Abstract
Abstract
In recent years, zebrafish have been proposed as a model for rapid analysis of gene function and biological activity due to high genetic similarities with humans. The aim of this study was to determine the effects of overfeeding-induced diabetes w/o glucose on inflammatory cytokine as well as insulin and glucose transporter-2 genes (GLUT2) genes expression in the pancreas in zebrafish.
Materials and methods
The experiment was performed on 120 zebrafish (duplicated sample) with a specific genetic mapping (AB-Wild type). A total of 8 tanks, each containing 15 fish per 2-liter water, were used and divided into four groups: (1) Control group, (2) regular diet with glucose,3) Only Artemia overfeeding and 4) Combined Artemia with glucose. We induced T2DM zebrafish using glucose monohydrate solution in water and repeated daily Artemia feeding. In this model, fasting blood glucose increase is preceded by obesity and glucose intolerance. The experiment lasted for two months. Blood glucose and fish biometrics were measured in two steps. The expression of TNFα, IFNγ, GLUT2 and Insulin genes were quantified by a Real-Time qPCR System (Applied Biosystems, USA) using a set of specific primers.
Results
The highest mortality rate was observed in combined Artemia and glucose (p < 0.05). We showed significantly higher expression of IL-1B and TNF-α as well as inhibitory cytokines such as IFNγ genes in overfeeding induced diabetes(OID) which was highest in the combined Artemia and glucose group.(p < 0.05)The GLUT2 gene expression was higher in the pure artemia group which decreased to a lower level by adding glucose to Artemia in the diet. (p < 0.05). Also, the lowest insulin gene expression was observed in the combined group (p < 0.05).
Conclusions
In zebrafish, diabetes induction with overfeeding and supraphysiological glucose in diet accompanied with higher expression of inflammatory cytokines genes in the pancreas as well as lower insulin and GLU2 genes. These epigenetic factors appeared to initiate pancreatic beta dysfunction alongside insulin resistance and could have a crucial role in the pathogenesis of overfeeding-induced diabetes using primitive animal models.
Keywords: Zebrafish, Obesity-induced diabetes, IL-1B gene, TNF-α, Insulin resistance
Introduction
Unhealthy lifestyle and genetic factors are known as common causes of obesity and predispose the human being to type 2 diabetes.[1–3] Zebrafish provides a suitable primitive animal model in metabolic diseases and is recognized as a cutting edge in diabetes research due to its 70 % genetic similarity to humans [4]. In order to tackle a higher life span with a more insidious pathologic process in humans, the zebrafish (Danio rerio) has been recognized as a promising model for research on diabetes and its complications. [4, 5]
Zang et al. developed a model of overfeeding-induced diabetes(OID) in zebrafishes overfed with Artemia [6]. In the present research, we modified the overfeeding method by adding glucose in the diet to determine the expression changes of insulin, GLUT2 and inflammatory cytokines genes expressions in pancreatic β-cells.
Materials and methods
Ethical statement
All experiments were conducted in accordance with the ethical guidelines of Endocrinology and Metabolism Research Institute (EMRI), Tehran University of Medical Sciences (TUMS), Tehran, Iran.
The ethical approval was obtained from the ethics committee of AJA university of medical science ( IR.AJAUMS.REC.1399.144).
Zebrafish (Danio rerio) maintenance
The AB (wild type) strain of adult zebrafish was used in the present research provided by the Zebrafish Core Facility, EMRI, (ZFIN ID: ZDB-LAB-190117-2).
The fish were acclimatized in designated incubators for ten days to temperature and light circulation of the zebrafish laboratory of EMRI before the breeding began. Specific incubators with water recirculation systems were used in order to keep the oxygenated water at 6.5 ± 0.5 mg/L oxygen, as well as the temperature of about 28 ± 1 °C, and hardness of 250 mg/L CaCO3.
Then, we exposed the fish to a 10:14 h light/dark cycle. Following the acclimatization, we transferred the males into the tank with the females at a 1:1 male to female ratio. Soon after mating, the newly fertilized eggs were placed in a tank with E3 water. The fish were then bred and kept in constant care according to standard protocol until becoming seven months old to initiate the experiment [4].
