Abstract
Purpose
To evaluate quantitative parafoveal microvascular changes using Optical Coherence Tomography Angiography (OCTA) by comparing the area of foveal avascular zone (FAZ) and vessel density (VD) between nondiabetic controls and patients with different levels of Diabetic Retinopathy (DR).
Methods
A systematic review was performed according to the recommendations of the “Cochrane Handbook for Systematic Reviews of Interventions” and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Three electronic databases including PubMed, Web of Science and Cochrane Library were systematically retrieved by using key terms with Boolean operators. The data extracted from each study included: first author, year of publication, study design, sample size and participant characteristics (mean age, type of diabetes mellitus and mean duration of diabetic disease). Outcome variables included: VD and area of FAZ, in superficial and deep capillary plexuses of parafovea.
Results
355 articles were identified from our search of databases and 10 studies were included in this systematic review. Patients with diabetes with or without clinical signs of DR have a significantly enlarged area of FAZ and decreased parafoveal VD compared to healthy controls, as well as an association between these microvascular changes and worsening DR.
Conclusion
OCTA can provide valuable information about early and subtle microvascular changes of parafoveal capillary plexuses in patients with diabetes and can identify preclinical DR before the manifestation of clinically apparent retinopathy. The non-invasive nature of OCTA allows routine imaging of the retinal vasculature, so this approach may be a promising tool for screening programmes of DR.
Keywords: Diabetic retinopathy, Optical coherence tomography angiography, Microvascular changes, Retinal imaging
Introduction
Diabetes Mellitus (DM) is considered an important public health problem because it is one of the most common chronic diseases and it is a cause of high morbidity and mortality [1, 2]. The global prevalence of DM has reached epidemic proportions [1]. According to WHO data, in 2014 around 422 million adults worldwide had diabetes [1] and it is estimated that in 2030, the number of people with DM worldwide will reach 439 million people [3].
Diabetic retinopathy (DR), one of the most frequent microvascular complication of DM, is the leading cause of blindness in people of working aged in many developed countries [4].
Multiple metabolic pathways induced by hyperglycemia are implicated in the pathogenesis of DR. These include the polyol and hexosamine pathways, advanced glycation end products (AGEs) formation, oxidative stress and activation of protein kinase C (PKC) isoforms [5]. Hyperglycemia can activate protein kinase C δ (PKCδ) and p38α mitogen-activated protein kinase (MAPK), increasing the expression of a protein tyrosine phosphatase, which in turn leads to the dephosphorylation of platelet derived growth factor receptor-β (PDGFR-β) contributing to reduction in downstream signaling from this receptor, resulting in pericyte apoptosis [6]. PDGF plays an important role in the angiogenesis cascade that is activated in retinochoroidal vascular diseases and in the maintenance of retinal vasculature. Since pericytes are responsible for providing structural support for capillaries [6], and as the blood–retina barrier requires proper pericyte function, loss of pericytes may contribute to vascular permeability [7]. This process is associated with microaneurysm formation, which is the earliest clinical sign of DR. Pericyte loss, apoptosis of endothelial cells and thickening of the basement membrane, collectively, contribute to the impairment of the blood retinal barrier. Furthermore, pronounced loss of pericytes and endothelial cells results in capillary occlusion, ischemia [5], appearance of acellular capillaries and neovascularization [7].
The major risk factors for DR have been reported from epidemiologic studies. The most consistent risk factors for the development of DR are long duration of diabetes, hyperglycaemia, hypertension, hyperlipidaemia, pregnancy and puberty [8–17].
However, DR is described as a microcirculatory disease, evidence suggests that retinal neurodegeneration is an early event in the pathogenesis of DR, which may precede, and also participates in the microcirculatory abnormalities that occur in DR [18].
Major neurodegenerative changes include an imbalance in retinal production of neuroprotective mediators, pro-inflammation cytokines, apoptosis and glial activation [18]. Production of neurotoxic factors, such as glutamate, oxidative stress, caspase-3 and nitrous oxide, which are all neurotoxic mediators, result in neuronal cell dysfunction as well as damage to pericytes and endothelial cells [19]. Diabetes also induces activation of microglia cells, located inside the retina, which migrate to the subretinal space and release cytokines, contributing to neuronal cell death [18].
Glutamate is the major excitatory neurotransmitter in the retina and is involved in neurotransmission from photoreceptors to bipolar cells and from bipolar cells to ganglion cells. There is evidence that the ganglion cell death in DR occurs via glutamate-mediated toxicity [20]. Elevated levels of glutamate are implicated in neurodegeneration in DR because it leads to cell death due to an intracellular increase in calcium [19, 20].
DR can be classified into two stages: nonproliferative (NPDR) and proliferative (PDR) [21].
In NPDR, which is sub-divided as mild, moderate or severe, symptoms may be mild or non-existent, and it is defined by early intra-retinal microvascular findings associated with damage to the structures of blood vessels [22]. The earliest clinically observable signs in NPDR are microaneurysms, dot and blot hemorrhages [23]. Progressive capillary nonperfusion is accompanied by development of hard exudates, cotton-wool spots, venous dilation and beading, and intraretinal microvascular abnormalities (IRMAs) [4, 23].
Microaneurysms appear as small circular red lesions on the fundus and represent saccular vascular weakness and typical focal point of vascular leakage, representing early signs of DR [24, 25]. The intraretinal hemorrhages can be flame-shaped or dot-blot–like in appearance, reflecting the architecture of the layer of the retina in which they occur [24, 25]. Flame-shaped hemorrhages occur in inner retina closer to the vitreous, and dot-blot hemorrhages occur deeper in the retina [24, 25]. Hard exudates are yellow irregular shaped lesions; represent intra-retinal lipids and protein deposition [24, 25]. When accompanied by retinal thickening, represent feature of diabetic macular edema (DME) [24, 25]. Cotton-wool spots are superficial feather-bordered white lesions; represent nerve fiber layer infarctions from capillary occlusion [24, 25]. IRMAs appear as large calibre tortuous vessels in areas of ischaemia and may represent attempted vascular remodelling [24, 25].
As the severity of DR progresses, capillary non-perfusion leads to retinal ischaemia, which, in turn, causes upregulation of pro-angiogenic cytokines that drive pathological intra-retinal and intravitreal neovascularization [24, 25].
Neovascularization (angiogenesis) and DME are the two major and sight threatening complications of DR [26].
PDR is characterized by the growth of new blood vessels on the surface of the retina or the optic disc [4, 23]. These abnormal vessels may bleed, resulting in vitreous hemorrhage, subsequent fibrosis, tractional retinal detachment and neovascular glaucoma, which are the main reasons for loss of vision [4, 23].
DME is the main cause of vision loss in persons with diabetes, which can occur at any stage of DR [27]. DME is characterized by increased vascular permeability and the deposition of hard exudates at the central retina [27].
In early stages DR may causes no symptoms but advances in retinal imaging and image processing techniques have allowed researchers to detect earlier signs of disease preceding classic NPDR [28, 29].
A variety of techniques have been developed to detect and classify DR. Dilated fundus examination, in daily clinical practice, is the gold standard tool to detect vascular changes in diabetic eye disease [30–34]. Colour fundus photography (CFP) has also proved useful as a complementary screening tool and current is the gold standard in DR screening programmes, which permits the detection of disease and monitoring of progression and treatment response [30–34].
Other imaging modalities such as fluorescein angiography (FA) can provide a more sensitive examination of the posterior pole detecting primary microvascular lesions, e.g. microaneurysms and advanced vascular abnormalities such as venous beading and IRMA [30–34]. Retinal non-perfusion, which represents intra-retinal capillary occlusion or dropout, can be visualized and neovascularization can be identified too [30–34]. However, FA is an invasive procedure, costly, time-consuming, the images have limited depth resolution and it is not able to clearly visualize small capillary vessels within various retinal layers and the images of the superficial capillaries and deep capillaries overlap because FA images are limited to two dimensions [30–34]. Furthermore, even in healthy subjects, dye injections can also occasionally cause nausea and, rarely but critically, anaphylaxis [35].
The introduction of optical coherence tomography (OCT) and its evolution has revolutionized retinal imaging [30] and supports the recent international expert grading of diabetic maculopathy [36]. Optical Coherence Tomography Angiography (OCTA) is a relatively new non-invasive imaging technique that visualizes retinal capillary blood flow without the need for intravenous dye and provides rapid scanning by newer spectral domain or swept source OCT, high‑resolution 3‑D images from the retinal and choroidal vasculature [22, 30, 33]. In contrast to FA, OCTA enables visualization of the microvasculature at different depths and uniquely visualizes individual retinal capillary plexuses, including the superficial capillary plexus (SCP) that includes capillary network located in the ganglion cell layer and/or the nerve fiber layer, deep capillary plexus (DCP) that consists of the capillary network in the inner nuclear layer, the choriocapillaris (CC) slab, and more recently, the middle capillary plexus (MCP)[30, 32, 37].
Some cross-sectional studies have identified alterations in OCTA metrics in diabetic patients without clinical DR signs where the most reported indices are the enlargement of the foveal avascular zone (FAZ) and reduced vessel density (VD)[30, 32, 33, 35].
FAZ, a capillary‑free area enclosed by foveal capillary circles, is located at the center of macula and it is widely accepted that the FAZ size reflects the health of the microcirculation in the retina and in case of abnormalities in the structure or perfusion of this area profoundly affect vision [30, 38]. A common feature of DR is the enlargement of FAZ that results from the occlusion of retinal capillaries and loss of precapillary arterioles near the fovea. This can occur in patients with diabetes without clinical DR [39]. So, FAZ measurement could serve an important role in the diagnosis and management of DR [38].
VD is defined as the percentage or proportion of blood vessels area over the total measured area [28]. Generally, VD values gradually become lower from healthy controls to patients with DM without DR to NPDR to PDR, but results vary based on the methods used to obtain the VD value [39].
This suggests that OCTA metrics could reflect early microvascular alterations in individual capillary plexuses and are potential biomarkers for DR [30, 33, 35]. The hope is that these markers will allow clinicians to diagnose disease and stratify patients according to their risk of complications earlier. This is important, since earlier treatment is associated with better outcomes [29].
