Abstract
Introduction
Globally, a metabolic disorder like Diabetes is considered as one of the largest global health issues, as it accounts for the majority of the disease burden and happens to be one of the leading causes of mortality as well as reduced life expectancy across the world. As in 2019, India is home to the second-largest number (77 million) of Diabetic adults and the number of people affected has been increasing rapidly over the years. Termed as “the diabetes capital of the world,” with every fifth diabetic in the world being an Indian, there is an urgent need to address many critically significant challenges posed by Diabetes in India, like, increasing prevalence among young people in urban areas, less awareness among people, high cost of disease management, limited healthcare facilities, suboptimal diabetes control etc. In Indian context, not enough attempts have been made to observe and understand the long-term pattern of diabetes incidence and mortality. This study aims to provide deep insights into the recent trends of diabetes incidence and mortality in India from 1990 to 2019.
Materials and methods
This is an observational study based on the most recent data from the Global Burden of Disease (GBD) Study 2019. We extracted numbers, age-specific and age-standardized incidence and mortality rates of diabetes (from 1990 to 2019) from the Global Health Data Exchange. The average annual percentage changes in incidence and mortality were analysed by joinpoint regression analysis; the net age, period, and cohort effects on the incidence and mortality were estimated by age-period-cohort analysis.
Results
During the study period, age-standardized incidence and mortality rates of diabetes in India experienced an upsurge in numbers, the incidence rate increased from 199.14 to 317.02, and consequently, mortality increased from 22.30 to 27.35 per 100,000 population. The joinpoint regression analysis showed that the age-standardized incidence significantly rose by 1.63 % (95 % CI: 1.57 %, 1.69 %) in Indian males and 1.56 % in Indian females (95 % CI: 1.49 %, 1.63 %) from 1990 to 2019. On the other hand, the age-standardized mortality rates rose by 0.77 % (95 % CI: 0.24 %, 1.31 %) in Indian males and 0.57 % (95 % CI: -0.54 %, 1.70 %) in Indian females. For age-specific rates, incidence increased in most age groups, with exception of age groups 5–9, 70–74, 75–79 and 80–84 in male, and age groups 5–9, 75–79 and 80–84 in female. Mortality in male saw a decreasing trend till age group 20–24, whereas in female, the rate decreased till age group 35–39. The age effect on incidence showed no obvious changes with advancing age, but the mortality significantly increased with advancing age; period effect showed that both incidence and mortality increased with advancing time period; cohort effect on diabetes incidence and mortality decreased from earlier birth cohorts to more recent birth cohorts, while incidence showed no material changes from 1975 to 1979 to 2000–2004 birth cohort.
Conclusions
Mortality of diabetes decreased in younger age groups but increased in older age groups; however, Incidence increased in most age groups for both male and female. The net age or period effect showed an unfavourable trend while the net cohort effect presented a favourable trend. Aging was likely to drive a continued increase in the mortality of diabetes. Timely population-level interventions aiming for health education, lifestyle modification with special emphasis on the promotion of physical activity and healthy diet should be conducted, especially for male and earlier birth cohorts at high risk of diabetes.
Keywords: Diabetes, Incidence, Mortality, Joinpoint regression analysis, Age Period Cohort, India, GBD
Introduction
Diabetes is a long-term condition which has a major impact on the lives and wellbeing of individuals, families and societies across the world. It is one of the largest global health issues and a leading cause of mortality and reduced life expectancy. Globally, an estimated 1.1 million children and teenagers younger than 20 years are suffering from type 1 diabetes. The diabetes prevalence is increasing rapidly across the globe at an alarming rate. As per the World Health Organization estimates, around 422 million people worldwide have diabetes (WHO webpage, last updated on 13th April 2021). There has been an upsurge in the number of cases and the prevalence of diabetes in the past few decades. According to the International Federation of Diabetes [1], diabetes is one of the fastest growing health challenges of the 21st century. The number of adults living with diabetes has tripled in the past 20 years. IDF statistics expects that there will be 578 million adults with diabetes by 2030, and 700 million by 2045 [1].
Over the years, the research findings related to Diabetes trends in different populations are plagued by contradictory results and interpretations. In this section, we try to mention some of the notable works and their findings related to Diabetes across countries from different income groups and regions. According to a very recent study, the incidence of clinically diagnosed Diabetes has continued to grow in only a small population in low and middle-income countries since 2006, with over one-third of the population experiencing a decline in incidence of Diabetes. Around 80 % of the people with Diabetes live in a developing country [2]. However, another recent publication indicates that the incidence rates of diabetes have been declining recently in some other countries as well [3–5]. Carstensen and colleagues found a decreasing incidence of type-2 diabetes with an increasing prevalence during 21-period in Denmark [6]. Diabetes contributes to 11.3 % of worldwide deaths from all causes among individuals in the age groups 20–79 years [1]. According to a study that inquired type 2 diabetes in early adulthood among Asians, Diabetes has been termed as an increasing epidemic, characterized by rapid rates of increase over short periods, and onset at a relatively young age and low body mass index in Asian countries. The study shows that an increasing trend of childhood obesity has resulted in many young individuals being at high risk for type 2 diabetes in early adulthood among Asians [7].
Some study suggests that since 1980, diabetes prevalence in adults has increased with higher ages in every country. The study asserts that in recent decades, the prevalence of both types of Diabetes has significantly increased. It has also been estimated that the prevalence of Diabetes in adults will increase in the next two decades, and the increase will be more in the population aged 45 to 64 years in a developing country [8]. Together with aging and population growth, the rise is expected to be nearly four times the number of adults with diabetes worldwide. Another popular and very recent evidence, based on pooled analysis of 751 population-based studies with 4.4 million participants, suggests that the Diabetic population has increased faster in low-income and middle-income countries than in high-income countries, both in terms of prevalence and number of adults affected by Diabetes [9]. The authors also assert that the adults aged 50–70 years have the highest diabetes prevalence among all age groups.
Next, if we look into the numbers manifested by India in respect of diabetes, the prevalence has been the highest in India among Asian countries. Worldwide, India is home to the second-largest number (77 million) of Diabetic adults [1]. India is expected to have 153 million diabetic population by 2045 [1]. The increasing prevalence of diabetes can be driven by growing urbanisation, changing lifestyle habits, rising levels of obesity and physical inactivity. In Asian countries, Diabetes complications are common, and the economic burden is very high, especially in an imperfect society [10]. A study has found that the peak prevalence of Diabetes in India is 10-year-younger in comparison with Chinese and Japanese subjects [11]. High prevalence of diabetes is a growing challenge in India with an estimated 8.7 % diabetic population in the age group of 20 and 70 years. IDF estimates shows that the diabetic population in India, Bangladesh, and Sri Lanka make up to 98.9 % of the total adult population with Diabetes in the South East-Asia Region [1]. The primary drivers of diabetes in urban and rural areas of India, are demographic, genetic, environmental, behavioural components and their mutual interactions [12]. Prevalence of diabetes is higher in urban areas and it is manifested by the increasing pattern with age. Around 54 % of Diabetes occurs before the age of 50 years. Some past studies have presented noticeable and intriguing results as they have found that the prevalence of diabetes tended to increase at ages 40 years onwards, maintained almost the same level in the ages of 40–60 and then showed a significant rise in subjects over the age of 60 years [13, 14]. The significant challenges posed by Diabetes in India are manifold, some of these critical challenges that found their mention in a study are the increasing prevalence among young people in urban areas less awareness among people, the high cost of disease management, limited healthcare facilities, and suboptimal diabetes control [15].
As we move towards capturing the regional diversity, a recent study has reported that in north India, the epidemic of Diabetes is growing at an alarming rate. Knowledge and awareness about Diabetes in India, particularly in northern rural areas, is very poor [16]. A high prevalence of Diabetes Mellitus (DM) was observed among permanently settled tribal individuals in tribal and urban areas of northern states, with lifestyle risk factors like physical inactivity, obesity, non-vegetarian diet, and hypertension [17]. The most recent three decades have seen an increase in the number of people having diabetes specifically, type 2 diabetes. Recent data shows that Diabetes causes one death among twenty deaths from all causes, and around four million people die annually due to complications related to diabetes [18]. In one of the studies, India has been termed as “the diabetes capital of the world” as it is estimated that 41 million Indians have the disease with every fifth diabetic in the world being an Indian [19].