Experimental design and fish group definition
The 7-month-old adult fish were randomly selected and 15 fish per 2-liter water were used. All 8 fish tanks were maintained with a constant concentration of dissolved oxygen and pH ~ 7 and a temperature of 28 C°. Each group scenario was repeated as a duplicate. Specifically, the first experimental group (CTRL) was fed with commercial diet. In the second group, in addition to the commercial diet, 18 g (50 mmolar) glucose monohydrate was added to water. The third group was fed only Artemia (ART) six times per day, each time 2 mL. Eventually, the fourth group (Glu + ART) received 18 g glucose (50 mmolar) plus Artemia six times per day (2 mL). The fish underwent a feeding regime by the Gemma micro (500) diet. Tanks were kept at a constant concentration of dissolved oxygen using pipes to supply oxygen and kept constant at 28 C°. Fresh water was supplied every other day.
Preparing induced hyperglycemia zebrafish
In this research, hyperglycemia was induced in zebrafish as a model through enhancing the glucose concentration in the tank water. After five days of acclimation, a group of fish started to become glucose intolerant by adding glucose into the respective tank. At first, induction was initiated with 50 mM glucose concentration (Merk, Germany) in a total of 2 L of water. The fish were then kept at this glucose concentration for four days when the freshwater was supplied daily. On the fifth day, the glucose concentration was enhanced up to 100 mM, with the fish kept for another three days while constantly monitoring the situation. The fish adapted to the new glucose concentration, after which the glucose level was again elevated, where the glucose-induced fish were subject to a 200 mM glucose.
Zebrafish biometric analysis
For measuring the biometric parameters, three fish from each tank were randomly selected. To reduce the stress during fish transport, resting was allowed for 1 min, and then three drops of Eugenol in 50 ml of water were used to euthanize the fish. The length and weight of fin-clipped fish were measured and recorded for future gene analysis and then immediately prepared for blood glucose measurement.
The length was measured from the anterior-most point of the upper jaw to the posterior-most region of the caudal peduncle using digital calipers. Subsequently, weight measurement was done using a microbalance (Mettler MT5, Mettler Toledo, Spain).
Gene expression analysis and real-time PCR
The total RNA was extracted from the zebrafish tail using RNAX_plus solution from sinaclon following the manufacturer’s protocol. The concentration and integrity of the total RNA were determined using NanoDrop 1000 (Thermo Scientific). For cDNA synthesis, a 100ng of RNA was reverse-transcribed (Pars Toos Kit, Iran). The cDNA was synthesized based on the manufacture protocol.
Before real-time PCR, one microliter of cDNA was used as a template in PCR reactions containing specific PCR primers.
The expression of TNFα, IFNγ, GLUT2 and Insulin genes were quantified by a Real-Time qPCR System (Applied Biosystems, USA) using a set of specific primers listed in Table 1 and SYBR Green PCR Master Mix (Applied Biosystems). The qRT-PCR measurements were carried out three times using cDNA obtained from three independent experiments.
Table 1.
Primers used for Real-Time PCR experiment: tumor necrosis factor (TNFα), Interferon gamma (IFNγ), Interleukin-1β (IL-1β), Beta actin (bACT)
| Gene Name | forward primer (5–3) Seq ( 5 − 3) | Gene bank accession number |
|---|---|---|
| TNFα- F | GCTGGATCTTCAAAGTCGGGTGTA | AY427649 |
| TNFα_R | TGTGAGTCTCAGCACACTTCCATC | AY427649 |
| IFNγ _F | GAATGGCTTGGCCGATACAGGATA | AB126869 |
| IFNγ _R | TCCTCCACCTTTGACTTGTCCATC | AB126869 |
| IL-1β _F | CATTTGCAGGCCGTCACA | CU695198 |
| IL-1β _R | GGACATGCTGAAGCGCACTT | CU695198 |
| GLUT2-F | CTGGCTATTGTCATTGGCATCC | Q102R8 |
| GLUT2-R | TGTCCTTAGAGGTGTCATAATCTCCC | Q102R8 |
| Insulin-F | GGTCCTGTTGGTCGTGTC | O73727 |
| Insulin –R | GTTGTAGAAGAAGCCTGTTGG | O73727 |
| bACT_F | ACAGGGAAAAGATGACACAGATCA | AF057040 |
| bACT_R | CAGCCTGGATGGCAACGTA | AF057040 |
The relative value of each mRNA expression was then obtained by the threshold of β actin cycle values, with the values of each cycle presented by the standard curve method. Data analysis was conducted via version 2.0 of iQ5 optical software, including Genex Macro iQ5 Conversion and Genex Macro iQ5 files. The primer sequences used are listed in Table 1.