So, the aim of this systematic review was to evaluate quantitative parafoveal microvascular changes using OCTA by comparing the area of FAZ and VD between nondiabetic controls and patients with DM with different levels of DR.
Materials and methods
We used the PICO strategy [40]; Participants/ population: Subjects with DM type 1 or 2 of all ages with no DR (NDR), NPDR and PDR were included; Intervention: We conducted a review aiming to collect all evidence related to quantification of retinal vascular changes in subjects with diabetes mellitus using OCTA; Comparator/ control: quantification of retinal vascular changes in subjects without DM and with healthy eyes; Outcome(s): To investigate differences in quantitative measurements of retinal vascular changes with OCTA such as FAZ area and VD in superficial and deep plexuses and where possible, in choriocapillaris plexus.
Literature search
This systematic review was performed according to the recommendations of the “Cochrane Handbook for Systematic Reviews of Interventions” [41] and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [42]. Three electronic databases including PubMed, Web of Science and Cochrane Library were systematically retrieved by using key terms with Boolean operators.
The search string used in PubMed was: “optical coherence tomography angiography” OR “OCT angiography” OR “OCTA” AND “diabetic retinopathy” OR “diabetes” OR “diabetes mellitus” OR “diabetes retinopathy”.
In Web of Science was: (TI = (optical coherence tomography angiography OR OCTA OR Angio OCT OR OCT Angiography) AND (diabetic retinopathy OR diabetes mellitus)).
The search used in Cochrane Central Register of Controlled Trials (CENTRAL) was: ("optical coherence tomography angiography":ti,ab,kw OR "Angio OCT":ti,ab,kw OR "OCTA":ti,ab,kw OR "OCT angiography":ti,ab,kw) AND ([Diabetic Retinopathy] explode all trees OR [Diabetes Mellitus] explode all trees). The electronic databases were last searched on May 4, 2020.
Eligibility criteria
The search was performed with the aim of identify all studies in which OCTA imaging had been used in patients with DM with specified stage of DR (NDR, NPDR or PDR) for measurement of FAZ area and VD in each study group.
The clinical application of OCTA was first reported in 2014, thus, the initial year of publication was confined between January 2014 and May 2020. Studies included were limited to those published in English and in human subjects.
The literature review was limited to original studies that aimed to quantify retinal vascular changes. The following types of studies will be included in the review: randomised controlled trials, controlled trials, prospective and retrospective cohort studies, observational studies and case-controlled studies.
The inclusion criteria were: (i) English used as language, (ii) human subjects, (iii) patients with DM as the investigated group, (iv) patients without DM and with healthy eyes as the control group, (v) specified stage of DR, (vi) OCTA as method for investigation, (vii) outcome measures in each study group (e.g. VD and FAZ area at SCP, DCP and in CC layer if information was available) and (viii) full article online.
The exclusion criteria were as follows: (1) conference abstracts, editorials, case reports, letters and reviews; (2) animal subjects; (3) full text of published articles was not written in English; (4) studies that did not report FAZ area and VD in diabetic and nondiabetic controls as an outcome measure; (5) studies that quantifies these retinal vascular changes by other imaging methods rather than OCTA technology.
Data collection process and data synthesis
Two reviewers (FP and PC) independently screened all acquired studies based on information found in the titles and abstracts.
Then we evaluated the full text of the selected articles for eligibility and any discrepancies were discussed and resolved by consensus. A data extraction form was prepared, and data were extracted, documented and entered into a formatted MS Excel® database by one researcher (FP) and verified by another (PC).
Data extraction
The data extracted from each study included: first author, year of publication, study design, sample size and participant characteristics (mean age with standard deviation or range, type of diabetes mellitus and mean duration of diabetic disease).
Outcome variables included: VD of parafovea (% or ratio) in superficial and deep capillary plexuses, (measurements in coriocapillary layer were included if information was available), FAZ area (mm2) in superficial and deep capillary plexuses of parafovea and other factors that have impact on measurements (e.g.: type of OCTA).
Quality assessment
We assessed the methodological quality of the included studies in the review with the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [43]. The choice for the applied tool was based on the study designs (cross-sectional). The two authors applied these tools; we independently evaluated the items of the tools as “yes”, “no”, “not applicable” (NA), “cannot determine” (CD) or “not reported” (NR). This was used to guide the overall rating for the quality of each study as “good”, “fair” or “poor”.
Results
Screening and selection of literature
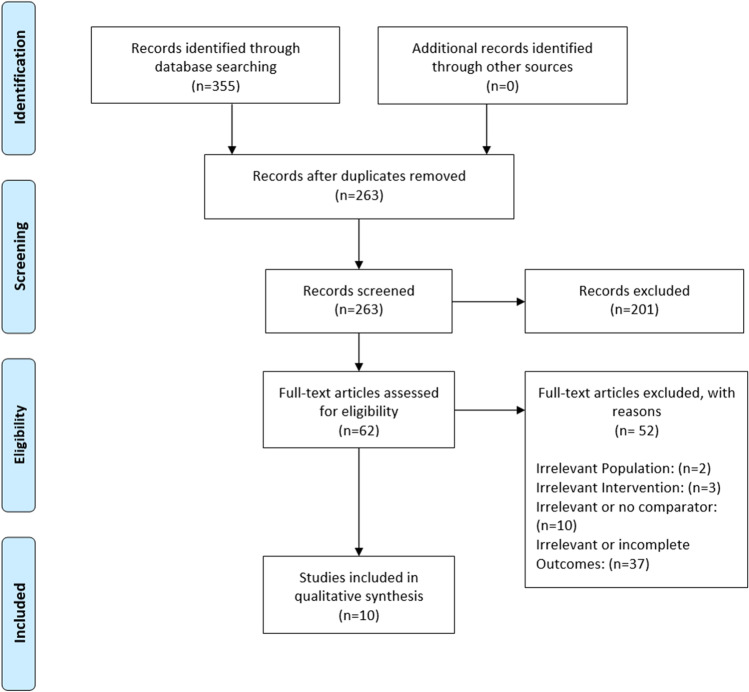
We identified 355 articles from our search of the PubMed (n = 247), Web of Science (n = 105) and CENTRAL (n = 3) databases. We removed 92 duplicate articles and then we screened 263 articles based on its abstract. Then, we assessed the full text of 62 studies and included 10 studies in our systematic review. The selection process is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram
Characteristics and methodological aspects of the included studies
The number of the included subjects, age, disease type, and disease duration of the patients and OCTA software of each study were summarized in Table 1. As shown in Table 1, among the 10 included studies 6 analysed NPD eyes, 7 studied NPDR eyes (mild, moderate or severe NPDR) and only 4 investigated PDR eyes. All included studies are cross-sectional, 7 prospective observational case–control clinical studies and 3 retrospective studies.
Table 1.
Characterization of the population in the included studies and type of OCTA software used
| Article | Type of Study | OCTA Software | Type of Diabetes | Duration of Diabetes (years) Mean ± SD | Age (years) Mean ± SD or range |
|---|---|---|---|---|---|
| Al-Sheikh et al. [44] (2016) | Cross-sectional | DRI OCTA Triton (Topcon Corporation) | NR |
Control: NA Patients with DR: NR |
Control: 39 (range 30–58) |
| Patients with DR: 72 (range 54–93) | |||||
| Bhanushali et al. [45] (2016) | Cross-sectional | AngioVue Avanti OCTA system (Optovue, Inc., Fremont, CA, USA) | Type 2 | Control: NA | Control:38.7 ± 1.68 |
| Mild NPDR: 11.0 ± 2.31 | Mild NPDR: 64.3 ± 2.16 | ||||
| Moderate NPDR: 15.8 ± 1.17 | Moderate NPDR: 61.1 ± 0.99 | ||||
| Severe NPDR: 26.7 ± 7.69 | Severe NPDR: 59.6 ± 1.56 | ||||
| PDR: 7.69 ± 6.0 | PDR: 59.1 ± 1.75 | ||||
| Carnevali et al. [46] (2017) | Cross-sectional | AngioPlex CIRRUS HD-OCT model 5000 (Carl Zeiss Meditec, Inc., Dublin, USA) | Type 1 | Control: NA | Control:23 ± 2 (range 18–26) |
| NDR: 11 ± 4 | NDR: 22 ± 2 (range 7–18) | ||||
| Dimitrova et al. [47] (2017) | Cross-sectional | AngioVue RTVue-XR Avanti OCTA (Optovue, Inc., Fremont, CA, USA) | Type 2 | Control: NA | Controls: 65 ± 11.35 |
| NDR: 7.37 ± 5.96 | NDR: 69 ± 9.01 | ||||
| Lee et al. [48] (2016) | Cross-sectional | Cirrus Angioplex (Carl Zeiss Meditec, Dublin, CA) | Type 2 | Control: NA | Control: 57.4 ± 11.2 |
| NDR: 9.6 ± 5.4 | NDR: 58.5 ± 12.1 | ||||
| Mild NPDR: 15.8 ± 11.0 | Mild NPDR: 63.7 ± 8.5 | ||||
| Moderate to severe NPDR: 11.6 ± 8.8 | Moderate to severe NPDR: 62.1 ± 7.4 | ||||
| PDR: 20.6 ± 14.6 | PDR: 59.5 ± 7.1 | ||||
| Nesper et al. [49] (2017) | Cross-sectional | AngioVue RTVue-XR Avanti OCTA (Optovue, Inc., Fremont, CA, USA) | Type 1 and 2 | Control: NA | Control: 50 ± 18 |
| NDR: 11 ± 15 | NDR: 57 ± 10 | ||||
| NPDR: 17 ± 12 | NPDR: 54 ± 12 | ||||
| PDR: 19 ± 10 | PDR: 49 ± 14 | ||||
| Simonett et al. [50] (2017) | Cross-sectional |
AngioVue RTVue-XR Avanti OCTA (Optovue, Inc., Fremont, CA, USA) |
Type 1 | Control: NA | Control: 39.6 ± 10.1 |
| Diabetic patients: 21.3 ± 10.6 | Diabetic patients: 42.3 ± 8.6 | ||||
|
Alam et al. [51] (2018) |
Cross-sectional | AngioVue RTVue-XR Avanti OCTA (Optovue, Inc., Fremont, CA, USA) | Type 2 | Control: NA | Control:42 ± 9.8 |
| Mild NPDR: 19.64 ± 13.27 | Mild NPDR: 50.1 ± 12.61 | ||||
| Moderate NPDR: 16.13 ± 10.58 | Moderate NPDR: 50.8 ± 8.39 | ||||
| Severe NPDR: 23.40 ± 11.95 | Severe NPDR: 57.84 ± 10.37 | ||||
| Cao et al. [52] (2018) | Cross-sectional | AngioVue RTVue-XR Avanti OCTA (Optovue, Inc., Fremont, CA, USA) | Type 2 | Control: NA | Control:53.7 ± 9.4 |
| NDR: 6.6 ± 1.9 | NDR: 57.4 ± 13.5 | ||||
| Ciloglu et al. [53] (2019) | Cross-sectional | AngioVue RTVue-XR Avanti OCTA (Optovue, Inc., Fremont, CA, USA) | Type 2 | Control: NA | Control:54.09 ± 1.49 |
| NDR: 13.65 ± 0.66 | NDR: 56.61 ± 1.33 |
DR Diabetic Retinopathy, NA not applicable, NDR No Diabetic Retinopathy, NPDR Nonproliferative Diabetic Retinopathy, NR not reported, OCTA Optical Coherence Tomography Angiography, PDR Proliferative Diabetic Retinopathy, SD Standard Deviation
Quality assessment
Quality assessment tool from the NIH was used to assess the methodological quality of the included studies (Table 3). 9 studies were rated fair and the remaining article was rated poor. All included studies were considered to have enough quality to be included in the systematic review.