Till now in India, we could not find even a single study which provides the detailed information regarding the age effect, period effect and cohort effect on Incidence and mortality. The primary aim of our study is to provide deep insights of up-to-date trends of diabetes incidence and mortality in India from 1990 to 2019.
Materials and methods
The Global Burden of Disease (GBD) Study offers a powerful resource to understand the changing health challenges being faced by the people across the world in the 21st century. Led by the Institute for Health Metrics and Evaluation (IHME), the GBD study is the most comprehensive worldwide observational epidemiological study to date. By tracking progress within and between countries, GBD provides an important tool to inform clinicians, researchers, and policy makers, promote accountability, and improve lives worldwide.
Over the past 3 decades, the IHME has developed a methodology to quantify the burden of diseases, injuries, and risk factors for informing health programs and policy-makers so that the performances of disease related interventions in the path of progress can be assessed unbiasedly. GBD regularly provides comparable estimates of the key indicators of disease burden assessment, including the incidence and mortality rates of Diabetes. This is an observational study that has utilized the GBD 2019 database, which is also the most recent data available to the researchers and demographers, in order to systematically summarize and analyse the Incidence and mortality of Diabetes and its changes since 2019 for India.
GBD 2019 estimates incidence, prevalence, mortality, years of life lost (YLLs), years lived with disability (YLDs), and disability-adjusted life-years (DALYs) due to 369 diseases and injuries, for two sexes, and for 204 countries and territories that are grouped in 21 regions and 7 super-regions. Cause-specific death rates for several causes and cause fractions are calculated using the Cause of Death Ensemble model (CODEm) and spatial-temporal Gaussian process regression. A detailed description about CODEm is reported here [20–22]. Cause-specific deaths are adjusted to match the total all-cause deaths calculated as part of the GBD population, fertility, and mortality estimates. Deaths are multiplied by the standard life expectancy at every age to calculate YLLs. DisMod-MR 2.1 which is a Bayesian meta-regression modelling tool, is used to ensure the consistency between incidence, prevalence, remission, excess mortality, and cause-specific mortality for most of the causes. The estimated prevalence is multiplied by disability weights for mutually exclusive sequelae of diseases and injuries to calculate YLDs for each cause. Uncertainty intervals are generated for every metric by taking the 25th and 975th ordered 1000 draw values of the posterior distribution [23].
The key sources of data to model the cause of death due to diabetes in India included causes of death studies by verbal autopsy (VA), Medical certification of cause of deaths of the country and various states, vital statistics, other surveys on cause of death and published scientific articles [23]. The case definition used in this paper has included all forms of Diabetes i.e., Diabetes mellitus type 1 and Diabetes mellitus type 2. The ICD-10 codes for Diabetes are E10-E10.1, E10.3-E11.1, E11.3-E11.9, P70.2. Data of incidence rate and Death rate of diabetes are extracted from a publicly available database from an online tool produced by the IHME called the GHDx (Global Health Data Exchange) query tool (http://ghdx.healthdata.org/gbd-results-tool) [24]. Ethics approval and consent to participate were not applicable in this study.
Statistical analysis
The variables obtained from GBD 2019 include population, year, age, gender and cause (Diabetes). The incidence and mortality are used as the primary metrics to assess the impacts on health within the population. Incidence rate (per 100,000) is defined as the number of new cases divided by the population size, multiplied by 100,000; the mortality rate (per 100,000) is defined as the number of annual deaths divided by the total population size, multiplied by 100,000. The age-standardized mortality rate (ASMR) and age standardized incidence rate (ASIR) (per 100,000 population) are computed to quantitatively estimate the trends of Diabetes in India.
Joinpoint regression analysis
Joinpoint regression analysis is used to compute the magnitude of the time trends in the age standardized incidence and mortality rates of diabetes, the Average Annual Percent Change (AAPC) and corresponding 95 % Confidence Interval (CI) for every age and sex group. In brief, by using rates as inputs, this method identifies the year(s) when a trend change is evident, it calculates the annual percentage change (APC) in rates between trend-change points, and it also estimates the average annual percentage change (AAPC) in the whole period of study.
To estimate the APC, the following model is used:
where log (Yx) is the natural logarithm of the rate in year x.
Then, the APC from year x to year x + 1 is:
In absence of any join points, join points (i.e., no changes in trend), APC becomes constant, and thus it equals the AAPC. Otherwise, the whole period is segmented by the points with trend change. AAPC is then estimated as a weighted average of the estimated APC in each segment, and we use the segment lengths as the weights. The Z test is utilised to assess whether an AAPC or APC is different from zero. The terms ‘increase’ and ‘decrease’ are used only when the slope (AAPC or APC) of the trend is statistically significant, while the term ‘stable’ refers to a non-significant slope of the trend. The average APC (AAPC) is estimated using the best model considering maximum 5 join-points referring to 6 segments for the full range of our study periods. This analysis is performed using ‘Joinpoint Regression Program’ software (version 4.9.0.0) from the Surveillance Research Program of the US National Cancer Institute.
Age-period-cohort analysis
Incidence and mortality rates of Diabetes not only represent the incidence and death risk of Diabetes experienced by the population in a specific year, but also manifest additional accumulation of risks to health and wellbeing since birth. An ordinary statistical analysis is therefore not able to identify these accumulated risks and health hazards while calculating incidence and mortality due to Diabetes [25, 26]. Age-Period-Cohort examination is a widely used statistical procedure that accesses the contribution of age, period and cohort effects on the observed age specific incidence and mortality rates. It is generally used in demographic, epidemiological and sociological studies [27, 28].
Holford [29] has proposed that if the trends in age, period and cohort are analysed orthogonal to their linear and nonlinear parts, then several useful functions [30–35] can be estimated. In our study, we solely focus on following estimable functions: (1) Longitudinal age curve, (2) Period and Cohort risk ratio, (3) Net drift, (4) Local drift. Longitudinal Age Curve indicates the adjusted longitudinal age specific rates in reference to the cohort adjusted for period deviations; Period (Cohort) Rate Ratio is the period (or cohort) relative risk adjusted for age and nonlinear cohort (or period) effect in a period (or cohort) versus the reference period (or cohort); Net Drift gives the overall log-linear trend for each age group by calendar period and birth cohort, and it indicates the overall annual percentage change; finally, Local Drift is defined as the log linear pattern for each age group by calendar period and birth cohort, and it indicates the annual percentage change [36, 37].
To conduct Age-Period-Cohort Analysis, the incidence and mortality of diabetes and population data are arranged into a 5-year period from 1990 to 2019, and successive 5-year age groups (from 5 to 9 to 80–84 years) are formed. Individuals over the age of 85-years were not included in the study since they are not recorded in one group in GBD data base and five-year calendar (1990–1994, 1995–1999, 2000–2004, 2005–2009, 2010–2014, 2015–2019) spanning 21 particularly overlapping five-year birth cohorts (1910-14, …., 2010-14).
The APC model can be expressed as-.
Where Mij denotes the incidence or mortality rate, Yijk denotes the response variable, the net effect on incidence or mortality of Diabetes for ith age group (i = 1,2,3,……. α group) at the jth time period (j = 1,2,………p time period) born in the kth cohort (k = 1,2, 3,……, α + p-1), αi, βj, and γk denote the effect of ith age group, jth period and kth cohort of Age-Period-Cohort model respectively. µ denotes the general effect and εijk denotes the residual of the Age-Period-Cohort model. Age-Period-Cohort model is performed through the Age-Period-Cohort Web Tool (Biostatistics Branch, National Cancer Institute, Bethesda, MD, USA). The central age group, period and birth cohort are taken as the reference in all analysis [37, 38].