Blood glucose measurement and enzyme determinations
The samples collection was duplicated. Before the blood collection experiment, three fish were randomly collected from each tank and kept fast in normal RO water for 12 h. The temperature and dissolved oxygen remained the same as other experimental tanks. The fish were euthanized in the same procedures as mentioned above. The posterior tail was cut and a drop of blood was directly applied to strip on the glucometer, and then blood glucose was recorded. The fish were then immediately dissected for the intestine sample collection. The fish organs were separated and homogenized using a homogenizer and then placed into 0.15 M of NaCl in ice-filled boxes. The samples were then centrifuged in a microtube at 2500 rpm (4 °C) for 15 min.
Statistical analysis
We used SPSS (version 16) for statistical analysis and results obtained from all data were represented as Mean ± SEM (n = 3). Comparison between groups was conducted using ANOVA Test. A p-value ≤ 0.05 was statistically considered significant.
Results
Baseline characteristics of four intervention groups and two months follow-up were showed in Table 2. The highest rate of mortality was observed in combined Artemia with glucose diet, which was significantly higher compared with control, as demonstrated in Fig. 1 (p < 0.05).
Table 2.
Baseline characteristics of zebra fishes and changes during the intervention in four groups
| Variable | Day 7 | Day 60 | P | |
|---|---|---|---|---|
| Weight (gr) | CTRL | 0.31 | 0.39 | < 0.001 |
| Glu | 0.36 | 0.45 | 0.01 | |
| ART | 0.32 | 0.48 | < 0.001 | |
| Glu + ART | 0.33 | 0.60 | 0.01 | |
| Hight (cm) | CTRL | 3 | 3.2 | < 0.001 |
| Glu | 2.8 | 3 | < 0.001 | |
| ART | 2.8 | 2.9 | < 0.001 | |
| Glu + ART | 2.9 | 3 | < 0.001 | |
| Fasting blood sugar/ (mg/dl) | CTRL | 69 | 75 | < 0.001 |
| Glu | 100 | 125 | 0.01 | |
| ART | 110 | 137 | 0.01 | |
| Glu + ART | 141 | 168 | < 0.001 |
Control group(CTRL), Regular diet with glucose (glu), Only Artemia overfeeding(ART), Combined glucose and Artemia(GLU + ART)
Fig. 1.
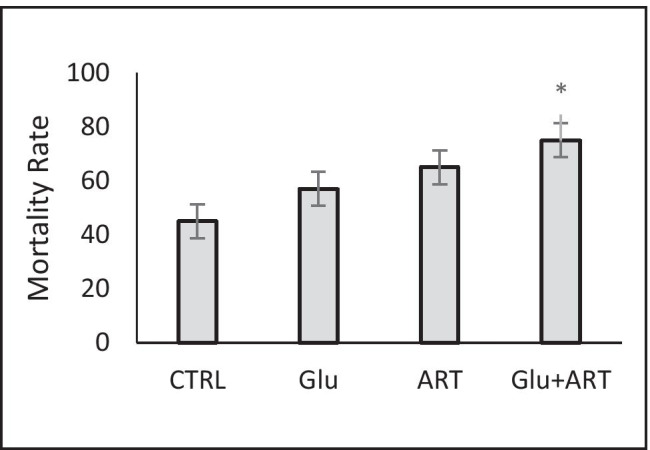
Comparison of Mortality rate among zebra fishes with four dietary protocols. Control group (CTRL), regular diet with glucose (glu), Only Artemia overfeeding( ART), Combined glucose and Artemia(GLU + ART).*p < 0.05
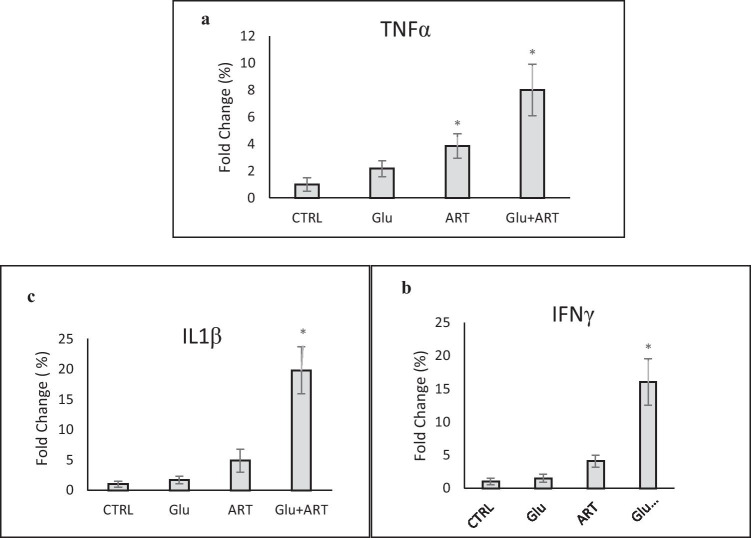
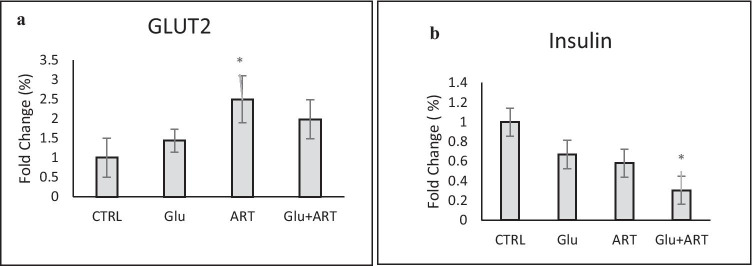
