 | 70 | 3 | 2–3 | 10 | 10 | 3 | 40 | 70 | 3 | 1 | 10 | 10 | 2 | |||
| 11–12 | 45 | 70 | 3 | 2–3 | 8 | 8 | 3 | 45 | 70 | 3 | 1 | 8 | 8 | 2 | |||
| 13–14 | 40 | 75 | 3 | 2–3 | 8 | 8 | 3 | 40 | 75 | 3 | 1 | 8 | 8 | 2 | |||
* The maximum heart rate achieved during the maximal treadmill exercise test performed at baseline
† The maximum weight that can be lifted the slated number of times while maintaining proper form. For example, 15 maximum repetitions are the maximum weight that can be lifted 15 times while maintaining proper form
The combined-training group did the three aerobic-training sessions every week (full aerobic-training program) plus two resistant-training sessions per week, with each session consisting of 1 set of each of the above nine exercises to ensure an adequate dose of each type of exercise. Control participants were asked to maintain their daily activity levels as same as pre-study activity levels [32, 33].
We prescribed a diet to all participants that would not cause weight loss to minimize dietary variability among groups. Dietary counseling was based on the Canadian Diabetes Association guidelines [34]. The dietitian interviewed each participant at baseline and three months later and reviewed a 3-day food diary. Letters were sent to participants’ physicians asking that therapy with antihypertensive, lipid-altering, or glucose-lowering medications not be initiated or altered during the 3-month intervention unless it was medically required. When medication changes were deemed necessary, we asked physicians and participants to inform us of these changes. There were no changes in any medications between enrollment and three months.
Heart rate variability measurement
Before run-in phase and at the end of the 3-month study period, we examined HRV between 06:30 a.m. and 11:00 a.m. for 20 min in a comfortable, temperature-controlled (22–24 °C) room with dimmed lighting and absent distraction from noise following a 12-h fasting period. Participants abstained from consuming caffeine-containing products and alcoholic beverages for 12 h and heavy physical activity for 48 h. We controlled respiration rate using a metronome at a fixed rate of 12 breaths/min (0.2 Hz). To measure HRV, each participant was connected to a 3-channel ECG digital Holter recorder (VX3 + Digital Holter Recorder, DMS Service Company, Made in the USA) and asked to lie down for 15 min. Then, a supine, resting 20-min electrocardiographic (ECG) recording was obtained and used to calculate time-domain and frequency-domain HRV parameters using Full Option software (DM Software, CARDIOSCAN II, Version: 12.2.0017a, Designed by the USA). The time and frequency HRV parameters used in the analysis are the standard deviation of all regular RR intervals (SDNN), which reflects total variability and carries the strongest prognostic information in heart disease, and the root means square of successive differences in normal RR intervals (RMSSD), which reflects the parasympathetic modulations in heart rate. Frequency-domain analysis included: low-frequency (LF) component (frequency range 0.04–0.15 Hz) and the high-frequency (HF) component (frequency range 0.15–0.4 Hz) in absolute units (ms2), low-frequency to a high-frequency ratio (LF/HF). The LF component is modulated by both the sympathetic and parasympathetic nervous system and thus indicates a mixture of both autonomic inputs. The HF component is commonly defined as a marker of vagal modulation. The LF/HF ratio provides a criterion of the sympathovagal balance, where an increase in the ratio reflects a predominance of sympathetic over vagal modulation [35, 36].
Blood sampling and analysis
Following a 10–12 h overnight fast, blood was drawn via an antecubital vein for analysis. All blood measurements were taken before the run-in phase and 48 h after the last training session. The biochemical examinations involved detection of fasting plasma glucose, cholesterol, triglyceride, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels and were conducted using enzymatic methods on a Mindray BS-480 Chemistry Analyzer (Shenzhen Mindray Bio-Medical Electronics Co., Ltd, China). Glycosylated hemoglobin (HbA1c) was assessed by the technical performance of the Bronate affinity assay using a Cera-Stat 2000-type analyzer (HbA1C POCT Analyzers kit, LabonaCheck A1C & Cera-Stat 2000, South Korea). The insulin levels were assessed by the electrochemiluminescent immunoassay method (Elecsys 2010 analyzer, Cobas, Roche Diagnostic, Germany).
Anthropometric measurements
Weight and height were measured and used to calculate the body mass index (BMI) (kg/m2). Also, body composition (body fat percentage and skeletal muscle mass) was measured by bioelectrical impedance analyzer (BIA), Inbody 230 (Biospace, Seoul, Korea) in a temperature-controlled (22–24 °C) room following a 12-h fast. Participants, abstained from heavy physical activity for 24 h. Blood pressure was checked after 10 min at rest (by Beurer BM20 Digital Blood Pressure Monitor); the mean of 2 readings obtained 2 min apart was used in the statistical analysis.
