Abstract
Perihilar cholangiocarcinoma (pCCA) is one of the most complex challenges for hepatobiliary surgeons. Poor results and high incidence of morbidity after Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) for pCCA discouraged this indication. It has been proposed that minimally invasive approach for ALPPS first stage, as well as combination of surgical liver partition and radiologic portal vein embolization (PVE), may improve outcomes reducing interstage morbidity. We report a case of right trisectionectomy with enbloc caudatectomy ALPPS scheduled for pCCA with robotic approach at stage-1, the full video is provided as supplementary material. Due to intraoperative presence of portal vein tumor infiltration during hilar dissection (no evidence in the pre-operative work-up), a radiologic right PVE was performed after stage-1 instead of portal vein ligation, followed by portal vein resection and biductal hepatico-jejunostomy at stage-2 with open approach. The patient was a 74-year-old female diagnosed with 3-cm mass-forming pCCA. The total clean liver volume was 1231 cc, with future liver remnant (FLR) volume of 25.1% (segments II and III). She was discharged in the interstage interval on postoperative day (POD) 4; CT scan on POD 12 showed that FLR increased up to 33% (369 cc) (Fig. 1). ALPPS was completed on POD 17, the postoperative course was uneventful, and the patient was discharged in good general condition on POD 19 after stage-2. Besides the already demonstrated advantages in terms of reduced interstage morbidity, robotic ALPPS represents a promising strategy to expand surgical indication in patients with pCCA. The combination of liver partition and PVE may increase the opportunities to perform radical resections in selected patients with pCCA and portal vein infiltration.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13304-021-01209-x.
Keywords: ALPPS, Klatskin, pCCA, Two-stage hepatectomy, Robotic hepatectomy
Introduction
Perihilar cholangiocarcinoma (pCCA) is one of the most complex challenges for hepatobiliary surgeons due to both anatomical and oncological reasons [1, 2]. The extent of liver resection needed for a radical resection may require the adoption of pre-operative strategies of liver hypertrophy to reach an adequate future liver remnant (FLR), in particular in patients with Bismuth’s grade > 3 [3]. Across the second decade of 2000s, a novel technique called Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) was reported, bringing an innovation in the concept of liver regeneration [4]. However, after the initial enthusiasm, poor results and high incidence of morbidity after ALPPS for pCCA discouraged this indication [5]. Recently, it has been proposed that a modified ALPPS approach with minimally invasive first stage, as well as the combination of surgical liver partition and radiologic portal vein embolization (PVE), may improve outcomes reducing interstage morbidity [6–8]. We report a case of right trisectionectomy and caudatectomy ALPPS scheduled for pCCA with robotic approach. Due to intraoperative presence of portal vein tumor infiltration during hilar dissection (no evidence in the pre-operative work-up), a radiologic right PVE was performed after stage-1 instead of portal vein ligation, followed by portal vein resection and biductal hepatico-jejunostomy at stage-2 with open approach.