Table 3.
Results of quality assessment of the included studies with the National Institutes of Health (NIH) Quality Assessment tool for Observational Cohort and Cross-Sectional Studies
| Al-Sheikh et al. (2016) | Bhanushali et al (2016) | Carnevali et al. (2017) | Dimitrova et al. (2017) | Lee et al. (2017) | Nesper et al. (2017) | Simonett et al. (2017) | Alam et al. (2018) | Cao et al. (2018) | Ciloglu et al. (2019) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 2. Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 3. Was the participation rate of eligible persons at least 50%? | CD | CD | CD | CD | CD | CD | CD | CD | CD | CD | |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | NR | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | |
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | No | No | No | No | No | No | No | No | No | No | |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | No | No | No | No | No | No | No | No | No | No | |
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | No | No | No | No | No | No | No | No | No | No | |
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 10. Was the exposure(s) assessed more than once over time? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 12. Were the outcome assessors blinded to the exposure status of participants? | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| 13. Was loss to follow-up after baseline 20% or less? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | No | Yes | Yes | Yes | Yes | Yes | No | NA | Yes | Yes | |
| Quality Rating (Good, Fair or Poor) | Fair | Fair | Fair | Fair | Fair | Fair | Poor | Fair | Fair | Fair | |
NA not applicable, CD cannot determine, NR not reported
Description of studies
The study of Al-Sheikh et al. [44] included 28 eyes of 18 diabetic patients with DR and 40 eyes of 22 healthy individuals. A swept-source OCTA device (DRI OCT Triton) was used to obtain the images and the scans were taken from 3 × 3 mm cubes centered on the fovea. The mean age of the patients with DR was 72 years (range, 54–93) and the average age of control subjects was 39 years (range, 30– 58) [44]. The authors did not mention the diabetes duration or even the type of DM. Of the eyes with DR, 10 had mild, 10 moderate, and 2 severe NPDR and 6 had PDR [44] (Table 1).
Table 2 shows the main results of the included studies and as we can see, in the study of Al-Sheikh et al. [44] the mean FAZ area in the SCP was 0.339 ± 0.118 mm2 in the control group (p = 0.003), 0.498 ± 0.401 mm2 (p = 0.301), 0.444 ± 0.191 mm2 (p = 0.199), 0.681 ± 0.006 mm2 (p < 0.001) and 0.619 ± 0.119 mm2 (p < 0.001), in mild NPDR, moderate NPDR, severe NPDR and PDR, respectively [44]. Comparing the subgroups of diabetic patients to healthy individuals, there was a statistically significant difference in the FAZ area in the SCP in patients with severe NPDR and PDR [44]. In the DCP, the mean FAZ area was 0.358 ± 0.105 mm2 in healthy individuals (p < 0.001), in mild NPDR was 0.608 ± 0.187 mm2 area (p < 0.001), in moderate NPDR was 0.546 ± 0.250 mm2 (p = 0.029), in severe NPDR was 0.643 ± 0.086 mm2 (p = 0.001) and in PDR was 0.734 ± 0.314 mm2 (p = 0.027) [44]. All subgroups showed a statistically significant difference in the FAZ area in DCP [44].
Table 2.
Main results of the included studies
| Article | Normal control eyes | NDR eyes | Mild NPDR eyes | Moderate NPDR eyes | Severe NPDR eyes | PDR eyes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonproliferative DR eyes | |||||||||||||||||
| Al-Sheikh et al. [44] (2016) | n = 40 | NA | n = 10 | n = 10 | n = 2 | n = 6 | |||||||||||
| FAZ -A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | |||||||||||||
| SCP: 0.339 ± 0.118 | p = 0.003 | SCP: 0.498 ± 0.401 | p = 0.301 | SCP: 0.444 ± 0.191 | p = 0.199 | SCP: 0.681 ± 0.006 | p < 0.001 | SCP: 0.619 ± 0.119 | p < 0.001 | ||||||||
| DCP: 0.358 ± 0.105 | p < 0.001 | DCP: 0.608 ± 0.187 | p < 0.001 | DCP: 0.546 ± 0.250 | p = 0.029 | DCP: 0.643 ± 0.086 | p = 0.001 | DCP: 0.734 ± 0.314 | p = 0.027 | ||||||||
| VD (ratio) | VD (ratio) | VD (ratio) | VD (ratio) | VD (ratio) | |||||||||||||
| SCP: 0.709 ± 0.038 | p < 0.001 | SCP: 0.591 ± 0.097 | p = 0.004 | SCP: 0.566 ± 0.114 | p = 0.003 | SCP: 0.589 ± 0.0043 | p = 0.148 | SCP: 0.519 ± 0.081 | p = 0.002 | ||||||||
| DCP: 0.714 ± 0.049 | p = 0.028 | DCP: 0.699 ± 0.076 | p = 0.544 | DCP: 0.649 ± 0.118 | p = 0.122 | DCP: 0.704 ± 0.079 | p = 0.773 | DCP: 0.636 ± 0.017 | p = 0.136 | ||||||||
| Bhanushali et al. [45] (2016) | n = 60 | NA | n = 35 | n = 95 | n = 57 | n = 22 | |||||||||||
| FAZ—A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | |||||||||||||
| 0.38 ± 0.01 | 0.46 ± 0.03 | 0.45 ± 0.01 | 0.46 ± 0.02 | 0.47 ± 0.02 | |||||||||||||
| VD (%) | VD (%) | VD (%) | VD (%) | VD (%) | |||||||||||||
| SCP: 49.7 ± 0.55 | SCP: 39.2 ± 1.21 | SCP: 40.1 ± 0.58 | SCP: 38.5 ± 0.76 | SCP: 38.9 ± 1.38 | |||||||||||||
| DCP: 53.1 ± 0.73 | DCP: 39.7 ± 1.57 | DCP: 40.2 ± 0.53 | DCP: 39.4 ± 0.68 | DCP: 39.2 ± 0.94 | |||||||||||||
| Carnevali et al. [46] (2017) | n = 25 | n = 25 | NA | NA | NA | NA | |||||||||||
| FAZ—A (mm2) | FAZ—A (mm2) | ||||||||||||||||
| SCP: 0.251 ± 0.104 | p = 0.341 | SCP: 0.223 ± 0.100 | p = 0.341 | ||||||||||||||
| DCP: 0.762 ± 0.231 | p = 0.808 | DCP: 0.747 ± 0.199 | p = 0.808 | ||||||||||||||
| VD (ratio) | VD (ratio) | ||||||||||||||||
| SCP: 0.430 ± 0.020 | p = 0.805 | SCP: 0.432 ± 0.023 | p = 0.805 | ||||||||||||||
| DCP: 0.477 ± 0.014 | p = 0.005 | DCP: 0.464 ± 0.016 | p = 0.005 | ||||||||||||||
| CC: 0.487 ± 0.015 | p = 0.359 | CC: 0.490 ± 0.013 | p = 0.359 | ||||||||||||||
| Dimitrova et al. [47] (2017) | n = 33 | n = 29 | NA | NA | NA | NA | |||||||||||
| FAZ—A (mm2) | FAZ—A (mm2) | ||||||||||||||||
| SCP: 0.31 ± 0.10 | p = 0.02 | SCP: 0.37 ± 0.11 | p = 0.02 | ||||||||||||||
| VD (%) | VD (%) | ||||||||||||||||
| SCP: 51.39 ± 13.05 | p = 0.04 | SCP: 44.35 ± 13.31 | p = 0.04 | ||||||||||||||
| DCP: 41.53 ± 14.08 | p < 0.01 | DCP: 31.03 ± 16.33 | p < 0.01 | ||||||||||||||
| Lee et al. [48] (2017) | n = 30 | n = 31 | n = 26 | n = 31 | n = 33 | ||||||||||||
| FAZ—A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | |||||||||||||
| SCP: 0.325 ± 0.084 | SCP: 0.354 ± 0.137 | SCP: 0.350 ± 0.114 | SCP: 0.431 ± 0.654 | SCP: 0.492 ± 0.207 | |||||||||||||
| DCP: 0.797 ± 0.213 | DCP: 0.822 ± 0.249 | DCP: 0.902 ± 0.294 | DCP: 1.174 ± 0.685 | DCP: 1.239 ± 0.769 | |||||||||||||
| VD (ratio) | VD (ratio) | VD (ratio) | VD (ratio) | VD (ratio) | |||||||||||||
| SCP: 0.425 ± 0.028 | SCP: 0.408 ± 0.039 | SCP: 0.399 ± 0.043 | SCP: 0.386 ± 0.043 | SCP: 0.355 ± 0.046 | |||||||||||||
| DCP: 0.272 ± 0.057 | DCP: 0.267 ± 0.064 | DCP: 0.240 ± 0.056 | DCP: 0.225 ± 0.054 | DCP: 0.193 ± 0.060 | |||||||||||||