Results
Descriptive analysis of incidence and mortality trends of diabetes in India
Overall trend in the Crude Incidence Rate (CIR), Crude Mortality Rate (CMR), Age-Standardized Incidence Rate (ASIR) and Age-Standardized Mortality Rate (ASMR) for diabetes among male and female in the period 1990–2019for India are depicted in Fig. 1. In all years, crude rates of both incidence and mortality are higher among males as compared to females. Both age-standardized and crude incidence rates of diabetes for male and female in India have increased continuously from 1990 to 2019. Although, age-standardized and crude mortality rates experienced a slight increase and decrease over the years for both male and female, but overall mortality rate due to diabetes has increased from year 1990 to year 2019 (Fig. 1).
Fig. 1.
Trends in the crude rates and age-standardized rates for diabetes mellitus among Indian males and females in the period 1990–2019. Note: CIR, Crude Incidence Rate; ASIR, Age-Standardized Incidence Rate; CMR, Crude Mortality Rate; and ASMR, Age-Standardized Mortality Rate
Trends in age standardized incidence and mortality rates of Diabetes in India for both sexes
Table 1 presents the calculated APC and AAPC of incidence and mortality of diabetes in India from 1990 to 2019. The age standardized diabetes incidence rate in India between 1990 and 2019 ranged from 199.14 to 317.02 per 100,000 population. The regression model showed a significant increasing pattern of incidence rate in India between 1990 and 2019 (AAPC: 1.59; 95 % CI: 1.51 to 1.67; p < 0.001). Similarly, mortality rate due to diabetes has also increased in 2019 (27.35 deaths per 100,000) as compared to 1990 (22.30 deaths per 100,000), however the regression analysis shows it as stable for period 1990 and 2019 (AAPC: 0.70; 95 % CI: -0.34 to 1.75; p = 0.186).
Table 1.
Trends in diabetes incidence and mortality of India from 1990 to 2019 using Joinpoint Regression Analysis
| Age standardised incidence rate | Age standardised mortality rate | ||||
|---|---|---|---|---|---|
| Segment | Year | APC* (95 % C.I.) | Segment | Year | APC* (95 % C.I.) |
| 1 | 1990–2001 | 1.34* (1.31,1.37) | 1 | 1990–1995 | 0.17 (-1.41,1.77) |
| 2 | 2001–2004 | 2.20* (1.77,2.63) | 2 | 1995–1998 | 4.41 (-2.74,12.10) |
| 3 | 2004–2011 | 0.65* (0.58,0.72) | 3 | 1998–2007 | 0.91* (0.13,1.69) |
| 4 | 2011–2014 | 2.16* (1.73,2.59) | 4 | 2007–2010 | -2.66 (-9.33,4.51) |
| 5 | 2014–2017 | 0.93* (0.50,1.35) | 5 | 2010–2019 | 0.71* (0.06,1.37) |
| 6 | 2017–2019 | 5.58* (5.14,6.02) | - | - | - |
| AAPC* | 1990–2019 | 1.59* (1.51,1.67) | AAPC* | 1990–2019 | 0.70 (-0.34,1.75) |
Note: *, Indicates that the Annual Percent Change (APC) is significantly different from zero at the alpha = 0.05 level; APC, annual percentage change; AAPC, average annual percent change; CI, confidence interval
Trends in age-specific incidence and mortality rates of diabetes in india using joinpoint regression analysis
Table 2 depicts the average annual percent change (AAPC) in the incidence and mortality of diabetes for both male and female in India from 1990 to 2019. Age-standardized incidence rate has increased significantly by 1.63 % (95 % CI: 1.57 %, 1.69 %) among male and 1.56 % (95 % CI: 1.49 %, 1.63 %) among female, whereas age-standardized mortality rate has increased significantly by 0.77 % (95 % CI: 0.24 %, 1.31 %) in male and a non-significant increase of 0.57 % (95 % CI: -0.54 %, 1.70 %) is obtained in females over the last three decades. Incidence rate has increased in most age groups (10–14 to 70–74) for both male and female, with exception of the 70–74 age group in male. Mortality in male has decreased for the younger age groups (from 5 to 9 to 20–24 age group) whereas it increased for the higher age groups (from 25 to 29 to80–84 age group). Mortality in females has almost decreased for all of the age groups below 40 years whereas it increased for the age group 40–44 to 80-84during the period.
Table 2.
Average Annual Percentage Change (AAPC) of Diabetes incidence and mortality of India by age and gender from 1990 to 2019 using Joinpoint Regression Analysis
| Age-Group (Year) | Incidence (95 % CI) | Mortality (95 % CI) | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| ASR | 1.63* (1.57,1.69) | 1.56* (1.49,1.63) | 0.77* (0.24,1.31) | 0.57 (-0.54,1.70) |
| 5–9 | -0.09* (-0.12, -0.05) | -0.08* (-0.13,-0.04) | -1.03* (-1.95,-0.09) | -1.24* (-1.92,-0.56) |
| 10–14 | 0.14* (0.11,0.17) | 0.28* (0.26,0.30) | -0.06 (-2.49,2.43) | -0.48 (-2.09, 1.16) |
| 15–19 | 1.83* (1.77,1.88) | 1.43* (1.21,1.65) | -1.31 (-4.16,1.63) | -1.71 (-3.78, 0.41) |
| 20–24 | 2.11* (2.01,2.21) | 1.76* (1.57,1.95) | -0.20 (-1.13,0.74) | -1.56* (-2.83,-0.26) |
| 25–29 | 2.08* (1.88,2.29) | 1.81* (1.61,2.02) | 0.37 (-0.31,1.07) | -0.81 (-1.98,0.38) |
| 30–34 | 1.90* (1.82,1.97) | 1.55* (1.32,1.78) | 0.93* (0.32,1.55) | -0.52 (-1.16,0.13) |
| 35–39 | 1.79* (1.52,2.07) | 1.22* (0.78,1.66) | 0.96 (-0.38,2.32) | -0.42 (-1.13,0.29) |
| 40–44 | 1.85* (1.44,2.26) | 1.56* (1.33,1.80) | 0.80* (0.47,1.13) | 0.17 (-0.68,1.03) |
| 45–49 | 2.14* (1.89,2.38) | 1.93* (1.86,2.00) | 0.52* (0.20,0.85) | 0.44 (-0.22,1.12) |
| 50–54 | 2.15* (1.96,2.33) | 2.12* (1.95,2.29) | 0.73* (0.40,1.06) | 1.08* (0.15,2.02) |
| 55–59 | 2.04* (1.87,2.22) | 2.30* (2.20,2.40) | 0.89 (-0.01,1.79) | 0.67* (0.23,1.12) |
| 60–64 | 1.60* (1.46,1.73) | 2.10* (2.00,2.21) | 0.46 (-0.59,1.52) | 0.78* (0.61,0.95) |
| 65–69 | 0.66* (0.62,0.69) | 1.39* (1.30,1.48) | 0.64 (-0.27,1.55) | 0.35 (-0.72,1.43) |
| 70–74 | -0.35* (-0.52, -0.18) | 0.12* (0.07,0.18) | 0.59 (-0.01,1.20) | 0.56* (0.24,0.89) |
| 75–79 | -1.19* (-1.37, -1.01) | -0.92* (-1.01, -0.83) | 0.80* (0.49,1.10) | 0.22 (-0.93,1.39) |
| 80–84 | -1.52* (-1.66, -1.38) | -1.25* (-1.34, -1.16) | 1.12 (-0.19,2.44) | 1.12* (0.02,2.23) |
Note: *, Indicates that the Average Annual Percent Change (AAPC) is significantly different from zero at the alpha = 0.05 level; AAPC, average annual percent change; CI, confidence interval
The age, period, cohort effects on the incidence and mortality rates of diabetes in India
Figure 2a and b indicate the existence of a period effect on incidence and mortality of Diabetes in both the sexes (males and females) respectively. While in case of incidence, period effect does not exist up-to age ≤ 29 years among males and females both. Further, among males’ recent cohorts such as 2000-04, 2005-09, 2010-14& 2015-19 show a clear difference in the incidence of diabetes with respect to older cohorts 1995-99& 1990-94. Whereas, in case of females, all cohorts are having different diabetes incidence. One more very interesting observation is that newer cohorts are having high incidence rates in both gender and in most of the age groups, irrespective of the fact that females in the age 75–79 years have the highest incidence (870.1 per 100,000) of diabetes in the period 2000-04. It is evident from the figure that up to some age incidence rate shows an inverted U shape (Male 30–34 years to 65–69, Female 40–44 years to 65–69 years).