Materials and methods
Methods
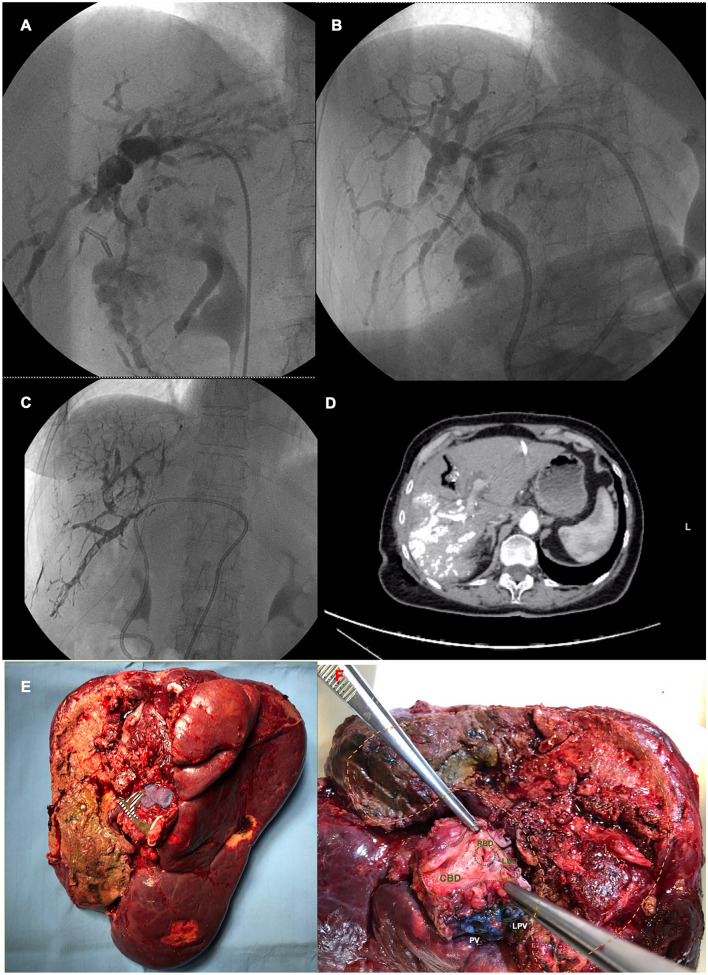
The patient was a 74-year-old female diagnosed with a 3-cm mass-forming pCCA, stage 3a according to Bismuth classification on pre-operative imaging. A CT scan revealed dilatation of the intrahepatic bile ducts; percutaneous transhepatic biliary drainage (PTBD) was placed in the left lobe (Fig. 1a,b) and endo-biliary biopsies confirmed the diagnosis of cholangiocarcinoma. The total clean liver volume was 1231 cc, with future liver remnant (FLR) volume of 25.1% (segments II and III). The decision to schedule her for ALPPS instead of other strategies was taken after multidisciplinary evaluation given the good general conditions of the patient (no relevant past medical history), and to obtain a faster volume growth. Robotic approach was chosen to mitigate the risk of postoperative complications and, in particular, interstage morbidity. A daVinci Si platform (Intuitive Inc. Sunnyvale, CA, US) was used for this procedure. Institutional Review Board (IRB) approval does not apply for this report; however, all patients in our Center sign an informed consent at least 24 h before and agree to the video recording of the surgical procedure.
Fig. 1.
Steps of ALPPS for pCCA. a, b Preoperative cholangiography and PTBD placement. c Radiologic PVE. d Interstage CT scan showing effects of PVE. e, f Details of the final specimen
Surgical technique
The video of the entire procedure is provided as Supplementary material to this manuscript (video clip: robotic liver partition and portal vein embolization for staged hepatectomy for perihilar cholangiocarcinoma). Figure 2 shows trocar disposition. For this kind of procedures, the patient is supine, with the table slightly rotated to the left and about 20° anti-Trendelenburg inclination. The surgeon at the table side has two assistant trocars for a more efficient support. The cart enters from the patient head side, shifted toward the right shoulder. Robotic stage 1 started with lymphadenectomy of the hepatic hilum extended to the celiac trunk. While approaching the portal vein, a suspect infiltration was found; therefore, we modified our surgical strategy and decided to perform the liver partition without right portal vein ligation to avoid tumor dissemination, and to schedule a radiologic right PVE after surgery. However, portal branches for the caudate lobe were ligated and divided to avoid portal flow stealing and increase the inflow to the FLR. The decision to combine liver partition and postoperative PVE was adopted to maximize the chances to obtain an adequate liver hypertrophy. After having prepared the hepatic vein cuff for an easier access during the transection, we performed the intrahepatic ultrasound, to identify and mark the middle hepatic vein (MHV). Parenchymal transection is conducted with a classical kelly-crush technique, combined with advanced sealing using Harmonic ACE. This technique allows to overcome the absence of CUSA in the robotic setting, as previously reported [9–11]. At the end of the full-thickness parenchymal transection, a hemostatic sealant was applied on both sides of the partition and a drain was left in place. Operative time of stage one ALPPS was 390 min including docking, without the need for Pringle or blood transfusion (estimated blood loss: 150 cc). The postoperative course was uneventful, and the patient was discharged in the interstage interval on postoperative day (POD) 4. On POD 8, the right PVE was performed to ensure adequate liver regeneration (Fig. 1c). On POD 12, she underwent a new CT scan, showing that FLR increased already up to 33% (369 cc) (Fig. 1d). Therefore, stage 2 ALPPS was scheduled as soon as possible according to Institutional limitations regarding operative room availability during COVID-19 pandemic, and the procedure was completed on POD 17 with open approach (Supplementary material). We decided to perform the second stage of the ALPPS with open approach due to the need for portal vein resection and reconstruction.