Adverse events
We used a standard form to log each adverse event. Subjects were questioned on adverse events by the research coordinator at each visit and by the exercise specialist if a scheduled exercise session was missed. In addition, adverse event forms were completed if a participant spontaneously reported an adverse event to the trainer. In this study, one combined exercise training participant, and one aerobic-training participant reported mild hypoglycemia.
Statistical analysis
The normality of the distribution was tested using a Kolmogorov–Smirnov test. Different anthropometric, biochemical characteristics, and HRV parameters between the four groups were compared before (baseline) and after exercise, using ANCOVA and data are presented as mean ± standard deviation. A post-hoc test (Bonferroni) was used to determine where significant differences. A p-value of < 0.05 was considered statistically significant. Data were analyzed with the 26th version of IBM SPSS statistical software.
Results
Participant characteristics
The anthropometric and biochemical characteristics of study participants are shown in Table 2. The inter-group variation of anthropometrics characteristics showed that systolic blood pressure in all three types of exercise groups (F3, 39 = 11.56, p < 0.01), BMI in the aerobic-training group and WHR in the aerobic and combined-training groups were diminished compared with the control group (p > 0.01). Changes in other anthropometrics and biochemical characteristics values weren’t significantly different in the exercise groups compared to the control group (p < 0.05).
Table 2.
The anthropometrics and biochemical characteristics of the participants
| Groups | Mean (SD) Value | Change | P-Value | Mean (SD) Value | Change | P-Value | ||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Baseline | 12 weeks | |||||
| Weight, kg | Percent Body Fat, % | |||||||
| AT | 91.74 ± 13.89 | 89.69 ± 12.80 | -2.05 ± 3.03 | 0.048* | 29.55 ± 7.12 | 24.18 ± 7.45 | -5.37 ± 3.85 | 0.001** |
| RT | 85.64 ± 8.60 | 84.60 ± 8.09 | -1.04 ± 2.12 | 0.134 | 28.00 ± 5.49 | 22.43 ± 8.30 | -5.58 ± 3.95 | 0.001** |
| CT | 88.76 ± 14.31 | 87.03 ± 13.91 | -1.74 ± 3.12 | 0.095 | 30.02 ± 7.94 | 25.97 ± 6.49 | -4.04 ± 4.22 | 0.010** |
| C | 91.79 ± 16.24 | 91.54 ± 17.23 | -0.25 ± 2.44 | 0.729 | 28.60 ± 10.10 | 27.49 ± 11.82 | -1.11 ± 4.05 | 0.362 |
| BMI, kg/m2 | WHR | |||||||
| AT | 30.39 ± 3.36 | 29.45 ± 3.21 | -0.94 ± 0.66 | 0.001** | 0.962 ± 0.033 | 0.940 ± 0.037 | -0.022 ± 0.019 | 0.005** |
| RT | 28.79 ± 2.59 | 28.42 ± 2.50 | -0.37 ± 0.68 | 0.101 | 0.949 ± 0.025 | 0.950 ± 0.035 | 0.001 ± 0.025 | 0.908 |
| CT | 30.36 ± 3.02 | 29.81 ± 2.56 | -0.55 ± 1.01 | 0.101 | 0.946 ± 0.023 | 0.928 ± 0.019 | -0.018 ± 0.021 | 0.020* |