Fig. 2.
Period effect on incidence and mortality of diabetes by gender in India
Figure 2c and d display the period effect of mortality for males and females respectively. Figures indicate that there is very minor change in the increase in mortality below the age of 50 years and after that mortality rate increased exponentially till the last age for both sexes. Male and female show the period effect after the age of 55 years and it is clearly seen that recent cohorts are having higher mortality with respect to older cohorts. Peak value of Mortality due to Diabetes is slightly higher among males.
Figure 3 advocates the existence of a cohort effect on incidence and mortality of Diabetes in India for both sexes in the respective age strata. In case of both males and females, older cohorts have higher rates of incidence as well as mortality in comparison to later cohorts. In spite of the fact that we are considering the diabetes incidence for females, we are getting a higher incidence rate in older cohorts in comparison to males (Fig. 3a and b). Wherever it is also true that incidence of diabetes is lower for males but mortality is lower among females irrespective of birth cohort. With the increase in the age, incidence and mortality rates increased primarily and then decreased within the same age group with advancing the birth cohort. For instance, the 80 to 84 years age group shows an increasing pattern of diabetes incidence among males in the 1910-14 to 1930-34 birth cohort and then decreases for the 1935-39 birth cohort. Additionally, birth cohort incidence rates among the population up to age 25 years is below 200 per 100,000 in both males and females. However, there has not been a significant change in the incidence rate of diabetes in the population below 20 years with the advancing birth cohort. Highest diabetes incidence is found in the age group 75–79 years in both males and females. In comparison to incidence, mortality rate is below50 per 100,000 up to age 60 years among both genders (Fig. 3c and d).
Fig. 3.
Cohort effect on incidence and mortality rates of diabetes by gender in India
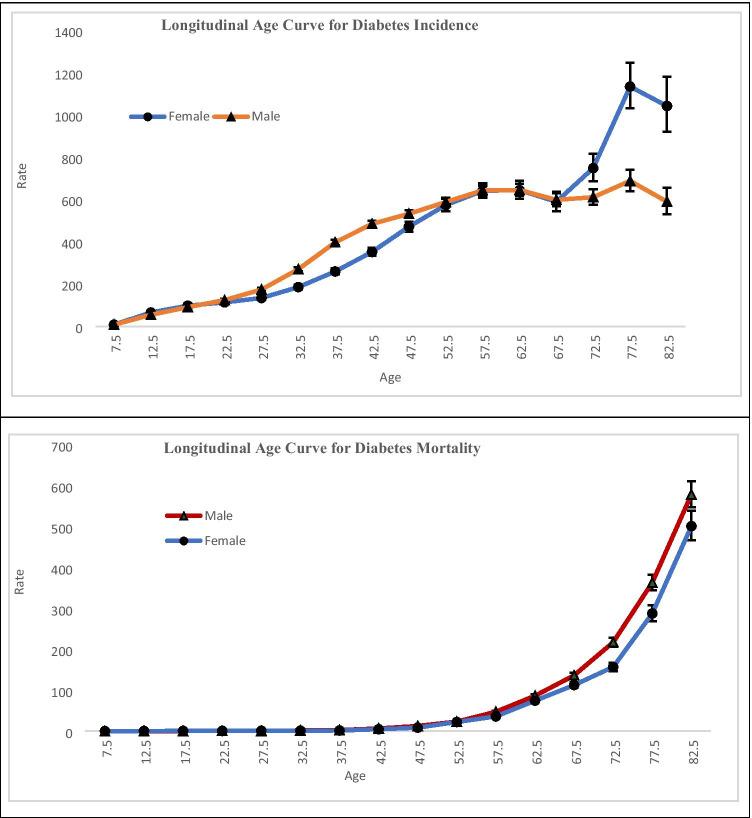
Longitudinal age curves of incidence and mortality of diabetes by sex are represented in Fig. 4, In the same birth cohort, the incidence of Diabetes showed a continuous increase up to the age interval55-59 years for male and 60–64 years for females just after that it decreases up to the age 65–69 years with a slight magnitude. Incidence of diabetes among females aged 65–69 years showed a rapid increase up to the age 75–79 years. Males also showed the increment of Diabetes incidence in the same age strata but with a slower pace in comparison to females. The incidence rate of Diabetes for both male and female starts with the value 7.6 for male and 7.9 for female in the age 5–9 years and reaches to the highest value in the age group 75–79 years which was 687.7 per 100,000 for male and 1134.2 per 100,000 for female.
Fig. 4.
Longitudinal age curve of Diabetes incidence and mortality rate of India under the Age-Period-Cohort framework
Generally speaking, the mortality rate of Diabetes for both sexes followed an upward trend with advancing age. It was noted that the fastest growth in mortality due to Diabetes starts from the age 35–39 years (2.9 per 100,000 for male, 1.8 per 100,000 for female) and reaches to its peak value at the age 80–84 years (579.3 per 100,000 for males, 501.7 per 100,000 for females) for both sexes.
The estimated period and cohort RRs by sex are shown in Tables 3 and 4. Year 2000-04 are taken as the year of reference for both sexes (male and female) and for both rates (incidence and mortality) (Table 3). Risk of diabetes incidence and mortality decreases while going back from the reference period 2000-04. Whereas incidence of diabetes among males and females increases by 8 % (RR: 1.08, CI: 1.05–1.11) and 11 % (RR: 1.11, CI: 1.07–1.16) respectively in the period 2015-19 with reference to the period 2000-04. Furthermore, mortality in both genders decreased by a few percentage points (3 % among males, 5 % among females) in the period 2015-19 in comparison to the reference period.
Table 3.
Period RRs of Diabetes incidence & mortality rate of India adjusted for age and birth cohort effects compared to the reference period (2000-04) and the corresponding 95 % CI
| Period | Incidence (RR 95 % CI) | Mortality (RR 95 % CI) | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| 1990–1994 | 0.85 (0.82–0.87) | 0.87 (0.83–0.91) | 0.89 (0.85–0.92) | 0.92 (0.87–0.97) |
| 1995–1999 | 0.91 (0.89–0.94) | 0.90 (0.86–0.95) | 0.93 (0.90–0.96) | 0.95 (0.91–0.99) |
| 2000–2004 | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) |
| 2005–2009 | 1.03 (1.00–1.06) | 1.06 (1.02–1.11) | 1.00 (0.97–1.03) | 0.94 (0.90–0.98) |
| 2010–2014 | 0.99 (0.97–1.02) | 1.03 (0.99–1.07) | 1.03 (1.00–1.07) | 0.93 (0.89–0.97) |
| 2015–2019 | 1.08 (1.05–1.11) | 1.11 (1.07–1.16) | 0.97 (0.93–1.01) | 0.95 (0.90–0.99) |
Table 4.