Fig. 2.
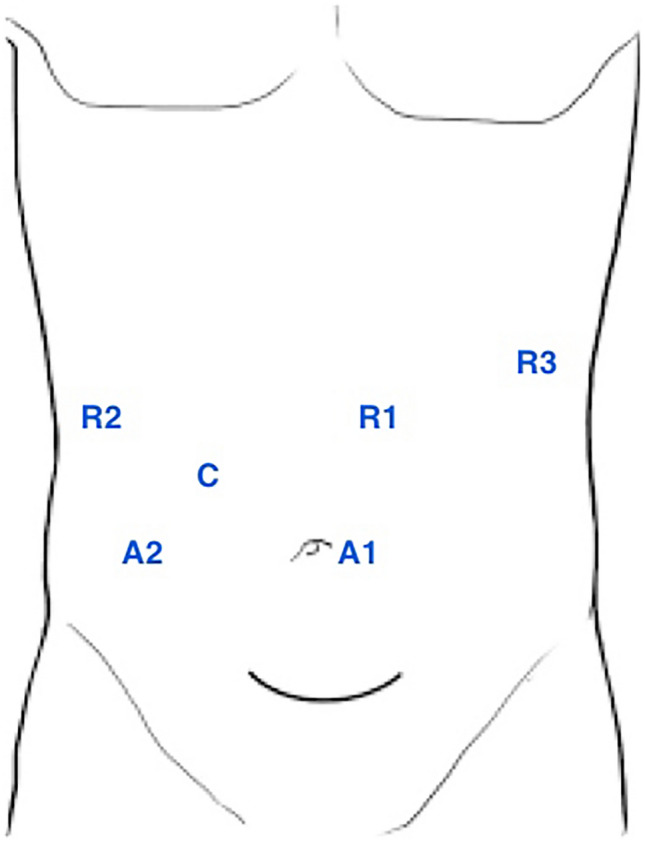
Trocar disposition for robotic approach with daVinci Si platform
The procedure started dividing the common bile duct; the distal margin was sent for frozen and resulted negative. The left hepatic duct was isolated in the transection plane, and divided and sent for frozen as well. The right hepatic artery was ligated and divided, and the transection was completed isolating the MHV and mobilizing the right lobe from the vena cava. Finally, MHV and right hepatic vein were divided with a 35 mm vascular stapler. The right trisectionectomy with enbloc caudatectomy was finalized by completely mobilizing the caudate lobe from the vena cava. Finally, left portal vein and main portal vein were clamped and divided. Portal inflow was reconstructed with a direct anastomosis with running 6-0 prolene suture. After having completed the anastomosis and released the clamps, the portal flow was assessed with flussimetry (Medistim, Norway) and an ultrasound is performed to check both inflow and outflow. The frozen sections of the left hepatic duct revealed atypical cells close to the margin; therefore, an additional extension on the left duct was sent and resulted negative. A biductal hepatico-jejunostomy was finally constructed. Stage 2 of the ALPPS took 370 min with an estimated blood loss of 450 cc, without the need of pringle or blood transfusion. Final pathology demonstrated the presence of a G3 cholangiocarcinoma infiltrating the portal vein bifurcation and main portal vein, signs of peri-nervous and microvascular infiltration were present, and 1 positive node (1/21) at station 12a1 was found, T4 N1 M0. The lesion involved the biliary duct for segment IV, showing biliary resection margin of 2 mm, and transection margin of 40 mm from the pCCA solid component. The patient was discharged in good general conditions on POD 19 after stage 2 (Table 1), and after 13 months, there are no signs of local or systemic recurrence.
Table 1.