| C | 30.93 ± 5.12 | 31.29 ± 5.32 | 0.36 ± 0.83 | 0.166 | 0.940 ± 0.035 | 0.954 ± 0.037 | 0.014 ± 0.014 | 0.006** |
| FBS, mg/dL | Hemoglobin A1c | |||||||
| AT | 142.64 ± 39.48 | 148 ± 40.76 | 5.36 ± 37.92 | 0.649 | 7.12 ± 1.17 | 6.29 ± 0.79 | -0.83 ± 0.93 | 0.014* |
| RT | 129.36 ± 37.45 | 121.91 ± 31.58 | -7.45 ± 40.04 | 0.551 | 6.95 ± 0.77 | 6.33 ± 0.59 | -0.62 ± 0.56 | 0.004** |
| CT | 155.73 ± 94.18 | 155.72 ± 64.38 | -0.01 ± 108.20 | 0.999 | 7.15 ± 1.11 | 6.84 ± 0.96 | -0.31 ± 1.11 | 0.378 |
| C | 156.75 ± 55.57 | 149.75 ± 61.59 | -7.00 ± 71.33 | 0.740 | 7.26 ± 1.01 | 6.82 ± 1.14 | -0.44 ± 0.71 | 0.055 |
| Insulin, µU/ml | Total Cholesterol, mg/dL | |||||||
| AT | 12.47 ± 10.74 | 13.32 ± 11.62 | 0.85 ± 3.23 | 0.402 | 136.27 ± 41.85 | 154.91 ± 52.21 | 18.64 ± 33.39 | 0.094 |
| RT | 15.85 ± 18.76 | 12.84 ± 13.32 | -3.01 ± 10.03 | 0.343 | 166.36 ± 29.56 | 162.36 ± 25.30 | -4.00 ± 27.73 | 0.665 |
| CT | 10.36 ± 6.62 | 15.74 ± 8.70 | 5.38 ± 6.85 | 0.026* | 138.82 ± 28.27 | 139.91 ± 25.32 | 1.09 ± 22.13 | 0.873 |
| C | 19.43 ± 14.14 | 21.42 ± 15.48 | 1.99 ± 5.24 | 0.215 | 137.33 ± 34.12 | 171.25 ± 61.37 | 33.92 ± 44.43 | 0.023* |
| Triglycerides, mg/dL | HDL cholesterol, mg/dL | |||||||
| AT | 170.63 ± 81.56 | 210.64 ± 125.24 | 40.01 ± 102.51 | 0.225 | 38.36 ± 6.68 | 38.18 ± 5.69 | -0.18 ± 5.44 | 0.914 |
| RT | 131.73 ± 72.93 | 132.18 ± 47.35 | 0.45 ± 38.75 | 0.970 | 46.82 ± 11.54 | 43.36 ± 7.98 | -3.46 ± 5.30 | 0.056 |
| CT | 127.55 ± 50.77 | 130.91 ± 58.98 | 3.36 ± 32.12 | 0.736 | 36.45 ± 7.10 | 36.73 ± 8.72 | 0.28 ± 4.00 | 0.826 |
| C | 205.42 ± 85.09 | 321.92 ± 89.36 | 116.5 ± 311.56 | 0.222 | 32.83 ± 9.79 | 31.92 ± 8.06 | -0.91 ± 3.87 | 0.430 |
| LDL cholesterol, mg/dL | Systolic blood pressure, mm Hg | |||||||
| AT | 69.54 ± 29.00 | 75.87 ± 27.08 | 6.33 ± 18.70 | 0.288 | 124.91 ± 12.01 | 118.91 ± 8.73 | -6.00 ± 10.95 | 0.099 |
| RT | 96.53 ± 22.40 | 83.09 ± 15.62 | -13.44 ± 19.17 | 0.042* | 124.91 ± 9.61 | 114.36 ± 11.20 | -10.55 ± 10.59 | 0.008** |
| CT | 78.09 ± 16.54 | 71.91 ± 19.08 | -6.18 ± 18.38 | 0.291 | 126.91 ± 11.94 | 116.91 ± 7.61 | -10.00 ± 6.57 | 0.001** |
| C | 76.27 ± 23.19 | 77.05 ± 21.02 | 0.78 ± 20.97 | 0.899 | 119.00 ± 6.83 | 134.82 ± 14.01 | 15.82 ± 15.97 | 0.008** |
| Diastolic blood pressure, mm Hg | ||||||||
| AT | 71.64 ± 7.89 | 69.82 ± 7.72 | -1.82 ± 11.54 | 0.613 | ||||
| RT | 72.27 ± 5.76 | 67.27 ± 11.39 | -5.00 ± 11.04 | 0.164 | ||||
| CT | 76.73 ± 7.60 | 67.91 ± 9.21 | -8.82 ± 8.11 | 0.005** | ||||
| C | 71.36 ± 5.77 | 75.91 ± 6.86 | 4.54 ± 6.77 | 0.050 | ||||
BMI, body mass index; WHR, waist-hip ratio; FBS, fasting blood sugar. Results are expressed as mean ± SD
** The p < 0.01 was considered statistically significant
* The p < 0.05 was considered statistically significant.