| Nesper et al.[49] (2017) | n = 44 | n = 45 | n = 52 | n = 40 | |||||||||||||
| FAZ—A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | ||||||||||||||
| SCP: 0.269 ± 0.086 | p < 0.01 | SCP: 0.309 ± 0.140 | p < 0.01 | SCP: 0.356 ± 0.207 | p < 0.01 | SCP: 0.493 ± 0.238 | p < 0.01 | ||||||||||
| VD (%) | VD (%) | VD (%) | VD (%) | ||||||||||||||
| SCP: 53.59 ± 3.18 | p < 0.01 | SCP: 53.02 ± 3.11 | p < 0.01 | SCP: 46.59 ± 4.88 | p < 0.01 | SCP: 43.43 ± 3.83 | p < 0.01 | ||||||||||
| DCP:60.82 ± 2.44 | p < 0.01 | DCP: 59.14 ± 2.80 | p < 0.01 | DCP: 53.76 ± 4.63 | p < 0.01 | DCP: 49.73 ± 3.66 | p < 0.01 | ||||||||||
| Simonett et al. [50] (2017) | n = 23 | n = 9 | n = 19 | NA | NA | NA | |||||||||||
| FAZ-A (mm2) | FAZ-A (mm2) | ||||||||||||||||
| SCP: 0.26 ± 0.11 | p = 0.821 | SCP: 0.26 ± 0.12 | p = 0.821 | ||||||||||||||
| DCP: 0.38 ± 0.15 | p = 0.510 | DCP: 0.40 ± 0.15 | p = 0.510 | ||||||||||||||
| VD (%) | VD (%) | ||||||||||||||||
| SCP: 51.5 ± 4.0 | p = 0.143 | SCP: 49.8 ± 4.2 | p = 0.143 | ||||||||||||||
| DCP: 60.7 ± 2.4 | p < 0.001 | DCP: 57.0 ± 3.3 | p < 0.001 | ||||||||||||||
| Alam et al. [51] (2018) | n = 40 | NA | n = 40 | n = 40 | n = 40 | NA | |||||||||||
| FAZ—A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | FAZ—A (mm2) | ||||||||||||||
| SCP: 0.29 ± 0.16 | p = 0.006 | SCP: 0.31 ± 0.17 | p = 0.006 | SCP: 0.36 ± 0.18 | p = 0.006 | SCP: 0.40 ± 0.21 | p = 0.006 | ||||||||||
| DCP: 0.46 ± 0.17 | p = 0.006 | DCP: 0.47 ± 0.15 | p = 0.006 | DCP: 0.52 ± 0.11 | p = 0.006 | DCP: 0.58 ± 0.26 | p = 0.006 | ||||||||||
| VD (%) | VD (%) | VD (%) | VD (%) | ||||||||||||||
| SCP: 48.16 ± 3.32 | p = 0.001 | SCP: 45.62 ± 2.11 | p = 0.001 | SCP: 40 ± 3.52 | p = 0.001 | SCP: 36.84 ± 2.45 | p = 0.001 | ||||||||||
| DCP: 49.72 ± 3.18 | p = 0.001 | DCP: 45.98 ± 3.09 | p = 0.001 | DCP: 41.78 ± 2.17 | p = 0.001 | DCP: 37.46 ± 3.16 | p = 0.001 | ||||||||||
| Cao et al. [52] (2018) | n = 67 | n = 71 | NA | NA | NA | NA | |||||||||||
| FAZ—A (mm2) | FAZ—A (mm2) | ||||||||||||||||
| SCP: 0.35 ± 0.09 | p = 0.253 | SCP: 0.32 ± 0.18 | p = 0.253 | ||||||||||||||
| VD (%) | VD (%) | ||||||||||||||||
| SCP: 55.72 ± 2.43 | p < 0.001 | SCP: 51.34 ± 4.09 | p < 0.001 | ||||||||||||||
| DCP: 62.10 ± 2.11 | p < 0.001 | DCP: 57.66 ± 5.73 | p < 0.001 | ||||||||||||||
| CC: 66.97 ± 1.23 | p = 0.006 | CC: 66.36 ± 1.29 | p = 0.006 | ||||||||||||||
| Ciloglu et al. [53] (2019) | n = 45 | NA | n = 49 | NA | |||||||||||||
| FAZ—A (mm2) | FAZ—A (mm2) | ||||||||||||||||
| SCP: 0.246 ± 0.022 | p < 0.001 | SCP: 0.438 ± 0.05 | p < 0.001 | ||||||||||||||
| DCP: 0.342 ± 0.022 | p < 0.001 | DCP: 0.732 ± 0.06 | p < 0.001 | ||||||||||||||
| VD (%) | VD (%) | ||||||||||||||||
| SCP: 52.17 ± 0.58 | p < 0.001 | SCP: 45.43 ± 0.56 | p < 0.001 | ||||||||||||||
| DCP: 60.68 ± 0.90 | p < 0.001 | DCP: 52.82 ± 0.85 | p < 0.001 | ||||||||||||||
DCP deep capillary plexus, CC choriocapillaris, FAZ-A foveal avascular zone area, NA not applicable, NDR No Diabetic Retinopathy, NPDR Nonproliferative Diabetic Retinopathy, PDR Proliferative Diabetic Retinopathy, SCP superficial capillary plexus, VD vessel density
VD was assessed as the ratio of area occupied by vessels [44]. In the SCP, the mean parafoveal VD ratio was 0.709 ± 0.038 in normal individuals (p < 0.001) and 0.591 ± 0.097 (p = 0.004), 0.566 ± 0.114 (p = 0.003), 0.589 ± 0.043 (p = 0.148), 0.519 ± 0.081 (p = 0.002), in mild, moderate, severe NPDR and PDR, respectively. Comparing those subgroups to healthy individuals, there was a statistically significant difference in the parafoveal VD ratio in the SCP in patients with mild or moderate NPDR as well as PDR [44]. In the DCP, the mean ratio was 0.714 ± 0.049 in normal individuals (p = 0.028), in mild was 0.699 ± 0.076 (p = 0.544), in moderate was 0.649 ± 0.118 (p = 0.122), in severe was 0.704 ± 0.079 (p = 0.773) and in PDR was 0.636 ± 0.017 (p = 0.136) [44]. There was no statistically significant difference in the parafoveal VD in the DCP compared to healthy individuals [44] (Table 2).
Bhanushali et al. [45] studied 209 eyes of 122 type 2 DM patients with DR and 60 eyes of 31 normal subjects. The DR patients were graded as having either NPDR or PDR using Early Treatment Diabetic Retinopathy Study classification. The number of eyes was 35 with mild, 95 with moderate, 57 with severe NPDR and 22 with PDR [45]. Mean age of control individuals was 38.7 ± 1.68 years, in mild NPDR group was 64.3 ± 2.16 years, 61.1 ± 0.99 years in moderate NPDR, 59.6 ± 1.56 years in severe NPDR and 59.1 ± 1.75 years in PDR [45]. Mean duration of DM was 11.0 ± 2.31 years in mild NPDR group, 15.8 ± 1.17 years in moderate NPDR group, 26.7 ± 7.69 years in severe NPDR group and 7.69 ± 6.0 years in PDR group [45]. All DR and normal subjects underwent imaging on a spectral-domain OCTA system (AngioVue) [45]. Analyses were performed on a scan area of 3 × 3 mm generated from the SCP and DCP around the fovea for all eyes [45]. The authors assessed the FAZ area by projecting the entire inner retina into one enface angiogram [45].
The FAZ area did not significantly change (p = 0.82) among the DR grades [45]. The mean FAZ area was 0.38 ± 0.01mm2 in healthy individuals, 0.46 ± 0.03mm2 in mild NPDR subjects, 0.45 ± 0.01mm2 in moderate NPDR, 0.46 ± 0.02mm2 in severe NPDR and 0.47 ± 0.02mm2 in PDR group [45].
Parafoveal VD in SCP was 49.7 ± 0.55% in normal eyes of control group, 39.2 ± 1.21% in mild NPDR, 40.1 ± 0.58% in moderate NPDR, 38.5 ± 0.76% in severe NPDR and 38.9 ± 1.38% in PDR group [45]. In DCP, VD in normal eyes was 53.1 ± 0.73%, 39.7 ± 1.57%, 40.2 ± 0.53%, 39.4 ± 0.68% and 39.2 ± 0.94% in mild, moderate, severe NPDR and PDR, respectively [45]. Among the DR grades, VD was similar (p > 0.05) in both superficial and deep retinal layers [45].
So, normal eyes had a lower FAZ area (p < 0.001) and higher VD (p < 0.001) compared with DR grades [45].
Carnevali et al. [46] studied a total of 25 eyes of 25 type 1 diabetic patients (mean age: 22 ± 2 years) without signs of DR with mean duration of the DM disease 11 ± 4 years [46]. They were compared to 25 healthy subjects (control eyes) with mean age of 23 ± 2 years [46]. All patients underwent Angioplex CIRRUS HD-OCT model 5000, and in all patients, a scanning area of 3 × 3 mm was adopted, centered on the fovea [46] (Table 1). All acquisitions were performed using FastTrac™ retinal-tracking technology to reduce motion artifacts [46].
Mean FAZ area at SCP was 0.223 ± 0.100 mm2 in diabetic eyes and 0.251 ± 0.104 mm2 in control eyes (p = 0.341) [46]. In DCP FAZ area was 0.747 ± 0.199 mm2 and 0.762 ± 0.231 mm2 in diabetic and healthy subjects, respectively (p = 0.808) [46]. So, no significant difference was found in FAZ area at both SCP and DCP by comparing diabetics with NDR and control group [46].