Lab exams trend after stage 1 and 2
| Preoperative | Stage 1 | Stage 2 | 4 months | |||||
|---|---|---|---|---|---|---|---|---|
| p.o.d. 1 | p.o.d. 3 | p.o.d. 5 | p.o.d. 1 | p.o.d. 3 | p.o.d. 5 | |||
| Bilirubin (mg/dl) | 0.66 | 2.87 | 2.16 | 1.98 | 4.78 | 4.55 | 5.6 | 0.92 |
| INR | 1.15 | 1.38 | 1.48 | 1.41 | 2 | 2 | 2.6 | 0.95 |
| Albumin (g/dl) | 4.2 | 2.7 | 2.6 | 3.3 | 2.6 | 2.6 | 3.5 | 3.5 |
| AST (u/l) | 50 | 1219 | 194 | – | 36 | 23 | 25 | 58 |
| ALT (u/l) | 48 | 882 | 327 | 71 | 18 | 14 | 13 | 34 |
| Creatinine (mg/dl) | 1.24 | 0.64 | 0.63 | 0.83 | 0.54 | 0.57 | 0.54 | 0.64 |
INR international normalized ratio; ALT alanine aminotransferase; AST aspartate aminotransferase
Discussion
ALPPS is an important resource in the armamentarium of the hepatobiliary surgeon, and should be integrated with other strategies to increase the curative options and provide a tailored approach to each patient. Several strategies have been implemented to predict and reduce the risk of liver failure after major resections, like hepatobiliary scintigraphy, liver hemodynamics, and indocyanine green clearance [12]. A wise combination of the available strategies can lead to oncological accurate results preserving patients’ safety. Recent evidences have highlighted that minimally invasive approach for ALPPS may reduce interstage complications, failure of stage 2 and in-hospital mortality, with the advantages of faster liver regeneration and FLR growth [13]. In this setting, the robot adds several advantages to standard laparoscopy thanks to its well-known characteristics, namely view magnification and tremor filtration. One of the most fearsome complications of ALPPS is bile leak in the interstage, along with the ischemia of segment IV [14]. The robotic platform allows for a precise dissection increasing the control of biliary and arterial branches at the umbilical groove and on the hilar plate, potentially reducing this risk. Although no comparative data are available in this specific setting, the robotic platform has been recently linked to a reduced rate of conversion in complex liver resections, as compared to laparoscopic hepatectomies [15]. Therefore, this kind of dissection, that requires exceptional accuracy and precision to preserve arterial vascularization to segment IV, may be better performed with the help of the robot. In selected cases of intraoperative diagnosis of portal vein infiltration, PVE may complete the ALPPS approach avoiding the risk of tumor dissemination. This experience highlights that patients’ safety and oncological radicality are the key points in surgery, and to achieve those two results the originally planned strategy may need timely corrections. Therefore, a flexible approach is needed in the interest of a good outcome for the patients. Minimally invasive approach in the field of pCCA is still in its exploratory phase, and should be reserved to experienced high-volume centers [16]; therefore, locally advanced tumors requiring vascular resection should be approached open to minimize the risk of tumor dissemination. To the best of our knowledge, this is the first report of robotic first-stage ALPPS for pCCA. This kind of approach may expand the field of available strategies when FLR hypertrophy is needed and should not be intended to replace other techniques. With the limitation of a single experience, this technique alongside with the already described ALLPS-PVE [7] expands the application of the surgical strategies based on the concept of liver partition, with the benefits of a minimally invasive approach at stage 1, ensuring a faster FLR growth and potentially reducing the risk of morbidity and mortality of ALPPS.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 Video clip: Robotic liver partition and portal vein embolization for staged hepatectomy for perihilar cholangiocarcinoma (MP4 115034 KB)
Acknowledgements
Authors want to thank Modena ARTS Foundation (Academy for Robotic and Transplant Surgery) for the support to clinical research.
Declarations
Conflict of interest
Prof. Fabrizio Di Benedetto, Dr. Paolo Magistri, Dr. Gian Piero Guerrini, and Prof. Stefano Di Sandro have no conflicts of interest or financial ties to disclose.