AT Aerobic-training group, RT Resistant-training group, CT Combined-training group, C control group
The results of intra-group changes indicated that body fat percent in all three types of exercise groups (aerobic (t10 = 4.63, p < 0.01, resistant (t10 = 4.68, p < 0.01 and combined (t10 = 3.18, p < 0.01), weight in the aerobic-training group (t10 = 2.25, p < 0.05), BMI in the aerobic-training group (t10 = 4.69, p < 0.01), WHR in the aerobic (t10 = 3.64, p < 0.01) and the combined-training groups (t10 = 2.76, p < 0.05), systolic blood pressure in the resistant (t10 = 3.30, p < 0.01) and the combined-training groups (t10 = 5.05, p < 0.01) and diastolic blood pressure in the combined-training groups (t10 = 3.61, p < 0.01) were declined after versus before exercise training. In addition, HbA1c was reduced after versus before exercise training in the aerobic (t10 = 2.95, p < 0.05) and the resistant-training groups (t10 = 3.65, p < 0.01). Insulin was incremented in the combined-training group (t10 = 2.61, p < 0.05), and LDL was decreased in the resistant-training group (t10 = 2.32, p < 0.05) after versus before exercise training. The control group showed escalation in WHR (t11 = 3.40, p < 0.01), total cholesterol (t11 = 2.64, p < 0.05) and systolic blood pressure (t10 = 3.29, p < 0.01) variables in posttest versus pretest (Table 1). In other variables of anthropometric measurements or biochemical indices, no significant difference was observed.
Frequency-domain measures
The inter-group variation of Frequency-domain parameters showed that HF power (F3, 37 = 83.56, p < 0.01) and LF/HF ratio (F3, 35 = 6.24, p < 0.01) values were significantly different in the training groups compared with the control group and HF power was increased, and LF/HF ratio was decreased in all three types of aerobic, resistant and combined-training compared with the control group at P < 0.05. The results showed no significant difference between HF power and LF/HF ratio values between training groups (see Table 3)
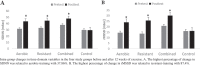
 .
.
Table 3.
Changes in HRV parameters in the four study groups before and after 12 weeks of exercise
| Variable | Mean (SD) Value | Change | Change Percent | Difference in Change from Baseline to 3 Months (95% CI) |
P-Value | |
|---|---|---|---|---|---|---|
| Baseline | 12 weeks | |||||
| SDNN, mSec, [n]† | ||||||
| AT | 31.70 ± 9.80 [10] | 43.70 ± 10.77 [10] | 12.00 ± 7.53 | 37.86 | 6.61 to 17.38 | 0.001** |
| RT | 33.00 ± 13.33 [11] | 44.64 ± 15.68 [11] | 11.64 ± 7.80 | 35.27 | 6.40 to 16.88 | 0.001** |
| CT | 38.00 ± 17.71 [11] | 47.82 ± 19.05 [11] | 9.82 ± 7.96 | 25.84 | 4.47 to 15.16 | 0.002** |
| C | 29.92 ± 15.38 [12] | 29.58 ± 13.62 [12] | 0.33 ± 6.41 | -1.10 | -4.41 to 3.74 | 0.860 |
| rMSSD, mSec | ||||||
| AT | 14.50 ± 4.97 [10] | 24.10 ± 7.03 [10] | 9.60 ± 4.88 | 66.21 | 6.11 to 13.09 | 0.000** |
| RT | 13.73 ± 5.83 [11] | 25.73 ± 12.12 [11] | 12.00 ± 11.85 | 87.40 | 4.04 to 19.96 | 0.007** |
| CT | 20.73 ± 17.40 [11] | 30.09 ± 19.50 [11] | 9.36 ± 7.00 | 45.15 | 4.66 to 14.07 | 0.001** |
| C | 16.00 ± 5.03 [12] | 16.17 ± 6.13 [12] | 0.17 ± 6.71 | 1.06 | -4.10 to 4.43 | 0.933 |
| LF Power, ms2 | ||||||
| AT | 257.48 ± 146.19 [10] | 319.83 ± 364.80 [10] | 62.35 ± 337.40 | 24.22 | -179.01 to 303.72 | 0.573 |
| RT | 184.39 ± 104.12 [10] | 289.74 ± 177.66 [10] | 105.35 ± 148.98 | 57.13 | -1.22 to 211.92 | 0.052 |
| CT | 268.18 ± 140.29 [11] | 328.27 ± 201.43 [11] | 60.09 ± 173.07 | 22.41 | -56.18 to 176.36 | 0.276 |
| C | 180.73 ± 159.51 [11] | 272.50 ± 392.22 [11] | 91.77 ± 304.23 | 50.22 | -112.61 to 296.16 | 0.341 |
| HF Power, ms2 | ||||||
| AT | 76.41 ± 25.32 [11] | 295.98 ± 69.00 [11] | 219.57 ± 60.40 | 287.36 | 178.99 to 260.15 | 0.000** |
| RT | 39.46 ± 26.99 [10] | 271.45 ± 59.50 [10] | 231.99 ± 38.39 | 587.91 | 204.53 to 259.45 | 0.000** |
| CT | 47.26 ± 32.07 [10] | 243.80 ± 67.63 [10] | 196.54 ± 38.27 | 415.87 | 169.16 to 223.91 | 0.000** |
| C | 46.46 ± 34.59 [11] | 43.86 ± 36.89 [11] | -2.60 ± 14.23 | -5.66 | -12.16 to 6.96 | 0.558 |
| LF/HF Ratio | ||||||
| AT | 3.31 ± 1.82 [10] | 1.21 ± 1.59 [10] | -2.10 ± 1.84 | -63.44 | -3.42 to -0.78 | 0.006** |
| RT | 6.09 ± 5.25 [9] | 1.01 ± 0.55 [9] | -5.09 ± 5.08 | -83.42 | -8.99 to -1.18 | 0.017* |