In the SCP, the parafoveal VD ratio in diabetic subjects was 0.432 ± 0.023 and in control subjects was 0.430 ± 0.020 (p = 0.805), so no difference was disclosed in VD between the two groups in SCP [46]. Parafoveal VD ratio in the DCP significantly decreased in diabetic eyes compared to control eyes of healthy subjects, 0.464 ± 0.016 and 0.477 ± 0.014, respectively (p = 0.005) [46]. Instead, regarding to CCP, no difference was disclosed in VD ratio between two groups analysed (0.490 ± 0.013 in diabetic eyes and 0.487 ± 0.015, in control eyes, p = 0.359) [46].
Dimitrova et al. [47] included 62 patients in the study: 33 control subjects with healthy eyes and 29 diabetic patients without DR. One eye of control subjects and of patients with diabetes with NDR was included in the study [47]. Macular blood flow parameters were obtained by using AngioVue OCTA system (RTVue-XR Avanti) with an split-spectrum amplitude decorrelation angiography (SSADA) software algorithm [47]. Image quality was considered, and images were categorized in three groups: good (absence of artifacts), fair (cumulative presence of artifacts in less than 1/3 of the image), and poor (cumulative presence of artifacts in more than 1/3 of the image) [47]. The mean age was 65 ± 11.35 years in control group and 69 ± 9.01 years in NDR group. Mean duration of diabetes was 7.37 ± 5.96 years [47].
FAZ area in the SCP was 0.37 ± 0.11 mm2 in patients with diabetes and 0.31 ± 0.10 mm2 in control subjects (p = 0.02) [47].
The VD in the SCP was 44.35 ± 13.31% in patients with diabetes and 51.39 ± 13.05% in control subjects (p = 0.04) [47]. VD at SCP was significantly decreased, and FAZ area at SCP was significantly increased in patients with diabetes in comparison to control subjects [47]. VD in the DCP significantly decreased in patients with diabetes (31.03 ± 16.33%) in comparison to control subjects (41.53 ± 14.08%, p < 0.01) [47].
So, results from this study indicated that in the parafovea of patients with NDR vessel density in the SCP and DCP decreased, whereas FAZ area in the SCP increased when compared to control subjects [47].
Lee et al. [48] analysed 30 healthy eyes and 121 eyes of type 2 diabetic subjects (31 with NDR, 26 with mild NPDR, 31 with moderate to severe NPDR and 33 with PDR). The mean age was 57.4 ± 11.2 years, 58.5 ± 12.1 years, 63.7 ± 8.5 years, 62.1 ± 7.4 years and 59.5 ± 7.1 years in healthy, NDR, mild, moderate to severe NPDR and PDR, respectively. Mean duration of DM was 9.6 ± 5.4 years in no DR group, 15.8 ± 11.0 years in mild NPDR, 11.6 ± 8.8 years in moderate to severe NPDR group and 20.6 ± 14.6 years in PDR subjects [48]. Spectral-domain OCTA data were acquired with a Cirrus Angioplex (Carl Zeiss Meditec) and a 3 × 3 mm square image was cropped to make circular areas with 3 mm and 1.5 mm diameter centered on the fovea [48].
FAZ area in the SCP was 0.325 ± 0.084 mm2 in healthy eyes, 0.354 ± 0.137 mm2 in NDR eyes, 0.350 ± 0.114 mm2 in mild NPDR group, 0.431 ± 0.654 mm2 in moderate to severe NPDR group and 0.492 ± 0.207 mm2 in PDR eyes [48]. In the DCP, FAZ area was 0.797 ± 0.213 mm2, 0.822 ± 0.249 mm2, 0.902 ± 0.294 mm2, 1.174 ± 0.685 mm2, 1.239 ± 0.769 mm2 in healthy, NDR, mild NPDR, moderate to severe NPDR and PDR group, respectively [48]. FAZ area increased as DR progressed in both SCP and DCP [48]. However, a statistically significant change from healthy eyes was observed only in DCP of PDR (p = 0.029) [48].
VD ratio in the SCP with 3 mm area was 0.425 ± 0.028, 0.408 ± 0.039, 0.399 ± 0.043, 0.386 ± 0.043 and 0.355 ± 0.046 in healthy, NDR, mild, moderate to severe NPDR and PDR, respectively [48]. In the DCP VD ratio with 3 mm area was 0.272 ± 0.057 in healthy eyes, 0.267 ± 0.064 in NDR group, 0.240 ± 0.056 in mild NPDR, 0.225 ± 0.054 in moderate to severe NPDR and 0.193 ± 0.060 in PDR group [48]. VD decreased as DR progressed in both SCP and DCP [48]. The statistically significant decrease from healthy eyes emerged at moderate to severe NPDR (p = 0.002 in SCP, p = 0.019 in DCP) [48].
The study of Nesper et al. [49] studied 137 eyes of 86 type 1 and 2 diabetic patients with different stages of DR (45 eyes with NDR, 52 eyes with NPDR and 40 eyes with PDR) and 44 eyes of 26 healthy controls. The mean age (years) was 50 ± 18 in healthy controls, 57 ± 10 in diabetic patients without DR, 54 ± 12 in NPDR subjects and 49 ± 14 in PDR group [49]. Mean duration of DM (years) was 11 ± 15 in NDR group, 17 ± 12 in NPDR and 19 ± 10 in PDR group [49]. Participants were imaged with RTVue-XR Avanti OCTA device with SSADA software and they obtained 3 × 3mm2 scans centered on the fovea [49]. The authors excluded images with significant artifactual components, such as enhanced decorrelation signal from excessive eye motion, or hyporeflectivity due to blockage of OCT signal from media opacity [49].
FAZ area in the SCP was 0.269 ± 0.086 mm2 in healthy controls, 0.309 ± 0.140 mm2 in NDR, 0.356 ± 0.207 mm2 in NPDR and 0.493 ± 0.238 mm2 in PDR group (p < 0.01) [49].
Parafoveal VD in the SCP was 53.59 ± 3.18%, 53.02 ± 3.11%, 46.59 ± 4.88%, 43.43 ± 3.83% in healthy controls, NDR, NPDR, PDR group, respectively (p < 0.01) [49]. In DCP, VD (%) was 60.82 ± 2.44 in healthy control eyes, 59.14 ± 2.80 in NDR group, 53.76 ± 4.63 in NPDR, 49.73 ± 3.66 in PDR group (p < 0.01) [49]. The FAZ area measured in the SCP increased significantly with DR severity [49]. Retinal parafoveal VD measured in the SCP and DCP decreased significantly with DR severity [49].
Simonett et al. [50] investigated 28 type 1 diabetic patients (9 eyes without clinical signs of DR and 19 had mild NPDR) and 23 healthy control subjects. There was no statistical difference in age (42.3 ± 8.6 years versus 39.6 ± 10.1 years, p = 0.33) between the diabetic patients and healthy controls [50]. Type 1 diabetic subjects had a mean disease duration of 21.3 ± 10.6 years and all patients were insulin dependent [50]. Images were obtained using the AngioVue OCTA software of the commercially available RTVue XR spectral domain OCT device. A SSADA algorithm, was used to detect erythrocyte movement and depict blood flow in a 3 × 3 mm scanning area centred on the fovea. Low-quality OCTA images, defined as signal strength index below 50 or presence of motion artefacts, were excluded from the study [50].
There was no significant difference in FAZ area in the DCP (0.40 ± 0.15 mm2 versus 0.38 ± 0.15 mm2, p = 0.510) or in the SCP (0.26 ± 0.12 mm2 versus 0.26 ± 0.11 mm2, p = 0.821) between type 1 diabetic eyes and controls [50].
Parafoveal VD in the DCP was significantly reduced in the type 1 diabetic patients compared to healthy controls (57.0 ± 3.3% versus 60.7 ± 2.4%, p < 0.001) [50]. There was no significant difference in parafoveal VD in the SCP between the two cohorts (49.8 ± 4.2% versus 51.5 ± 4.0%, p = 0.143) [50]. In the DCP, there was no significant difference in parafoveal VD between diabetic patients with no DR and diabetic patients with mild NPDR (58.4 ± 2.9% versus 56.3 ± 3.3%, p = 0.10) [50]. When comparing these subgroups to the control cohort, diabetic patients with mild NPDR had a significant reduction in DCP parafoveal VD (56.3 ± 3.3% versus 60.7 ± 2.4%, p < 0.001), while only a trend in the same direction was seen in diabetic patients with no DR group (58.4 ± 2.9% versus 60.7 ± 2.4%, p = 0.052) [50].
Compared to controls, this cohort had a decreased parafoveal VD in the DCP, while no difference was found in the SCP [50]. As this type 1 diabetic patients was limited to eyes with no or mild signs of diabetic retinopathy, these findings suggest that a decrease in parafoveal capillary density is an early process in the disease and occurs initially at the level of the DCP [50]. Additionally, as there was no difference in FAZ area between cohorts, the authors speculated that the decrease in parafoveal VD is most likely a result of diffuse capillary loss or nonperfusion, rather than FAZ enlargement or remodelling [50].
Alam et al. [51] performed a retrospective study of 20 control subjects (40 eyes) and 60 patients (120 eyes) with type 2 DM who underwent OCTA images of both eyes to quantify OCTA features for different DR stages. The patients were classified by severity of DR (40 eyes with mild, 40 moderate and 40 severe) according to the Early Treatment Diabetic Retinopathy Study (ETDRS) staging system [51]. OCTA images with a 6 mm × 6 mm field of view were used for extracting all vascular and foveal features of both control and NPDR eyes. Data were acquired using an AngioVue spectral domain OCTA system. To account for light and contrast image variation, the authors performed multiple preprocessing steps for image standardization before feature extraction and classification [51]. Mean age was 42 ± 9.8 years in control group, 50.1 ± 12.61 years, 50.8 ± 8.39 years and 57.84 ± 10.37 years in mild, moderate and severe NPDR, respectively [51]. Mean duration of DM was 19.64 ± 13.27 years in mild group, 16.13 ± 10.58 years in moderate group and 23.40 ± 11.95 years in severe group [51].