We showed significantly higher expression of IL-1B as a major pro-inflammatory cytokine and TNF-α as well as inhibitory cytokine such as IFγ genes in experimental groups compared with the control group, which was highest in combined Artemia and glucose group as presented in Fig. 2(a,b,c) (p < 0.05). We showed that the highest GLUT2 gene expression was observed in the Pure Artemia overfeeding group compared with other groups (p < 0.05between group with ANOVA test). In addition, the lowest insulin gene expression was determined in the combined Artemia and glucose diet group, as demonstrated in Fig. 3(a,b).
Fig. 2.
Comparison of changes in (a) Alpha TNF gene expression (b) interferon gamma gene (c) IL-1B gene expression among zebra fishes with four dietary protocols. Control group (CTRL), regular diet with glucose (glu), Only Artemia overfeeding ( ART), Combined glucose and Artemia(GLU + ART).*p < 0.05 between groups
Fig. 3.
Comparison of changes in (a) Glucose transporter 2(GLUT2) expression (b) Insulin gene expression among zebra fishes with four dietary protocols. Control group(CTRL), regular diet with glucose (glu), Only Artemia overfeeding( ART), Combined glucose and Artemia(GLU + ART)
Discussion
Type 2 diabetes is a chronic metabolic disease accompanies by insulin secretion defect and insulin resistance. The molecular mechanism of insulin resistance should be investigated carefully based on RNAs regulating gene expression. [7, 8] Chronic low-level inflammation may be a pivotal link among obesity, insulin resistance, and type 2 diabetes [9]. It is supported by studies showing associations between elevated inflammatory markers and insulin resistance in obese animals and humans [10, 11] and in vitro studies showing that the addition of inflammatory cytokines inhibits the insulin signaling cascade [12]. Obesity-induced insulin resistance is mediated through inflammatory factors, including TNF-α and IL-1β. [13, 14].
IL-1β as a master pro-inflammatory mediator induces the production of other cytokines and it is upregulated in pancreatic islets of patients with T2DM as well as overfeeding-induced diabetes[15, 16]. Our study results were consistent with the above findings in overfeeding induced diabetes especially in combined with a glucose diet. Also, we showed that the higher Interferon-γ gene expression was present in OID. Interferon gamma inhibits the specific response of the antigen by preventing the elevation of inflammatory responses generated by cytokines [8]. It appears that both devastating and protective inflammatory responses could be exaggerated in overfeeding-induced diabetes with higher glucose consumption in the diet.