Ethical standards and informed consent
Institutional Review Board (IRB) approval does not apply for this report; however, all patients in our Center sign an informed consent at least 24 h before and agree to the video recording of the surgical procedure.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Balci D, McCormack L. Perihilar cholangiocarcinoma: a difficult surgery in a difficult patient where experience matters most. Surgery. 2021 doi: 10.1016/j.surg.2021.02.049. [DOI] [PubMed] [Google Scholar]
- 2.Ebata T, Kamiya J, Nishio H, et al. The concept of perihilar cholangiocarcinoma is valid. Br J Surg. 2009;96:926–934. doi: 10.1002/bjs.6655. [DOI] [PubMed] [Google Scholar]
- 3.Petrowsky H, Fritsch R, Guckenberger M, et al. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat Rev Gastroenterol Hepatol. 2020;17:755–772. doi: 10.1038/s41575-020-0314-8. [DOI] [PubMed] [Google Scholar]
- 4.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 5.Balci D, Sakamoto Y, Li J, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure for cholangiocarcinoma. Int J Surg Lond Engl. 2020;82S:97–102. doi: 10.1016/j.ijsu.2020.06.045. [DOI] [PubMed] [Google Scholar]
- 6.Balci D, Kirimker EO, Üstüner E, et al. Stage I-laparoscopy partial ALPPS procedure for perihilar cholangiocarcinoma. J Surg Oncol. 2020;121:1022–1026. doi: 10.1002/jso.25868. [DOI] [PubMed] [Google Scholar]
- 7.Linecker M, Kron P, Lang H, et al. Too many languages in the ALPPS: preventing another tower of babel? Ann Surg. 2016;263:837–838. doi: 10.1097/SLA.0000000000001632. [DOI] [PubMed] [Google Scholar]
- 8.Lang H, de Santibanes E, Clavien PA. Outcome of ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB. 2017;19:379–380. doi: 10.1016/j.hpb.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Di Benedetto F, Assirati G, Magistri P. Full robotic ALPPS for HCC with intrahepatic portal vein thrombosis. Int J Med Robot Comput Assist Surg MRCAS. 2020;16:e2087. doi: 10.1002/rcs.2087. [DOI] [PubMed] [Google Scholar]
- 10.Di Benedetto F, Magistri P. First case of full robotic ALPPS for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2020 doi: 10.1245/s10434-020-08794-y. [DOI] [PubMed] [Google Scholar]
- 11.Magistri P, Assirati G, Ballarin R, et al. Major robotic hepatectomies: technical considerations. Updat Surg. 2021;73:989–997. doi: 10.1007/s13304-020-00940-1. [DOI] [PubMed] [Google Scholar]
- 12.Tomassini F, D’Asseler Y, Giglio MC, et al. Hemodynamic changes in ALPPS influence liver regeneration and function: results from a prospective study. HPB. 2019;21:557–565. doi: 10.1016/j.hpb.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Melandro F, Giovanardi F, Hassan R, et al. Minimally invasive approach in the setting of ALPPS procedure: a systematic review of the literature. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2019 doi: 10.1007/s11605-018-04092-x. [DOI] [PubMed] [Google Scholar]
- 14.Lang H, de Santibañes E, Schlitt HJ, et al. 10th anniversary of ALPPS-lessons learned and quo vadis. Ann Surg. 2019;269:114–119. doi: 10.1097/SLA.0000000000002797. [DOI] [PubMed] [Google Scholar]
- 15.Cipriani F, Fiorentini G, Magistri P, et al. Pure laparoscopic versus robotic liver resections: multicentric propensity score-based analysis with stratification according to difficulty scores. J Hepato-Biliary-Pancreat Sci. 2021 doi: 10.1002/jhbp.1022. [DOI] [PubMed] [Google Scholar]
- 16.Ratti F, Fiorentini G, Cipriani F, et al. Perihilar cholangiocarcinoma: are we ready to step towards minimally invasiveness? Updat Surg. 2020;72:423–433. doi: 10.1007/s13304-020-00752-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file2 Video clip: Robotic liver partition and portal vein embolization for staged hepatectomy for perihilar cholangiocarcinoma (MP4 115034 KB)