| CT | 7.90 ± 6.14 [10] | 1.31 ± 0.90 [10] | -6.60 ± 5.58 | -83.42 | -10.58 to -2.61 | 0.005** |
| C | 5.29 ± 4.86 [10] | 5.72 ± 5.82 [10] | 0.43 ± 4.96 | 8.13 | -3.11 to 3.98 | 0.788 |
† Values in brackets are the numbers of patients with complete data
** The p < 0.01 was considered statistically significant
* The p < 0.05 was considered statistically significant
SDNN the standard deviation of all normal RR intervals, rMSSD the root means square of successive differences in normal RR intervals, HF high-frequency power, LF low-frequency power, LF: HF Ratio the ratio of low frequency to high-frequency power, AT Aerobic-training group, RT Resistant-training group, CT Combined-training group, C control group
Discussion
This study was designed to determine the effects of three different types of exercise on cardiac autonomic modulation in patients with type 2 diabetic neuropathy. The major finding of the present study shows that cardiac autonomic modulation as measured by HRV was significantly improved through increasing cardio-vagal tone and decreasing cardio- sympathetic tone in all three types of aerobic, resistant and combined-training groups compared with the control group.
In the current study, rMSSD and SDNN parameters, both were statistically increased after all three types of exercise; the highest rate of the increase in rMSSD parameter was related to resistant training, and in SDNN parameter, the highest percentage of its increase was related to aerobic training.
Using more specific information obtained from frequency-domain measures, HF power was raised and LF/HF ratio was declined in all three types of exercise but the highest percentage of the increment in HF power was related to resistant-training and the highest percentage of decline in LF/HF ratio was related to both resistant and combined-training, whereas LF (ms2) was not different after training. Our findings are in agreement with those from past studies that have examined the impact of exercise training on HRV [26, 27, 37–40]. Those studies have revealed that HRV raised significantly after training, despite studies used either time-domain measure or frequency-domain measure for the analysis of HRV. Nonetheless, Loimaala et al. and Kang et al. [41, 42] reported that although the exercise program did not markedly improve HRV after 12 weeks of training, SDNN, rMSSD, LF, and HF were boosted, and LF/HF was reduced. Thus, exercise may conduce to the prevention and management of cardiac autonomic neuropathy in patients with T2DM.
The mechanism by which exercise training improves HRV is not well understood. It is believed, however, that exercise training enlarges vagal tone and decreases the sympathetic cardiac influence, causing in an amelioration in HRV [36, 43]. At least two moderators are proposed to play a role in increasing the cardiac vagal tone in return to exercise training: nitric oxide (NO) and angiotensin II. NO is assumed to have a direct impact on cardiac vagal tone and an indirect effect on sympathetic cardiac influence [44]. Exercise training has been found to boost NO bioavailability and endothelial function [45]. The second mediator, angiotensin II, is an accepted inhibitor of cardiac vagal activity [25, 46]. Athletes and physically trained individuals have been indicated to have reduced levels of plasma renin activity, and therefore, decreased angiotensin II levels, than non-athletes and untrained people [47]. In addition, several biological mechanisms by which exercise training ameliorates HRV have been proposed. They could likely be associated with subsequent changes in body-fat distribution, atherogenic lipoprotein profiles, and blood pressure, as well as favorable effects on muscular capillary density and autonomic nervous system balance [25, 48]. In diabetes mellitus, physical activity has favorable effects on both glucose metabolism and insulin sensitivity. These involve expanded sensitivity to insulin, declined production of glucose by the liver, an extensive number of muscle cells that use more glucose than adipose tissue, and reduced obesity [23, 49]. Nonetheless, detecting the specific mechanism by which exercise training promotes HRV and autonomic cardiac regulation needs additional research studies.