At the early stages of type 2 diabetes, a compensation phase exists when β-cells respond by enhancing their mass and insulin production [17–19]. We showed increase in GLUT2 gene expression in early stage of OID followed by deteriation in insulin and GLUT genes expression with usage of glucose in diet as more metabolic stress.
Produced pro-inflammatory cytokines clearly enhance the number of apoptotic pancreatic β-cells [20, 21]. This metabolic disorder causes insulin resistance, impaired beta cell function and insulin production and Vice versa [21, 22]. Our study results showed that Insulin gene expression was impaired alongside high inflammatory cytokine genes expression in OID, especially in the combination of overfeeding with excessive glucose intake, which could be due to more beta cell dysfunction in response to the inflammatory milieu.
In diabetic rats, impaired GSIS (glucose stimulated insulin secretion) is associated with markedly reduced expression of GLUT2 [22, 23]. Although, GLUT2 with high Km for glucose gives glucose unrestricted access to glucokinase, the enzyme that catalyzes the rate-controlling step in GSIS, its role in transducing an intracellular glucose-dependent signal, independent from its glucose transport activity, cannot be excluded [24]. We demonstrated that the highest GLUT2 gene expression was present in Artemia over feeding group, but this gene expression was decreased by adding glucose to overfeeding diet. The glucolipotoxicity and higher metabolic stress could explain this detrimental effect of more glucose consumption in the diet on GLUT2 gene expression.
On the other hand, The concentrations out of the physiological glucose stimulatory range, a ‘‘GSIS range’’ are harmful, especially when exposed to β-cells for a prolonged time [25–28] Our study results were consistent with pieces of evidence shown that excessive glucose consumption with over feeding can exaggerate impairment in beta cell function and causes lower insulin production.
Finally, we observed the highest rate of mortality in the group overfed with Artemia plus glucose, which could be due to the presence of a higher inflammatory milieu and more severe major organ damage in this group.
Our study had some limitations, such as we could not directly measure insulin resistance as well as the oxidative stress process in zebrafish. Further researches are required to shed light on the pathogenesis of overfeeding-induced diabetes as well as histopathological changes in the liver and intestine by using the primitive animal as diabetes experimental models.
Conclusion
In zebrafish, diabetes induction with overfeeding and diet with supraphysiological glucose accompanied higher expression of inflammatory cytokines genes in the pancreas as well as lower insulin and GLU2 genes. These epigenetic factors appeared to initiate pancreatic beta dysfunction alongside insulin resistance and could have a crucial role in the pathogenesis of overfeeding-induced diabetes using primitive animal models.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hassan Mohammadi, Email: Babolmohamadi@gmail.com.
Radina Eshtiaghi, Email: r.eshtiaghi@gmail.com.
Sattar Gorgani, Email: Gorgani59@gmail.com.
Mohammadreza Khoramizade, Email: khorami@yahoo.com.