Cohort RRs of diabetes incidence & mortality rates of India adjusted for age and period effects compared to the reference cohort (1960-64) and the corresponding 95 % CI
| Cohort | Incidence (RR 95 % CI) | Mortality (RR 95 %CI) | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| 1910-14 | 0.75 (0.54–1.02) | 0.71 (0.50–1.01) | 0.50 (0.45–0.56) | 0.62 (0.55–0.71) |
| 1915-19 | 0.73 (0.61–0.87) | 0.70 (0.58–0.86) | 0.55 (0.51–0.60) | 0.66 (0.60–0.72) |
| 1920-24 | 0.75 (0.67–0.84) | 0.71 (0.61–0.82) | 0.60 (0.57–0.64) | 0.69 (0.63–0.74) |
| 1925-29 | 0.78 (0.72–0.85) | 0.71 (0.63–0.80) | 0.65 (0.62–0.69) | 0.73 (0.68–0.79) |
| 1930-34 | 0.80 (0.75–0.85) | 0.69 (0.62–0.76) | 0.69 (0.65–0.73) | 0.75 (0.70–0.81) |
| 1935-39 | 0.80 (0.75–0.84) | 0.67 (0.62–0.73) | 0.74 (0.70–0.77) | 0.77 (0.72–0.83) |
| 1940-44 | 0.78 (0.75–0.82) | 0.69 (0.64–0.75) | 0.77 (0.73–0.81) | 0.80 (0.75–0.85) |
| 1945-49 | 0.78 (0.75–0.82) | 0.76 (0.71–0.81) | 0.83 (0.79–0.87) | 0.88 (0.83–0.94) |
| 1950-54 | 0.82 (0.79–0.85) | 0.83 (0.79–0.89) | 0.85 (0.81–0.89) | 0.90 (0.85–0.96) |
| 1955-59 | 0.89 (0.86–0.93) | 0.91 (0.86–0.96) | 0.91 (0.87–0.95) | 0.93 (0.87–0.99) |
| 1960-64 | 1.0 (1.0– 1.0) | 1.0 (1.0– 1.0) | 1.0 (1.0– 1.0) | 1.0 (1.0– 1.0) |
| 1965-69 | 1.12 (1.08–1.15) | 1.13 (1.07–1.19) | 1.01 (0.95–1.07) | 1.12 (1.04–1.21) |
| 1970-74 | 1.22 (1.18–1.26) | 1.25 (1.19–1.33) | 1.06 (0.99–1.14) | 1.05 (0.95–1.16) |
| 1975-79 | 1.28 (1.23–1.32) | 1.31 (1.23–1.39) | 1.07 (0.98–1.17) | 1.04 (0.92–1.18) |
| 1980-84 | 1.29 (1.24–1.35) | 1.30 (1.22–1.39) | 1.04 (0.93–1.17) | 0.92 (0.79–1.09) |
| 1985-89 | 1.32 (1.26–1.38) | 1.33 (1.23–1.43) | 1.03 (0.89–1.18) | 0.87 (0.72–1.05) |
| 1990-94 | 1.34 (1.28–1.42) | 1.33 (1.22–1.45) | 0.97 (0.80–1.16) | 0.76 (0.61–0.94) |
| 1995-99 | 1.37 (1.29–1.45) | 1.30 (1.18–1.43) | 0.85 (0.68–1.06) | 0.64 (0.49–0.83) |
| 2000-04 | 1.36 (1.26–1.47) | 1.25 (1.11–1.40) | 0.71 (0.51–0.98) | 0.58 (0.42–0.80) |
| 2005-09 | 0.99 (0.87–1.11) | 0.87 (0.72–1.04) | 0.72 (0.44–1.17) | 0.54 (0.34–0.83) |
| 2010-14 | 1.48 (1.12–1.96) | 1.46 (0.96–2.22) | 0.54 (0.26–1.11) | 0.58 (0.31–1.07) |
Year 1960-64 is taken as the year of reference cohort for both sexes (male and female) while estimating the risk of incidence and mortality (Table 4). Lower risk of incidence and mortality were found prior to the reference cohort 1960-64, whereas, after the reference period risk of diabetes incidence for both genders increases significantly. Furthermore, mortality risk decreases after a certain cohort. For males, cohort RRs for mortality increases from the birth cohort (1960-64) to (1985-89) and then decreases up to 2010-14. Additionally, among females the mortality due to diabetes primarily increases after the reference period and then starts decreasing till the end of the period. In reference to the 1960-64 birth cohort, cohort 2010-14 shows 42 % (RR: 0.58, CI: 0.31–1.07) decrease in mortality due to Diabetes among females and 46 % (RR: 0.54, CI: 0.26–1.11) decrease among males. While comparing incidence and mortality after the reference period, risk of diabetes incidence shows an increasing pattern but mortality shows the decreasing pattern. Further, the decrement is observed to be higher among females.
Figure 5 indicates the net drifts and local drift for incidence and mortality of diabetes among both sexes from 1990 to 2019. The overall net drifts per year are found to be 0.86 % (95 % CI, 0.76 to 0.96) for males and 0.97 % (95 % CI, 0.82 to 1.13) for females in case of diabetes incidence. For females, local drift values are below net drift value (0.97 %) except in the age 30 to 70. In the case of male, there are two age groups having the value greater than net drift value (0.86 %) which are 30–55 years and 75 + years.
Fig. 5.
Local drift with net drift and 95 % confidence intervals in incidence & mortality of diabetes by gender in India
In case of mortality, net drift value for males and females are 0.44 (95 % CI, 0.22 to 0.67) and 0.01 (95 % CI, -0.26 to 0.27) respectively. The curve for local drifts for mortality due to diabetes shows an upward trend almost for all ages. It is also found that for both sexes the local drift value crosses the net drift at the same age strata which is 30 to 35 years.
In addition, the results of Wald test demonstrated that cohort and period RRs for both sex in case of incidence as well as for mortality and the net drifts and local drifts were statistically significant at p < 0.05.
Discussion
We have used Joinpoint regression analysis to determine the magnitude of the time trends in the age standardized incidence and mortality rates of diabetes, the Average Annual Percent Change (AAPC) and corresponding 95 % Confidence Interval (CI). Prior to the development of the joinpoint and AAPC methodology, in order to characterize a trend over a fixed interval, a single regression line (on a log scale) over the fixed interval was fit, and the slope coefficient was then transformed to an APC. This older methodology has two disadvantages over the AAPC. First, the older methodology assumes linearity of the trend (on a log scale) over the interval, while the AAPC does not. Secondly, the AAPC can be used to characterize a short segment based on a joinpoint model fit over a much longer series. This is especially advantageous for situations when the data are sparse.
In order to determine the Age, Period and Cohort effects on Incidence and Mortality of Diabetes in India, we have utilized Age Period Cohort analysis technique. The earliest version of this model [39], though could be used to describe the complex social, historical, and environmental elements that affect individual and populations at the same time [40], was plagued by multicollinearity problem due to the linear relationship between Age, Period and Cohort (Age = Period - Cohort), and hence the real deal was to identify all three of the Age, Period and Cohort effects uniquely. Later in 2004, Intrinsic Estimator (IE) method was proposed [41], where the dimension of the design matrix was full rank minus one, so that a unique solution could be extracted from the Age-Period-Cohort model. The IE method being just a variant of principal components regression model, provides a unique unbiased estimator with smaller variance than constrained models [41]. However, in a very recent review article of existing methods [42], it has been recommended that, due to the nature of the identification problem, there should be a possible combination of Age-Period-Cohort Effects which can be estimated empirically. It has been concluded that none of the existing methodologies can actually solve the identification problem, rather these methods are based on strong and explicit theory-based assumptions [42]. Hence, Age-Period-Cohort analysis despite being a widely used statistical methodology that involves following a person or a community across time to investigate age, period, and cohort effects, must be used with caution while handling the potentially strong model assumptions.
Next, in light of the other recent studies on Diabetes around the globe and across different regions of India, as we attempt to validate and interpret the findings from our study that utilized longitudinal data on diabetes in the period 1990–2019 from the GBD with an aim to investigate the age, period and cohort effects of incidence and mortality rates in India, it is observed that the incidence of diabetes and the related mortality have increased in the period 1990–2019. Numerous epidemiological studies from southern and eastern India [13] have shown an increasing trend for the prevalence of diabetes since 2000. A similar trend was observed in a study from Denmark which reported an increase in the prevalence of type-2 diabetes [6]. However, the incidence of diabetes and the mortality due to diabetes did not differ much by gender.