In SCP the FAZ area was 0.29 ± 0.16 mm2 in control group, 0.31 ± 0.17 mm2, 0.36 ± 0.18 mm2 and 0.40 ± 0.21 mm2 in mild, moderate and severe NPDR groups respectively (p = 0.006) [51]. According the authors in DCP the FAZ area was 0.46 ± 0.17 mm2 in control group, 0.47 ± 0.15 mm2 in mild NPDR, 0.52 ± 0.11 mm2 in moderate NPDR and 0.58 ± 0.26 mm2 in severe NPDR patients (p = 0.006) [51].
Regarding to parafoveal VD, in SCP was 48.16 ± 3.32% in control group, 45.62 ± 2.11%, 40 ± 3.52% and 36.84 ± 2.45% in mild, moderate and severe group, respectively [51]. In DCP, the parafoveal VD in control group was 49.72 ± 3.18%, in mild NPDR patients was 45.98 ± 3.09%, in moderate NPDR was 41.78 ± 2.17% and in severe NPDR patients was 37.46 ± 3.16% (p = 0.006) [51].
A total of 71 eyes of type 2 diabetic patients and 67 eyes of healthy control subjects were included in the study of Cao et al. [52]. All subjects underwent OCTA examination with AngioVue OCTA system (RTVue-XR Avanti) and the 6 × 6 mm scan was performed [52]. A SSADA software algorithm was used for evaluation of VD and FAZ area [52]. The mean age of type 2 diabetic patients with NDP was 57.4 ± 13.5 years and 53.7 ± 9.4 years in normal controls. The mean duration of DM was 6.6 ± 1.9 years [52].
FAZ area in diabetic patients was 0.32 ± 0.18mm2 and in normal controls was 0.35 ± 0.09mm2 (p = 0.253) [52].
The average parafoveal VD in SCP was 55.72 ± 2.43% in normal controls and 51.34 ± 4.09% in type 2 diabetic patients with no DR (p < 0.001) [52]. In DCP, average VD was 62.10 ± 2.11% in normal controls and 57.66 ± 5.73% in type 2 diabetic patients with no DR, respectively (p < 0.001) [52]. Relatively to choriocapillaris the average of parafoveal VD in normal controls was 66.97 ± 1.23% and in diabetic individuals with no DR was 66.36 ± 1.29% (p = 0.006) [52].
So, parafoveal VD in both SCP and DCP decreased in the eyes with NDR compared to normal controls (p < 0.001) [52]. Type 2 diabetic patients with NDR also had a significant reduction in average VD of SCP, DCP and choriocapillaris (p < 0.001, p < 0.001 and p = 0.006, respectively) [52]. There was no significant difference in FAZ area in the SCP between type 2 diabetic eyes and healthy controls (p = 0.253) [52].
The study of Ciloglu et al. [53] included 49 type 2 diabetic patients with NPDR and 45 persons with healthy eyes as the control group. The right eyes of the control subjects and of type 2 DM patients with NPDR were included in the study [53]. The mean age was 56.61 ± 1.33 years in the NPDR group and 54.09 ± 1.49 years in the control group. DM subjects had a mean disease duration of 13.65 ± 0.66 years [53]. OCTA Optovue RTVue XR Avanti was used for macular retinal vascularization assessment [53]. The image was set at scale 3 × 3 mm in the software parameters [53].
FAZ area in the SCP was 0.438 ± 0.05 mm2 in the NPDR group, and 0.246 ± 0.022 mm2 in the control group (p < 0.001) [53]. FAZ area in the DCP was 0.732 ± 0.06 mm2 in the NPDR group, and 0.342 ± 0.022 mm2 in the control group (p < 0.001) [53].
Parafoveal VD in the SCP was 45.43 ± 0.56% in NPDR group and 52.17 ± 0.58% in control group [53]. In the DCP, parafoveal VD was 52.82 ± 0.85% and 60.68 ± 0.90% in NPDR and control group, respectively (p < 0.001)[53].
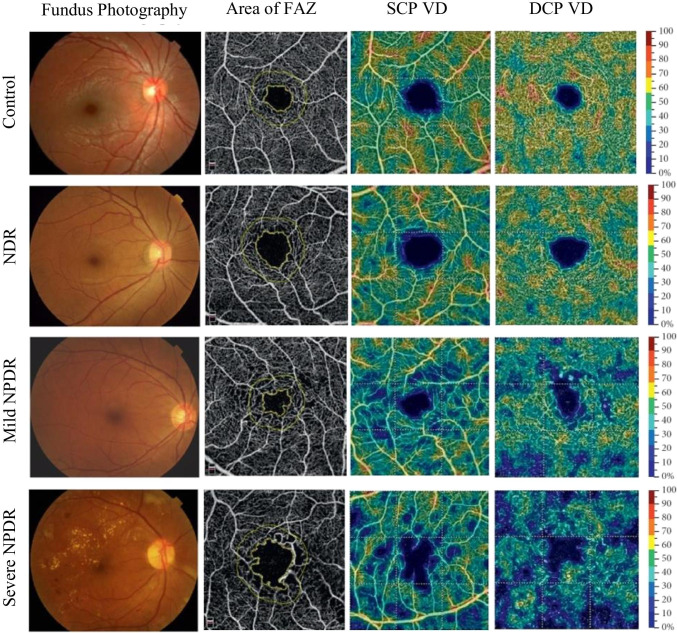
Parafoveal VD in the SCP and DCP decreased and parafoveal FAZ area in the SCP and DCP increased in NPDR group when compared to the control subjects [53] Fig. 2.
Fig. 2.
Representative samples of fundus photography and OCTA images of eyes with different stages of DR. The first column illustrates fundus photographs increasing severity of DR from top to bottom. The second column shows the area of foveal avascular zone (FAZ) increasing from top to bottom. The third column reveals the parafoveal vessel density (VD) in the superficial capillary plexus (SCP) decreasing from top to bottom and the fourth column exhibits the parafoveal vessel density in the deep capillary plexus (DCP) decreasing from top to bottom, using color maps
Discussion
OCTA provides high resolution images and depth resolved information in a non-invasive way and allows the evaluation of microvascular changes in the superficial, deep and choriocapillaris plexuses individually which is essential to improve our knowledge about DR [35, 51, 52].
The studies illustrated the importance of quantitative analysis alongside qualitative observation in DR staging because we observed a statistically significant enlargement of the FAZ area and a lower VD of the capillary network in both superficial and deep capillary plexuses in diabetic patients with clinically signs of DR, as well as diabetic patients without DR, compared with healthy controls [44, 45, 47–49, 51–53].
Glycaemic control, duration of diabetes and age may influence the area of FAZ and VD and may be the cause of different results between the studies. In the study of Cao et al. [52] the authors found that the VD of SCP and DCP was not significantly associated with duration of diabetes. The group of type 2 diabetic patients with NDR had decreased parafoveal VD in superficial and deep capillary plexus as well as in choriocapillaris in comparison with the control group, indicating that both retinal and choroidal circulation may be affected before clinical manifestation of DR [52].
Carnevali et al. [46] demonstrated a significant decreased VD in the DCP, in patients with type 1 diabetes without DR compared to healthy subjects. As in the study of Cao et al. [52] no correlation between duration of diabetes and VD of DCP was disclosed but the authors believed that this lack of association was due to the small standard deviation of the duration of the disease, to the homogeneity of the sample and to the relative small sample size [46]. Moreover, the authors hypothesized that an alteration of the VD in parafoveal capillaries is a sign that precedes the enlargement and remodeling of FAZ because there were no significant differences in the analysis of the FAZ area between the two study groups [46].
Ciloglu et al. [53] after further subdivision of the DR group based on the duration of diabetes observed no difference at FAZ area, VD in the SCP and DCP between the groups. Although the two subgroups had different durations of diabetes, subjects from the two groups had similar baseline clinical findings like NPDR at the beginning [53].
In the study of Dimitrova et al. [47] beyond the decrease of VD in SCP and DCP, the choriocapillaris VD (measurement data not shown) tended to decrease too in patients with NDR in comparison to control subjects [47]. As in the study of Cao et al. [52], these findings may indicate that both retinal and choroidal circulation are affected before clinical manifestation of DR and, the pathophysiologic process in both vascular systems may be interrelated [47]. One more time, duration of diabetes was not associated significantly with any of the OCTA parameters in diabetic patients’ parafovea [47]. Age was significantly correlated with superficial VD in control subjects and with deep VD in both study groups [47]. On the other hand, Bhanushali et al. [45] were not able to demonstrate a correlation between age and VD and area of FAZ.
As in the study of Simonett et al. [50] some other authors think that microvascular changes related to DR progression occur at DCP earlier than SCP [35]. Previous histologic studies also indicated that the DCP is more vulnerable to injury and preferentially affected. A growing body of evidence supports the differential involvement of distinct retinal capillary layers in diabetic eyes [35].
It is worth to mention the importance of other metrics in other plexuses (in addition to FAZ area and parafoveal VD) and to establish their relationship with DR, its severity and as potential biomarkers for early detection of the pathology. In addition to the parameters studied, other parameters must be considered in the future, such as vessel tortuosity and fractal dimension; vessel perfusion density; vessel spacing/inter-capillary area; vascular length density or skeleton density; vessel diameter index; total length of vessels (vessel length fraction) [28].
OCTA seems to be a very useful technology in evaluating microvascular changes in healthy as well as diseased eyes. However, a lot of improvement, understanding and linking with structural findings (e.g. ellipsoid zone, myoid zone, disorganization of retinal inner layers and retinal thickness) [36] but also with expression levels of genes and proteins and different DR phenotypes [54], is still needed to correctly interpret the data and its clinical significance. It is necessary to correlate these microvascular changes with multiple sources of information using algorithms that include risk factors of DM (e.g. metabolic control, HbA1c, DM duration and age) and biomarkers for the prediction and detection of patients with higher risk of developing DR and sight threatening complications.