Considering so, regular exercise modulates cardiac autonomic control by increasing vagal tone and decreasing sympathetic influence [39]. This transfer toward greater vagal modulation may beneficially affect the prognosis of subjects with a variety of morbidities [50]. Therefore, patients with type 2 diabetic neuropathy may attenuate or obviate the cardiac autonomic neuropathy complication with the beneficial effects of exercise on HRV and other cardiovascular health indicators in those individuals with this comorbidity.
Conclusions
In conclusion, 3–5 times a week, 3-month, aerobic, resistant and combined-training program in type 2 diabetic neuropathy patients who are clinically free of the cardiovascular disease causes considerable advances in cardiac autonomic modulation of HRV through enhancing cardio-vagal tone and reducing cardio- sympathetic tone. Furthermore, based on the present findings, it seems that the inclusion of complementary resistant-training in physical exercise programs for patients with T2DM may be applicable, but further studies are required. As the ahead study was limited by restricted sample size, further studies in a large number of people with type 2 diabetic neuropathy are necessary to support these favorable effects detected in the biochemical and autonomic variables after the training period have beneficial effects on the clinical outcome of the subjects.
Acknowledgements
We are grateful to the University of Mohaghegh Ardabili and the University of Tabriz, for their laboratory equipment, exercise facilities, and technical support. We thank the patients and trainers for their participation in this study.
Authors’ contributions
EP, MS, SDN, and LSP were involved in study design. EP, MS, MK, and SDN were involved in data collection. MS and SDN were responsible for cleaning and running statistical analysis. EP, MS, SDN, LSP, MS and MK were responsible for interpretation of results. EP was responsible for writing the first draft. All authors contributed equally to editing draft versions and accept full responsibility for the content of the manuscript.
Funding
The funds for this study were provided three quarters by Dr. Elaheh Piralaiyand a quarter by University of Mohaghegh Ardabili. These funds were used to purchase the necessary supplies.
Data availability (data transparency)
Data are all contained within the article.
Declarations
Ethics approval
This study was approved by the research ethics committee (REC) of Tabriz University of Medical Sciences (TUOMS) (IR.TBZMED.REC.1395.754).
Consent to participate
Written informed consent was obtained from all the participants after necessary explanations at the beginning of the study.
Consent for publication
All the participants gave their consent for the publication of identifiable details, which can include photograph(s) and/or videos and/or case history and/or details within the text (“Material”) to be published in the Journal of diabetes and metabolic disorders and the present article.
Conflicts of interest/Competing interests
There are no competing conflicts of interest to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elaheh Piralaiy and Mostafa Khani contributed equally to this paper
Contributor Information
Elaheh Piralaiy, Email: epiralaiy@tabrizu.ac.ir.
Marefat Siahkuhian, Email: m_siahkohian@uma.ac.ir.
Saeed Dabbagh Nikookheslat, Email: nikookheslat@tabrizu.ac.ir.
Linda S. Pescatello, Email: Linda.Pescatello@uconn.edu
Mahboub Sheikhalizadeh, Email: m-sheykhalizadeh@iau-ahar.ac.ir.
Mostafa Khani, Email: khani_ms@tabrizu.ac.ir.
References
- 1.Henson J, Dunstan DW, Davies MJ, et al. Sedentary behaviour as a new behavioural target in the prevention and treatment of type 2 diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):213–220. doi: 10.1002/dmrr.2759. [DOI] [PubMed] [Google Scholar]
- 2.Shah SM, Ali R, Loney T, et al. Prevalence of diabetes among migrant women and duration of residence in the United Arab Emirates: a cross sectional study. PLoS One. 2017;12(1):e0169949. doi: 10.1371/journal.pone.0169949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayan A, Dubey RK, Padmavati P, et al. Association of lipid profile with fasting and post prandial glucose level in type 2 diabetic patients. J Univers Coll Med Sci. 2015;3(1):2–5. [Google Scholar]
- 4.Maser RE, Mitchell BD, Vinik AI, et al. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26(6):1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 5.Pop-Busui R, Lu J, Lopes N, et al. Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J Peripher Nerv Syst. 2009;14(1):1–13. doi: 10.1111/j.1529-8027.2009.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benichou T, Pereira B, Mermillod M, et al. Heart rate variability in type 2 diabetes mellitus: a systematic review and meta–analysis. PLoS One. 2018;13(4):e0195166. doi: 10.1371/journal.pone.0195166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olafsdottir E, Andersson DK, Dedorsson I, et al. Early detection of type 2 diabetes mellitus and screening for retinopathy are associated with reduced prevalence and severity of retinopathy. Acta Ophthalmol (Copenh) 2016;94(3):232–239. doi: 10.1111/aos.12954. [DOI] [PubMed] [Google Scholar]