Several other studies in India have also reported the incidence of diabetes to be independent of gender [43, 13, 44], although a few studies have shown higher prevalence among male [6, 45, 46]. This implies that the incidence of diabetes in India has increased and requires immediate attention. This finding corroborates and contrasts with the findings of First Nations [47]. However, a clinically diagnosed incidence of type-2 diabetes has witnessed a decline in the United Kingdom [48]. Our study has found the incidence of diabetes to be at increasing trend with increasing age, while mortality due to diabetes has decreased in younger ages and increased among the older population. In another study, Little et al. has reported a high prevalence of type 2 diabetes (10.8 %) among adults population aged more than 19 years in rural parts of South India [49]. A study based on large cross-sectional study in Punjab by Tripathy et al., has found the prevalence of diabetes to be significantly higher among people aged 45–69 years (18.0 %), with hypertension (14.3 %), with obesity (14.4 %) and having a family history of DM (11.9 %) [43]. Advancements in treatment of diabetes has been identified as a factor which might have led to the increases in prevalence by decreasing mortality rates among those with diabetes [50, 51]. In our results, the incidence of the disease shows a rising trend with advancing ages after the age of 35–39 years, and a decreasing trend after age of 60 years for male and female. The incidence rate ratios are found to be decreasing in the older ages which have witnessed a decline after 2011 in the United Kingdom [48]. Likewise, the net period effect showed that the mortality increased with advancing ages and it was highest for the period 2015-19 for both male and female. It followed a J-shaped curve. The net cohort effect presented the incidence and mortality for both genders. However, a decline in the mortality among diabetic patients has been reported in several countries [6]. According to our findings, the incidence and mortality decreased from earlier birth cohorts to more recent birth cohorts. This finding is in line with the findings from first nations and Ontario [47]. However, in our findings, the age-standardised incidence rate is found to increase from 199.14 to 1990 to 317.02 in 2019 per 100,000 population. Similarly, mortality due to diabetes increased from 22.30 deaths in 1990 to 27.35 deaths per 100,000 in 2019. In this context, we can quote a very recent study, that has reported that across strata of increasing age, the diabetes related Mortality Risk ratios decreased considerably [52].
When standardized for age, by applying joint point regression the Annual Percentage Change depicts that there has been a significant increase in the incidence rates of diabetes over the study period with highest increase in the year 2017–2019. However, the mortality of diabetes is stable over the period 1990–2019.The longitudinal age curve of Diabetes mortality rate of diabetes under the Age-Period-Cohort framework showed an increasing trend for ages up to 55–59 for males and 50–54 years females. Through the Age-Period-Cohort model, it was observed that the net period effect showed significant changes in incidence with increasing age. The period RRs for diabetes incidence in both sexes increased initially and then decreased. Newer cohorts have high incidence rates irrespective of gender in most of the ages. For females, the highest incidence is observed in age group 75–79 in the period 2000-04 (870.1 per 100,000).
Cohort RRs for both genders show an increasing pattern for incidence of Diabetes for females but a decreasing pattern for mortality. The age, period and cohort effect on the incidence and mortality of diabetes are discussed in the following sections which would be helpful in providing epidemiological evidence to understand the reasons behind the rise in the prevalence of diabetes in the country.
Age effect: Age effect on the mortality of diabetes show a declining pattern with advancing age for both male and female but increased after age group 60–64 and 55–59 and above for males and females respectively. This may be attributed to the increase in the old age population. Anecdotal evidence shows that older people are more likely to develop different kinds of disease, especially cardiovascular disease. They are also at higher risk of mortality due to diabetes mellitus [53]. With the increasing age and decreased physical activity coupled with decreased immunity people are likely to become weak and fragile [54]. It is often associated with increased mortality. Apart from that, complications related to diabetes and co-morbidities are more prevalent in older ages than in their younger counterparts [55]. They are also likely to suffer from visual and cognitive impairments [56]. These factors may impact the net age effect on mortality and incidence of diabetes in Indian population.
Period effect: Period effect is affected by the historical events and environmental factors that may have taken place throughout the period. As evident from the literature, the rapid epidemiological transition and associated changes in dietary patterns especially in urban areas is considered to play an important role in the epidemic of diabetes [46]. Risk factors of diabetes mainly included age, family history of diabetes mellitus, obesity, and physical inactivity. Strong association exists between over-weight and the increased prevalence of diabetes as evident from the literature [57, 58]. Overweight, in turn, is correlated with the lack of physical activity [57]. Overall, there has been a rapid increase in the incidence and mortality of diabetes in the last few decades. This rapid increase has been attributed to change in lifestyle resulting in rise in obese population [59], physical inactivity, population ageing and urbanization. However, the pattern of incidence and mortality in younger age groups has remained more or less the same. Improved socio-economic status has resulted in an explosion of metabolic disorders, such as CVD, diabetes and hypertension among Indians [60, 61]. Point worth noting is that incidence and mortality of diabetes is higher in older ages irrespective of gender. A number of studies have highlighted that common risk factors of diabetes include hypertension, smoking, and obesity [60, 62]. These findings are similar to the studies who have reported a rising trend in prevalence of diabetes across different parts of India.
Cohort effect: Cohort effect explains the variations across age groups of individuals born in the same year. These variations occur when a cohort carries the imprints of the physical and social exposures received in different ages. Cohort effect on the incidence and mortality of diabetes decreased from earlier birth cohorts to more recent birth cohorts. These findings are compatible with the study from China [63]. In reference to the 1960-64 birth cohort, cohort 2010-14 shows 58 % decrement in mortality due to Diabetes among females and 54 % decrement among males. After the reference period, risk of diabetes incidence shows an increasing pattern but mortality has decreased and the highest pace of decrement is observed among females. India has several-fold higher prevalence of diabetes compared with European populations, as reported by the International Diabetes Epidemiology Group when considered for all age groups [64]. In the earlier birth cohorts, the incidence of the disease is higher, which have decreased in the later birth cohorts. The diabetic proportion in ages less than 40 years of age has increased considerably over a decade [60]. Cohort RRs for male mortality increased for the birth cohort (1910 − 114) to (1975-79) and then decreased up to 2010-14. Although, the Female mortality follows a similar pattern, it started declining in birth cohort 1970-75. In reference to 1960-64 birth cohorts, cohort 2010-14 shows a 42 and 46 % decrement in mortality due to Diabetes resp. among females and males. One of the probable reasons for the decline in mortality is good education, better access to health and more awareness regarding health in comparison to their counterparts in earlier birth cohorts [65].
The overall net drifts per year were found to be 0.86 % for females and 0.97 % for males in case of diabetes incidence. However, in case of mortality, net drift value for males and females were calculated to be 0.44 and 0.01 respectively. Additionally, Wald test results demonstrated that the cohort and period RRs for both sexes (p < 0.05, for all) were statistically significant in case of incidence as well as for mortality also and similarly for the net drifts and local drifts (at p < 0.05).
Our study has several limitations. First, Although the GBD study has incorporated methods to adjust for missing or incomplete data, there still may be a possibility of some inaccuracy in the mortality data. Secondly, we have performed Age-Period-Cohort analysis in periods which are multiples of five years as GBD provides data in five-year intervals. This may lead to the smoothening of certain subtle variations in age, period and cohort effects. Thirdly, being an ecological study, the interpretations derived here are true at population levels but do not necessarily hold for individuals. Finally, while there are many approaches for aggregated data, there is a need to develop more sophisticated techniques for a better and complete use of the rising availability of huge, individual-level datasets [66].