References
- 1.Casazza K, et al. Beyond thriftiness: independent and interactive effects of genetic and dietary factors on variations in fat deposition and distribution across populations. Am J Phys Anthropol. 2011;145(2):181–91. doi: 10.1002/ajpa.21483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalili L, et al. Probiotic assisted weight management as a main factor for glycemic control in patients with type 2 diabetes: a randomized controlled trial. Diabetol Metab Syndr. 2019;11:5. doi: 10.1186/s13098-019-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Pastors JG, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25(3):608–13. doi: 10.2337/diacare.25.3.608. [DOI] [PubMed] [Google Scholar]
- 4.Capiotti KM, et al. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp Biochem Physiol B: Biochem Mol Biol. 2014;171:58–65. doi: 10.1016/j.cbpb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Zang L, Shimada Y, Nishimura N. Development of a novel zebrafish model fortype 2 diabetes mellitus. Sci Rep. 2017;7(1):1–11. [DOI] [PMC free article] [PubMed]
- 6.Zang L, Shimada Y, Nishimura Y, Tanaka T,and N Nishimura. A novel reliable method for repeated blood collection from aquarium fish. Zebrafish. 2013;10(3):425–432. [DOI] [PubMed]
- 7.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance many choices on the menu. Genes Dev. 2007;21(12):1443–55. [DOI] [PubMed]
- 8.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 9.Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GD, O’Reilly DS, Packard CJ, Sattar N. West of Scotland Coronary Prevention Study. C-reactive protein is an independent predictor of risk for the. development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596–600. [DOI] [PubMed]
- 10.Tateya S, Tamori Y, Kawaguchi T, Kanda H, Kasuga M. An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of. adipose tissue inflammation in mice. Endocrinology. 2010;151:971–9. [DOI] [PubMed]
- 11.Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos Proietto SJ, Go¨ rgu¨ n CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4:465–74. [DOI] [PubMed]
- 12.Vandanmagsar B, et al. Te NLRP3 infammasome instigates obesity-induced infammation and insulin resistance. Nat Med. 2011;17:179-188. 10.1038/nm.2279. [DOI] [PMC free article] [PubMed]
- 13.Stienstra R, et al. Te infammasome-mediated caspase-1 activation controls adipocyte diferentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed]
- 14.Boni-Schnetzler M, et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93:4065–74. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maedler K, et al. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Investig. 2002;110:851–860. 10.1172/JCI15318. [DOI] [PMC free article] [PubMed]
- 16.Bensellam M, Jonas JC, Laybutt DR. Mechanisms of b-cell dedifferentiation in diabetes: recent findings and future research directions. J Endocrinol. 2018;236:R109–43. doi: 10.1530/JOE-17-0516. [DOI] [PubMed] [Google Scholar]
- 17.Swisa A, Glaser B, Dor Y. Metabolic stress and compromised identity of pancreatic beta cells. Front Genet. 2017;8:21. doi: 10.3389/fgene.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic b cell dedifferentiation as a mechanism of diabetic b cell failure. Cell. 2012;150:1223–34. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cnop M, Abdulkarim B, Bottu G, Cunha DA, IgoilloEsteve M, et al. RNA sequencing identifies dysregulation of the human pancreatic islet transcriptome by the saturated fatty acid palmitate. Diabetes. 2014;63:1978–93. doi: 10.2337/db13-1383. [DOI] [PubMed] [Google Scholar]
- 20.Jager J, Gre´meaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1b-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148:241–51. [DOI] [PMC free article] [PubMed]
- 21.Janikiewicz J, Hanzelka K, Kozinski K, Kolczynska K, Dobrzyn A. Islet b-cell failure in type 2 diabetes— within the network of toxic lipids. Biochem Biophys Res Commun. 2015;460:491–496. [DOI] [PubMed]
- 22.Orci L, Unger RH, Ravazzola M, et al. Reduced b-cell glucose transporter in new onset diabetic BB rats. J Clin Investig. 1990;86:1615–22. doi: 10.1172/JCI114883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohtsubo K, Takamatsu S, Gao C, Korekane H, Kurosawa TM, Taniguchi N. N-Glycosylation modulates the membrane sub-domain distribution and activity of glucose transporter 2 in pancreatic beta cells. Biochem Biophys Res Commun. 2013;434:346–51. doi: 10.1016/j.bbrc.2013.03.076. [DOI] [PubMed] [Google Scholar]
- 24.Ohtsubo K, Chen MZ, Olefsky JM, Marth JD. Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat Med. 2011;17:1067–75. doi: 10.1038/nm.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58:221–32. doi: 10.1007/s00125-014-3451-1. [DOI] [PubMed] [Google Scholar]
- 26.Sarre A, Gabrielli J, Vial G, Leverve XM, andAssimacopoulos-Jeannet F. Reactive oxygen species are produced at low glucose and contribute to the activation of AMPK in insulin-secreting cells. Free Radic Biol Med. 2012;52:142–50. doi: 10.1016/j.freeradbiomed.2011.10.437. [DOI] [PubMed] [Google Scholar]
- 27.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 28.Westwell-Roper CY, et al. IL-1 mediates amyloid-associated islet dysfunction and inflammation in human islet amyloid polypeptide transgenic mice. Diabetologia. 2015;58:575–585. [DOI] [PubMed]