- 8.Ewing DJ, Martyn CN, Young RJ, et al. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8(5):491–498. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- 9.Hufnagel C, Chambres P, Bertrand PR, et al. The need for objective measures of stress in autism. Front Psychol. 2017;8:64. doi: 10.3389/fpsyg.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudet G, Walther G, Courteix D, et al. Paradoxical dissociation between heart rate and heart rate variability following different modalities of exercise in individuals with metabolic syndrome: The RESOLVE study. Eur J Prev Cardiol. 2017;24(3):281–296. doi: 10.1177/2047487316679523. [DOI] [PubMed] [Google Scholar]
- 11.Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clin Exp Hypertens. 2001;23(1–2):45–55. doi: 10.1081/ceh-100001196. [DOI] [PubMed] [Google Scholar]
- 12.Singleton JR, Smith AG, Marcus RL. Exercise as therapy for diabetic and prediabetic neuropathy. Curr Diab Rep. 2015;15(12):120. doi: 10.1007/s11892-015-0682-6. [DOI] [PubMed] [Google Scholar]
- 13.Balducci S, Iacobellis G, Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20(4):216–223. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Allet L, Armand S, De Bie R, et al. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia. 2010;53(3):458–466. doi: 10.1007/s00125-009-1592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Heijden M, van Dooren FE, Pop V, et al. Effects of exercise training on quality of life, symptoms of depression, symptoms of anxiety and emotional well-being in type 2 diabetes mellitus: a systematic review. Diabetologia. 2013;56(6):1210–1225. doi: 10.1007/s00125-013-2871-7. [DOI] [PubMed] [Google Scholar]
- 16.Mann S, Beedie C, Balducci S, et al. Changes in insulin sensitivity in response to different modalities of exercise: a review of the evidence. Diabetes Metab Res Rev. 2014;30(4):257–268. doi: 10.1002/dmrr.2488. [DOI] [PubMed] [Google Scholar]
- 17.Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94(2):146–150. doi: 10.1038/icb.2015.101. [DOI] [PubMed] [Google Scholar]
- 18.Kluding PM, Pasnoor M, Singh R, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26(5):424–429. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singleton JR, Marcus RL, Lessard MK, et al. Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann Neurol. 2015;77(1):146–153. doi: 10.1002/ana.24310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huikuri HV, Stein PK. Clinical application of heart rate variability after acute myocardial infarction. Front Physiol. 2012;3:41. doi: 10.3389/fphys.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira NL, Ribeiro F, Alves AJ, et al. Heart rate variability in myocardial infarction patients: effects of exercise training. Revista Portuguesa de Cardiologia (English Edition) 2013;32(9):687–700. doi: 10.1016/j.repc.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Fong SS, Wong JY, Chung LM, et al. Changes in heart-rate variability of survivors of nasopharyngeal cancer during Tai Chi Qigong practice. J Phys Ther Sci. 2015;27(5):1577–1579. doi: 10.1589/jpts.27.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park M, Chu M, Song Y, et al. Glycemic Control Affects Heart-Rate Variability in Adults with Type 1 Diabetes. Am Diabetes Assoc. 2020;69(Supplement 1):43–LB.
- 24.Kanaley J, Goulopoulou S, Franklin R, et al. Plasticity of heart rate signalling and complexity with exercise training in obese individuals with and without type 2 diabetes. Int J Obes. 2009;33(10):1198. doi: 10.1038/ijo.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhati P, Shenoy S, Hussain ME. Exercise training and cardiac autonomic function in type 2 diabetes mellitus: a systematic review. Diabetes Metab Syndr. 2018;12(1):69–78. doi: 10.1016/j.dsx.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Sacre JW, Jellis CL, Jenkins C, et al. A six-month exercise intervention in subclinical diabetic heart disease: effects on exercise capacity, autonomic and myocardial function. Metabolism. 2014;63(9):1104–1114. doi: 10.1016/j.metabol.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Baynard T, Goulopoulou S, Sosnoff RF, et al. Cardiovagal modulation and efficacy of aerobic exercise training in obese individuals. Med Sci Sports Exerc. 2014;46(2):369. doi: 10.1249/MSS.0b013e3182a66411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGee J. Effects of aerobic exercise training on arterial stiffness and autonomic function in obese adults at-risk of cardiovascular disease and type 2 diabetes. 2020. East Carolina University. Doctoral Dissertation.