If FAZ alterations and VD are truly early markers of DR, this could have implications for disease classification in clinical practice and clinical trials as most clinicians and research investigators currently use the ETDRS grading system or the International Clinical DR and Diabetic Macular Edema Severity Scales, which use microaneurysms as the first clinical sign of retinopathy.
Recently, an international panel of experts elaborated and proposed a Spectral Domain OCT (SD-OCT) based classification, centered on standard figures, which considers specific morphologic features and quantitative indices of the entire spectrum of macular involvement in DR [36]. So, if was needed almost twenty years to create this new classification based on SD- OCT, similarly, OCTA may have a long way ahead.
Further studies with long-term follow-up are needed to confirm these findings and a direct comparison with CFP is essential to determine the value of OCTA as a screening tool. This also includes work to optimize the risk-prediction algorithm to determine how it may influence clinical decision making.
Current treatment strategies for DR include intravitreal pharmacologic agents, laser photocoagulation and vitreous surgery. Intravitreal administration of anti-VEGF agents is currently the mainstay of therapy for both early and advanced stages of DR [5]. While the conventional laser therapy only provides stabilization of visual acuity, anti-VEGF therapy can result in visual improvement with less ocular adverse effects [5].
One endogenous inhibitor of angiogenesis is endostatin [55, 56]. Endostatin is a carbon terminal protein fragment obtained after cleavage from the carbon terminus of collagen XVIII (coll XVIII). Coll XVIII is an integral proteoglycan in endothelial and epithelial basement membranes [55]. In previously published articles, endostatin has been demonstrated to inhibit retinal neovascularization [57] by downregulation of Vascular Endothelial Growth Factor (VEGF) [58], it blocks MAPK activation [59] in endothelial cells and inhibit vasopermeability by stabilizing endothelial cell junctions [55, 57].
Limitations of OCTA
A qualitative assessment of the OCTA images is almost always possible and can provide the clinician with valuable insights into the morphology and perfusion status of the choroid and retina. Despite the advantages, imaging with OCTA can only provide a limited view of the peripheral retina and is unable to demonstrate leakage, staining, or pooling. OCTA requires patients to maintain good fixation to obtain high-resolution images which can be a challenge for those with severe macular disease [60, 61].
As in any other imaging technology of the retina and choroid, various artifacts appear in OCTA images with diferent frequencies and can have multiple causes ranging from technical to clinical factors. Artifacts in OCTA may be caused due to data processing algorithms, the method of data acquisition, intrinsic properties of the eye, pathological alterations, and insufficient cooperation of the patient during image capture [60, 61].
OCTA projection artifacts also occur from superficial retinal vessels which can appear in deeper retinal layers, or retinal and choroidal vessels which can even appear deep in the sclera.
When the segmentation algorithms fail from any cause, layers of vessels are visualized together in ways that do not reflect actual anatomy. The segmentation lines should therefore be checked for plausibility in the corresponding B-scan and, if necessary, adjusted to ensure correct clinical evaluation [60, 61].
Masking artifacts are caused by dense media that may lead to signal loss in underlying layers and impede their visualization. Vitreous hemorrhages or vitreous floaters can cause masking artifacts in the upper retinal layers, while subretinal hemorrhages can affect the visualization of vessels in the choriocapillaris and choroid. Also pronounced edema and even highly reflective layers or fibroses can make the visualization of the underlying layers more difficult due to masking effects [60, 61].
Motion artifacts are seen as very thin white horizontal lines resulting in an illusive interruption or displacement of the vessels and they are a result of eye movements. Even small movements of the patient in general, or the eye in particular, can produce dramatic changes from one B-scan to the next. To reduce the effects of motion several strategies can be employed, depending on what type of motion is present. Motion of the retina and choroid can occur in the axial direction can result from pulsations related to the cardiac cycle, breathing, tremors, and microsaccades [60, 61].
In order to maximize the benefit from OCTA images, operator interaction is required to reduce the frequency of artifacts. A good knowledge of possible artifacts and a critical analysis of the complete OCTA dataset are essential for correct clinical interpretation and precise clinical diagnosis [60, 61].
Study strengths and limitations
Several studies used different OCTA devices and this could be the reason why we found some discrepancies in the available results.
OCTA devices use different algorithms for image acquisition and processing and different methods for layer segmentation.
The study design in the current literature (cross-sectional), the limited field of view and movement artifacts resulting in image quality degradation are the main limitations. Most of the studies only include information of eyes with good-quality images, which may have introduced selection bias and limited the generalizability of results.
As we included 10 articles with relative small samples sizes, we can’t do a general conclusion because it involves limited data available.
Conclusion
The high prevalence of DR and the negative impact it can have on visual function make this pathology of major importance for ophthalmology and for society, so it is essential to invest both in the prevention and diagnosis, as well as in the treatment and monitoring of the disease.
In recent years, ophthalmology has experienced significant developments in imaging modalities. OCTA is one such technology that seeks to improve diagnostics for retinal diseases as DR.
OCTA has the unique ability to visualize, quantify, and distinguish functional and structural changes in all retinal and choroidal layers.
Patients with DM have a significantly enlarged FAZ area and decreased parafoveal VD compared to healthy controls, as well as an association between these microvascular changes and worsening DR.
Even patients with DM without clinical DR have a significantly enlarged FAZ and decreased VD compared to healthy controls.
In conclusion, this systematic review suggests that OCTA is an emerging and promising technology that has the potential to reveal valuable information about early and subtle microvascular changes of parafoveal capillary plexuses in patients with diabetes and can identify preclinical DR even before the manifestation of clinically apparent retinopathy.
Nevertheless, these results should be explored in comparison to the structural findings of the classic SD-OCT.
The non-invasive nature of OCTA allows routine imaging of the retinal vasculature, so this technology may be a promising tool for screening programmes of DR.
Abbreviations
- AGEs
Advanced Glycation End products
- CC
Choriocapillaris
- CD
Cannot Determine
- CFP
Colour Fundus Photography
- DCP
Deep Capillary Plexus
- DME
Diabetic Macular Edema
- DR
Diabetic Retinopathy
- FA
Fluorescein Angiography
- FAZ
Foveal Avascular Zone
- FAZ—A
Foveal Avascular Zone Area
- IRMA
Intraretinal Microvascular Abnormalities
- MAPK
Mitogen-activated protein kinase
- MCP
Middle Capillary Plexus
- NA
Not Applicable
- NDR
No Diabetic Retinopathy
- NIH
National Institutes of Health
- NPDR
Nonproliferative Diabetic Retinopathy
- NR
Not Reported
- OCT
Optical Coherence Tomography
- OCTA
Optical Coherence Tomography Angiography
- PDGF
Platelet derived growth factor
- PDR
Proliferative Diabetic Retinopathy
- PICO
Population, Intervention, Comparison, Outcomes
- PKC
Protein kinase C
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- SSADA
Split-Spectrum Amplitude Decorrelation Angiography
- SD
Standard Deviation
- SCP
Superficial Capillary Plexus
- VEGF
Vascular Endothelial Growth Factor
- VD
Vessel Density
- WHO
World Health Organization
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. GLOBAL REPORT ON DIABETES. 2016.
- 2.World Health Organization. CLASSIFICATION OF DIABETES MELLITUS. 2019.