Conclusions
This study shows the incidence of diabetes and the related mortality has increased over in the past few decades in India. The incidence of diabetes increased with increasing age, while diabetes mortality has decreased in younger age groups and increased in older age groups. Age effect on the mortality of diabetes has increased with advancing age for both male and females. Period effects showed an increase in the incidence and mortality of diabetes with advancing periods. There is a decreasing trend in the mortality and incidence of diabetes from earlier birth cohorts to more recent birth cohorts in India. Community based health promotional activities like health education, lifestyle modification with special emphasis on physical activity, healthy diet and environmental modification should be promoted, especially for male and earlier birth cohorts at high risk of diabetes.
Author contributions
RPJ, DD and MS contributed in conceptualizing the study. RPJ, NS, PP, DD, KB and MS were responsible for the analysis. All authors contributed to the interpretation of the data, and critically revised all versions of the manuscript and approved the final version.
Declarations
Conflict of interest
None declared.
Footnotes
The original online version of this article was revised: The references have been updated
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/12/2021
A Correction to this paper has been published: 10.1007/s40200-021-00865-5
Contributor Information
Ravi Prakash Jha, Email: ravijha0292@gmail.com.
Neha Shri, Email: nshri793@gmail.com.
Priyanka Patel, Email: patelpriyankacsb@gmail.com.
Deepak Dhamnetiya, Email: drdeepakdhamnetiya@gmail.com.
Krittika Bhattacharyya, Email: krittikabhattacharyya.federer@gmail.com.
Mayank Singh, Email: singhmayank.251195@gmail.com.
References
- 1.International diabetes federation. IDF diabetes Atlas, D, 9th ed; 2019.
- 2.Nanditha A, Ma RC, Ramachandran A, Snehalatha C, Chan JC, Chia KS, Zimmet PZ. Diabetes in Asia and the Pacific: implications for the global epidemic. Diabetes Care. 2016;39(3):472–85. doi: 10.2337/dc15-1536. [DOI] [PubMed] [Google Scholar]
- 3.Norhammar A, Bodegård J, Nyström T, Thuresson M, Eriksson JW, Nathanson D. Incidence, prevalence and mortality of type 2 diabetes requiring glucose-lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006–2013. Diabetologia. 2016;59(8):1692–701. [DOI] [PubMed]
- 4.McAllister DA, Read SH, Kerssens J, Livingstone S, McGurnaghan S, Jhund P, Wild SH. Incidence of hospitalization for heart failure and case-fatality among 3.25 million people with and without diabetes mellitus. Circulation. 2018;138(24):2774–86. [DOI] [PMC free article] [PubMed]
- 5.Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, Gregg EW. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–26. [DOI] [PubMed]
- 6.Carstensen B, Rønn PF, Jørgensen ME. Prevalence, incidence and mortality of type 1 and type 2 diabetes in Denmark 1996–2016. BMJ Open Diabetes Res Care. 2020;8(1):e001071. [DOI] [PMC free article] [PubMed]
- 7.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–40. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 8.Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269. doi: 10.5001/omj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou B, Lu Y, Hajifathalian K, Bentham J, Di Cesare M, Danaei G, Lo WC. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4· 4 million participants. Lancet. 2016;387(10027):1513–30. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramachandran A, Snehalatha C, Shetty AS, Nanditha A. Trends in prevalence of diabetes in Asian countries. World J Diabetes. 2012;3(6):110. doi: 10.4239/wjd.v3.i6.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao Q. Age–and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care. 2003;26(6):1770–80. doi: 10.2337/diacare.26.6.1770. [DOI] [PubMed] [Google Scholar]
- 12.Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, Bhansali A. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5(8):585–96. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, Das AK, Diabetes Epidemiology Study Group in India (DESI). High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia. 2001;44(9):1094–101. [DOI] [PubMed]
- 14.Sadikot SM, Nigam A, Das S, Bajaj S, Zargar AH, Prasannakumar KM, Jamal A. The burden of diabetes and impaired glucose tolerance in India using the WHO 1999 criteria: prevalence of diabetes in India study (PODIS). Diabetes Res Clin Pract. 2004;66(3):301–7. [DOI] [PubMed]
- 15.Hwang CK, Han PV, Zabetian A, Ali MK, Narayan KV. Rural diabetes prevalence quintuples over twenty-five years in low-and middle-income countries: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2012;96(3):271–85. doi: 10.1016/j.diabres.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Gutch M, Razi SM, Kumar S, Gupta KK. Diabetes mellitus: trends in northern India. Indian J Endocrinol Metab. 2014;18(5):731. doi: 10.4103/2230-8210.139219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapoor D, Bhardwaj AK, Kumar D, Raina SK. Prevalence of diabetes mellitus and its risk factors among permanently settled tribal individuals in tribal and urban areas in northern state of sub-Himalayan region of India. Int J Chronic Dis. 2014;2014:380597. [DOI] [PMC free article] [PubMed]
- 18.Vision Rehabilitation Centre. Diabetes and the eye. Newsletter 12 [document on Internet]. Hyderabad (India): Vision Rehabilitation Centre; 2003.
- 19.Joshi SR, Parikh RM. India; the diabetes capital of the world: now heading towards hypertension. J Assoc Physicians India. 2007;55(Y):323. [PubMed]
- 20.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. 2012;10:1. doi: 10.1186/1478-7954-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray CJ, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–6. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 23.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020. 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed]
- 24.Global Burden of Disease Collaborative Network. Global burden of disease study 2019 (GBD 2019) results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME); 2020. Available from http://ghdx.healthdata.org/gbd-results-tool. Accessed 30 Jan 2021.
- 25.Luo G, Zhang Y, Guo P, Wang L, Huang Y, Li K. Global patterns and trends in stomach cancer incidence: age, period and birth cohort analysis. Int J Cancer. 2017;141(7):1333–44. doi: 10.1002/ijc.30835. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Bai Z, Wang Z, Yu C. Comparison of secular trends in cervical cancer mortality in China and the United States: an age-period-cohort analysis. Int J Environ Res Public Health. 2016;13(11):1148. [DOI] [PMC free article] [PubMed]
- 27.Wang L, Yu C, Liu Y, Wang J, Li C, Wang Q, Zhang ZJ. Lung cancer mortality trends in China from 1988 to 2013: new challenges and opportunities for the government. Int J Environ Res Public Health. 2016;13(11):1052. doi: 10.3390/ijerph13111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y. Social inequalities in happiness in the United States, 1972 to 2004: an age-period-cohort analysis. Am Sociol Rev. 2008;73(2):204–26. [Google Scholar]
- 29.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983. pp. 311–24. [PubMed]
- 30.Robertson C, Gandini S, Boyle P. Age-period-cohort models: a comparative study of available methodologies. J Clin Epidemiol. 1999;52(6):569–83. doi: 10.1016/s0895-4356(99)00033-5. [DOI] [PubMed] [Google Scholar]
- 31.Mbulaiteye SM, Anderson WF, Ferlay J, Bhatia K, Chang C, Rosenberg PS, Parkin DM. Pediatric, elderly, and emerging adult-onset peaks in Burkitt’s lymphoma incidence diagnosed in four continents, excluding Africa. Am J Hematol. 2012;87(6):573–8. [DOI] [PMC free article] [PubMed]