- 29.Kingsley JD, Figueroa A. Acute and training effects of resistance exercise on heart rate variability. Clin Physiol Funct Imaging. 2016;36(3):179–187. doi: 10.1111/cpf.12223. [DOI] [PubMed] [Google Scholar]
- 30.Turri-Silva N, Garner D, Moosavi S, et al. Effects of resistance training protocols on nonlinear analysis of heart rate variability in metabolic syndrome. Braz J Med Biol Res. 2018;51(8):e7459. doi: 10.1590/1414-431X20187459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavin JR, III, Alberti K, Davidson MB, et al. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 32.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304(20):2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 34.Wolever T, Barbeau M, Charron S, et al. Guidelines for the nutritional management of diabetes mellitus in the new millennium: a position statement by the Canadian Diabetes Association. Can J Diabetes Care. 1999;23(3):56–69. [Google Scholar]
- 35.Peçanha T, Bartels R, Brito LC, et al. Methods of assessment of the post-exercise cardiac autonomic recovery: a methodological review. Int J Cardiol. 2017;227:795–802. doi: 10.1016/j.ijcard.2016.10.057. [DOI] [PubMed] [Google Scholar]
- 36.Routledge FS, Campbell TS, McFetridge-Durdle JA, et al. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26(6):303–312. doi: 10.1016/s0828-282x(10)70395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faulkner MS, Michaliszyn SF, Hepworth JT, et al. Personalized exercise for adolescents with diabetes or obesity. Biol Res Nurs. 2014;16(1):46–54. doi: 10.1177/1099800413500064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faulkner MS, Quinn L, Rimmer JH, et al. Cardiovascular endurance and heart rate variability in adolescents with type 1 or type 2 diabetes. Biol Res Nurs. 2005;7(1):16–29. doi: 10.1177/1099800405275202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goit RK, Paudel BH, Khadka R, et al. Mild-to-moderate intensity exercise improves cardiac autonomic drive in type 2 diabetes. J Diabetes Investig. 2014;5(6):722–727. doi: 10.1111/jdi.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jisu K, Hun-Young P, Kiwon L. Effects of 12 weeks of combined exercise on heart rate variability and dynamic pulmonary function in obese and elderly Korean women. Iran J Public Health. 2018;47(1):74–81. [PMC free article] [PubMed] [Google Scholar]
- 41.Loimaala A, Huikuri HV, Kööbi T, et al. Exercise training improves baroreflex sensitivity in type 2 diabetes. Diabetes. 2003;52(7):1837–1842. doi: 10.2337/diabetes.52.7.1837. [DOI] [PubMed] [Google Scholar]
- 42.Kang S-J, Ko K-J, Baek U-H. Effects of 12 weeks combined aerobic and resistance exercise on heart rate variability in type 2 diabetes mellitus patients. J Phys Ther Sci. 2016;28(7):2088–2093. doi: 10.1589/jpts.28.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter JB, Banister EW, Blaber AP. The effect of age and gender on heart rate variability after endurance training. Med Sci Sports Exerc. 2003;35(8):1333–1340. doi: 10.1249/01.MSS.0000079046.01763.8F. [DOI] [PubMed] [Google Scholar]
- 44.Chowdhary S, Townend JN. Role of nitric oxide in the regulation of cardiovascular autonomic control. Clin Sci. 1999;97(1):5–17. [PubMed] [Google Scholar]
- 45.Kingwell BA. Nitric oxide as a metabolic regulator during exercise: effects of training in health and disease. Clin Exp Pharmacol Physiol. 2000;27(4):239–250. doi: 10.1046/j.1440-1681.2000.03232.x. [DOI] [PubMed] [Google Scholar]
- 46.Townend JN, Al-Ani M, West JN, et al. Modulation of cardiac autonomic control in humans by angiotensin II. Hypertension. 1995;25(6):1270–1275. doi: 10.1161/01.hyp.25.6.1270. [DOI] [PubMed] [Google Scholar]
- 47.Fagard R, Grauwels R, Groeseneken D, et al. Plasma levels of renin, angiotensin II, and 6-ketoprostaglandin F1 alpha in endurance athletes. J Appl Physiol. 1985;59(3):947–952. doi: 10.1152/jappl.1985.59.3.947. [DOI] [PubMed] [Google Scholar]
- 48.Ruderman N, Devlin JT. The health professional’s: Guide to diabetes and exercise. Amer Diabetes Assn. 1995;ISBN: 094544852X.
- 49.Malfatto G, Facchini M, Sala L, et al. Effects of cardiac rehabilitation and beta-blocker therapy on heart rate variability after first acute myocardial infarction. Am J Cardiol. 1998;81(7):834–840. doi: 10.1016/s0002-9149(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 50.Cowan MJ. Measurement of heart rate variability. West J Nurs Res. 1995;17(1):32–48. doi: 10.1177/019394599501700104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are all contained within the article.