- 3.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Wong TY, Cheung CMG, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Prim [Internet]. 2016;2:17. Available from: http://www.nature.com/articles/nrdp201612 [DOI] [PubMed]
- 5.Wang W, C.Y.LO A. Diabetic retinopathy: Pathophysiology and treatments. Int J Mol Sci. 2018;19(6). [DOI] [PMC free article] [PubMed]
- 6.Geraldes P, Hiraoka-yamamoto J, Matsumoto M, Clermont A. Activation of PKCδ and SHP1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2012;15(11):1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonetti D, Klein R, Gardner TW. Mechanisms of disease diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 8.UKPDS Group. Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53. [PubMed]
- 9.UKPDS Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–13. [PMC free article] [PubMed]
- 10.Varma R, Torres M, Pena F, Klein R, Azen SP. Prevalence of diabetic retinopathy in Adult Latinos The Los Angeles Latino Eye Study. Ophthalmology. 2004;111(7):1298–1306. doi: 10.1016/j.ophtha.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Klein BEK, Moss SE, Klein R, Surawicz TS. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XIII. Relationship of Serum Cholesterol to Retinopathy and Hard Exudate. Ophthalmology [Internet]. 1989;98(8):1261–5. Available from: 10.1016/S0161-6420(91)32145-6 [DOI] [PubMed]
- 12.Klein R, Klein BEK, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII The 14-year Incidence and Progression of Diabetic Retinopathy and Associated Risk Factors in Type 1 Diabetes. Ophthalmology. 1998;105(10):1801–1815. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Klein BEK, Moss SE, Davis MD, Demets DL. The Wisconsin epidemiologic of diabetic retinopathy study III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527–32. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BEK, Moss SE, Davis MD, Demets DL. The Wisconsin epidemiologic study of diabetic retinopathy II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520–6. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 15.Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, et al. UKPDS 50: Risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–163. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 16.DCCT Research Group Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1998;116(7):874–886. doi: 10.1001/archopht.116.7.874. [DOI] [PubMed] [Google Scholar]
- 17.ACCORD Study Group Effects of Intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martins T. Diabetic retinopathy: a neuropathy. Einstein. 2021;19:eED6110. [DOI] [PMC free article] [PubMed]
- 19.Altmann C, Schmidt MHH. The role of microglia in diabetic retinopathy: Inflammation, microvasculature defects and neurodegeneration. Int J Mol Sci. 2018;19(1). [DOI] [PMC free article] [PubMed]
- 20.Smith SB. Diabetic retinopathy and the NMDA receptor. Drug News Perspect. 2002;15(4):226–232. doi: 10.1358/dnp.2002.15.4.840055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong D, Lloyd A, Garden TW, King GL, Blankenship G, Cavallerano JD, et al. Diabetic Retinopathy. Diabetes Care. 2003;26(1). [DOI] [PubMed]
- 22.Eleonora FB, Adriano C, Pierro CL. Optical coherence tomography angiography of diabetic retinopathy. Dev Ophthalmol. 2016;56:107–112. doi: 10.1159/000442801. [DOI] [PubMed] [Google Scholar]
- 23.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet [Internet]. 2010;376(9735):124–36. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0140673609621243 [DOI] [PubMed]
- 24.Hendrick, Andrew M., Gibson, Maria V., Kulshreshtha A. Diabetic retinopathy. Prim Care Clin Off Pr. 2015;42(8):451–64. [DOI] [PubMed]
- 25.Lechner J, Leary OEO, Stitt AW. The pathology associated with diabetic retinopathy. Vision Res [Internet]. 2017;139:7–14. Available from: 10.1016/j.visres.2017.04.003 [DOI] [PubMed]
- 26.Praidou A, Androudi S, Brazitikos P, Karakiulakis G, Dimitrakos S. Angiogenic Growth Factors and their Inhibitors in Diabetic Retinopathy. Curr Diabetes Rev. 2010;304–12. [DOI] [PubMed]
- 27.Wu L. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4(6):290. doi: 10.4239/wjd.v4.i6.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tey KY, Teo K, Tan ACS, Devarajan K, Tan B, Tan J, et al. Optical coherence tomography angiography in diabetic retinopathy: a review of current applications. Eye Vis. 2019;6(37):1–10. doi: 10.1186/s40662-019-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen RB, Andrade Romo JS, Krawitz BD, Mo S, Fawzi AA, Linderman RE, et al. Earliest evidence of preclinical diabetic retinopathy revealed using optical coherence tomography angiography perfused capillary density. Am J Ophthalmol. 2019;203:103–115. doi: 10.1016/j.ajo.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khadamy J, Aghdam KA, Falavarjani KG. Review Article An Update on Optical Coherence Tomography Angiography in Diabetic Retinopathy. J Ophthalmic Vis Res. 2018;487–97. [DOI] [PMC free article] [PubMed]
- 31.Ishibazawa A, Nagaoka T, Takahashi A, Omae T, Tani T, Sogawa K, et al. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Stud. Am J Ophthalmol [Internet]. 2015; Available from: 10.1016/j.ajo.2015.04.021 [DOI] [PubMed]
- 32.Kim K, Kim ES, Kim DG, Yu SY. Progressive retinal neurodegeneration and microvascular change in diabetic retinopathy: longitudinal study using OCT angiography. Acta Diabetol [Internet]. 2019;56(12):1275–82. Available from: 10.1007/s00592-019-01395-6 [DOI] [PubMed]
- 33.Thompson IA, Durrani AK, Patel S. Optical coherence tomography angiography characteristics in diabetic patients without clinical diabetic retinopathy. Eye [Internet]. 2018;33(4):648–52. Available from: 10.1038/s41433-018-0286-x [DOI] [PMC free article] [PubMed]
- 34.Falavarjani KG, Sarraf D. Optical coherence tomography angiography of the retina and choroid; current applications and future directions. J Curr Ophthalmol. 2017;29:2–5. doi: 10.1016/j.joco.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Z, Tang F, Wong R, Lok J, Szeto SKH, Chan JCK, et al. OCT Angiography Metrics Predict Progression of Diabetic Retinopathy and Development of Diabetic Macular Edema: A Prospective Study. Ophthalmology [Internet]. 2019;126(12):1675–84. Available from: 10.1016/j.ophtha.2019.06.016 [DOI] [PubMed]
- 36.Panozzo G, Cicinelli MV, Augustin AJ, BattagliaParodi M, Cunha-Vaz J, Guarnaccia G, et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: The European School for Advanced Studies in Ophthalmology classification. Eur J Ophthalmol. 2020;30(1):8–18. doi: 10.1177/1120672119880394. [DOI] [PubMed] [Google Scholar]
- 37.Ashraf M, Nesper PL, Jampol LM, Yu F, Fawzi AA. Statistical model of optical coherence tomography angiography parameters that correlate with severity of diabetic retinopathy. Investig Ophthalmol Vis Sci. 2018;59(10):4292–4298. doi: 10.1167/iovs.18-24142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim K, Kim ES, Yu SY. Optical coherence tomography angiography analysis of foveal microvascular changes and inner retinal layer thinning in patients with diabetes. Br J Ophthalmol. 2018;102(9):1226–1231. doi: 10.1136/bjophthalmol-2017-311149. [DOI] [PubMed] [Google Scholar]
- 39.Nesper PL, Soetikno BT, Zhang HF, Fawzi AA. OCT angiography and visible-light OCT in diabetic retinopathy. Vision Res. 2017;1(139):191–203. doi: 10.1016/j.visres.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas J, Kneale D, McKenzie J, Brennan S, Bhaumik S. Determining the scope of the review and the questions it will address. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). In: Cochrane Handbook for Systematic Reviews of Interventions version 60 [Internet]. Available from: https://training.cochrane.org/handbook/current/chapter-02
- 41.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ WV. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 [Internet]. Available from: https://training.cochrane.org/handbook/current
- 42.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7). [DOI] [PMC free article] [PubMed]
- 43.National Heart Lung and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [Internet]. 2018. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 44.Al-Sheikh M, Akil H, Pfau M, Sadda SR. Swept-source OCT angiography imaging of the foveal avascular zone and macular capillary network density in diabetic retinopathy. Investig Ophthalmol Vis Sci. 2016;57(8):3907–3913. doi: 10.1167/iovs.16-19570. [DOI] [PubMed] [Google Scholar]
- 45.Bhanushali D, Anegondi N, Gadde SGK, Srinivasan P, Chidambara L, Yadav NK, et al. Linking Retinal Microvasculature Features With Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Investig Opthalmology Vis Sci [Internet]. 2016;57(9). Available from: http://iovs.arvojournals.org/article.aspx?doi=10.1167/iovs.15-18901 [DOI] [PubMed]
- 46.Carnevali A, Sacconi R, Corbelli E, Tomasso L, Querques L, Zerbini G, et al. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017;54(7):695–702. doi: 10.1007/s00592-017-0996-8. [DOI] [PubMed] [Google Scholar]
- 47.Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Investig Ophthalmol Vis Sci. 2017;58(1):190–196. doi: 10.1167/iovs.16-20531. [DOI] [PubMed] [Google Scholar]
- 48.Lee H, Lee M, Chung H, Kim HC. Quantification of Retinal Vessel Tortuosity in Diabetic Retinopathy Using Optical Coherence Tomography Angiography. J Retin Vitr Dis. 2017;0(0):1–10. [DOI] [PubMed]
- 49.Nesper PL, Roberts PK, Onishi AC, Chai H, Liu L, Jampol LM, et al. Quantifying microvascular abnormalities with increasing severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58(6):BIO307–15. [DOI] [PMC free article] [PubMed]
- 50.Simonett JM, Scarinci F, Picconi F, Giorno P, De Geronimo D, Di Renzo A, et al. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017;95(8):e751–e755. doi: 10.1111/aos.13404. [DOI] [PubMed] [Google Scholar]
- 51.Alam M, Zhang Y, Lim JI, Chan R, Yang Y, Yao X. Quantitative optical coherence features for objective classification and staging of diabetic retinopathy. J Retin Vitr Dis. 2018;00(00):1–11. doi: 10.1097/IAE.0000000000002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao D, Yang D, Huang Z, Zeng Y, Wang J, Hu Y, et al. Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic retinopathy. Acta Diabetol [Internet]. 2018;55(5):469–77. Available from: http://link.springer.com/10.1007/s00592-018-1115-1 [DOI] [PubMed]
- 53.Ciloglu E, Unal F, Sukgen EA, Koçluk Y. Evaluation of foveal avascular zone and capillary plexuses in diabetic patients by optical coherence tomography angiography. Korean J Ophthalmol. 2019;33(4):359. doi: 10.3341/kjo.2018.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marques IP, Madeira MH, Messias AL, Santos T, Martinho AC-V, Figueira J, et al. Retinopathy phenotypes in type 2 diabetes with different risks for macular edema and proliferative retinopathy. J Clin Med. 2020;9(5):1433. [DOI] [PMC free article] [PubMed]
- 55.Bhutto IA, Kim SY, Mcleod DS, Merges C, Fukai N, Olsen BR, et al. Localization of Collagen XVIII and the Endostatin Portion of Collagen XVIII in aged human control eyes and eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(5):1544–1552. doi: 10.1167/iovs.03-0862. [DOI] [PubMed] [Google Scholar]
- 56.Noma H, Funatsu H, Yamashita H, Kitano S, Mishima H, Hori S. Regulation of Angiogenesis in Diabetic Retinopathy Possible Balance Between Vascular Endothelial Growth Factor and Endostatin. Arch Ophthalmol. 2002;120. [DOI] [PubMed]
- 57.Bai YJ, Huang LZ, Zhou AY, Zhao M, Yu WZ, Li XX. Antiangiogenesis effects of endostatin in retinal neovascularization. J Ocul Pharmacol Ther. 2013;29(7):619–626. doi: 10.1089/jop.2012.0225. [DOI] [PubMed] [Google Scholar]
- 58.Behl T, Kotwani A. Possible role of endostatin in the antiangiogenic therapy of diabetic retinopathy. Life Sci [Internet]. 2015;135:131–7. Available from: 10.1016/j.lfs.2015.06.017 [DOI] [PubMed]
- 59.Kim Y, Hwang S, Kim Y, Pyun B, Kim T, Lee S, et al. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR / Flk-1. J Biol Chem. 2002;277(31):27872–27879. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- 60.Spaide R, Fujimoto J, Waheed N. Image artifacts in optical coherence angiography. Retina. 2015;35(11):2163–2180. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enders C, Lang GE, Dreyhaupt J, Loidl M, Lang GK, Werner JU. Quantity and quality of image artifacts in optical coherence tomography angiography. PLoS ONE. 2019;14(1):1–9. doi: 10.1371/journal.pone.0210505. [DOI] [PMC free article] [PubMed] [Google Scholar]