- 32.Anderson WF, Rosenberg PS, Menashe I, Mitani A, Pfeiffer RM. Age-related crossover in breast cancer incidence rates between black and white ethnic groups. J Natl Cancer Inst. 2008;100(24):1804–14. doi: 10.1093/jnci/djn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pastor-Barriuso R, López-Abente G. Changes in period and cohort effects on haematological cancer mortality in Spain, 1952–2006. BMC Cancer. 2014;14(1):250. doi: 10.1186/1471-2407-14-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang HP, Anderson WF, Rosenberg PS, Trabert B, Gierach GL, Wentzensen N, Sherman ME. Ovarian cancer incidence trends in relation to changing patterns of menopausal hormone therapy use in the United States. J Clin Oncol. 2013;31(17):2146. doi: 10.1200/JCO.2012.45.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jemal A, Ma J, Rosenberg PS, Siegel R, Anderson WF. Increasing lung cancer death rates among young women in southern and midwestern States. J Clin Oncol. 2012;30(22):2739. doi: 10.1200/JCO.2012.42.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Hu S, Sang S, Luo L, Yu C. Age–period–cohort analysis of stroke mortality in China: data from the Global Burden of Disease Study 2013. Stroke. 2017;48(2):271–5. doi: 10.1161/STROKEAHA.116.015031. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg PS, Check DP, Anderson WF. A web tool for age–period–cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Prev Biomark. 2014;23(11):2296–302. doi: 10.1158/1055-9965.EPI-14-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Lin Z, Liu Z, He H, Bai L, Lyu J (2020) Secular trends in the incidence of eating disorders in China from 1990 to 2017: a joinpoint and age–period–cohort analysis. Psychol Med;1–11. 10.1017/S0033291720002706. [DOI] [PubMed]
- 39.Mason KO, Mason WM, Winsborough HH, Poole WK. Some methodological issues in cohort analysis of archival data. Am Sociol Rev. 1973;38(2):242–58. [Google Scholar]
- 40.Yang Y, Land KC. Age-period-cohort analysis: new models, methods, and empirical applications. Abingdon: Taylor & Francis; 2013. [Google Scholar]
- 41.Yang Y, Fu WJ, Land KC. A methodological comparison of age-period-cohort models: the intrinsic estimator and conventional generalized linear models. Sociol Methodol. 2004;34(1):75–110. [Google Scholar]
- 42.Bell A. Age period cohort analysis: a review of what we should and shouldn’t do. Ann Hum Biol. 2020;47(2):208–17. doi: 10.1080/03014460.2019.1707872. [DOI] [PubMed] [Google Scholar]
- 43.Tripathy JP, Thakur JS, Jeet G, Chawla S, Jain S, Pal A, Saran R. Prevalence and risk factors of Diabetes in a large community-based study in North India: results from a STEPS survey in Punjab, India. Diabetol MetabSyndr. 2017;9(1):8. doi: 10.1186/s13098-017-0207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barik A, Mazumdar S, Chowdhury A, Rai RK. Physiological and behavioral risk factors of type 2 diabetes mellitus in rural India. BMJ Open Diabetes Res Care. 2016;4(1):e000255. [DOI] [PMC free article] [PubMed]
- 45.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research–INdiaDIABetes (ICMR–INDIAB) study. Diabetologia. 2011;54(12):3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 46.Meshram II, Rao MVV, Rao VS, Laxmaiah A, Polasa K. Regional variation in the prevalence of overweight/obesity, hypertension and diabetes and their correlates among the adult rural population in India. Br J Nutr. 2016;115(7):1265–72. doi: 10.1017/S0007114516000039. [DOI] [PubMed] [Google Scholar]
- 47.Walker JD, Slater M, Jones CR, Shah BR, Frymire E, Khan S, Green ME. Diabetes prevalence, incidence and mortality in First Nations and other people in Ontario, 1995–2014: a population-based study using linked administrative data. CMAJ. 2020;192(6):E128–35. doi: 10.1503/cmaj.190836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pal K, Horsfall L, Sharma M, Nazareth I, Petersen I. Time trends in the incidence of clinically diagnosed type 2 diabetes and pre-diabetes in the UK 2009–2018: a retrospective cohort study. BMJ Open Diabetes Res Care. 2021;9(1):e001989. [DOI] [PMC free article] [PubMed]
- 49.Little M, Humphries S, Patel K, Dodd W, Dewey C. Factors associated with glucose tolerance, pre-diabetes, and type 2 diabetes in a rural community of south India: a cross-sectional study. DiabetolMetab Syndr. 2016;8(1):21. doi: 10.1186/s13098-016-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magliano DJ, Islam RM, Barr EL, Gregg EW, Pavkov ME, Harding JL, Shaw JE. Trends in incidence of total or type 2 diabetes: systematic review. BMJ. 2019;366:l5003. [DOI] [PMC free article] [PubMed]
- 51.Magliano DJ, Chen L, Pavkov ME, Gregg EW, Shaw JE. Trends in diabetes incidence worldwide: are the findings real? Maturitas. 2020;137:63–4. doi: 10.1016/j.maturitas.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt C, Reitzle L, Heidemann C, Paprott R, Ziese T, Scheidt-Nave C, Baumert J. Excess mortality in adults with documented diabetes in Germany: routine data analysis of all insurance claims in Germany 2013–2014. BMJ Open. 2021;11(1):e041508. [DOI] [PMC free article] [PubMed]
- 53.Chi MJ, Liang CK, Lee WJ, Peng LN, Chou MY, Chen LK. Association of new-onset diabetes mellitus in older people and mortality in Taiwan: a 10-year nationwide population-based study. J Nutr Health Aging. 2017;21(2):227–32. doi: 10.1007/s12603-016-0751-9. [DOI] [PubMed] [Google Scholar]
- 54.Cobo A, Vázquez LA, Reviriego J, Rodríguez-Mañas L. Impact of frailty in older patients with diabetes mellitus: an overview. Endocrinol Nutr (Engl Ed). 2016;63(6):291–303. [DOI] [PubMed]
- 55.Zhang ZJ, Zhao G, Song Y. Attention is needed for women in the control of diabetic complications in China. Acta Diabetol. 2014;51(6):1081. doi: 10.1007/s00592-014-0589-8. [DOI] [PubMed] [Google Scholar]
- 56.Chentli F, Azzoug S, Mahgoun S. Diabetes mellitus in elderly. Indian J Endocrinol Metab. 2015;19(6):744. doi: 10.4103/2230-8210.167553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Type 2 diabetes in Asian-Indian urban children. Diabetes Care. 2003;26(4):1022–5. doi: 10.2337/diacare.26.4.1022. [DOI] [PubMed] [Google Scholar]
- 58.Ehtisham S, Barrett TG, Shaw NJ. Type 2 diabetes mellitus in UK children–an emerging problem. Diabet Med. 2000;17(12):867–71. doi: 10.1046/j.1464-5491.2000.00409.x. [DOI] [PubMed] [Google Scholar]
- 59.Mohan V, Mathur P, Deepa R, Deepa M, Shukla DK, Menon GR, Thankappan KR, et al. Urban rural differences in prevalence of self-reported diabetes in India—The WHO–ICMR Indian NCD risk factor surveillance. Diabetes Res Clin Pract. 2008;80(1):159–68. doi: 10.1016/j.diabres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 60.Ramachandran A, Mary S, Yamuna A, Murugesan N, Snehalatha C. High prevalence of diabetes and cardiovascular risk factors associated with urbanization in India. Diabetes Care. 2008;31(5):893–8. doi: 10.2337/dc07-1207. [DOI] [PubMed] [Google Scholar]
- 61.Ramachandran A, Snehalatha C, Latha E, Manoharan M, Vijay V. Impacts of urbanisation on the lifestyle and on the prevalence of diabetes in native Asian Indian population. Diabetes Res Clin Pract. 1999;44(3):207–13. doi: 10.1016/s0168-8227(99)00024-8. [DOI] [PubMed] [Google Scholar]
- 62.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362(12):1090–101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 63.Liu X, Yu C, Wang Y, Bi Y, Liu Y, Zhang ZJ. Trends in the incidence and mortality of diabetes in China from 1990 to 2017: a joinpoint and age-period-cohort analysis. Int J Environ Res Public Health. 2019;16(1):158. doi: 10.3390/ijerph16010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Decode-Decoda Study Group, & European Diabetes Epidemiology Group. Age, body mass index and type 2 diabetes—associations modified by ethnicity. Diabetologia. 2003;46(8):1063–70. [DOI] [PubMed]
- 65.Cohen AK, Syme SL. Education: a missed opportunity for public health intervention. Am J Public Health. 2013;103(6):997–1001. doi: 10.2105/AJPH.2012.300993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Debiasi E. Age-period-cohort analysis: a summary of analytical approaches and results. Centre for Economic Demography and Department of Economic History, Lund University; 2018.